Abstract
Laser photocoagulation is a well-established treatment modality for retinal disease. Discrete laser burns can be placed anywhere in the retina, singly or multiply, and the burn intensity is controllable. This study investigates the effect of prior laser photocoagulation on the retinal transduction properties of intravitreally administered adeno-associated viral (AAV) vectors. C57BL/6J mice were subjected to unilateral laser photocoagulation 48 hr before bilateral intravitreal injection of self-complementary cytomegaloviral enhanced green fluorescent protein (EGFP) vectors packaged in AAV type 2, 5, and 8 capsids. The eyes were enucleated 4 weeks after injection and examined by histochemistry and quantitative image analysis. Laser pretreatment resulted in substantially increased localized transduction around the burn site for all AAV capsid types. Without laser pretreatment, the vectors transduced only ganglion cells (AAV2) or sporadic cells around the optic nerve head (AAV5 and AAV8). Laser pretreatment increased AAV2 vector expression throughout the entire retina and focally at the burn site. Transduced cells at the burn site included retinal pigment epithelium (RPE), photoreceptors, Müller cells, inner nuclear layer cells, and retinal ganglion cells. The AAV5 vector showed increased RPE transduction at the burn site only. The AAV8 vector showed augmented expression in RPE, photoreceptors, and Müller cells around the burn site. Migrating RPE cells, present in the neural retina near the burn site, were also transduced by all three capsid types as evidenced by colocalization of EGFP and cytokeratin. Laser photocoagulation can be used to precisely direct AAV vector transduction to discrete locations in the retina. A combination of laser and AAV-mediated gene expression may allow the development of improved therapies for diabetic retinopathy, branch and central vein occlusion, and age-related macular degeneration.
Introduction
Clinical successes with adeno-associated viral (AAV) vectors designed to treat Leber congenital amaurosis suggest that AAV vectors may be a platform technology for a variety of ocular therapeutics (Bainbridge et al., 2008; Hauswirth et al., 2008; Maguire et al., 2008). Two methods of vector administration are used for retinal transduction: subretinal and intravitreal injection. The two routes produce transduction in different parts of the retina and differ in degree of invasiveness; subretinal injection is a surgical procedure and intravitreal injection can be done on an outpatient basis. In primates, subretinal injection can produce efficient transduction of the outer retina, including the photoreceptors, retinal pigmented epithelium (RPE), and Müller cells (Weber et al., 2003). It is typically limited to about 25% of the total retinal area in humans to minimize the risk of uncontrolled retinal detachment. Intravitreal injection in primates results in transduction of the parafoveal ganglion cells, with little significant transduction of the peripheral retina (Yin et al., 2011). The efficiency of transduction and the cell types transduced for both routes are dependent on the promoters and capsids of the vectors, but both methods produce gene expression uniformly over broad regions of the retina (e.g., the outer retina) or entire structures (e.g., parafoveal ganglion cells). Neither is capable of mediating directed transduction of discrete subareas within a region or structure.
Laser photocoagulation is a well-established therapeutic modality for retinal disease. Discrete laser burns can be placed anywhere in the retina, either singly or multiply, and the diameter, depth, and intensity of the burn are controllable. Panretinal photocoagulation (PRP) is the mainstay for treatment of neovascularization in proliferative diabetic retinopathy (PDR). Laser burns are placed one burn width apart over the entire peripheral retina outside of the vascular arcades to reduce the density of highly metabolic photoreceptors, thereby reducing the ischemia that drives neovascular proliferation. Focal and grid laser treatments are used in cases of diabetic macular edema (DE) and branch vein occlusion (BRVO) that do not respond to anti-vascular endothelial growth factor (VEGF) drugs (i.e., ranibizumab) and for patients that cannot or will not comply with monthly anti-VEGF therapy. A focal laser is used to seal leaky aneurysms in and around the macula and a grid laser is used between the vascular arcades and the macula to treat diffuse areas of edema that threaten to spread centrally. The focal laser is also used as a more convenient alternative to anti-VEGF drugs for extrafoveal choroidal neovascularization (Macular Photocoagulation Study Group, 1991a; Virgili and Menchini, 2005).
Although current laser therapies are effective, they are not without limitations and side effects. Panretinal photocoagulation essentially sacrifices night vision to preserve central vision. A grid laser can adversely affect the retina and choroid near the macula, leading to a significant loss of peripheral vision (McDonald and Schatz, 1985; Branch Vein Occlusion Study Group, 1986; Kleiner et al., 1988). Focal laser treatment in and around the macula has proven to be beneficial to the diabetic population, but its usefulness is limited by side effects (Fong, 2002). In short, all of these laser therapies have room for improvement.
Expression of therapeutic proteins in the areas of retinal pathology may augment the therapeutic effects of laser therapies and may reduce the number of burns required for beneficial effect. To begin to investigate this idea, we evaluated the effect of prior laser photocoagulation on retinal transduction of intravitreally administered enhanced green florescent protein (EGFP) reporter vectors, employing AAV2, AAV5, and AAV8 capsids. Substantial augmentation of transduction was observed around burn sites for all capsid types. In addition, capsid types that showed little transduction of untreated retina after intravitreal injection were able to transduce cells in all retinal layers around the laser burn site.
Materials and Methods
Animals
Forty C57BL/6J mice (OrientBio, Sungnam, Korea) at 8 weeks of age were used. Animals were handled according to the Association for Research in Vision and Ophthalmology (ARVO) Statement for the Use of Animals in Ophthalmic and Vision Research and approved by the Animal Care Committee of Soonchunhyang University Bucheon Hospital.
