Abstract
Development, growth and adult survival are coordinated with available metabolic resources, ascertaining that the organism responds appropriately to environmental conditions. MicroRNAs are short (21–23 nt) regulatory RNAs that confer specificity on the RNA-induced silencing complex (RISC) to inhibit a given set of mRNA targets. We profiled changes in miRNA expression during adult life in Drosophila melanogaster and determined that miR-277 is downregulated during adult life. Molecular analysis revealed that this miRNA controls branched-chain amino acid (BCAA) catabolism and as a result it can modulate the activity of the TOR kinase, a central growth regulator, in cultured cells. Metabolite analysis in cultured cells as well as flies suggests that the mechanistic basis may be an accumulation of branched-chain α-keto-acids (BCKA), rather than BCAAs, thus avoiding potentially detrimental consequences of increased branched chain amino acid levels on e.g., translational fidelity. Constitutive miR-277 expression shortens lifespan and is synthetically lethal with reduced insulin signaling, indicating that metabolic control underlies this phenotype. Transgenic inhibition with a miRNA sponge construct also shortens lifespan, in particular on protein-rich food. Thus, optimal metabolic adaptation appears to require tuning of cellular BCAA catabolism by miR-277.
Keywords: miRNA, miRNA target validation, longevity, ageing, maple syrup urine disease (MSUD), metabolic syndrome, diabetes
Introduction
MicroRNAs are short, single-stranded RNA molecules that confer specificity to the RNA-induced silencing complex (RISC) through partial sequence complementarity with specific mRNAs known as their targets. Recruitment of RISC most often results in repression of the mRNA by increased turnover and/or translational inhibition. During development, miRNAs frequently engage in negative feedback loops, thereby setting gene expression thresholds,1,2 and conferring robustness against environmental perturbations during embryonic development.3 Likewise, miRNAs may be involved in robustness of the adult organisms against extrinsic and/or intrinsic perturbations as suggested by miRNA expression profiling experiments.4-8 Genetic studies in C. elegans have shown that reduced expression and/or overexpression of certain miRNAs can affect lifespan via modulation of the DNA damage and insulin/IGF pathways.6 Furthermore, a mutant of Drosophila miR-14 displayed abnormal lipid metabolism and a shortened lifespan.9
The insulin/IGF and TOR signaling pathways are the main coordinators of development with nutrient availability. Their activity is controlled by a network of cross-regulations to ensure that growth signals on the cellular (TOR) and the organismic (insulin/IGF) levels are concordant. Together, they promote resource allocation toward growth and influence the timing of key developmental steps.10-12 In addition to their role in developmental decisions, these pathways help adult organisms to respond to metabolic challenges in a variable environment.13 Perhaps not surprisingly, the extent of insulin/IGF and TOR signaling correlates with life expectancy (reviewed in ref. 14). Gene expression studies comparing young and old flies have demonstrated that aging correlates with an increase in immune-response gene expression (potentially due to a life history of infection) and a downregulation of reproductive and metabolic genes.15,16 However, neither metabolic rate nor hydrogen peroxide generation seems to be altered upon aging or the application of lifespan extending conditions.17,18
A major physiological activator of TOR signaling as well as insulin secretion are the branched-chain amino acids (BCAAs), in particular leucine.19-22 Though the exact mechanism through which amino acids are sensed is still not fully understood, it is clear that in addition to leucine itself, the transamination product α-keto-isocaproic acid (KIC) can activate TOR23,24 and stimulate insulin secretion.25 Food supplementation with branched-chain amino acids (BCAAs) increases muscle mass and this anabolic effect is used by the livestock industry. Furthermore, in a rodent model of elderly malnutrition, BCAA supplementation delayed age-associated morbidities by improving mitochondrial function.26 On the other hand, a high level of circulating BCAAs and their metabolites has been associated with a higher risk of developing insulin resistance or diabetes27-29 and mutations affecting BCAA catabolism, specifically the branched-chain-α-keto-acid dehydrogenase enzyme complex, cause the severe metabolic disorder maple syrup urine disease (MSUD). MSUD patients suffer from severe neurological problems and, if untreated, die in early childhood. This paradox role of BCAAs is currently not well understood30 and indicates that the metabolic context is essential for determining whether their anabolic signaling function is beneficial or detrimental to the organism.
We describe a miRNA-mediated control of the BCAA degradation pathway in Drosophila downstream of the branched-chain aminotransferase enzyme. The miRNA responsible for this effect, miR-277, is normally downregulated during adult life. Upon constitutive miR-277 expression, flies are short lived and concomitant reduction of insulin signaling results in synthetic lethality. A mechanistic basis for this lifespan shortening may be activation of the TOR kinase due to accumulation of branched-chain α-keto acids (BCKAs). This implies that the metabolic fate of BCAAs is key to understanding their physiological importance, e.g., in the search for predictive biomarkers of metabolic syndrome27-29 or their role in neurological disorders.31
Results
Age-induced changes of miRNA expression
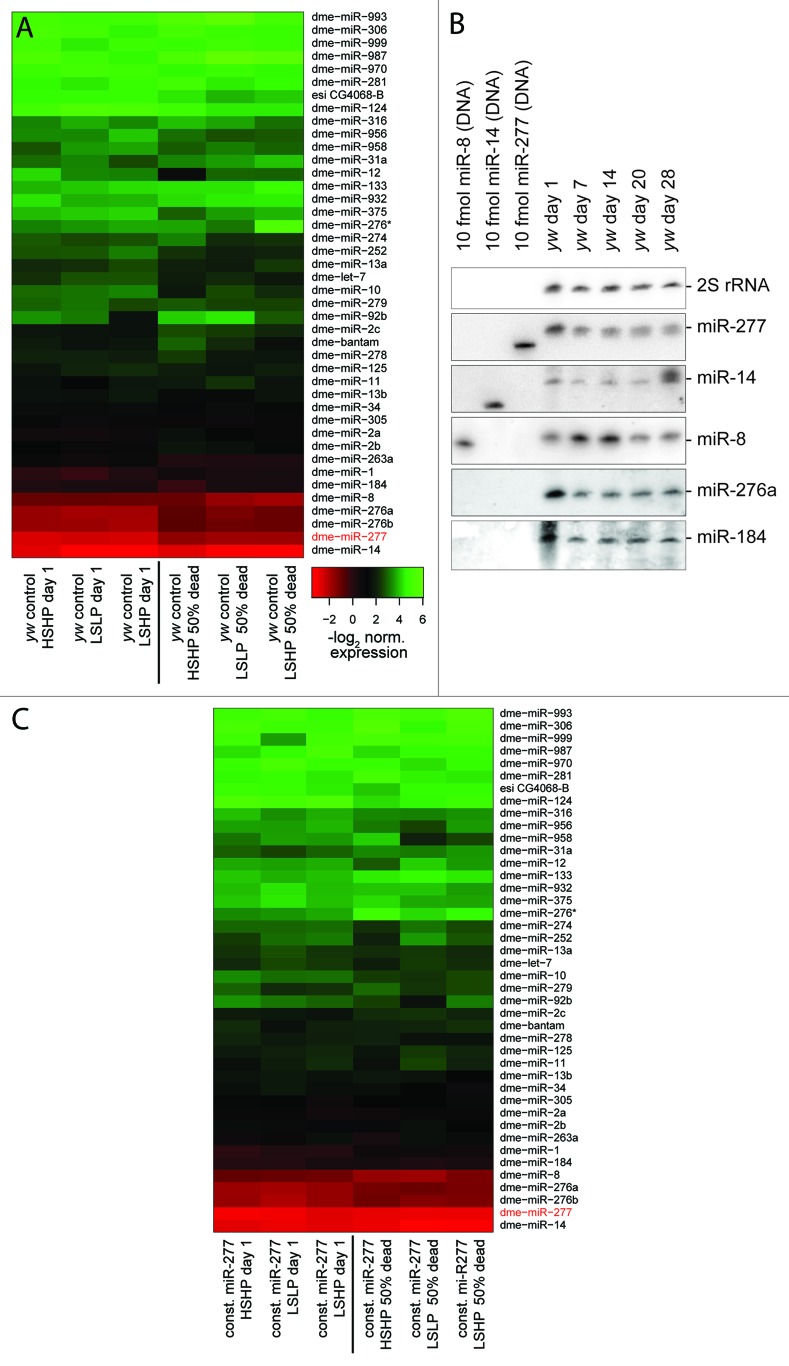
As organisms age, changes in physiology take place that are reflected in part in their miRNA profile.6 We collected control flies (genotype yw) on the day of eclosion and recorded their chronologic lifespan on three different diets (low sugar/low protein = LSLP, low sugar/high protein = LSHP and high sugar/high protein = HSHP). From parallel cohorts, total RNA was isolated on the first day and again on the day when 50% of the population had died. Since female flies can adjust their egg production rate according to age and nutrient availability,32 likely leading to changes in the relative contribution of somatic and germ-line tissue to the isolated RNA, we examined only male flies. As expected, we observed an extension of median lifespan by dietary restriction compared with rich food (27 d on LSLP vs. 22 d on HSHP food, p = 0.005 log-rank test, Fig. S1A). In our hands, food that was rich in protein but low in sugar resulted in the lowest median lifespan (22 d on HSHP vs. 17 d on LSHP, p = 2.8 × 10−8, Fig. S1B).
We measured the abundance of 80 miRNAs in young and old flies by qRT-PCR (see Table S1 for custom primer sequences), roughly 40 miRNAs were reliably detected in all samples. When comparing expression levels between young and old flies, we noticed that there is a general trend toward reduced expression with increased age despite the use of presumably invariant control RNAs for normalization. Since global downregulation of RNA production with age has been described in male fruit flies,33 we decided conservatively to retain only the changes that differ from the general trend. Overall, the profiles revealed consistently altered expression levels for several miRNAs as flies age (Fig. 1A). Food composition, on the other hand, had only a minor impact on the miRNA profile. We note that our strategy sampled physiologically rather than chronologically equivalent time points (50% dead rather than e.g., 20 d). A clear change that occurred after eclosion was downregulation of miR-277 (between 2 and 4-fold), one of the most abundant miRNAs in adult flies. Further changes include downregulation of miR-276a/b and miR-1. For the more abundant miRNAs, we validated expression changes with age by northern blot in a time-series for female flies (Fig. 1B), confirming that downregulation of miR-277 and miR-276 also occurs in females. The chronologic sampling points in this experiment suggest that much of this downregulation occurs within the first week of adult life.
