Abstract
Medial arterial calcification is accelerated in patients with CKD and strongly associated with increased arterial rigidity and cardiovascular mortality. Recently, a novel in vitro blood test that provides an overall measure of calcification propensity by monitoring the maturation time (T50) of calciprotein particles in serum was described. We used this test to measure serum T50 in a prospective cohort of 184 patients with stages 3 and 4 CKD, with a median of 5.3 years of follow-up. At baseline, the major determinants of serum calcification propensity included higher serum phosphate, ionized calcium, increased bone osteoclastic activity, and lower free fetuin-A, plasma pyrophosphate, and albumin concentrations, which accounted for 49% of the variation in this parameter. Increased serum calcification propensity at baseline independently associated with aortic pulse wave velocity in the complete cohort and progressive aortic stiffening over 30 months in a subgroup of 93 patients. After adjustment for demographic, renal, cardiovascular, and biochemical covariates, including serum phosphate, risk of death among patients in the lowest T50 tertile was more than two times the risk among patients in the highest T50 tertile (adjusted hazard ratio, 2.2; 95% confidence interval, 1.1 to 5.4; P=0.04). This effect was lost, however, after additional adjustment for aortic stiffness, suggesting a shared causal pathway. Longitudinally, serum calcification propensity measurements remained temporally stable (intraclass correlation=0.81). These results suggest that serum T50 may be helpful as a biomarker in designing methods to improve defenses against vascular calcification.
The extent of vascular calcification is an established predictor of cardiovascular events and mortality risk in patients with CKD.1 Mineral deposition within the medial layer of the large- and medium-sized muscular arteries is commonly seen with normal aging,2 but it is markedly accelerated in patients with CKD, representing a key element of the premature vascular aging seen in this population.3 Calcification of the tunica media leads to stiffening of the arterial wall,4 which is associated with increased pulse pressure, pulse wave velocity (PWV), and wave reflection.5 These hemodynamic changes can result in increased cardiac afterload, left ventricular hypertrophy and fibrosis, reduced diastolic coronary blood flow and subendocardial ischemia, abnormal endothelial function, and damage to the microcirculation of the kidney and brain.6
Exposure of the vasculature to the uremic milieu triggers cellular processes closely resembling physiologic bone formation within the vascular wall and cardiac valves.7 A key event in this phenomenon is the transdifferentiation of contractile vascular smooth muscle cells to an osteochondrocytic mineralizing phenotype.8 A number of systemic and paracrine factors important in regulating mineralization in bone have also been identified as potentiators of this process in the arterial wall. Less attention, however, has been paid to the common final pathway of the mineralization process itself—the formation of hydroxyapatite, which may occur as a cell-dependent or purely physiochemical process. In uremic serum, crystalline hydroxyapatite is present in spindle-shaped high molecular weight protein–mineral complexes termed secondary calciprotein particles (CPP), which are derived from spherical, amorphous calcium phosphate-containing primary CPP.9
The main protein component in both primary and secondary CPP is fetuin-A (Fet-A), a liver-derived regulator of extracellular matrix mineralization.10 Serum CPP levels can be estimated indirectly by calculating the difference between Fet-A concentrations before and after sedimentation of the high molecular weight species with high-speed centrifugation, leaving only monomeric or free Fet-A in solution.11 Higher serum Fet-A reduction ratios (CPP Fet-A) have been associated with declining renal function, increased systemic inflammation (high-sensitivity C-reactive protein [hsCRP]), and procalcific cytokine production in addition to increased coronary calcification scores and aortic stiffness.11,12 However, although CPP Fet-A shows promise as a marker of extraosseous mineral stress, there is currently no data linking higher CPP Fet-A levels to patient outcome.
Recently, a novel test was developed that measures the overall calcification propensity of serum.13 This test is based on timing the transformation of amorphous calcium phosphate–containing primary CPP to crystalline hydroxyapatite–containing secondary CPP. Primary and secondary CPP are formed sequentially in vitro on the addition of supraphysiologic concentrations of buffered calcium and phosphate solutions to patient serum. The balance of potentiating and inhibitory factors present in each serum sample governs the transformation time (serum calcium phosphate precipitation time [T50]).
In this study, we provide the first analysis of the clinical and biochemical determinants of serum calcification propensity (T50) in a well described prospective cohort of patients with stages 3 and 4 CKD. We examined the relationship of T50 with longitudinal changes in aortic stiffness and its association with all-cause mortality in this population. We hypothesized that increased serum calcification propensity (i.e., reduced serum T50) would be associated with progressive aortic stiffening and predict poor survival.
Results
Characteristics of the Study Population
Baseline characteristics of 184 individuals in whom T50 measurements were performed are summarized in Table 1. Mean±SD estimated GFR (eGFR) was 33±11 ml/min per 1.73 m2 in this predominantly elderly Caucasian male cohort with prevalent systolic hypertension and cardiovascular comorbidity. Conventional serum mineral parameters appeared well controlled and within population-based reference intervals for most patients (adjusted calcium=93%, phosphate=89%, magnesium=78%, and intact parathyroid hormone=50%).
Table 1.