Recombinant adeno-associated viral vector preparation and titration
Self-complementary AAV (scAAV) vectors expressing EGFP under the control of the cytomegalovirus (CMV) promoter were generated as previously described (Shin et al., 2008). Briefly, 293T cells were transfected by the calcium phosphate method with the following plasmids: pHpa-trs-SK-EGFP vector plasmid; AAV helper plasmid pHLP 2, pHLP 5, or pHLP 8; and the adenoviral helper plasmid pHelper (Stratagene, Kirkland, WA). The vectors were purified by polyethylene glycol (PEG) precipitation followed by CsCl gradient ultracentrifugation (a step gradient followed by a linear gradient). The purified vectors were then dialyzed against 50 mM Tris-HCl, 1 mM MgCl2, 10% sorbitol (pH 7.4) and stored at −80°C. The vectors were titered by TaqMan real-time PCR with the following primers and probe: 5′-CGTTACATAACTTACGGTAAATG-3′ (forward primer), 5′-ATACGTCATTATTGACGTCAATG-3′ (reverse primer), 5′-FAM-CCTGGCTGACCGCCCAACGAC-TAMRA-3′ (TaqMan probe) (TaqMan universal master mix; Applied Biosystems, Foster City, CA). The vector concentrations of the preparations were 1.27×1010 viral genomes (VG)/ml for scAAV2-EGFP, 3.38×1010 VG/ml for scAAV5-EGFP, and 3.65×1010 VG/ml for scAAV8-EGFP.
Laser photocoagulation
Animals were anesthetized by intraperitoneal injection of tiletamine and zolazepam (Zoletil; Virbac, Carros, France), followed by pupil dilation with 0.5% tropicamide and 2.5% phenylephrine (Mydrin-P; Santen, Emeryville, CA). Laser photocoagulation (spot size, 200 μm; duration, 0.04 sec; laser power, 100 mW) was performed with the slit-lamp delivery system of a PASCAL diode laser (OptiMedica, Santa Clara, CA). Ten laser spots were distributed in a concentric pattern around the optic nerve head of the right eye (Fig. 1A). A handheld coverslip was used as a contact lens to view the retina. If a lesion produced a gaseous bubble, indicating Bruch's membrane rupture, or hemorrhage, the mice were excluded from the experiment.
FIG. 1.
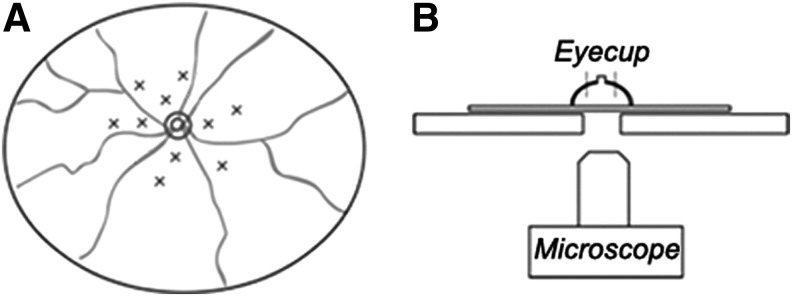
Schematic of a mouse fundus with laser burns distributed around the optic nerve head (A), and method for eyecup examination with fluorescence microscope (B).
Intravitreal administration of AAV vectors
Three groups of 10 mice received 1-μl bilateral, intravitreal injections of scAAV2-CMV-EGFP, scAAV5-CMV-EGFP, or scAAV8-CMV-EGFP, at the concentrations mentioned previously. Vector was administered 48 hr after laser photocoagulation of the right eye. A fourth group of 10 mice was not injected before unilateral laser pretreatment and served as a control. Intravitreal injection was performed under deep anesthesia with pupil dilation, using a 10-μl Hamilton syringe with a 33-gauge beveled-tip needle (Hamilton, Reno, NV). The needle was inserted through the sclera posterior to the limbus. All intravitreal injections were performed with a condensing lens system on the surgical microscope and a small plastic ring filled with 0.5% methylcellulose (GenTeal; Novartis, Basel, Switzerland), which allowed direct visualization of the fundus during the procedure.
Tissue preparation and fluorescence detection
Four weeks after injection, mice were killed by CO2 inhalation and the eyeballs were enucleated. Eye tissues were trimmed by the removal of the anterior segment and the lens by cutting through the limbal cornea. To determine the orientation of the eyecups, nictitating membrane was left attached to the limbus. Eyecups were fixed with 4% paraformaldehyde in 0.1 M phosphate buffer (PB) at pH 7.4 for 2 hr. The eyecups were then transferred to 30% sucrose in phosphate-buffered saline incubated overnight. Immediately before embedding the eyecups, they were examined and photographed with an Axioplan microscope at ×50 magnification (Carl Zeiss, Oberkochen, Germany) and a 1500-msec exposure time. The images were digitalized with a monochromatic charge-coupled device (CCD) camera (AxioCam MRm; Carl Zeiss) and a frame grabber (Fig. 1B). To compare transduction efficiency between both eyes, the optic nerve head was positioned at the center of all images, because laser photocoagulation was performed around the optic nerve head of the right eye. Eyecups were then embedded in O.C.T. compound (Sakura FineTek, Torrance, CA), sectioned at 10 μm, mounted on adhesion microscope slides (Histobond; Marienfeld-Superior, Lauda-Königshofen, Germany), and stored at −80°C for future study.
Immunolabeling
Sections were permeabilized for 1 hr with 0.1% Triton X-100 in 5% goat serum, and incubated overnight at 4°C with primary antibodies against cell markers for glial cells (Eng et al., 1985; Tuccari et al., 1986; Lewis et al., 2003) and retinal pigment epithelium (Owaribe et al., 1988; Turksen et al., 1989). The primary antibodies used in the study were as follows: a rabbit polyclonal anti-glial fibrillary acidic protein (GFAP) antibody (NG1817590; Millipore, Billerica, MA) at 1:2000 dilution, and a mouse anti-human cytokeratin antibody (M3515; Dako, Glostrup, Denmark) at 1:1000 dilution. After antibody incubation, the samples were washed with 0.1 M PB three times for 15 min each, and incubated with secondary antibodies: Alexa Fluor 568-conjugated goat anti-mouse IgG (Molecular Probes, Grand Island, NY) and Alexa Fluor 568-conjugated goat anti-rabbit IgG (Molecular Probes) for 2 hr at room temperature. Using 0.1 M PB, samples were again rinsed three times for 15 min each, and 4′,6-diamidino-2-phenylindole dihydrochloride (DAPI) (D9542; Sigma-Aldrich, St. Louis, MO) stain was used to label cell nuclei. The results were examined with the Axioplan microscope with a ×20 objective lens and a ×10 eyepiece.
