Abstract
There is currently no effective prophylactic regimen available to prevent contrast-induced AKI (CI-AKI), a frequent and life-threatening complication after cardiac catheterization. Therefore, novel treatment strategies are required to decrease CI-AKI incidence and to improve clinical outcomes in these patients. Remote ischemic preconditioning (rIPC), defined as transient brief episodes of ischemia at a remote site before a subsequent prolonged ischemia/reperfusion injury of the target organ, is an adaptational response that protects against ischemic and reperfusion insult. Indeed, several studies demonstrated the tissue-protective effects of rIPC in various target organs, including the kidneys. In this regard, rIPC may offer a novel noninvasive and virtually cost-free treatment strategy for decreasing CI-AKI incidence. This review evaluates the current experimental and clinical evidence for rIPC as a potential renoprotective strategy, and discusses the underlying mechanisms and key areas for future research.
Ischemic preconditioning (IPC), transient brief episodes of ischemia before a subsequent prolonged ischemia/reperfusion injury, has been shown to reduce the extent of organ damage. IPC can be induced locally when the preconditioning stimulus is applied to the same organ or tissue incurring the ischemic injury. The concept of IPC was introduced in 1986 by Murry et al., who first described the cardioprotective effect of multiple brief ischemic episodes before subsequent sustained ischemic insult in dogs with myocardial infarction.1
However, this protection not only acts locally but can also protect distant tissues, a phenomenon known as remote IPC (rIPC). rIPC was first demonstrated in cardiac tissue in which brief episodes of myocardial ischemia and reperfusion applied to one vascular territory reduced the infarct size of the adjacent tissue that had not undergone any preconditioning.2
rIPC has primarily been applied to the myocardium as a target organ, but subsequent studies showed that brief ischemia induced in nontarget tissue, most commonly in the limb or arm, confers protection at a remote site such as the brain, lung, kidney, intestine, or skeletal muscle.3–5rIPC causes a similar degree of tissue protection, as does IPC.6
Kidneys are one of the major organs of interest for clinical application of rIPC. Due to their high energy demand and complex microvascular network, kidneys are especially sensitive to ischemic injury, which is a major pathophysiologic basis of acute renal dysfunction, including contrast-induced AKI (CI-AKI), in patients with pre-existing heart disease.7,8 Accordingly, experimental and clinical evidence suggests that rIPC might be an effective tool to protect kidneys from ischemic injury. In this regard, we and others recently demonstrated that rIPC may offer a novel noninvasive and virtually cost-free treatment strategy to decrease acute renal impairment incidence in patients undergoing cardiac catheterization.5,9This article evaluates the current evidence for rIPC as a potential renoprotective strategy and discusses its possible clinical applications.
Potential Mechanisms in RIPC
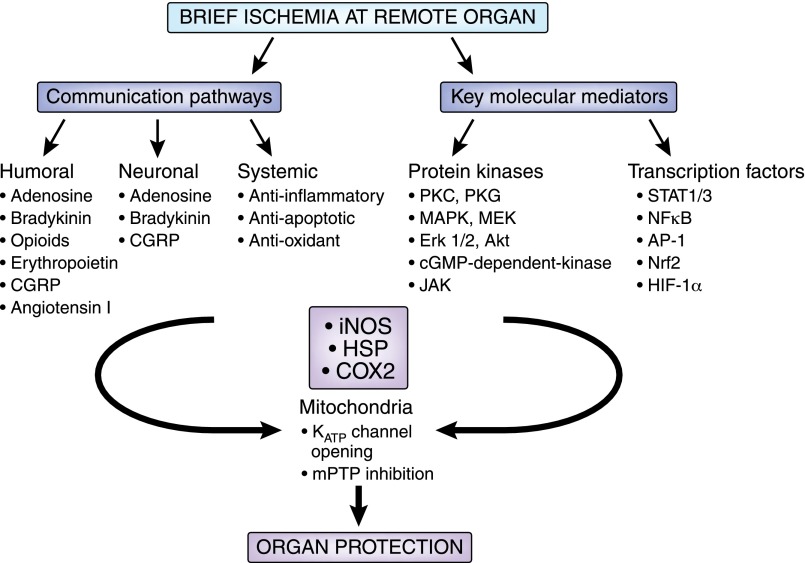
The underlying mechanisms of rIPC are very complex and not yet fully defined. It has been hypothesized that rIPC predominantly involves systemic multifactorial anti-inflammatory, neuronal, and humoral signaling pathways, which may differ in response to various ischemic stimuli and are likely to interact with each other (Figure 1).
Figure 1.
Mechanisms of rIPC. AP-1, activator protein-1; cGMP, cyclic guanosine monophosphate; CGRP, calcitonin gene-related peptide; COX2, cyclooxygenase 2; HIF-1α, hypoxia-inducible factor 1α; HSP, heat shock protein; iNOS, inducible nitric oxide synthase; JAK, Janus kinase; MEK, MAPK kinase; mPTP, mitochondrial permeability transition pore; Nrf2, nuclear factor (erythroid-derived 2)-like 2; STAT1/3, signal transducer and activator of transcription.
