Abstract
Since its discovery in 2001, our understanding of fragile X-associated tremor/ataxia syndrome (FXTAS) has undergone a remarkable transformation. Initially characterized rather narrowly as an adult-onset movement disorder, the definition of FXTAS is broadening; moreover, the disorder is now recognized as only one facet of a much broader clinical pleiotropy among children and adults who carry premutation alleles of the FMR1 gene. Furthermore, the intranuclear inclusions of FXTAS, once thought to be a CNS-specific marker of the disorder, are now known to be widely distributed in multiple non-CNS tissues; this observation fundamentally changes our concept of the disease, and may provide the basis for understanding the diverse medical problems associated with the premutation. Recent work on the pathogenic mechanisms underlying FXTAS indicates that the origins of the late-onset neurodegenerative disorder actually lie in early development, raising the likelihood that all forms of clinical involvement among premutation carriers have a common underlying mechanistic basis. There has also been great progress in our understanding of the triggering event(s) in FXTAS pathogenesis, which is now thought to involve sequestration of one or more nuclear proteins involved with microRNA biogenesis. Moreover, there is mounting evidence that mitochondrial dysregulation contributes to the decreased cell function and loss of viability, evident in mice even during the neonatal period. Taken together, these recent findings offer hope for early interventions for FXTAS, well before the onset of overt disease, and for the treatment of other forms of clinical involvement among premutation carriers.
Keywords: fragile X, premutation, RNA toxicity, microRNA, DGCR8, neurodegeneration
The fragile X gene
The fragile X mental retardation 1 (FMR1) gene, located near the end of the long arm of the X chromosome (Xq27.3), is one of a growing number of genes with dynamic mutations that involve expansion of a trinucleotide repeat element [128,160]. For the FMR1 gene, the CGG trinucleotide repeat lies in the 5′ untranslated region (5′UTR) of the gene (Fig. 1). Within the general population, the trinucleotide repeat element ranges from ~5 to 40 CGG repeats, with a mode of 29–30 repeats. Interestingly, this same mode and distribution is observed for rhesus monkeys [4]; however, repeat sizes are generally much smaller in non-primate mammals (e.g., 9–11 CGG repeats for the mouse Fmr1 gene). One possible explanation for the increase in repeat size may be a mediating role of FMRP in the improvement of cognition during primate evolution.
Fig. 1.
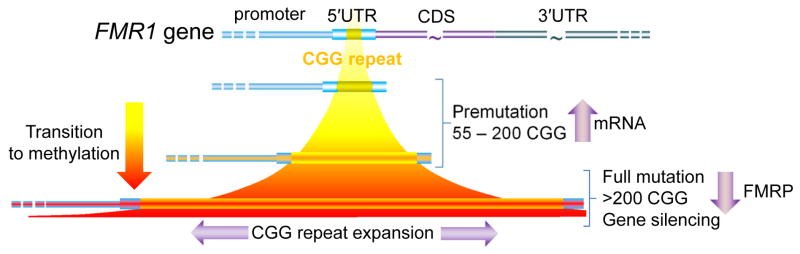
Schematic of the CGG-repeat element within the 5′ untranslated region (5′UTR) of the FMR1 gene. The modal number of CGG repeats in the general population is approximately 30; 45–54 CGG repeats are generally referred to as “gray zone” alleles. Premutation alleles are generally unmethylated (yellow); however, partially methylated alleles are observed near the upper end of the premutation range (indicated by red shading). Full mutation alleles are generally, although not always methylated. CDS, protein coding sequence.
The significance of the FMR1 gene lies in the fact that expansions of the repeat element to more than 200 CGG repeats (full mutation range) give rise to fragile X syndrome, the leading heritable form of intellectual disability, through a process of hypermethylation of the promoter and repeat region, and consequent gene silencing. The resulting absence of the FMR1 protein (FMRP), important for synaptic development and plasticity [103], is the proximal cause of the disorder.
Smaller expansions, from 55 to 200 CGG repeats (premutation range), paradoxically result in much higher levels of gene activity, with mRNA levels in lymphocytes that can exceed the levels found for normal alleles by 5–10-fold [152–154]. Prior to the discovery of the elevated mRNA levels, the importance of the premutation allele was generally thought to be its propensity for expansion to the full mutation range during matrilineal transmission. One remarkable feature of this expansion is its dependence on the size of the maternal allele, and on the number of AGG (interruptions) within the CGG-repeat element [43,45,46,105,124,169]. Indeed, for maternal alleles in the 70–80 CGG-repeat range, two AGG interruptions, spaced approximately 10 repeats apart, are associated with a nearly eight-fold reduction in transmission of a full mutation allele relative to the same size allele with no interruptions (~10%, 2 AGGs versus 80%, no AGGs)[168]. The basis of this dramatic effect is not understood, though it is likely due to altered structural features of the AGG-containing DNA that reduce the propensity of the CGG region to slip during replication and/or repair [122,126,163]. However, the influence of AGG interruptions on the risk of repeat expansion is of critical importance for genetic counseling [168], and demonstrates an intriguing complexity of the allelic instability mechanism.
The puzzle of clinical involvement in premutation carriers
Following the discovery of the FMR1 gene in 1991, the basis for genetic anticipation for fragile X syndrome—termed the “Sherman paradox”—was resolved: expansion of the unstable CGG-repeat element leads to more cases of the syndrome with succeeding generations (Fig. 2). From this perspective, the significance of the “premutation” is that it defines a range of repeat sizes that are increasingly unstable (favoring further expansion) during transmission. For many years, there was no a priori expectation for clinical involvement in the premutation range, since the gene is generally not methylated. However, it is now clear that there are multiple, distinct clinical phenotypes among carriers of premutation alleles (Fig. 2); at least two, fragile X-associated primary ovarian insufficiency (FXPOI) and fragile X-associated tremor/ataxia syndrome (FXTAS), are specific to the premutation range. Because these clinical phenotypes are largely limited to the premutation range (i.e., not present in those who lack FMRP production), they must have a distinct pathogenic mechanism from that of fragile X syndrome. This singular observation, coupled with the elevated expression of the CGG-repeat–containing FMR1 mRNA [101,152,153], led to the proposal that the premutation disorders are the direct result of a gain-of-function toxicity of the FMR1 mRNA [94,96, recent reviews: 66,111,132,151].
Fig. 2.
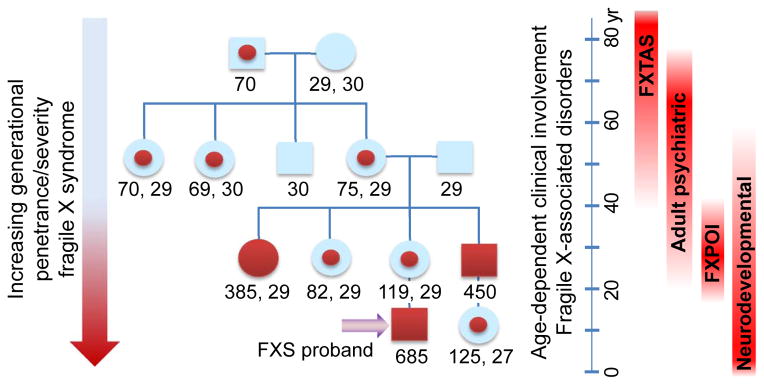
Representative fragile X pedigree displaying intergenerational shift from premutation to full mutation alleles, and consequent increase in disease penetrance and severity (genetic anticipation), due to CGG-repeat instability during transmission. Numbers below family members indicate CGG-repeat size (two alleles for females); red dots, premutation; solid red symbols, full mutation/fragile X syndrome. Premutation alleles are associated with at least four phenotypic domains, with typical age ranges as indicated to the right side of the figure.
Clinical presentations among premutation carriers
There are at least four domains of clinical involvement seen in some, but not all carriers of premutation alleles (Fig. 2): (i) neurodevelopmental problems, including developmental delay, social and behavioral deficits (e.g., ADHD; autism spectrum disorder; intellectual disability (ID)), and childhood seizures; (ii) FXPOI; (iii) increased psychiatric problems among adults; (iv) FXTAS, and associated neurological and medical problems, including hypertension, autonomic dysfunction, migraine headaches, sleep apnea, and immune-mediated disorders [166,6,20,69,70]. Since all of these forms of involvement have been extensively reviewed, their features will only be briefly summarized in the following paragraphs.
Neurodevelopmental phenotypes
There is mounting evidence that some children who are carriers of premutation alleles experience a range of developmental problems; including ID, attention problems or ADHD, autism and autism spectrum disorder (ASD), and increased frequency of seizures in childhood [11,21,27,28,49,57,66,114,131]. In a study of autism and ASD in premutation boys, Farzin et al. [49] found a higher rate of ASD in boys with the premutation presenting as probands compared with sibling (non-premutation) controls (79% versus 0%; p < 0.001); probands also had more ADHD symptoms than controls (93% versus 13%, p < 0.0001). Non-proband premutation carriers, identified through cascade testing, also had elevated levels of ASD and ADHD relative to controls.
As noted, childhood seizures constitute a significant co-morbidity of the premutation, and more broadly, a co-morbidity of ASD. In a survey of 57 premutation males and 199 premutation females, Bailey et al. [11] found that 8% of males (> 6 yr) had been treated for seizures. In their survey, premutation males were also more likely to have presented with developmental delay, and attention and behavioral problems than age-matched controls. More recently, Chonchaiya et al. [27] found that seizures occurred more frequently among premutation carrier probands (28%) than for non-proband carriers (0%), controls (0%), or population estimates (1%).
The developmental presentations raise a very important question regarding mechanism: is the developmental involvement due to an earlier manifestation of the same RNA-toxicity mechanism that leads to FXTAS, and likely to FXPOI; or is it caused by incipient FMRP deficiency (fragile X-spectrum disorder); or a combination of the two mechanisms? In a small cohort (n=10) of boys with either premutation or intermediate CGG-triplet expansions, Aziz et al. [8] observed a striking resemblance in clinical presentation to boys with fragile X syndrome. Using a semi-qualitative measure of protein (hair root analysis), the authors observed that FMRP expression was essentially normal in all participants where it was assessed. By contrast, Goodlin-Jones et al. [57] and Tassone et al. [155] found lowered FMRP levels in premutation carriers with developmental delays. More recent measurements with animal models of the premutation have provided evidence for lowered FMRP with higher CGG-repeat number in the premutation range [22, review: 14], raising the possibility of FMRP insufficiency as an added contribution to developmental involvement in some premutation carriers.
Fragile X-associated premature ovarian insufficiency (FXPOI)
Just prior to the discovery of the FMR1 gene, Cronister et al. [35] had observed that there is an approximately 20-fold increase in the incidence of premature ovarian failure (infertility prior to age 40) in heterozygous carriers (~20% versus 1% in the general population). Now known as FXPOI [reviewed in: 148], the reproductive condition is recognized as the leading heritable form of early ovarian insufficiency/infertility. The association of FXPOI with premutation CGG-repeat expansions raises the prospect that this condition is also (along with FXTAS) due to an RNA gain-of-function toxicity [32,82,167]. Studying the mechanism of FXPOI has proven to be more difficult than for FXTAS, due to the relative paucity of suitable human samples or appropriate animal models. However, Hoffman et al. [82] have utilized a knock-in (KI; premutation CGG repeat) mouse model that displays features of FXTAS, suggesting that it should be useful, from a mechanistic standpoint, for studying FXPOI as well. Using the KI model, the authors investigated the basis for altered hormone levels that are suggestive of a reduced residual follicle pool in the premutation carriers. Hoffman et al. [82] demonstrated that the development and establishment of the primordial follicle pool is grossly normal, but that there is an accelerated loss of follicles of all follicle classes, suggesting that the problem is intrinsic to the ovary. In addition, there are abnormalities of the follicles, including reduced size and number of granulosa cells. These observations provide an important foundation for further investigation of the pathogenic mechanism of FXPOI, and the determination of the extent to which there is mechanistic overlap with FXTAS.
Adult-onset psychiatric problems
Psychiatric involvement in adult premutation carriers, particularly depression and anxiety [144,53,52], was initially thought to be related to the stress of raising an affected (FXS) child. This involvement is now understood to be an intrinsic feature associated with premutation carriers (~40%)[133,19,20,79,80,134,142], although such problems can be exacerbated by the difficulty of raising an affected child or dealing with a parent with FXTAS [141,10]. Psychiatric problems generally intensify for those individuals who develop features of FXTAS [9]. Younger adult male carriers also manifest lowered activity of the amygdala on functional MRI (fMRI) compared to controls [78]; this lowered activity has also been shown to correlate with mild deficits in FMRP [80].
Fragile X-associated tremor/ataxia syndrome (FXTAS)
The neurodegenerative phenotype in older adult carriers of premutation alleles comprises core features of intention tremor and gait ataxia [67,94]; as well as associated white matter changes and global brain atrophy (Fig. 3); intranuclear neuronal and astrocytic inclusions; parkinsonism; peripheral neuropathy; and cognitive decline [1,23,30,59,60,67,97,116,146,2,63,114, reviews: 15,3,25,109,110].
Fig. 3.

MRI images of white matter and structural abnormalities associated with FXTAS [1,3,23,30,162]. For each pair of images, a normal control (left) and FXTAS case (right) are represented. The white arrows indicate increased signal intensity on T2 turbo spin-echo sequences in the middle cerebellar peduncle (MCP) (a), sub-insular white matter (c), and cerebral white matter (d). Thinning and increased signal of the trunk and splenium of the corpus callosum (b; arrows) are from the T2 FLAIR sequence. Cases depicted in this figure are males, as follows: (a) 70 yr, FXTAS stage 3, 96 CGG repeats; (b) same as a; (c) 65 yr, stage 3, 91 CGG repeats; (d) 78 yr, stage 4, 116 CGG repeats. The control used for all images and comparisons was a 68-year-old man with 32 CGG repeats. Images kindly provided by Patrick Adams.