Image analysis and statistical analysis
To quantify the areas of fluorescence on mouse retinas, image capture software (AxioVision; Carl Zeiss) was used. Areas were calculated in pixels, using the histogram function in ImageJ image analysis software (National Institutes of Health, Bethesda, MD). Each histogram showed one major peak along the gray scale, and pixels counted from and above the major peak were considered to be the fluorescence-positive area. The ratio of fluorescence area to total retina area was determined. The Wilcoxon signed-rank test was conducted to compare area ratios (fluorescence area/total area pixel intensity) between the control group (laser-untreated/vector-uninjected, laser-treated/vector-uninjected) and the vector-injected groups (laser-untreated/vector-injected group, laser-treated/vector-injected group), as well as between the various vector-injected groups, with and without laser treatment. Statistical analysis was conducted with SPSS software version 15.0 for Windows (SPSS, Chicago, IL), and p<0.05 was considered statistically significant.
Results
Photocoagulation increases vector transduction
The effect of prior laser photocoagulation on the retinal transduction of intravitreally administered AAV2, AAV5, and AAV8 vectors was examined in C57BL/6J mice. Four groups of 10 mice were subjected to unilateral retinal laser photocoagulation. The laser-treated right eyes received 10 burns per eye, arranged in a concentric pattern around the optic nerve head, using a spot size of 200 μm, a duration of 0.04 sec, and laser power at 100 mW. Forty-eight hours after laser treatment, 3 groups of 10 mice received bilateral intravitreal injections of scAAV CMV EGFP vectors packaged in AAV type 2, 5, or 8 capsids. The remaining group did not receive vector administration and served as control to assess autofluorescence. The vector doses for the scAAV CMV EGFP type 2, 5, and 8 vectors were 1.27×107, 3.38×107, and 3.65×107 VG/eye, respectively. All eyes were enucleated 4 weeks after injection and examined by histochemistry and quantitative image analysis (Fig. 1). Vector transduction in laser-treated right eyes was compared with transduction in contralateral left eyes, which did not receive laser treatment.
The eyecups from uninjected eyes demonstrated only scant autofluorescence around the optic nerve head, both with and without laser treatment (Fig. 2A and E). When the eyecups from the laser-untreated/vector-injected group were examined, the AAV2 vector produced scattered fluorescence spots around the entire fundus and fluorescence in the retinal nerve fiber/ganglion cell layer (Fig. 2B), whereas AAV5 and AAV8 vector-treated eyes showed occasional fluorescent spots around the optic nerve head (Fig. 2C and D). Laser pretreatment caused a substantial increase in local transduction around the laser photocoagulation sites for all capsid types (Fig. 2F–H). AAV2 and AAV8 vector transduction was strongly increased around the photocoagulation site whereas the increase in local AAV5 vector transduction was more moderate. AAV2 vector transduction appeared augmented throughout the entire retina. Dark, hypofluorescent areas near laser burn sites were also visible in the photocoagulated retinas (Fig. 2G and H).
FIG. 2.
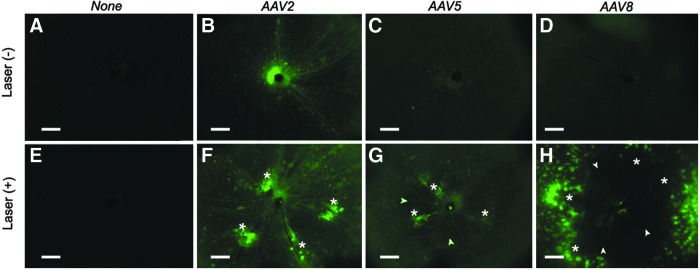
Fluorescence microscope images of the control (laser-untreated/vector-uninjected and laser-treated/vector-uninjected) and scAAV type 2, 5, and 8 CMV EGFP vector-transduced fundi. The vector doses were 1.27×107, 3.38×107, and 3.65×107 VG/eye, respectively, and the exposure time was 1500 msec. The optic nerve head is positioned at the center of the each image. The fundi of the control demonstrated only scant autoflurescence around the optic nerve head, regardless of the laser pretreatment (A and E). In the laser-untreated/vector-injected group, AAV2 vector resulted in scattered fluorescence spots around the entire fundus and fluorescence in retinal nerve fiber/ganglion cell layer (B), while AAV 5 and 8 vector treated eyes showed occasional fluorescent spots around the optic nerve head (C and D). Laser pretreatment produced increased transduction around the laser sites in all capsid types (F–H). Dark, hypofluorescent areas around laser burn site were due to RPE cell proliferation (G and H, arrowheads). Asterisk (*), laser burn sites. Scale bars: 200 μm.
