Abstract
Since the recent publication of data showing favorable outcomes for patients with HIV-1 and ESRD, kidney transplantation has become a therapeutic option in this population. However, reports have documented unexplained reduced allograft survival in these patients. We hypothesized that the unrecognized infection of the transplanted kidney by HIV-1 can compromise long-term allograft function. Using electron microscopy and molecular biology, we examined protocol renal transplant biopsies from 19 recipients with HIV-1 who did not have detectable levels of plasma HIV-1 RNA at transplantation. We found that HIV-1 infected the kidney allograft in 68% of these patients. Notably, HIV-1 infection was detected in either podocytes predominately (38% of recipients) or tubular cells only (62% of recipients). Podocyte infection associated with podocyte apoptosis and loss of differentiation markers as well as a faster decline in allograft function compared with tubular cell infection. In allografts with tubular cell infection, epithelial cells of the proximal convoluted tubules frequently contained abnormal mitochondria, and both patients who developed features of subclinical acute cellular rejection had allografts with tubular cell infection. Finally, we provide a novel noninvasive test for determining HIV-1 infection of the kidney allograft by measuring HIV-1 DNA and RNA levels in patients’ urine. In conclusion, HIV-1 can infect kidney allografts after transplantation despite undetectable viremia, and this infection might influence graft outcome.
More than 30 years after the first description of AIDS, kidney disease remains an important contributor to morbidity and mortality among HIV-infected patients.1,2 The introduction of highly active antiretroviral therapy (HAART) 15 years ago has completely changed the prognosis of HIV-infected patients, significantly reducing mortality and increasing life expectancy.3 However, ESRD remains common in this population.2 Indeed, HIV represents the third most common cause of ESRD among African Americans aged <65 years, and approximately 900 HIV-infected patients per year start dialysis in the United States.2
The kidney lesions that develop during HIV infection are primarily due to a particularly aggressive form of FSGS called HIV-associated nephropathy (HIVAN). This disease is directly related to the infection of kidney cells by HIV.2,4 Until recently, long-term dialysis was the only treatment available for this group of patients and was associated with a poor prognosis. Since the recent publication of reports showing favorable outcomes, kidney transplantation has become a therapeutic option for these patients.5–7 Most centers have defined eligibility criteria for transplantation such as a CD4 count >200 cells/ml and an undetectable plasma level of HIV RNA.8 When using these criteria, the survival rate of HIV-infected transplant recipients is similar to that of non–HIV-infected transplant recipients, but intriguingly, the 3-year allograft survival rate is reduced among the HIV-infected transplant recipients.5 The mechanisms responsible for this reduced allograft survival rate are unknown, and at least two explanations are usually suggested.5 The first explanation is the poorly understood high rate of acute cellular rejection observed early after transplantation. The second explanation is the greater exposure to calcineurin inhibitor blood levels due to pharmacokinetic interactions with some antiretroviral drugs. Nevertheless, these explanations do not completely explain the observations, and very little attention has been paid to the potential role of infection of the kidney allograft by HIV, despite the fact that the kidney is a well recognized reservoir for HIV-1 in patients receiving efficient antiretroviral therapy.9 In 2006, we started to transplant kidneys into HIV-positive patients and observed that few patients had early unexplained allograft dysfunction. We then hypothesized that the unrecognized reinfection of the transplanted kidney by HIV-1 can compromise allograft function.
Using protocol biopsies, electron microscopy, and molecular biology assays, we demonstrate that although plasma HIV-1 RNA is undetectable, HIV-1 infects kidney allografts either asymptomatically or with clinical manifestations, thereby affecting the allograft prognosis. In addition, we developed a new and simple urinary assay to detect the HIV-1 infection in kidneys.
Results
HIV-1 Reinfects Kidney Allografts
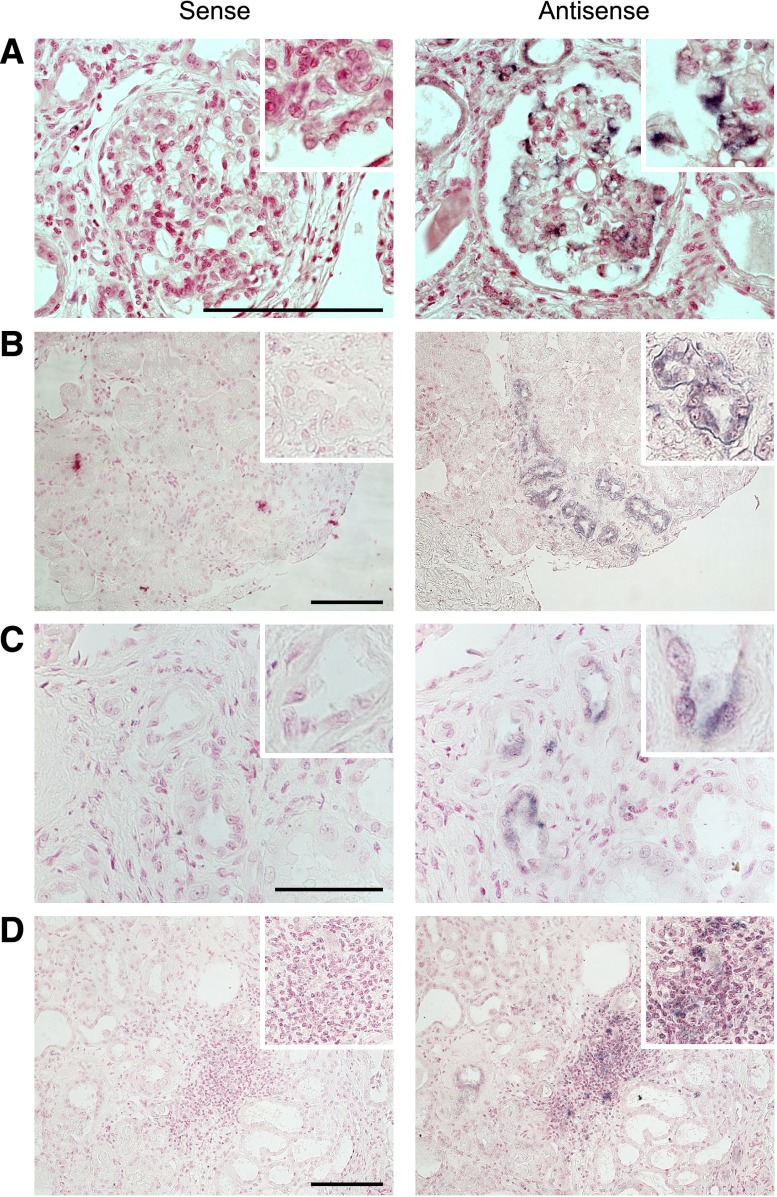
During the study period, 939 kidney transplantations were performed in our center. Nineteen of the transplants went to HIV-1–infected patients. We first investigated whether HIV-1 could infect the allograft with undetectable plasma HIV-1 RNA. We performed PCR for HIV-1 DNA in protocol biopsies after transplantation. Surprisingly, we observed that 68% of HIV-1 transplant recipients had detectable HIV-1 DNA in biopsies performed at 3 months after transplantation. Interestingly, HIV-1 DNA remained persistent in biopsies performed at 12 months after transplantation. To determine whether the presence of HIV-1 DNA in the allograft biopsies was caused by infiltrating inflammatory cells or by a true infection of the kidney cells, in situ hybridization (ISH) for HIV-1 RNA was performed in the kidney biopsies. ISH revealed that HIV-1 RNA was detectable in all biopsies. Importantly, we could determine two different forms of allograft infection by HIV-1. In the first group of patients (38% of HIV-1 transplant recipients, n=5), HIV-1 RNA was detectable in the glomeruli and only in very few tubular cells of transplant biopsies at 3 and 12 months after transplantation (Figure 1A, Supplemental Figure 1). The positive cells were outlying glomerular cells with morphologic features typical of podocytes (Figure 1A, Supplemental Figure 1). Interestingly, the rate of positive glomeruli for ISH at 3 months was 21.1%±4% and increased to 36.9%±6% at 12 months. In the second group of patients (62% of HIV-1 transplant recipients, n=8), HIV-1 RNA was detectable only in tubular cells and some vascular sections but not in glomeruli of transplant biopsies performed at 3 and 12 months after transplantation (Figure 1, B and C, Supplemental Figure 1). ISH showed also positive infiltrating cells at 3 and 12 months after transplantation in 25% (n=2) of patients in the latest group (Figure 1D, Supplemental Figure 1). We therefore concluded that HIV-1 is able to infect kidney allografts even when the level of plasma HIV-1 RNA is undetectable.