Baseline characteristics of the study group according to tertile of serum T50
| Characteristic | Overall (n=184) | Low (n=61) | Intermediate (n=62) | High (n=61) | P for Trenda |
|---|---|---|---|---|---|
| Serum T50 (min) | 329±95 | 227±44 | 326±26 | 434±58 | — |
| Clinical and hemodynamic parameters | |||||
| Age (yr) | 69±11 | 71±10 | 69±12 | 68±12 | 0.48 |
| Sex (% men) | 73 | 61 | 73 | 85 | 0.009 |
| Cardiovascular comorbidity (%) | 46 | 57 | 44 | 37 | 0.05 |
| History of diabetes (%) | 24 | 28 | 24 | 21 | 0.36 |
| Alcohol intake (units/wk) | 7.7±9.3 | 7.9±9.3 | 7.6±9.1 | 7.6±9.4 | 0.98 |
| Smoking (pack/yr) | 17.1±27.1 | 18.0±27.2 | 14.1±21.0 | 19.2±32.1 | 0.55 |
| Body mass index (kg/m2) | 28.4 (25.5–33.6) | 27.8 (24.9–29.7) | 29.7 (26.3–34.1) | 28.4 (25.7–33.8) | 0.15 |
| Systolic BP (mmHg) | 151±21 | 153±21 | 150±19 | 152±24 | 0.75 |
| Diastolic BP (mmHg) | 81±11 | 79±10 | 82±11 | 83±12 | 0.12 |
| MAP (mmHg) | 105±13 | 105±12 | 104±11 | 102±15 | 0.33 |
| Pulse pressure (mmHg) | 70±19 | 74±20 | 69±21 | 68±16 | 0.07 |
| Heart rate (bpm) | 71±12 | 69±12 | 72±13 | 72±12 | 0.51 |
| APWV (m/s) | 12.9±2.6 | 13.7±2.7 | 13.1±2.6 | 12.0±2.2 | 0.002 |
| Laboratory measurements | |||||
| Hemoglobin (g/dl) | 12.7±1.7 | 11.8±1.7 | 13.0±1.6 | 13.3±1.5 | <0.001 |
| eGFR (ml/min per 1.73 m2)b | 32.9±10.8 | 30.1±10.3 | 32.8±10.7 | 35.7±10.9 | 0.02 |
| Proteinuria (mg/mmol) | 27 (13–68) | 23 (14–83) | 23 (12–76) | 29 (13–57) | 0.70 |
| Albumin (g/L) | 43±3 | 41±3 | 43±3 | 44±3 | 0.05 |
| Total calcium (mmol/L) | 2.34±0.13 | 2.37±0.13 | 2.36±0.12 | 2.31±0.12 | 0.07 |
| Adjusted calcium (mmol/L)c | 2.28±0.12 | 2.27±0.11 | 2.31±0.13 | 2.29±0.11 | 0.19 |
| Ionized calcium (mmol/L) | 1.25±0.11 | 1.27±0.10 | 1.24±0.10 | 1.23±0.11 | 0.04 |
| Phosphate (mmol/L) | 1.08±0.20 | 1.17±0.18 | 1.07±0.22 | 0.99±0.15 | <0.001 |
| Magnesium (mmol/L) | 0.95±0.11 | 0.95±0.10 | 0.95±0.09 | 0.94±0.12 | 0.89 |
| Pyrophosphate (μmol/L) | 3.12±0.63 | 3.01±0.65 | 3.04±0.59 | 3.33±0.57 | 0.02 |
| Parathyroid hormone (ng/L) | 75 (50–114) | 82 (49–125) | 78 (50–111) | 68 (50–110) | 0.11 |
| CTx (μg/L) | 0.44 (0.33–0.57) | 0.51 (0.40–0.65) | 0.41 (0.32–0.57) | 0.42 (0.28–0.52) | 0.004 |
| Total cholesterol (mmol/L) | 4.36±0.96 | 4.20±0.89 | 4.26±1.00 | 4.29±0.95 | 0.14 |
| Triglycerides (mmol/L) | 1.46±0.44 | 1.48±0.45 | 1.51±0.41 | 1.40±0.47 | 0.36 |
| Inflammatory markers | |||||
| hsCRP (mg/L) | 2.27 (0.94–5.80) | 2.75 (1.01–6.29) | 2.01 (0.93–6.08) | 1.98 (0.90–3.98) | 0.04 |
| TNF-α (pg/ml) | 16.8 (12.7–21.2) | 17.5 (13.5–22.9) | 16.6 (13.2–22.4) | 16.4 (11.6–18.3) | 0.02 |
| Fet-A components | |||||
| Total Fet-A (mg/L) | 208±63 | 175±49 | 220±63 | 229±63 | <0.001 |
| Mono Fet-A (mg/L) | 176±63 | 128±39 | 191±57 | 209±60 | <0.001 |
| CPP Fet-A (mg/L) | 32±24 | 47±28 | 29±19 | 20±13 | <0.001 |
| Medication use | |||||
| ACEi/ARB (%) | 67 | 67 | 68 | 66 | 0.97 |
| Calcium channel blocker (%) | 47 | 51 | 43 | 47 | 0.66 |
| Diuretic (%) | 54 | 44 | 55 | 62 | 0.10 |
| β-Blocker (%) | 33 | 31 | 33 | 35 | 0.93 |
| Statin (%) | 59 | 61 | 57 | 61 | 0.86 |
| Calcium supplementation (%) | 8 | 15 | 5 | 3 | 0.02 |
| Vitamin D supplementation (%) | 12 | 18 | 7 | 7 | 0.06 |
Data are mean±SD or median (25th to 75th percentile). ACEi/ARB, angiotensin-converting enzyme inhibitor/angiotensin II receptor blocker.
P for trend was calculated by one-way ANOVA with Tukey post hoc test for continuous variables and chi-squared test for categorical variables.
eGFR was calculated according to the Chronic Kidney Disease Epidemiology Collaboration equation.
Adjusted for plasma albumin concentration according to the following equation: measured calcium (mmol/L)+0.02 (40−albumin [g/L]).
Determinants of Serum T50
Descending tertiles of serum T50 were associated with female gender and lower eGFR, hemoglobin, serum albumin, and plasma pyrophosphate levels as well as higher serum phosphate and ionized calcium concentrations (Table 1). Notably, lower serum T50 was associated with higher inflammatory marker concentrations (hsCRP and TNF-α) as well as the bone resorption marker, C-terminal telopeptide (CTx). Lower serum T50 showed only a weak association with the prevalence of preexisting cardiovascular disease (P=0.05) but was not associated with diabetic status (P=0.36). With respect to medication use, there was a significant association between descending tertiles of serum T50 and calcium supplementation, although the number of individuals on therapy was small. When considering total, monomeric (mono), and CPP-associated Fet-A components separately, total and mono Fet-A concentrations were markedly correlated (r=0.94, P<0.001). Serum T50 was more strongly associated with mono Fet-A concentrations (r=0.54, P<0.001) than total serum Fet-A levels (r=0.35, P<0.001). Serum CPP Fet-A concentrations were also strongly correlated with serum T50 (r=−0.47, P<0.001) but only weakly and inversely correlated with mono Fet-A (r=−0.18, P=0.01). After multivariate modeling, serum T50 remained associated with albumin, phosphate, ionized calcium, pyrophosphate, mono Fet-A, and CTx, explaining more than 49% of the variation in T50 at baseline (Table 2). Scatter plots for these covariates are shown in Figure 1. Although serum magnesium concentration was not associated with serum T50, even in univariate analysis, inclusion in the final model improved fit (F test, P=0.02). Cardiovascular comorbidity, lower eGFR, and higher CPP Fet-A concentrations showed marginal significance in this fully adjusted model.
Table 2.