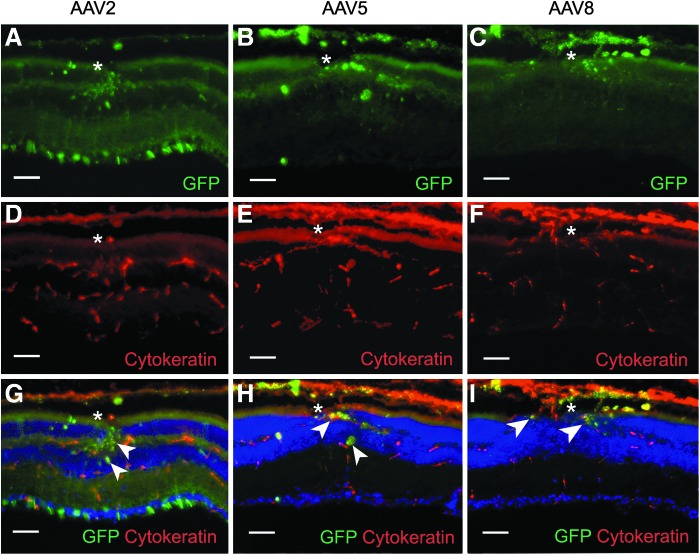
Transverse sections allowed identification of the cell types transduced by the vectors. Consistent with eyecup images, there was no sign of transduction in eyes that did not receive vector, with or without laser treatment (Fig. 3A and E). In the laser-untreated/vector-injected group, AAV2 vector transduction was restricted to the ganglion cells and sporadic cells in the inner nuclear layer (Fig. 3B). AAV5 and AAV8 vectors produced little transduction (Fig. 3C and D) except for occasional cells in the inner nuclear layer around the optic nerve head. Laser pretreatment resulted in localized transduction changes that were capsid type dependent. AAV2 vectors transduced RPE, photoreceptors, Müller cells, inner nuclear layer (INL) cells, and ganglion cells near the photocoagulation site. AAV8 vectors transduced photoreceptors, Müller cells, and RPE, and AAV5 vectors transduced RPE only. A number of EGFP-positive, irregularly shaped, pigmented cells of uncertain type were also visible in the neurosensory retina (Fig. 3F and G). The cells correspond to the hypofluorescent areas in the eyecup images.
FIG. 3.
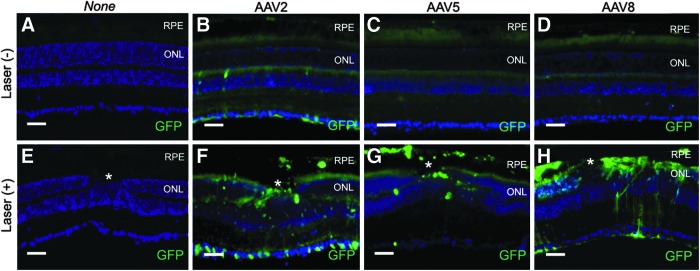
Fluorescence microscope images of transverse retinal sections from the control (laser-untreated/vector-uninjected and laser-treated/vector-uninjected) and scAAV type 2, 5, and 8 CMV EGFP vector-transduced mice. The vector doses were 1.27×107, 3.38×107, and 3.65×107 VG/eye, respectively and the exposure time was 1500 msec. There was no sign of transduction in the control eyes (A and E), and the laser-untreated/vector-injected group produced limited transduction (B–D). When treated with laser pretreatment, AAV2 vectors transduced retinal pigment epithelium (RPE), photoreceptors, Müller cells, inner nuclear layer (INL) cells, and ganglion cells (F), while AAV8 vectors transduced photoreceptors, Müller cells and RPE (H). On the other hand, AAV5 vectors transduced RPE only (G). Nonspecific fluorescent areas are visible in the inner and outer segments of the photoreceptor layers (A–E). 4′,6-diamidino-2-phenylindole dihydrochloride (DAPI) was used for nuclei staining to visualize retinal layers (shown in blue). Asterisk (*), laser burn sites. GFP, green fluorescent protein; ONL, outer nuclear layer; RPE, retinal pigment epithelium. Scale bars: 50 μm.
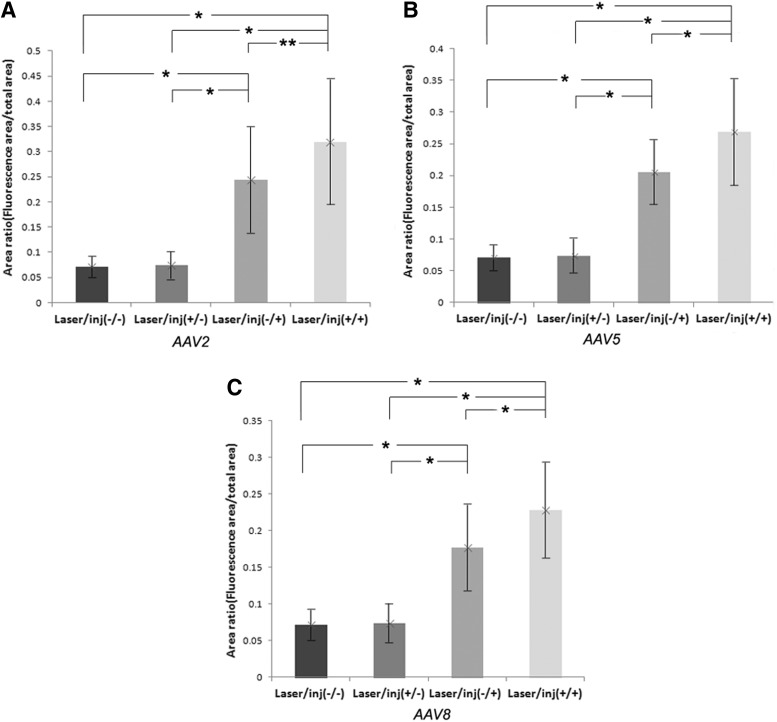
The total laser-mediated transduction increase in the fundus was measured by comparing the ratio of fluorescence area with the total fundus area in laser-treated and laser-untreated groups. The mean values for this ratio for each injected capsid type were (laser-untreated, laser-treated) as follows: AAV2: 0.24±0.10, 0.32±0.12; AAV5: 0.21±0.05, 0.26±0.08; and AAV8: 0.17±0.05, 0.31±0.06 (Fig. 4). There was a statistically significant difference in the AAV5 and AAV8 vector-treated animals (p=0.028) but not in the AAV2 vector-treated animals (p=0.075).