Figure 1.
HIV-1 reinfects the kidney allograft after transplantation. (A) Representative ISH of HIV-1 RNA performed with either sense (negative control) or antisense probes on kidney transplant biopsy performed at the onset of proteinuria. ISH reveals the presence of HIV-1 RNA in podocytes. (B) Representative ISH of HIV-1 RNA performed with either sense (negative control) or antisense probes on kidney transplant biopsy performed at 3 months after transplantation. ISH reveals the presence of HIV-1 RNA in the tubular compartment. (C) Representative ISH of HIV-1 RNA performed with either sense (negative control) or antisense probes on kidney transplant biopsy performed at 3 months after transplantation. ISH reveals the presence of HIV-1 RNA in tubular and vascular sections. (D) Representative ISH of HIV-1 RNA performed with either sense (negative control) or antisense probes on kidney transplant biopsy performed at 3 months after transplantation. ISH reveals the presence of HIV-1 RNA in the inflammatory infiltrates. Infiltrating cells and tubular cells are indistinguishable. Scale bar, 50 μm.
Distinct Clinical Features and Allograft Outcome
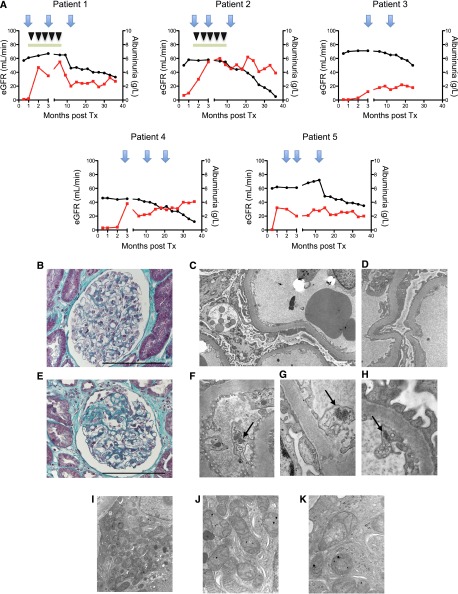
We then evaluated the clinical characteristics and transplant biopsies of the different patient groups. We observed that patients in the first group (i.e., with podocyte infection) developed nephrotic-range proteinuria early after transplantation (between day 15 and 3 months after transplantation) (Figure 2A). Demographic characteristics of these patients are presented in Table 1. Transplant biopsies performed for each patient at the onset of proteinuria revealed normal histology as determined by light microscopy and no deposit of Igs or complement proteins by immunofluorescence (Figure 2B). However, electron microscopy revealed effacement of the podocyte foot processes and vacuolization of the podocyte cytoplasm (Figure 2, C and D). To note, before we had ISH results, we initiated our current therapeutic protocol for recurrent FSGS in the first two patients10 without any improvement of the proteinuria (Figure 2A, patients 1 and 2). Subsequent biopsies performed at 3 and 12 months after transplantation in the group with podocyte infection (patients 1–5) revealed progressive constitution of FSGS lesions in all patients (Figure 2E). In addition, electron microscopy revealed the presence of tubuloreticular inclusions within the cytoplasm of endothelial cells (Figure 2, F–H). These inclusions consist of structures located within the dilated cisternae of the endoplasmic reticulum and are frequently, but not specifically, observed during HIVAN.11 Finally, these patients had a rapid decline in renal function (Figure 2A) and 40% (n=2) reached ESRD in the first 3 years of transplantation.
Figure 2.
Distinct clinical and morphologic features between the two groups of patients. (A) Representative graphics for eGFR (black plot) and albuminuria (red plot) for each patient. The first two patients are treated with plasmapheresis (inverted black triangles) and high-dose cyclosporine (green rectangles). The blue arrows represent transplant biopsies. (B) Representative trichrome staining of glomerular structures at the onset of albuminuria in patients with HIV-1 podocyte infection. The optical microscopy observations are considered normal. (C) Representative electron microscopy image in patients with HIV-1 podocyte infection. Electron microscopy reveals effacement of the foot processes, thickening of the glomerular basement membrane, and vacuolization of the cytoplasm of podocytes. (D) High-power view of the foot process effacement. (E) Representative trichrome staining of the focal and segmental lesions observed in protocol biopsies at 12 months after transplantation in patients with HIV-1 podocyte infection. (F–H) Representative electron microscopy images for patients 1, 2, and 3, respectively. High-power images of a capillary loop showing tubuloreticular inclusions (virus-like particles) within the cytoplasm of endothelial cells (arrows). (I– K) Representative electron microscopy images in patients with HIV-1 tubular cell infection. The epithelial cells of the proximal convoluted tubules frequently contain abnormal mitochondria (i.e., significant variability in size and shape, annulated or distorted cristae, increase in mitochondrial matrix). Scale bar, 100 μm. Original magnification, ×12,000 in C; ×30,000 in D; ×48,000 in F–H; ×15,000 in I; ×53,000 in J; ×70,000 in K. eGFR, estimated GFR; Tx, transplantation.
Table 1.
Demographic characteristics of the HIV-1 kidney transplant recipients with podocyte infection
| Patient No. | Sex | Ethnicity | Cause of ESRD | Nadir CD4+ Count (per mm3) | CD4+ Count at Day 0 (per mm3) | HIV-1 DNA per PBMC at Day 0 (log copies/106 cells) | Duration of Undetectable Plasma HIV-1 RNA before Transplantation (yr) | AIDS Stage | Age at HD (yr) | Hemodialysis Duration (yr) | HCV Status | Induction | Donor |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | M | Caucasian | MPGN | 155 | 359 | 2.9 | 3.5 | No | 53 | 2 | Negative | Basiliximab | DC |
| 2 | M | Caucasian | IgAN | 453 | 1216 | 2.4 | 1.7 | No | 36 | 2 | Negative | Basiliximab | DC |
| 3 | F | Sub-Saharan African | HIVAN | 245 | 906 | 2.5 | 8.3 | No | 30 | 11 | Negative | Basiliximab | DC |
| 4 | M | Sub-Saharan African | HIVAN | 340 | 575 | <1.7 | 2.4 | No | 50 | 3 | Positive | Basiliximab | DC |
| 5 | F | Sub-Saharan African | HIVAN | 107 | 382 | 3.6 | 8.0 | No | 32 | 7 | Negative | Basiliximab | LR |
HD, hemodialysis; HCV, hepatitis C virus; M, male; MPGN, membranoproliferative GN; DC, deceased donor; F, female; LR, living related donor.
Demographic characteristics and post-transplantation outcomes of the second group of patients, with only tubular and vascular cell infection by HIV-1, are listed Table 2 and Supplemental Figure 2. Interestingly, optical analysis of transplant biopsies at 3 and 12 months after transplantation did not show significant abnormalities and electron microscopy revealed that the epithelial cells of the proximal convoluted tubules frequently contained abnormal mitochondria (Figure 2, J–K). However, patients with infiltrating positive ISH cells on biopsies performed at 3 months after transplantation had features of subclinical acute cellular rejection. In this setting, before we had ISH results, we treated these individuals infiltrate with high-dose steroids. Interestingly, these infiltrates remained persistent on biopsies performed at 12 months after transplantation. In this group, only 12% of participants (n=1) reached ESRD during the early follow-up.
Table 2.
