Abstract
Systemic lupus erythematosus (SLE) is a devastating autoimmune disease characterized by chronic inflammation and systemic destruction of host organs or tissue. A key feature of SLE is T cell dysfunction characterized by hyperresponsive antigen receptor signaling. In this issue of the JCI, McDonald and colleagues provide evidence that homeostasis of a subset of lipids, the glycosphingolipids (GSLs), is severely perturbed in the membranes of T cells from SLE patients. Furthermore, normalization of GSLs restored TCR signaling and ameliorated T cell dysfunction. These data suggest that targeting host metabolism may be an effective means of reinforcing self-tolerance and attenuating autoimmunity.
Systemic lupus erythematosus (SLE) is a systemic autoimmune disease characterized by immune hyperactivity and loss of immunologic tolerance to self-antigens. Clinical manifestations often include symptoms of chronic inflammation, such as fatigue and fevers, as well as more specific features, including skin rashes, renal disease, arthritis, cardiovascular disease, vasculopathies, coagulopathies, and CNS involvement. The etiology of SLE is not well understood and likely includes both environmental and genetic factors. However, it is clear that dysfunction of multiple facets of host immunity underlies SLE pathogenesis, resulting in inflammatory immune cell infiltrates, autoantibody production, and deposition of pathogenic antibodies in target organs (reviewed in ref. 1).
Lipid dysfunction in autoimmune disease
Lipid dysfunction is a common clinical observation in many patients with rheumatic diseases and results in a heightened risk of cardiovascular disease independent of therapy (2–4). Genetic deletion of key proteins, such as apolipoprotein E, involved in lipid homeostasis has also been shown to exacerbate SLE pathogenesis in a broad array of model systems (5–7). Furthermore, T cells isolated from SLE patients have intrinsic alterations in lipid composition, especially in specialized microdomains of the plasma membrane (also known as lipid rafts) that contain the T cell antigen receptor (TCR), and alterations in associated signaling molecules (8, 9). The combined weight of these observations argues that lipid metabolism influences self-tolerance and autoimmune pathogenesis; however, it is not known how defects in lipid metabolic programs direct the fate and function of immune cells in autoimmunity. In this issue, McDonald and colleagues shed new light on the complex crosstalk between lipid metabolism and T cell dysfunction by demonstrating that resetting glycosphingolipid (GSL) homeostasis partially restores TCR signaling and normalizes function in T cells isolated from patients with lupus (10).
Alterations in T cell responsiveness associated with SLE
It has long been appreciated that T cells purified from individuals with SLE are dysfunctional, particularly in patients with active disease (reviewed in ref. 1). Perhaps the best-characterized changes in T cells from SLE patients are profound alterations in TCR signaling. Detailed studies indicate that T cells from SLE patients display heightened calcium flux in response to antigen receptor stimulation. The molecular mechanisms underlying the acquisition of this “exaggerated” signaling phenotype in SLE patients appears to be multifactorial. Biochemical characterization of the TCR signaling apparatus in T cells isolated from SLE patients indicates that changes in the assembly of the antigen receptor signal transduction machinery are mediated by the replacement of some signaling proteins (8, 11). The consequence of protein replacement is an alteration of the biochemical signaling pattern downstream of antigen receptor cross-linking and a subsequent perturbation of gene expression programs.
Multiple studies, including the current study by McDonald and colleagues, indicate that the alterations in TCR signaling are attributable, in part, to disruption of lipid homeostasis in the plasma membrane of SLE T cells (8–10). Specialized signal transduction localities within the plasma membrane, termed lipid rafts, rely on lipid composition to facilitate efficient TCR signaling. The lipid raft is specifically enriched with lipids, including cholesterol, sphingolipids, in particular sphingomyelin, and GSL species (Figure 1). The biochemical and biophysical properties of these lipids within the lipid raft are thought to facilitate the aggregation (or exclusion) of signal transduction machinery, and disruption of these microdomains can substantially alter TCR signaling (12). Interestingly, the characterization of T cells purified from SLE patients revealed alterations in GSLs and cholesterol within the plasma membrane, correlating with perturbations in lipid raft function (8, 9). These studies imply that lipid raft dysfunction may be a root cause of the exaggerated TCR signaling that is commonly observed in lymphocytes from SLE patients. In support of this assertion, disruption of lipid rafts or inhibition of cholesterol or GSL biosynthesis appears to be able to normalize TCR signaling and attenuate excessive cytokine production from autoimmune lymphocytes (8, 9, 13). However, identification of the molecular mechanism(s) that drive lipid metabolic dysfunction in T cells from patients with rheumatic diseases has remained elusive.