Figure 1. miRNA profiling during Drosophila adult life. (A) Heat-map of normalized expression values in young and aged male flies (50% of population dead). Flies living on three different food compositions were analyzed (LSLP, dietary restricted; HSHP, rich food; LSHP, food with an excess of protein over sugar). (B) Northern blot validation of expression changes identified in (A). Female flies were maintained on standard yeast-molasses-cornmeal growth medium, 28 d of age corresponds to a time point when about 50% of the population has died. The membrane was probed, stripped and re-probed sequentially for the miRNAs indicated on the right. The 30 nt long 2S rRNA served as a loading control. (C) Experiment analogous to (A) with transgenic male flies expressing miR-277 under the control of a strong, ubiquitous promotor. Downregulation of miR-277 was prevented, but other age-associated changes in miRNA-levels remain unperturbed. A common color scale was employed for (A and C).
Constitutive expression of miR-277 shortens lifespan
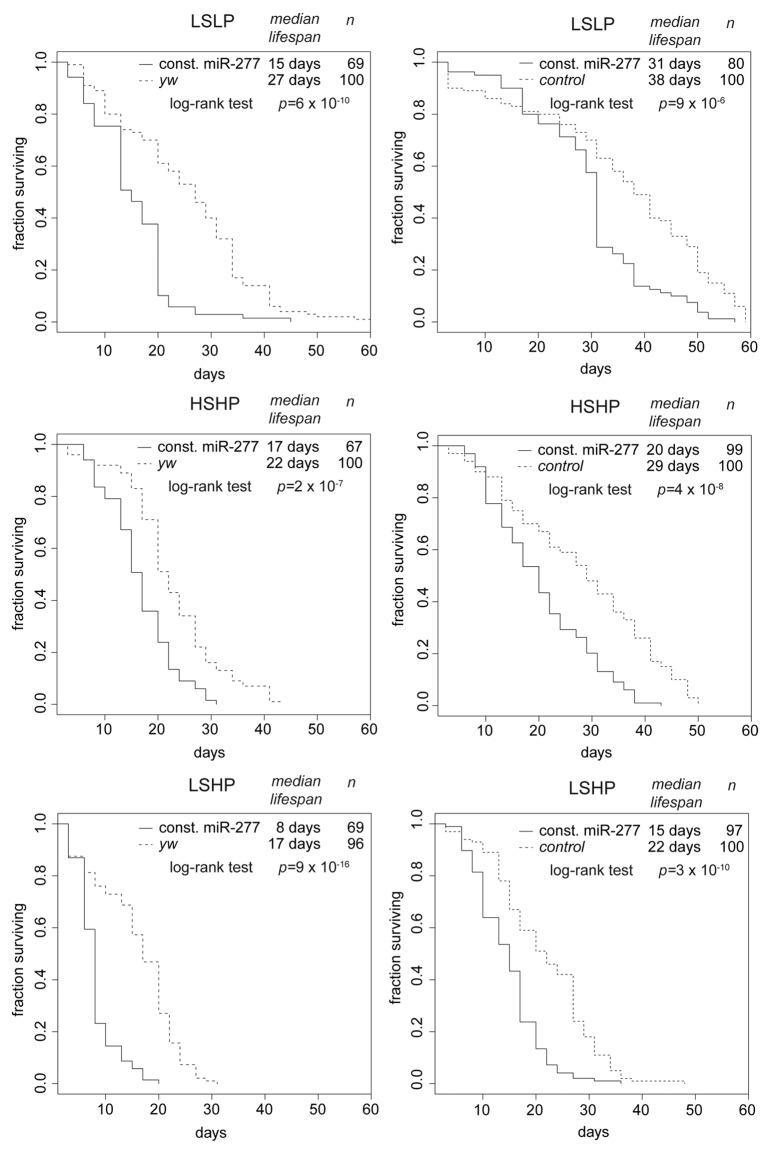
We created transgenic flies that express the pre-miR-277 hairpin under the control of the constitutively and ubiquitously expressed ubi63E promotor34 and repeated our lifespan and miRNA profiling experiment. As expected, miR-277 levels were now essentially constant, while the remainder of the mRNA profile changes with age was unperturbed (Fig. 1C). Compared with the controls, flies with constitutive miR-277 expression were short-lived on all three food regimes (Fig. 2, left panels). The lifespan shortening effect of constitutive miR-277 expression was consistent between independent transgenic lines, observable in both males and females and could also be reproduced by yet another transgenic line that was established in w1118, then backcrossed into yw (Fig. 2, right panels; as well as Fig. S2).
Figure 2. Constitutive expression of miR-277 shortens lifespan. Comparison of survival curves under different food conditions. The panels on the left side represent a transgenic fly line established in the yw background, while the panels on the right represent an independent transgenic line established in the w1118 background, then backcrossed into yw. The control flies for these experiments were w1118 flies backcrossed into yw. Median lifespan and statistical significance were calculated in R! using the package “survival.” The different food compositions are indicated on top of each diagram. While differences due to genetic background are clearly visible, constitutive expression of miR-277 consistently shortens lifespan under all food conditions.
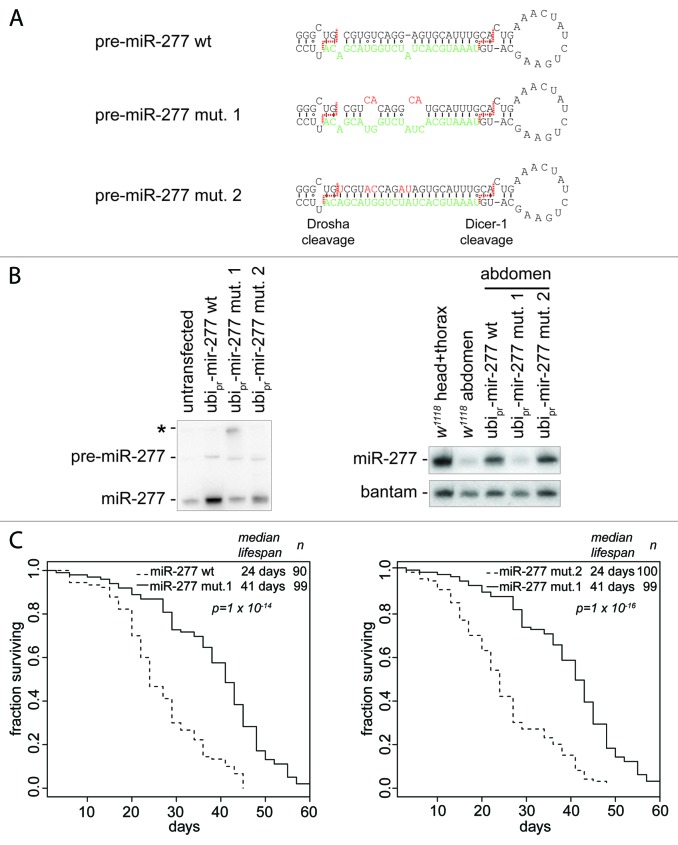
Overexpression of a non-coding RNA may have detrimental consequences that shorten lifespan independently of miR-277 target repression. We detected no additional changes in the mature miRNA profile in our miR-277 transgenic flies (Fig. 1C), arguing that processing by Dcr-1 or Drosha was not saturated. However, other functional RNAs might derive from our construct and provoke the lifespan-shortening phenotype. The most likely candidate for such an RNA is the star-strand of miR-277. We therefore used site-directed mutagenesis and converted the miR-277 hairpin to a perfectly base-paired dsRNA, changing only nucleotides along the star-strand sequence (Fig. 3A, mut. 2). As a control, we introduced additional bulges into the miR-277 hairpin (Fig. 3A, mut. 1) that should interfere with processing by Drosha.35 We transfected mutant and wild-type constructs of miR-277 into S2-cells, isolated total RNA and tested miR-277 expression by northern blot. While both wild-type and the perfectly base-paired miR-277 construct were efficiently processed into mature miRNAs, the mutant with extra bulges only moderately increased mature miR-277 levels (Fig. 3B, left panel). Furthermore, a larger RNA species that hybridized with our miR-277 probe was detectable in this transfection (asterisk in Fig. 3B), consistent with the notion that the primary transcript derived from this construct is not efficiently processed by Drosha.
Figure 3. Lifespan shortening is due to expression of mature miR-277. (A) The sequence and secondary structure of wild-type and mutant pre-miR-277 hairpins; the sequence of mature miR-277 is depicted in green and the the cleavage sites for Drosha and Dcr-1 are indicated by a dashed red line. (B) Expression of mature miR-277 from wild-type and mutant pre-miR-277 hairpins; left panel, the constructs were transiently transfected into S2 cells right panel, RNA was extracted from the abdomen of transgenic flies and miR-277 levels were compared with wild-type flies. (C) Constitutive expression of wild-type pre-miR-277 as well as the perfect dsRNA hairpin (mut. 2) results in lifespan shortening compared with constitutive expression of the hairpin with additional bulges (mut. 1). The transgenic lines used for these experiments were established in a w1118 background but not backcrossed into yw (as in Fig. 2), food type was HSHP.
We tested whether transgenic flies generated with the mutant constructs overexpressed miR-277. For maximum sensitivity, we extracted RNA from the abdomen of female flies since miR-277 is normally not expressed in the ovary,34 while the ubiquitin-promotor used to drive our constructs is active in the germ line. Again, we saw efficient overexpression of the perfectly base-paired pre-miR277 version but no increase in miR-277 levels with the construct containing the extra bulges (Fig. 3B, right panel). To determine whether mature miR-277 was correctly processed from all constructs we deep sequenced small RNAs from the abdomen of female flies, then mapped the reads to wild-type as well as the corresponding mutant pre-miR-277 hairpins (Fig. S3). We saw efficient and correct processing of wild-type miR-277; a low level of miR-277* was also detected. The pre-miR-277 mutant with additional bulges did not yield any reads that match the mutant star-strand sequence, while wild-type miR-277* could be seen in the same data set. This is consistent with our northern blot data and argues that most, if not all, of the mature miR-277 present in this sequencing library is derived from the endogenous source. The perfectly base-paired miR-277 hairpin was efficiently processed as it produced an excess of mutant miR-277* reads over the corresponding wild-type reads. We also detected that processing by the Dicer enzyme (presumably Dcr-1) was partially shifted by 2 nt toward the loop. Due to the shifted seed sequence, the resulting miRNA is unlikely to repress the same targets as miR-277. Since the mutant versions of miR-277 performed essentially as we had desired, we measured the lifespan of transgenic flies on HSHP medium (Fig. 3C). Both wild-type and the perfectly base-paired pre-miR277 constructs showed a substantially shortened lifespan when compared with the mutant that contained the additional bulges impairing Drosha processing. We conclude that lifespan shortening is due to the expression of mature miR-277 and unlikely to be caused by indirect effects of other functional RNAs derived from our transgenes.
