Abstract
Background
Surface populations of A. mexicanus, living in rivers like their common ancestors, school, while several, independent derived cave populations of the same species have lost schooling behavior.
Results
We quantify schooling behavior in individual A. mexicanus and identify quantitative trait loci (QTL) for this trait. We find that the evolutionary modulation of schooling has both vision-dependent and independent components. We also quantify differences in the lateral line and vision between cavefish and surface fish and relate these differences to the evolutionary loss of schooling behavior. We provide evidence that a monoamine may have played a role in the evolution of schooling behavior.
Conclusions
We find that vision is essential for schooling tendency in A. mexicanus, while the lateral line has a small effect on this behavior. Schooling behavior in A. mexicanus has evolved both through changes in sensory systems and through changes in genetic loci that likely act downstream of sensory inputs.
Introduction
Most species of fish exhibit schooling behavior during some phase of their life cycle [1]. Schooling benefits fish in a variety of ways, including predator avoidance and foraging [2-5]. However, there are some situations in which schooling behavior is less advantageous. For example, when food is scarce, fish tend to school less [6, 7]. Schooling fish rely on the ability to sense one another. The visual system and the ability to sense water pressure and current through the lateral line have been implicated in schooling behavior [2, 8, 9].
Little is known about how schooling behavior evolves, with the exception of studies in laboratory strains of zebrafish [10]. The Mexican tetra, Astyanax mexicanus, provides an excellent opportunity to examine this question. A. mexicanus exists in two forms, a sighted surface-dwelling form, and a blind cave-dwelling form. Morphological adaptations to life in the caves include an increased number and distribution of taste buds and cranial superficial neuromasts, regressed eyes and decreased or absent melanin pigmentation [11-13]. Cavefish also have a variety of modified behaviors, including decreases in aggression and in time spent sleeping, a depressed response to alarm substance, an enhanced attraction to vibrations in their environment, modified feeding behaviors, and the absence of schooling [14-19]. While many of these behaviors have been studied to some extent, little is known about their genetic architecture.
Cave and surface forms of A. mexicanus are interfertile, allowing for the genetic analysis of cave traits [11]. In particular, quantitative trait locus (QTL) mapping has been used successfully to identify loci underlying the evolution of several morphological traits in these fish [20-25]. Another advantage of studying evolution in A. mexicanus is the existence of a number of independently evolved cave populations (reviewed in [26]) (Supplemental Figure 1A) with similar morphological characteristics and behaviors, making A. mexicanus an ideal system in which to study parallel and convergent evolution (though this is beyond the scope of this paper).
While the surface form of A. mexicanus actively aggregate into schools and shoals, the cave forms have reduced this behavior [19, 27, 28]. The apparent absence of macroscopic predators in the caves relieves one selective pressure favoring schooling, suggesting that the loss of schooling behavior could be the result of relaxed selection. Alternatively, the scarcity of food resources in most caves potentially renders clustering of the fish disadvantageous. Thus, the loss of this behavior could be adaptive in the caves. The absence of schooling could also be a secondary consequence of the loss of vision and/or changes in the lateral line system in cavefish, or a pleiotropic consequence of other adaptive neurological or morphological changes.
Results
Loss of schooling behavior in cavefish
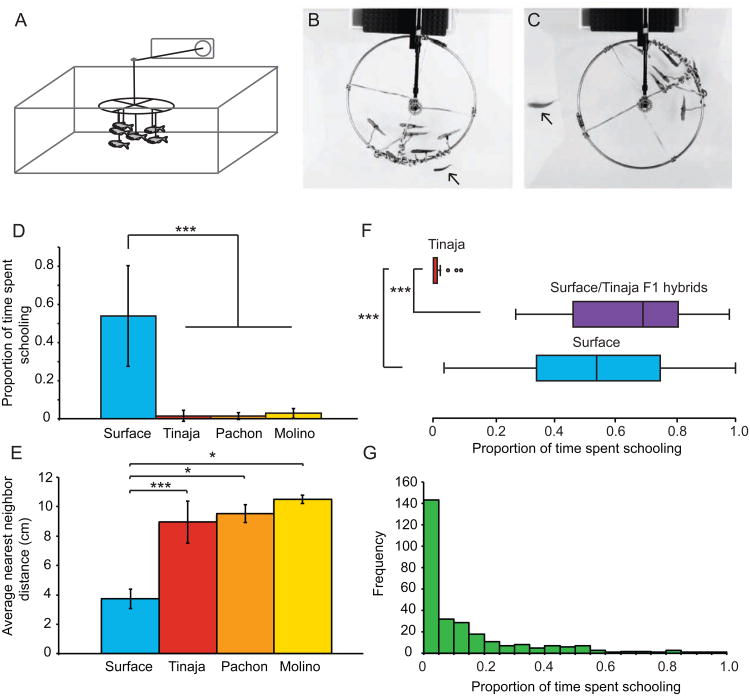
Schooling and shoaling behaviors occur when individual fish, perceiving and responding to their local environment, interact in the context of larger groups. By following a set of relatively simple rules on the local scale, individuals' behaviors can result in complex group patterns of collective motion (reviewed in [29]). In order to quantify differences in this behavior, we use a relatively simple definition of schooling, the tendency of fish to synchronize their behavior, and swim in an oriented manner relative to one another [30]. To quantify schooling behavior we measured the tendency of fish to follow a model school of plastic fish [31] (Figure 1A). Surface fish follow the model school (Figure 1B and D). In contrast, three independently evolved cave populations (reviewed in [26]) from the Tinaja, Pachón, and Molino caves were significantly different from surface fish, and did not display schooling behavior (Kruskal Wallis: H4=63.6, p<0.001; Mann-Whitney compared to surface: Tinaja: U=3, z=-6, p<0.001; Pachón: U=1, z=-4.6, p<0.001; Molino: U=4, z=-4.6, p<0.001; Surface: n=34, Tinaja: n=19, Pachón: n=9, Molino: n=10, F1s: n=12; Figure 1C and D).
Figure 1. Cavefish have lost the tendency to school.
A. Diagram of the model school behavioral assay. Images from videos of a B. surface and C. Tinaja cave fish following the model school. Arrows indicate the position of the live fish. D. Schooling tendency was quantified as the proportion of time during the trial that each fish spent following the model school. Average time spent following the school was recorded for surface fish (n=34), and cavefish populations – Tinaja (n=19), Pachón (n=10) and Molino (n=10). Asterisks indicate p-values in a Mann-Whitney test. E. Shoaling as the average of the nearest neighbor distances (in centimeters) for each fish in a group. Groups of six fish each were measured for surface (9 groups), Tinaja (9 groups), Pachón (3 groups), and Molino (3 groups) fish. Asterisks indicate p-values in a Mann-Whitney test. F. Distribution of the proportion of time spent schooling in surface fish (n=34), surface/Tinaja F1 hybrid fish (n=12), Tinaja cavefish (n=19). Asterisks indicate p-values in a Mann-Whitney test. G. The distribution of the average proportion of time spent schooling across five trials of 287 F2 fish from a surface/Tinaja F1 hybrid intercross. All error bars indicate standard deviation. *p<0.05, **p<0.01, ***p<0.001.