Neuropathology
Based on studies of post-mortem CNS tissue from nineteen cases (18 cases with FXTAS, 1 premutation carrier without FXTAS), three general features of the neuropathology of FXTAS have emerged [59,60,150]: (i) significant cerebellar and cerebral white matter disease, consistent with MRI findings (Fig. 3); (ii) associated astrocyte pathology, particularly evident in sub-cortical cerebral white matter; (iii) the presence of solitary, generally spherical intranuclear inclusions, detected with either H&E or ubiquitin immunohistochemistry (IHC), present in both neurons and astrocytes (but not oligodendroglia), and in broad distribution throughout the brain and brainstem. Further details of the neuropathology are summarized in Table 1; however, several observations are noteworthy [59,60,150]. First, although both males and females with FXTAS possess inclusions, there tend to be far fewer inclusions in females, presumably due to the effects of the normal X chromosome; we also note that one female carrier without clinical evidence of FXTAS nevertheless possesses small numbers of inclusions in various brain regions. Second, the inclusions are exclusively located in the nuclei of cells; that is, no cytoplasmic inclusions have ever been observed in any brain tissue from a human case of FXTAS; this property of FXTAS inclusions is in stark contrast to the substantial numbers of cytoplasmic inclusions in the fly model of the premutation [96]. Third, although white matter changes in the MCP are observed in only ~60% of cases, there is histopathological evidence of disease of the MCP in nearly all cases (7/8) where there is available tissue. Fourth, there is a highly significant association between the number of inclusions in both neurons and astrocytes, and the number of CGG repeats within the premutation range. Finally, although MCP disease and Purkinje cell (PC) dropout are prominent features of the neuropathology of FXTAS, there is a paucity of inclusions in pontine nuclei; however, there is substantial pontine volume loss and disease, and despite substantial PC dropout, inclusions are only rarely observed in PCs. These last observations suggest that the presence of inclusions is not necessary for cellular dysfunction.
Table 1.
Summary of CNS pathology in FXTAS(a)
| Inclusions | CNS pathology |
|---|---|
Physical characteristics
|
Gray and white matter pathology
|
Location within cell types/CNS
| |
Composition(c)
|
Contemporaneous with the discovery of the clinical disorder was the observation of elevated, CGG-repeat-containing FMR1 mRNA [101,152,153], indicating that FXTAS is due to a very different molecular mechanism than the protein-deficiency mechanism responsible for FXS. The remainder of this review will address this mechanism and recent findings regarding FXTAS pathology that have revised our thinking on a disorder thought to be limited to the CNS.
Current understanding of the pathogenesis of FXTAS
The discovery of FXTAS [67], and the mounting evidence supporting a role for the CGG-repeat-containing mRNA in triggering the disorder, has raised a number of questions related more broadly to the family of premutation-associated disorders. First, the role of the RNA may well be restricted to the initial triggering events in FXTAS pathogenesis, thus leaving open the question of what happens next; that is, what downstream processes are actually involved with cellular dysregulation and loss of neuronal-cell viability? Second, although FXTAS has been the principal focus of research on pathogenic mechanisms in the premutation range; it is likely that most, and perhaps all of the additional forms of clinical involvement in the premutation range will involve the same RNA-toxicity mechanism, at least initially. In particular, FXPOI displays the same apparent restriction to the premutation range, suggesting that FMRP insufficiency is not a primary driver of the loss of ovarian function. Third, although little is known regarding the basis for partial penetrance of FXTAS in the aging premutation population, genetic background effects are doubtless influencing the appearance and severity of the disorder. In this regard, it is interesting that risk factors for related neurodegenerative disorders may also influence risk for FXTAS (e.g., APOE4 allelotype, [143]) or related adult clinical involvement (e.g., corticotrophin releasing hormone receptor 1 polymorphisms, [91]). Fourth, one puzzling feature of clinical involvement in FXTAS is the greater frequency of associated medical findings, such as hypothyroidism and fibromyalgia [134,29], in women with FXTAS relative to males of similar age and stage of neurological involvement.
Finally, whereas the initial focus of FXTAS has been in the aging adult population, recent animal studies indicate that many features of the neuronal and astrocytic cellular pathology are occurring in the neonatal period [24,26,36]. These findings suggest that FXTAS may simply be one end-stage outcome of a cellular dysregulation that may be life-long, and that the developmental problems experienced by some children who are premutation carriers may be manifestations of this early, non-degenerative process. All of these considerations will be important for developing treatment approaches for the premutation-associated disorders, including FXTAS, and raise the possibility that the sub-clinical features of cellular dysregulation may be remediable long before any neurodegenerative features develop.
Initial events in the pathogenesis of FXTAS and other premutation-associated disorders
The neurodegenerative disorder, FXTAS, principally affects carriers of premutation alleles of the FMR1 gene; the pathogenic mechanism, gain-of-function “toxicity” of the elevated levels of CGG-repeat-containing mRNA, is completely distinct from the gene-silencing mechanism (FMRP insufficiency) leading to fragile X syndrome [recent reviews: 151,132,111,66]. As currently envisioned, toxicity is thought to arise through partial sequestration of one or more RNA-binding proteins by the expanded CGG repeat, thus leading to a functional insufficiency of the sequestered proteins (Fig. 4). The paradigm for such a process is myotonic dystrophy, wherein the mRNA produced by the myotonic dystrophy protein kinase (DMPK) gene harbors a CUG repeat in its 3′UTR region, which, when substantially expanded, gives rise to many of the features of myotonic dystrophy (DM) [44,120,158]. Notably, no mutations causing DM have been reported within the DMPK coding region, arguing against a direct role of the protein in the pathogenesis of DM; nor have coding mutations in the FMR1 gene been linked to FXTAS, though such mutations do lead to fragile X syndrome [31,38,64,119]. Furthermore, an intronic CCTG-repeat expansion in an unrelated gene, ZNF9, leads to a highly similar form of myotonic dystrophy (DM2) [112,130].
Fig. 4.
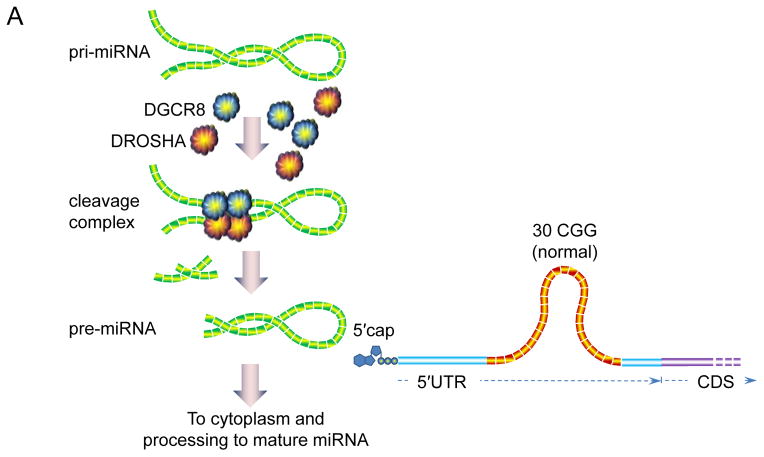
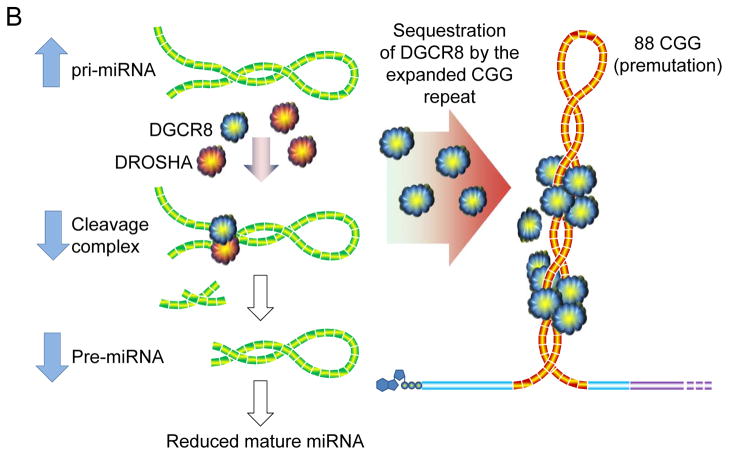
Schematic of the role of DGCR8 in the pathogenesis of FXTAS [139]. (a) DGCR8 binds to pri-miRNA in the nucleus, recruiting DROSHA (and other proteins) to form the “microprocessor” complex; binding of DGCR8 is cooperative and targets a specific (imperfect) helix region of the pri-miRNAs. Cleavage yields precursor miRNAs (pre-miRNAs), which exit the nucleus for further processing into mature miRNAs. Normal FMR1 alleles do not interact appreciably with DGCR8. (b) Expanded CGG-repeat RNA forms stable helical stems, which recruit (sequester) DGCR8, thus preventing normal levels of pri-miRNA processing. Consequently, nuclear pri-miRNA levels are increased and mature, cytoplasmic miRNAs are decreased. The 88 CGG-repeat allele depicted in the figure is near the modal value for individuals with FXTAS.
For the CUG-repeat element in the DMPK gene, expression in an unrelated reporter gene was capable of recapitulating a number of key phenotypic domains of DM, including defects in a muscle chloride channel and the formation of CUG-RNA–containing nuclear “foci.” Thus, the expanded CUG-containing mRNA itself appeared to be responsible for the phenotype; the presence of the foci suggested that the CUG-repeat element was interacting with one or more proteins, such that the abnormal interaction would produce both clinical and histopathological features of DM.
The preponderance of evidence suggests that the triggering event in FXTAS pathogenesis involves the sequestration of one or more proteins by the expanded CGG repeat in the FMR1 mRNA 5′UTR [recent reviews: 132,111,65]. Candidates for the sequestered proteins include hnRNP A2/B1 [93,145], one of the most abundant members of the family of heterogeneous nuclear ribonucleoproteins, and thought to play a role in binding to and translocating the myelin basic protein mRNA [98]; Purα [95,7], a transcriptional activator also thought to be involved in the control DNA replication; Sam68 [140], an RNA-binding protein that belongs to the “signal transduction and activation of RNA” (STAR) family [16]; and DGCR8, part of the “microprocessor” complex that processes micro (mi)RNA precursors in the nucleus [42,139].
For all of the above-mentioned proteins, there is evidence for in vitro association with CGG-repeat-containing RNA. Although both hnRNP A2/B1 and Purα appear to bind preferentially, at least in vitro, to shorter (~20 CGG) repeat RNAs [139], both proteins do appear to have mediating effects on Drosophila models of CGG-repeat–induced neurodegeneration [95,145]. Notwithstanding their role(s) in mitigating the phenotypes in animal models, roles for Purα and hnRNP A2/B1 have yet to be demonstrated in human disease or in murine models of FXTAS. For Sam68, there is clear evidence of insufficiency in animals and humans; with altered splicing noted in FXTAS patients [140]. However, a critical test of these candidate proteins will be whether they have any direct primary or secondary role in FXTAS pathogenesis in humans.
DGCR8 sequestration and dysregulation of microRNA biogenesis
As an extension of their initial study [140] of CGG-binding proteins identified through in vitro association with CGG-repeat RNA, Sellier and co-workers identified DGCR8 (DiGeorge syndrome critical region 8; [48,127,147]) as another protein that binds with relatively high specificity to CGG-repeat RNA [139]. Among the more than 30 RNA-binding proteins derived from mouse brain nuclei that were identified using their binding protocol, including the aforementioned hnRNP A2/B1, Purα, and Sam68 [7,93,95,140,145], 10 proteins were found to bind preferentially to premutation CGG repeats (60 and 100 CGG repeats). Of these 10, only three colocalized within the nuclear RNA aggregates that were associated with premutation alleles, and only one, DGCR8, was specific for such aggregates. Interestingly, both hnRNP A2/B1 and Purα were preferentially recruited to the short (20 CGG-repeat) RNAs, and neither protein demonstrated any significant colocalization with the CGG RNA aggregates. For Purα, absence of colocalization in the RNA aggregates is consistent with its absence from the nuclear inclusions in mouse models of FXTAS [54].
The potential significance of DGCR8 lies in its central role in micro(mi)RNA biogenesis [40,51,62,71,107,108,161]. DGCR8 acts as an anchor for the type III RNase, DROSHA, which cleaves primary miRNAs (pri-miRNAs) in the nucleus to produce precursor miRNAs (pre-miRNAs)[40,51,62,71,107,108,161]; the pre-miRNAs are subsequently exported from the nucleus to the cytoplasm, where they are converted into mature miRNAs by DICER. The DGCR8-DROSHA complex is referred to as the “microprocessor,” and is a key player in the production of the vast array of miRNAs in the cell. Insufficiency of the microprocessor function, as would be expected if DGCR8 were partially sequestered, would be expected to dampen production of mature miRNAs, and in the current context would lead to neuronal dysfunction and cell loss [37,39,50,72,77,83,136,138,147].
In their study of the DGCR8-CGG-repeat interaction, Sellier et al. [139] made several key observations that support a central role of sequestration of DGCR8 in the pathogenesis of FXTAS; specifically, they demonstrated the following:
DGCR8 preferentially binds to the expanded CGG-repeat RNAs, consistent with a CGG-repeat threshold for RNA toxicity, and for clinical involvement, at the lower end of the premutation range [81,149].
miRNA levels are globally depressed both in cultured cells expressing the expanded-repeat RNA and in human brain tissue from FXTAS patients. Moreover, consistent with reduced function of the DGCR8-DROSHA microprocessor, levels of the corresponding pri-miRNAs were elevated in the human brain samples.
Overexpression of DGCR8 reversed both the decreased dendritic complexity and the lowered neuronal cell viability [e.g., 26] resulting from expression of expanded CGG repeats.