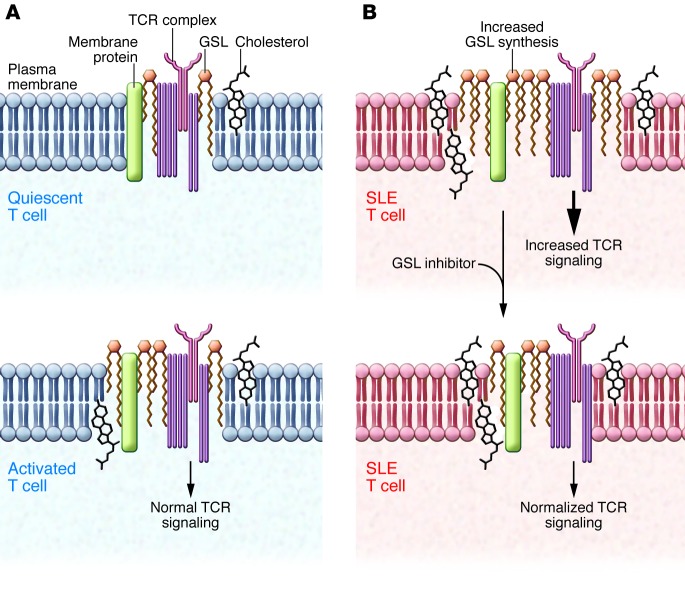
Figure 1. Resetting GSL homeostasis restores SLE T cell dysfunction.
(A) Lipid rafts are cholesterol- and GSL-rich microdomains in the plasma membrane that play important roles in regulating TCR signaling. In healthy individuals, the amount of cholesterol and GSLs is tightly regulated in quiescence. Activation increases both GSL and cholesterol levels in T cells, which then return to quiescent levels following removal of activation signals. (B) SLE T cells have altered GSL and cholesterol homeostasis in lipid rafts that results in abnormal TCR signaling. Pharmacologic inhibition of GSL synthesis in SLE T cells restores GSL homeostasis, normalizes TCR signaling, and attenuates lupus T cell dysfunction, indicating crosstalk between lipid metabolism and T cell function.
GSL changes: at the heart of autoimmune T cell dysfunction?
The first clue to identification of the players in SLE-associated T cell lipid dysfunction came from a detailed assessment of GSL composition. GSLs are a structurally diverse class of glycerolipids that are defined by the type of carbohydrate moiety bound to the lipid backbone (14). Using HPLC, McDonald and colleagues found that T cells from autoimmune patients have heightened GSL levels, which is consistent with previous findings (8, 9); however, HPLC analysis revealed that the composition of the GSLs was distinct from that found in activated T cells from healthy individuals. Furthermore, the SLE-associated GSL pattern remained in place even when SLE T cells were cultured under “resting” conditions, suggesting that the unique GSL composition in autoimmune T cells is not a consequence of activation per se. Perhaps more provocatively, serum collected from SLE patients was able to increase GSL levels in the plasma membrane of T cells cultured from healthy individuals. These data clearly suggest that the lipid metabolic program of SLE T cells is a consequence of signals emanating from the host environment and imply that a generalized disruption of host lipid homeostasis underlies SLE-associated T cell dysfunction.