Signal Transduction Pathways
As reviewed in more detail elsewhere,10considerable interest has focused on the role of protein kinases (PKs) as the key point of convergence for a range of preconditioning triggers, including adenosine, bradykinin, and opioids. Armstrong et al. were the first to identify PKC as a potential mediator of ischemia-induced protection.11The current concept of signal transduction in IPC suggests activation of the signaling cascades through the phosphoinositide 3-kinase/Akt/endothelial nitric oxide synthase (NOS)/cyclic guanosine monophosphate/PKG pathways, eventually leading to the opening of the ATP-dependent mitochondrial potassium (KATP) channel, which is believed to be a downstream target of PKG/PKC activation.10,12,13The activated mitochondrial KATP channels have the ability to limit the opening of mitochondrial permeability transition pores, thus causing a marked improvement in cell survival.14
Nitric oxide (NO) is emerging as an important cytoprotective agent and may play a pivotal role in rIPC both as a trigger and mediator of rIPC. Supporting evidence for the role of NO in mediating the protection against ischemic injury comes from experiments showing that inhibition of NOS isoforms by the nonselective NOS inhibitor L-NAME (NG-nitro-L-arginine methyl ester hydrochloride) results in abrogation of the protective effects of IPC. 15In addition, IPC was shown to induce NOS expression with a subsequent increase in the NO oxidation products nitrite and nitrate. 16,17Similarly, infusion of L-NAME before hind limb rIPC abolished its protective effects against subsequent abdominal adipocutaneous flap ischemia.18
Neuronal and Humoral Pathways
Involvement of neuronal pathways is mostly based on the finding that blockade of the autonomic ganglion reversed the cardioprotective effects of rIPC when the preconditioning ischemic insult is performed via mesenteric artery occlusion.19 This concept appears to also apply to the rIPC-associated neuroprotection in cerebral tissue, as was recently demonstrated.20 In addition, adenosine receptors, particularly the subtype A1, have been implicated as the mediators of neuroprotection in rIPC, likely through increased production of specific antioxidants and NO.21,22
Organ protection by remote ischemia may also be related to a catecholamine effect, because pretreatment with certain catecholamines can mimic the effect of preconditioning.23,24 Other underlying mechanisms may include humoral factors, such as adenosine, bradykinin, erythropoietin, δ 1-opioid, and free radicals, released into the systemic circulation, which subsequently protect the remote organ.25–29
Anti-Inflammatory Pathways
Some studies suggested that protective effect of rIPC may be due to the beneficial anti-inflammatory or antioxidant effects, including decreased extracellular levels of noxious metabolites, such as protons and lactate.25,30 In support of this concept, rIPC reduced neutrophil activation through expression of neutrophil CD11b and platelet neutrophil complexes.31 Moreover, all three key kinases involved in TNF synthesis, mitogen-activated protein kinase (MAPK)–activated protein kinase 2, MAPK kinase kinase 2, and MAPK kinase kinase 8, were suppressed, whereas ischemic preconditioning activated TNF-R1, which promotes the production of manganese SOD, a strong antioxidant and protector against reactive oxygen species. The suppression of anti-inflammatory genes extended to proapoptotic, chemotactic, and cell adhesion molecules that promote cellular extravasation.32 Other immunologic changes include the suppression of genes encoding key proteins involved in cytokine synthesis, leukocyte chemotaxis, adhesion, migration, exocytosis, innate immunity signaling pathways, and apoptosis.32,33
Contrast-Induced Nephropathy: Incidence, Pathophysiology, and Therapy
Contrast agents are being widely utilized in diagnostic and interventional procedures, resulting in increased incidence of contrast-induced renal impairment. CI-AKI after cardiac intervention is associated with significant morbidity and mortality, with an in-hospital mortality rate of 20% in unselected patients and a 1-year mortality rate of up to 66% in patients with acute myocardial infarction and renal dysfunction.34–36
CI-AKI, defined most commonly as an increase in serum creatinine by >25% or >0.5 mg/dl (>44 μmol/L) above baseline within 48 days after administration of contrast agents in the absence of an alternative etiology, is one of the most common causes of hospital-acquired ARF.37 The incidence of CI-AKI varies substantially among studies due to the lack of a uniform definition of CI-AKI.38,39 Indeed, the CI-AKI rate may be as high as >50%, depending on the presence of risk factors.34,39–41
CI-AKI is very much dependent on the patient’s risk profile. The main predictor of CI-AKI is preexisting renal dysfunction with an estimated GFR (eGFR)<60 ml/min per 1.73 m2, and its severity directly correlates with the incidence of CI-AKI.34,42 Other risk factors for CI-AKI include diabetes mellitus, major cardiovascular comorbidities, hypovolemia, and administration of high doses of contrast medium and nephrotoxic drugs such as aminoglycosides or nonsteroidal anti-inflammatory drugs.43
The mechanisms of contrast-induced renal impairment are not fully understood. There is solid experimental evidence that renal ischemia, resulting from an imbalance of various vasodilator and vasoconstrictor factors, is a key factor in the pathogenesis of CI-AKI.38,44–46 This mechanism causes subsequent ischemia and hypoxia in the renal medulla, a region with extreme susceptibility to ischemic injury. In addition, oxygen free radicals contribute at least in part to the renal tubular cellular injury.47
Various treatment strategies have been investigated in an effort to decrease CI-AKI incidence in patients undergoing cardiac catheterization. Dopamine, fenoldopam, furosemide, mannitol, aminophylline, atrial natriuretic peptide, captopril, calcium channel blockers, alprostadil, and N-acetylcysteine were not effective in preventing contrast-induced nephropathy.48–53 To date, periprocedural hydration remains the most effective prophylactic measure to prevent CI-AKI. Although clinical studies have not uniformly shown that dehydration is a definite risk factor, iodinated contrast agents increase urine volume and osmolar clearance, and their effect on the kidney is prolonged by the decrease in both renal blood flow and GFR, as seen in dehydrated states.54 Therefore, by increasing the renal perfusion and subsequently diminishing the tubular fluid viscosity, adequate hydration may counteract some of the putative hemodynamic effects that may lead to CI-AKI. The positive effect of hydration has consistently been reported in several studies.52,55,56
RIPC-Induced Renoprotection
Animal Studies
Although the majority of studies to date have demonstrated protection by rIPC against ischemia/reperfusion injury to the myocardium of animals and humans, a small number of studies have investigated the potential of rIPC to protect the kidney. In animal models, the rIPC application was associated with the striking renoprotection.
Earlier investigations demonstrated improved kidney resistance to ischemia by preceding ischemic events,57,58 suggesting potential beneficial effects of rIPC on renal function. Ateş et al. assessed the beneficial effect of brief liver ischemia and reperfusion on rat kidney function as a remote organ.59 Biochemical determination, TNF-α, and tissue thiobarbituric acid–reactive substance levels and histopathologic findings were evaluated. A 10-minute hepatic ischemia with 10-minute reperfusion afforded functional and morphologic protection in rat kidney that underwent a subsequent 45-minute ischemic insult before rIPC, as confirmed by biochemical, histopathologic, and ultrastructural findings at 24 hours of reperfusion.
The corroborative evidence that application of brief small intestinal ischemia attenuates renal ischemia and subsequent reperfusion injury was provided by Song et al., who investigated the effect of small intestinal rIPC on renal function in rats.60 Renal ischemic injury was induced by a 45-minute renal artery occlusion and reperfusion for 2 or 24 hours in rats with a previous contralateral nephrectomy, and rIPC was induced by three cycles of 8-minute ischemia and 5-minute reperfusion of the small intestine. Indeed, pretreatment with intestinal IPC significantly alleviated renal ischemic impairment.