Univariate and multivariate linear regression analyses of factors associated with serum T50 at baseline (n=184)
| Variablea | SD Increment | Univariateb | Multivariateb | ||
|---|---|---|---|---|---|
| β (95% CI) | P Value | β (95% CI) | P Value | ||
| Men | Men=1 | 42.5 (11.9 to 73.2) | 0.007 | — | — |
| CVD comorbidity | Yes=1 | −14.2 (−34.3 to −6.5) | 0.04 | −10.3 (−29.0 to 0.0) | 0.05 |
| eGFR | 10.8 ml/min per 1.73 m2 | 23.9 (10.6 to 37.2) | 0.001 | 8.5 (−3.3 to 22.9) | 0.07 |
| Albumin | 3 g/L | 23.8 (10.3 to 37.2) | 0.004 | 14.3 (3.51 to 25.3) | 0.04 |
| Phosphate | 0.20 mmol/L | −37.9 (−50.6 to −25.2) | <0.001 | −27.0 (−42.3 to −7.47) | 0.01 |
| Magnesium | 0.11 mmol/L | 4.29 (−9.66 to 18.2) | 0.55 | 8.45 (−2.34 to 19.2) | 0.09 |
| Pyrophosphate | 0.63 μmol/L | 26.7 (13.1 to 40.3) | <0.001 | 17.5 (5.9 to 28.4) | 0.02 |
| Total calciumc | 0.13 mmol/L | −14.1 (−27.9 to −0.29) | 0.05 | — | — |
| Ionized calciumc | 0.11 mmol/L | −16.5 (−39.9 to −9.00) | 0.007 | −9.2 (−24.5 to 0.00) | 0.05 |
| Hemoglobin | 1.7 g/dl | 30.4 (17.5 to 43.3) | <0.001 | — | — |
| Total Fet-Ad | 63 mg/L | 35.0 (21.5 to 48.6) | <0.001 | — | — |
| Mono Fet-Ad | 63 mg/L | 53.4 (41.3 to 65.5) | <0.001 | 39.4 (27.7 to 51.1) | <0.001 |
| CPP Fet-A | 24 mg/L | −47.4 (−59.2 to −35.6) | <0.001 | −20.3 (−50.3 to 4.5) | 0.06 |
| CTxe | 0.19 μg/L | −41.3 (−53.7 to −29.0) | <0.001 | −13.9 (−29.6 to −3.22) | 0.04 |
| TNF-αe | 1.46 pg/ml | −25.1 (−54.3 to −5.83) | 0.02 | — | — |
| hsCRPe | 3.78 mg/L | −14.8 (−27.0 to −3.21) | 0.05 | — | — |
| Ca supplementation | Yes=1 | 31.7 (11.7 to 51.6) | 0.01 | — | — |
CVD, cardiovascular disease.
Per 1 SD increase in each continuous independent variable.
All baseline variables with P<0.10.
Only ionized calcium was entered into the multivariate model because of colinearity with total calcium concentration.
Only mono Fet-A was entered into the final multivariate model because of colinearity with total Fet-A.
Natural log transformed.
Figure 1.
Serum T50 associates with mineral and bone markers in CKD. Bivariate correlation analysis of baseline serum T50 with (A) mono Fet-A, (B) phosphate, (C) albumin, (D) CTx, and (E) PPi concentrations. Pearson’s coefficient (r) is given for each pairwise combination. The continuous line indicates least-square linear regression. PPi, pyrophosphate.
Association of Serum T50 with Progressive Aortic Stiffening
At baseline, descending tertiles of serum T50 were associated with higher aortic PWV (APWV) (Table 1). Linear regression analysis also revealed an inverse association between APWV and serum T50 that was maintained after adjustment for age, mean arterial pressure (MAP), heart rate, and eGFR (per 1 SD decrease in T50, 0.56 m/s; 95% confidence interval [95% CI], 0.17 to 0.95 m/s; P=0.005). We previously reported that CPP Fet-A was a significant determinant of APWV12; however, despite the strong association between serum T50 and CPP Fet-A, the effect of serum T50 on APWV was not significantly attenuated by the addition of CPP Fet-A to the model (0.51 m/s; 95% CI, 0.16 to 0.87 m/s; P=0.005). Together with age, MAP, eGFR, and CPP Fet-A concentration, serum T50 explained 39% of the variation in baseline APWV in the whole cohort of 184 individuals.
The longitudinal measurement of APWV at 6-month intervals, available in 93 patients, allowed us to evaluate whether baseline serum T50 was also predictive of changes in APWV over time (APWV slope). APWV slope was based on four measurements from baseline to 30 months, and a ≥20% increase in APWV from baseline was considered evidence of progressive aortic stiffening (equivalent to a mean increase of 1.0 m/s per year). Compared with the overall study cohort, this subgroup was younger (mean age=64±9 years), had lower baseline systolic BP (145±12 mmHg), had higher eGFR (36±8 ml/min per 1.73 m2), and had less cardiovascular comorbidity (40%). Progressive aortic stiffening was observed in 31 (33%) patients, and it was associated with lower baseline eGFR, pyrophosphate, and T50 and higher baseline age, MAP, APWV, phosphate, hsCRP, CTx, and CPP Fet-A (Table 3). In multivariable-adjusted logistic regression analysis, a lower baseline serum T50 remained associated with increased likelihood of aortic stiffening along with higher age, MAP, hsCRP, APWV, CTx and lower pyrophosphate concentration (Table 4). In this adjusted analysis, the association of aortic stiffening with baseline eGFR and CPP Fet-A concentration lost significance.
Table 3.
Comparison of baseline variables in cases with qualifying longitudinal APWV measurements over 30 months (n=93)
| Variablea | ΔAPWV<20% from Baselineb (n=62) | ΔAPWV≥20% from Baselineb (n=31) | P Value |
|---|---|---|---|
| Age (yr) | 66.9±11.9 | 74.2±11.4 | 0.02 |
| eGFR (ml/min per 1.73 m2) | 35.3±10.5 | 30.3±10.7 | 0.04 |
| MAP (mmHg) | 101±10 | 109±13 | 0.003 |
| APWV (m/s) | 12.1±2.4 | 12.6±2.6 | 0.05 |
| Phosphate (mmol/L) | 1.02±0.19 | 1.11±0.19 | 0.04 |
| Pyrophosphate (μmol/L) | 3.24±0.61 | 2.92±0.60 | 0.02 |
| CTx (μg/L) | 0.44 (0.31–0.60) | 0.51 (0.37–0.72) | 0.02 |
| hsCRP (mg/L) | 1.90 (0.78–4.04) | 2.99 (1.25–6.87) | 0.009 |
| CPP Fet-A (mg/L) | 23±16 | 29±15 | 0.09 |
| T50 (min) | 352±106 | 292±74 | 0.007 |
Data are mean±SD or median (25th to 75th percentile).
All variables with P<0.10.
APWV slope expressed as percent change from baseline; ≥20% increase from baseline value at 30 months was considered significant progression in aortic stiffness.
Table 4.
Multivariable-adjusted odds ratios of APWV progression (≥20% from baseline) in 93 patients
| Variablea | Odds Ratio of APWV Progression (95% CI) | P Value |
|---|---|---|
| Age | 1.89 (1.02 to 3.71) | 0.02 |
| eGFR | 0.91 (0.80 to 1.01) | 0.05 |
| MAP | 1.74 (1.14 to 2.66) | 0.002 |
| Baseline APWV | 2.33 (1.44 to 3.79) | 0.001 |
| Pyrophosphate | 0.95 (0.71 to 0.99) | 0.04 |
| CTx | 1.64 (1.06 to 2.54) | 0.02 |
| hsCRP | 1.24 (1.07 to 2.61) | 0.03 |
| CPP Fet-A | 1.51 (0.98 to 2.32) | 0.06 |
| T50 | 0.52 (0.31 to 0.85) | 0.01 |
All variables with P<0.10.
Odds ratio expressed per 1 SD increase in each independent variable.