Shoaling behavior is defined as the tendency of fish to aggregate with other fish of the same species [30] (including schooling). We next measured shoaling for groups of fish, quantifying the average nearest neighbor distance (NND) and the average inter-individual distance (IID), (Supplemental Figures 1B and C). Surface fish swam significantly closer together than fish from any of the cave populations by NND (Kruskal-Wallis: H3=18.8, p<0.001; Mann-Whitney test compared to surface: Tinaja: U<0.001, z=-3.6, p<0.001; Pachón: U<0.001, z=-2.5, p<0.05; Molino: U<0.001, z=-2.5, p<0.05; Surface: n=9 groups, Tinaja: n=9 groups, Pachón: n=3 groups, Molino: n=3 groups; Figure 1E) and by IID (Kruskal Wallis: H3=17.4, p<0.001; Mann-Whitney compared to surface: Tinaja: U<0.001, z=-3.6, p<0.001; Pachón: U<0.001, z=-2.5, p<0.05; Molino: U<0.001, z=-2.5, p<0.05; Supplemental Figure 1E). Thus, in multiple, independently evolved natural populations, cavefish have lost the tendency to swim oriented to one another, or school, as well as decreased the tendency to congregate in a group, or shoal.
Genetics of schooling behavior
Surface fish raised in isolation follow the model school, responding similarly in the assay to group-raised fish (t36=-0.5, p=0.61; group-raised n=34, isolation-raised n=4; Supplemental Figure 1D). Thus, this behavior is not learned and likely has a genetic basis. To study the inheritance of schooling, we crossed surface fish and Tinaja cavefish to generate F1 hybrid fish. F1 fish follow the model school, similar to surface fish (Mann-Whitney compared to F1: surface: U=155.5, z=-1.2, p=1.0; Tinaja: U<0.001, z=-4.8, p<0.001; Surface: n=34, Tinaja: n=19, F1: n=12), indicating that tendency to school segregates as a dominant trait (Figure 1F).
To probe the genetic architecture of this trait more deeply, F1 fish were intercrossed to generate F2 fish. F2 fish vary widely in their behavior (Figure 1G). These results strongly indicate a polygenic basis for this behavior. Tendency to school in F2 fish differed based on sex (Mann-Whitney: U=6669, z=-2.1, p<0.05, n=252; Supplemental Figure 1H) and was not correlated with size (Spearman's rho=0.05, p=0.37, n=271; Supplemental Figure 1I).
An enhanced lateral line in cavefish does not contribute significantly to loss of schooling behavior
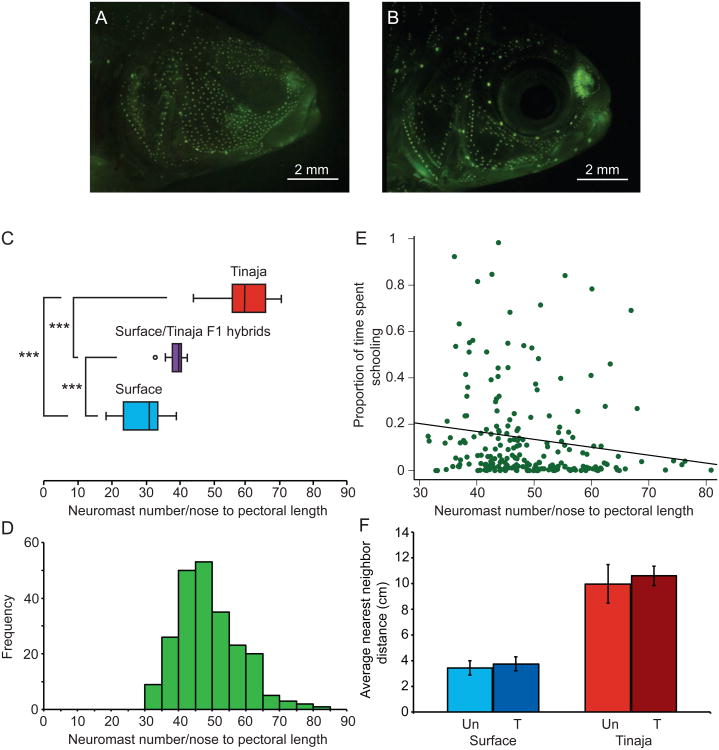
The lateral line and the visual system have been implicated in schooling behavior in other fish species [2, 8, 9]. Cavefish have enhanced the size and number of superficial cranial neuromasts, the sensory organ of the lateral line system, relative to surface fish [12, 32] (Figure 2A and B). It was possible that the larger numbers of cranial neuromasts in cavefish provide a sensory input that repels them from one another, leading to avoidance of conspecifics and a decrease in the tendency to school.
Figure 2. Relationship between schooling behavior and the lateral line system.
A. Cranial neuromasts in a Tinaja cavefish. B. Cranial neuromasts in a surface fish. Neuromasts are visualized using DASPEI. C. Distribution of cranial neuromast number corrected for size in surface fish (n=21), surface/Tinaja F1 hybrid fish (n=7), and Tinaja fish (n=21). D. Distribution of cranial neuromast number corrected for size in the F2 population (n=227). E. Proportion of the time spent schooling as a function of number of cranial neuromasts corrected for size in the F2 population (n=214). F. Nearest neighbor distances (in centimeters) in groups of surface (n=6) and Tinaja cavefish (n=6) treated with 0.002% gentamicin (T) or untreated (Un). *p<0.05, **p<0.01, ***p<0.001.
Surface fish indeed have significantly fewer cranial neuromasts than cavefish (one-way ANOVA: F2,46=99.2, p<0.001; Surface: n=21, Tinaja: n=21, F1: n=12; Games-Howell Surface compared to Tinaja: p<0.001, Figure 2A-C). F1 fish have an intermediate number of cranial neuromasts, significantly different from both surface (Games-Howell p<0.001) and cavefish (Games-Howell p<0.001). The F2 population (n=227) ranges in number of cranial neuromasts (Figure 2D).
To determine if the number of cranial neuromasts has an effect on schooling behavior, we compared the number of neuromasts to the proportion of time spent schooling for each fish in the F2 population (Figure 2E). The number of neuromasts in F2 fish accounted for a statistically significant amount of variation in schooling behavior, but the size of this effect was small (Spearman's rho=-0.22, p<0.001, n=214). In addition to superficial neuromast number, we also measured superficial neuromast diameter in F2 fish, and found no correlation between this measure and the schooling behavior (Spearman's rho=0.04, p=0.64, n=154, Supplemental Figure 2C). Thus, the increased number and size of neuromasts that evolved in response to the cave environment did not have a large effect on the evolutionary loss of schooling.