miR-277 tunes BCAA catabolism
What is the molecular basis for the lifespan shortening induced by constitutive miR-277 expression? It is well established that miRNAs de-stabilize their cognate target mRNAs in addition to translational inhibition.36-38 We isolated mRNA from male flies at the time when 50% of the cohort had died, then compared the transcriptome of flies with constitutive miR-277 expression to controls. With a threshold of 95% confidence, we found that 237 mRNAs were upregulated (excluding the w marker gene associated with our transgenic construct) and 376 mRNAs downregulated (Table S2). Analysis of miRNA target predictions and GO annotations with g:profiler39 revealed a significant enrichment of predicted miR-277 targets among the downregulated mRNAs (p = 1.7 × 10−19) as well as the annotation “branched chain amino acid catabolism” (p = 3.2 × 10−15, g:SCS statistics according to ref. 39). Only one other miRNA, miR-184*, showed significant enrichment for its predicted targets, albeit at a far lower confidence level (p = 4.6 × 10−4). Eight (of 34) cytochrome P450 genes were downregulated in the flies with constitutive miR-277 expression, leading to enrichment of GO-terms associated with xenobiotic metabolism and lipid peroxidation (p = 10−4 to 10−5). None of these regulated P450 enzymes are involved in ecdysone biogenesis or otherwise known to influence lifespan. Furthermore, ketone body and fatty acid metabolism were significantly enriched (p = 1.6 × 10−4 for both), but the annotations partially overlap with BCAA metabolism (see Table S3) due to the structural similarity of BCAAs with short-chain fatty acids and the potential of BCAAs to yield precursors for ketone body synthesis. In contrast to the downregulated mRNAs, no GO-term or miRNA target prediction was significantly enriched among the upregulated genes.
While our results agree well with miR-277 target predictions40 in the sense that many of the downregulated mRNAs are predicted miR-277 targets, the validation rate for each predicted target was moderate: Only 52 of the more than 600 predicted miR-277 targets were significantly downregulated, arguing against the notion that our constitutive miR-277 expression leads to a non-physiologic increase in miR-277 repressive capacity. This would be accompanied by downregulation of many mRNAs with seed complementarity to miR-277, in particular those predicted as potential targets. On the other hand, transcriptional co-regulation of the BCAA metabolic cascade could also explain the preferential recovery of miR-277 targets within the BCAA degradation pathway through a compensatory change in response to lifespan shortening. We therefore prepared RNA samples from two week old flies, a time point where miR-277 downregulation has become obvious in control flies (see Fig. 1) but the majority of the population is still alive. Using quantitative RT-PCR assays we could confirm that the miR-277 responsive BCAA degradation genes were downregulated in flies with constitutive miR-277 expression relative to controls, while a collection of genes known to be up- or downregulated with age did not show comparable expression differences (see Fig. S4). Thus, miR-277 induced changes precede aging induced changes, which is consistent with the notion that repression of BCAA degradation enzymes in response to miR-277 occurs independently of other age-associated transcriptional changes. Again, not all predicted miR-277 targets responded to the constitutive expression, corroborating the notion that miR-277 mediated repression is not exaggerated.
To directly measure transcriptional and post-transcriptional effects imposed by miR-277, we made use of a 4-thio-uridine pulse-labeling strategy in S2-cells that allows separation of newly transcribed mRNA from the population present before the beginning of the 4-tU pulse.41-44 In this dynamic transcriptome analysis (DTA), changes in mRNA degradation rate are most obvious in the older mRNA fraction, whereas transcription rate changes are reflected in the newly transcribed fraction of mRNA.42,43 Since miR-277 is expressed endogenously at moderate levels in Schneider cells, we used 2’-O-methyl antisense oligonucleotides to inhibit the endogenously expressed miR-277.45 Three days after transfection of the inhibitor, we pulse-labeled the mRNA and analyzed the fractions by DTA. In total RNA, only 20 genes were significantly upregulated and 17 genes were downregulated (Table 1A and 1B). Half of the upregulated genes but only one of the downregulated genes were predicted miR-277 targets. Again, genes required for the catabolism of BCAAs were prominent among the upregulated genes (eight out of 20, see Fig. S5 for a view of regulated genes within the BCAA catabolic pathway). Upon fractionation of the mRNA, we determined that their increased steady-state level was due to a stabilization of the corresponding mRNAs rather than an increase in their rate of transcription (Table 1). This strongly argues that they represent direct miR-277 targets and that co-regulation of the enzymatic cascade occurs post-transcriptionally. The extent of regulation is relatively modest, as expected for the effects mediated by a moderately expressed miRNA and, thus, likely more relevant for long-term adaptations rather than the rapid changes required e.g., after feeding. We detected several genes that changed in abundance in only one of our RNA fractions, potentially indicating that they are false positives. Furthermore, we saw no expression changes for cytochrome P450 family enzymes upon inhibition of endogenous miR-277 in S2-cells.
Table 1A. Upregulated upon inhibition of miR-277 (log2 fold change).
| Gene | Total RNA | > 1 h | < 1 h | Pred. target |
|---|---|---|---|---|
| CG8199 | 1.51 | 1.73 | n.s. | yes |
| CG5599 | 1.67 | 1.69 | n.s. | yes |
| CG15093 | 1.17 | 1.01 | n.s. | yes |
| CG3267 | 1.11 | 1.24 | n.s. | yes |
| CG2118 | 1.65 | 1.93 | 0.72 | yes |
| CG5044 | 0.63 | 0.55 | n.s. | yes |
| CG6984 | 0.87 | 1.19 | n.s. | yes |
| CG6543 | 1.25 | 1.25 | n.s. | yes |
| CG9867 | 0.65 | 0.70 | n.s. | yes |
| Usp7 | 0.58 | 0.58 | n.s. | - |
| GlcAT-S | 0.53 | 0.55 | n.s. | - |
| Sin1 | 0.65 | 0.65 | n.s. | - |
| CG5180 | 0.59 | 0.74 | 0.86 | - |
| CG32043 | 0.61* | 0.84 | 0.97 | - |
| edin | 1.99 | n.s. | 2.25 | - |
| CG4594 | 0.84 | n.s. | n.s. | yes |
| CG18273 | 0.76 | n.s. | n.s. | - |
| CG4942 | 0.70 | n.s. | n.s. | - |
| CG9793 | 0.40 | n.s. | n.s. | - |
| Kap3 | 0.47 | n.s. | n.s. | - |
| SelR | 0.43 | n.s. | n.s. | - |
| Amph | n.s. | 0.80 | n.s. | - |
| bchs | n.s. | 1.01 | n.s. | - |
| by | n.s. | 0.48 | n.s. | - |
| CG11398 | n.s. | 0.75 | n.s. | - |
| CG11658 | n.s. | 0.76 | n.s. | - |
| CG18273 | n.s. | 0.86 | n.s. | - |
| CG2202 | n.s. | 0.52 | n.s. | - |
| CG3407 | n.s. | 0.67 | n.s. | - |
| CG4925 | n.s. | 0.82 | n.s. | - |
| mus308 | n.s. | 0.85 | n.s. | - |
| nmdyn-D6 | n.s. | 0.63 | n.s. | - |
| nuf | n.s. | 0.51 | n.s. | - |
| Oat | n.s. | 1.29 | n.s. | - |
| CG32813 | n.s. | n.s. | 0.65 | - |
| CG6656 | n.s. | n.s. | 0.56 | - |
| Drs | n.s. | n.s. | 1.21 | - |
p = 0.057
Table 1B. Downregulated upon inhibition of miR-277 (log2 fold change).
| Gene | Total RNA | > 1 h | < 1 h | Pred. target |
|---|---|---|---|---|
| ogre | -1.09 | -0.85 | n.s. | |
| C9194 | -0.88 | -0.61 | n.s. | |
| bab2 | -1.11 | n.s. | n.s. | yes |
| RhoGAP54D | -0.50 | n.s. | n.s. | |
| CG16896 | -0.69 | n.s. | n.s. | |
| CG6175 | -0.90 | n.s. | n.s. | |
| Grip163 | -0.70 | n.s. | n.s. | |
| CG10948 | -0.85 | n.s. | n.s. | |
| CG7510 | -0.48 | n.s. | n.s. | |
| CG7646 | -0.48 | n.s. | n.s. | |
| Fas1 | -0.85 | n.s. | n.s. | |
| Sirt7 | -0.69 | n.s. | n.s. | |
| CG15523 | -0.68 | n.s. | n.s. | |
| zfh2 | -0.53 | n.s. | n.s. | |
| CG3191 | -0.73 | n.s. | n.s. | |
| CG1636 | -0.74 | n.s. | n.s. | |
| CG32666 | -0.87 | n.s. | n.s. | |
| CG8349 | n.s. | -0.97 | n.s. | |
| Pcf11 | n.s. | -0.59 | n.s. | |
| CG14102 | n.s. | -0.59 | n.s. | |
| CG14252 | n.s. | n.s. | -0.58 | |
| CecC | n.s. | n.s. | -1.48 | |
| CG34319 | n.s. | n.s. | -0.68 |
While much of the expression changes we detected in cell culture could be explained by predicted targeting through miR-277, one notable exception in both flies and S2-cells is the first enzyme of the BCAA degradation cascade: CG1673, the branched-chain amino transferase enzyme (BCAT) that converts e.g., leucine to α-keto isocaproic acid (KIC). We therefore validated our DTA analysis with luciferase reporter constructs containing the 3′-UTRs of CG1673, CG8199 and CG5599 (Fig. S6). Upon co-transfection of a miR-277 expression plasmid we saw that the UTRs of CG8199 and CG5599 efficiently repressed expression of Renilla luciferase, whereas the 3′-UTR of CG1673 was only moderately affected. Inhibition of endogenous miR-277 by contransfection of a 2’-O-methyl antisense RNA oligonucleotide did not reveal any significant changes. This is potentially due to saturation of the moderate endogenous miR-277 levels in S2 cells by abundant reporter mRNAs. Nonetheless, we observed a trend consistent with the results from miR-277 overexpression.
