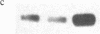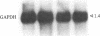Abstract
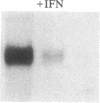
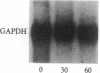
The recent observation made in our laboratory that cellular myc (c-myc) mRNA has a very short half-life in a variety of normal and transformed human cells emphasized the potential importance of post-transcriptional regulation of c-myc gene expression. Jonak and Knight [Jonak, G. J. & Knight, E., Jr. (1984) Proc. Natl. Acad. Sci. USA 81, 1747-1750] have reported a selective reduction of c-myc mRNA accumulation in lymphoblastoid Daudi cells treated with human beta interferon. This provided a suitable situation in which to examine a possible action of negative modulators of c-myc expression at the level of mRNA stability. Our results confirm the observation by Jonak and Knight that c-myc mRNA level is depressed in cells treated with beta interferon and extend it to alpha 2 interferon. Furthermore, we now demonstrate that interferon has no effect on c-myc transcription rate in isolated nuclei but rather reduces the half-life of its mRNA. Conversely, we show that it increases the level of HLA-A2 mRNA by stimulating its transcription.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Auffray C., Rougeon F. Purification of mouse immunoglobulin heavy-chain messenger RNAs from total myeloma tumor RNA. Eur J Biochem. 1980 Jun;107(2):303–314. doi: 10.1111/j.1432-1033.1980.tb06030.x. [DOI] [PubMed] [Google Scholar]
- Baglioni C., Nilsen T. W. Mechanisms of antiviral action of interferon. Interferon. 1983;5:23–42. [PubMed] [Google Scholar]
- Bantle J. A., Maxwell I. H., Hahn W. E. Specificity of oligo (dT)-cellulose chromatography in the isolation of polyadenylated RNA. Anal Biochem. 1976 May 7;72:413–427. doi: 10.1016/0003-2697(76)90549-2. [DOI] [PubMed] [Google Scholar]
- Basham T. Y., Bourgeade M. F., Creasey A. A., Merigan T. C. Interferon increases HLA synthesis in melanoma cells: interferon-resistant and -sensitive cell lines. Proc Natl Acad Sci U S A. 1982 May;79(10):3265–3269. doi: 10.1073/pnas.79.10.3265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burrone O. R., Milstein C. Control of HLA-A,B,C synthesis and expression in interferon-treated cells. EMBO J. 1982;1(3):345–349. doi: 10.1002/j.1460-2075.1982.tb01172.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chebath J., Merlin G., Metz R., Benech P., Revel M. Interferon-induced 56,000 Mr protein and its mRNA in human cells: molecular cloning and partial sequence of the cDNA. Nucleic Acids Res. 1983 Mar 11;11(5):1213–1226. doi: 10.1093/nar/11.5.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colonno R. J., Pang R. H. Induction of unique mRNAs by human interferons. J Biol Chem. 1982 Aug 25;257(16):9234–9237. [PubMed] [Google Scholar]
- Dani C., Blanchard J. M., Piechaczyk M., El Sabouty S., Marty L., Jeanteur P. Extreme instability of myc mRNA in normal and transformed human cells. Proc Natl Acad Sci U S A. 1984 Nov;81(22):7046–7050. doi: 10.1073/pnas.81.22.7046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dani C., Piechaczyk M., Audigier Y., El Sabouty S., Cathala G., Marty L., Fort P., Blanchard J. M., Jeanteur P. Characterization of the transcription products of glyceraldehyde 3-phosphate-dehydrogenase gene in HeLa cells. Eur J Biochem. 1984 Dec 3;145(2):299–304. doi: 10.1111/j.1432-1033.1984.tb08552.x. [DOI] [PubMed] [Google Scholar]
- Dron M., Tovey M. G. Isolation of Daudi cells with reduced sensitivity to interferon. I. Characterization. J Gen Virol. 1983 Dec;64(Pt 12):2641–2647. doi: 10.1099/0022-1317-64-12-2641. [DOI] [PubMed] [Google Scholar]
- Farrell P. J., Broeze R. J., Lengyel P. Accumulation of an mRNA and protein in interferon-treated Ehrlich ascites tumour cells. Nature. 1979 Jun 7;279(5713):523–525. doi: 10.1038/279523a0. [DOI] [PubMed] [Google Scholar]
- Fellous M., Kamoun M., Gresser I., Bono R. Enhanced expression of HLA antigens and beta 2-microglobulin on interferon-treated human lymphoid cells. Eur J Immunol. 1979 Jun;9(6):446–449. doi: 10.1002/eji.1830090606. [DOI] [PubMed] [Google Scholar]
- Fellous M., Nir U., Wallach D., Merlin G., Rubinstein M., Revel M. Interferon-dependent induction of mRNA for the major histocompatibility antigens in human fibroblasts and lymphoblastoid cells. Proc Natl Acad Sci U S A. 1982 May;79(10):3082–3086. doi: 10.1073/pnas.79.10.3082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman R. L., Manly S. P., McMahon M., Kerr I. M., Stark G. R. Transcriptional and posttranscriptional regulation of interferon-induced gene expression in human cells. Cell. 1984 Oct;38(3):745–755. doi: 10.1016/0092-8674(84)90270-8. [DOI] [PubMed] [Google Scholar]
- Grosso L. E., Pitot H. C. Modulation of c-myc expression in the HL-60 cell line. Biochem Biophys Res Commun. 1984 Mar 15;119(2):473–480. doi: 10.1016/s0006-291x(84)80273-9. [DOI] [PubMed] [Google Scholar]