Neuromast ablation does not have a significant effect on aggregation behaviors
To determine the extent to which the lateral line system is required for schooling and shoaling activity in Astyanax, fish were treated with 0.002% gentamicin to ablate neuromast function [17, 33, 34]. Surface fish did not show a significant difference in behavior in the absence of neuromasts, as assessed either by shoaling (NND: t10=-1.03, p=0.33; IID: t10=-1.01, p=0.34; treated: n=6 groups, untreated: n=6 groups), or by schooling (Mann-Whitney: U=191, z=-0.5, p=0.63; treated: n=21, untreated: n=21; Figure 2F, Supplemental Figures 2A and B). Treated Tinaja cavefish were not significantly different by NND (t10=-1.69, p=0.12; treated: n=6, untreated: n=6, Figure 2F) and IID (t10=-1.15, p=0.28, Supplemental Figure 2B). While not significant, NND and IID in both surface and Tinaja fish were greater in treated fish compared to controls. Therefore, it is unlikely that an enhanced lateral line in cavefish drives the evolution of loss of schooling or shoaling behavior.
Vision is essential for schooling behavior
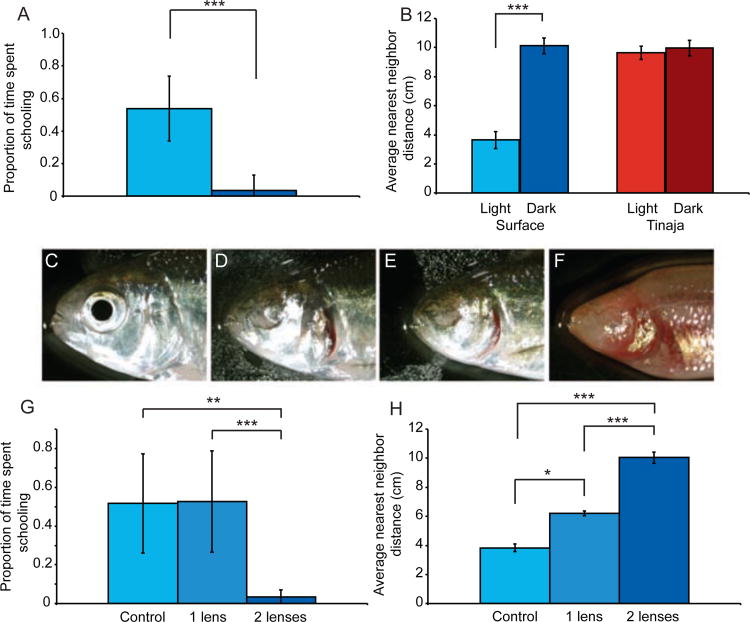
Visual function is important for schooling and shoaling behavior in a variety of fish species, either independent of or in conjunction with lateral line function (for example [8, 35]). It has been previously reported that in A. mexicanus, surface fish placed in the dark show a reduction in shoaling [35]. We verified this result in our shoaling assay. Groups of surface fish in the dark swam significantly farther apart compared to the same groups in the light (NND: paired t-test: t4=-17.2, p<0.001, n=5 groups; IID: paired t-test: t4=-15.2, p<0.001; Figure 3B, Supplemental Figure 3A). Cavefish were unaffected by the change in lighting conditions (NND: paired t-test: t4=-1.2, p=0.31, n=5 groups; IID: paired t-test: t4=0.45, p=0.67; Figure 3B, Supplemental Figure 3A). Schooling behavior in surface fish was lost in the dark compared to in the light (Mann-Whitney: U=1, z=-4, p<0.001; light: n=12, dark: n=10, Figure 3A).
Figure 3. Vision is required for schooling and shoaling behavior.
A. Proportion of time spent schooling of surface fish in the light (n=12) versus the dark (n=10). Asterisks indicate p-value in a Mann-Whitney test. B. Shoaling NND measured in groups of six of surface fish (5 groups) and cavefish (5 groups) in the light and the dark. Asterisks indicate p-value in a paired t-test. C. Eye size in control surface fish. D. Partial eye degradation in surface fish with lenses removed. E. Complete eye degradation in surface fish with lenses removed. F. Eye degradation in cavefish. G. Surface fish with zero (n=7), one (n=12) or two (n=8) lenses removed were assayed with the model school. Asterisks indicate p-value in a Games-Howell test. H. One group each of one- (5 trials), two-lenses (5 trials) removed, or control fish (2 trials) were assayed for shoaling by NND. Asterisks indicate p-value in a Games-Howell test. Error bars indicate standard deviation. *p<0.05, **p<0.01, ***p<0.001.
Loss of schooling in the dark could be due to a learned reliance on vision for schooling behavior. If this were the case, fish that lose vision early in development might school in the absence of sight. Cavefish develop eyes, which undergo apoptosis and degenerate [11, 36, 37]. Cavefish eye degradation can be phenocopied in surface fish [38]. In order to test if loss of schooling could be rescued if fish lost visual function during development, one, two, or no lenses were removed in surface fish larvae, resulting in a range of adult eye morphology (Figure 3C-F).
Lens removal had a significant effect on both schooling (one-way ANOVA: F2,24=13.9, p<0.001; control: n=8, one lens removed: n=12, two lenses removed: n=7) and shoaling (NND: Welch ANOVA: F=253.9, p<0.01; IID: ANOVA: F2,9=127.1, p<0.001; control n=2 trials, one lens: n=5 trials, no lenses: n=5 trials). Surface fish with both lenses removed schooled significantly differently from control fish and fish with one lens removed (planned-contrast test: t14.1=-7.8, p<0.001; Games-Howell test compared to control fish: p<0.01 and one-lens removed fish: p<0.001, Figure 3G) and swam significantly farther away from one another compared to control fish (NND: Games-Howell test: p<0.001; IID: Games-Howell test: p<0.001; Figure 3H, Supplemental Figure 3B). Surface fish retaining one eye were indistinguishable from control fish in the model school assay (Games-Howell test: p=0.996, Figure 3G). However, these fish were significantly different from both control (NND: Games-Howell test: p<0.05; IID Games-Howell test: p<0.001) and two-lenses removed fish (NND: Games-Howell test: p<0.001; IID: Games-Howell test: p<0.001; Figure 3H, Supplemental Figure 3B) in the shoaling assay. These results demonstrate that visual function is necessary for schooling and shoaling in surface forms of Astyanax mexicanus.