miR-277 tunes BCAA catabolism and activates TOR
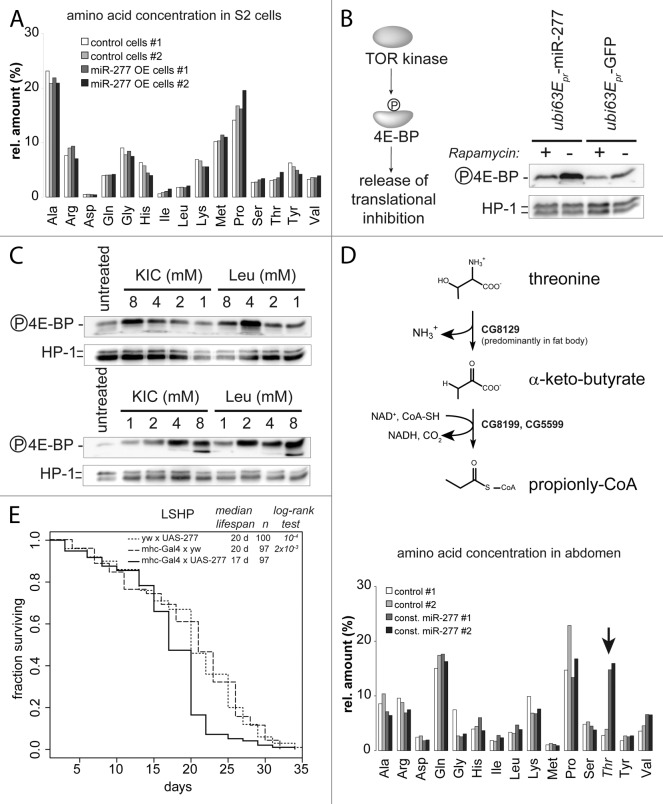
How can a metabolic change in branched chain amino acid catabolism affect lifespan? Based on our analysis, we predict that constitutive expression of miR-277 reduces the capacity for metabolic clearance of BCAAs. The resulting increase in BCAA concentration might stimulate the TOR kinase14,19 and thereby shorten lifespan. As a first step toward evaluating this hypothesis, we started with a homogeneous tissue source in cell culture. We examined the metabolic consequences of modulated miR-277 levels by measuring amino acid concentrations in S2 cell extracts after transfection with a miR-277 expression plasmid. Surprisingly, this did not increase the concentration of BCAAs (Fig. 4A). Yet, overexpression of miR-277 in S2-cells led to increased phosphorylation of eIF4E-binding protein (4EBP, Fig. 4B), a substrate of the TOR kinase. This phosphorylation could be blunted by the specific TORC1 inhibitor rapamycin. According to our transcriptomic and luciferase reporter analysis, the first catabolic enzyme (BCAT) is not efficiently targeted by miR-277; consequently, transamination of BCAAs to BCKAs may occur but further degradation by the branched-chain α-keto acid dehydrogenase complex (BCKDH) is diminished (see Fig. S5). As a result of this, it is conceivable that the branched chain α-keto acids (BCKAs) accumulate as a metabolic intermediate. The derivative of leucine (KIC) is known to be a strong growth-promoting component in mammalian cells25,46,47 and is able to activate the vertebrate TOR kinase.23,24,48 To test whether this hypothesis is plausible in Drosophila, we added increasing amounts of KIC or leucine to the culture medium. After 24 h, both KIC and leucine were able to stimulate TOR activity (Fig. 4C). Thus, manipulating the levels of KIC has the potential to activate TOR in Drosophila as well.
Figure 4. Activation of TOR by miR-277 likely occurs via increased KIC levels. (A) Amino acid measurement of S2-cell extracts. Control cells were transfected with a GFP expression construct instead of the miR-277 hairpin expression plasmid. To correct for small differences in extract concentration, values were normalized to the sum of all amino acid concentrations in the sample and displayed as relative proportion. (B) Detection of TOR kinase activity with an antibody specific for the phosphorylated form of the TOR substrate 4E-BP. Phosphorylation inactivates 4E-BP and releases translational inhibition. The effect of miR-277 expression on 4E-BP phosphorylation can be blunted by rapamycin, confirming that the TOR kinase is acting on 4E-BP. (C) Western blots for phosphorylated 4E-BP and HP1 as a loading control; both KIC and Leucine can activate the TOR kinase when added to the cell culture medium. The two blots show independent experiments to illustrate that the apparent maximal phospho-4E-BP levels at 4 mM KIC or Leu are within the experimental variation. (D) Upper panel: schematic representation of the threonine degradation pathway via threonine-ammonia-lyase that produced α-keto-butyrate, an alternative substrate of the BCKDH complex that is repressed by miR-277. Lower panel, the Drosophila homolog of threonine ammonia lyase is predominantly expressed in fat body and the spermatheca but absent in S2-cells. Increased threonine levels in female abdomen in response to constitutive miR-277 expression indicate that the BCKDH complex is indeed less active. (F) Muscle-specific transgenic expression of miR-277 is sufficient to shorten lifespan. While neither the driver transgene (the myosin heavy chain promotor controlling expression of the Gal4 transcription factor) nor the UAS-mir277 construct alone show abnormal lifespan, a combination of both leads to lifespan shortening on medium with an excess of protein over sugar (LSHP). The p values for the log-rank test between either of the controls and the mhc-gal4 × UAS-277 survival curve are indicated in the graph. There was no significant differences between the two control curves (p = 0.352, log-rank test).
To validate that miR-277 induces changes on the metabolite level in vivo, we demonstrated inhibition of the BCKDH-complex by an alternative substrate: In addition to branched-chain α-keto acids, the mitochondrial BCKDH complex can also oxidize α-keto-butyrate, a metabolite derived from threonine in the reaction catalyzed by threonine-ammonia-lyase (see Fig. 4D, upper panel).49,50 The Drosophila homolog of this enzyme (CG8129) carries no predicted miR-277 target site and its mRNA level did not change in response to miR-277 in our experiments (see Table 1; Table S2). It is expressed predominantly in fat body and the female spermatheca, while transcript levels are very low in S2-cells (see Fig. S7 and Flybase data). We therefore measured amino acid concentrations in extracts prepared from the abdomen of wt and transgenic miR-277 female flies. We observed increased threonine levels in flies with constitutive miR-277 expression (Fig. 4D, lower panel). Consistent with the low expression levels of threonine-ammonia lyase in S2-cells, threonine levels were unchanged in extracts prepared from cultured S2 cells upon overexpression of miR-277 (Fig. 4A). Thus, we could confirm functionally relevant downregulation of the BCKDH complex in response to miR-277 on the metabolite level.
Muscle tissue is an important player in metabolic homeostasis, and decreased muscle function contributes to age-related pathologies. Since miR-277 is highly expressed in the thorax (Fig. 4B; Fig. S8) its function may be to regulate BCAA metabolism in the flight muscles. We thus made use of the Gal4-UAS system to express miR-277 specifically in muscles using the mhc-Gal4 driver line,51 then recorded the resulting lifespan changes. When both the driver and the miR-277 expression construct were present, we observed significant lifespan shortening (Fig. 4E). Either component alone did not affect lifespan, indicating that differences in genetic background are not responsible for the effect. Rather, muscle-specific increases in miR-277 levels appear to be sufficient to cause lifespan shortening.
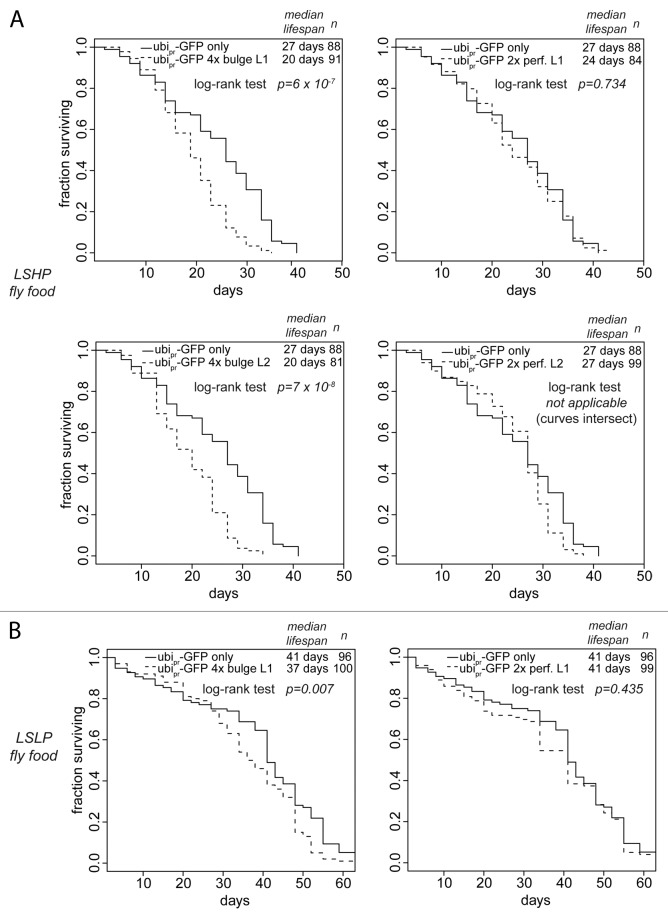
We examined the life-span of transgenic flies that express GFP with or without artificial miR-277 target sites from a strong, ubiquitously expressed promotor. Such artificial miR-277 targets are predicted to compete with endogenous miR-277 targets for regulation by the miRNA, resulting in inhibition of miR-277 action on its endogenous targets by a so-called sponge-effect.52,53 Based on the number of target sites and the fact that miR-277 may repress the perfect matches in a catalytic mode by cleavage and turnover, we predict that the bulged-match artificial target is the more potent repressor of miR-277. On protein-rich LSHP food, we observed a clear lifespan shortening in two independent transgenic lines expressing the artificial miRNA-target with four bulged matches to miR-277, while two independent lines expressing an artificial target with two perfect matches did not shorten lifespan compared with flies expressing only GFP (Fig. 5A). We tested whether this effect could be modulated by food composition and measured the lifespan of one set of transgenic lines under dietary restriction (LSLP food). This alleviated the lifespan-shortening phenotype of the artificial target with the bulged matches, whereas the artificial target with perfect matches still showed no lifespan shortening compared with the controls. Apparently, tuning of BCAA catabolism by miR-277 is required for optimal longevity since both overexpression as well as inhibition reduce lifespan, in particular on food that has an excess of dietary protein.