Expression of DROSHA does not reverse the RNA toxicity, suggesting that DGCR8, but not DROSHA, is limiting microprocessor function in the presence of the expanded CGG-repeat RNA.
Taken together, the results of Sellier et al. [139] strongly implicate DGCR8 sequestration as a central, if not sole mode of transduction of CGG-repeat “toxicity” (Fig. 4). However, several things should be considered regarding mechanism. First, whereas DGCR8 undoubtedly plays an important role in mediating RNA toxicity, additional proteins, including the above-mentioned hnRNP A2/B1, Purα, and Sam68, may still play ancillary roles in the initiation of FXTAS pathogenesis. Although, as noted above, Sam68 sequestration does lead to splicing alterations in humans—and hence may play some role in the development and/or the domains of clinical involvement in FXTAS—its overexpression is not capable of reversing the abnormal cell phenotype [139]; thus, Sam68 sequestration is not sufficient for neuronal pathogenesis.
Possible alternative mechanisms for the initiation of FXTAS pathogenesis
The foregoing section has described a model for the initial events in FXTAS pathogenesis whereby the toxicity of the expanded CGG-repeat RNA is due to its partial sequestration of one or more RNA-binding proteins. In the case of DGCR8 sequestration, the consequent depression of miRNA production likely has multiple, as-yet-unspecified downstream effects leading to cellular dysregulation, though the haploinsufficiency of DGCR8 in 22q deletion syndrome [147] may provide a useful foundation for understanding the consequences of depressed miRNA production. However, despite the preponderance of evidence supporting such a sequestration model, several additional processes have been proposed as candidates for an initial event in the disease process.
RNA-mediated protein aggregation
Renoux and Todd [132] have suggested that the CGG-repeat–containing RNA could initiate a conformational transition in one or more proteins that possess prion-like domains [104,132], triggering a cascade of aggregation reminiscent of amyloid plaque formation in Alzheimer’s disease [104,132]. Indeed, King et al. [104] observed that hnRNP A2/B1, which is found in the intranuclear inclusions of FXTAS [60,59,93,145], ranks among RNA-recognition–motif proteins as highly “prionogenic.” Such a model, if demonstrated to participate in FXTAS pathogenesis, would address one potential problem with a sequestration model involving hnRNP A2/B1; namely, its very high abundance in cells, which would seem to rule out its participation in a sequestration process, but would actually favor a prion-like nucleation process. One lingering concern with the prion-domain model is that nearly all of the proteins with prion domains listed by King et al. [104] tend to form cytoplasmic inclusions; whereas the inclusions in FXTAS are exclusively nuclear in humans [60,59] and the premutation mouse [14,159,164]. A second concern with the aggregation model, at least with respect to the participation of hnRNP A2/B1, is the observation of Sofola et al. [145] that overexpression of hnRNP A2/B1 actually reduces the neurodegenerative phenotype.
Antisense-RNA effects on expression
One interesting, albeit unresolved issue regarding FXTAS pathogenesis is what role, if any, is played by the antisense transcripts generated at the FMR1 locus [106,102,116]. Although the initial reports by Ladd et al. [106] and Khalil et al. [102] differed in some details regarding the exact nature of the antisense transcripts, the two studies were remarkably consistent in their general observations regarding the transcripts. The RNA species are produced by RNA pol II, are spliced and polyadenylated, are exported from the nucleus, and may code for one or more small peptides. Moreover, levels of the antisense transcripts generally track with relative production of the sense FMR1 transcript—elevated in the premutation range and absent in hypermethylated, full mutation alleles. Thus, the CGG-repeat element appears to regulate the expression of both sense and antisense transcripts, a remarkable finding in light of the fact that the more 5′ of the two antisense promoters (upstream in the antisense direction) lies more than 10 kb upstream (in the second intron of the FMR1 RNA) of the CGG-repeat element.
Two observations raise the possibility of a functional role for the antisense RNA in FXTAS pathogenesis. First, Ladd et al. [106] observed that a specific splice isoform of the antisense transcript is only found with transcripts from premutation alleles. Determination of what role, if any, this isoform plays in FXTAS pathogenesis will necessarily require additional study. However, the altered isoform distribution could reflect a dysregulation of splicing either by Sam68 [140], or by the splice modulator, MBNL1, which is sequestered in DM; both Sam68 and MBNL1 are found in the intranuclear inclusions of FXTAS [93,140]. Second, Khalil et al. [102] observed that the presence of the antisense transcript markedly affected cell proliferation in culture; siRNA-mediated knockdown of the transcript altered both cell-cycle progression and increased apoptosis, whereas overexpression of the antisense RNA resulted in cell proliferation. Taken together, these intriguing results point to some functional importance of the antisense transcript; however, its precise role remains to be determined.
Role of reduced FMRP levels
Although the moderately reduced FMRP levels observed for large premutation alleles (> 120–150 CGG repeats) in both humans and in animal models cannot be playing a primary role in FXTAS pathogenesis, a functional FMRP insufficiency in some carriers may contribute to aspects of the premutation phenotypes, particularly with respect to domains of reduced cognition and abnormal behavior observed in both adults and children with premutation alleles [155,57,80]. Moreover, there could well be focal deficits of FMRP in the CNS that would not be properly gauged by the study of peripheral tissues (e.g., [47,129]). More recently, Iliff et al. [92] demonstrated impaired activity-dependent FMRP translation and enhanced mGluR-dependent long term depression in premutation mice, suggesting the possibility of a mixed phenotype, particularly in the upper portion of the premutation range. In this regard, in an elegant study in which an expanded CGG repeat was expressed ectopically in mouse cerebellar Purkinje cells, Hashem et al. [73] demonstrated that expression of the CGG-repeat RNA is both necessary and sufficient to produce the intranuclear inclusions of FXTAS and the associated neuronal cell death, where FMRP levels remain at wildtype levels.
RAN-mediated expression of alternative peptides
Todd, Oh et al. [157] have recently proposed an alternative model for FXTAS pathogenesis in which “toxic” peptides are produced through a process of repeat-associated, non-AUG–initiated (RAN) translation [125,170]. The authors provide evidence that translation initiation upstream of the CGG-repeat element results in the production of truncated peptides that possess a polyglycine (polyGly) stretch (GGC-codon repeat in the +1 frame), and that such peptides are toxic to the cell. The polyGly peptides are shown to accumulate in the intranuclear inclusions in both fly models and in human FXTAS cases. RAN translation has also been observed in association with the G4C2 hexanucleotide repeat expansion (C9orf72 locus) that causes amyotrophic lateral sclerosis and frontotemporal dementia [5]. The importance of this intriguing mechanism to FXTAS neurodegeneration remains to be determined. One puzzling aspect of the RAN translation model is that the “NIH” mouse [47](Table 2), which does not support RAN translation [157], nevertheless displays substantial Purkinje cell dropout, similar to FXTAS in humans; whereas the “Dutch” mouse model, which does support RAN translation, does not exhibit Purkinje cell loss. Clearly, additional studies are warranted, and it may turn out that both RAN translation and RNA toxicity play roles in specific features of the FXTAS phenotype.
Table 2.
Comparison of the neuropathology for two principal premutation knock-in mouse lines used to model FXTAS
| Feature | NIH mouse(a) | Dutch mouse(a) |
|---|---|---|
| Mouse strain(s) | C57/BL6 | C57/BL6J |
| Constructs(b) | Expanded CGG-repeat elements inserted into mouse 5′UTR; NIH construct retains more of the mouse sequence proximal to the CGG repeat |
|
| Inclusion formation | Inclusions present; morphologically and immunochemically similar to those in the Dutch mouse, although in much lower numbers | Inclusion numbers comparable to or exceeding those found in humans with FXTAS; broad distribution |
| Fmr1 mRNA and FMRP | Both mouse lines have comparable mRNA elevations; however, FMRP levels appear to be lower in the NIH mouse – as low as 15% of WT in some brain regions | |
| Cerebellar pathology | Evidence of Purkinje cell pathology: abnormal calbindin staining, swollen axons and torpedoes, Purkinje cell dropout | No evidence of Purkinje cell dropout; no Bergmann gliosis, though inclusions are in Bergmann glia |
| Prenatal neocortical development | Not examined | Embryonic premutation mice display migration defects in the neocortex and altered expression of neuronal lineage markers |
| Dendrite and spine morphology | Reduced complexity of dendritic branching patterns and increased spine density | Reduced complexity of dendritic branching patterns but no changes in spine density |
| Anxiety | Lower anxiety (Open field) | Increased anxiety (Plus maze) |
Designations follow Todd et al. [157]. (NIH mouse: [47,82,129]; Dutch mouse: [7,13,17,22,36,137,164,165]; general review: [84])
Human reference, GenBank: L19476.1; NIH and Dutch constructs following summary in Todd et al. [157]. For the Dutch mouse, upper case letters indicates identity with the human sequence (30 nt 5′, and 12 nt 3′ of the CGG repeat); lower case, mouse sequence. Note that the corresponding sequence in Todd et al. [157] contains an extra C adjacent to the CGG repeat (removed: underscore, gray highlight). Red italics indicate a Xho I site. For the NIH mouse, lower case represents mouse sequence; additional, inserted non-mouse sequence is underlined; double underline indicates SfiI sites. Again, an extra C adjacent to the CGG repeat (underscore, gray highlight) in Todd et al. (2013) has been removed. The gray-highlighted “GG” dinucleotide in the NIH sequence is subject to some uncertainty (GG vs CC).
The TAA stop codon (red) prevents upstream translation in the +1 codon frame
Cellular dysregulation
Although there is now compelling support for an RNA-toxicity/sequestration model for the initial triggering step in FXTAS pathogenesis, beyond the general notion that miRNA dysregulation (e.g., DGCR8 sequestration) would disrupt numerous additional cell functions, the RNA-toxicity model does not provide a specific explanation for why neuronal cells sicken and ultimately die. For such an explanation, one would need either to identify specific miRNAs that, when up-regulated, reverse the loss of neuronal viability; or to directly identify the more critical modes of dysregulation. Although the loss of the normal nuclear lamin architecture has been described in cell culture [4], the consequences of this altered architecture have not been further identified. Two additional, more promising modes of cellular dysregulation have now been identified, namely, altered mitochondrial function [123,135,99], and altered neuronal morphology and electrical activity [24].
Mitochondrial dysfunction
Premutation-associated mitochondrial dysfunction has now been demonstrated in both human (fibroblast) and mouse (hippocampal neurons) cultured cells, and in the post-mortem CNS tissues from individuals who have died with FXTAS [99,123,135]. In addition to a lowered oxidative phosphorylation capacity, there is evidence for a deficit in mitochondrial import of nuclear-encoded mitochondrial proteins, and this latter deficit appears to be due to reduced loading of zinc into the metalloproteases that process proteins for import [123]. General mitochondrial dysfunction could explain the higher level of oxidative stress observed in CNS tissue [135]. Taken together, these observations would provide one explanation for the reduced neuronal viability, since neuronal cells are exquisitely sensitive to mitochondrial dysfunction due to their high metabolic demands. Using hippocampal neurons obtained from neonatal premutation KI mice, Kaplan et al. [99] were able to demonstrate that the premutation neurons possessed fewer mitochondria and that the mobility of these mitochondria was reduced. This form of dysfunction would be expected to have a strong negative impact on the growth of dendritic arbors as well as of synaptic function, consistent with the earlier observations of Chen et al. [26] of reduced dendritic growth for premutation (mouse) neurons.
Dysregulation of neuronal activity
Another form of dysregulation observed in the neonatal mice (cultured hippocampal neurons) is altered neuronal electrical activity, characterized by burst-type network firing behavior and altered Ca2+ regulation [26,24]; the latter is also observed in iPSC fibroblast-derived neurons from human premutation carriers [113]. The burst-type patterns of firing across networks of cultured mouse premutation neurons, likely related to the increased seizure activity in children who are premutation carriers, is completely reversed with either mGluR5 inhibitors, or the GABAA receptor positive modulator, allopregnanolone [24]. These observations are encouraging from the perspective of potential therapeutic intervention, since many of the drugs now in trials for fragile X syndrome may also prove efficacious for the premutation disorders.
Mouse models reveal early developmental phenotypes for premutation Fmr1 alleles
One of the major unresolved questions regarding the diverse phenotypes among premutation carriers is whether there is a common underlying molecular mechanism. Specifically, does the RNA toxicity that appears to trigger the late-adult-onset neurodegenerative disorder, FXTAS, also initiate the early developmental, decidedly non-degenerative, domains of involvement (e.g., autism, seizures) and the ovarian dysfunction in carrier females? The most revealing observations thus far have come from mouse models in which the native CGG-repeat element (~9–11 repeats) has been replaced by a much larger repeat in the premutation range [17,165](Table 2). Two recent studies of premutation mice suggest that primary ovarian dysfunction could, indeed, be the result of an RNA-toxicity mechanism [82,118]; results that would be consistent with the infertility phenotype in women with expanded CGG-repeat alleles being restricted to the premutation range.
With regard to the neurodevelopmental phenotypes, the mouse models have been transformative, having provided substantial evidence that expression of the premutation CGG-repeat RNA leads to early molecular and behavioral phenotypes. Much of the work at the molecular level has involved studies of cultured neuronal and astrocytic cells derived from neonatal mice, where defects in both neuronal morphology (reduced dendritic growth; altered mitochondrial number and distribution) and electrical activity have been noted [24,26,99,129]. Moreover, Cunningham et al. [36] have demonstrated in vivo in mouse embryos that premutation alleles lead to impaired neocortical development, as reflected by both delayed neuronal maturation and altered neuronal migration. Thus, from the molecular perspective, the premutation influences CNS development and function at a very early stage, and could therefore be causal in the developmental involvement, including the propensity for seizures, experienced by children who carry a premutation allele.