Demographic characteristics of the HIV-1 kidney transplant recipients without nephrotic-range proteinuria
| Patient No. | Sex | Ethnicity | Cause of ESRD | Nadir CD4+ Count (per mm3) | CD4+ Count at Day 0 (per mm3) | HIV-1 DNA per PBMC at Day 0 (log copies/106 cells) | Duration of Undetectable Plasma HIV-1 RNA before Transplantation (yr) | AIDS Stage | Age at HD (yr) | Duration of HD (yr) | HCV Status | Induction | Donor |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| With HIV-1 infection of kidney tubular cells | |||||||||||||
| 6 | M | Sub-Saharan African | HIVAN | 250 | 560 | 2.6 | 5.6 | No | 23 | 19 | Negative | Basiliximab | DC |
| 7 | M | Sub-Saharan African | HIVAN | 234 | 356 | 2 | 11.1 | No | 41 | 10 | Negative | Basiliximab | DC |
| 8 | F | Sub-Saharan African | HIVAN | 146 | 189 | 1.4 | 8.3 | Yes | 39 | 11 | Negative | Basiliximab | DC |
| 9 | F | Sub-Saharan African | HIVAN | 49 | 344 | 1.9 | 12.4 | Yes | 36 | 17 | Negative | Antithymocyte globulins | DC |
| 10 | M | Sub-Saharan African | HIVAN | 274 | 453 | 2.8 | 2.5 | Yes | 32 | 10 | Negative | Basiliximab | DC |
| 11 | M | North African | HIVAN | 455 | 346 | 1.8 | 4.9 | No | 52 | 2 | Positive | Basiliximab | DC |
| 12 | F | Sub-Saharan African | HIVAN | 286 | 564 | 2.8 | 0.3 | No | 26 | 3 | Negative | Basiliximab | DC |
| 13 | F | Sub-Saharan African | HIVAN | 130 | 401 | 2.1 | 1.5 | No | 27 | 6 | Negative | Antithymocyte globulins | LR |
| With no allograft HIV-1 infection | |||||||||||||
| 14 | M | Sub-Saharan African | HIVAN | 185 | 708 | 3.2 | 7.6 | No | 37 | 7 | Negative | Antithymocyte globulins | DC |
| 15 | M | Caucasian | ADPKD | 162 | 331 | 2.5 | 3.5 | No | 58 | 3 | Negative | Antithymocyte globulins | DC |
| 16 | F | Sub-Saharan African | HIVAN | 534 | 404 | 3 | 5.2 | Yes | 36 | 8 | Negative | Antithymocyte globulins | DC |
| 17 | F | Sub-Saharan African | HIVAN | 4 | 486 | <1.7 | 5.6 | No | 42 | 6 | Negative | Basiliximab | DC |
| 18 | F | North African | HIVAN | 246 | 367 | 2.6 | 2.1 | No | 33 | 3 | Positive | Antithymocyte globulins | DC |
| 19 | F | Sub-Saharan African | HIVAN | 167 | 428 | 2 | 1.1 | No | 51 | 5 | Positive | Basiliximab | DC |
HD, hemodialysis; HCV, hepatitis C virus; M, male; DC, deceased donor; F, female; LR, living related donor; ADPKD, autosomal dominant polycystic kidney disease.
To note, we explored the presence of other viruses (e.g., cytomegalovirus, BK virus, and hepatitis) in all patients and failed to detect any of these viruses in biopsy samples.
Podocyte Depletion
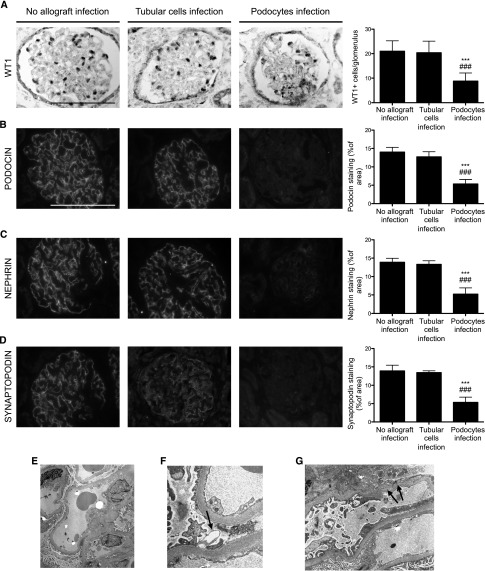
Because podocyte infection by HIV-1 seems to be more severe, we next aimed to better characterize the mechanisms underlying the high rate of allograft failure in this group. In rodents, podocyte depletion is associated with the development of FSGS lesions.12 We therefore monitored the number of podocytes in the different patient groups. We observed that the number of Wilms tumor protein 1 (WT1)-positive cells was dramatically reduced in patients with podocyte infection at 12 months after transplantation, whereas the count of WT1-positive cells was not affected in patients with tubular cell infection (Figure 3A). Importantly, transmission electron microscopy revealed the presence of many apoptotic podocytes only in patients with podocyte infection (Figure 3, E–G). To characterize podocyte differentiation, we next explored the expression of different podocyte proteins. Interestingly, we observed that compared with the groups of HIV-1 transplantation recipients without proteinuria, transplantation recipients with HIV-1 with podocyte infection had reduced expression of podocin, nephrin, and synaptopodin at 12 months after transplantation, suggesting a state of dedifferentiation (Figure 3, B–D). Finally, analysis of the protocol biopsies performed 3 months after transplantation revealed that these events appeared quite early after transplantation as podocyte rarefaction and reduced expression of podocyte proteins was already detectable (Supplemental Figure 3).
Figure 3.
Podocyte infection is associated with podocyte dedifferentiation and depletion at 12 months after transplantation (transplantation). (A) WT1 immunostaining and quantification of WT1-positive glomerular cells in biopsies from HIV-1 transplantation recipients without allograft infection, HIV-1 transplantation recipients with tubular cell infection, and HIV-1 transplantation recipients with podocyte infection at 12 months after transplantation. (B) Podocin immunostaining and quantification of podocin glomerular area in biopsies from HIV-1 transplantation recipients without allograft infection, HIV-1 transplantation recipients with tubular cell infection, and HIV-1 transplantation recipients with podocyte infection at 12 months after transplantation. (C) Nephrin immunostaining and quantification of the nephrin glomerular area in biopsies from HIV-1 transplantation recipients without allograft infection, HIV-1 transplantation recipients with tubular cell infection, and HIV-1 transplantation recipients with podocyte infection at 12 months after transplantation. (D) Synaptopodin immunostaining and quantification of synaptopodin glomerular area in biopsies from HIV-1 transplantation recipients without allograft infection, HIV-1 transplantation recipients with tubular cell infection, and HIV-1 transplantation recipients with podocyte infection at 12 months after transplantation. (E) Representative electron microscopy image in patients with HIV-1 podocyte infection. Low-power view showing slight increase of mesangial matrix, diffuse foot process effacement, and focal podocyte necrosis. (F) High-power field of a necrotic foot process (arrow) between two viable foot processes. (G) Podocytes are necrotic with detachment of foot processes from glomerular basement membrane (arrows). Data are the mean±SD from ANOVA followed by the Tukey-Kramer test. ***P<0.001, HIV-1 transplantation recipients without allograft infection versus HIV-1 transplantation recipients with podocyte infection; ###P<0.001, HIV-1 transplantation recipients with tubular cell infection versus HIV-1 transplantation recipients with podocyte infection. Scale bar, 100 μm. Original magnification, ×2000 in E; ×30,000 in F; ×12,500 in G.
Risk Factors for Graft Infection
We next explored whether any risk factors for graft infection could be identified. Analysis of the demographic characteristics revealed no significant differences between patients with podocyte or tubular cell reinfection and patients without any reinfection (Tables 1–3). The G1 and G2 haplotypes in the APOL1 gene were recently identified as risk factors for the development of HIVAN in African Americans.13 APOL1 encodes a protein component of HDL that has trypanolytic activity. We explored whether the APOL1 variants in donors and transplant recipients could explain the recurrence rate and tissue distribution of reinfection. Although the allele frequencies of the three single-nucleotide polymorphisms (SNPs) in African patients was similar to those described in African-American patients with HIVAN,14 we did not observe any correlation between the APOL1 genotype and reinfection of the kidney allograft by HIV-1 (Table 4).
Table 3.