Prediction of All-Cause Mortality by T50
During a median follow-up of 5.3 years (interquartile range=3.0–5.9), 43 patients died. Univariate survival analyses according to tertiles of serum T50 and related determinants are presented in Supplemental Figure 1. Descending tertiles of serum T50 remained associated with an increased risk of all-cause mortality after sequential adjustment for patient demographics (model 1), eGFR and proteinuria (model 2), serum phosphate (model 3), and cardiovascular-related risk factors (model 4). Additional adjustment for baseline determinants of serum T50 (mono Fet-A, albumin, ionized calcium, magnesium, pyrophosphate, and CTx concentrations) failed to significantly attenuate the association with risk of all-cause death (model 5) (Table 5). Finally, in another exploratory modeling step, the addition of MAP-adjusted APWV to the model was found to completely attenuate the association between descending tertiles of T50 and outcome (model 6). APWV, however, remained associated with all-cause mortality after these adjustments (high versus low tertile; hazard ratio, 2.35; 95% CI, 1.03 to 5.37; P=0.04). Considering serum T50 on a continuous scale, each 1 SD reduction (95 minutes) was associated with a 39% (95% CI, 10%–87%) increase in the risk of all-cause mortality independent of demographic, renal, phosphate, and other baseline determinants (albumin, magnesium, pyrophosphate, ionized calcium, mono Fet-A, and CTx) as well as cardiovascular risk factors (not including APWV). Applying a similar hierarchical modeling procedure to CPP Fet-A, increasing CPP Fet-A concentrations and ascending tertiles of CPP Fet-A remained associated with outcome after adjustment for demographic, renal, and cardiovascular covariates but lost significance after additional adjustment for serum hsCRP concentration (Supplemental Table 1).
Table 5.
Crude and multivariable-adjusted hazard ratios for all-cause mortality according tertiles of baseline serum T50
| Tertile | High | Intermediate HR (95% CI) | Low HR (95% CI) | P Valuea |
|---|---|---|---|---|
| Crude | Referent | 1.86 (0.76 to 5.24) | 4.86 (1.99 to 11.8) | 0.01 |
| Model 1b | Referent | 1.84 (0.79 to 5.71) | 4.70 (1.82 to 11.8) | 0.01 |
| Model 2c | Referent | 1.80 (0.77 to 5.70) | 4.70 (1.80 to 11.0) | 0.01 |
| Model 3d | Referent | 1.52 (0.59 to 3.01) | 2.95 (1.28 to 7.89) | 0.02 |
| Model 4e | Referent | 1.50 (0.58 to 3.06) | 2.94 (1.29 to 7.53) | 0.02 |
| Model 5f | Referent | 1.36 (0.56 to 2.39) | 2.24 (1.13 to 5.37) | 0.04 |
| Model 6g | Referent | 1.19 (0.52 to 1.74) | 2.01 (0.96 to 3.72) | 0.07 |
The highest tertile was used as the reference group. HR, hazard ratio.
P value for linear trend.
Model 1 including age and sex.
Model 2 including covariates from model 1 plus eGFR (Chronic Kidney Disease Epidemiology Collaboration equation) and proteinuria.
Model 3 including covariates from model 2 plus phosphate.
Model 4 including covariates from model 3 plus cardiovascular disease comorbidity, systolic BP, and smoking history.
Model 5 including covariates from model 4 plus albumin, magnesium, pyrophosphate, ionized calcium, mono Fet-A, and CTx.
Model 6 including covariates from model 5 plus MAP-adjusted APWV.
Apart from serum CTx concentrations, the other determinants of baseline serum T50 were not associated with outcome, even in univariate analysis (Supplemental Figure 1). However, we found that phosphate, magnesium, pyrophosphate, mono Fet-A, and CTx concentrations (stratified by the median value) significantly modified the association between serum T50 and mortality, whereas ionized calcium concentration did not (Table 6).
Table 6.
Modification of the association between baseline serum T50 (per 1 SD decrease) and all-cause mortality by selected covariates dichotomized by the median value
| Variable | n | Hazard Ratio (95% CI) | P Valuea |
|---|---|---|---|
| Overall | |||
| Crude | 184 | 1.89 (1.38 to 2.60) | — |
| +eGFR, age | 184 | 1.67 (1.20 to 2.33) | |
| Albuminb (g/L) | |||
| <43 | 92 | 1.62 (1.13 to 2.29) | 0.45 |
| ≥43 | 92 | 1.60 (1.11 to 2.26) | |
| Phosphateb (mmol/L) | |||
| <1.07 | 92 | 1.55 (1.14 to 2.63) | 0.008 |
| ≥1.07 | 92 | 1.38 (1.08 to 2.24) | |
| Magnesiumb (mmol/L) | |||
| <0.96 | 92 | 1.60 (1.11 to 2.53) | 0.02 |
| ≥0.96 | 92 | 1.72 (1.26 to 2.95) | |
| Pyrophosphateb (μmol/L) | |||
| <3.20 | 92 | 1.49 (1.09 to 2.17) | 0.01 |
| ≥3.20 | 92 | 1.62 (1.16 to 2.55) | |
| Ionized calciumb (mmol/L) | |||
| <1.25 | 92 | 1.54 (1.11 to 2.01) | 0.44 |
| ≥1.25 | 92 | 1.50 (1.10 to 1.95) | |
| Monomeric Fet-Ab (mg/L) | |||
| <174 | 92 | 1.51 (1.13 to 2.35) | 0.03 |
| ≥174 | 92 | 1.60 (1.17 to 2.71) | |
| CTxb (μg/L) | |||
| <0.44 | 92 | 1.63 (1.16 to 2.78) | 0.03 |
| ≥0.44 | 92 | 1.53 (1.13 to 2.40) |
Likelihood test for interaction.
Adjusted for age and eGFR.
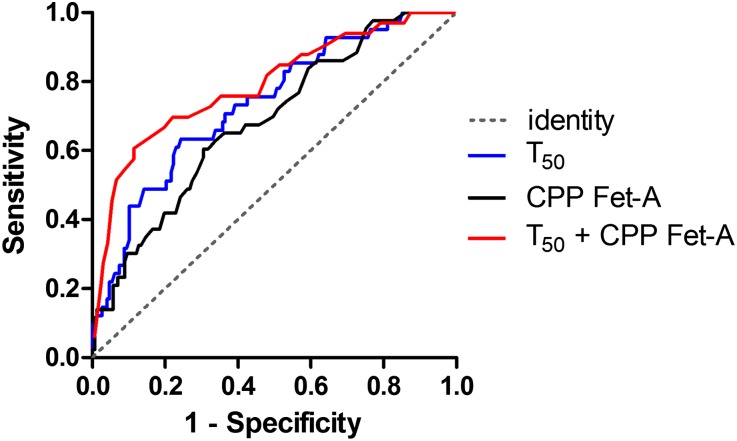
To evaluate the discriminative performance of serum T50 in predicting the risk of all-cause mortality, we constructed receiver operating characteristic curves as depicted in Figure 2. The area under the curve (AUC) for T50 was 0.74 (95% CI, 0.65 to 0.82; P=0.001), which was significantly higher than for CPP Fet-A (0.69; 95% CI, 0.60 to 0.77; P=0.002). Compared with serum T50 alone, a combined model of serum T50 and CPP Fet-A yielded a small but significant increment in the AUC (0.77; 95% CI, 0.70 to 0.88; P<0.001). Substitution of CPP Fet-A with hsCRP in this combined model gave a similar AUC (0.80; 95% CI, 0.71 to 0.89; P<0.001).