FIG. 4.
The fundi of animals, receiving laser photocoagulation and/or scAAV CMV EGFP vectors, were evaluated for area of fluorescence. The area of fluorescence of each fundus was normalized relative to the total fundus area. The fractional areas of fundus fluorescence were compared between groups. The mean values for this ratio for each capsid type were (laser-untreated, laser-treated) as follows: AAV2: 0.24±0.10, 0.32±0.12; AAV5: 0.21±0.05, 0.26±0.08; and AAV8: 0.17±0.05, 0.31±0.06. There was a statistically significant difference in the AAV5 and AAV8 vector-treated animals (p=0.028) but not in the AAV2 vector-treated animals (p=0.075). The mean values for area ratio for the laser-untreated/vector-uninjected and laser-treated/vector-uninjected eyes were 0.07±0.02 and 0.07±0.03, respectively. (A) Control versus scAAV2-EGFP injected group; (B) control versus scAAV5-EGFP-injected group; (C) control versus scAAV8-EGFP-injected group. *p<0.05, **p>0.05, tested by Wilcoxon signed-rank test.
The uninjected control group showed fluorescence values far below any of the values of mice receiving vectors. Uninjected eyes showed little difference with or without laser treatment, and had fluorescence area/total fundus area ratios of 0.07±0.02 and 0.07±0.03, respectively. There was a statistically significant difference between the control group and the vector-injected eyes with or without laser treatment for all capsid types (p=0.028).
Identification of ambiguous pigmented cell types
The irregularly shaped, ambiguously pigmented cells observed in the transverse sections of laser-treated eyes were probed with an anti-cytokeratin antibody to determine whether they were RPE cells. Cytokeratin expression has been shown to be specific for RPE cells in the retina (Hiscott et al., 1984; Kasper et al., 1988). The ambiguous pigmented cells stained robustly with the antibody; most likely they are RPE cells that migrated into the neurosensory retina in response to laser treatment (Fig. 5).
FIG. 5.
Cryosection images reveal irregularly shaped, EGFP- and cytokeratin-positive pigmented cells migrating into inner retinal layers in all three serotype groups (A–F). Combined images showing colocalization of EGFP and cytokeratin identify the pigmented cells as migrating RPE cells after laser photocoagulation (G–I, arrowheads). Nuclei are stained with DAPI (shown in blue). Asterisk (*), laser burn sites. Scale bars: 50 μm.
Laser stimulates Müller cell activation and vector transduction
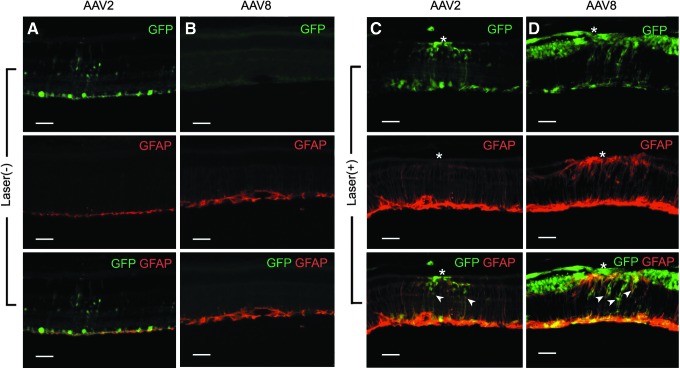
Müller cell transduction by intravitreally administered CMV EGFP vectors was evaluated with and without laser pretreatment. Müller cell activation was assessed with an antibody to GFAP (Fig. 6). Only scAAV2 and scAAV8 vectors were analyzed, because the scAAV5 vector did not transduce Müller cells. In the absence of laser pretreatment, GFAP expression was restricted to the ganglion cell layer and no transduced Müller cells were observed with either vector (Fig. 6A and B). Laser pretreatment produced extensive gliosis, upregulation of Müller cell GFAP, and a substantial increase in Müller cell transduction with both capsid types (Fig. 6C and D). Only GFAP-positive Müller cells showed transduction.
FIG. 6.
Colocalization of glial fibrillary acidic protein (GFAP) expression with EGFP expression in Müller cells, with and without prior laser treatment. Vector transduction/EGFP expression is shown in green, and anti-GFAP antibody staining is shown in red. Without laser pretreatment, no transduced Müller cells were detected, and GFAP expression was limited to the ganglion cell layer with both AAV2 and AAV8 vector (A and B). Laser pretreatment resulted in Müller cell GFAP up-regulation and a substantial increase in Müller cell transduction with both capsid types (C and D) Arrowheads indicate colocalization sites of EGFP and GFAP, which indicate transduced Müller cells. Asterisk (*), laser burn sites. Scale bars: 50 μm.
Discussion
Laser pretreatment of retinas resulted in robust transduction by intravitreally administered vectors around the burn sites. This was true for all capsid types tested, including those that showed little transduction after intravitreal delivery to untreated retinas (types 5 and 8). Vectors had access to all retinal layers around the burn site as evidenced by the transduction of RPE, photoreceptor cells, Müller cells, INL cells, and retinal ganglion cells. This study demonstrates that laser photocoagulation can be used to direct AAV vector-mediated expression to any part of the retina in a discrete manner. This is particularly true when using capsids that show little ocular transduction after intravitreal injection in the absence of photocoagulation.
The majority of vectors examined so far do not transduce retina after intravitreal injection. This group includes AAV capsid types 1, 4, 5, 8, and 9. Dalkara and colleagues demonstrated that the vitreoretinal junction acts as a barrier for several of these capsid types (Dalkara et al., 2009). Laser treatment appears to overcome this blockade. The mechanism is not clear but could be due to simple thermal disruption of the junction or may also involve indirect mechanisms such as Müller cell stress response. Previous studies have shown enhanced Müller cell proliferation around laser burn sites (Paulus et al., 2008; Tackenberg et al., 2009). This causes increased movement of Müller cell endfeet, which may disrupt the internal limiting membrane. The enhanced transduction of the AAV8 reporter vector extends beyond the burn site, suggesting that mechanisms other than simple thermal disruption are likely. The robust vector expression in the burn region may also be due to the cellular stress response and upregulation of capsid receptors (Ie et al., 1994; Yamamoto et al., 1996; Ishida et al., 1998; Ozaki et al., 2000; Nagineni et al., 2005).