Figure 5. Inhibition of miR-277 activity by sponge transgenes shortens lifespan. (A) The lifespan of transgenic flies with ubiquitous expression of a GFP construct containing either none, two perfectly base-paired or four bulged match miR-277 target sites was measured on food with high protein and low sugar content. Two independent lines were tested for each sponge transgene. (B) Dietary restriction alleviates the life span shortening effect of miR-277 inhibition.
miR-277 genetically interacts with the insulin signaling system
If TOR signaling is tuned by miR-277, then there should be genetic interaction of miR-277 with other growth signaling pathways. To coordinate nutrient availability with cellular and organismal growth, the TOR pathway shares extensive cross-regulation with insulin signaling, leading to concordant output (reviewed in ref. 21). We disrupted this concordance by combining constitutive miR-277 expression, presumably resulting in TOR activation, with a mutation in the insulin receptor substrate chico that reduces insulin signaling. While a control cross of chico1/CyO flies gave offspring in the expected Mendelian ratio of 2:1 for heterozygous vs. homozygous animals (62 vs. 36 animals; homozygous CyO flies are lethal), the combination of our transgenic ubi-63Epr- > miR-277 construct was synthetic lethal with a homozygous chico1 mutation (only heterozygous adult offspring observed, n = 57).
Is activation of TOR required to shorten lifespan in response to miR-277? In principle, it should be possible to answer this question by testing whether feeding of the TOR inhibitor rapamycin reverses the lifespan shortening effect of constitutive miR-277 expression. However, conflicting results about the lifespan extending effect of rapamycin on adult flies have been published;54,55 genetic inhibition of TOR signaling extended lifespan.56 Since macrolide antibiotics are unstable in aqueous solutions, we prepared rapamycin-containing food by spreading a freshly made dilution of rapamycin in water onto the food one day before transferring the flies, thus making sure that the compound was present in its active form. As a control, we used an identical dilution containing only the DMSO used as solvent for the rapamycin stock solution. We found that with this administration scheme, a concentration of 200 µM rapamycin in the spreading solution was highly toxic. At a rapamycin concentration of 20 µM, we saw a delay of larval development and a corresponding delay in eclosion, indicating that the TOR kinase was effectively inhibited (Fig. S9A). However, neither male control flies nor male flies with constant miR-277 expression displayed any lifespan extension induced by the drug, both on protein-rich LSHP food as well as standard fly food (Fig. S9B). If anything, rapamycin treatment slightly exacerbated the lifespan-shortening effect of constant miR-277 expression, but this was inconsistent between the two different food types. We note that even in the publication where Drosophila lifespan was successfully prolonged with rapamycin, the extension was much stronger in female than in male flies, indicating that effects on e.g., egg production may, in part, mediate the rapamycin effect. The effect of TOR inhibition on lifespan, therefore remains controversial and we cannot draw any conclusion concerning the interaction of constitutive miR-277 expression with rapamycin feeding.
Discussion
Using a novel experimental miRNA target validation technique, we have demonstrated that miR-277 controls the degradation of branched-chain amino acids (BCAA) downstream of the transaminase enzyme. As a result, miR-277 appears to tune the BCAA/BCKA ratio rather than the BCAA concentration per se. The physiologic function of mir-277 may be to support metabolic adaptation as flies age: Expression of miR-277 is downregulated during adult life and constitutive expression of the miRNA shortens lifespan. This lifespan reduction may be due to TOR activation via modulation of BCAA catabolism. Genetic interaction of modified miR-277 activity with insulin signaling and food composition demonstrates that precise tuning of the BCAA catabolism is an important aspect of metabolic homeostasis.
DTA as a tool for miRNA target validation
The reliable prediction of miRNA, target mRNA interactions is hampered by their limited complementarity: Roughly eight nucleotides of complementarity in the so-called seed region can suffice for a productive interaction. Even when additional parameters such as evolutionary conservation or absence of strong secondary structure are considered, usually many targets have the potential to be regulated by a given miRNA in silico.40,57-63 As a consequence, about 30% of all transcripts are predicted miRNA targets, and the relevance of such a large number of predicted interactions has been questioned.64 The need for experimental validation has been recognized early on and since miRNA-mediated repression also stimulates mRNA degradation, powerful technologies available for transcriptome analysis can be employed. While the relative importance of translational inhibition vs. mRNA degradation remains a matter of debate, parallel analysis via quantitative proteomics and transcriptomics have demonstrated that both approaches will in most cases identify the same targets.36-38,65 Thus, inhibition of a given miRNA will increase and overexpression will reduce steady-state levels of its target mRNAs. A straightforward interpretation of transcriptomic data are hindered, however, by the presence of indirect effects, i.e., secondary changes that occur as a consequence of loss of miRNA function. One successful strategy to focus on miRNA targets is to recover transcripts that are physically associated with RISC.66,67 However, this will enrich for the targets of all miRNAs expressed in a given tissue or cell line and predictions still need to be employed to describe the individual miRNA, target mRNA interactions. Including a cross-linking step prior to RISC immunoprecipitation, followed by deep sequencing, has been a further improvement of the method since individual target sites can be identified based on characteristic mutations of the sequences obtained, making prediction of the associated miRNA fairly unambiguous.68-70 Nonetheless, functional validation of in silico predictions remains a bottleneck in current miRNA research. The approach of miRNA inhibition followed by dynamic transcriptome analysis (DTA) allows focusing on those mRNAs that show increased steady-state levels due to reduced transcript degradation rates. Indirect effects that occur via transcriptional induction can thus be detected as such and the complexity is reduced, as can be seen in Table 1 (compare column “total RNA” with column “> 1 h”). Our mRNA pulse-labeling approach has thus allowed us to conclude that Drosophila miR-277 defines a post-transcriptional regulation unit for the enzymes that degrade branched-chain amino acids. One obvious limitation remains, though: Since only mRNA levels are examined, miRNA targets that are repressed predominantly on the translational level will not be detected.
While many of the miR-277 targets we validated were indeed predicted (especially in the case of S2-cells, see Table 1), relatively few of the predicted targets could be experimentally confirmed: There are 691 predicted mRNA targets for miR-277 in the targetscan database (version 6.0) and 358 were expressed in our S2-cells, but only 10 showed significant changes upon inhibition of miR-277. It may well be that in tissues with much higher miR-277 expression levels, more mRNA targets are affected. On the other hand, a high-target abundance together with low seed-match base-pairing stability (miR-277 has a high A/U content in the seed) may regulate the silencing efficiency of miRNAs,71 a concept that has also been referred to as pseudotargets.64 In this scenario, many inefficiently repressed targets serve to regulate the miRNA, rather than the respective mRNAs. Whether this is the case for miR-277 remains to be examined.
Even though the indirect effects due to changes in transcription rates observed in transcriptome studies hamper the assignment of miRNA, target mRNA interactions, they constitute a potentially rich source of information on the physiological relevance of a given miRNA. How does the cell try to compensate the induced loss of a given miRNA’s regulation? In our experiment, one gene (edin) was transcriptionally upregulated upon inhibition of miR-277. While obviously no mechanistic insight can be derived from the indirect effect on a single gene, it proves the principle that such an analysis is possible and may be of value for other miRNAs. Since DTA is not limited to Drosophila cells, we propose that it is a useful strategy to bridge the gap between target identification and physiological relevance of miRNAs in other cell culture systems as well. Conveniently, the same modified nucleoside can be used for both DTA and PAR-clip (though with different incubation times).
Gain-of-function genetics with microRNAs
It is a prevalent conception that miRNAs target hundreds of mRNAs and that gain-of-function, i.e., overexpression, studies will likely induce many non-physiological miRNA-mRNA interactions. This is of course a possible outcome and certainly a concern when a physiological function needs to be derived from a gain-of-function phenotype. In the case of miRNAs, recent screening approaches with transgenic miRNA overexpression libraries72,73 indicate that very specific phenotypes can nonetheless be discovered. We describe a lifespan reduction in the context of miR-277, which is certainly a phenotype that may be affected by many influences. Nonetheless, our molecular analysis demonstrates a striking coalescence of the miR-277 effects within the BCAA degradation cascade. Furthermore, cell culture reporter studies demonstrate that even upon co-overexpression of miR-277 and the non-reactive, yet predicted, target 3′-UTR of CG1673, only a mild repression can be observed. We employed constitutive expression of the miRNA, but not the corresponding targets, for our in vivo approach. We therefore expect that the derived target specificity largely represents physiologic interactions. Genetic interaction with insulin signaling and the alleviation of the miR-277 transgenic sponge phenotype on food with lower protein content further indicate that metabolic control is a physiologic function of miR-277. On the other hand, we certainly do not want to exclude that mir-277 has additional functions that are specific to certain cell types. These may not have produced a significant effect in our molecular analysis based on whole fly RNA and cultured S2-cells.
Leucine vs. KIC in TOR activation
Our finding that miR-277 represses the BCKDH enzyme complex and activates TOR in cell culture suggests that a change in the BCAA/BCKA ratio may have physiologic consequences, a novel aspect in TOR regulation. Both components were previously known TOR activators, but their interconversion via the BCAT reaction is generally assumed to be rapid and bi-directional, thus the compounds were considered “synonymous.” Yet, our data demonstrates that at least in cultured S2-cells TOR can be activated by increased miR-277 levels in the absence of changes in BCAA concentration. We were unfortunately unable to quantify BCKA levels directly using enzymatic assays due to their low concentration, forcing us to search for a surrogate metabolite marker. The very pronounced increase of threonine levels in the abdomen of flies with constitutive miR-277 expression indicates that the BCKDH complex is indeed substantially repressed. The effect on BCAAs was again much weaker, consistent with the notion that miR-277 action does not primarily augment BCAA levels.
If the BCAT transamination reaction is readily reversible, then how can BCKAs accumulate selectively? The metabolite flux through this pathway is complex and depends not only on the levels and activity state of the enzymes involved, but also on the distribution of the metabolites among intra- and extracellular compartments. Since both BCAAs and BCKAs can be released from the cell, it has been previously suggested that in a situation where transamination capacity is high, modulation of BCKDH activity will influence the rate of BCKA, rather than BCAA, release.74 The most dramatic form of this effect can be observed in patients suffering from maple syrup urine disease (MSUD), a hereditary deficiency resulting in complete loss of the BCKDH activity. During episodes of metabolic crisis, MSUD patients were reported to have plasma concentrations of up to 5 mM leucine, 1 mM valine and 1 mM isoleucine but 4.6 mM KIC, 1.5 mM α-keto-β-methyl-valeric acid (KMV) and 0.35 mM α-keto-isovaleric acid (KIV).75,76 While all compounds are substantially elevated, the proportional increase is much higher for the BCKAs and the normally 8‒10-fold excess of BCAAs over BCKAs is reduced to essentially equal levels. In summary, it is reasonable to assume that repression of BCKDH enzymes in the absence of BCAT repression can increase BCKA concentration more than BCAA concentration. How BCKA concentration is relayed to the activation of TOR will be a subject of future studies. We note that depletion of the BCKDH E2 subunit by RNAi increased translation activity as well as phosphorylation of the TOR substrate p70S6K in murine C2C12 cells, indicating that such a mechanism may be conserved in mammals.77
It must be clearly stated, however, that we could not unambiguously demonstrate that the lifespan-shortening phenotype of constitutive miR-277 expression is dependent on TOR activation (see Fig. S9), and we want to propose this only as a hypothesis that is consistent with our results.