Vision-dependent and independent loss of schooling tendency in F2 fish
Since visual function is required for schooling behavior in A. mexicanus, the ancestral fish would have lost the ability to school immediately upon entering the pitch-dark cave environment. Thus, cavefish may have evolved their decreased tendency to school in our assays solely as a consequence of their loss of eyes. Alternatively, loss of schooling behavior may have become fixed in these fish through additional changes, independent of the loss of vision. To distinguish between these possibilities, visual function was assayed in the F2 population.
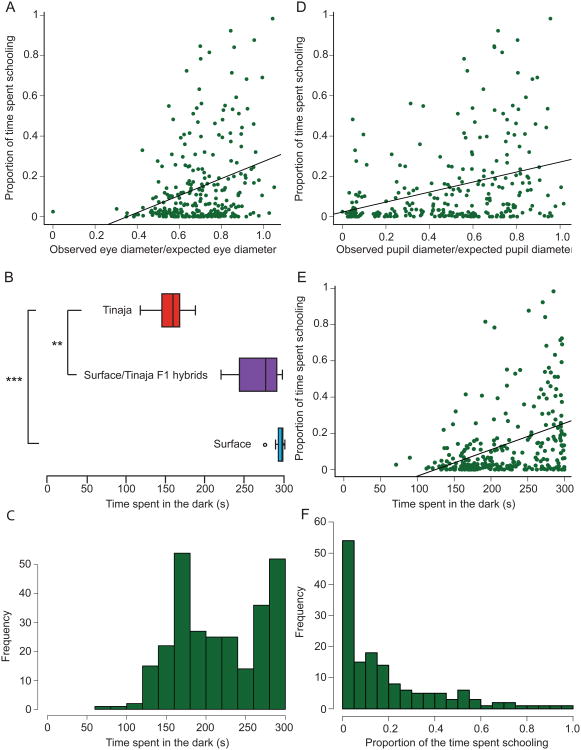
Visual function in the F2 population can be approximated using the external morphology of the eyes. Both eye (t8.7=-13.1, p<0.001; Surface: n=20, F1: n=5) and pupil diameters (t4.9=-9.0, p<0.001) are significantly reduced in F1 fish compared to surface fish (Supplemental Figure 4A). Nearly all F2 fish (n=283) have eyes, although most of them are smaller than surface fish eyes (Supplemental Figures 4B and C). Eye and pupil diameters are highly correlated in the F2 population (Pearson's R = 0.81, p<0.001, n=283, Supplemental Figure 4D). Proportion of time schooling in the F2 population is weakly to moderately positively correlated with both eye (Spearman's rho=0.27, p<0.001, n=270) and pupil (Spearman's rho=0.35, p<0.001, n=270, Figure 4A and D) diameters. However, there are individual fish with very large eyes and pupils who still do not school. This suggests that while schooling requires visual function, there may be an independent genetic basis for loss of schooling.
Figure 4. Measures of the visual system correlate with schooling behavior in F2 fish.
A. Average proportion of time schooling in the F2 population (n=270) as a function of eye size. B. Average proportion of the time schooling in the F2 population (n=270) as a function of pupil size. Both eye and pupil diameters were corrected for the expected size of the eye or pupil of a surface fish of the individual's body length. C. Surface (n=9), Tinaja (n=14), and Surface/Tinaja hybrid F1 (n=4) individuals in an assay for dark preference. Dark preference was quantified as the number of seconds spent in the dark out of a total of 300 seconds. Asterisks indicate p-values in a Mann-Whitney test. D. Distribution of average time spent in the dark across 3 trials for F2 population of fish (n=275). E. Average proportion of the time spent schooling as a function of dark preference in the F2 population (n=266). F. The distribution of the tendency to school in seeing F2 fish, defined as spending an average of 200 seconds in the dark (n=151). *p<0.05, **p<0.01, ***p<0.001.
F2 fish with large eyes and pupils may still lack visual function, and fish with smaller eyes may be able to see. Therefore, fish were tested for their ability to sense light. Surface fish display strong negative phototaxis, spending nearly all of their time in the dark. Tinaja cavefish behave significantly differently (Kruskal Wallis: H2=175.6, p<0.001; Surface: n=9, Tinaja: n=14, F1: n=4; Mann-Whitney: U<0.001, z=-4, p<0.001; Figure 4B), showing no preference for either the dark or the light. F1 hybrids display strong negative phototaxis, not significantly different from surface fish (Mann-Whitney: U=5, z=-2, p=0.15) and significantly different from Tinaja fish (U<0.001, z=-3, p<0.01; Figure 4B). Dark preference in the F2 fish population has a bimodal distribution (n=275, Figure 4C). Dark preference in the F2 population was moderately positively correlated with both eye diameter (Speaman's rho=0.36, p<0.001, n=265) and pupil diameter (Spearman's rho=0.47, p<0.001, n=265, Supplemental Figures 4E and F).
Dark preference was also moderately positively correlated with schooling in the F2 population (Spearman's rho=0.42, p<0.001, n=266, Figure 4E). A large proportion of non-schooling fish had no dark preference, indicating that many of the F2 fish that displayed no tendency to school have little visual function. However, some F2 individuals that showed a strong dark preference did not show any tendency to school, suggesting that there has been a loss of schooling in cavefish independent of vision.
In order to test for factors that affect schooling behavior independently of vision, we defined a population of F2 fish with visual perception as those fish that spent at least 2/3 of their time in the dark. This cutoff would include F1 and surface fish, but exclude Tinaja cavefish. Light-perceiving fish (n=151) were then analyzed for their propensity to school. Interestingly, within this group of light-perceiving F2 fish, many do not display schooling behavior (Figure 4F).
Groups of light-perceiving and non-light-perceiving F2 fish were tested in the shoaling assay. Light-perceiving, schooling groups of fish swam significantly closer to one another compared to light-perceiving, non-schooling fish groups and non-light-perceiving, non-schooling groups by NND (ANOVA: F2,10=7.65, p<0.05; planned-contrast test: t10=-3.91, p<0.01; Schooling: n=6 groups, Light-perceiving, non-schooling: n=4 groups, Non-light-perceiving, non-schooling: n=3 groups; Supplemental Figure 3C). These groups were not significantly different by IID, although they trended in the same direction (one-way ANOVA: F2,10=3.5, p=0.07, Supplemental Figure 3D). Furthermore, a mixed group of F2s, containing 2 fish from each of these categories, had a NND of 10.17 and an IID of 22.27. This NND is outside the range of the light-perceiving, schooling F2 fish (Supplemental Table 1). This confirms that a subset of F2 fish maintain visual function, but do not have a tendency to aggregate.
The roles of monoamine neurotransmitters in schooling behavior
Recent research has shown that there are differences in levels of monoamine neurotransmitters between cave and surface Astyanax mexicanus [39, 40]. In order to determine if these differences could have an effect on schooling behavior, we treated cave and surface fish with two inhibitors, (R)-(-)-Deprenyl hydrochloride and fluoxetine hydrochloride. Both of these drugs result in an increase in serotonin levels in the brain [40]. However, (R)-(-)-Deprenyl hydrochloride targets monoamine oxidase (MAO), inhibiting the breakdown of multiple monoamines.