Young premutation (KI) mice have also demonstrated problems both in motor control/coordination and in various cognitive functions. The mice display early (mild) deficits in both a ladder rung task designed to detect gait/coordination problems [89], and in a forelimb reaching task designed to probe forelimb coordination [41]. Perhaps the most exciting developments in the mouse studies have come in the study of specific domains of cognition that were designed to mirror deficits observed in humans. In a series of elegant studies, the premutation mice (mainly female heterozygotes) were shown to have deficits in spatiotemporal processing in which distinct domains became manifest at different times, and in a manner that also depended on the size of the CGG repeat [18,41,85,90,88]. These results are particularly significant in light of parallel studies in young women with the premutation who demonstrated both attention problems and deficits in magnitude estimation tasks [58]. Boys and young-adult men with the premutation had earlier been shown to have deficits in executive function, working memory, and attention tasks [34,33], which is similar to the findings of Moore et al. [121] in older adult carriers without FXTAS.
Developments in the pathology of FXTAS
Beyond the CNS
FXTAS had until recently been considered fundamentally to be a CNS disorder; with principal neuropathologic findings of intranuclear neuronal and astrocytic inclusions [18,41,59,60,85,90], as well as white matter disease and loss of brain volume [1,23,30,60,94,100,74–76,162] that point to subtle alterations in brain structure and white matter that may precede overt symptoms of the disorder [12,115]. However, an early indication that the pathology of FXTAS may extend beyond the CNS were the observations that intranuclear inclusions were present in both the anterior and posterior pituitary [61,117], and in testicular Leydig and myoid cells in two men who had died with FXTAS [61]. Gokden et al. [56] later reported the presence of intranuclear inclusions in broad distribution in the peripheral nervous system, including ganglion cells of the adrenal medulla, myenteric ganglia of the stomach, and subepicardial autonomic ganglion of the heart.
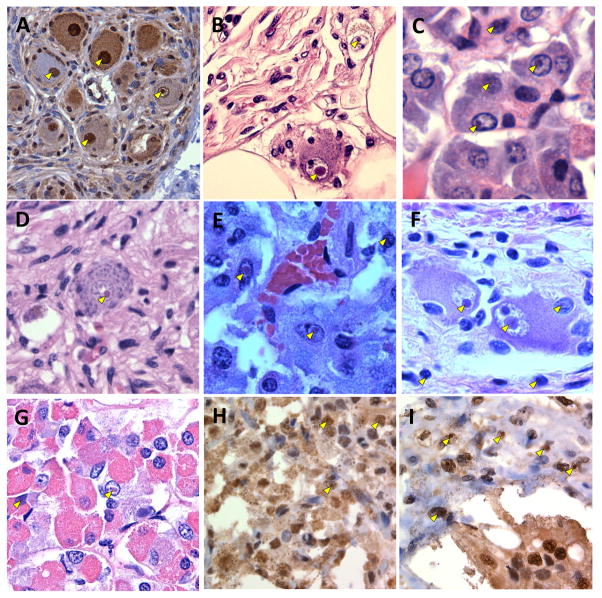
More recently, in a comprehensive analysis of the distribution of non-CNS inclusions, Hunsaker et al. [86] reported inclusions in many peripheral locations, including in numerous neuroendocrine and somatic organs, in premutation carriers who had died with FXTAS (Fig. 5). Importantly, they demonstrated a very similar distribution in the premutation KI mouse, indicating that the animal model may prove useful for the study of non-CNS aspects of the premutation disorders. Recognition of this broad, non-CNS distribution of inclusions is of importance for at least two reasons. First, the formation of morphologically similar inclusions, in an exclusively nuclear distribution and in a wide range of cell types, argues that the molecular mechanisms for inclusion formation are present in many types of non-neural cells. This is useful both for molecular studies in non-CNS tissues such as fibroblasts [4,55,123,135], and for the use of more convenient cell types to study the effects of various candidate therapeutic agents. Second, the distribution of inclusions may reflect non-CNS co-morbid features associated with, or preceding the onset of, FXTAS. Thus, the hypothyroidism in many women with FXTAS [29] could reflect a direct disease process within the thyroid. Similar arguments could also be made for peripheral neuropathy [114]; cardiac arrhythmias [67,94]; hypertension [29,69]; and various forms of autonomic dysfunction, including erectile dysfunction [61], sleep apnea [70], migraine headaches [6], and immune mediated disorders [166].
Fig. 5.
Representative examples of intranuclear inclusions in post-mortem, non-CNS tissues/organs from FXTAS patients; for more details of the range of non-CNS tissues bearing inclusions in both humans and premutation KI mice, see text and Table 2 of Hunsaker et al. [86]. For each image, arrowheads point to inclusions. (a) Cardiomyocytes (ubiquitin; 400x); (b) cardiac autonomic ganglion cell (H&E, 600x); (c) pancreas (ubiquitin, 1000x); (d) intestinal wall (H&E, 400x); (e) adrenal medulla (H&E, 600x); (f) myenteric plexus, stomach (H&E, 400x); (g) anterior pituitary (H&E, 400x); (h) testicular Leydig cells (ubiquitin, 400x); (i) renal distal tubule (ubiquitin, 400x). Images kindly provided by Claudia Greco.
Beyond the premutation
One of the long-standing predictions of the RNA-toxicity model is that, strictly speaking, this pathogenic mechanism should not be limited to the premutation, but only to expanded alleles where there is significant, continued FMR1 mRNA production. This prediction raises the possibility that those individuals who are mosaic for unmethylated, full mutation alleles [152–154] may retain an increased risk of developing features of FXTAS. Recently, intranuclear inclusions have been identified post-mortem in three full mutation males [87]. Although the inclusions were present at very low levels, as were mRNA levels, and none of the three cases displayed overt features of FXTAS; these cases demonstrate the possibility that some individuals may present with FXTAS even though they have alleles that lie within the full mutation range. Moreover, Loesch et al. [116] have described a male with an unmethylated, full-mutation allele, who presented with neurological (significant gait ataxia, intention tremor and parkinsonism), cognitive, and radiological (periventricular and middle cerebellar peduncle white matter disease) features supporting a diagnosis of definite FXTAS (diagnostic criteria: [94]). Several cases of FXTAS have also been reported for individuals with gray-zone alleles (45 to 54 CGG repeats), particularly in association with elevated FMR1 mRNA levels [68,113]. Therefore, the range of CGG-repeat lengths giving rise to FXTAS must extend beyond the premutation range, albeit still in accord with the RNA-toxicity mechanism.
Conclusions
Our view of FXTAS has evolved rapidly over the past several years, as the data from animal models, large clinical cohorts, and more sophisticated imaging methods help to form a more detailed picture of the nature of the disorder. Although it is becoming increasingly evident that the initial triggering event for FXTAS involves the expanded CGG-repeat mRNA, what has been more surprising, from the animal models, is how early those events lead to dysregulation during development. This emerging picture of a unifying pathogenic mechanism—for neurodevelopment, for loss of fertility, and for the eventual neurodegenerative process leading to FXTAS—will radically change our thinking as to when, and by what means, to treat all of the premutation-associated disorders. Such a unifying concept is quite encouraging, since it suggests that therapeutic intervention for FXTAS could occur long before any symptoms emerge.
Despite the encouraging picture revealed through mechanistic unification, there is a lingering difficulty with our understanding of FXTAS and the other premutation-associated disorders; that is, we cannot predict who will develop any of the particular phenotypes. In the mouse models, nearly every animal displays abnormal mitochondrial function, and altered morphological and electrical properties of neurons; however, only a minority of children will develop behavioral or cognitive impairment, only ~20% of women will suffer FXPOI, and only about half of older males will experience the neurodegenerative phenotype. What we really need to understand is why some individuals develop these premutation disorders, whereas others with identical CGG repeats do not. It is likely, although not established, that part of the answer is to be found in genetic background effects, and with differences in intrinsic susceptibility. However, there are likely to be additional determinants, such as environmental effects (smoking, exposures to environmental toxins, etc.) that will help to determine who will or will not experience these disorders. Therefore, part of the task in understanding the pathogenesis of FXTAS is to identify those factors that determine penetrance; such knowledge will be critical for ultimate success in treatment.
Acknowledgments
This work was supported by a National Institutes of Health grant, R01 HD040661. The author wishes to thank Dr. Claudia Greco for comments/suggestions pertaining to Table 1; Drs. Renate Hukema, Karen Usdin, and Peter Todd for input on sequences for the Dutch and NIH mice (Table 2); for Drs. Ryan Hunsaker and Robert Berman for their comments/corrections pertaining to Table 2; Dr. Rivera for providing her manuscript prior to publication; and Dr. Randi Hagerman and Elisabeth Makhoul for critically reading and editing this manuscript. The author also wishes to thank the many patients with FXTAS and other types of premutation involvement who have participated in FXTAS research. He is grateful for the private donations from families who support FXTAS and fragile X syndrome research.
Footnotes
Conflicts: Dr. Hagerman is an uncompensated collaborator with Pacific Biosciences regarding new FMR1 sequencing strategies; he holds patents for FMR1 genotyping and protein tests.
References
- 1.Adams JS, Adams PE, Nguyen D, Brunberg JA, Tassone F, Zhang W, Koldewyn K, Rivera SM, Grigsby J, Zhang L, DeCarli C, Hagerman PJ, Hagerman RJ. Volumetric brain changes in females with fragile X-associated tremor/ataxia syndrome (FXTAS) Neurology. 2007;69 (9):851–859. doi: 10.1212/01.wnl.0000269781.10417.7b. [DOI] [PubMed] [Google Scholar]
- 2.Adams PE, Adams JS, Nguyen DV, Hessl D, Brunberg JA, Tassone F, Zhang W, Koldewyn K, Rivera SM, Grigsby J, Zhang L, Decarli C, Hagerman PJ, Hagerman RJ. Psychological symptoms correlate with reduced hippocampal volume in fragile X premutation carriers. Am J Med Genet B Neuropsychiatr Genet. 2010;153B (3):775–785. doi: 10.1002/ajmg.b.31046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Apartis E, Blancher A, Meissner WG, Guyant-Marechal L, Maltete D, De Broucker T, Legrand AP, Bouzenada H, Thanh HT, Sallansonnet-Froment M, Wang A, Tison F, Roue-Jagot C, Sedel F, Charles P, Whalen S, Heron D, Thobois S, Poisson A, Lesca G, Ouvrard-Hernandez AM, Fraix V, Palfi S, Habert MO, Gaymard B, Dussaule JC, Pollak P, Vidailhet M, Durr A, Barbot JC, Gourlet V, Brice A, Anheim M. FXTAS: new insights and the need for revised diagnostic criteria. Neurology. 2012;79 (18):1898–1907. doi: 10.1212/WNL.0b013e318271f7ff. [DOI] [PubMed] [Google Scholar]
- 4.Arocena DG, Iwahashi CK, Won N, Beilina A, Ludwig AL, Tassone F, Schwartz PH, Hagerman PJ. Induction of inclusion formation and disruption of lamin A/C structure by premutation CGG-repeat RNA in human cultured neural cells. Hum Mol Genet. 2005;14 (23):3661–3671. doi: 10.1093/hmg/ddi394. [DOI] [PubMed] [Google Scholar]
- 5.Ash PE, Bieniek KF, Gendron TF, Caulfield T, Lin WL, Dejesus-Hernandez M, van Blitterswijk MM, Jansen-West K, Paul JW, 3rd, Rademakers R, Boylan KB, Dickson DW, Petrucelli L. Unconventional translation of C9ORF72 GGGGCC expansion generates insoluble polypeptides specific to c9FTD/ALS. Neuron. 2013;77 (4):639–646. doi: 10.1016/j.neuron.2013.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Au J, Akins R, Berkowitz-Sutherland L, Tang HT, Chen Y, Boyd A, Tassone F, Nguyen D, Hagerman R. Prevalence and risk of migraine headaches in adult fragile X premutation carriers. Clin Genet. 2013 doi: 10.1111/cge.12109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aumiller V, Graebsch A, Kremmer E, Niessing D, Forstemann K. Drosophila Pur-alpha binds to trinucleotide-repeat containing cellular RNAs and translocates to the early oocyte. RNA Biol. 2012;9 (5):633–643. doi: 10.4161/rna.19760. [DOI] [PubMed] [Google Scholar]
- 8.Aziz M, Stathopulu E, Callias M, Taylor C, Turk J, Oostra B, Willemsen R, Patton M. Clinical features of boys with fragile X premutations and intermediate alleles. Am J Med Genet B Neuropsychiatr Genet. 2003;121B (1):119–127. doi: 10.1002/ajmg.b.20030. [DOI] [PubMed] [Google Scholar]