Is tuning of the BCAA/BCKA ratio conserved?
Although miR-277 is not conserved, an analogous modulation of BCKA levels can be achieved by controlling the action of BCDKH-kinase on the BCKDH complex78 (summarized in Fig. 6). BCKDH-kinase-knockout mice have hyperactive BCKA clearance and are viable with lower BCAA blood concentrations, neurological abnormalities and show reduced growth.79 Furthermore, mutations in the human BCKDH kinase gene result in autism, epilepsy and intellectual disability and reduced serum BCAA and BCKA levels.31 Pharmacological inhibition of BCKDH-kinase with clofibric acid (also leading to hyperactivation of BCAA clearance) decreased steady-state plasma BCAA concentrations and blunted stimulation of TOR by a leucine pulse.80 However, none of these studies distinguished whether the observed effects were due to changes in BCAA or BCKA levels. Analysis of serum BCKA levels in humans revealed a significant decrease in institutionalized old (> 60 y) vs. healthy young (19–59 y) subjects,81 analogous to the likely consequences of miR-277 downregulation in adult fruit flies. In addition to the regulation of BCKDH by its associated kinase, human miR-29b has been proposed to control BCKDH mRNA levels analogous to Drosophila miR-277.82 In conclusion, regulation of the BCKDH enzyme complex certainly occurs in mammals as well.
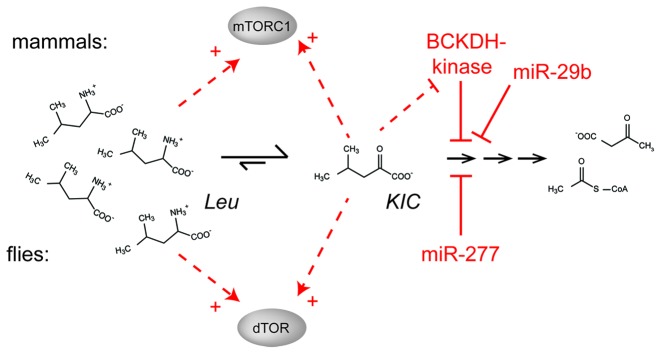
Figure 6. Activation of TOR via control of BCAA catabolism. While flies appear to use miR-277 for the control of branched-chain amino acid catabolism and signaling, a specific protein kinase can phosphorylate the branched-chain α keto acid dehydrogenase (BCKDH) enzyme in mammals. This kinase inhibits the enzyme and is itself inhibited by KIC, a substrate for the BCKDH reaction. This regulatory loop can work both as a feed-forward activation mechanism but also as a negative feed-back loop to prevent excessive accumulation of KIC. In addition, human miR-29b was reported to regulate the BCKDH complex.
Outlook
Would there be any benefit from activating TOR via BCKAs rather than BCAAs? At this point we can only speculate, but chronically upregulated intracellular BCAA levels may result in adverse effects on translation fidelity, which are avoided if KIC levels are modulated in the context of long-term adaptations. Deficient coordination between TOR and insulin signaling may be a mediator of metabolic abnormalities or disease predispositions in humans, analogous to the synthetic lethality we observed in flies. Consistent with this notion, elevated BCAA blood concentrations are indicators for a higher risk of developing insulin resistance or diabetes.27-29 If the metabolic fate of BCAAs determines the extent of TOR activation, simultaneous monitoring of both BCKA and BCAA concentrations may improve the predictive value of this biomarker. Furthermore, MSUD patients may also suffer from chronic TOR activation. Clearly, these hypotheses are speculative at best, but future research may clarify whether long-term tuning of BCAA catabolism leads to physiologically relevant changes in humans as well.
Materials and Methods
Fly husbandry and lifespan analysis
Flies were maintained on standard yeast/cornmeal/molasses medium at 25°C with a 12 h light/dark cycle, defined sucrose/yeast extract media83 were used for lifespan measurements. Transgenic flies with constitutive miR-277 expression were generated by injection of ubi63E-pre-miR277 (pKF84, ref. 34) into either w1118 embyros (Rainbow Transgencis) and backcrossed into yw background or directly into yw embyros (kindly provided by P. D. Zamore). During lifespan measurements, male flies were kept at an initial density of 10 flies per vial, counted and transferred to fresh food three times per week. Parallel cohorts for RNA isolation were kept and transferred under identical conditions.
RNA isolation, miRNA profiling, northern blotting and microarray analysis
RNA was extracted either with Trizol (Invitrogen) according to the manufacturer’s instructions or using the QIAGEN miRNeasy Kit (Qiagen) and quantified using spectrophotometry. For miRNA profiling, tailing and reverse transcription was performed with the Qiagen miScript reverse transcription kit according to the manufacturer’s instruction. PCR amplification was performed on an ABI prism 7000 instrument with the Qiagen miScript qPCR kit using miRNA-specific primers from a custom primer plate (see Table S1 for primer sequences). The expression levels were first expressed relative to U6 RNA, then the general trend of RNA reduction with age was determined by linear regression and subtracted to retain only those differences that diverge from a general trend. Northern blots for small RNAs were performed as previously described.84 Microarray analysis was performed using the GeneChip 3′IVT labeling assay (Affymetrix) with 100 ng input RNA. Samples were hybridized to GeneChip Drosophila Genome 2.0 microarrays following the instructions from the supplier (Affymetrix), computational analysis of differential expression was performed using R!/Bioconductor. Labeling of nascent RNA with 4-thio-uridine and RNA fractionation was performed as previously described.42 Real-time quantitative PCR analysis for aging-related and miR-277 target genes was performed using an ABI prism 7000 instrument with the DynamoFlash Sybr green kit and a custom primer plate (see Table S4 for primer sequences). The microarray data have been submitted to ArrayExpress with the accession numbers E-MEXP-3785 and E-MEXP-3784 for the fly and cell culture data, respectively.
The microarray and sequencing data were submitted to the following databases:
Deep sequencing analysis
RNA was extracted from dissected female abdomina by grinding the tissue in Trizol with a micor-pestle, then following the manufacturer’s instructions. Linker ligation and library construction were performed as described,85 then deep sequenced using the Illumina platform (Fasteris, Geneva/CH). For data analysis, the reads were sorted and pre-processed using PERL scripts (available upon request), then mapped to target sequences using BOWTIE86 with no mismatch allowed. The sequence data has been submitted to NCBI GEO with the accession number GSE42442.
Metabolite measurements
Cultured Drosophila cells were transfected with ubi63E-pre-miR-277 (pKF84) or an analogous GFP-expression construct (pKF63) using Fugene HD (Promega), split 1:4 on day 4 and harvested on day 6. A total of 18 ml of culture volume, corresponding to roughly 5 × 107 cells, was harvested, washed three times in PBS and extracted with 100 μl of 0.1 N HCl. Precipitated proteins were removed by centrifugation and the extract was neutralized with an equal volume of 0.1 N NaOH. Extracts from fly tissue were prepared analogously. After appropriate dilution, samples were analyzed on a Dionex DX 500 IC HPLC system equipped with an AminoPac PA-10 column (2 mm ID) and an ED40 electrochemical detector operating in pulse mode with potential switching between -1.67 and 0.93V. Five point calibrations were measured using a commercial set of amino acid standards (Sigma). Detailed chromatographic conditions are available upon request.
Western blotting
Protein extraction, gel electrophoresis, transfer and incubation of the membranes were performed essentially as previously described42 but using an extraction buffer suuplemented with a cocktail containing both protease and phosphatase inhibitors (Phos-Stop, Roche). Antibodies directed against phosphorylated 4E-BP were from cell signaling technologies (mAB#2855), the HP1-antibody87 was obtained from the Developmental Studies Hybridoma Bank.
Supplementary Material
Acknowledgments
We want to thank Romy Böttcher for help with the experiments. Stefan Sigrist kindly provided the mhc-Gal4 driver line and Phillip Zamore the ubi63E-miR277 line established directly in the yw background. The HP-1 antibody developed by S.E. was obtained from the Developmental Studies Hybridoma Bank developed under the auspices of the NICHD and maintained by The University of Iowa, Department of Biolog. Funding for this work was provided by an LMU excellent grant to KF, the Munich Center for Advanced Protein Science (CIPSM) and the DFG Sonderforschungsbereich 646. K.F. was the recipient of a Human Frontier Science Program Career Development Award and AT was supported by the LMU guest professorship “Computational Biochemistry.”