Renoprotective effects of brief hind limb occlusion were reported in rats.61 Rats underwent either unilateral or bilateral rIPC. After 24 hours of reperfusion, renal function was improved in both the bilateral rIPC group and in the fractionated unilateral group, albeit bilateral rIPC was more effective than unilateral rIPC. Treatment with the adenosine receptor blocker 8-(p-sulfophenyl)theophylline had no effect on fractionated or continuous rIPC.
A recently published meta-analysis of experimental data obtained in animal models evaluated three outcome measures: serum creatinine, BUN, and histologic renal damage in renal ischemic/reperfusion injury.62 IPC-associated protective effects were reported for all three parameters. Interestingly, renoprotection was not evident in the female subgroup, stressing the need for future studies in female subjects.
Furthermore, analysis of the gene expression profile in murine heart at 24 hours after brief cycles of occlusion of the superior mesenteric artery revealed that rIPC significantly induced the expression of many genes, including anti-inflammatory and DNA repair genes.63
Clinical Evidence
In addition to experimental evidence obtained in animal models, substantial progress has been made in translating the concept of rIPC from experimental models into clinical practice. Several clinical studies have been performed thus far that predominantly (but, importantly, not all) support the concept that rIPC can be used to reduce renal damage in humans (Table 1). Concerning the safety and tolerability of the methodology, no relevant adverse events related to the rIPC application were described in the clinical studies performed to date. The inflation of the BP cuff may cause pain or a tingling sensation of the arm or leg, but this has never been so severe as to warrant abandoning the preconditioning protocol.
Table 1.
Major clinical trials on renal outcomes in rIPC
| Reference | Clinical Setting | Patients (N) | rIPC Protocol | Renal Outcomes |
| Ali et al. (2007)64 | Elective open abdominal aortic aneurysm repair. | 82 | Two cycles of intermittent cross-clamping of the common iliac artery with 10-min ischemia and 10-min reperfusion. | rIPC reduced the incidence of renal impairment, defined as peak serum creatinine level >2.0 mg/dl (30% versus 7%; P=0.01). |
| Walsh et al. (2009)66 | Endovascular aneurysm repair. | 40 | Lower limb ischemia was used as the rIPC stimulus. After 10 min, the cuff was deflated, and the procedure was repeated on the other leg. | Reduction in urinary albumin/creatinine ratio and urinary retinol binding protein. No differences in the rates of renal impairment. |
| Walsh et al. (2010)65 | Elective open abdominal aortic aneurysm repair. | 51 | Right common iliac clamping for 10 min. After 10 min, the right iliac territory was reperfused and the clamp applied to the left common iliac artery for 10 min. | No statistically significant differences in renal outcome indices (median urinary retinol binding protein, albumin/creatinine ratios, serum creatinine, or GFR values). |
| Rahman et al. (2010)67 | Elective cardiac surgery (CABG). | 162 | 3×5-min cycles of upper-limb cuff inflation to 200 mmHg separated by 5-min periods of cuff deflation. | No significant differences in renal outcome indices (rates of dialysis, peak creatinine, and urinary albumin/creatinine ratio). |
| Venugopal et al. (2010)70 | Elective cardiac surgery (CABG) in nondiabetic patients. | 78 | 3×5-min cycles of right forearm ischemia, induced by inflating a BP cuff to 200 mmHg, after 5 min of reperfusion. | rIPC decreased the incidence of AKI (AKI stages 1, 2, and 3 in rIPC group were 3%, 8%, and 0% compared with 25%, 0%, and 0% in control group, respectively; P=0.01). |
| Zimmermann et al. (2011)71 | Elective cardiac surgery (CABG). | 120 | Thigh tourniquet consisting of 3×5-min intervals of ischemia separated by 5-min intervals of reperfusion. | rIPC decreased the rate of AKI compared with the control group (20% versus 47%; P=0.004). |
| Choi et al. (2011)74 | Elective complex valvular heart surgery. | 76 | 3×10-min cycles of lower limb ischemia and reperfusion with an automated cuff inflator. | No significant differences in serum levels of renal injury biomarkers or incidence of AKI. |
| Pedersen et al. (2012)73 | Children undergoing surgery for complex congenital heart disease. | 113 | Intermittent leg ischemia through four cycles of 5-min BP cuff inflation to 40 mmHg above the systolic pressure and 5-min deflation. | No statistically significant differences in AKI incidence and levels of the renal biomarkers. |
| Er et al. (2012)5 | Elective coronary angiography in patients with impaired renal function. | 100 | Intermittent arm ischemia through four cycles of 5-min inflation and 5-min deflation of a BP cuff. | Decrease in CI-AKI by rIPC (40% control group versus 12% rIPC group; P=0.002) and the incidence of composite cardiovascular endpoint (death, hospitalization, or hemodialysis) (38% versus 16%; P=0.02). |
| Deftereos et al. (2013)72 | Patients with NSTEMI undergoing PCI. | 225 | Remote ischemic postconditioning by cycles of inflation and deflation of the stent balloon during PCI. | rIPC decreased the rate of AKI (12.4% versus 29.5%; P=0.002) and reduced the 30-d mortality or rehospitalization for any cause (12.4% versus 22.3%; P=0.05) compared with the control group. |
| Huang et al. (2013)77 | Patients undergoing laparoscopic partial nephrectomy. | rIPC consisted of three 5-min cycles of right lower limb ischemia and 5 min of reperfusion during each cycle. | rIPC decreased the rate of GFR reduction at 1 mo compared with the control group (8.8% versus 15%; P=0.03). No differences in the GFR change at 6 mo. |
NSTEMI, non–ST-segment elevation myocardial infarction; PCI, percutaneous coronary intervention.
rIPC-mediated effects on the kidney have been extensively investigated in the setting of adult cardiac or vascular surgery. In a landmark study, rIPC-induced cardioprotection and renoprotection were evaluated in 82 adults undergoing abdominal aortic aneurysm repair.64 rIPC, induced by two cycles of intermittent cross-clamping of the common iliac artery, was associated with a 23% decrease in AKI (30% versus 7%; P=0.01). In addition, rIPC significantly reduced the incidence of myocardial infarction.
A separate study in the same clinical scenario, but with fewer patients (N=51) and a different type of rIPC stimulus (common iliac artery clamping), did not find statistically significant differences in renal outcome indices.65 In another randomized clinical trial, the same authors aimed to determine whether rIPC can reduce renal injury in a smaller number of patients (N=40) after endovascular aneurysm repair.66 rIPC was induced by sequential lower limb ischemia. Although there were no significant differences in the rates of renal impairment, rIPC reduced renal injury during procedure, as demonstrated by a reduction in postoperative urinary biomarker levels.