- 9.Bacalman S, Farzin F, Bourgeois JA, Cogswell J, Goodlin-Jones BL, Gane LW, Grigsby J, Leehey MA, Tassone F, Hagerman RJ. Psychiatric Phenotype of the Fragile X-Associated Tremor/Ataxia Syndrome (FXTAS) in Males: Newly Described Fronto-Subcortical Dementia. J Clin Psychiatry. 2006;67 (1):87–94. doi: 10.4088/jcp.v67n0112. [DOI] [PubMed] [Google Scholar]
- 10.Bailey DB, Jr, Raspa M, Bishop E, Mitra D, Martin S, Wheeler A, Sacco P. Health and economic consequences of fragile X syndrome for caregivers. J Dev Behav Pediatr. 2012;33(9):705–712. doi: 10.1097/DBP.0b013e318272dcbc00004703-201211000-00004. [pii] [DOI] [PubMed] [Google Scholar]
- 11.Bailey DB, Jr, Raspa M, Olmsted M, Holiday DB. Co-occurring conditions associated with FMR1 gene variations: findings from a national parent survey. Am J Med Genet A. 2008;146A (16):2060–2069. doi: 10.1002/ajmg.a.32439. [DOI] [PubMed] [Google Scholar]
- 12.Battistella G, Niederhauser J, Fornari E, Hippolyte L, Gronchi Perrin A, Lesca G, Forzano F, Hagmann P, Vingerhoets FJ, Draganski B, Maeder P, Jacquemont S. Brain structure in asymptomatic FMR1 premutation carriers at risk for fragile X-associated tremor/ataxia syndrome. Neurobiol Aging. 2013 doi: 10.1016/j.neurobiolaging.2012.12.001. [DOI] [PubMed] [Google Scholar]
- 13.Berman RF, Murray KD, Arque G, Hunsaker MR, Wenzel HJ. Abnormal dendrite and spine morphology in primary visual cortex in the CGG knock-in mouse model of the fragile X premutation. Epilepsia. 2012;53(Suppl 1):150–160. doi: 10.1111/j.1528-1167.2012.03486.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Berman RF, Willemsen R. Mouse models of fragile X-associated tremor ataxia. J Investig Med. 2009;57 (8):837–841. doi: 10.231/JIM.0b013e3181af59d6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Berry-Kravis E, Abrams L, Coffey SM, Hall DA, Greco C, Gane LW, Grigsby J, Bourgeois JA, Finucane B, Jacquemont S, Brunberg JA, Zhang L, Lin J, Tassone F, Hagerman PJ, Hagerman RJ, Leehey MA. Fragile X-associated tremor/ataxia syndrome: clinical features, genetics, and testing guidelines. Mov Disord. 2007;22 (14):2018–2030. doi: 10.1002/mds.21493. quiz 2140. [DOI] [PubMed] [Google Scholar]
- 16.Bielli P, Busa R, Paronetto MP, Sette C. The RNA-binding protein Sam68 is a multifunctional player in human cancer. Endocr Relat Cancer. 2011;18 (4):R91–R102. doi: 10.1530/ERC-11-0041. [DOI] [PubMed] [Google Scholar]
- 17.Bontekoe CJ, de Graaff E, Nieuwenhuizen IM, Willemsen R, Oostra BA. FMR1 premutation allele (CGG)81 is stable in mice. Eur J Hum Genet. 1997;5 (5):293–298. [PubMed] [Google Scholar]
- 18.Borthwell RM, Hunsaker MR, Willemsen R, Berman RF. Spatiotemporal processing deficits in female CGG KI mice modeling the fragile X premutation. Behav Brain Res. 2012;233 (1):29–34. doi: 10.1016/j.bbr.2012.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bourgeois JA, Coffey SM, Rivera SM, Hessl D, Gane LW, Tassone F, Greco C, Finucane B, Nelson L, Berry-Kravis E, Grigsby J, Hagerman PJ, Hagerman RJ. A review of fragile X premutation disorders: expanding the psychiatric perspective. J Clin Psychiatry. 2009;70 (6):852–862. doi: 10.4088/JCP.08m04476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bourgeois JA, Seritan AL, Casillas EM, Hessl D, Schneider A, Yang Y, Kaur I, Cogswell JB, Nguyen DV, Hagerman RJ. Lifetime prevalence of mood and anxiety disorders in fragile X premutation carriers. J Clin Psychiatry. 2011;72 (2):175–182. doi: 10.4088/JCP.09m05407blu. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Boyle L, Kaufmann WE. The behavioral phenotype of FMR1 mutations. Am J Med Genet C Semin Med Genet. 2010;154C (4):469–476. doi: 10.1002/ajmg.c.30277. [DOI] [PubMed] [Google Scholar]
- 22.Brouwer JR, Huizer K, Severijnen LA, Hukema RK, Berman RF, Oostra BA, Willemsen R. CGG-repeat length and neuropathological and molecular correlates in a mouse model for fragile X-associated tremor/ataxia syndrome. J Neurochem. 2008;107 (6):1671–1682. doi: 10.1111/j.1471-4159.2008.05747.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brunberg JA, Jacquemont S, Hagerman RJ, Berry-Kravis EM, Grigsby J, Leehey MA, Tassone F, Brown WT, Greco CM, Hagerman PJ. Fragile X premutation carriers: characteristic MR imaging findings of adult male patients with progressive cerebellar and cognitive dysfunction. AJNR Am J Neuroradiol. 2002;23 (10):1757–1766. [PMC free article] [PubMed] [Google Scholar]
- 24.Cao Z, Hulsizer S, Tassone F, Tang HT, Hagerman RJ, Rogawski MA, Hagerman PJ, Pessah IN. Clustered burst firing in FMR1 premutation hippocampal neurons: amelioration with allopregnanolone. Hum Mol Genet. 2012;21(13):2923–2935. doi: 10.1093/hmg/dds118. dds118 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Capelli LP, Goncalves MR, Kok F, Leite CC, Nitrini R, Barbosa ER, Vianna-Morgante AM. Fragile X-associated tremor/ataxia syndrome: intrafamilial variability and the size of the FMR1 premutation CGG repeat. Mov Disord. 2007;22 (6):866–870. doi: 10.1002/mds.21347. [DOI] [PubMed] [Google Scholar]
- 26.Chen Y, Tassone F, Berman RF, Hagerman PJ, Hagerman RJ, Willemsen R, Pessah IN. Murine hippocampal neurons expressing Fmr1 gene premutations show early developmental deficits and late degeneration. Hum Mol Genet. 2010;19 (1):196–208. doi: 10.1093/hmg/ddp479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chonchaiya W, Au J, Schneider A, Hessl D, Harris SW, Laird M, Mu Y, Tassone F, Nguyen DV, Hagerman RJ. Increased prevalence of seizures in boys who were probands with the FMR1 premutation and co-morbid autism spectrum disorder. Hum Genet. 2012;131 (4):581–589. doi: 10.1007/s00439-011-1106-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Clifford S, Dissanayake C, Bui QM, Huggins R, Taylor AK, Loesch DZ. Autism spectrum phenotype in males and females with fragile X full mutation and premutation. J Autism Dev Disord. 2007;37 (4):738–747. doi: 10.1007/s10803-006-0205-z. [DOI] [PubMed] [Google Scholar]
- 29.Coffey SM, Cook K, Tartaglia N, Tassone F, Nguyen DV, Pan R, Bronsky HE, Yuhas J, Borodyanskaya M, Grigsby J, Doerflinger M, Hagerman PJ, Hagerman RJ. Expanded clinical phenotype of women with the FMR1 premutation. Am J Med Genet A. 2008;146A (8):1009–1016. doi: 10.1002/ajmg.a.32060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cohen S, Masyn K, Adams J, Hessl D, Rivera S, Tassone F, Brunberg J, DeCarli C, Zhang L, Cogswell J, Loesch D, Leehey M, Grigsby J, Hagerman PJ, Hagerman RJ. Molecular and imaging correlates of the fragile X-associated tremor/ataxia syndrome. Neurology. 2006;67 (8):1426–1431. doi: 10.1212/01.wnl.0000239837.57475.3a. [DOI] [PubMed] [Google Scholar]
- 31.Collins SC, Bray SM, Suhl JA, Cutler DJ, Coffee B, Zwick ME, Warren ST. Identification of novel FMR1 variants by massively parallel sequencing in developmentally delayed males. Am J Med Genet A. 2010;152A (10):2512–2520. doi: 10.1002/ajmg.a.33626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Conway GS, Payne NN, Webb J, Murray A, Jacobs PA. Fragile X premutation screening in women with premature ovarian failure. Hum Reprod. 1998;13 (5):1184–1187. doi: 10.1093/humrep/13.5.1184. [DOI] [PubMed] [Google Scholar]
- 33.Cornish KM, Kogan CS, Li L, Turk J, Jacquemont S, Hagerman RJ. Lifespan changes in working memory in fragile X premutation males. Brain Cogn. 2009;69 (3):551–558. doi: 10.1016/j.bandc.2008.11.006. S0278-2626(08)00327-8 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cornish KM, Li L, Kogan CS, Jacquemont S, Turk J, Dalton A, Hagerman RJ, Hagerman PJ. Age-dependent cognitive changes in carriers of the fragile X syndrome. Cortex. 2008;44 (6):628–636. doi: 10.1016/j.cortex.2006.11.002. S0010-9452(07)00108-6 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cronister A, Schreiner R, Wittenberger M, Amiri K, Harris K, Hagerman RJ. Heterozygous fragile X female: historical, physical, cognitive, and cytogenetic features. Am J Med Genet. 1991;38 (2–3):269–274. doi: 10.1002/ajmg.1320380221. [DOI] [PubMed] [Google Scholar]
- 36.Cunningham CL, Martinez Cerdeno V, Navarro Porras E, Prakash AN, Angelastro JM, Willemsen R, Hagerman PJ, Pessah IN, Berman RF, Noctor SC. Premutation CGG-repeat expansion of the Fmr1 gene impairs mouse neocortical development. Hum Mol Genet. 2011;20 (1):64–79. doi: 10.1093/hmg/ddq432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Davis TH, Cuellar TL, Koch SM, Barker AJ, Harfe BD, McManus MT, Ullian EM. Conditional loss of Dicer disrupts cellular and tissue morphogenesis in the cortex and hippocampus. J Neurosci. 2008;28 (17):4322–4330. doi: 10.1523/JNEUROSCI.4815-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.De Boulle K, Verkerk AJ, Reyniers E, Vits L, Hendrickx J, Van Roy B, Van den Bos F, de Graaff E, Oostra BA, Willems PJ. A point mutation in the FMR-1 gene associated with fragile X mental retardation. Nat Genet. 1993;3 (1):31–35. doi: 10.1038/ng0193-31. [DOI] [PubMed] [Google Scholar]
- 39.De Pietri Tonelli D, Pulvers JN, Haffner C, Murchison EP, Hannon GJ, Huttner WB. miRNAs are essential for survival and differentiation of newborn neurons but not for expansion of neural progenitors during early neurogenesis in the mouse embryonic neocortex. Development. 2008;135 (23):3911–3921. doi: 10.1242/dev.025080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Denli AM, Tops BB, Plasterk RH, Ketting RF, Hannon GJ. Processing of primary microRNAs by the Microprocessor complex. Nature. 2004;432 (7014):231–235. doi: 10.1038/nature03049. [DOI] [PubMed] [Google Scholar]
- 41.Diep AA, Hunsaker MR, Kwock R, Kim K, Willemsen R, Berman RF. Female CGG knock-in mice modeling the fragile X premutation are impaired on a skilled forelimb reaching task. Neurobiol Learn Mem. 2012;97 (2):229–234. doi: 10.1016/j.nlm.2011.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Disney MD, Liu B, Yang WY, Sellier C, Tran T, Charlet-Berguerand N, Childs-Disney JL. A Small Molecule That Targets r(CGG)(exp) and Improves Defects in Fragile X-Associated Tremor Ataxia Syndrome. ACS Chem Biol. 2012;7 (10):1711–1718. doi: 10.1021/cb300135h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dombrowski C, Levesque S, Morel ML, Rouillard P, Morgan K, Rousseau F. Premutation and intermediate-size FMR1 alleles in 10572 males from the general population: loss of an AGG interruption is a late event in the generation of fragile X syndrome alleles. Hum Mol Genet. 2002;11 (4):371–378. doi: 10.1093/hmg/11.4.371. [DOI] [PubMed] [Google Scholar]
- 44.Echeverria GV, Cooper TA. RNA-binding proteins in microsatellite expansion disorders: mediators of RNA toxicity. Brain Res. 2012;1462:100–111. doi: 10.1016/j.brainres.2012.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Eichler EE, Hammond HA, Macpherson JN, Ward PA, Nelson DL. Population survey of the human FMR1 CGG repeat substructure suggests biased polarity for the loss of AGG interruptions. Hum Mol Genet. 1995;4 (12):2199–2208. doi: 10.1093/hmg/4.12.2199. [DOI] [PubMed] [Google Scholar]
- 46.Eichler EE, Holden JJ, Popovich BW, Reiss AL, Snow K, Thibodeau SN, Richards CS, Ward PA, Nelson DL. Length of uninterrupted CGG repeats determines instability in the FMR1 gene. Nat Genet. 1994;8 (1):88–94. doi: 10.1038/ng0994-88. [DOI] [PubMed] [Google Scholar]
- 47.Entezam A, Biacsi R, Orrison B, Saha T, Hoffman GE, Grabczyk E, Nussbaum RL, Usdin K. Regional FMRP deficits and large repeat expansions into the full mutation range in a new Fragile X premutation mouse model. Gene. 2007;395 (1–2):125–134. doi: 10.1016/j.gene.2007.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Faller M, Toso D, Matsunaga M, Atanasov I, Senturia R, Chen Y, Zhou ZH, Guo F. DGCR8 recognizes primary transcripts of microRNAs through highly cooperative binding and formation of higher-order structures. RNA. 2010;16 (8):1570–1583. doi: 10.1261/rna.2111310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Farzin F, Perry H, Hessl D, Loesch D, Cohen J, Bacalman S, Gane L, Tassone F, Hagerman P, Hagerman R. Autism spectrum disorders and attention-deficit/hyperactivity disorder in boys with the fragile X premutation. J Dev Behav Pediatr. 2006;27 (2 Suppl):S137–144. doi: 10.1097/00004703-200604002-00012. [DOI] [PubMed] [Google Scholar]