Submitted
02/12/13
Revised
04/10/13
Accepted
04/25/13
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Cohen SM, Brennecke J, Stark A. Denoising feedback loops by thresholding--a new role for microRNAs. Genes Dev. 2006;20:2769–72. doi: 10.1101/gad.1484606. [DOI] [PubMed] [Google Scholar]
- 2.Li X, Carthew RW. A microRNA mediates EGF receptor signaling and promotes photoreceptor differentiation in the Drosophila eye. Cell. 2005;123:1267–77. doi: 10.1016/j.cell.2005.10.040. [DOI] [PubMed] [Google Scholar]
- 3.Li X, Cassidy JJ, Reinke CA, Fischboeck S, Carthew RW. A microRNA imparts robustness against environmental fluctuation during development. Cell. 2009;137:273–82. doi: 10.1016/j.cell.2009.01.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ibáñez-Ventoso C, Yang M, Guo S, Robins H, Padgett RW, Driscoll M. Modulated microRNA expression during adult lifespan in Caenorhabditis elegans. Aging Cell. 2006;5:235–46. doi: 10.1111/j.1474-9726.2006.00210.x. [DOI] [PubMed] [Google Scholar]
- 5.Kato M, Chen X, Inukai S, Zhao H, Slack FJ. Age-associated changes in expression of small, noncoding RNAs, including microRNAs, in C. elegans. RNA. 2011;17:1804–20. doi: 10.1261/rna.2714411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Lencastre A, Pincus Z, Zhou K, Kato M, Lee SS, Slack FJ. MicroRNAs both promote and antagonize longevity in C. elegans. Curr Biol. 2010;20:2159–68. doi: 10.1016/j.cub.2010.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Williams AE, Perry MM, Moschos SA, Lindsay MA. microRNA expression in the aging mouse lung. BMC Genomics. 2007;8:172. doi: 10.1186/1471-2164-8-172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Drummond MJ, McCarthy JJ, Sinha M, Spratt HM, Volpi E, Esser KA, et al. Aging and microRNA expression in human skeletal muscle: a microarray and bioinformatics analysis. Physiol Genomics. 2011;43:595–603. doi: 10.1152/physiolgenomics.00148.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xu P, Vernooy SY, Guo M, Hay BA. The Drosophila microRNA Mir-14 suppresses cell death and is required for normal fat metabolism. Curr Biol. 2003;13:790–5. doi: 10.1016/S0960-9822(03)00250-1. [DOI] [PubMed] [Google Scholar]
- 10.Weinkove D, Neufeld TP, Twardzik T, Waterfield MD, Leevers SJ. Regulation of imaginal disc cell size, cell number and organ size by Drosophila class I(A) phosphoinositide 3-kinase and its adaptor. Curr Biol. 1999;9:1019–29. doi: 10.1016/S0960-9822(99)80450-3. [DOI] [PubMed] [Google Scholar]
- 11.Brogiolo W, Stocker H, Ikeya T, Rintelen F, Fernandez R, Hafen E. An evolutionarily conserved function of the Drosophila insulin receptor and insulin-like peptides in growth control. Curr Biol. 2001;11:213–21. doi: 10.1016/S0960-9822(01)00068-9. [DOI] [PubMed] [Google Scholar]
- 12.Mirth C, Truman JW, Riddiford LM. The role of the prothoracic gland in determining critical weight for metamorphosis in Drosophila melanogaster. Curr Biol. 2005;15:1796–807. doi: 10.1016/j.cub.2005.09.017. [DOI] [PubMed] [Google Scholar]
- 13.Grewal SS. Insulin/TOR signaling in growth and homeostasis: a view from the fly world. Int J Biochem Cell Biol. 2009;41:1006–10. doi: 10.1016/j.biocel.2008.10.010. [DOI] [PubMed] [Google Scholar]
- 14.Katewa SD, Kapahi P. Role of TOR signaling in aging and related biological processes in Drosophila melanogaster. Exp Gerontol. 2011;46:382–90. doi: 10.1016/j.exger.2010.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pletcher SD, Macdonald SJ, Marguerie R, Certa U, Stearns SC, Goldstein DB, et al. Genome-wide transcript profiles in aging and calorically restricted Drosophila melanogaster. Curr Biol. 2002;12:712–23. doi: 10.1016/S0960-9822(02)00808-4. [DOI] [PubMed] [Google Scholar]
- 16.Landis GN, Abdueva D, Skvortsov D, Yang J, Rabin BE, Carrick J, et al. Similar gene expression patterns characterize aging and oxidative stress in Drosophila melanogaster. Proc Natl Acad Sci USA. 2004;101:7663–8. doi: 10.1073/pnas.0307605101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cochemé HM, Quin C, McQuaker SJ, Cabreiro F, Logan A, Prime TA, et al. Measurement of H2O2 within living Drosophila during aging using a ratiometric mass spectrometry probe targeted to the mitochondrial matrix. Cell Metab. 2011;13:340–50. doi: 10.1016/j.cmet.2011.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hulbert AJ, Clancy DJ, Mair W, Braeckman BP, Gems D, Partridge L. Metabolic rate is not reduced by dietary-restriction or by lowered insulin/IGF-1 signalling and is not correlated with individual lifespan in Drosophila melanogaster. Exp Gerontol. 2004;39:1137–43. doi: 10.1016/j.exger.2004.04.006. [DOI] [PubMed] [Google Scholar]
- 19.Dann SG, Thomas G. The amino acid sensitive TOR pathway from yeast to mammals. FEBS Lett. 2006;580:2821–9. doi: 10.1016/j.febslet.2006.04.068. [DOI] [PubMed] [Google Scholar]
- 20.Nicklin P, Bergman P, Zhang B, Triantafellow E, Wang H, Nyfeler B, et al. Bidirectional transport of amino acids regulates mTOR and autophagy. Cell. 2009;136:521–34. doi: 10.1016/j.cell.2008.11.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zoncu R, Efeyan A, Sabatini DM. mTOR: from growth signal integration to cancer, diabetes and ageing. Nat Rev Mol Cell Biol. 2011;12:21–35. doi: 10.1038/nrm3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Avruch J, Long X, Ortiz-Vega S, Rapley J, Papageorgiou A, Dai N. Amino acid regulation of TOR complex 1. Am J Physiol Endocrinol Metab. 2009;296:E592–602. doi: 10.1152/ajpendo.90645.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Patti ME, Brambilla E, Luzi L, Landaker EJ, Kahn CR. Bidirectional modulation of insulin action by amino acids. J Clin Invest. 1998;101:1519–29. doi: 10.1172/JCI1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xu G, Kwon G, Cruz WS, Marshall CA, McDaniel ML. Metabolic regulation by leucine of translation initiation through the mTOR-signaling pathway by pancreatic beta-cells. Diabetes. 2001;50:353–60. doi: 10.2337/diabetes.50.2.353. [DOI] [PubMed] [Google Scholar]
- 25.Zawalich WS. Time-dependent potentiation of insulin release induced by alpha-ketoisocaproate and leucine in rats: possible involvement of phosphoinositide hydrolysis. Diabetologia. 1988;31:435–42. doi: 10.1007/BF00271588. [DOI] [PubMed] [Google Scholar]
- 26.D’Antona G, Ragni M, Cardile A, Tedesco L, Dossena M, Bruttini F, et al. Branched-chain amino acid supplementation promotes survival and supports cardiac and skeletal muscle mitochondrial biogenesis in middle-aged mice. Cell Metab. 2010;12:362–72. doi: 10.1016/j.cmet.2010.08.016. [DOI] [PubMed] [Google Scholar]
- 27.Newgard CB, An J, Bain JR, Muehlbauer MJ, Stevens RD, Lien LF, et al. A branched-chain amino acid-related metabolic signature that differentiates obese and lean humans and contributes to insulin resistance. Cell Metab. 2009;9:311–26. doi: 10.1016/j.cmet.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tai ES, Tan ML, Stevens RD, Low YL, Muehlbauer MJ, Goh DL, et al. Insulin resistance is associated with a metabolic profile of altered protein metabolism in Chinese and Asian-Indian men. Diabetologia. 2010;53:757–67. doi: 10.1007/s00125-009-1637-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang TJ, Larson MG, Vasan RS, Cheng S, Rhee EP, McCabe E, et al. Metabolite profiles and the risk of developing diabetes. Nat Med. 2011;17:448–53. doi: 10.1038/nm.2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Valerio A, D’Antona G, Nisoli E. Branched-chain amino acids, mitochondrial biogenesis, and healthspan: an evolutionary perspective. Aging (Albany NY) 2011;3:464–78. doi: 10.18632/aging.100322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Novarino G, El-Fishawy P, Kayserili H, Meguid NA, Scott EM, Schroth J, et al. Mutations in BCKD-kinase lead to a potentially treatable form of autism with epilepsy. Science. 2012;338:394–7. doi: 10.1126/science.1224631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Terashima J, Bownes M. Translating available food into the number of eggs laid by Drosophila melanogaster. Genetics. 2004;167:1711–9. doi: 10.1534/genetics.103.024323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tahoe NM, Mokhtarzadeh A, Curtsinger JW. Age-related RNA decline in adult Drosophila melanogaster. J Gerontol A Biol Sci Med Sci. 2004;59:B896–901. doi: 10.1093/gerona/59.9.B896. [DOI] [PubMed] [Google Scholar]
- 34.Förstemann K, Horwich MD, Wee L, Tomari Y, Zamore PD. Drosophila microRNAs are sorted into functionally distinct argonaute complexes after production by dicer-1. Cell. 2007;130:287–97. doi: 10.1016/j.cell.2007.05.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Han J, Lee Y, Yeom KH, Nam JW, Heo I, Rhee JK, et al. Molecular basis for the recognition of primary microRNAs by the Drosha-DGCR8 complex. Cell. 2006;125:887–901. doi: 10.1016/j.cell.2006.03.043. [DOI] [PubMed] [Google Scholar]
- 36.Guo H, Ingolia NT, Weissman JS, Bartel DP. Mammalian microRNAs predominantly act to decrease target mRNA levels. Nature. 2010;466:835–40. doi: 10.1038/nature09267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Selbach M, Schwanhäusser B, Thierfelder N, Fang Z, Khanin R, Rajewsky N. Widespread changes in protein synthesis induced by microRNAs. Nature. 2008;455:58–63. doi: 10.1038/nature07228. [DOI] [PubMed] [Google Scholar]
- 38.Baek D, Villén J, Shin C, Camargo FD, Gygi SP, Bartel DP. The impact of microRNAs on protein output. Nature. 2008;455:64–71. doi: 10.1038/nature07242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Reimand J, Arak T, Vilo J. g:Profiler--a web server for functional interpretation of gene lists (2011 update) Nucleic Acids Res. 2011;39(Web Server issue):W307-15. doi: 10.1093/nar/gkr378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stark A, Brennecke J, Russell RB, Cohen SM. Identification of Drosophila MicroRNA targets. PLoS Biol. 2003;1:E60. doi: 10.1371/journal.pbio.0000060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dölken L, Ruzsics Z, Rädle B, Friedel CC, Zimmer R, Mages J, et al. High-resolution gene expression profiling for simultaneous kinetic parameter analysis of RNA synthesis and decay. RNA. 2008;14:1959–72. doi: 10.1261/rna.1136108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hartig JV, Esslinger S, Böttcher R, Saito K, Förstemann K. Endo-siRNAs depend on a new isoform of loquacious and target artificially introduced, high-copy sequences. EMBO J. 2009;28:2932–44. doi: 10.1038/emboj.2009.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Miller C, Schwalb B, Maier K, Schulz D, Dümcke S, Zacher B, et al. Dynamic transcriptome analysis measures rates of mRNA synthesis and decay in yeast. Mol Syst Biol. 2011;7:458. doi: 10.1038/msb.2010.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rabani M, Levin JZ, Fan L, Adiconis X, Raychowdhury R, Garber M, et al. Metabolic labeling of RNA uncovers principles of RNA production and degradation dynamics in mammalian cells. Nat Biotechnol. 2011;29:436–42. doi: 10.1038/nbt.1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hutvágner G, Simard MJ, Mello CC, Zamore PD. Sequence-specific inhibition of small RNA function. PLoS Biol. 2004;2:E98. doi: 10.1371/journal.pbio.0020098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Escobar J, Frank JW, Suryawan A, Nguyen HV, Van Horn CG, Hutson SM, et al. Leucine and alpha-ketoisocaproic acid, but not norleucine, stimulate skeletal muscle protein synthesis in neonatal pigs. J Nutr. 2010;140:1418–24. doi: 10.3945/jn.110.123042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Amaral AU, Leipnitz G, Fernandes CG, Seminotti B, Schuck PF, Wajner M. Alpha-ketoisocaproic acid and leucine provoke mitochondrial bioenergetic dysfunction in rat brain. Brain Res. 2010;1324:75–84. doi: 10.1016/j.brainres.2010.02.018. [DOI] [PubMed] [Google Scholar]