Laser-directed vector transduction may be useful for the treatment of several major retinal diseases including proliferative diabetic retinopathy, diabetic macular edema, branch and central retinal vein occlusion, and age-related macular degeneration (AMD). PRP has been the standard treatment for proliferative diabetic retinopathy since the publications of the Diabetic Retinopathy Study (Diabetic Retinopathy Study Research Group, 1976, 1978, 1981). The function of laser photocoagulation in proliferative diabetic retinopathy is to ablate nonperfused areas of the retina to reduce the oxygen demand and lower the production of proangiogenic cytokines, such as VEGF, which cause neovascularization, vascular permeability, and macular edema. Although laser treatment has been widely used for PDR and high-risk PDR, side effects are still a concern. These include the destruction of functional retinal neurons, which results in decreased peripheral visual field and dark adaptation, and the loss of visual acuity due to exacerbation of macular edema. Augmentation of laser treatment with gene therapy may allow a reduction in the number, size, or intensity of the laser burns required for effective treatment of neovascularization and vascular permeability and thereby reduce laser damage. Prolonged, vector-mediated expression of antiangiogenic proteins at the site of pathology may also prevent further development of neovascularization and DE and relapse. In addition, a more sparing use of the laser may allow treatment to begin earlier, before macular involvement and the associated damage.
Grid laser photocoagulation is a treatment option for DE and BRVO. However, several clinical studies have demonstrated limitations of laser treatment for treating macular edema. Grid laser photocoagulation is beneficial only to small fraction of patients meeting a particular set of clinical criteria, and its usefulness is limited by side effects (Fong, 2002; McIntosh et al., 2007). A combination of AAV-mediated ocular gene therapy and laser photocoagulation would provide simultaneous ablation of leaky sites and directed transduction of problematic areas. The continuous expression of antiangiogenic proteins in these areas may prevent further progression of macular edema. In the case of central retinal vein occlusion (CRVO), laser photocoagulation does not have any beneficial effect on angiographically proven macular edema (Central Vein Occlusion Study Group, 1995). A multicenter clinical trial demonstrated the efficacy of ranibizumab (Lucentis) therapy in patients with CRVO with macular edema (Brown et al., 2010); however, the effect was evaluated only for a 6-month period. Laser-directed gene transfer would act to reduce angiographic leakage by laser photocoagulation while simultaneously targeting prolonged expression of anti-VEGF proteins to the areas of pathology.
Before the era of anti-VEGF therapy, laser photocoagulation was the only treatment choice available for exudative AMD (Macular Photocoagulation Study Group, 1991b–d). Laser therapy was limited by collateral damage to the neurosensory retina and retinal pigment epithelium and had a high incidence of lesion recurrence (Macular Photocoagulation Study Group, 1986). The technique successfully sealed the microvascular networks of choroidal neovascularization (CNV), but did little to inhibit further angiogenesis. Anti-VEGF therapy was introduced in 2006 (Brown et al., 2006; Rosenfeld et al., 2006) and is now the standard treatment for exudative AMD. However, there are cases which respond minimally to anti-VEGF therapy. One example of this is idiopathic polypoidal choroidal vasculopathy (PCV), the predominant form of CNV in Asia, which has a CNV pattern but with a branching choroidal vascular network and polypoidal dilatation. Several studies have shown that anti-VEGF therapy, although effective for decreasing exudation and retinal thickness, is not an effective treatment for eliminating leaky polyps in PCV (Song et al., 2009; Kokame et al., 2010). This limitation may be overcome by combined therapy of laser photocoagulation and AAV vector-mediated anti-VEGF expression, which can effectively ablate the vascular network while reducing the exudative problem with persistently secreted anti-VEGF. Laser treatment can be applied without the risk of damaging the fovea, because polyps are typically located in the peripapillary and extrafoveal region (Kwok et al., 2002; Uyama et al., 2002). Moreover, multiple studies have shown that anti-VEGF therapy has a limited effect on stabilized abnormal blood vessels, suggesting that they become less dependent on VEGF for survival as vessel maturation proceeds (Benjamin et al., 1999; Gee et al., 2003). VEGF withdrawal causes the regression of new sprouts of naked vascular endothelial cells, but not vessels with pericyte coverage (Bergers et al., 2003; Cursiefen et al., 2004). The combination of PRP and an AAV vector expressing a VEGF inhibitor may address this problem. In addition, laser photocoagulation would be used to ablate pericyte-covered core CNV while the continuous expression of the VEGF inhibitor would induce regression of adjacent pericyte-free vessels and prevent the formation of new ones. This approach may be particularly useful in the extrafoveal and juxtafoveal areas, where judicious use of laser is necessary. However, it is not applicable in the cases of subfoveal lesions, because laser photocoagulation could cause direct damage to the fovea and produce irreversible vision loss.
Gene therapy has begun to show clear clinical promise for treating inherited retinal disease and may be a broadly generalizable approach for treating several types of retinal disease. The technology is still in its infancy and vector delivery methods have substantial limitations. In this study, we demonstrate enhanced, localized transduction of AAV vectors around laser burn sites and present a method for directing the therapeutic effect of gene therapy to discrete regions of retina. This may allow the development of improved therapies for several major retinal diseases.
Author Disclosure Statement
This work was supported by a Basic Science Research Program through the National Research Foundation of Korea (NRF), funded by the Ministry of Education, Science, and Technology (grant 2011-0014354).