In a prospective randomized placebo-controlled trial (N=162), Rahman et al. tested whether rIPC improves myocardial or other end-organ protection after on-pump coronary surgery.67 Renal outcomes were among the secondary end points. The results showed that in patients undergoing multivessel coronary artery bypass graft (CABG) surgery, the incidence of AKI in those who received rIPC was similar to that of controls. However, this study was performed in anesthetized and, thus, patients were pain free. On the other hand, there is evidence from several experimental studies that pain may be a strong trigger of preconditioning,68 and rIPC is dependent on intact local neural pathways.69 In this context, cautious interpretation of these results is needed, warranting further rIPC efficacy studies in anesthetized versus nonanesthetized subgroups.
In 2010, another retrospective study of nondiabetic patients undergoing elective CABG surgery found that rIPC using transient ischemia of the forearm decreased the incidence of AKI.70 A total of 78 consented patients were randomly assigned to either rIPC (N=38) or control (N=40) groups before CABG surgery. Of 40 patients in the control group, 10 (25%) developed stage 1 AKI and none developed stage 2 or 3 AKI. In contrast, only 1 of 38 patients (3%) in the rIPC group developed stage 1 AKI, although 3 patients developed stage 2 AKI. The overall difference in AKI between the two groups was statistically significant (P=0.01). There was no difference in duration of hospital stay.
The study group was at lower risk of AKI than a standard cardiac surgery cohort, because patients with diabetes and established kidney failure were excluded from this study. Therefore, it is reasonable to hypothesize that any protective effect of rIPC on kidney function may be even more pronounced in a higher-risk cohort. Indeed, our group recently addressed the effects of rIPC in a high-risk patient populations in the RenPro trial.5 This study included 100 adults (mean age 73.2 years) with impaired renal function (serum creatinine>124 μmol/L and/or eGFR<60 ml/min per 1.73 m2; mean Mehran score 13) who underwent elective coronary angiography. Enrolled patients were randomly assigned to either control group (N=50) or to rIPC before cardiac intervention (N=50). The primary study outcome, CI-AKI (defined as a serum creatinine increase of >44 μmol/L or a relative increase of ≥25% from baseline within 48 hours after exposure to contrast medium), occurred in significantly fewer patients in the rIPC group than in the control group (12% versus 40%; P=0.002). No major adverse events related to the procedure were reported. Overall, there was a substantial decrease in the number of patients developing CI-AKI in individuals who received rIPC before coronary angiography, suggesting that rIPC was particularly renoprotective in high-risk patients.
Similar results were obtained in another study of lower limb preconditioning in patients undergoing elective CABG.71 The primary end point was AKI defined as an elevation of serum creatinine of ≥0.3 mg/dl or ≥50% within 48 hours after surgery. Sixty patients were randomized to rIPC or control groups. Significantly fewer patients in the rIPC group had AKI within 48 hours after surgery compared with the control group (20% versus 47%, P=0.004), reflecting an absolute risk reduction of 0.27 (95% confidence interval, 0.24–0.76) and a significantly reduced relative risk due to preconditioning of 0.43 (95% confidence interval, 0.10–0.42).
The effect of rIPC on renal impairment was also investigated in patients undergoing laparoscopic partial nephrectomy. The primary outcome was the absolute change in GFR of the affected kidney by renal scintigraphy from baseline to 6 months. rIPC, which consisted of three 5-minute cycles of right lower limb ischemia and 5 minutes of reperfusion during each cycle, was associated with the lower incidence of GFR reduction at 1 month after the procedure (8.8% versus 15% in the control group, P=0.03). However, no differences in the GFR change of the affected kidney or serum creatinine were observed at 6 months of follow-up.
Deftereos et al. recently provided additional evidence that remote ischemic postconditioning may also be effective in preventing acute kidney damage in intermediate-risk patients (mean Mehran risk score 10).72 The authors evaluated the renoprotective effect of remote ischemic postconditioning in patients with a non–ST-segment elevation myocardial infarction undergoing percutaneous coronary intervention (N=225). In the intervention group, four 1-minute cycles were performed, each consisting of 30-second inflation of the stent balloon and 30-second deflation. The CI-AKI rate in the rIPC group was significantly lower than in the control group (12.4% versus 29.5%, P=0.002). Furthermore, the 30-day rate of death or rehospitalization for any cause was 22.3% in the control group versus 12.4% in rIPC patients (P=0.05).
Some studies have failed to demonstrate a beneficial effect of rIPC on renal function. Thus, the trial of leg preconditioning in children undergoing surgery for complex congenital cardiac disease found no evidence that rIPC protected renal function.73 End points were AKI development, initiation of dialysis, plasma creatinine, eGFR, plasma cystatin C, plasma and urinary neutrophil gelatinase-associated lipocalin, and urinary output. Similar results were reported by another study on 76 patients undergoing complex valvular heart surgery.74 rIPC consisted of three 10-minute cycles of lower limb ischemia and reperfusion with an automated cuff inflator. Primary end points were comparisons of biomarkers of renal injury including serum creatinine, cystatin C and neutrophil gelatinase–associated lipocalin, and incidence of AKI. There were no significant differences in serum levels of renal markers or eGFR between the groups throughout the study period. The AKI incidences also did not differ between the groups, and none of the patients required hemodialysis. Despite the lack of a renal protective effect, rIPC was associated with a significantly lower creatinine kinase MB level at 24 hours after surgery and with a shorter stay in the intensive care unit compared with the control.
Strategies to attenuate ischemic-reperfusion injury are particularly important in transplantation medicine, given the high incidence of dialysis-requiring renal dysfunction in the setting of kidney transplantation and the easier applicability of the rIPC procedure in both organ donors and recipients on the other side. Despite experimental evidence demonstrating a decrease in renal allograft injury and improvement of allograft function,75 only one small-sized study thus far has evaluated the role of rIPC in human renal transplantation. rIPC, consisting of three 5-minute cycles of leg ischemia and 5 minutes of reperfusion in donors or recipients, failed to improve early renal function in patients receiving living-donor renal transplantation.76 One possible explanation for inefficiency of the procedure might be a unilaterally performed rIPC stimulus in donors or recipients. Conversely, simultaneously applying rIPC in both donors and recipients may yield different results and should be addressed in future trials in solid organ transplantation.