- 48.Christie GR, Hajduch E, Hundal HS, Proud CG, Taylor PM. Intracellular sensing of amino acids in Xenopus laevis oocytes stimulates p70 S6 kinase in a target of rapamycin-dependent manner. J Biol Chem. 2002;277:9952–7. doi: 10.1074/jbc.M107694200. [DOI] [PubMed] [Google Scholar]
- 49.House JD, Hall BN, Brosnan JT. Threonine metabolism in isolated rat hepatocytes. Am J Physiol Endocrinol Metab. 2001;281:E1300–7. doi: 10.1152/ajpendo.2001.281.6.E1300. [DOI] [PubMed] [Google Scholar]
- 50.Paxton R, Scislowski PW, Davis EJ, Harris RA. Role of branched-chain 2-oxo acid dehydrogenase and pyruvate dehydrogenase in 2-oxobutyrate metabolism. Biochem J. 1986;234:295–303. doi: 10.1042/bj2340295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schuster CM, Davis GW, Fetter RD, Goodman CS. Genetic dissection of structural and functional components of synaptic plasticity. I. Fasciclin II controls synaptic stabilization and growth. Neuron. 1996;17:641–54. doi: 10.1016/S0896-6273(00)80197-X. [DOI] [PubMed] [Google Scholar]
- 52.Loya CM, Lu CS, Van Vactor D, Fulga TA. Transgenic microRNA inhibition with spatiotemporal specificity in intact organisms. Nat Methods. 2009;6:897–903. doi: 10.1038/nmeth.1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ebert MS, Neilson JR, Sharp PA. MicroRNA sponges: competitive inhibitors of small RNAs in mammalian cells. Nat Methods. 2007;4:721–6. doi: 10.1038/nmeth1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bjedov I, Toivonen JM, Kerr F, Slack C, Jacobson J, Foley A, et al. Mechanisms of life span extension by rapamycin in the fruit fly Drosophila melanogaster. Cell Metab. 2010;11:35–46. doi: 10.1016/j.cmet.2009.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Harrison B, Tran TT, Taylor D, Lee SD, Min KJ. Effect of rapamycin on lifespan in Drosophila. Geriatr Gerontol Int. 2010;10:110–2. doi: 10.1111/j.1447-0594.2009.00569.x. [DOI] [PubMed] [Google Scholar]
- 56.Kapahi P, Zid BM, Harper T, Koslover D, Sapin V, Benzer S. Regulation of lifespan in Drosophila by modulation of genes in the TOR signaling pathway. Curr Biol. 2004;14:885–90. doi: 10.1016/j.cub.2004.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lewis BP, Burge CB, Bartel DP. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell. 2005;120:15–20. doi: 10.1016/j.cell.2004.12.035. [DOI] [PubMed] [Google Scholar]
- 58.Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136:215–33. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Friedman RC, Farh KK, Burge CB, Bartel DP. Most mammalian mRNAs are conserved targets of microRNAs. Genome Res. 2009;19:92–105. doi: 10.1101/gr.082701.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Krek A, Grün D, Poy MN, Wolf R, Rosenberg L, Epstein EJ, et al. Combinatorial microRNA target predictions. Nat Genet. 2005;37:495–500. doi: 10.1038/ng1536. [DOI] [PubMed] [Google Scholar]
- 61.Rajewsky N. microRNA target predictions in animals. Nat Genet. 2006;38(Suppl):S8–13. doi: 10.1038/ng1798. [DOI] [PubMed] [Google Scholar]
- 62.Brennecke J, Stark A, Russell RB, Cohen SM. Principles of microRNA-target recognition. PLoS Biol. 2005;3:e85. doi: 10.1371/journal.pbio.0030085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Stark A, Lin MF, Kheradpour P, Pedersen JS, Parts L, Carlson JW, et al. Harvard FlyBase curators. Berkeley Drosophila Genome Project Discovery of functional elements in 12 Drosophila genomes using evolutionary signatures. Nature. 2007;450:219–32. doi: 10.1038/nature06340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Seitz H. Redefining microRNA targets. Curr Biol. 2009;19:870–3. doi: 10.1016/j.cub.2009.03.059. [DOI] [PubMed] [Google Scholar]
- 65.Esslinger S, Förstemann K. MicroRNAs repress mainly through mRNA decay. Angew Chem Int Ed Engl. 2009;48:853–5. doi: 10.1002/anie.200805127. [DOI] [PubMed] [Google Scholar]
- 66.Beitzinger M, Peters L, Zhu JY, Kremmer E, Meister G. Identification of human microRNA targets from isolated argonaute protein complexes. RNA Biol. 2007;4:76–84. doi: 10.4161/rna.4.2.4640. [DOI] [PubMed] [Google Scholar]
- 67.Easow G, Teleman AA, Cohen SM. Isolation of microRNA targets by miRNP immunopurification. RNA. 2007;13:1198–204. doi: 10.1261/rna.563707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hafner M, Landthaler M, Burger L, Khorshid M, Hausser J, Berninger P, et al. Transcriptome-wide identification of RNA-binding protein and microRNA target sites by PAR-CLIP. Cell. 2010;141:129–41. doi: 10.1016/j.cell.2010.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chi SW, Zang JB, Mele A, Darnell RB. Argonaute HITS-CLIP decodes microRNA-mRNA interaction maps. Nature. 2009;460:479–86. doi: 10.1038/nature08170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhang C, Darnell RB. Mapping in vivo protein-RNA interactions at single-nucleotide resolution from HITS-CLIP data. Nat Biotechnol. 2011;29:607–14. doi: 10.1038/nbt.1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Garcia DM, Baek D, Shin C, Bell GW, Grimson A, Bartel DP. Weak seed-pairing stability and high target-site abundance decrease the proficiency of lsy-6 and other microRNAs. Nat Struct Mol Biol. 2011;18:1139–46. doi: 10.1038/nsmb.2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Schertel C, Rutishauser T, Förstemann K, Basler K. Functional characterization of Drosophila microRNAs by a novel in vivo library. Genetics. 2012;192:1543–52. doi: 10.1534/genetics.112.145383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bejarano F, Bortolamiol-Becet D, Dai Q, Sun K, Saj A, Chou YT, et al. A genome-wide transgenic resource for conditional expression of Drosophila microRNAs. Development. 2012;139:2821–31. doi: 10.1242/dev.079939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hutson SM. Regulation of substrate availability for the branched-chain alpha-keto acid dehydrogenase enzyme complex. Ann N Y Acad Sci. 1989;573:230–9. doi: 10.1111/j.1749-6632.1989.tb15000.x. [DOI] [PubMed] [Google Scholar]
- 75.Tanaka K, Rosenberg L. Disorders of branched chain amino acid and organic acid metabolism. The metabolic bases of inherited disease, 5th ed., McGraw-Hill New York, 1982: p. 440-473. [Google Scholar]
- 76.Bodner-Leidecker A, Wendel U, Saudubray JM, Schadewaldt P. Branched-chain L-amino acid metabolism in classical maple syrup urine disease after orthotopic liver transplantation. J Inherit Metab Dis. 2000;23:805–18. doi: 10.1023/A:1026708618507. [DOI] [PubMed] [Google Scholar]
- 77.Nakai N, Shimomura Y, Tamura T, Tamura N, Hamada K, Kawano F, et al. Leucine-induced activation of translational initiation is partly regulated by the branched-chain alpha-keto acid dehydrogenase complex in C2C12 cells. Biochem Biophys Res Commun. 2006;343:1244–50. doi: 10.1016/j.bbrc.2006.03.074. [DOI] [PubMed] [Google Scholar]
- 78.Paxton R, Harris RA. Regulation of branched-chain alpha-ketoacid dehydrogenase kinase. Arch Biochem Biophys. 1984;231:48–57. doi: 10.1016/0003-9861(84)90361-8. [DOI] [PubMed] [Google Scholar]
- 79.Joshi MA, Jeoung NH, Obayashi M, Hattab EM, Brocken EG, Liechty EA, et al. Impaired growth and neurological abnormalities in branched-chain alpha-keto acid dehydrogenase kinase-deficient mice. Biochem J. 2006;400:153–62. doi: 10.1042/BJ20060869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ishiguro H, Katano Y, Nakano I, Ishigami M, Hayashi K, Honda T, et al. Clofibrate treatment promotes branched-chain amino acid catabolism and decreases the phosphorylation state of mTOR, eIF4E-BP1, and S6K1 in rat liver. Life Sci. 2006;79:737–43. doi: 10.1016/j.lfs.2006.02.037. [DOI] [PubMed] [Google Scholar]
- 81.Pailla K, Blonde-Cynober F, Aussel C, De Bandt JP, Cynober L. Branched-chain keto-acids and pyruvate in blood: measurement by HPLC with fluorimetric detection and changes in older subjects. Clin Chem. 2000;46:848–53. [PubMed] [Google Scholar]
- 82.Mersey BD, Jin P, Danner DJ. Human microRNA (miR29b) expression controls the amount of branched chain alpha-ketoacid dehydrogenase complex in a cell. Hum Mol Genet. 2005;14:3371–7. doi: 10.1093/hmg/ddi368. [DOI] [PubMed] [Google Scholar]
- 83.Mair W, Piper MD, Partridge L. Calories do not explain extension of life span by dietary restriction in Drosophila. PLoS Biol. 2005;3:e223. doi: 10.1371/journal.pbio.0030223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Förstemann K, Tomari Y, Du T, Vagin VV, Denli AM, Bratu DP, et al. Normal microRNA maturation and germ-line stem cell maintenance requires Loquacious, a double-stranded RNA-binding domain protein. PLoS Biol. 2005;3:e236. doi: 10.1371/journal.pbio.0030236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hartig JV, Förstemann K. Loqs-PD and R2D2 define independent pathways for RISC generation in Drosophila. Nucleic Acids Res. 2011;39:3836–51. doi: 10.1093/nar/gkq1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Langmead B, Trapnell C, Pop M, Salzberg SL. Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol. 2009;10:R25. doi: 10.1186/gb-2009-10-3-r25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.James TC, Elgin SC. Identification of a nonhistone chromosomal protein associated with heterochromatin in Drosophila melanogaster and its gene. Mol Cell Biol. 1986;6:3862–72. doi: 10.1128/mcb.6.11.3862. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.