Based on their identification of a differential GSL expression pattern in SLE T cells, McDonald and colleagues examined whether the SLE-associated alterations were a function of altered GSL synthesis or turnover. Under normal conditions, cellular GSL levels are achieved through the combined effects of de novo synthesis, turnover, and recycling (15). The extent to which T cells in normal or disease states preferentially rely on one or more of these pathways remains largely unknown. Using a combination of fluorescent lipid tracers, organelle labeling probes, and pharmacologic inhibitors of organelle trafficking, McDonald et al. concluded that SLE T cells have both heightened GSL biosynthesis and increased trafficking to and from the plasma membrane, resulting in an aberrant accumulation of GSLs. Perhaps more importantly, pharmacologic inhibition of GSL biosynthesis in vitro with the clinically approved competitive inhibitor of glucosylceramide synthase NB-DNJ (16), a drug used to ameliorate lysosomal storage diseases, normalized GSL levels to those of healthy individuals and partially restored signaling defects in SLE T cells. Moreover, correction of GSL homeostasis in vitro ameliorated multiple facets of dysfunction and diminished the ability of these cells to drive autoantibody production from cocultured B cells.
Liver X receptors: regulators of lipid homeostasis and self-tolerance?
So how do lupus T cells acquire this abnormal GSL metabolic phenotype? The observation that serum from SLE patients could induce lipid dysfunction in otherwise healthy T cells provided a clue, suggesting that a signal emanating from the host environment likely drives the metabolic program. McDonald and colleagues ruled out inflammatory signals in SLE-associated alterations of GSL biology and observed that the addition of oxidized lipoproteins (LDL) induced disease-associated GSL patterns in healthy T cells. Internalization of oxidized LDL is known to affect metabolism and inflammation through the actions of the lipid-regulated transcription factors liver X receptors (LXRα and LXRβ). LXRs are members of the nuclear receptor superfamily that have an ever-expanding role in the transcriptional regulation of cellular lipid homeostasis (17). The LXRs are best known for their ability to transactivate genes involved in cellular cholesterol efflux (18); however, newer data indicate that LXRs also reduce lipoprotein import via the action of the E3 ligase IDOL (19) and induce phospholipid remodeling in membranes by the acetyl transferase LPCAT3 (20). Interestingly, LXRs are also able to repress inflammatory gene expression (e.g., IL6 and INOS), providing crosstalk between cellular metabolic status and inflammatory signaling (18).
Genetic ablation of both Lxra and Lxrb in mice results in a lupus-like disease, whereas pharmacologic activation of LXRs can attenuate lympho-proliferative disease and ameliorate autoantibody-mediated glomerulonephritis in spontaneous models of murine lupus (21). The molecular mechanisms underlying LXR-dependent mediation of self-tolerance are cell type dependent and multifactorial. Work from our lab and others suggests that LXR signaling facilitates the clearance of apoptotic bodies while suppressing inflammatory gene expression (21, 22). Likewise, LXRs can suppress proliferative capacity and differentiation of T lymphocytes (23, 24), likely through their ability to regulate cellular cholesterol. Thus, LXRs appear to be critical for coordination between self-tolerance and lipid metabolism.
McDonald and colleagues further asked whether LXRs could mediate the GSL dysregulation observed in human SLE T cells. Examination of LXR target genes indicated that LXRB and genes involved in cellular lipid transport, such as the Niemann-Pick (NPC) proteins NPC1 and NPC2, were upregulated in isolated SLE T cells. Pharmacologic activation of LXRs transiently increased GSL expression in both healthy and SLE T cells; however, canonical LXR target genes, including ABCA1 and ABCG1, were not upregulated in SLE T cells, indicating an unusual pattern of LXR activity. Moreover, McDonald and colleagues noted that genes involved in cholesterol homeostasis, such as the sterol regulatory element–binding protein 2 (SREBP2) were also upregulated in SLE T cells. SREBPs are considered to be the master transcriptional regulators of lipid homeostasis through their ability to transactivate numerous genes involved in cholesterol biosynthesis and transport, including those encoding the NPC proteins (25, 26). GSL levels in lipid rafts are intimately tied to cholesterol homeostasis (15), and previous studies from Jury and colleagues have shown that inhibition of cholesterol synthesis can ameliorate many of the signaling abnormalities and dysfunction of SLE T cells (13). Thus, the question remains whether LXR signaling truly drives SLE-associated perturbations in GSL homeostasis, or whether LXR is activated to compensate for the dysregulation of other aspects of cholesterol metabolism in autoimmune T cells. Thus, it will be of interest in future experiments to dissect the apparent crosstalk between the SREBP and LXR pathways in T cells and the combined influence of these pathways on lipid raft function. It will also be intriguing to further investigate the potential of targeting GSL homeostasis in individuals with SLE as a therapeutic approach for attenuating autoimmune pathology. In conclusion, the provocative studies by McDonald and colleagues support the growing notion that metabolic reprogramming could provide a therapeutic avenue for ameliorating complex rheumatic diseases.