References
- Bainbridge J.W., Smith A.J., Barker S.S., et al. (2008). Effect of gene therapy on visual function in Leber's congenital amaurosis. N. Engl. J. Med. 358, 2231–2239 [DOI] [PubMed] [Google Scholar]
- Benjamin L.E., Golijanin D., Itin A., et al. (1999). Selective ablation of immature blood vessels in established human tumors follows vascular endothelial growth factor withdrawal. J. Clin. Invest. 103, 159–165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergers G., Song S., Meyer-Morse N., et al. (2003). Benefits of targeting both pericytes and endothelial cells in the tumor vasculature with kinase inhibitors. J. Clin. Invest. 111, 1287–1295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branch Vein Occlusion Study Group (1986). Argon laser scatter photocoagulation for prevention of neovascularization and vitreous hemorrhage in branch vein occlusion: A randomized clinical trial. Arch. Ophthalmol. 104, 34–41 [DOI] [PubMed] [Google Scholar]
- Brown D.M., Campochiaro P.A., Singh R.P., et al. (2010). Ranibizumab for macular edema following central retinal vein occlusion: Six-month primary end point results of a phase III study. Ophthalmology 117, 1124–1133.e1. [DOI] [PubMed] [Google Scholar]
- Brown DM, Kaiser PK, Michels M, Soubrane G, et al. (2006). Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N Engl J Med 355, 1432–1444 [DOI] [PubMed] [Google Scholar]
- Central Vein Occlusion Study Group (1995). Evaluation of grid pattern photocoagulation for macular edema in central vein occlusion: The Central Vein Occlusion Study Group M report. Ophthalmology 102, 1425–1433 [DOI] [PubMed] [Google Scholar]
- Cursiefen C., Chen L., Borges L.P., et al. (2004). VEGF-A stimulates lymphangiogenesis and hemangiogenesis in inflammatory neovascularization via macrophage recruitment. J. Clin. Invest. 113, 1040–1050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalkara D., Kolstad K.D., Caporale N., et al. (2009). Inner limiting membrane barriers to AAV-mediated retinal transduction from the vitreous. Mol. Ther. 17, 2096–2102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diabetic Retinopathy Study Research Group (1976). Preliminary report on effects of photocoagulation therapy. Am. J. Ophthalmol. 81, 383–396 [DOI] [PubMed] [Google Scholar]
- Diabetic Retinopathy Study Research Group (1978). Photocoagulation treatment of proliferative diabetic retinopathy: The second report of diabetic retinopathy study findings. Ophthalmology 85, 82–106 [DOI] [PubMed] [Google Scholar]
- Diabetic Retinopathy Study Research Group (1981). Photocoagulation treatment of proliferative diabetic retinopathy: Clinical application of Diabetic Retinopathy Study (DRS) findings, DRS Report Number 8. Ophthalmology 88, 583–600 [PubMed] [Google Scholar]
- Eng L.F., Reich S.J., Fosnot J., et al. (1985). Glial fibrillary acidic protein (GFAP): The major protein of glial intermediate filaments in differentiated astrocytes. J. Neuroimmunol. 8, 203–214 [DOI] [PubMed] [Google Scholar]
- Fong D.S. (2002). Changing times for the management of diabetic retinopathy. Surv. Ophthalmol. 47(Suppl. 2), S238–S245 [DOI] [PubMed] [Google Scholar]
- Gee M.S., Procopio W.N., Makonnen S., et al. (2003). Tumor vessel development and maturation impose limits on the effectiveness of anti-vascular therapy. Am. J. Pathol. 162, 183–193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauswirth W.W., Aleman T.S., Kaushal S., et al. (2008). Treatment of Leber congenital amaurosis due to RPE65 mutations by ocular subretinal injection of adeno-associated virus gene vector: Short-term results of a phase I trial. Hum. Gene Ther. 19, 979–990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiscott P.S., Grierson I., and McLeod D. (1984). Retinal pigment epithelial cells in epiretinal membranes: An immunohistochemical study. Br. J. Ophthalmol. 68, 708–715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ie D., Gordon L.W., Glaser B.M., and Pena R.A. (1994). Transforming growth factor-β2 levels increase following retinal laser photocoagulation. Curr. Eye Res. 13, 743–746 [DOI] [PubMed] [Google Scholar]
- Ishida K., Yoshimura N., Yoshida M., and Honda Y. (1998). Upregulation of transforming growth factor-β after panretinal photocoagulation. Invest. Ophthalmol. Vis. Sci. 39, 801–807 [PubMed] [Google Scholar]
- Kasper M., Moll R., Stosiek P., and Karsten U. (1988). Patterns of cytokeratin and vimentin expression in the human eye. Histochemistry 89, 369–377 [DOI] [PubMed] [Google Scholar]
- Kleiner R.C., Elman M.J., Murphy R.P., and Ferris F.L., III., (1988). Transient severe visual loss after panretinal photocoagulation. Am. J. Ophthalmol. 106, 298–306 [DOI] [PubMed] [Google Scholar]
- Kokame G.T., Yeung L., and Lai J.C. (2010). Continuous anti-VEGF treatment with ranibizumab for polypoidal choroidal vasculopathy: 6-month results. Br. J. Ophthalmol. 94, 297–301 [DOI] [PubMed] [Google Scholar]
- Kwok A.K., Lai T.Y., Chan C.W., et al. (2002). Polypoidal choroidal vasculopathy in Chinese patients. Br. J. Ophthalmol. 86, 892–897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis G.P., Fisher S.K., Maguire A.M., et al. (2003). Up-regulation of glial fibrillary acidic protein in response to retinal injury: Its potential role in glial remodeling and a comparison to vimentin expression. Int. Rev. Cytol. 230, 263–290 [DOI] [PubMed] [Google Scholar]