Figure 2.
Discriminative performance of serum T50. Receiver operating characteristics curve analysis of serum T50 and CPP Fet-A concentration alone or combined for the prediction of all-cause mortality. Black, CPP Fet-A; blue, T50; red, T50 plus CPP Fet-A. The dashed gray line indicates identify (no discrimination; AUC=0.5).
Longitudinal Variability of Serum T50
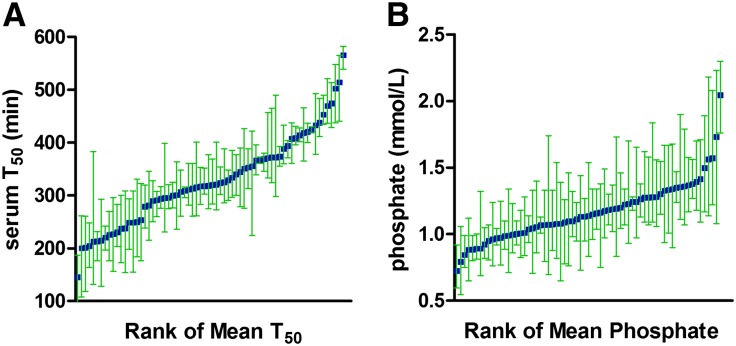
Finally, we considered the longitudinal variability of serum T50 in those patients who completed 2 years of follow-up (four serial readings) and also had concomitant measurement of serum phosphate concentration at the same 6-month time points (n=68). This subgroup was enriched for survivors, and compared with the overall study cohort, individuals in this group were generally younger (mean age=65±10 years), had better baseline renal function (eGFR=36±10 ml/min per 1.73 m2), and had fewer cardiovascular comorbidities (38%). The total variation of each variable comprises between- and within-subject components, with the latter arising from a combination of true biologic variability and analytical imprecision.14 To assess the range of within-subject variance for serum T50 (Figure 3A) and phosphate (Figure 3B), measurements were rank ordered by each individual’s mean value. Overall, the within-subject variability for serum T50 was less pronounced than for serum phosphate concentration, and it showed a trend to greater variability at lower values. Within- (σw) and between-subject (σb) components of variance were estimated using a random effects ANOVA model, and they are depicted in Table 7. Intraclass correlation coefficients (ICC) were also derived for each variable, which expresses the fraction of total variation explained by between-subject variation. A higher ICC indicates greater measurement stability in individual patients over time. Serum T50 measurements yielded a significantly higher ICC compared with phosphate as indicated by the almost nonoverlapping 95% CIs.
Figure 3.
Temporal stability of serum T50 measurements. Longitudinal variation in (A) serum T50 and (B) phosphate concentration over 24 months of follow-up (n=68). Measurements for each individual are rank-ordered by mean value. Plots show mean values (blue squares) along with upper and lower range values (green whiskers).
Table 7.
Variance components for serum T50 and phosphate
| Variable | Mean (Range) | σb (% Total Variance) | σw (% Total Variance) | ICC (95% CI) |
|---|---|---|---|---|
| Serum T50 (min) | 317 (166–547) | 86.2 (81.3) | 19.8 (18.7) | 0.81 (0.68 to 0.91) |
| Serum phosphate (mmol/L) | 1.16 (0.73–2.05) | 0.25 (62.5) | 0.15 (37.5) | 0.63 (0.34 to 0.71) |
Discussion
In this prospective long-term study of predominantly elderly hypertensive patients with stable mild to moderate CKD, increased serum calcification propensity (reduced T50) was independently associated with an increased future risk of all-cause mortality. Serum T50 was also independently associated with progressive aortic stiffness, an important intermediate cardiovascular end point and downstream consequence of enhanced vascular calcification. CPP Fet-A concentration, however, was found to be an inflammation-associated risk factor for future risk of death.
In baseline cross-sectional analysis, reduced serum T50 was associated with lower Fet-A, magnesium, and pyrophosphate concentrations—three inhibitors of vascular calcification15–17—and higher phosphate and calcium concentrations—promoters of vascular calcification.18 Variation in this novel measure of overall serum calcification propensity, therefore, seems consistent with the putative action of these factors. Reduced serum T50, like CPP Fet-A,12 was, furthermore, independently associated with increased serum CTx concentrations, an indicator of bone oesteoclastic activity. This finding is consistent with epidemiologic data in dialysis cohorts, where loss of bone mineral density has been correlated with increased mineral deposition within the arterial wall and soft tissues.19–21
Fet-A is a potent regulator of extracellular matrix mineralization and the major serum-based inhibitor of calcium phosphate precipitation.10 In serum, Fet-A is a heterogeneous mixture of monomeric species either as free protein or in complex with calcium phosphate ion clusters and in macromolecular structures called CPP. Here, we have assessed these mono and polymeric species independently of one another. In the present study, serum T50 was most convincingly associated with the mono Fet-A fraction. This finding makes mechanistic sense, because only the free unbound protein would be expected to participate in de novo CPP formation, as triggered by the addition of high calcium and phosphate in this ex vivo test of serum. Similarly, ionized calcium, rather than total or albumin-adjusted concentration, was most closely related to serum T50.
The association between CPP Fet-A and all-cause mortality was lost after adjustment for hsCRP, suggesting CPP Fet-A to be an inflammation-related risk factor. Our recent in vitro work adds biologic plausibility to this idea, where we found that exposure of murine macrophages to high levels of CPP induced a sustained proinflammatory response.9 Indeed, proinflammatory cytokines, such as TNF-α, are considered important drivers of vascular smooth muscle cell osteochondrocytic conversion and mineralization.22 Unlike CPP Fet-A,12 however, serum T50, was only weakly associated with TNF-α and hsCRP concentrations, and inclusion of either of these parameters in Cox regression analysis did not attenuate the strength of the relationship between serum T50 and death (data not shown). On the contrary, inclusion of hsCRP into a combined model with T50 yielded a significant increment in the AUC of the receiver operating characteristic curve. Serum T50 may, therefore, more accurately reflect the physiochemical determinants of mineral crystal growth and aggregation in solution rather than the proposed cellular inflammation-driven pathways of arterial calcification. Since the relative importance of cellular involvement and physiochemical processes in mineral deposition in CKD is unclear, additional work in human arteries from adult CKD patients is needed to address these questions and determine their presence and activity in older patients with longer cumulative exposure to injurious vascular toxins.
After multivariate adjustment for other baseline covariates, the association between serum T50 and CPP Fet-A failed to maintain significance (P=0.06). Thus, endogenous CPP load does not seem to be a major determinant of current serum calcification propensity as determined by this test. This finding seems logical, because the T50 result is based on the maturation times of primary to secondary CPP,13 whereas the endogenous CPP Fet-A concentration mainly reflects the abundance of CPP already circulating in uremic serum.9 Importantly, this finding does not preclude the possibility that CPP may initiate or drive mineralization in the arterial wall, and this hypothesis needs to be tested.