Overall, particularly in view of the latest published reports, it seems that rIPC is beneficial in patients at intermediate or high risk, whereas no significant renoprotective effect is detectable in patients at low risk or patients with absent renal impairment. However, further studies are necessary to establish the therapeutic value of rIPC in the clinical setting.
Future Directions
Since the first evidence of rIPC was reported almost 20 years ago, this simple procedure has been the focus of extensive experimental and clinical research. However, the exact mode of communication between the site of the ischemia application and the target tissue remains unknown. Several cellular, neurogenic, and humoral pathways have been proposed to be the candidate mechanisms involved as a complex cascade in transduction of the preconditioning stimulus with overlap between various signaling pathways.
Nevertheless, substantial work has been done that expanded the paradigm of rIPC beyond the initial observation of intracardiac protection and yielded novel insights into both the spatial and temporal characteristics of this phenomenon. With regard to the kidney, several recently published proof-of-concept clinical studies have reported encouraging results. The results of some other clinical studies, however, have been disappointing for a number of reasons. Thus, different stimulus protocols in terms of the number, duration, and timing of cycles as well as the heterogenous patient population (high versus low risk) may explain discrepant results obtained in clinical studies. Furthermore, additional studies are needed to elucidate the optimal choice of application site (e.g., arm versus leg versus internal organs such as the intestine or kidney). Large multicenter randomized clinical trials are now underway to investigate the effect of rIPC on clinical outcomes in patients with renal impairment, and to prove that rIPC, a type of “distant healing,” may indeed be a new renoprotective strategy.
Disclosures
None.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
References
- 1.Murry CE, Jennings RB, Reimer KA: Preconditioning with ischemia: A delay of lethal cell injury in ischemic myocardium. Circulation 74: 1124–1136, 1986 [DOI] [PubMed] [Google Scholar]
- 2.Przyklenk K, Bauer B, Ovize M, Kloner RA, Whittaker P: Regional ischemic ‘preconditioning’ protects remote virgin myocardium from subsequent sustained coronary occlusion. Circulation 87: 893–899, 1993 [DOI] [PubMed] [Google Scholar]
- 3.Jensen HA, Loukogeorgakis S, Yannopoulos F, Rimpiläinen E, Petzold A, Tuominen H, Lepola P, Macallister RJ, Deanfield JE, Mäkelä T, Alestalo K, Kiviluoma K, Anttila V, Tsang V, Juvonen T: Remote ischemic preconditioning protects the brain against injury after hypothermic circulatory arrest. Circulation 123: 714–721, 2011 [DOI] [PubMed] [Google Scholar]
- 4.Tapuria N, Kumar Y, Habib MM, Abu Amara M, Seifalian AM, Davidson BR: Remote ischemic preconditioning: A novel protective method from ischemia reperfusion injury—a review. J Surg Res 150: 304–330, 2008 [DOI] [PubMed] [Google Scholar]
- 5.Er F, Nia AM, Dopp H, Hellmich M, Dahlem KM, Caglayan E, Kubacki T, Benzing T, Erdmann E, Burst V, Gassanov N: Ischemic preconditioning for prevention of contrast medium-induced nephropathy: Randomized pilot RenPro Trial (Renal Protection Trial). Circulation 126: 296–303, 2012 [DOI] [PubMed] [Google Scholar]
- 6.Przyklenk K, Whittaker P: Remote ischemic preconditioning: Current knowledge, unresolved questions, and future priorities. J Cardiovasc Pharmacol Ther 16: 255–259, 2011 [DOI] [PubMed] [Google Scholar]
- 7.Katholi RE, Woods WT, Jr, Taylor GJ, Deitrick CL, Womack KA, Katholi CR, McCann WP: Oxygen free radicals and contrast nephropathy. Am J Kidney Dis 32: 64–71, 1998 [DOI] [PubMed] [Google Scholar]
- 8.Gleeson TG, Bulugahapitiya S: Contrast-induced nephropathy. AJR Am J Roentgenol 183: 1673–1689, 2004 [DOI] [PubMed] [Google Scholar]
- 9.Igarashi G, Iino K, Watanabe H, Ito H: Remote ischemic pre-conditioning alleviates contrastinduced acute kidney injury in patients with moderate chronic kidney disease [published online ahead of print August 29, 2013. Circ J 10.1253/circj.CJ-13-0171 [DOI] [PubMed] [Google Scholar]
- 10.Hausenloy DJ, Yellon DM: Survival kinases in ischemic preconditioning and postconditioning. Cardiovasc Res 70: 240–253, 2006 [DOI] [PubMed] [Google Scholar]
- 11.Armstrong S, Downey JM, Ganote CE: Preconditioning of isolated rabbit cardiomyocytes: Induction by metabolic stress and blockade by the adenosine antagonist SPT and calphostin C, a protein kinase C inhibitor. Cardiovasc Res 28: 72–77, 1994 [DOI] [PubMed] [Google Scholar]
- 12.Xu Z, Ji X, Boysen PG: Exogenous nitric oxide generates ROS and induces cardioprotection: Involvement of PKG, mitochondrial KATP channels, and ERK. Am J Physiol Heart Circ Physiol 286: H1433–H1440, 2004 [DOI] [PubMed] [Google Scholar]
- 13.Costa AD, Garlid KD, West IC, Lincoln TM, Downey JM, Cohen MV, Critz SD: Protein kinase G transmits the cardioprotective signal from cytosol to mitochondria. Circ Res 97: 329–336, 2005 [DOI] [PubMed] [Google Scholar]
- 14.Ma H, Huang X, Li Q, Guan Y, Yuan F, Zhang Y: ATP-dependent potassium channels and mitochondrial permeability transition pores play roles in the cardioprotection of theaflavin in young rat. J Physiol Sci 61: 337–342, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Caban A, Oczkowicz G, Abdel-Samad O, Cierpka L: Influence of ischemic preconditioning and nitric oxide on microcirculation and the degree of rat liver injury in the model of ischemia and reperfusion. Transplant Proc 38: 196–198, 2006 [DOI] [PubMed] [Google Scholar]