- 50.Fenelon K, Mukai J, Xu B, Hsu PK, Drew LJ, Karayiorgou M, Fischbach GD, Macdermott AB, Gogos JA. Deficiency of Dgcr8, a gene disrupted by the 22q11.2 microdeletion, results in altered short-term plasticity in the prefrontal cortex. Proc Natl Acad Sci U S A. 2011;108 (11):4447–4452. doi: 10.1073/pnas.1101219108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Finnegan EF, Pasquinelli AE. MicroRNA biogenesis: regulating the regulators. Crit Rev Biochem Mol Biol. 2013;48 (1):51–68. doi: 10.3109/10409238.2012.738643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Franke P, Leboyer M, Gansicke M, Weiffenbach O, Biancalana V, Cornillet-Lefebre P, Croquette MF, Froster U, Schwab SG, Poustka F, Hautzinger M, Maier W. Genotype-phenotype relationship in female carriers of the premutation and full mutation of FMR-1. Psychiatry Res. 1998;80 (2):113–127. doi: 10.1016/s0165-1781(98)00055-9. [DOI] [PubMed] [Google Scholar]
- 53.Franke P, Maier W, Hautzinger M, Weiffenbach O, Gansicke M, Iwers B, Poustka F, Schwab SG, Froster U. Fragile-X carrier females: evidence for a distinct psychopathological phenotype? Am J Med Genet. 1996;64 (2):334–339. doi: 10.1002/(SICI)1096-8628(19960809)64:2<334::AID-AJMG20>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 54.Galloway JN, Nelson DL. Evidence for RNA-mediated toxicity in the fragile X-associated tremor/ataxia syndrome. Future Neurol. 2009;4 (6):785. doi: 10.2217/fnl.09.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Garcia-Arocena D, Yang JE, Brouwer JR, Tassone F, Iwahashi C, Berry-Kravis EM, Goetz CG, Sumis AM, Zhou L, Nguyen DV, Campos L, Howell E, Ludwig A, Greco C, Willemsen R, Hagerman RJ, Hagerman PJ. Fibroblast phenotype in male carriers of FMR1 premutation alleles. Hum Mol Genet. 2010;19 (2):299–312. doi: 10.1093/hmg/ddp497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gokden M, Al-Hinti JT, Harik SI. Peripheral nervous system pathology in fragile X tremor/ataxia syndrome (FXTAS) Neuropathology. 2009;29 (3):280–284. doi: 10.1111/j.1440-1789.2008.00948.x. [DOI] [PubMed] [Google Scholar]
- 57.Goodlin-Jones BL, Tassone F, Gane LW, Hagerman RJ. Autistic spectrum disorder and the fragile X premutation. J Dev Behav Pediatr. 2004;25 (6):392–398. doi: 10.1097/00004703-200412000-00002. [DOI] [PubMed] [Google Scholar]
- 58.Goodrich-Hunsaker NJ, Wong LM, McLennan Y, Tassone F, Harvey D, Rivera SM, Simon TJ. Enhanced manual and oral motor reaction time in young adult female fragile X premutation carriers. J Int Neuropsychol Soc. 2011;17 (4):746–750. doi: 10.1017/S1355617711000634. S1355617711000634 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Greco CM, Berman RF, Martin RM, Tassone F, Schwartz PH, Chang A, Trapp BD, Iwahashi C, Brunberg J, Grigsby J, Hessl D, Becker EJ, Papazian J, Leehey MA, Hagerman RJ, Hagerman PJ. Neuropathology of fragile X-associated tremor/ataxia syndrome (FXTAS) Brain. 2006;129 (Pt 1):243–255. doi: 10.1093/brain/awh683. [DOI] [PubMed] [Google Scholar]
- 60.Greco CM, Hagerman RJ, Tassone F, Chudley AE, Del Bigio MR, Jacquemont S, Leehey M, Hagerman PJ. Neuronal intranuclear inclusions in a new cerebellar tremor/ataxia syndrome among fragile X carriers. Brain. 2002;125 (Pt 8):1760–1771. doi: 10.1093/brain/awf184. [DOI] [PubMed] [Google Scholar]
- 61.Greco CM, Soontrapornchai K, Wirojanan J, Gould JE, Hagerman PJ, Hagerman RJ. Testicular and pituitary inclusion formation in fragile X associated tremor/ataxia syndrome. J Urol. 2007;177 (4):1434–1437. doi: 10.1016/j.juro.2006.11.097. [DOI] [PubMed] [Google Scholar]
- 62.Gregory RI, Yan KP, Amuthan G, Chendrimada T, Doratotaj B, Cooch N, Shiekhattar R. The Microprocessor complex mediates the genesis of microRNAs. Nature. 2004;432 (7014):235–240. doi: 10.1038/nature03120. [DOI] [PubMed] [Google Scholar]
- 63.Grigsby J, Brega AG, Engle K, Leehey MA, Hagerman RJ, Tassone F, Hessl D, Hagerman PJ, Cogswell JB, Bennett RE, Cook K, Hall DA, Bounds LS, Paulich MJ, Reynolds A. Cognitive profile of fragile X premutation carriers with and without fragile X-associated tremor/ataxia syndrome. Neuropsychology. 2008;22 (1):48–60. doi: 10.1037/0894-4105.22.1.48. [DOI] [PubMed] [Google Scholar]
- 64.Gronskov K, Brondum-Nielsen K, Dedic A, Hjalgrim H. A nonsense mutation in FMR1 causing fragile X syndrome. Eur J Hum Genet. 2011;19 (4):489–491. doi: 10.1038/ejhg.2010.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hagerman PJ. Current Gaps in Understanding the Molecular Basis of FXTAS. Tremor Other Hyperkinet Mov (N Y) 2012:2. doi: 10.7916/D80C4TH0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hagerman R, Au J, Hagerman P. FMR1 premutation and full mutation molecular mechanisms related to autism. J Neurodev Disord. 2011;3 (3):211–224. doi: 10.1007/s11689-011-9084-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hagerman RJ, Leehey M, Heinrichs W, Tassone F, Wilson R, Hills J, Grigsby J, Gage B, Hagerman PJ. Intention tremor, parkinsonism, and generalized brain atrophy in male carriers of fragile X. Neurology. 2001;57 (1):127–130. doi: 10.1212/wnl.57.1.127. [DOI] [PubMed] [Google Scholar]
- 68.Hall D, Tassone F, Klepitskaya O, Leehey M. Fragile X-associated tremor ataxia syndrome in FMR1 gray zone allele carriers. Mov Disord. 2012;27 (2):296–300. doi: 10.1002/mds.24021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hamlin A, Liu Y, Nguyen DV, Tassone F, Zhang L, Hagerman RJ. Sleep apnea in fragile X premutation carriers with and without FXTAS. Am J Med Genet B Neuropsychiatr Genet. 2011;156B (8):923–928. doi: 10.1002/ajmg.b.31237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hamlin AA, Sukharev D, Campos L, Mu Y, Tassone F, Hessl D, Nguyen DV, Loesch D, Hagerman RJ. Hypertension in FMR1 premutation males with and without fragile X-associated tremor/ataxia syndrome (FXTAS) Am J Med Genet A. 2012;158A (6):1304–1309. doi: 10.1002/ajmg.a.35323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Han J, Lee Y, Yeom KH, Kim YK, Jin H, Kim VN. The Drosha-DGCR8 complex in primary microRNA processing. Genes Dev. 2004;18 (24):3016–3027. doi: 10.1101/gad.1262504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Haramati S, Chapnik E, Sztainberg Y, Eilam R, Zwang R, Gershoni N, McGlinn E, Heiser PW, Wills AM, Wirguin I, Rubin LL, Misawa H, Tabin CJ, Brown R, Jr, Chen A, Hornstein E. miRNA malfunction causes spinal motor neuron disease. Proc Natl Acad Sci U S A. 2010;107 (29):13111–13116. doi: 10.1073/pnas.1006151107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hashem V, Galloway JN, Mori M, Willemsen R, Oostra BA, Paylor R, Nelson DL. Ectopic expression of CGG containing mRNA is neurotoxic in mammals. Hum Mol Genet. 2009;18(13):2443–2451. doi: 10.1093/hmg/ddp182. ddp182 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hashimoto R, Backer KC, Tassone F, Hagerman RJ, Rivera SM. An fMRI study of the prefrontal activity during the performance of a working memory task in premutation carriers of the fragile X mental retardation 1 gene with and without fragile X-associated tremor/ataxia syndrome (FXTAS) J Psychiatr Res. 2011;45 (1):36–43. doi: 10.1016/j.jpsychires.2010.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hashimoto R, Javan AK, Tassone F, Hagerman RJ, Rivera SM. A voxel-based morphometry study of grey matter loss in fragile X-associated tremor/ataxia syndrome. Brain. 2011;134 (Pt 3):863–878. doi: 10.1093/brain/awq368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hashimoto R, Srivastava S, Tassone F, Hagerman RJ, Rivera SM. Diffusion tensor imaging in male premutation carriers of the fragile X mental retardation gene. Mov Disord. 2011;26 (7):1329–1336. doi: 10.1002/mds.23646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hebert SS, Papadopoulou AS, Smith P, Galas MC, Planel E, Silahtaroglu AN, Sergeant N, Buee L, De Strooper B. Genetic ablation of Dicer in adult forebrain neurons results in abnormal tau hyperphosphorylation and neurodegeneration. Hum Mol Genet. 2010;19 (20):3959–3969. doi: 10.1093/hmg/ddq311. [DOI] [PubMed] [Google Scholar]
- 78.Hessl D, Rivera S, Koldewyn K, Cordeiro L, Adams J, Tassone F, Hagerman PJ, Hagerman RJ. Amygdala dysfunction in men with the fragile X premutation. Brain. 2007;130 (Pt 2):404–416. doi: 10.1093/brain/awl338. [DOI] [PubMed] [Google Scholar]
- 79.Hessl D, Tassone F, Loesch DZ, Berry-Kravis E, Leehey MA, Gane LW, Barbato I, Rice C, Gould E, Hall DA, Grigsby J, Wegelin JA, Harris S, Lewin F, Weinberg D, Hagerman PJ, Hagerman RJ. Abnormal elevation of FMR1 mRNA is associated with psychological symptoms in individuals with the fragile X premutation. Am J Med Genet B Neuropsychiatr Genet. 2005;139B (1):115–121. doi: 10.1002/ajmg.b.30241. [DOI] [PubMed] [Google Scholar]
- 80.Hessl D, Wang JM, Schneider A, Koldewyn K, Le L, Iwahashi C, Cheung K, Tassone F, Hagerman PJ, Rivera SM. Decreased fragile X mental retardation protein expression underlies amygdala dysfunction in carriers of the fragile X premutation. Biol Psychiatry. 2011;70 (9):859–865. doi: 10.1016/j.biopsych.2011.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hoem G, Raske CR, Garcia-Arocena D, Tassone F, Sanchez E, Ludwig AL, Iwahashi CK, Kumar M, Yang JE, Hagerman PJ. CGG-repeat length threshold for FMR1 RNA pathogenesis in a cellular model for FXTAS. Hum Mol Genet. 2011;20 (11):2161–2170. doi: 10.1093/hmg/ddr101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hoffman GE, Le WW, Entezam A, Otsuka N, Tong ZB, Nelson L, Flaws JA, McDonald JH, Jafar S, Usdin K. Ovarian abnormalities in a mouse model of fragile X primary ovarian insufficiency. J Histochem Cytochem. 2012;60 (6):439–456. doi: 10.1369/0022155412441002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Huang T, Liu Y, Huang M, Zhao X, Cheng L. Wnt1-cre-mediated conditional loss of Dicer results in malformation of the midbrain and cerebellum and failure of neural crest and dopaminergic differentiation in mice. J Mol Cell Biol. 2010;2 (3):152–163. doi: 10.1093/jmcb/mjq008. [DOI] [PubMed] [Google Scholar]
- 84.Hunsaker MR, Arque G, Berman RF, Willemsen R, Hukema RK. Mouse models of the fragile x premutation and the fragile X associated tremor/ataxia syndrome. Results Probl Cell Differ. 2012;54:255–269. doi: 10.1007/978-3-642-21649-7_14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hunsaker MR, Goodrich-Hunsaker NJ, Willemsen R, Berman RF. Temporal ordering deficits in female CGG KI mice heterozygous for the fragile X premutation. Behav Brain Res. 2010;213 (2):263–268. doi: 10.1016/j.bbr.2010.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hunsaker MR, Greco CM, Spath MA, Smits AP, Navarro CS, Tassone F, Kros JM, Severijnen LA, Berry-Kravis EM, Berman RF, Hagerman PJ, Willemsen R, Hagerman RJ, Hukema RK. Widespread non-central nervous system organ pathology in fragile X premutation carriers with fragile X-associated tremor/ataxia syndrome and CGG knock-in mice. Acta Neuropathol. 2011;122 (4):467–479. doi: 10.1007/s00401-011-0860-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hunsaker MR, Greco CM, Tassone F, Berman RF, Willemsen R, Hagerman RJ, Hagerman PJ. Rare intranuclear inclusions in the brains of 3 older adult males with fragile x syndrome: implications for the spectrum of fragile x-associated disorders. J Neuropathol Exp Neurol. 2011;70 (6):462–469. doi: 10.1097/NEN.0b013e31821d3194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hunsaker MR, Kim K, Willemsen R, Berman RF. CGG trinucleotide repeat length modulates neural plasticity and spatiotemporal processing in a mouse model of the fragile X premutation. Hippocampus. 2012;22 (12):2260–2275. doi: 10.1002/hipo.22043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hunsaker MR, von Leden RE, Ta BT, Goodrich-Hunsaker NJ, Arque G, Kim K, Willemsen R, Berman RF. Motor deficits on a ladder rung task in male and female adolescent and adult CGG knock-in mice. Behav Brain Res. 2011;222 (1):117–121. doi: 10.1016/j.bbr.2011.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hunsaker MR, Wenzel HJ, Willemsen R, Berman RF. Progressive spatial processing deficits in a mouse model of the fragile X premutation. Behav Neurosci. 2009;123 (6):1315–1324. doi: 10.1037/a0017616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hunter JE, Leslie M, Novak G, Hamilton D, Shubeck L, Charen K, Abramowitz A, Epstein MP, Lori A, Binder E, Cubells JF, Sherman SL. Depression and anxiety symptoms among women who carry the FMR1 premutation: impact of raising a child with fragile X syndrome is moderated by CRHR1 polymorphisms. Am J Med Genet B Neuropsychiatr Genet. 2012;159B (5):549–559. doi: 10.1002/ajmg.b.32061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Iliff AJ, Renoux AJ, Krans A, Usdin K, Sutton MA, Todd PK. Impaired activity-dependent FMRP translation and enhanced mGluR-dependent LTD in Fragile X premutation mice. Hum Mol Genet. 2013;22 (6):1180–1192. doi: 10.1093/hmg/dds525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Iwahashi CK, Yasui DH, An HJ, Greco CM, Tassone F, Nannen K, Babineau B, Lebrilla CB, Hagerman RJ, Hagerman PJ. Protein composition of the intranuclear inclusions of FXTAS. Brain. 2006;129 (Pt 1):256–271. doi: 10.1093/brain/awh650. [DOI] [PubMed] [Google Scholar]