Taken together, the available data suggest that CPP Fet-A measurement provides a current or retrospective measure of extraosseous mineralization stress, whereas serum T50 better defines the future calcification risk largely related to common mineral and bone factors. Therefore, serum T50 and CPP Fet-A potentially capture different but complementary aspects of CKD patient risk. Indeed, the significant increment in AUC observed with serum T50 combined with CPP Fet-A points to the value of considering both parameters concurrently.
Serum calcification propensity is evidently governed by the activity of numerous humoral and cellular factors that either promote or inhibit mineralization through their effects on crystal nucleation and growth.7 The balance between these opposing forces is, therefore, inherently difficult to gauge by measurement of a single protein or molecule because of the multifactorial nature of the mechanisms driving mineralization. Rather than focus on individual elements, calcium phosphate precipitation may be a more attractive target and a functional and direct measure of this terminal event itself. Timing the transformation of nascent primary CPP to mature hydroxyapatite-containing secondary CPP, a nanoscale mineralization phenomenon, may represent such a tool and a window to the physiochemical disturbances within the arterial environment. In a single measure, T50 ascertainment provides a readout of the equilibrium point of calcification processes within the extracellular fluid; thus, it may represent a more powerful predictor of patient outcome, because it better defines the overall tendency to calcify. Consistent with this notion, apart from CTx concentration, the major determinants of serum T50 (namely phosphate, albumin, ionized calcium, mono Fet-A, and pyrophosphate concentrations) were also not by themselves predictive of the study end point. Although this finding might reflect an inability to detect the small effect associated with each of these variables on outcome because of the moderate study size/event rate, it also supports the utility of the functional composite measure T50. Importantly, however, phosphate, pyrophosphate, magnesium, mono Fet-A, and CTx concentration were found to significantly modulate the association between T50 and outcome. In this context, it is also noteworthy that the aforementioned baseline determinants of serum T50 only accounted for approximately 50% of the variation in this parameter; thus, other species that modulate CPP maturation await identification. At least in the stages 3 and 4 CKD setting, another characteristic that may favor the use of serum T50 as a predictive risk marker is its marked between-individual variance but apparent stability over time in the same individual.
A limitation of the present study is the lack of vascular imaging to provide a correlate of serum T50 and the severity of arterial calcification. Instead, we have shown a robust association with APWV, the gold standard measure of aortic stiffness,23 at baseline and despite survivor bias, longitudinally as well. Aortic calcification has been strongly associated with APWV,4,24–26 and as confirmed here, it is itself independently predictive of patient outcome in predialysis CKD.27 Importantly, our analysis suggests that the risk associated with reduced T50 and all-cause mortality is on the same causal pathway as the risk associated with elevated APWV, because inclusion of APWV fully attenuated the relationship of T50 and outcome. Nonetheless, we readily acknowledge that analysis of arterial calcification scores may have provided additional mechanistic insight in this context.
Another limitation is that the moderate study size/event rate did not permit evaluation of the relationship of serum T50 with cardiovascular and noncardiovascular causes of death separately. Furthermore, it should be acknowledged that CPP Fet-A measurement itself is constrained by stringent analytical requirements, necessitating very high precision and rigorous control of preparative ultracentrifugation. Even with these efforts, 14 (7%) patients had CPP Fet-A concentrations below the limit of quantitation (7.5 mg/L), which may have contributed to the loss of signal observed in multivariable-adjusted survival analysis.
In summary, we have provided evidence that serum T50, a novel and global measure of extracellular calcification potential, is principally dependent on serum phosphate and mono Fet-A concentrations in human CKD. We also report here, for the first time, that increased serum calcification propensity was independently associated with progressive aortic stiffening and an increased risk of all-cause mortality in this setting. Serum T50 ascertainment seems to improve prognostication beyond single protein or molecule measurements. Additional studies are needed to corroborate these findings and evaluate whether therapeutic targeting or manipulation of serum T50 and its determinants might provide patient benefit.
Concise Methods
Study Design and Population
The Arterial Compliance And oxiDant strEss as predictors of rate of loss of renal function, morbidity and Mortality In CKD study was a single-center, prospective, observational study examining aspects of cardiovascular risk in a cohort of 200 individuals with stages 3 and 4 CKD.28,29 A detailed study description with inclusion and exclusion criteria is provided in Supplemental Material. These patients were recruited from nephrology outpatient clinics at Brighton and Sussex University Hospitals National Health Service Trust, United Kingdom, between March of 2006 and September of 2009. Patients were followed prospectively until they died, they started renal replacement therapy, or the observation period ended. Samples were collected at entry to the study and biannual follow-up visits concomitant with clinical and vascular assessments. Samples taken for standard clinical practice were analyzed immediately at the central Brighton and Sussex University Hospitals pathology laboratory, and additional aliquots were stored for future testing. Standard biochemical analysis was performed using routine automated analyzers as described in Supplemental Material. Because of limited availability of stored serum samples at the study enrollment visit, the present analysis is restricted to participants who had samples available for measurement of serum calcification risk factors at the 6-month visit (n=184). We used clinical and laboratory data collected at this 6-month visit as covariates in all subsequent analyses. Underlying causes of renal dysfunction in this cohort were hypertension (n=70), diabetic nephropathy (n=10), chronic GN (n=28), vasculitis (n=14), interstitial nephritis (n=7), cystic kidney disease (n=8), obstructive or congenital disease (n=21), and unknown (n=26). Participants gave written informed consent, and the study was approved by the West Sussex Research Ethics Committee (5/Q1911/89) and conducted in accordance with the Declaration of Helsinki.
APWV Assessment
APWV measurement was performed using Complior (Colson, Les Lilas, France) according to best practice guidelines as previously described.29,30 The progression of aortic stiffening (ΔAPWV) was evaluated in individuals with APWV readings at four consecutive study visits from baseline using linear mixed modeling. APWV slopes were non-normally distributed, and qualifying patients (n=93) were categorized into two groups: patients with an APWV slope≥20% (progressors) and patients with an APWV<20% (nonprogressors).
Serum Calcification Propensity Test
The serum calcification propensity test was performed using a Nephelostar nephelometer (BMG Labtech, Offenburg, Germany) as previously described.13 All serum samples were measured in a blinded manner at the Department of Nephrology and Hypertension, University Hospital Bern, Bern, Switzerland. Data were processed by calculating the precipitation time T50 from nonlinear regression curves. Samples were measured in triplicate. The analytical coefficient of variation of a pooled serum precipitating at 270 minutes was 8.3%.