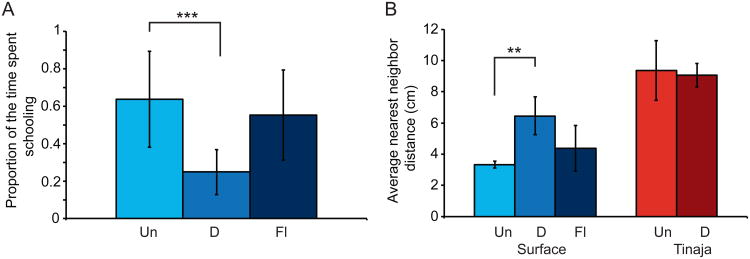
The treatments resulted in significant differences in schooling behavior (Kruskal Wallis: H2=18.4, p<0.001; Untreated: n=36, Deprenyl: n=12, Fluoxetine: n=22). (R)-(-)-Deprenyl treatment results in a significant decrease in schooling behavior (Mann-Whitney: U=46, z=-4, p<0.001) while fluoxetine does not significantly affect schooling relative to control fish (Mann-Whitney: U=313, z=-1.3, p=0.38, Figure 5A). In addition, (R)-(-)-Deprenyl (Welch ANOVA: H2=18.4, p<0.01; Untreated: n=6 groups, R-Deprenyl: n=6 groups, Fluoxetine: n=5 groups; Games-Howell: p<0.01) but not fluoxetine (Games-Howell: p=0.35) results in significantly greater separation between fish in the shoaling assay using NND (Figure 5B) and IDD (Welch ANOVA: H2=21.6, p<0.01; R-Deprenyl Games-Howell: p<0.01, Fluoxetine Games-Howell: p=0.21; Supplemental Figure 5). In contrast, the Tinaja cavefish in the shoaling assay were not affected by treatment with (R)-(-)-Deprenyl hydrochloride (NND: Mann-Whitney: U=0.9, z=-1.4, p=0.18; IID: Mann-Whitney: U=12, z=-0.96, p=0.39; Untreated: n=6 groups, Treated: n=6 groups; Figure 5B, Supplemental Figure 5). This data suggests that an increase in brain monoamine levels, but not specifically brain serotonin levels, decreases the tendency to school.
Figure 5. The effects of increased brain monoamine levels on schooling behavior.
A. Proportion of the time spent schooling in untreated (Un, n=21), 10uM Deprenyl treated (D, n=12), and 14 uM Fluoxetine treated (Fl, n=21) surface fish. Asterisks indicate p-values in a Mann-Whitney test. B. Average nearest neighbor distance in groups of untreated (Un, n=6 groups), 10uM Deprenyl treated (D, n=6 groups), and 14 uM Fluoxetine treated (Fl, n=5 groups) surface fish and untreated (Un, n=6 groups) and 10 uM Deprenyl treated (D, n=6 groups) Tinaja cavefish. Asterisks indicate p-values in a Games-Howell test. All error bars indicate the standard deviation. *p<0.05, **p<0.01, ***p<0.001.
QTL mapping of schooling behavior
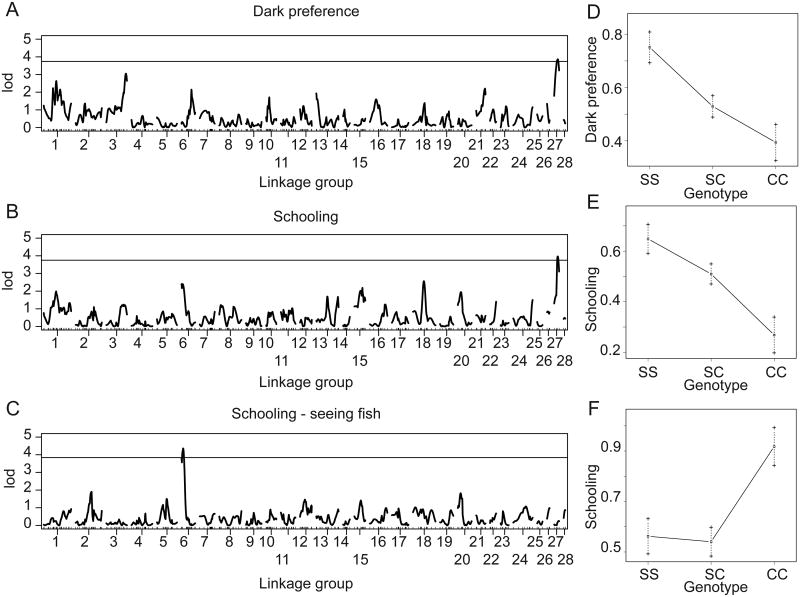
Finally, we performed QTL analysis to map the regions of the genome underlying the loss of schooling behavior in the Tinaja cavefish. Using a binary measure of schooling behavior, we mapped a single significant QTL on linkage group 27 that explained 6.4% of the variance (n=276, p<0.05, Figure 6B, Table 1). Homozygous cave alleles at a marker underlying this QTL result in a decrease in schooling behavior, and a heterozygous genotype result in an intermediate tendency to school (Figure 6E).
Figure 6. Visual and non-visual QTL for evolutionary loss of the tendency to school in cavefish.
A. Linkage map derived from SNPs in a Tinaja/Surface intercross. B. QTL for a binary measure of dark preference (n=267) where fish spending greater than 200 seconds were scored as preferring the dark. The line indicates a significant LOD score for a p-value < 0.05. C. QTL for a binary measure of the tendency to follow the model school (n=276). Fish were scored as schooling if they spent more than 5% of their time following the model on average. The line indicates a genome-wide significance LOD score for a p-value < 0.05. D. QTL for a binary measure of the tendency to follow the model school for the subset of fish that preferred the dark (n=143). E. Effect plot for the QTL for dark preference measured as a binary of time the dark (1=preferring the dark, 0=no dark preference). F. Effect plot for the QTL for schooling, measured as proportion of time following the model schooling and then made into a binary trait (1=schooling, 0=non-schooling). G. Effect plot for the schooling QTL in light-responsive fish, measured as proportion of time following the model schooling and then made into a binary trait (1=schooling, 0=non-schooling). Genotypes are for homozygous surface (SS), heterozygous (SC) or homozygous cave (CC) alleles.
Table 1.