- 94.Jacquemont S, Hagerman RJ, Leehey M, Grigsby J, Zhang L, Brunberg JA, Greco C, Des Portes V, Jardini T, Levine R, Berry-Kravis E, Brown WT, Schaeffer S, Kissel J, Tassone F, Hagerman PJ. Fragile X premutation tremor/ataxia syndrome: molecular, clinical, and neuroimaging correlates. Am J Hum Genet. 2003;72 (4):869–878. doi: 10.1086/374321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Jin P, Duan R, Qurashi A, Qin Y, Tian D, Rosser TC, Liu H, Feng Y, Warren ST. Pur alpha binds to rCGG repeats and modulates repeat-mediated neurodegeneration in a Drosophila model of fragile X tremor/ataxia syndrome. Neuron. 2007;55 (4):556–564. doi: 10.1016/j.neuron.2007.07.020. S0896-6273(07)00541-7 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Jin P, Zarnescu DC, Zhang F, Pearson CE, Lucchesi JC, Moses K, Warren ST. RNA-mediated neurodegeneration caused by the fragile X premutation rCGG repeats in Drosophila. Neuron. 2003;39 (5):739–747. doi: 10.1016/s0896-6273(03)00533-6. [DOI] [PubMed] [Google Scholar]
- 97.Juncos JL, Lazarus JT, Graves-Allen E, Shubeck L, Rusin M, Novak G, Hamilton D, Rohr J, Sherman SL. New clinical findings in the fragile X-associated tremor ataxia syndrome (FXTAS) Neurogenetics. 2011;12 (2):123–135. doi: 10.1007/s10048-010-0270-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kamma H, Horiguchi H, Wan L, Matsui M, Fujiwara M, Fujimoto M, Yazawa T, Dreyfuss G. Molecular characterization of the hnRNP A2/B1 proteins: tissue-specific expression and novel isoforms. Exp Cell Res. 1999;246 (2):399–411. doi: 10.1006/excr.1998.4323. [DOI] [PubMed] [Google Scholar]
- 99.Kaplan ES, Cao Z, Hulsizer S, Tassone F, Berman RF, Hagerman PJ, Pessah IN. Early mitochondrial abnormalities in hippocampal neurons cultured from Fmr1 pre-mutation mouse model. J Neurochem. 2012;123 (4):613–621. doi: 10.1111/j.1471-4159.2012.07936.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kasuga K, Ikeuchi T, Arakawa K, Yajima R, Tokutake T, Nishizawa M. A patient with fragile x-associated tremor/ataxia syndrome presenting with executive cognitive deficits and cerebral white matter lesions. Case Rep Neurol. 2011;3 (2):118–123. doi: 10.1159/000328838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kenneson A, Zhang F, Hagedorn CH, Warren ST. Reduced FMRP and increased FMR1 transcription is proportionally associated with CGG repeat number in intermediate-length and premutation carriers. Hum Mol Genet. 2001;10 (14):1449–1454. doi: 10.1093/hmg/10.14.1449. [DOI] [PubMed] [Google Scholar]
- 102.Khalil AM, Faghihi MA, Modarresi F, Brothers SP, Wahlestedt C. A novel RNA transcript with antiapoptotic function is silenced in fragile X syndrome. PLoS One. 2008;3 (1):e1486. doi: 10.1371/journal.pone.0001486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kim M, Ceman S. Fragile X mental retardation protein: past, present and future. Curr Protein Pept Sci. 2012;13 (4):358–371. doi: 10.2174/138920312801619420. [DOI] [PubMed] [Google Scholar]
- 104.King OD, Gitler AD, Shorter J. The tip of the iceberg: RNA-binding proteins with prion-like domains in neurodegenerative disease. Brain Res. 2012;1462:61–80. doi: 10.1016/j.brainres.2012.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kunst CB, Leeflang EP, Iber JC, Arnheim N, Warren ST. The effect of FMR1 CGG repeat interruptions on mutation frequency as measured by sperm typing. J Med Genet. 1997;34 (8):627–631. doi: 10.1136/jmg.34.8.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ladd PD, Smith LE, Rabaia NA, Moore JM, Georges SA, Hansen RS, Hagerman RJ, Tassone F, Tapscott SJ, Filippova GN. An antisense transcript spanning the CGG repeat region of FMR1 is upregulated in premutation carriers but silenced in full mutation individuals. Hum Mol Genet. 2007;16 (24):3174–3187. doi: 10.1093/hmg/ddm293. [DOI] [PubMed] [Google Scholar]
- 107.Landthaler M, Yalcin A, Tuschl T. The human DiGeorge syndrome critical region gene 8 and Its D. melanogaster homolog are required for miRNA biogenesis. Curr Biol. 2004;14 (23):2162–2167. doi: 10.1016/j.cub.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 108.Lee Y, Ahn C, Han J, Choi H, Kim J, Yim J, Lee J, Provost P, Radmark O, Kim S, Kim VN. The nuclear RNase III Drosha initiates microRNA processing. Nature. 2003;425 (6956):415–419. doi: 10.1038/nature01957. [DOI] [PubMed] [Google Scholar]
- 109.Leehey MA. Fragile X-Associated Tremor/Ataxia Syndrome: Clinical Phenotype, Diagnosis, and Treatment. J Investig Med. 2009 doi: 10.231/JIM.0b013e3181af59c4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Leehey MA, Hagerman PJ. Fragile X-associated tremor/ataxia syndrome. In: Vinken PJ, Bruyn GW, editors. Handb Clin Neurol. Vol. 103. 2012. pp. 373–386. 2011/08/11 edn. [DOI] [PubMed] [Google Scholar]
- 111.Li Y, Jin P. RNA-mediated neurodegeneration in fragile X-associated tremor/ataxia syndrome. Brain Res. 2012;1462:112–117. doi: 10.1016/j.brainres.2012.02.057. S0006-8993(12)00389-7 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Liquori CL, Ricker K, Moseley ML, Jacobsen JF, Kress W, Naylor SL, Day JW, Ranum LP. Myotonic dystrophy type 2 caused by a CCTG expansion in intron 1 of ZNF9. Science. 2001;293 (5531):864–867. doi: 10.1126/science.1062125. [DOI] [PubMed] [Google Scholar]
- 113.Liu J, Koscielska KA, Cao Z, Hulsizer S, Grace N, Mitchell G, Nacey C, Githinji J, McGee J, Garcia-Arocena D, Hagerman RJ, Nolta J, Pessah IN, Hagerman PJ. Signaling defects in iPSC-derived fragile X premutation neurons. Hum Mol Genet. 2012;21 (17):3795–3805. doi: 10.1093/hmg/dds207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Loesch DZ, Bui QM, Dissanayake C, Clifford S, Gould E, Bulhak-Paterson D, Tassone F, Taylor AK, Hessl D, Hagerman R, Huggins RM. Molecular and cognitive predictors of the continuum of autistic behaviours in fragile X. Neurosci Biobehav Rev. 2007;31 (3):315–326. doi: 10.1016/j.neubiorev.2006.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Loesch DZ, Cook M, Litewka L, Gould E, Churchyard A, Tassone F, Slater HR, Storey E. A low symptomatic form of neurodegeneration in younger carriers of the FMR1 premutation, manifesting typical radiological changes. J Med Genet. 2008;45 (3):179–181. doi: 10.1136/jmg.2007.054171. [DOI] [PubMed] [Google Scholar]
- 116.Loesch DZ, Godler DE, Evans A, Bui QM, Gehling F, Kotschet KE, Trost N, Storey E, Stimpson P, Kinsella G, Francis D, Thorburn DR, Venn A, Slater HR, Horne M. Evidence for the toxicity of bidirectional transcripts and mitochondrial dysfunction in blood associated with small CGG expansions in the FMR1 gene in patients with parkinsonism. Genet Med. 2011;13 (5):392–399. doi: 10.1097/GIM.0b013e3182064362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Louis E, Moskowitz C, Friez M, Amaya M, Vonsattel JP. Parkinsonism, dysautonomia, and intranuclear inclusions in a fragile X carrier: a clinical-pathological study. Mov Disord. 2006;21 (3):420–425. doi: 10.1002/mds.20753. [DOI] [PubMed] [Google Scholar]
- 118.Lu C, Lin L, Tan H, Wu H, Sherman SL, Gao F, Jin P, Chen D. Fragile X premutation RNA is sufficient to cause primary ovarian insufficiency in mice. Hum Mol Genet. 2012;21 (23):5039–5047. doi: 10.1093/hmg/dds348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Lugenbeel KA, Peier AM, Carson NL, Chudley AE, Nelson DL. Intragenic loss of function mutations demonstrate the primary role of FMR1 in fragile X syndrome. Nat Genet. 1995;10 (4):483–485. doi: 10.1038/ng0895-483. [DOI] [PubMed] [Google Scholar]
- 120.Mahadevan MS. Myotonic dystrophy: is a narrow focus obscuring the rest of the field? Curr Opin Neurol. 2012;25 (5):609–613. doi: 10.1097/WCO.0b013e328357b0d9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Moore CJ, Daly EM, Tassone F, Tysoe C, Schmitz N, Ng V, Chitnis X, McGuire P, Suckling J, Davies KE, Hagerman RJ, Hagerman PJ, Murphy KC, Murphy DG. The effect of pre-mutation of X chromosome CGG trinucleotide repeats on brain anatomy. Brain. 2004;127 (12):2672–2681. doi: 10.1093/brain/awh256. [DOI] [PubMed] [Google Scholar]
- 122.Napierala M, Michalowski D, de Mezer M, Krzyzosiak WJ. Facile FMR1 mRNA structure regulation by interruptions in CGG repeats. Nucleic Acids Res. 2005;33 (2):451–463. doi: 10.1093/nar/gki186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Napoli E, Ross-Inta C, Wong S, Omanska-Klusek A, Barrow C, Iwahashi C, Garcia-Arocena D, Sakaguchi D, Berry-Kravis E, Hagerman R, Hagerman PJ, Giulivi C. Altered zinc transport disrupts mitochondrial protein processing/import in fragile X-associated tremor/ataxia syndrome. Hum Mol Genet. 2011;20 (15):3079–3092. doi: 10.1093/hmg/ddr211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Nolin SL, Brown WT, Glicksman A, Houck GE, Jr, Gargano AD, Sullivan A, Biancalana V, Brondum-Nielsen K, Hjalgrim H, Holinski-Feder E, Kooy F, Longshore J, Macpherson J, Mandel JL, Matthijs G, Rousseau F, Steinbach P, Vaisanen ML, von Koskull H, Sherman SL. Expansion of the fragile X CGG repeat in females with premutation or intermediate alleles. Am J Hum Genet. 2003;72 (2):454–464. doi: 10.1086/367713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Pearson CE. Repeat associated non-ATG translation initiation: one DNA, two transcripts, seven reading frames, potentially nine toxic entities! PLoS Genet. 2011;7(3):e1002018. doi: 10.1371/journal.pgen.1002018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Pearson CE, Eichler EE, Lorenzetti D, Kramer SF, Zoghbi HY, Nelson DL, Sinden RR. Interruptions in the triplet repeats of SCA1 and FRAXA reduce the propensity and complexity of slipped strand DNA (S-DNA) formation. Biochemistry. 1998;37 (8):2701–2708. doi: 10.1021/bi972546c. [DOI] [PubMed] [Google Scholar]
- 127.Perron MP, Provost P. Protein components of the microRNA pathway and human diseases. Methods Mol Biol. 2009;487:369–385. doi: 10.1007/978-1-60327-547-7_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Pieretti M, Zhang FP, Fu YH, Warren ST, Oostra BA, Caskey CT, Nelson DL. Absence of expression of the FMR-1 gene in fragile X syndrome. Cell. 1991;66(4):817–822. doi: 10.1016/0092-8674(91)90125-i. 0092-8674(91)90125-I [pii] [DOI] [PubMed] [Google Scholar]
- 129.Qin M, Entezam A, Usdin K, Huang T, Liu ZH, Hoffman GE, Smith CB. A mouse model of the fragile X premutation: effects on behavior, dendrite morphology, and regional rates of cerebral protein synthesis. Neurobiol Dis. 2011;42 (1):85–98. doi: 10.1016/j.nbd.2011.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Radvanszky J, Surovy M, Polak E, Kadasi L. Uninterrupted CCTG tracts in the myotonic dystrophy type 2 associated locus. Neuromuscul Disord. 2013 doi: 10.1016/j.nmd.2013.02.013. [DOI] [PubMed] [Google Scholar]