- 16.Koti RS, Tsui J, Lobos E, Yang W, Seifalian AM, Davidson BR: Nitric oxide synthase distribution and expression with ischemic preconditioning of the rat liver. FASEB J 19: 1155–1157, 2005 [DOI] [PubMed] [Google Scholar]
- 17.Barrier A, Olaya N, Chiappini F, Roser F, Scatton O, Artus C, Franc B, Dudoit S, Flahault A, Debuire B, Azoulay D, Lemoine A: Ischemic preconditioning modulates the expression of several genes, leading to the overproduction of IL-1Ra, iNOS, and Bcl-2 in a human model of liver ischemia-reperfusion. FASEB J 19: 1617–1626, 2005 [DOI] [PubMed] [Google Scholar]
- 18.Claytor RB, Aranson NJ, Ignotz RA, Lalikos JF, Dunn RM: Remote ischemic preconditioning modulates p38 MAP kinase in rat adipocutaneous flaps. J Reconstr Microsurg 23: 93–98, 2007 [DOI] [PubMed] [Google Scholar]
- 19.Gho BC, Schoemaker RG, van den Doel MA, Duncker DJ, Verdouw PD: Myocardial protection by brief ischemia in noncardiac tissue. Circulation 94: 2193–2200, 1996 [DOI] [PubMed] [Google Scholar]
- 20.Malhotra S, Naggar I, Stewart M, Rosenbaum DM: Neurogenic pathway mediated remote preconditioning protects the brain from transient focal ischemic injury. Brain Res 1386: 184–190, 2011 [DOI] [PubMed] [Google Scholar]
- 21.Nayak GH, Prentice HM, Milton SL: Neuroprotective signaling pathways are modulated by adenosine in the anoxia tolerant turtle. J Cereb Blood Flow Metab 31: 467–475, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hu S, Dong H, Zhang H, Wang S, Hou L, Chen S, Zhang J, Xiong L: Noninvasive limb remote ischemic preconditioning contributes neuroprotective effects via activation of adenosine A1 receptor and redox status after transient focal cerebral ischemia in rats. Brain Res 1459: 81–90, 2012 [DOI] [PubMed] [Google Scholar]
- 23.Bankwala Z, Hale SL, Kloner RA: Alpha-adrenoceptor stimulation with exogenous norepinephrine or release of endogenous catecholamines mimics ischemic preconditioning. Circulation 90: 1023–1028, 1994 [DOI] [PubMed] [Google Scholar]
- 24.Hale SL, Kloner RA: Protection of myocardium by transient, preischemic administration of phenylephrine in the rabbit. Coron Artery Dis 5: 605–610, 1994 [DOI] [PubMed] [Google Scholar]
- 25.Vinten-Johansen J, Yellon DM, Opie LH: Postconditioning: A simple, clinically applicable procedure to improve revascularization in acute myocardial infarction. Circulation 112: 2085–2088, 2005 [DOI] [PubMed] [Google Scholar]
- 26.Patel HH, Moore J, Hsu AK, Gross GJ: Cardioprotection at a distance: Mesenteric artery occlusion protects the myocardium via an opioid sensitive mechanism. J Mol Cell Cardiol 34: 1317–1323, 2002 [DOI] [PubMed] [Google Scholar]
- 27.Weinbrenner C, Schulze F, Sárváry L, Strasser RH: Remote preconditioning by infrarenal aortic occlusion is operative via delta1-opioid receptors and free radicals in vivo in the rat heart. Cardiovasc Res 61: 591–599, 2004 [DOI] [PubMed] [Google Scholar]
- 28.Liem DA, Verdouw PD, Ploeg H, Kazim S, Duncker DJ: Sites of action of adenosine in interorgan preconditioning of the heart. Am J Physiol Heart Circ Physiol 283: H29–H37, 2002 [DOI] [PubMed] [Google Scholar]
- 29.Diwan V, Kant R, Jaggi AS, Singh N, Singh D: Signal mechanism activated by erythropoietin preconditioning and remote renal preconditioning-induced cardioprotection. Mol Cell Biochem 315: 195–201, 2008 [DOI] [PubMed] [Google Scholar]
- 30.Kin H, Zhao ZQ, Sun HY, Wang NP, Corvera JS, Halkos ME, Kerendi F, Guyton RA, Vinten-Johansen J: Postconditioning attenuates myocardial ischemia-reperfusion injury by inhibiting events in the early minutes of reperfusion. Cardiovasc Res 62: 74–85, 2004 [DOI] [PubMed] [Google Scholar]
- 31.Kharbanda RK, Peters M, Walton B, Kattenhorn M, Mullen M, Klein N, Vallance P, Deanfield J, MacAllister R: Ischemic preconditioning prevents endothelial injury and systemic neutrophil activation during ischemia-reperfusion in humans in vivo. Circulation 103: 1624–1630, 2001 [DOI] [PubMed] [Google Scholar]
- 32.Konstantinov IE, Arab S, Kharbanda RK, Li J, Cheung MM, Cherepanov V, Downey GP, Liu PP, Cukerman E, Coles JG, Redington AN: The remote ischemic preconditioning stimulus modifies inflammatory gene expression in humans. Physiol Genomics 19: 143–150, 2004 [DOI] [PubMed] [Google Scholar]
- 33.Liang J, Wang J, Saad Y, Warble L, Becerra E, Kolattukudy PE: Participation of MCP-induced protein 1 in lipopolysaccharide preconditioning-induced ischemic stroke tolerance by regulating the expression of proinflammatory cytokines. J Neuroinflammation 8: 182, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rihal CS, Textor SC, Grill DE, Berger PB, Ting HH, Best PJ, Singh M, Bell MR, Barsness GW, Mathew V, Garratt KN, Holmes DR, Jr: Incidence and prognostic importance of acute renal failure after percutaneous coronary intervention. Circulation 105: 2259–2264, 2002 [DOI] [PubMed] [Google Scholar]
- 35.Shlipak MG, Heidenreich PA, Noguchi H, Chertow GM, Browner WS, McClellan MB: Association of renal insufficiency with treatment and outcomes after myocardial infarction in elderly patients. Ann Intern Med 137: 555–562, 2002 [DOI] [PubMed] [Google Scholar]