Summary of QTL. CI = Confidence Interval. PVE = Percent variance explained.
| Trait | LG | cM | LOD | CI | PVE |
|---|---|---|---|---|---|
| Schooling | 27 | 20 | 4.0 | 16-26 cM | 6.4 |
| Dark preference | 27 | 20 | 3.9 | 9-27 cM | 6.4 |
| Schooling – seeing fish | 6 | 9 | 4.4 | 0-14 cM | 12 |
| Eye diameter | 3 | 74.1 | 4.9 | 65-113 cM | 7.9 |
| Pupil diameter | 3 | 74.1 | 4.6 | 67-77 cM | 7.3 |
In addition to schooling behavior, we mapped a binary measure of dark preference to one significant QTL on linkage group 27 that explains 6.4% of the variance (n=267, p<0.05, Figure 6A, Table 1). Homozygous cave alleles at a marker underlying this QTL result in less time spent in the dark, while heterozygous genotypes result in an intermediate percentage of time spent in the dark (Figure 6D). This QTL marker mapped to the same location as the schooling QTL.
In order to map the genetic basis of schooling behavior that is independent of visual function, a binary measure of schooling behavior in light-perceiving fish (defined as described above) was mapped. This resulted in one significant QTL on linkage group 6 that explains 12% of the variance (n=143, p<0.05, Figure 6C, Table 1). Somewhat surprisingly, homozygous cave genotypes at a marker underlying this QTL resulted in an increase in schooling behavior, while fish with homozygous surface or heterozygous genotypes schooled a similar amount of time (Figure 6F). This QTL does not fall in the same place as the QTL for dark preference, eye size, or pupil size (Figure 6A, Supplemental Figure 6A-D, Table 1). Thus this QTL identifies a vision-independent genetic contribution to the evolution of schooling behavior.
Discussion
Here, we determine that while both the visual system and the lateral line affect schooling in surface fish to some extent, it is the loss of sight in cavefish that plays the most significant role in the loss of schooling behavior. In contrast, lateral line enhancement in cavefish plays at most a minor role in schooling behavior loss. Our results suggest that loss of schooling evolved by multiple genetic changes, only some of which are vision-dependent.
The visual system is essential in schooling behavior in surface fish
It has been proposed that while the visual system allows fish to swim closer to one another during schooling, the lateral line provides a repulsive force [8]. We find that only vision, and not lateral line sensation, played a key role in the evolution of this behavior in the cave population of Astyanax mexicanus. Surface Astyanax do not school and have reduced shoaling in the dark or when they are blinded at 36 hpf. While there remains a possibility that surface fish choose not to school without visual cues, vision is likely necessary for both schooling and shoaling behavior.
We also tested the effect of loss of one eye on schooling and shoaling behavior. Interestingly, while fish with one eye could follow the model school, fish with one eye shoaled farther apart from one another. This could result from the importance of two functional eyes in tracking fish swimming in a disorganized manner, while other sensory organs, such as the lateral line, may compensate for the loss of one eye during schooling behavior.
Loss of vision has a large effect on the evolutionary loss of schooling behavior, while enhancement of the lateral line plays a minor role
We examined the role of visual function in the evolution of schooling behavior by examining these traits in the F2 population. We found that both morphological and behavioral measures of the visual system were correlated with schooling behavior in the F2 population. Additionally, the QTL for dark preference maps to the same region as a QTL for schooling behavior. This QTL for schooling behavior may explain the proportion of loss of schooling behavior explained by loss of visual function. Alternatively, since the QTL for dark preference does not fall in the same location as QTL for eye or pupil size, it is plausible that the behavioral difference in the dark preference assay mapped to this QTL has to do with a loss of dark preference per se, and not to perception of light. However, it could be related to an eye-size independent aspect of visual processing still related to light perception, such as retinal degeneration or lens degeneration.
While we found a significant correlation between number of neuromasts and schooling in F2 fish, the correlation was weak, and ablation of neuromasts in surface and cavefish was not sufficient to drive fish to swim closer together, or to increase schooling behavior. Therefore, it is unlikely that neuromasts play a large role in the evolution of schooling behavior.
Potential effects of increase of brain dopamine levels on decrease of schooling
Both dopamine and serotonin have been implicated in Pachón cavefish evolution [39, 40]. There is an increase in the amount of brain serotonin in Pachón cavefish compared to surface fish [40]. In addition, tyrosinase 3-monooxygenase/tryptophan 5-monooxygenase activation protein epsilon polypeptide 1 (YWHAE), an enzyme involved in dopamine biosynthesis, is upregulated in Pachón cavefish brains [39]. Both of these pathways are hypothesized to function in cavefish by increasing the amount of time spent foraging relative to surface fish [39, 40]. Therefore, these pathways may have been selected for in cavefish for other behavior purposes, and have a pleiotropic effect on schooling behavior.
We found that potentially increasing levels of multiple monoamines, presumably including both serotonin and dopamine, with (R)-(-)-Deprenyl hydrochloride, decreased both schooling and shoaling tendency in surface fish. Although (R)-(-)-Deprenyl hydrochloride affects brain levels of serotonin in A. mexicanus [40], it is unlikely that changing serotonin levels alone affects schooling behavior given the insignificant effect of fluoxetine. This indicates that another monoamine, not serotonin, plays a role in schooling behavior in A. mexicanus. Given the evidence that a molecule involved in the synthesis of dopamine is upregulated in at least one population of cavefish, our results are consistent with an increase in the amount of brain dopamine affecting schooling behavior in cavefish. Thus, brain neurotransmitter levels that have evolved to change adaptive behaviors, such as feeding behavior, may have a secondary, pleiotropic effect on schooling behavior. Testing whether modulation of dopamine specifically affects schooling and shoaling behavior, if R-deprenyl can induce increased levels of dopamine, and whether cavefish have increased amounts of dopamine compared to surface fish, would be an interesting complement to this work.
Evolution of schooling behavior independent of loss of vision
While loss of vision plays an important role in loss of schooling behavior, we also found evidence for a vision-independent loss of schooling behavior. Many F2 fish with a strong response to light still do not follow the model school. This is similar to what was previously seen in shoaling assays in Astyanax mexicanus [19]. Interestingly, when the effects of vision are removed by performing QTL analysis on only those fish that are light responsive, a second QTL, which does not fall in the same location as the vision, eye size, or pupil size QTL, emerges. We also performed QTL analysis for neuromast number, and neither of the schooling QTL fall in the same place as the neuromast QTL (data not shown). This suggests that the second QTL for the loss of schooling is vision and lateral line independent. Markers located under this QTL map to zebrafish chromosome 5 (Table 2). Fine scale mapping, combined with detailed analysis of the genes within this interval, will be necessary to identify the specific genetic changes responsible for the schooling QTL.