- 131.Renda MM, Voigt RG, Babovic-Vuksanovic D, Highsmith WE, Vinson SS, Sadowski CM, Hagerman RJ. Neurodevelopmental Disabilities in Children With Intermediate and Premutation Range Fragile X Cytosine-Guanine-Guanine Expansions. J Child Neurol. 2012 doi: 10.1177/0883073812469723. [DOI] [PubMed] [Google Scholar]
- 132.Renoux AJ, Todd PK. Neurodegeneration the RNA way. Prog Neurobiol. 2012;97 (2):173–189. doi: 10.1016/j.pneurobio.2011.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Roberts JE, Bailey DB, Jr, Mankowski J, Ford A, Sideris J, Weisenfeld LA, Heath TM, Golden RN. Mood and anxiety disorders in females with the FMR1 premutation. Am J Med Genet B Neuropsychiatr Genet. 2009;150B (1):130–139. doi: 10.1002/ajmg.b.30786. [DOI] [PubMed] [Google Scholar]
- 134.Rodriguez-Revenga L, Madrigal I, Alegret M, Santos M, Mila M. Evidence of depressive symptoms in fragile-X syndrome premutated females. Psychiatr Genet. 2008;18 (4):153–155. doi: 10.1097/YPG.0b013e3282f97e0b. [DOI] [PubMed] [Google Scholar]
- 135.Ross-Inta C, Omanska-Klusek A, Wong S, Barrow C, Garcia-Arocena D, Iwahashi C, Berry-Kravis E, Hagerman RJ, Hagerman PJ, Giulivi C. Evidence of mitochondrial dysfunction in fragile X-associated tremor/ataxia syndrome. Biochem J. 2010;429 (3):545–552. doi: 10.1042/BJ20091960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Schaefer A, O’Carroll D, Tan CL, Hillman D, Sugimori M, Llinas R, Greengard P. Cerebellar neurodegeneration in the absence of microRNAs. J Exp Med. 2007;204 (7):1553–1558. doi: 10.1084/jem.20070823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Schluter EW, Hunsaker MR, Greco CM, Willemsen R, Berman RF. Distribution and frequency of intranuclear inclusions in female CGG KI mice modeling the fragile X premutation. Brain Res. 2012;1472:124–137. doi: 10.1016/j.brainres.2012.06.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Schofield CM, Hsu R, Barker AJ, Gertz CC, Blelloch R, Ullian EM. Monoallelic deletion of the microRNA biogenesis gene Dgcr8 produces deficits in the development of excitatory synaptic transmission in the prefrontal cortex. Neural Dev. 2011;6:11. doi: 10.1186/1749-8104-6-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Sellier C, Freyermuth F, Tabet R, Tran T, He F, Ruffenach F, Alunni V, Moine H, Thibault C, Page A, Tassone F, Willemsen R, Disney MD, Hagerman PJ, Todd PK, Charlet-Berguerand N. Sequestration of DROSHA and DGCR8 by Expanded CGG RNA Repeats Alters MicroRNA Processing in Fragile X-Associated Tremor/Ataxia Syndrome. Cell Rep. 2013;3 (3):869–880. doi: 10.1016/j.celrep.2013.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Sellier C, Rau F, Liu Y, Tassone F, Hukema RK, Gattoni R, Schneider A, Richard S, Willemsen R, Elliott DJ, Hagerman PJ, Charlet-Berguerand N. Sam68 sequestration and partial loss of function are associated with splicing alterations in FXTAS patients. EMBO J. 2010;29 (7):1248–1261. doi: 10.1038/emboj.2010.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Seltzer MM, Barker ET, Greenberg JS, Hong J, Coe C, Almeida D. Differential sensitivity to life stress in FMR1 premutation carrier mothers of children with fragile X syndrome. Health Psychol. 2012;31(5):612–622. doi: 10.1037/a0026528. 10.1037/a0026528 2011-28412-001. [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Seritan A, Bourgeois J, Schneider A, Mu Y, Hagerman R, Nguyen DV. Ages of Onset of Mood and Anxiety Disorders in Fragile X Premutation Carriers. Curr Psychiat Rev. 2013 doi: 10.2174/157340013805289662. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Silva F, Rodriguez-Revenga L, Madrigal I, Alvarez-Mora MI, Oliva R, Mila M. High apolipoprotein E4 allele frequency in FXTAS patients. Genet Med. 2013 doi: 10.1038/gim.2013.12. [DOI] [PubMed] [Google Scholar]
- 144.Sobesky WE, Taylor AK, Pennington BF, Bennetto L, Porter D, Riddle J, Hagerman RJ. Molecular/clinical correlations in females with fragile X. Am J Med Genet. 1996;64 (2):340–345. doi: 10.1002/(SICI)1096-8628(19960809)64:2<340::AID-AJMG21>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 145.Sofola OA, Jin P, Qin Y, Duan R, Liu H, de Haro M, Nelson DL, Botas J. RNA-binding proteins hnRNP A2/B1 and CUGBP1 suppress fragile X CGG premutation repeat-induced neurodegeneration in a Drosophila model of FXTAS. Neuron. 2007;55 (4):565–571. doi: 10.1016/j.neuron.2007.07.021. S0896-6273(07)00542-9 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Soontarapornchai K, Maselli R, Fenton-Farrell G, Tassone F, Hagerman PJ, Hessl D, Hagerman RJ. Abnormal nerve conduction features in fragile X premutation carriers. Arch Neurol. 2008;65 (4):495–498. doi: 10.1001/archneur.65.4.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Stark KL, Xu B, Bagchi A, Lai WS, Liu H, Hsu R, Wan X, Pavlidis P, Mills AA, Karayiorgou M, Gogos JA. Altered brain microRNA biogenesis contributes to phenotypic deficits in a 22q11-deletion mouse model. Nat Genet. 2008;40 (6):751–760. doi: 10.1038/ng.138. [DOI] [PubMed] [Google Scholar]
- 148.Sullivan SD, Welt C, Sherman S. FMR1 and the continuum of primary ovarian insufficiency. Semin Reprod Med. 2011;29 (4):299–307. doi: 10.1055/s-0031-1280915. [DOI] [PubMed] [Google Scholar]
- 149.Tassone F, Beilina A, Carosi C, Albertosi S, Bagni C, Li L, Glover K, Bentley D, Hagerman PJ. Elevated FMR1 mRNA in premutation carriers is due to increased transcription. RNA. 2007;13 (4):555–562. doi: 10.1261/rna.280807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Tassone F, Greco CM, Hunsaker MR, Seritan AL, Berman RF, Gane LW, Jacquemont S, Basuta K, Jin LW, Hagerman PJ, Hagerman RJ. Neuropathological, clinical and molecular pathology in female fragile X premutation carriers with and without FXTAS. Genes Brain Behav. 2012;11 (5):577–585. doi: 10.1111/j.1601-183X.2012.00779.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Tassone F, Hagerman R. The fragile X-associated tremor ataxia syndrome. Results Probl Cell Differ. 2012;54:337–357. doi: 10.1007/978-3-642-21649-7_18. [DOI] [PubMed] [Google Scholar]
- 152.Tassone F, Hagerman RJ, Chamberlain WD, Hagerman PJ. Transcription of the FMR1 gene in individuals with fragile X syndrome. Am J Med Genet. 2000;97 (3):195–203. doi: 10.1002/1096-8628(200023)97:3<195::AID-AJMG1037>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 153.Tassone F, Hagerman RJ, Loesch DZ, Lachiewicz A, Taylor AK, Hagerman PJ. Fragile X males with unmethylated, full mutation trinucleotide repeat expansions have elevated levels of FMR1 messenger RNA. Am J Med Genet. 2000;94 (3):232–236. doi: 10.1002/1096-8628(20000918)94:3<232::aid-ajmg9>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 154.Tassone F, Hagerman RJ, Taylor AK, Hagerman PJ. A majority of fragile X males with methylated, full mutation alleles have significant levels of FMR1 messenger RNA. J Med Genet. 2001;38 (7):453–456. doi: 10.1136/jmg.38.7.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Tassone F, Hagerman RJ, Taylor AK, Mills JB, Harris SW, Gane LW, Hagerman PJ. Clinical involvement and protein expression in individuals with the FMR1 premutation. Am J Med Genet. 2000;91 (2):144–152. doi: 10.1002/(sici)1096-8628(20000313)91:2<144::aid-ajmg14>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 156.Tassone F, Iwahashi C, Hagerman PJ. FMR1 RNA within the intranuclear inclusions of fragile X-associated tremor/ataxia syndrome (FXTAS) RNA Biol. 2004;1 (2):103–105. doi: 10.4161/rna.1.2.1035. [DOI] [PubMed] [Google Scholar]
- 157.Todd PK, Oh SY, Krans A, He F, Sellier C, Frazer M, Renoux AJ, Chen KC, Scaglione KM, Basrur V, Elenitoba-Johnson K, Vonsattel JP, Louis ED, Sutton MA, Taylor JP, Mills RE, Charlet-Berguerand N, Paulson HL. CGG Repeat-Associated Translation Mediates Neurodegeneration in Fragile X Tremor Ataxia Syndrome. Neuron. 2013 doi: 10.1016/j.neuron.2013.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Udd B, Krahe R. The myotonic dystrophies: molecular, clinical, and therapeutic challenges. Lancet Neurol. 2012;11 (10):891–905. doi: 10.1016/S1474-4422(12)70204-1. [DOI] [PubMed] [Google Scholar]
- 159.Van Dam D, Errijgers V, Kooy RF, Willemsen R, Mientjes E, Oostra BA, De Deyn PP. Cognitive decline, neuromotor and behavioural disturbances in a mouse model for fragile-X-associated tremor/ataxia syndrome (FXTAS) Behav Brain Res. 2005;162 (2):233–239. doi: 10.1016/j.bbr.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 160.Verkerk AJ, Pieretti M, Sutcliffe JS, Fu YH, Kuhl DP, Pizzuti A, Reiner O, Richards S, Victoria MF, Zhang FP, Eussen BE, van Ommen GJB, Blonden LAJ, Riggins GJ, Chastain JL, Kunst CB, Galjaard H, Caskey CT, Nelson DL, Oostra BA, Warren ST. Identification of a gene (FMR-1) containing a CGG repeat coincident with a breakpoint cluster region exhibiting length variation in fragile X syndrome. Cell. 1991;65 (5):905–914. doi: 10.1016/0092-8674(91)90397-h. [DOI] [PubMed] [Google Scholar]
- 161.Wang H, Wang M, Reiss K, Darbinian-Sarkissian N, Johnson EM, Iliakis G, Amini S, Khalili K, Rappaport J. Evidence for the involvement of Puralpha in response to DNA replication stress. Cancer Biol Ther. 2007;6(4):596–602. 3889. doi: 10.4161/cbt.6.4.3889. [pii] [DOI] [PubMed] [Google Scholar]
- 162.Wang JY, Hessl DH, Hagerman RJ, Tassone F, Rivera SM. Age-dependent structural connectivity effects in fragile x premutation. Arch Neurol. 2012;69 (4):482–489. doi: 10.1001/archneurol.2011.2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Weisman-Shomer P, Cohen E, Fry M. Interruption of the fragile X syndrome expanded sequence d(CGG)(n) by interspersed d(AGG) trinucleotides diminishes the formation and stability of d(CGG)(n) tetrahelical structures. Nucleic Acids Res. 2000;28 (7):1535–1541. doi: 10.1093/nar/28.7.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Wenzel HJ, Hunsaker MR, Greco CM, Willemsen R, Berman RF. Ubiquitin-positive intranuclear inclusions in neuronal and glial cells in a mouse model of the fragile X premutation. Brain Res. 2010;1318:155–166. doi: 10.1016/j.brainres.2009.12.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Willemsen R, Hoogeveen-Westerveld M, Reis S, Holstege J, Severijnen LA, Nieuwenhuizen IM, Schrier M, van Unen L, Tassone F, Hoogeveen AT, Hagerman PJ, Mientjes EJ, Oostra BA. The FMR1 CGG repeat mouse displays ubiquitin-positive intranuclear neuronal inclusions; implications for the cerebellar tremor/ataxia syndrome. Hum Mol Genet. 2003;12 (9):949–959. doi: 10.1093/hmg/ddg114. [DOI] [PubMed] [Google Scholar]
- 166.Winarni TI, Chonchaiya W, Sumekar TA, Ashwood P, Morales GM, Tassone F, Nguyen DV, Faradz SMH, Van de Water J, Cook K, Hamlin A, Mu Y, Hagerman PJ, Hagerman RJ. Immune-mediated disorders among women carriers of fragile X premutation alleles. Am J Med Genet A. 2012;158A (10):2473–2481. doi: 10.1002/ajmg.a.35569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Wittenberger MD, Hagerman RJ, Sherman SL, McConkie-Rosell A, Welt CK, Rebar RW, Corrigan EC, Simpson JL, Nelson LM. The FMR1 premutation and reproduction. Fertil Steril. 2007;87 (3):456–465. doi: 10.1016/j.fertnstert.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 168.Yrigollen CM, Durbin-Johnson B, Gane L, Nelson DL, Hagerman R, Hagerman PJ, Tassone F. AGG interruptions within the maternal FMR1 gene reduce the risk of offspring with fragile X syndrome. Genet Med. 2012;14 (8):729–736. doi: 10.1038/gim.2012.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Zhong N, Yang W, Dobkin C, Brown WT. Fragile X gene instability: anchoring AGGs and linked microsatellites. Am J Hum Genet. 1995;57 (2):351–361. [PMC free article] [PubMed] [Google Scholar]
- 170.Zu T, Gibbens B, Doty NS, Gomes-Pereira M, Huguet A, Stone MD, Margolis J, Peterson M, Markowski TW, Ingram MA, Nan Z, Forster C, Low WC, Schoser B, Somia NV, Clark HB, Schmechel S, Bitterman PB, Gourdon G, Swanson MS, Moseley M, Ranum LP. Non-ATG-initiated translation directed by microsatellite expansions. Proc Natl Acad Sci U S A. 2011;108 (1):260–265. doi: 10.1073/pnas.1013343108. [DOI] [PMC free article] [PubMed] [Google Scholar]