- 36.Best PJ, Lennon R, Ting HH, Bell MR, Rihal CS, Holmes DR, Berger PB: The impact of renal insufficiency on clinical outcomes in patients undergoing percutaneous coronary interventions. J Am Coll Cardiol 39: 1113–1119, 2002 [DOI] [PubMed] [Google Scholar]
- 37.Nash K, Hafeez A, Hou S: Hospital-acquired renal insufficiency. Am J Kidney Dis 39: 930–936, 2002 [DOI] [PubMed] [Google Scholar]
- 38.Goldenberg I, Matetzky S: Nephropathy induced by contrast media: Pathogenesis, risk factors and preventive strategies. CMAJ 172: 1461–1471, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sudarsky D, Nikolsky E: Contrast-induced nephropathy in interventional cardiology. Int J Nephrol Renovasc Dis 4: 85–99, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McCullough PA, Wolyn R, Rocher LL, Levin RN, O’Neill WW: Acute renal failure after coronary intervention: Incidence, risk factors, and relationship to mortality. Am J Med 103: 368–375, 1997 [DOI] [PubMed] [Google Scholar]
- 41.Mehran R, Aymong ED, Nikolsky E, Lasic Z, Iakovou I, Fahy M, Mintz GS, Lansky AJ, Moses JW, Stone GW, Leon MB, Dangas G: A simple risk score for prediction of contrast-induced nephropathy after percutaneous coronary intervention: Development and initial validation. J Am Coll Cardiol 44: 1393–1399, 2004 [DOI] [PubMed] [Google Scholar]
- 42.Gruberg L, Mintz GS, Mehran R, Gangas G, Lansky AJ, Kent KM, Pichard AD, Satler LF, Leon MB: The prognostic implications of further renal function deterioration within 48 h of interventional coronary procedures in patients with pre-existent chronic renal insufficiency. J Am Coll Cardiol 36: 1542–1548, 2000 [DOI] [PubMed] [Google Scholar]
- 43.Maeder M, Klein M, Fehr T, Rickli H: Contrast nephropathy: Review focusing on prevention. J Am Coll Cardiol 44: 1763–1771, 2004 [DOI] [PubMed] [Google Scholar]
- 44.Hofmann L, Simon-Zoula S, Nowak A, Giger A, Vock P, Boesch C, Frey FJ, Vogt B: BOLD-MRI for the assessment of renal oxygenation in humans: Acute effect of nephrotoxic xenobiotics. Kidney Int 70: 144–150, 2006 [DOI] [PubMed] [Google Scholar]
- 45.Zhang Y, Wang J, Yang X, Wang X, Zhang J, Fang J, Jiang X: The serial effect of iodinated contrast media on renal hemodynamics and oxygenation as evaluated by ASL and BOLD MRI. Contrast Media Mol Imaging 7: 418–425, 2012 [DOI] [PubMed] [Google Scholar]
- 46.Tepel M, Aspelin P, Lameire N: Contrast-induced nephropathy: A clinical and evidence-based approach. Circulation 113: 1799–1806, 2006 [DOI] [PubMed] [Google Scholar]
- 47.Persson PB, Hansell P, Liss P: Pathophysiology of contrast medium-induced nephropathy. Kidney Int 68: 14–22, 2005 [DOI] [PubMed] [Google Scholar]
- 48.Abizaid AS, Clark CE, Mintz GS, Dosa S, Popma JJ, Pichard AD, Satler LF, Harvey M, Kent KM, Leon MB: Effects of dopamine and aminophylline on contrast-induced acute renal failure after coronary angioplasty in patients with preexisting renal insufficiency. Am J Cardiol 83: 260–263, A5, 1999 [DOI] [PubMed] [Google Scholar]
- 49.Mueller C, Buerkle G, Buettner HJ, Petersen J, Perruchoud AP, Eriksson U, Marsch S, Roskamm H: Prevention of contrast media-associated nephropathy: Randomized comparison of 2 hydration regimens in 1620 patients undergoing coronary angioplasty. Arch Intern Med 162: 329–336, 2002 [DOI] [PubMed] [Google Scholar]
- 50.Bailey SR: Past and present attempts to prevent radiocontrast nephropathy. Rev Cardiovasc Med 2[Suppl 1]: S14–S18, 2001 [PubMed] [Google Scholar]
- 51.Stone GW, McCullough PA, Tumlin JA, Lepor NE, Madyoon H, Murray P, Wang A, Chu AA, Schaer GL, Stevens M, Wilensky RL, O’Neill WW; CONTRAST Investigators: Fenoldopam mesylate for the prevention of contrast-induced nephropathy: A randomized controlled trial. JAMA 290: 2284–2291, 2003 [DOI] [PubMed] [Google Scholar]
- 52.ACT Trial Investigators: Rationale, design, and baseline characteristics of the Acetylcystein for Contrast-Induced nephropaThy (ACT) Trial: A pragmatic randomized controlled trial to evaluate the efficacy of acetylcysteine for the prevention of contrast-induced nephropathy. Trials 10: 38, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Webb JG, Pate GE, Humphries KH, Buller CE, Shalansky S, Al Shamari A, Sutander A, Williams T, Fox RS, Levin A: A randomized controlled trial of intravenous N-acetylcysteine for the prevention of contrast-induced nephropathy after cardiac catheterization: Lack of effect. Am Heart J 148: 422–429, 2004 [DOI] [PubMed] [Google Scholar]
- 54.Katzberg RW: Urography into the 21st century: New contrast media, renal handling, imaging characteristics, and nephrotoxicity. Radiology 204: 297–312, 1997 [DOI] [PubMed] [Google Scholar]
- 55.Solomon R, Werner C, Mann D, D’Elia J, Silva P: Effects of saline, mannitol, and furosemide to prevent acute decreases in renal function induced by radiocontrast agents. N Engl J Med 331: 1416–1420, 1994 [DOI] [PubMed] [Google Scholar]
- 56.Trivedi HS, Moore H, Nasr S, Aggarwal K, Agrawal A, Goel P, Hewett J: A randomized prospective trial to assess the role of saline hydration on the development of contrast nephrotoxicity. Nephron Clin Pract 93: C29–C34, 2003 [DOI] [PubMed] [Google Scholar]
- 57.Zager RA, Baltes LA, Sharma HM, Jurkowitz MS: Responses of the ischemic acute renal failure kidney to additional ischemic events. Kidney Int 26: 689–700, 1984 [DOI] [PubMed] [Google Scholar]
- 58.Zager RA, Jurkowitz MS, Merola AJ: Responses of the normal rat kidney to sequential ischemic events. Am J Physiol 249: F148–F159, 1985 [DOI] [PubMed] [Google Scholar]