Potential evidence for relaxed selection on schooling in the cave
Once the ancestors of cavefish entered caves, they would not be able to school due to lack of light, and this could relax selection on schooling behavior. In addition, the ecology of the cave habitat suggests that there would be no counter-selection to maintain schooling behavior, in spite of the loss of vision. A likely lack of macroscopic predators in the caves removes one major selective pressure for schooling in the cave environment. One possibility for the evolution of schooling behavior is that once vision was impaired by the lack of light, schooling was no longer under selection, and alterations in genes affecting this behavior would be neutral in consequence. This could be an explanation for identification of a locus where F2 fish with a homozygous cave genotype show an increase in schooling behavior. Since a large percentage of seeing F2 fish stil do not school, there are likely other loci with cave alleles contributing to loss of schooling behavior. We expect that decreased schooling behavior is caused by many genetic changes, and that many of these have effects too small to detect in our current analysis.
Convergence on a decreased tendency to school in multiple cavefish populations and different fish species
Here, we demonstrate that multiple, independently evolved cavefish populations have lost the tendency to school. Previous work on A. mexicanus also showed a loss of schooling and reduction in shoaling behavior in cave populations [19, 27, 28]. Our work corroborates this previous work. Schooling behavior is also lost in other species of cave populations (reviewed in [28, 41]). The importance of the loss of the visual system for loss of schooling behavior in A. mexicanus may be general in cave populations, and it would be interesting to know if other cavefish species have reduced schooling behavior due to lack of visual cues.
In addition to cavefish, benthic threespine sticklebacks (Gasterosteus aculeatus) display reduced schooling behavior [31]. Greenwood et al. explore the genetics of this loss of schooling behavior in marine versus benthic stickleback populations (cosubmitted). While cave Astyanax and benthic sticklebacks both have a reduced tendency to school, the mechanisms that lead to loss of schooling behavior may be different in these two species. Benthic sticklebacks, which have intact visual systems, still show some tendency to follow a model school, but position themselves differently within it. In contrast, cave Astyanax have lost all tendency to follow a model school. This may be due to differences in habitats and selective pressures. Once they have entered the cave environment, Astyanax could no longer school due to loss of visual cues necessary for this behavior. In addition, cavefish do not encounter predators within the cave, and have thus lost a selective pressure usually associated with schooling behavior. In contrast, benthic sticklebacks are still confronted with predators, but display a shelter seeking behavior rather than a schooling behavior [31].
Interestingly, both cave Astyanax and benthic sticklebacks appear to have evolved differences in schooling behavior through modifications of sensory systems. Loss of vision contributes to the evolutionary loss of schooling tendency in cave Astyanax and lateral line evolution contributes to the evolution of schooling position in sticklebacks. Thus, convergent reduction of schooling behavior can occur through modulation of different sensory systems and different behavioral components. Together, these studies demonstrate the contribution of sensory system evolution to the evolution of complex behaviors.
Conclusion
In conclusion, we report the results of two behavioral assays for social grouping in one surface and three cave populations of Astyanax mexicanus. We show that the loss of schooling behavior in a cave population of Astyanax has a genetic basis and is a complex trait, influenced by at least two loci. Vision, but not the lateral line, is important for schooling behavior in surface fish, and vision is not a learned cue for schooling, but instead is required for this behavior. However, vision is not sufficient for schooling behavior. Loss of schooling behavior in cavefish has a genetic basis independent of eye loss. Additionally, we offer evidence that schooling was likely lost due to relaxed selection, as opposed to selection against schooling behavior in cave populations.
Experimental Procedures
Methods are described in the Supplemental Experimental Procedures
Supplementary Material
Highlights.
Multiple, independently evolved cave populations have lost the tendency to school
Vision is necessary for schooling behavior
Cavefish have lost the tendency to school regardless of vision
Acknowledgments
This work was supported by RO1 HD047360 from the NIH to CJT. JEK was supported by an NSF Graduate Research Fellowship and NR was supported by a Research Fellowship of the DFG. Abby Wark, Anna Greenwood and Katie Peichel provided useful discussions on developing the schooling assay. Kelly O'Quin mapped the Astyanax mexicanus sequences back to zebrafish. Jessica Lehoczky performed BLAST analysis comparing stickleback and Astyanax sequence. Wes Warren provided preliminary sequence of the Astyanax genome. Meredith Protas provided useful discussion on phenotyping the cavefish. Jessica Whited and Jeff Trimarchi provided useful discussion on the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Shaw E. Schooling Fishes. American Scientist. 1978;66:166–175. [Google Scholar]
- 2.Partridge BL. The structure and function of fish schools. Scientific American. 1982;246:114–123. doi: 10.1038/scientificamerican0682-114. [DOI] [PubMed] [Google Scholar]
- 3.Landeau L, Terborgh J. Oddity and the ‘confusion effect’ in predation. Animal Behavior. 1986;34:1372–1380. [Google Scholar]
- 4.Magurran A, Oulton W, Pitcher TJ. Vigilant behavior and shoal size in minnows. Zeitschrist Fur Tierpsychologie-Journal of Comparative Ethology. 1985;67:167–178. [Google Scholar]
- 5.Baird TA, Ryer CH, Olla BL. Social enhancement of foraging on an ephemeral food source in juvenile walleye pollock, Theragra chalcogramma. Environmental Biology of Fishes. 1991;31:307–311. [Google Scholar]
- 6.Plath M, Schlupp I. Parallel evolution leads to reduced shoaling behavior in two cave dwelling populations of Atlantic mollies (Poecilia mexicana, Poeciliidae, Teleostei) Environmental Biology of Fishes. 2008;82:289–297. [Google Scholar]
- 7.Krause J. The influence of hunger on shoal size choice by 3-spined sticklebacks, Gasterosteus-aculeatus. Journal of Fish Biology. 1993;43:775–780. [Google Scholar]
- 8.Partridge BL, Pitcher TJ. The sensory basis of fish schools: Relative roles of lateral line and vision. Journal of Comparative Physiololgy. 1980;135:315–325. [Google Scholar]
- 9.Hemmings CC. Olfaction and vision in fish schooling. J Exp Biol. 1966;45:449–&. [Google Scholar]