Acknowledgments
The authors were supported by grants from the NIH (AI093768, to S.J. Bensinger) and the Sontag Foundation (to S.J. Bensinger).
Footnotes
Conflict of interest: The authors have declared that no conflict of interest exists.
Citation for this article: J Clin Invest. 2014;124(2):482–485. doi:10.1172/JCI74141.
See the related article beginning on page 712.
References
- 1.Tsokos GC. Systemic lupus erythematosus. N Engl J Med. 2011;365(22):2110–2121. doi: 10.1056/NEJMra1100359. [DOI] [PubMed] [Google Scholar]
- 2.Roman MJ, et al. Prevalence and correlates of accelerated atherosclerosis in systemic lupus erythematosus. N Engl J Med. 2003;349(25):2399–2406. doi: 10.1056/NEJMoa035471. [DOI] [PubMed] [Google Scholar]
- 3.Asanuma Y, et al. Premature coronary-artery atherosclerosis in systemic lupus erythematosus. N Engl J Med. 2003;349(25):2407–2415. doi: 10.1056/NEJMoa035611. [DOI] [PubMed] [Google Scholar]
- 4.Hahn BH, McMahon M. Atherosclerosis and systemic lupus erythematosus: the role of altered lipids and of autoantibodies. Lupus. 2008;17(5):368–370. doi: 10.1177/0961203308089989. [DOI] [PubMed] [Google Scholar]
- 5.Feng X, et al. ApoE–/–Fas–/– C57BL/6 mice: a novel murine model simultaneously exhibits lupus nephritis, atherosclerosis, and osteopenia. . J Lipid Res. 2007;48(4):794–805. doi: 10.1194/jlr.M600512-JLR200. [DOI] [PubMed] [Google Scholar]
- 6.Gautier EL, et al. Enhanced immune system activation and arterial inflammation accelerates atherosclerosis in lupus-prone mice. Arterioscler Thromb Vasc Biol. 2007;27(7):1625–1631. doi: 10.1161/ATVBAHA.107.142430. [DOI] [PubMed] [Google Scholar]
- 7.Ma Z, Choudhury A, Kang SA, Monestier M, Cohen PL, Eisenberg RA. Accelerated atherosclerosis in ApoE deficient lupus mouse models. Clin Immunol. 2008;127(2):168–175. doi: 10.1016/j.clim.2008.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Krishnan S, et al. Alterations in lipid raft composition and dynamics contribute to abnormal T cell responses in systemic lupus erythematosus. J Immunol. 2004;172(12):7821–7831. doi: 10.4049/jimmunol.172.12.7821. [DOI] [PubMed] [Google Scholar]
- 9.Jury EC, Kabouridis PS, Flores-Borja F, Mageed RA, Isenberg DA. Altered lipid raft-associated signaling and ganglioside expression in T lymphocytes from patients with systemic lupus erythematosus. J Clin Invest. 2004;113(8):1176–1187. doi: 10.1172/JCI20345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McDonald G, et al. Normalizing glycosphingolipids restores function in CD4+ T cells from lupus patients. . J Clin Invest. 2014;124(2):712–724. doi: 10.1172/JCI69571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Krishnan S, et al. Differential expression and molecular associations of Syk in systemic lupus erythematosus T cells. J Immunol. 2008;181(11):8145–8152. doi: 10.4049/jimmunol.181.11.8145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jury EC, Flores-Borja F, Kabouridis PS. Lipid rafts in T cell signalling and disease. Semin Cell Dev Biol. 2007;18(5):608–615. doi: 10.1016/j.semcdb.2007.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jury EC, Isenberg DA, Mauri C, Ehrenstein MR. Atorvastatin restores Lck expression and lipid raft-associated signaling in T cells from patients with systemic lupus erythematosus. J Immunol. 2006;177(10):7416–7422. doi: 10.4049/jimmunol.177.10.7416. [DOI] [PubMed] [Google Scholar]