Donor and recipient HLA groups
| Patient No. | Recipient HLA Group | Donor HLA Group | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A1 | A2 | B1 | B2 | DR1 | DR2 | DQ1 | DQ2 | A1 | A2 | B1 | B2 | DR1 | DR2 | DQ1 | DQ2 | |
| With podocyte infection | ||||||||||||||||
| 1 | 2 | 0 | 15 | 51 | 3 | 8 | 0 | 0 | 1 | 2 | 51 | 8 | 3 | 8 | 2 | 4 |
| 2 | 2 | 26 | 15 | 44 | 7 | 15 | 2 | 6 | 3 | 30 | 63 | 13 | 7 | 0 | 2 | 0 |
| 3 | 23 | 30 | 7 | 44 | 15 | 16 | 6 | 5 | 2 | 0 | 38 | 44 | 13 | 15 | 6 | 0 |
| 4 | 2 | 24 | 53 | 81 | 7 | 8 | 2 | 7 | 1 | 3 | 18 | 57 | 7 | 8 | 3 | 4 |
| 5 | 2 | 2 | 18 | 58 | 7 | 13 | 2 | 5 | 1 | 2 | 51 | 8 | 17 | 4 | 2 | 4 |
| With tubular cell infection | ||||||||||||||||
| 6 | 2 | 31 | 44 | 51 | 4 | 13 | 0 | 0 | 2 | 30 | 62 | 53 | 13 | 0 | 6 | 3 |
| 7 | 24 | 33 | 37 | 81 | 11 | 15 | 6 | 0 | 1 | 24 | 27 | 40 | 11 | 14 | 1 | 7 |
| 8 | 2 | 0 | 35 | 51 | 7 | 11 | 7 | 7 | 1 | 3 | 14 | 27 | 1 | 11 | 1 | 7 |
| 9 | 34 | 68 | 78 | 82 | 7 | 9 | 2 | 2 | 1 | 26 | 8 | 13 | 7 | 9 | 2 | 9 |
| 10 | 2 | 0 | 44 | 60 | 4 | 11 | 7 | 8 | 2 | 0 | 44 | 0 | 4 | 0 | 12 | 7 |
| 11 | 30 | 33 | 7 | 53 | 8 | 9 | 2 | 7 | 30 | 23 | 7 | 72 | 7 | 9 | 2 | 0 |
| 12 | 2 | 34 | 72 | 41 | 7 | 14 | 2 | 6 | 2 | 68 | 60 | 72 | 13 | 0 | 1 | 0 |
| 13 | 2 | 0 | 45 | 49 | 1 | 11 | 5 | 0 | 24 | 0 | 62 | 7 | 1 | 11 | 1 | 7 |
| Without allograft infection | ||||||||||||||||
| 14 | 23 | 36 | 15 | 58 | 11 | 13 | 0 | 0 | 1 | 3 | 35 | 49 | 11 | 18 | 3 | 4 |
| 15 | 23 | 29 | 15 | 58 | 10 | 10 | 5 | 0 | 2 | 29 | 18 | 57 | 3 | 11 | 2 | 7 |
| 16 | 1 | 23 | 35 | 78 | 10 | 13 | 0 | 0 | 1 | 2 | 14 | 57 | 7 | 13 | 2 | 3 |
| 17 | 26 | 0 | 44 | 49 | 4 | 0 | 8 | 0 | 1 | 29 | 44 | 0 | 1 | 7 | 2 | 5 |
| 18 | 2 | 36 | 7 | 53 | 11 | 13 | 6 | 6 | 2 | 68 | 14 | 45 | 7 | 11 | 2 | 7 |
| 19 | 2 | 33 | 45 | 78 | 11 | 13 | — | — | 1 | 3 | 35 | 37 | 4 | 17 | 2 | 3 |
Table 4.
Donor and recipient polymorphisms in APOL1
| Patient No. | Donor Group | Recipient Group | ||
|---|---|---|---|---|
| G1 | G2 | G1 | G2 | |
| With podocyte infection | ||||
| 1 | — | — | — | — |
| 2 | — | — | — | — |
| 3 | — | — | Homozygous | — |
| 4 | — | — | — | — |
| 5 | Heterozygous | — | Heterozygous | — |
| With tubular cell infection | ||||
| 6 | — | — | Heterozygous | Heterozygous |
| 7 | — | — | — | Heterozygous |
| 8 | — | — | Heterozygous | Heterozygous |
| 9 | — | — | Homozygous | |
| 10 | — | — | Heterozygous | Heterozygous |
| 11 | — | — | — | — |
| 12 | — | — | Heterozygous | — |
| 13 | Heterozygous | — | Heterozygous | — |
| Without allograft infection | ||||
| 14 | — | — | — | Heterozygous |
| 15 | — | — | — | — |
| 16 | — | — | Homozygous | — |
| 17 | — | — | — | Homozygous |
| 18 | — | — | — | Homozygous |
| 19 | — | — | Homozygous | — |
The G1 haplotype comprises two missense variants p.Ser342Glyand p.Ile384Met (rs73885319 and rs60910145, respectively), whereas the G2 haplotype comprises a 6 bp deletion p.del Asn388/Tyr389 (rs71785313).
We then tested whether HIV coreceptor tropism could predict recurrence. For each patient, virus tropism of the C-C chemokine receptor type 5 and C-X-C chemokine receptor type 4 was determined. No significant differences were observed between patients with or without recurrence (Table 5).
Table 5.
HIV-1 coreceptor tropism for each patient
| Patient No. | Coreceptor for HIV Entry |
|---|---|
| With podocyte infection | |
| 1 | CCR5 |
| 2 | CCR5 |
| 3 | ND |
| 4 | CCR5 |
| 5 | CCR5 |
| With tubular cell infection | |
| 6 | CCR5 |
| 7 | CCR5 |
| 8 | CCR5 |
| 9 | CCR5 |
| 10 | CCR5 |
| 11 | CCR5 |
| 12 | CCR5 |
| 13 | CCR5 |
| Without allograft infection | |
| 14 | CCR5 |
| 15 | CCR5 |
| 16 | CCR5 |
| 17 | CXCR4 |
| 18 | CCR5 |
| 19 | CCR5 |
CCR5, C-C chemokine receptor type 5; CXCR4, C-X-C chemokine receptor type 4; ND, not determined.
Finally, a SNP 35 kb upstream of the HLA-C locus (rs9264942) was recently shown to be associated with viral load control in a recent genome-wide association study,15 and this SNP correlates with the level of HLA-C cell surface protein expression.16 Therefore, we tested whether this SNP would be helpful in predicting recurrence. Unfortunately, no significant differences were observed in patients with or without reinfection (Table 6).
Table 6.
HLA-C-35 SNP genotyping
| Patient No. | HLA-C rs9264942 | |
|---|---|---|
| Donor | Recipient | |
| With podocyte infection | ||
| 1 | ND | CC |
| 2 | CC | TT |
| 3 | CC | TT |
| 4 | CC | CT |
| 5 | TT | CC |
| With tubular cell infection | ||
| 6 | CC | TT |
| 7 | CT | TT |
| 8 | TT | CT |
| 9 | CT | CT |
| 10 | TT | CT |
| 11 | CC | TT |
| 12 | TT | TT |
| 13 | TT | TT |
| Without allograft infection | ||
| 14 | CC | TT |
| 15 | CC | TT |
| 16 | CT | CT |
| 17 | TT | CC |
| 18 | CT | CT |
| 19 | TT | CC |
ND, not determined.
Predictive Urinary Test for Infection
Considering the high incidence of allograft infection and the difficulty of performing routine ISH, we thought that the development of a noninvasive diagnostic test for HIV-1 renal allograft infection would be of considerable value. To this end, we performed quantitative PCR of HIV-1 RNA and HIV-1 DNA in urine. We collected urine samples from patients just before protocol biopsies (3 and 12 months after transplantation) and biopsies for cause. After the fresh urine was centrifuged, RNA was extracted from the supernatant and the level of HIV-1 RNA was quantified. The pellets were used for DNA extraction. Sequentially collected urine samples showed that HIV-1 RNA and/or HIV-1 DNA were detectable only in the group of patients with an HIV-infected allograft. Importantly, the presence of HIV-1 RNA and/or HIV-1 DNA in the urine was strictly associated with positive ISH results for the biopsies (Table 7). Moreover, in some of these biopsies, we observed positive ISH tubular cells sloughing into the tubular lumen (Supplemental Figure 4). HIV-1 RNA and DNA were detectable at 3 months after transplantation and persisted at 12 months. Furthermore, to exclude any positivity due to infiltrating leukocytes, we performed hematoxylin and eosin staining and CD3 and CD68 immunostaining on cells isolated from the pellet, and we did not observe the presence of inflammatory cells (not shown). Finally, we tried to figure out why some patients develop podocytes or tubular epithelial cells infection by comparing the virus sequences obtained from the urine, but the low viral load did not allow us to succeed in virus sequences.