- 10.Wright D, Nakamichi R, Krause J, Butlin RK. QTL analysis of behavioral and morphological differentiation between wild and laboratory zebrafish (Danio rerio) Behavior genetics. 2006;36:271–284. doi: 10.1007/s10519-005-9029-4. [DOI] [PubMed] [Google Scholar]
- 11.Wilkens H. Evolution and genetics of epigean and cave astyanax-fasciatus (Characidae, Pisces) - support for the neutral mutation theory. Evolutionary Biology. 1988;23:271–367. [Google Scholar]
- 12.Teyke T. Morphological differences in neuromasts of the blind cave fish Astyanax hubbsi and the sighted river fish Astyanax mexicanus. Brain, behavior and evolution. 1990;35:23–30. doi: 10.1159/000115853. [DOI] [PubMed] [Google Scholar]
- 13.Schemmel C. Genetische Untersuchungen zur Evolution des Geschmacksapparates bei cavernicolen Fischen. Z Zool Syst Evolutionforsch. 1974;12:196–215. [Google Scholar]
- 14.Burchards H, Dolle A, Parzefall J. Aggressive behavior of an epigean population of Astyanax mexicanus (Characidae, Pisces) and some observations of three subterranean populations. Behavioral Processes. 1985;11:225–235. doi: 10.1016/0376-6357(85)90017-8. [DOI] [PubMed] [Google Scholar]
- 15.Duboue ER, Keene AC, Borowsky RL. Evolutionary convergence on sleep loss in cavefish populations. Current biology: CB. 2011;21:671–676. doi: 10.1016/j.cub.2011.03.020. [DOI] [PubMed] [Google Scholar]
- 16.Fricke D. Reaction to Alarm Substance in Cave Populations of Astyanax fasciatus (Characidae, Pisces) Ethology. 1987;76:305–308. [Google Scholar]
- 17.Yoshizawa M, Goricki S, Soares D, Jeffery WR. Evolution of a behavioral shift mediated by superficial neuromasts helps cavefish find food in darkness. Current biology: CB. 2010;20:1631–1636. doi: 10.1016/j.cub.2010.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schemmel C. Studies on the Genetics of Feeding Behavior in the Cave Fish Astyanax mexicanus F. anoptichthys. Z Tierpsychol. 1980;53:9–22. doi: 10.1111/j.1439-0310.1980.tb00730.x. [DOI] [PubMed] [Google Scholar]
- 19.Parzefall J, Fricke D. Alarm reaction and schooling in population hybrids of Astyanax fasciatus (Pisces, Characidae) Memoires e Biospeologie. 1991:29–32. [Google Scholar]
- 20.Protas ME, Hersey C, Kochanek D, Zhou Y, Wilkens H, Jeffery WR, Zon LI, Borowsky R, Tabin CJ. Genetic analysis of cavefish reveals molecular convergence in the evolution of albinism. Nature genetics. 2006;38:107–111. doi: 10.1038/ng1700. [DOI] [PubMed] [Google Scholar]
- 21.Protas M, Conrad M, Gross JB, Tabin C, Borowsky R. Regressive evolution in the Mexican cave tetra, Astyanax mexicanus. Current biology: CB. 2007;17:452–454. doi: 10.1016/j.cub.2007.01.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Protas M, Tabansky I, Conrad M, Gross JB, Vidal O, Tabin CJ, Borowsky R. Multi-trait evolution in a cave fish, Astyanax mexicanus. Evolution & development. 2008;10:196–209. doi: 10.1111/j.1525-142X.2008.00227.x. [DOI] [PubMed] [Google Scholar]
- 23.Gross JB, Borowsky R, Tabin CJ. A novel role for Mc1r in the parallel evolution of depigmentation in independent populations of the cavefish Astyanax mexicanus. PLoS genetics. 2009;5:e1000326. doi: 10.1371/journal.pgen.1000326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yoshizawa M, Yamamoto Y, O'Quin KE, Jeffery WR. Evolution of an adaptive behavior and its sensory receptors promotes eye regression in blind cavefish. BMC biology. 2012;10:108. doi: 10.1186/1741-7007-10-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.O'Quin KE, Yoshizawa M, Doshi P, Jeffery WR. Quantitative genetic analysis of retinal degeneration in the blind cavefish Astyanax mexicanus. PloS one. 2013;8:e57281. doi: 10.1371/journal.pone.0057281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gross JB. The complex origin of Astyanax cavefish. BMC evolutionary biology. 2012;12:105. doi: 10.1186/1471-2148-12-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Parzefall J. Field observation in epigean and cave populations of the Mexican Characid Astyanax mexicanus (Pisces, Characidae) Mem Biospelo. 1983;X:171–176. [Google Scholar]
- 28.Parzefall J. On the heredity of behavior patterns in cave animals and their epigean relatives. Nat Spel Soc Bull. 1985;47:128–135. [Google Scholar]
- 29.Couzin ID, Krause J. Self-Organizatoin and Collective Behavior in Vertebrates. Advances in the Study of Behavior. 2003;32:1–75. [Google Scholar]
- 30.Pitcher TJ. Heuristic definitions of fish shoaling behavior. Animal Behavior. 1983;31:611–613. [Google Scholar]
- 31.Wark AR, Greenwood AK, Taylor EM, Yoshida K, Peichel CL. Heritable differences in schooling behavior among threespine stickleback populations revealed by a novel assay. PloS one. 2011;6:e18316. doi: 10.1371/journal.pone.0018316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jeffery W, Strickler A, Guiney S, Heyser D, Tomarev S. Prox 1 in eye degeneration and sensory organ compensation during development and evolution of the cavefish Astyanax. Development genes and evolution. 2000;210:223–230. doi: 10.1007/s004270050308. [DOI] [PubMed] [Google Scholar]
- 33.Song J, Yan HY, Popper AN. Damage and recovery of hair cells in fish canal (but not superficial) neuromasts after gentamicin exposure. Hearing research. 1995;91:63–71. doi: 10.1016/0378-5955(95)00170-0. [DOI] [PubMed] [Google Scholar]
- 34.Van Trump WJ, Coombs S, Duncan K, McHenry MJ. Gentamicin is ototoxic to all hair cells in the fish lateral line system. Hearing research. 2010;261:42–50. doi: 10.1016/j.heares.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 35.Gregson JNS, De Perera TB. Shoaling in eyed and blind morphs of the characin Astyanax fasciatus under light and dark conditions. Journal of Fish Biology. 2007;70:1615–1619. [Google Scholar]
- 36.Jeffery W. Cavefish as a model system in evolutionary developmental biology. Developmental biology. 2001;231:1–12. doi: 10.1006/dbio.2000.0121. [DOI] [PubMed] [Google Scholar]
- 37.Jeffery WR, Martasian DP. Evolution of eye regression in the cavefish Astyanax: Apoptosis and the Pax-6 Gene. Amer Zool. 1998;38:685–696. [Google Scholar]
- 38.Yamamoto Y, Jeffery WR. Central role for the lens in cave fish eye degeneration. Science. 2000;289:631–633. doi: 10.1126/science.289.5479.631. [DOI] [PubMed] [Google Scholar]
- 39.Strickler AG, Soares D. Comparative genetics of the central nervous system in epigean and hypogean Astyanax mexicanus. Genetica. 2011;139:383–391. doi: 10.1007/s10709-011-9557-1. [DOI] [PubMed] [Google Scholar]
- 40.Elipot Y, H H, J C, S R. Evolutionary shift from fighting to foraging in blind cavefish through changes in the serotonin network. Current biology: CB. 2013;23:1–10. doi: 10.1016/j.cub.2012.10.044. [DOI] [PubMed] [Google Scholar]
- 41.Parzefall J. A review of morphological and behavioral changes in the cave molly, Poecilia mexicana, from Tabasco, Mexico. Environmental Biology of Fishes. 2001;62:263–275. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.