- 59.Ateş E, Genç E, Erkasap N, Erkasap S, Akman S, Firat P, Emre S, Kiper H: Renal protection by brief liver ischemia in rats. Transplantation 74: 1247–1251, 2002 [DOI] [PubMed] [Google Scholar]
- 60.Song T, Peng YF, Guo SY, Liu YH, Liul LY: Brief small intestinal ischemia lessens renal ischemia-reperfusion injury in rats. Comp Med 57: 200–205, 2007 [PubMed] [Google Scholar]
- 61.Wever KE, Warlé MC, Wagener FA, van der Hoorn JW, Masereeuw R, van der Vliet JA, Rongen GA: Remote ischaemic preconditioning by brief hind limb ischaemia protects against renal ischaemia-reperfusion injury: The role of adenosine. Nephrol Dial Transplant 26: 3108–3117, 2011 [DOI] [PubMed] [Google Scholar]
- 62.Wever KE, Menting TP, Rovers M, van der Vliet JA, Rongen GA, Masereeuw R, Ritskes-Hoitinga M, Hooijmans CR, Warlé M: Ischemic preconditioning in the animal kidney, a systematic review and meta-analysis. PLoS ONE 7: e32296, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Huda R, Chung DH, Mathru M: Ischemic preconditioning at a distance: Altered gene expression in mouse heart and other organs following brief occlusion of the mesenteric artery. Heart Lung Circ 14: 36–43, 2005 [DOI] [PubMed] [Google Scholar]
- 64.Ali ZA, Callaghan CJ, Lim E, Ali AA, Nouraei SA, Akthar AM, Boyle JR, Varty K, Kharbanda RK, Dutka DP, Gaunt ME: Remote ischemic preconditioning reduces myocardial and renal injury after elective abdominal aortic aneurysm repair: A randomized controlled trial. Circulation 116[Suppl]: I98–I105, 2007 [DOI] [PubMed] [Google Scholar]
- 65.Walsh SR, Sadat U, Boyle JR, Tang TY, Lapsley M, Norden AG, Gaunt ME: Remote ischemic preconditioning for renal protection during elective open infrarenal abdominal aortic aneurysm repair: Randomized controlled trial. Vasc Endovascular Surg 44: 334–340, 2010 [DOI] [PubMed] [Google Scholar]
- 66.Walsh SR, Boyle JR, Tang TY, Sadat U, Cooper DG, Lapsley M, Norden AG, Varty K, Hayes PD, Gaunt ME: Remote ischemic preconditioning for renal and cardiac protection during endovascular aneurysm repair: A randomized controlled trial. J Endovasc Ther 16: 680–689, 2009 [DOI] [PubMed] [Google Scholar]
- 67.Rahman IA, Mascaro JG, Steeds RP, Frenneaux MP, Nightingale P, Gosling P, Townsend P, Townend JN, Green D, Bonser RS: Remote ischemic preconditioning in human coronary artery bypass surgery: From promise to disappointment? Circulation 122[Suppl]: S53–S59, 2010 [DOI] [PubMed] [Google Scholar]
- 68.Steensrud T, Li J, Dai X, Manlhiot C, Kharbanda RK, Tropak M, Redington A: Pretreatment with the nitric oxide donor SNAP or nerve transection blocks humoral preconditioning by remote limb ischemia or intra-arterial adenosine. Am J Physiol Heart Circ Physiol 299: H1598–H1603, 2010 [DOI] [PubMed] [Google Scholar]
- 69.Zhong B, Wang DH: TRPV1 gene knockout impairs preconditioning protection against myocardial injury in isolated perfused hearts in mice. Am J Physiol Heart Circ Physiol 293: H1791–H1798, 2007 [DOI] [PubMed] [Google Scholar]
- 70.Venugopal V, Laing CM, Ludman A, Yellon DM, Hausenloy D: Effect of remote ischemic preconditioning on acute kidney injury in nondiabetic patients undergoing coronary artery bypass graft surgery: A secondary analysis of 2 small randomized trials. Am J Kidney Dis 56: 1043–1049, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zimmerman RF, Ezeanuna PU, Kane JC, Cleland CD, Kempananjappa TJ, Lucas FL, Kramer RS: Ischemic preconditioning at a remote site prevents acute kidney injury in patients following cardiac surgery. Kidney Int 80: 861–867, 2011 [DOI] [PubMed] [Google Scholar]
- 72.Deftereos S, Giannopoulos G, Tzalamouras VD, Raisakis K, Kossyvakis C, Kaoukis A, Panagopoulou V, Karageorgiou S, Avramides D, Toutouzas K, Hahalis G, Pyrgakis V, Manolis AS, Alexopoulos D, Stefanadis C, Cleman MW: Renoprotective effect of remote ischemic post-conditioning by intermittent balloon inflations in patients undergoing percutaneous coronary intervention. J Am Coll Cardiol 61: 1949–1955, 2013 [DOI] [PubMed] [Google Scholar]
- 73.Pedersen KR, Ravn HB, Povlsen JV, Schmidt MR, Erlandsen EJ, Hjortdal VE: Failure of remote ischemic preconditioning to reduce the risk of postoperative acute kidney injury in children undergoing operation for complex congenital heart disease: A randomized single-center study. J Thorac Cardiovasc Surg 143: 576–583, 2012 [DOI] [PubMed] [Google Scholar]
- 74.Choi YS, Shim JK, Kim JC, Kang KS, Seo YH, Ahn KR, Kwak YL: Effect of remote ischemic preconditioning on renal dysfunction after complex valvular heart surgery: A randomized controlled trial. J Thorac Cardiovasc Surg 142: 148–154, 2011 [DOI] [PubMed] [Google Scholar]
- 75.Selzner N, Boehnert M, Selzner M: Preconditioning, postconditioning, and remote conditioning in solid organ transplantation: basic mechanisms and translational applications. Transplant Rev (Orlando) 26: 115–124, 2012 [DOI] [PubMed] [Google Scholar]
- 76.Chen Y, Zheng H, Wang X, Zhou Z, Luo A, Tian Y: Remote ischemic preconditioning fails to improve early renal function of patients undergoing living-donor renal transplantation: A randomized controlled trial. Transplantation 95: e4–e6, 2013 [DOI] [PubMed] [Google Scholar]
- 77.Huang J, Chen Y, Dong B, Kong W, Zhang J, Xue W, Liu D, Huang Y: Effect of remote ischemic preconditioning on renal protection in patients undergoing laparoscopic partial nephrectomy: a ‘blinded’ randomised controlled trial. BJU Int 112: 74–80, 2013 [DOI] [PubMed] [Google Scholar]