Table 7.
Detection of HIV-1 RNA and/or DNA in the urine at 3 and 12 months after transplantation
| Patient No. | Month 3 | Month 12 | ||
|---|---|---|---|---|
| HIV-1 RNA (log copies/ml) | HIV-1 DNA (log copies/106 urinary cells) | HIV-1 RNA (log copies/ml) | HIV-1 DNA (log copies/106 urinary cells) | |
| With podocyte infection | ||||
| 1 | Undetectable | 2 | Undetectable | 2.5 |
| 2 | 3.3 | 1.4 | 3.1 | 1.2 |
| 3 | 2.1 | Undetectable | 3 | Undetectable |
| 4 | 3 | 2.1 | 2.2 | 2.2 |
| 5 | 2.2 | 1.8 | 2.3 | 2.4 |
| With tubular cell infection | ||||
| 6 | 2.4 | 1.1 | 2.5 | 1.4 |
| 7 | 2.5 | 2.3 | 2.3 | 2.3 |
| 8 | Undetectable | 3.2 | Undetectable | 3 |
| 9 | Undetectable | 2.5 | 2.4 | 2.6 |
| 10 | 3 | 1.2 | 3.4 | 1.4 |
| 11 | 3.3 | 1.2 | 3.6 | 1.3 |
| 12 | 2.5 | Undetectable | 2.4 | Undetectable |
| 13 | 2 | Undetectable | 2.1 | Undetectable |
| Without allograft infection | ||||
| 14 | Undetectable | Undetectable | Undetectable | Undetectable |
| 15 | Undetectable | Undetectable | Undetectable | Undetectable |
| 16 | Undetectable | Undetectable | Undetectable | Undetectable |
| 17 | Undetectable | Undetectable | Undetectable | Undetectable |
| 18 | Undetectable | Undetectable | Undetectable | Undetectable |
| 19 | Undetectable | Undetectable | Undetectable | Undetectable |
Discussion
Here we demonstrate that HIV-1 can reinfect kidney epithelial cells after transplantation, even though plasma HIV-1 RNA is undetectable. Importantly, we describe two different forms of allograft infection. In the first case, podocytes are the main target of HIV-1 and infection is associated with nephrotic-range proteinuria, progressive development of FSGS, and poor transplant outcome. In the second case, HIV-1 can infect tubular cells from the kidney allograft with fewer clinical manifestations. Finally, we provide a new tool to easily and noninvasively assess transplant kidney cell reinfection by the virus.
Infection of the kidney allograft while viral replication was undetectable in plasma was an unexpected finding. Indeed, all of these HIV-1 patients were registered on the transplant list as patients with controlled disease and thus were expected to benefit from kidney transplantation. Interestingly, the development of HIV nephropathy on native kidneys in patients with undetectable viremia was previously described in several studies.17–21 Furthermore, several studies involving HIV-1 transplant kidney recipients have also reported the occurrence of HIVAN in few patients without detectable viremia.5,6 How the virus reinfects kidney cells soon after kidney transplantation is an unsolved issue because repeated blood samples failed to detect viremia; however, we have a few hypotheses. Indeed, we cannot exclude that transient episodes of viremia may likely occur (HIV-1 blips) even in patients with successful viral suppression.22 We also hypothesize that the virus could be directly transfected from recipient-infected T cells to donor kidney cells, as was recently shown in vitro.23 Of note, it is still unclear how HIV-1 enters in podocytes and tubular cells because no HIV-1 receptors have been described in the kidney.2 Finally, it remains possible that kidney infection could be the consequence of immunosuppressive drugs and/or insufficient HAART with suboptimal antiretroviral diffusion in the graft.
Due to the small number of patients in our study, we were unable to establish the particular factors that either favor allograft reinfection or determine the surprising dichotomy of nephron segment reinfection (i.e., podocytes versus tubular cells). We provide evidence that transplant reinfection by HIV-1, at least in podocytes, shortens the allograft survival rate. Interestingly, we did not observe any HIVAN on the allografted kidney, whereas most of the recipients developed these collapsing lesions on native kidneys. In fact, we observed here that HIV-1 infection in podocytes was associated with podocyte dedifferentiation, assessed by the loss of specific markers (podocin, synaptopodin, and nephrin), apoptosis, and development of FSGS lesions. Interestingly, this sequence of events is observed in different models of podocyte injury leading to the development of FSGS.12,24,25 The exact mechanism is unknown, but certainly the genetic background of the donor and others parameters may affect the course of glomerular lesions. Indeed, on native kidneys, HIVAN has been linked to G1 and/or G2 APOL1 variants present in the African-American population,13 whereas Caucasians, who do not express these variants, display other various forms of glomerular lesions but usually have no features of HIVAN. Consistently, we observed that 87.5% of our African-African recipients that developed HIVAN on native kidneys displayed either a G1 and/or G2 APOL1 polymorphism, but none of our Caucasian recipients did. Importantly, we found a very low rate of the APOL1 polymorphism in our donor cohort, suggesting that donor APOL1 may play a local role in HIVAN pathophysiology, as was also suggested by the recent report of kidney APOL1 expression in normal and pathologic conditions.26 Besides genetic predisposition, we cannot exclude that immunosuppressive drugs, by either interacting with the immune system or directly with podocytes (calcineurin inhibitors27 and steroids28), may modify the classical pattern of kidney lesions observed with HIV-1.
Tubular reinfection seems to have less functional effect in our cohort, but the follow-up is certainly too short. We cannot exclude that tubular cell infection contributes to the shortened allograft survival observed in previous reports. A high rate of acute cellular rejection has been described in renal allografts of this patient population. Here, we observed that the two patients who developed what we called acute cellular rejection had ISH results showing the presence of HIV-1 RNA either in infiltrating leukocytes or in adjacent tubular cells. Persisting virus in infiltrating inflammatory cells (whereas plasma HIV-1 RNA is undetectable) was previously described29; however, the infiltration of inflammatory cells could be the result of an immune reaction against tubular cells infected by HIV-1. Therefore, we hypothesize that the high rate of acute cellular rejection described in HIV transplantation recipients might be related to, at least in part, tubular cell infection by the HIV-1.
To facilitate monitoring of transplant infection with HIV-1, we provide evidence that the detection of HIV-1 DNA, and RNA in urine samples by quantitative PCR seems to be a consequence of kidney cell infection. Indeed, urinary HIV-1 DNA and/or HIV-1 RNA were exclusively detected in patients with positive RNA ISH either in podocytes or in tubular cells. Furthermore, light microscopy examination and immunocytochemistry of urine pellets exclude that HIV-1 DNA and/or HIV-1 RNA derived from infiltrating inflammatory cells. We cannot formally exclude that HIV-1 could be filtered from the plasma into the urine, even in the absence of detectable viremia; however, its detection only in the setting of a positive ISH in the graft supports our hypothesis.
In conclusion, our work reveals the capacity of HIV-1 to infect the kidney allograft despite undetectable viremia. Urine testing appears to be a promising noninvasive method of diagnosing HIV-1 reinfection, although it remains to be confirmed in a larger cohort. Finally, our data strongly support the need for close proteinuria monitoring in assessing the outcome of HIV-infected kidney transplant recipients.
Concise Methods
Patients
This study was performed in the Department of Kidney Transplantation of the Necker Hospital from June 1, 2006, to October 31, 2011. Patients with HIV-1 infection were eligible for transplantation if, before transplantation, they had a CD4+ T cell count of at least 200 cells/mm3, had undetectable plasma HIV RNA levels (<50 copies/ml), and had been stable on HAART for at least 6 months. Patients with stage C HIV (according to the US Centers for Disease Control and Prevention classification) were also included in the program, with the exception of those patients with a previous history of progressive multifocal leukoencephalopathy, primary central nervous system lymphoma, or visceral Kaposi’s sarcoma.