Fet-A Measurements
Serum Fet-A was measured by ELISA (Biovendor, Brno, Czech Republic) as previously described.12 Briefly, total Fet-A concentration was measured after centrifugation of clotted blood samples (10 minutes at 2000×g at 4°C). Aliquots of each serum sample were then subjected to additional centrifugation at 24,000×g for 2 hours at 4°C in sealed tubes, and the supernatant was reanalyzed for Fet-A using the same ELISA assay. For total serum Fet-A measurements, samples were diluted 1:10,000 in dilution buffer as recommended by the manufacturer. Supernatants were assayed after 1:8500 dilution in the same buffer. CPP Fet-A was then calculated by the difference in total serum Fet-A and supernatant mono Fet-A concentration: CPP Fet-A=total Fet-A−mono Fet-A. Between-batch imprecision was 2.6% at 30 mg/L, and the limit of detection was 1.1 mg/L. All measurements were made in triplicate. Sample dilutions, reagent additions, incubations, and photomeric readings were performed using an automated DS2 ELISA processing system equipped with disposable tips (Dynex, Chantilly, VA). The limit of quantitation for CPP Fet-A estimation was 7.5 mg/L.
Exposures and Outcomes
The primary exposure was baseline serum T50, and the secondary exposure was CPP Fet-A concentration. The outcome measure for this analysis was time to death from any cause that occurred after the 6-month follow-up visit and before censoring in November of 2012. Survival data were gathered prospectively during the study. The survival status of patients was then confirmed using electronic hospital computer records.
Statistical Analyses
Demographic, cardiovascular, and biochemical factors were compared across tertiles of baseline serum T50 using one-way ANOVA, Kruskal–Wallis test, and chi-squared test as appropriate. Parathyroid hormone, CTx, hsCRP, and TNF-α concentrations showed a skewed distribution and were natural log transformed before additional analysis. Determinants of baseline serum T50 were evaluated using linear regression models. Continuous baseline predictors were standardized and expressed per 1 SD increase. Because clinical correlates of serum T50 have not been previously defined, all covariates with a P value <0.10 in univariate analysis were entered simultaneously into the final multivariable model. Models were tested for colinearity using variance inflation factors and stability of the regression coefficients. We observed significant colinearity between total and mono Fet-A and between total and ionized calcium concentrations, and these variables were modeled separately. The association between baseline APWV and serum T50 was analyzed using multiple linear regression adjusted for known determinants of APWV: age, eGFR, MAP, and heart rate.30 Multiple logistic regression was used to evaluate the relationship between APWV progression (dichotomized into <20% or ≥20% increase from baseline over 30 months) and baseline serum T50 adjusted for the above prespecified covariates and other factors significantly associated with APWV progression in univariate analysis: baseline APWV, hsCRP, CPP Fet-A, phosphate, pyrophosphate, and CTx concentrations. We analyzed the risk of all-cause death according to these exposures on a continuous scale (per 1 SD increment) and across tertiles to account for nonlinear effects. The Kaplan–Meier method was used to present unadjusted univariate analyses, and we tested for trends with log-rank tests. After confirming the proportionality assumption using Schoenfeld residual and log-minus-log survival plots, Cox proportional hazard models were used to adjust for confounding. The multivariable modeling strategy was hierarchical, prespecified, and consistent for both primary and secondary exposures. For analysis according to tertiles, the highest tertile served as the reference category for serum T50, and the lowest tertile served as the reference group for CPP Fet-A. Five sequential sets of covariates were considered: model 1 included age and sex; model 2 included covariates from model 1 plus eGFR and proteinuria; model 3 included covariates from model 2 plus phosphate concentration; model 4 included covariates from model 3 plus history of preexisting cardiovascular disease, systolic BP, and smoking status; and model 5 included covariates from model 4 plus previously identified determinants of each exposure (for serum T50, analyses were further adjusted for albumin, magnesium, pyrophosphate, ionized calcium, mono Fet-A, and CTx; for CPP Fet-A, analyses were adjusted for hsCRP concentration). In separate analyses, exclusion of those patients receiving calcium supplementation did not significantly affect the modeling (data not shown). Potential modifiers of the association between serum T50 and mortality by selected baseline confounders were evaluated by introducing interaction terms into the model and tested by the likelihood ratio test. The AUC was calculated to compare prognostic value of each exposure using the nonparametric method of DeLong et al.31 Variance components (within-subject variance [σ2w]; between-subject variance [σ2b]) were estimated using random effects ANOVA models as previously described, and ICC were derived using the following formula: σ2b/(σ2b+σ2w).32 All analyses were performed using Stata Release 12/IC (College Station, TX), and two-sided values of P<0.05 were considered statistically significant.
Disclosures
E.R.S., L.P.M., and S.G.H. have received research funding from Amgen and Baxter. E.R.S. has received honoraria from Shire. L.P.M. has received honoraria from Amgen and Roche. S.G.H. has received honoraria from Amgen, Baxter, Gilead, and Shire. A.P. has received research funding from Köhler Chemie. M.L.F., L.A.T., E.B., S.F., and C.R. do not have any competing financial interests.
Supplementary Material
Acknowledgments
We thank Beatrix Blanchard for excellent technical assistance with the T50 and magnesium assays.
We acknowledge support from the Sussex Kidney Unit and the Clinical Investigation and Research Unit, Brighton and Sussex University Hospitals and the Department of Renal Medicine, Eastern Health Clinical School, Monash University. E.R.S. was funded in part by an unrestricted investigator-initiated grant from Amgen Australia. Part of this work was supported by a Jacquot Foundation grant (to S.G.H.) and a research grant from the Swiss National Centre of Competence in Research Kidney (to A.P.).
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2013060635/-/DCSupplemental.