- Macular Photocoagulation Study Group (1986). Recurrent choroidal neovascularization after argon laser photocoagulation for neovascular maculopathy. Arch. Ophthalmol. 104, 503–512 [DOI] [PubMed] [Google Scholar]
- Macular Photocoagulation Study Group (1991a). Argon laser photocoagulation for neovascular maculopathy: Five-year results from randomized clinical trials. Arch. Ophthalmol. 109, 1109–1114 [PubMed] [Google Scholar]
- Macular Photocoagulation Study Group (1991b). Laser photocoagulation of subfoveal neovascular lesions in age-related macular degeneration: Results of a randomized clinical trial. Arch. Ophthalmol. 109, 1220–1231 [DOI] [PubMed] [Google Scholar]
- Macular Photocoagulation Study Group (1991c). Laser photocoagulation of subfoveal recurrent neovascular lesions in age-related macular degeneration: Results of a randomized clinical trial. Arch. Ophthalmol. 109, 1232–1241 [DOI] [PubMed] [Google Scholar]
- Macular Photocoagulation Study Group (1991d). Subfoveal neovascular lesions in age-related macular degeneration: Guidelines for evaluation and treatment in the macular photocoagulation study. Arch. Ophthalmol. 109, 1242–1257 [PubMed] [Google Scholar]
- Maguire A.M., Simonelli F., Pierce E.A., et al. (2008). Safety and efficacy of gene transfer for Leber's congenital amaurosis. N. Engl. J. Med. 358, 2240–2248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald H.R., and Schatz H. (1985). Visual loss following panretinal photocoagulation for proliferative diabetic retinopathy. Ophthalmology 92, 388–393 [DOI] [PubMed] [Google Scholar]
- McIntosh R.L., Mohamed Q., Saw S.M., and Wong T.Y. (2007). Interventions for branch retinal vein occlusion: An evidence-based systematic review. Ophthalmology 114, 835–854 [DOI] [PubMed] [Google Scholar]
- Nagineni C.N., Kutty V., Detrick B., and Hooks J.J. (2005). Expression of PDGF and their receptors in human retinal pigment epithelial cells and fibroblasts: Regulation by TGF-β. J. Cell. Physiol. 203, 35–43 [DOI] [PubMed] [Google Scholar]
- Owaribe K., Kartenbeck J., Rungger-Brandle E., and Franke W.W. (1988). Cytoskeletons of retinal pigment epithelial cells: Interspecies differences of expression patterns indicate independence of cell function from the specific complement of cytoskeletal proteins. Cell Tissue Res. 254, 301–315 [DOI] [PubMed] [Google Scholar]
- Ozaki S., Radeke M.J., and Anderson D.H. (2000). Rapid upregulation of fibroblast growth factor receptor 1 (Flg) by rat photoreceptor cells after injury. Invest. Ophthalmol. Vis. Sci. 41, 568–579 [PubMed] [Google Scholar]
- Paulus Y.M., Jain A., Gariano R.F., et al. (2008). Healing of retinal photocoagulation lesions. Invest. Ophthalmol. Vis. Sci. 49, 5540–5545 [DOI] [PubMed] [Google Scholar]
- Rosenfeld PJ, Brown DM, Heier JS, Boyer DS, et al. (2006). Ranibizumab for neovascular age-related macular degeneration. N Engl J Med 355, 1419–1431 [DOI] [PubMed] [Google Scholar]
- Shin O., Kim S.J., Lee W.I., et al. (2008). Effective transduction by self-complementary adeno-associated viruses of human dendritic cells with no alteration of their natural characteristics. J. Gene Med. 10, 762–769 [DOI] [PubMed] [Google Scholar]
- Song J.H., Byeon S.H., Lee S.C., et al. (2009). Short-term safety and efficacy of a single intravitreal bevacizumab injection for the management of polypoidal choroidal vasculopathy. Ophthalmologica 223, 85–92 [DOI] [PubMed] [Google Scholar]
- Tackenberg M.A., Tucker B.A., Swift J.S., et al. (2009). Muller cell activation, proliferation and migration following laser injury. Mol. Vis. 15, 1886–1896 [PMC free article] [PubMed] [Google Scholar]
- Tuccari G., Trombetta C., Giardinelli M.M., et al. (1986). Distribution of glial fibrillary acidic protein in normal and gliotic human retina. Basic Appl. Histochem. 30, 425–432 [PubMed] [Google Scholar]
- Turksen K., Opas M., and Kalnins V.I. (1989). Cytoskeleton, adhesion, and extracellular matrix of fetal human retinal pigmented epithelial cells in culture. Ophthalmic Res. 21, 56–66 [DOI] [PubMed] [Google Scholar]
- Uyama M., Wada M., Nagai Y., et al. (2002). Polypoidal choroidal vasculopathy: Natural history. Am. J. Ophthalmol. 133, 639–648 [DOI] [PubMed] [Google Scholar]
- Virgili G., and Menchini F. (2005). Laser photocoagulation for choroidal neovascularisation in pathologic myopia. Cochrane Database Syst. Rev. 4, CD004765. [DOI] [PubMed] [Google Scholar]
- Weber M., Rabinowitz J., Provost N., et al. (2003). Recombinant adeno-associated virus serotype 4 mediates unique and exclusive long-term transduction of retinal pigmented epithelium in rat, dog, and nonhuman primate after subretinal delivery. Mol. Ther. 7, 774–781 [DOI] [PubMed] [Google Scholar]
- Yamamoto C., Ogata N., Matsushima M., et al. (1996). Gene expressions of basic fibroblast growth factor and its receptor in healing of rat retina after laser photocoagulation. Jpn. J. Ophthalmol. 40, 480–490 [PubMed] [Google Scholar]
- Yin L., Greenberg K., Hunter J.J., et al. (2011). Intravitreal injection of AAV2 transduces macaque inner retina. Invest. Ophthalmol. Vis. Sci. 52, 2775–2783 [DOI] [PMC free article] [PubMed] [Google Scholar]