- 14.Lahiri S, Futerman AH. The metabolism and function of sphingolipids and glycosphingolipids. Cell Mol Life Sci. 2007;64(17):2270–2284. doi: 10.1007/s00018-007-7076-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Degroote S, Wolthoorn J, van Meer G. The cell biology of glycosphingolipids. Semin Cell Dev Biol. 2004;15(4):375–387. doi: 10.1016/j.semcdb.2004.03.007. [DOI] [PubMed] [Google Scholar]
- 16.Butters TD, Dwek RA, Platt FM. Imino sugar inhibitors for treating the lysosomal glycosphingolipidoses. Glycobiology. 2005;15(10):43R–52R. doi: 10.1093/glycob/cwi076. [DOI] [PubMed] [Google Scholar]
- 17.Calkin AC, Tontonoz P. Transcriptional integration of metabolism by the nuclear sterol-activated receptors LXR and FXR. Nat Rev Mol Cell Biol. 2012;13(4):213–224. doi: 10.1038/nrm3312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bensinger SJ, Tontonoz P. Integration of metabolism and inflammation by lipid-activated nuclear receptors. Nature. 2008;454(7203):470–477. doi: 10.1038/nature07202. [DOI] [PubMed] [Google Scholar]
- 19.Zelcer N, Hong C, Boyadjian R, Tontonoz P. LXR regulates cholesterol uptake through Idol-dependent ubiquitination of the LDL receptor. Science. 2009;325(5936):100–104. doi: 10.1126/science.1168974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rong X, et al. LXRs regulate ER stress and inflammation through dynamic modulation of membrane phospholipid composition. Cell Metab. 2013;18(5):685–697. doi: 10.1016/j.cmet.2013.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.A-Gonzalez N, et al. Apoptotic cells promote their own clearance and immune tolerance through activation of the nuclear receptor LXR. Immunity. 2009;31(2):245–258. doi: 10.1016/j.immuni.2009.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hong C, et al. Coordinate regulation of neutrophil homeostasis by liver X receptors in mice. J Clin Invest. 2012;122(1):337–347. doi: 10.1172/JCI58393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bensinger SJ, et al. LXR signaling couples sterol metabolism to proliferation in the acquired immune response. Cell. 2008;134(1):97–111. doi: 10.1016/j.cell.2008.04.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Solt LA, Kamenecka TM, Burris TP. LXR-mediated inhibition of CD4+ T helper cells. . PLoS One. 2012;7(9):e46615. doi: 10.1371/journal.pone.0046615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gévry N, Schoonjans K, Guay F, Murphy BD. Cholesterol supply and SREBPs modulate transcription of the Niemann-Pick C-1 gene in steroidogenic tissues. J Lipid Res. 2008;49(5):1024–1033. doi: 10.1194/jlr.M700554-JLR200. [DOI] [PubMed] [Google Scholar]
- 26.Horton JD, Goldstein JL, Brown MS. SREBPs: activators of the complete program of cholesterol and fatty acid synthesis in the liver. J Clin Invest. 2002;109(9):1125–1131. doi: 10.1172/JCI15593. [DOI] [PMC free article] [PubMed] [Google Scholar]