References
- 1.Blacher J, Guerin AP, Pannier B, Marchais SJ, London GM: Arterial calcifications, arterial stiffness, and cardiovascular risk in end-stage renal disease. Hypertension 38: 938–942, 2001 [DOI] [PubMed] [Google Scholar]
- 2.Monckeberg JG: Über die reine Mediaverkalkung der Extremitätenarterien und ihr Verhalten zur Arteriosklerose. Virchows Arch A Pathol Anat Histol 171: 141–167, 1903 [Google Scholar]
- 3.London GM, Pannier B, Marchais SJ: Vascular calcifications, arterial aging and arterial remodeling in ESRD. Blood Purif 35: 16–21, 2013 [DOI] [PubMed] [Google Scholar]
- 4.Guérin AP, London GM, Marchais SJ, Metivier F: Arterial stiffening and vascular calcifications in end-stage renal disease. Nephrol Dial Transplant 15: 1014–1021, 2000 [DOI] [PubMed] [Google Scholar]
- 5.O’Rourke MF: Arterial aging: Pathophysiological principles. Vasc Med 12: 329–341, 2007 [DOI] [PubMed] [Google Scholar]
- 6.Mitchell GF: Arterial stiffness and wave reflection: Biomarkers of cardiovascular risk. Artery Res 3: 56–64, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shroff R, Long DA, Shanahan C: Mechanistic insights into vascular calcification in CKD. J Am Soc Nephrol 24: 179–189, 2013 [DOI] [PubMed] [Google Scholar]
- 8.Shanahan CM, Cary NR, Salisbury JR, Proudfoot D, Weissberg PL, Edmonds ME: Medial localization of mineralization-regulating proteins in association with Mönckeberg’s sclerosis: Evidence for smooth muscle cell-mediated vascular calcification. Circulation 100: 2168–2176, 1999 [DOI] [PubMed] [Google Scholar]
- 9.Smith ER, Hanssen E, McMahon LP, Holt SG: Fetuin-A-containing calciprotein particles reduce mineral stress in the macrophage. PLoS One 8: e60904, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jahnen-Dechent W, Heiss A, Schäfer C, Ketteler M: Fetuin-A regulation of calcified matrix metabolism. Circ Res 108: 1494–1509, 2011 [DOI] [PubMed] [Google Scholar]
- 11.Hamano T, Matsui I, Mikami S, Tomida K, Fujii N, Imai E, Rakugi H, Isaka Y: Fetuin-mineral complex reflects extraosseous calcification stress in CKD. J Am Soc Nephrol 21: 1998–2007, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smith ER, Ford ML, Tomlinson LA, Rajkumar C, McMahon LP, Holt SG: Phosphorylated fetuin-A-containing calciprotein particles are associated with aortic stiffness and a procalcific milieu in patients with pre-dialysis CKD. Nephrol Dial Transplant 27: 1957–1966, 2012 [DOI] [PubMed] [Google Scholar]
- 13.Pasch A, Farese S, Gräber S, Wald J, Richtering W, Floege J, Jahnen-Dechent W: Nanoparticle-based test measures overall propensity for calcification in serum. J Am Soc Nephrol 23: 1744–1752, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fraser CG, Harris EK: Generation and application of data on biological variation in clinical chemistry. Crit Rev Clin Lab Sci 27: 409–437, 1989 [DOI] [PubMed] [Google Scholar]
- 15.Jahnen-Dechent W, Schäfer C, Heiss A, Grötzinger J: Systemic inhibition of spontaneous calcification by the serum protein alpha 2-HS glycoprotein/fetuin. Z Kardiol 90[Suppl 3]: 47–56, 2001 [DOI] [PubMed] [Google Scholar]
- 16.O’Neill WC, Lomashvili KA, Malluche HH, Faugere MC, Riser BL: Treatment with pyrophosphate inhibits uremic vascular calcification. Kidney Int 79: 512–517, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Louvet L, Büchel J, Steppan S, Passlick-Deetjen J, Massy ZA: Magnesium prevents phosphate-induced calcification in human aortic vascular smooth muscle cells. Nephrol Dial Transplant 28: 869–878, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shanahan CM, Crouthamel MH, Kapustin A, Giachelli CM: Arterial calcification in chronic kidney disease: Key roles for calcium and phosphate. Circ Res 109: 697–711, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Toussaint ND, Lau KK, Strauss BJ, Polkinghorne KR, Kerr PG: Associations between vascular calcification, arterial stiffness and bone mineral density in chronic kidney disease. Nephrol Dial Transplant 23: 586–593, 2008 [DOI] [PubMed] [Google Scholar]
- 20.Toussaint ND, Lau KK, Strauss BJ, Polkinghorne KR, Kerr PG: Relationship between vascular calcification, arterial stiffness and bone mineral density in a cross-sectional study of prevalent Australian haemodialysis patients. Nephrology (Carlton) 14: 105–112, 2009 [DOI] [PubMed] [Google Scholar]
- 21.London GM, Marchais SJ, Guérin AP, Boutouyrie P, Métivier F, de Vernejoul MC: Association of bone activity, calcium load, aortic stiffness, and calcifications in ESRD. J Am Soc Nephrol 19: 1827–1835, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tintut Y, Patel J, Parhami F, Demer LL: Tumor necrosis factor-alpha promotes in vitro calcification of vascular cells via the cAMP pathway. Circulation 102: 2636–2642, 2000 [DOI] [PubMed] [Google Scholar]
- 23.Laurent S, Cockcroft J, Van Bortel L, Boutouyrie P, Giannattasio C, Hayoz D, Pannier B, Vlachopoulos C, Wilkinson I, Struijker-Boudier H, European Network for Non-Invasive Investigation of Large Arteries : Expert consensus document on arterial stiffness: Methodological issues and clinical applications. Eur Heart J 27: 2588–2605, 2006 [DOI] [PubMed] [Google Scholar]
- 24.Raggi P, Bellasi A, Ferramosca E, Islam T, Muntner P, Block GA: Association of pulse wave velocity with vascular and valvular calcification in hemodialysis patients. Kidney Int 71: 802–807, 2007 [DOI] [PubMed] [Google Scholar]
- 25.Sigrist MK, Taal MW, Bungay P, McIntyre CW: Progressive vascular calcification over 2 years is associated with arterial stiffening and increased mortality in patients with stages 4 and 5 chronic kidney disease. Clin J Am Soc Nephrol 2: 1241–1248, 2007 [DOI] [PubMed] [Google Scholar]
- 26.McEniery CM, McDonnell BJ, So A, Aitken S, Bolton CE, Munnery M, Hickson SS, Yasmin, Maki-Petaja KM, Cockcroft JR, Dixon AK, Wilkinson IB, Anglo-Cardiff Collaboration Trial Investigators : Aortic calcification is associated with aortic stiffness and isolated systolic hypertension in healthy individuals. Hypertension 53: 524–531, 2009 [DOI] [PubMed] [Google Scholar]
- 27.Karras A, Haymann JP, Bozec E, Metzger M, Jacquot C, Maruani G, Houillier P, Froissart M, Stengel B, Guardiola P, Laurent S, Boutouyrie P, Briet M, Nephro Test Study Group : Large artery stiffening and remodeling are independently associated with all-cause mortality and cardiovascular events in chronic kidney disease. Hypertension 60: 1451–1457, 2012 [DOI] [PubMed] [Google Scholar]
- 28.Smith ER, Tomlinson LA, Ford ML, McMahon LP, Rajkumar C, Holt SG: Elastin degradation is associated with progressive aortic stiffening and all-cause mortality in predialysis chronic kidney disease. Hypertension 59: 973–978, 2012 [DOI] [PubMed] [Google Scholar]
- 29.Ford ML, Tomlinson LA, Chapman TP, Rajkumar C, Holt SG: Aortic stiffness is independently associated with rate of renal function decline in chronic kidney disease stages 3 and 4. Hypertension 55: 1110–1115, 2010 [DOI] [PubMed] [Google Scholar]
- 30.Benetos A, Adamopoulos C, Bureau JM, Temmar M, Labat C, Bean K, Thomas F, Pannier B, Asmar R, Zureik M, Safar M, Guize L: Determinants of accelerated progression of arterial stiffness in normotensive subjects and in treated hypertensive subjects over a 6-year period. Circulation 105: 1202–1207, 2002 [DOI] [PubMed] [Google Scholar]
- 31.DeLong ER, DeLong DM, Clarke-Pearson DL: Comparing the areas under two or more correlated receiver operating characteristic curves: A nonparametric approach. Biometrics 44: 837–845, 1988 [PubMed] [Google Scholar]
- 32.Ockene IS, Matthews CE, Rifai N, Ridker PM, Reed G, Stanek E: Variability and classification accuracy of serial high-sensitivity C-reactive protein measurements in healthy adults. Clin Chem 47: 444–450, 2001 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.