- Gupta S. L., Rubin B. Y., Holmes S. L. Interferon action: induction of specific proteins in mouse and human cells by homologous interferons. Proc Natl Acad Sci U S A. 1979 Oct;76(10):4817–4821. doi: 10.1073/pnas.76.10.4817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heron I., Hokland M., Berg K. Enhanced expression of beta2-microglobulin and HLA antigens on human lymphoid cells by interferon. Proc Natl Acad Sci U S A. 1978 Dec;75(12):6215–6219. doi: 10.1073/pnas.75.12.6215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huffaker T. C., Robbins P. W. Yeast mutants deficient in protein glycosylation. Proc Natl Acad Sci U S A. 1983 Dec;80(24):7466–7470. doi: 10.1073/pnas.80.24.7466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonak G. J., Knight E., Jr Selective reduction of c-myc mRNA in Daudi cells by human beta interferon. Proc Natl Acad Sci U S A. 1984 Mar;81(6):1747–1750. doi: 10.1073/pnas.81.6.1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly K., Cochran B. H., Stiles C. D., Leder P. Cell-specific regulation of the c-myc gene by lymphocyte mitogens and platelet-derived growth factor. Cell. 1983 Dec;35(3 Pt 2):603–610. doi: 10.1016/0092-8674(83)90092-2. [DOI] [PubMed] [Google Scholar]
- Knight E., Jr, Anton E. D., Fahey D., Friedland B. K., Jonak G. J. Interferon regulates c-myc gene expression in Daudi cells at the post-transcriptional level. Proc Natl Acad Sci U S A. 1985 Feb;82(4):1151–1154. doi: 10.1073/pnas.82.4.1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Land H., Parada L. F., Weinberg R. A. Cellular oncogenes and multistep carcinogenesis. Science. 1983 Nov 18;222(4625):771–778. doi: 10.1126/science.6356358. [DOI] [PubMed] [Google Scholar]
- Larner A. C., Jonak G., Cheng Y. S., Korant B., Knight E., Darnell J. E., Jr Transcriptional induction of two genes in human cells by beta interferon. Proc Natl Acad Sci U S A. 1984 Nov;81(21):6733–6737. doi: 10.1073/pnas.81.21.6733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leder P., Battey J., Lenoir G., Moulding C., Murphy W., Potter H., Stewart T., Taub R. Translocations among antibody genes in human cancer. Science. 1983 Nov 18;222(4625):765–771. doi: 10.1126/science.6356357. [DOI] [PubMed] [Google Scholar]
- Marcu K. B., Harris L. J., Stanton L. W., Erikson J., Watt R., Croce C. M. Transcriptionally active c-myc oncogene is contained within NIARD, a DNA sequence associated with chromosome translocations in B-cell neoplasia. Proc Natl Acad Sci U S A. 1983 Jan;80(2):519–523. doi: 10.1073/pnas.80.2.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsen T. W., Maroney P. A., Baglioni C. Synthesis of (2'-5')oligoadenylate and activation of an endoribonuclease in interferon-treated HeLa cells infected with reovirus. J Virol. 1982 Jun;42(3):1039–1045. doi: 10.1128/jvi.42.3.1039-1045.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabbitts T. H., Forster A., Hamlyn P., Baer R. Effect of somatic mutation within translocated c-myc genes in Burkitt's lymphoma. Nature. 1984 Jun 14;309(5969):592–597. doi: 10.1038/309592a0. [DOI] [PubMed] [Google Scholar]
- Reitsma P. H., Rothberg P. G., Astrin S. M., Trial J., Bar-Shavit Z., Hall A., Teitelbaum S. L., Kahn A. J. Regulation of myc gene expression in HL-60 leukaemia cells by a vitamin D metabolite. Nature. 1983 Dec 1;306(5942):492–494. doi: 10.1038/306492a0. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Schibler U., Hagenbüchle O., Wellauer P. K., Pittet A. C. Two promoters of different strengths control the transcription of the mouse alpha-amylase gene Amy-1a in the parotid gland and the liver. Cell. 1983 Jun;33(2):501–508. doi: 10.1016/0092-8674(83)90431-2. [DOI] [PubMed] [Google Scholar]
- St Laurent G., Yoshie O., Floyd-Smith G., Samanta H., Sehgal P. B., Lengyel P. Interferon action: two (2'-5')(A)n synthetases specified by distinct mRNAs in Ehrlich ascites tumor cells treated with interferon. Cell. 1983 May;33(1):95–102. doi: 10.1016/0092-8674(83)90338-0. [DOI] [PubMed] [Google Scholar]
- Staeheli P., Colonno R. J., Cheng Y. S. Different mRNAs induced by interferon in cells from inbred mouse strains A/J and A2G. J Virol. 1983 Sep;47(3):563–567. doi: 10.1128/jvi.47.3.563-567.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taub R., Moulding C., Battey J., Murphy W., Vasicek T., Lenoir G. M., Leder P. Activation and somatic mutation of the translocated c-myc gene in burkitt lymphoma cells. Cell. 1984 Feb;36(2):339–348. doi: 10.1016/0092-8674(84)90227-7. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl G. M., Stern M., Stark G. R. Efficient transfer of large DNA fragments from agarose gels to diazobenzyloxymethyl-paper and rapid hybridization by using dextran sulfate. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3683–3687. doi: 10.1073/pnas.76.8.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshie O., Schmidt H., Reddy E. S., Weissman S., Lengyel P. Mouse interferons enhance the accumulation of a human HLA RNA and protein in transfected mouse and hamster cells. J Biol Chem. 1982 Nov 25;257(22):13169–13172. [PubMed] [Google Scholar]