The immunosuppressive regimen was similar for all patients and included steroids, tacrolimus, and mycophenolate mofetil. Induction therapy was performed with the anti–IL-2 receptor antibody basiliximab for all patients except those with preformed donor-specific antibodies, for whom the induction therapy was changed to rabbit antithymocyte globulin. In the particular case of sensitized patients, intravenous Ig was added. The trough levels of tacrolimus were carefully monitored due to pharmacokinetic drug interactions. The antiretroviral drugs doses were adjusted based on kidney function, with frequent adjustments required particularly during the early post-transplantation period and during periods of graft dysfunction.
The antiretroviral combination therapy was similar for all patients and consisted of two nucleoside analog reverse-transcriptase inhibitors and one protease inhibitor. All patients continued their HAART regimen after transplantation and for the entire follow-up period. All patients with a functioning allograft underwent protocol biopsies at 3 and 12 months after transplantation. Furthermore, in the case of ARF and/or proteinuria, a transplant biopsy was performed.
Renal Function
The serum creatinine level was measured monthly during the first year and every 3 months thereafter using a Synchron Cx4 autoanalyzer (Beckman Coulter). The GFR was estimated using the Modification of Diet in Renal Disease study formula.30
Biopsy Samples and Morphologic Analyses
Transplant kidney biopsy specimens were fixed in alcohol-formalin-acetic acid solution and embedded in paraffin. Four-micrometer sections were stained with periodic acid–Schiff, Masson’s trichrome, and hematoxylin and eosin. Electron microscopy analyses were performed as previously described.31
Immunohistochemistry and Immunofluorescence
Four-micrometer sections of paraffin-embedded kidneys were incubated with anti-nephrin antibody (Progen), anti-WT1 antibody (Dako), anti-podocin antibody (Sigma-Aldrich) and anti-synaptopodin antibody (Novus Biologicals). The primary antibodies were revealed with the appropriate Alexa 488– or Alexa 555–conjugated secondary antibodies (Molecular Probes). Immunofluorescence staining was visualized using the Zeiss LSM 700 confocal microscope. The podocyte-stained area was automatically quantified using a Nikon digital camera Dx/m/1200 and ImageJ software and expressed as the percentage of the podocyte-stained area upon the total glomerular area. All glomerular sections per biopsy were quantified.
Plasma HIV-1 RNA Level and Blood Cell–Associated HIV-1 DNA Level
The plasma HIV-1 RNA level was quantified as previously described using the COBAS AmpliPrep/COBAS TaqMan HIV-1 RT-PCR assay (version 2.0; Roche Diagnostics), which has a detection limit of 20 copies/ml. The level of cell-associated HIV-1 DNA was quantified using whole-blood samples and a real-time HIV-1 DNA assay32 (Biocentric) that has a detection limit of 1.7 log copies/106 cells. Plasma HIV-1 RNA levels were determined on days 0 and 7 and then monthly until 6 months after transplantation.33 The HIV-1 RNA levels were monitored every 3 months thereafter.
HIV-1 DNA Detection in Frozen Biopsy Samples
Total DNA was extracted from renal tissues using the QIAamp tissue kit (Qiagen) according to the manufacturer’s instructions. The level of cell-associated HIV-1 DNA was quantified using a real-time HIV-1 DNA assay32 (Biocentric).
ISH
Alcohol-formalin-acetic acid solution–fixed, paraffin-embedded tissues were assayed for HIV-1 RNA expression using a previously described digoxygenin–antidigoxygenin technique.34 The digoxigenin-UTP-labeled riboprobe that was used spans the whole genome of HIV-1 (Lofstrand Labs Ltd). Nitroblue tetrazolium-5-bromo-4-chloro-3-indolyl phosphate toluidinium was used to visualize infected cells in the tissues. The specificity of the hybridization signal was systematically checked by hybridizing sense probes with parallel sections and antisense probes with uninfected renal tissues. ISH-stained tissues were visualized and photographed with an Olympus Proxis microscope and a Zeiss AxioCam ICc1 camera. For each biopsy, we determined the number of positive glomeruli in all fields of the section, which are expressed as the number of positive glomeruli for the total number of glomeruli.
Urine Collection and HIV-1 RNA/DNA Testing
Urine samples were collected longitudinally for each patient at 3 and 12 months after transplantation and when a biopsy for cause was performed. Fifty milliliters of freshly collected urine was immediately centrifuged at 1000×g for 10 minutes at 4°C. RNA was extracted from 3.2 ml of supernatant using a Qiagen kit, and the level of HIV-1 RNA was quantified using a Biocentric ultrasensitive assay. Pellets were used for DNA extraction with the Qiagen QIAamp DNA mini kit according to the manufacturer’s instructions. The level of HIV-1 DNA was then quantified using an ultrasensitive assay from Biocentric.32,33
HIV-1 Coreceptor Analyses
The coreceptor usage of viruses was determined using the SVMGeno2pheno algorithm (available at http://coreceptor.bioinf.mpi-sb.mpg.de/cgi-bin/coreceptor.pl) with a 10% false positive rate.
APOL1 SNP Genotyping
PCR amplification of the end of APOL1 exon 6 was performed for all individuals with the following primers: forward, 5′– 3′AAG CGG TGA ACA GGT GGA GA; and reverse, 5′– 3′CCC CTG CCA GGC ATA TCT CT. Sequencing was performed using a Big Dye terminator cycle sequencing kit and an ABI Prism 3130 XL DNA analyzer (Life Technologies). Direct Sanger sequencing allowed the detection of three SNPs: the two missense variants p.Ser342Gly and p.Ile384Met (rs73885319 and rs60910145) called the G1 risk allele and the 6 bp deletion p.del Asn388/Tyr389 (rs71785313) called G2.
Written informed consent was obtained from each patient.
HLA-C -35 SNP Genotyping
PCR amplification of rs9264942 variant, localized 35 kb upstream of the HLA-C gene, was performed for all individuals with the primers forward (GTC CCA ATT CCT TGA TTC AGT) and reverse (GTG GAG ATT CCT GCT GTG). Sequencing was carried out using a Big Dye terminator cycle sequencing kit and was analyzed with an ABI Prism 3130 XL DNA analyzer (Life Technologies).
Statistical Analyses
The data were expressed as the mean±SD. Differences among the experimental groups were evaluated using ANOVA, followed by the Tukey–Kramer test when significant. When only two groups were compared, the Mann–Whitney test was used. P values <0.05 were considered statistically significant. Analyses were performed with GraphPad Prism 5 software.
Disclosures
None.
Supplementary Material
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “Kidney Infection with HIV-1 Following Kidney Transplantation,” on pages 212–215.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2013050564/-/DCSupplemental.
References
- 1.Adih WK, Selik RM, Hu X: Trends in diseases reported on US death certificates that mentioned HIV infection, 1996-2006. J Int Assoc Physicians AIDS Care (Chic) 10: 5–11, 2011 [DOI] [PubMed] [Google Scholar]
- 2.Wyatt CM, Meliambro K, Klotman PE: Recent progress in HIV-associated nephropathy. Annu Rev Med 63: 147–159, 2012 [DOI] [PubMed] [Google Scholar]
- 3.Cohen MS, Shaw GM, McMichael AJ, Haynes BF: Acute HIV-1 Infection. N Engl J Med 364: 1943–1954, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marras D, Bruggeman LA, Gao F, Tanji N, Mansukhani MM, Cara A, Ross MD, Gusella GL, Benson G, D’Agati VD, Hahn BH, Klotman ME, Klotman PE: Replication and compartmentalization of HIV-1 in kidney epithelium of patients with HIV-associated nephropathy. Nat Med 8: 522–526, 2002 [DOI] [PubMed] [Google Scholar]
- 5.Stock PG, Barin B, Murphy B, Hanto D, Diego JM, Light J, Davis C, Blumberg E, Simon D, Subramanian A, Millis JM, Lyon GM, Brayman K, Slakey D, Shapiro R, Melancon J, Jacobson JM, Stosor V, Olson JL, Stablein DM, Roland ME: Outcomes of kidney transplantation in HIV-infected recipients. N Engl J Med 363: 2004–2014, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kumar MS, Sierka DR, Damask AM, Fyfe B, McAlack RF, Heifets M, Moritz MJ, Alvarez D, Kumar A: Safety and success of kidney transplantation and concomitant immunosuppression in HIV-positive patients. Kidney Int 67: 1622–1629, 2005 [DOI] [PubMed] [Google Scholar]
- 7.Touzot M, Pillebout E, Matignon M, Tricot L, Viard JP, Rondeau E, Legendre C, Glotz D, Delahousse M, Lang P, Peraldi MN: Renal transplantation in HIV-infected patients: The Paris experience. Am J Transplant 10: 2263–2269, 2010 [DOI] [PubMed] [Google Scholar]
- 8.Solid organ transplantation in the HIV-infected patient. Am J Transplant 4[Suppl 10]: 83–88, 2004 [DOI] [PubMed] [Google Scholar]
- 9.Wyatt CM, Klotman PE: HIV-associated nephropathy in the era of antiretroviral therapy. Am J Med 120: 488–492, 2007 [DOI] [PubMed] [Google Scholar]
- 10.Canaud G, Zuber J, Sberro R, Royale V, Anglicheau D, Snanoudj R, Gaha K, Thervet E, Lefrère F, Cavazzana-Calvo M, Noël LH, Méjean A, Legendre C, Martinez F: Intensive and prolonged treatment of focal and segmental glomerulosclerosis recurrence in adult kidney transplant recipients: A pilot study. Am J Transplant 9: 1081–1086, 2009 [DOI] [PubMed] [Google Scholar]
- 11.Strauss J, Abitbol C, Zilleruelo G, Scott G, Paredes A, Malaga S, Montané B, Mitchell C, Parks W, Pardo V: Renal disease in children with the acquired immunodeficiency syndrome. N Engl J Med 321: 625–630, 1989 [DOI] [PubMed] [Google Scholar]
- 12.Wharram BL, Goyal M, Wiggins JE, Sanden SK, Hussain S, Filipiak WE, Saunders TL, Dysko RC, Kohno K, Holzman LB, Wiggins RC: Podocyte depletion causes glomerulosclerosis: Diphtheria toxin-induced podocyte depletion in rats expressing human diphtheria toxin receptor transgene. J Am Soc Nephrol 16: 2941–2952, 2005 [DOI] [PubMed] [Google Scholar]
- 13.Genovese G, Friedman DJ, Ross MD, Lecordier L, Uzureau P, Freedman BI, Bowden DW, Langefeld CD, Oleksyk TK, Uscinski Knob AL, Bernhardy AJ, Hicks PJ, Nelson GW, Vanhollebeke B, Winkler CA, Kopp JB, Pays E, Pollak MR: Association of trypanolytic ApoL1 variants with kidney disease in African Americans. Science 329: 841–845, 2010 [DOI] [PMC free article] [PubMed]
- 14.Papeta N, Kiryluk K, Patel A, Sterken R, Kacak N, Snyder HJ, Imus PH, Mhatre AN, Lawani AK, Julian BA, Wyatt RJ, Novak J, Wyatt CM, Ross MJ, Winston JA, Klotman ME, Cohen DJ, Appel GB, D’Agati VD, Klotman PE, Gharavi AG: APOL1 variants increase risk for FSGS and HIVAN but not IgA nephropathy. J Am Soc Nephrol 22: 1991–1996, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pereyra F, Jia X, McLaren PJ, Telenti A, de Bakker PI, Walker BD, Ripke S, Brumme CJ, Pulit SL, Carrington M, Kadie CM, Carlson JM, Heckerman D, Graham RR, Plenge RM, Deeks SG, Gianniny L, Crawford G, Sullivan J, Gonzalez E, Davies L, Camargo A, Moore JM, Beattie N, Gupta S, Crenshaw A, Burtt NP, Guiducci C, Gupta N, Gao X, Qi Y, Yuki Y, Piechocka-Trocha A, Cutrell E, Rosenberg R, Moss KL, Lemay P, O’Leary J, Schaefer T, Verma P, Toth I, Block B, Baker B, Rothchild A, Lian J, Proudfoot J, Alvino DM, Vine S, Addo MM, Allen TM, Altfeld M, Henn MR, Le Gall S, Streeck H, Haas DW, Kuritzkes DR, Robbins GK, Shafer RW, Gulick RM, Shikuma CM, Haubrich R, Riddler S, Sax PE, Daar ES, Ribaudo HJ, Agan B, Agarwal S, Ahern RL, Allen BL, Altidor S, Altschuler EL, Ambardar S, Anastos K, Anderson B, Anderson V, Andrady U, Antoniskis D, Bangsberg D, Barbaro D, Barrie W, Bartczak J, Barton S, Basden P, Basgoz N, Bazner S, Bellos NC, Benson AM, Berger J, Bernard NF, Bernard AM, Birch C, Bodner SJ, Bolan RK, Boudreaux ET, Bradley M, Braun JF, Brndjar JE, Brown SJ, Brown K, Brown ST, Burack J, Bush LM, Cafaro V, Campbell O, Campbell J, Carlson RH, Carmichael JK, Casey KK, Cavacuiti C, Celestin G, Chambers ST, Chez N, Chirch LM, Cimoch PJ, Cohen D, Cohn LE, Conway B, Cooper DA, Cornelson B, Cox DT, Cristofano MV, Cuchural G, Jr, Czartoski JL, Dahman JM, Daly JS, Davis BT, Davis K, Davod SM, DeJesus E, Dietz CA, Dunham E, Dunn ME, Ellerin TB, Eron JJ, Fangman JJ, Farel CE, Ferlazzo H, Fidler S, Fleenor-Ford A, Frankel R, Freedberg KA, French NK, Fuchs JD, Fuller JD, Gaberman J, Gallant JE, Gandhi RT, Garcia E, Garmon D, Gathe JC, Jr, Gaultier CR, Gebre W, Gilman FD, Gilson I, Goepfert PA, Gottlieb MS, Goulston C, Groger RK, Gurley TD, Haber S, Hardwicke R, Hardy WD, Harrigan PR, Hawkins TN, Heath S, Hecht FM, Henry WK, Hladek M, Hoffman RP, Horton JM, Hsu RK, Huhn GD, Hunt P, Hupert MJ, Illeman ML, Jaeger H, Jellinger RM, John M, Johnson JA, Johnson KL, Johnson H, Johnson K, Joly J, Jordan WC, Kauffman CA, Khanlou H, Killian RK, Kim AY, Kim DD, Kinder CA, Kirchner JT, Kogelman L, Kojic EM, Korthuis PT, Kurisu W, Kwon DS, LaMar M, Lampiris H, Lanzafame M, Lederman MM, Lee DM, Lee JM, Lee MJ, Lee ET, Lemoine J, Levy JA, Llibre JM, Liguori MA, Little SJ, Liu AY, Lopez AJ, Loutfy MR, Loy D, Mohammed DY, Man A, Mansour MK, Marconi VC, Markowitz M, Marques R, Martin JN, Martin HL, Jr, Mayer KH, McElrath MJ, McGhee TA, McGovern BH, McGowan K, McIntyre D, Mcleod GX, Menezes P, Mesa G, Metroka CE, Meyer-Olson D, Miller AO, Montgomery K, Mounzer KC, Nagami EH, Nagin I, Nahass RG, Nelson MO, Nielsen C, Norene DL, O’Connor DH, Ojikutu BO, Okulicz J, Oladehin OO, Oldfield EC, 3rd, Olender SA, Ostrowski M, Owen WF, Jr, Pae E, Parsonnet J, Pavlatos AM, Perlmutter AM, Pierce MN, Pincus JM, Pisani L, Price LJ, Proia L, Prokesch RC, Pujet HC, Ramgopal M, Rathod A, Rausch M, Ravishankar J, Rhame FS, Richards CS, Richman DD, Rodes B, Rodriguez M, Rose RC, 3rd, Rosenberg ES, Rosenthal D, Ross PE, Rubin DS, Rumbaugh E, Saenz L, Salvaggio MR, Sanchez WC, Sanjana VM, Santiago S, Schmidt W, Schuitemaker H, Sestak PM, Shalit P, Shay W, Shirvani VN, Silebi VI, Sizemore JM, Jr, Skolnik PR, Sokol-Anderson M, Sosman JM, Stabile P, Stapleton JT, Starrett S, Stein F, Stellbrink HJ, Sterman FL, Stone VE, Stone DR, Tambussi G, Taplitz RA, Tedaldi EM, Telenti A, Theisen W, Torres R, Tosiello L, Tremblay C, Tribble MA, Trinh PD, Tsao A, Ueda P, Vaccaro A, Valadas E, Vanig TJ, Vecino I, Vega VM, Veikley W, Wade BH, Walworth C, Wanidworanun C, Ward DJ, Warner DA, Weber RD, Webster D, Weis S, Wheeler DA, White DJ, Wilkins E, Winston A, Wlodaver CG, van’t Wout A, Wright DP, Yang OO, Yurdin DL, Zabukovic BW, Zachary KC, Zeeman B, Zhao M, International HIV Controllers Study : The major genetic determinants of HIV-1 control affect HLA class I peptide presentation. Science 330: 1551–1557, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Apps R, Qi Y, Carlson JM, Chen H, Gao X, Thomas R, Yuki Y, Del Prete GQ, Goulder P, Brumme ZL, Brumme CJ, John M, Mallal S, Nelson G, Bosch R, Heckerman D, Stein JL, Soderberg KA, Moody MA, Denny TN, Zeng X, Fang J, Moffett A, Lifson JD, Goedert JJ, Buchbinder S, Kirk GD, Fellay J, McLaren P, Deeks SG, Pereyra F, Walker B, Michael NL, Weintrob A, Wolinsky S, Liao W, Carrington M: Influence of HLA-C expression level on HIV control. Science 340: 87–91, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Izzedine H, Wirden M, Launay-Vacher V: Viral load and HIV-associated nephropathy. N Engl J Med 353: 1072–1074, 2005 [DOI] [PubMed] [Google Scholar]
- 18.Hegde S, Singh C, Ohare B: HIV-associated nephropathy in the setting of maximal virologic suppression. Pediatr Nephrol 26: 973–977, 2011 [DOI] [PubMed] [Google Scholar]
- 19.Lescure FX, Flateau C, Pacanowski J, Brocheriou I, Rondeau E, Girard PM, Ronco P, Pialoux G, Plaisier E: HIV-associated kidney glomerular diseases: Changes with time and HAART. Nephrol Dial Transplant 27: 2349–2355, 2012 [DOI] [PubMed] [Google Scholar]
- 20.Bigé N, Lanternier F, Viard JP, Kamgang P, Daugas E, Elie C, Jidar K, Walker-Combrouze F, Peraldi MN, Isnard-Bagnis C, Servais A, Lortholary O, Noël LH, Bollée G: Presentation of HIV-associated nephropathy and outcome in HAART-treated patients. Nephrol Dial Transplant 27: 1114–1121, 2012 [DOI] [PubMed] [Google Scholar]
- 21.Audard V, Avouac J, Wirden M, Pardon A, Matignon M, Remy P, Desvaux D, Lang P, Grimbert P: HIV-related nephropathies associated with changes in blood and kidney tissue virus load. Kidney Int 73: 651–655, 2008 [DOI] [PubMed] [Google Scholar]
- 22.Nettles RE, Kieffer TL, Kwon P, Monie D, Han Y, Parsons T, Cofrancesco J, Jr, Gallant JE, Quinn TC, Jackson B, Flexner C, Carson K, Ray S, Persaud D, Siliciano RF: Intermittent HIV-1 viremia (Blips) and drug resistance in patients receiving HAART. JAMA 293: 817–829, 2005 [DOI] [PubMed] [Google Scholar]
- 23.Chen P, Chen BK, Mosoian A, Hays T, Ross MJ, Klotman PE, Klotman ME: Virological synapses allow HIV-1 uptake and gene expression in renal tubular epithelial cells. J Am Soc Nephrol 22: 496–507, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Matsusaka T, Xin J, Niwa S, Kobayashi K, Akatsuka A, Hashizume H, Wang QC, Pastan I, Fogo AB, Ichikawa I: Genetic engineering of glomerular sclerosis in the mouse via control of onset and severity of podocyte-specific injury. J Am Soc Nephrol 16: 1013–1023, 2005 [DOI] [PubMed] [Google Scholar]
- 25.Canaud G, Bienaimé F, Viau A, Treins C, Baron W, Nguyen C, Burtin M, Berissi S, Giannakakis K, Muda AO, Zschiedrich S, Huber TB, Friedlander G, Legendre C, Pontoglio M, Pende M, Terzi F: AKT2 is essential to maintain podocyte viability and function during chronic kidney disease. Nat Med 19: 1288–1296, 2013 [DOI] [PubMed] [Google Scholar]
- 26.Madhavan SM, O’Toole JF, Konieczkowski M, Ganesan S, Bruggeman LA, Sedor JR: APOL1 localization in normal kidney and nondiabetic kidney disease. J Am Soc Nephrol 22: 2119–2128, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Faul C, Donnelly M, Merscher-Gomez S, Chang YH, Franz S, Delfgaauw J, Chang JM, Choi HY, Campbell KN, Kim K, Reiser J, Mundel P: The actin cytoskeleton of kidney podocytes is a direct target of the antiproteinuric effect of cyclosporine A. Nat Med 14: 931–938, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wada T, Pippin JW, Marshall CB, Griffin SV, Shankland SJ: Dexamethasone prevents podocyte apoptosis induced by puromycin aminonucleoside: Role of p53 and Bcl-2-related family proteins. J Am Soc Nephrol 16: 2615–2625, 2005 [DOI] [PubMed] [Google Scholar]
- 29.Zhang L, Ramratnam B, Tenner-Racz K, He Y, Vesanen M, Lewin S, Talal A, Racz P, Perelson AS, Korber BT, Markowitz M, Ho DD: Quantifying residual HIV-1 replication in patients receiving combination antiretroviral therapy. N Engl J Med 340: 1605–1613, 1999 [DOI] [PubMed] [Google Scholar]
- 30.Stevens LA, Levey AS: Measured GFR as a confirmatory test for estimated GFR. J Am Soc Nephrol 20: 2305–2313, 2009 [DOI] [PubMed] [Google Scholar]
- 31.Mollet G, Ratelade J, Boyer O, Muda AO, Morisset L, Lavin TA, Kitzis D, Dallman MJ, Bugeon L, Hubner N, Gubler MC, Antignac C, Esquivel EL: Podocin inactivation in mature kidneys causes focal segmental glomerulosclerosis and nephrotic syndrome. J Am Soc Nephrol 20: 2181–2189, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Avettand-Fènoël V, Chaix ML, Blanche S, Burgard M, Floch C, Toure K, Allemon MC, Warszawski J, Rouzioux C, French Pediatric Cohort Study ANRS-CO 01 Group : LTR real-time PCR for HIV-1 DNA quantitation in blood cells for early diagnosis in infants born to seropositive mothers treated in HAART area (ANRS CO 01). J Med Virol 81: 217–223, 2009 [DOI] [PubMed] [Google Scholar]
- 33.Avettand-Fenoel V, Prazuck T, Hocqueloux L, Melard A, Michau C, Kerdraon R, Agoute E, Rouzioux C: HIV-DNA in rectal cells is well correlated with HIV-DNA in blood in different groups of patients, including long-term non-progressors. AIDS 22: 1880–1882, 2008 [DOI] [PubMed] [Google Scholar]
- 34.Hirsch VM, Dapolito G, Johnson PR, Elkins WR, London WT, Montali RJ, Goldstein S, Brown C: Induction of AIDS by simian immunodeficiency virus from an African green monkey: Species-specific variation in pathogenicity correlates with the extent of in vivo replication. J Virol 69: 955–967, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.