Abstract
Background
Abdominoperineal Resection (APR) for low rectal adenocarcinoma is a common procedure with high morbidity including perineal wound complications.
Objective
To determine risk factors for perineal wound dehiscence and to investigate the effect of wound dehiscence on survival.
Design
Retrospective medical record review.
Settings
Tertiary care, university medical center (Division of Surgery, Massachusetts General Hospital, Boston, USA).
Patients
Patients with low rectal adenocarcinoma who underwent APR from January 2001 to June 2012.
Main Outcomes Measures
We assessed the incidence of perineal wound dehiscence as well as survival following surgery.
Results
249 patients underwent APR for rectal carcinoma. The mean age was 62.6 years (range 23–98), 159 (63.8%) were male and the mean body mass index (BMI) was 27.9 (range 16.7–58.5). 153 (61.1%) patients survived for 5 years after surgery. 69 patients (27.7%) developed wound dehiscence. Multivariable analysis revealed the following associations with dehiscence: BMI (OR 1.09; 95% CI 1.03–1.15; P=0.002), inflammatory bowel disease (IBD) (OR 6.6; 95% CI 1.4 –32.5; P=0.02), history of other malignant neoplasm (OR 3.1; 95% CI 1.5–6.6) and APR for cancer recurrence (OR 2.8; 95% CI 1.2–6.3; P=0.01). In the survival analysis, wound dehiscence was associated with decreased survival (mean survival time for dehiscence vs. no dehiscence: 66.6 mo vs. 76.6 mo; P= 0.01). This relationship persisted in the multivariable analysis (HR 1.7; 95% CI 1.1–2.8; P=0.02).
Limitations
This was a retrospective, observational study from a single center.
Conclusions
The adjusted risk of death was 1.7 times higher in patients who experienced dehiscence than in those who did not. Attention to perineal wound closure with consideration of flap creation should at least be given to patients with a history of malignant neoplasm, those with IBD, those with rectal cancer recurrence, and females undergoing posterior vaginectomy. Pre-operative weight loss should also reduce dehiscence risk.
Keywords: rectal adenocarcinoma, survival, abdominoperineal resection, dehiscence, complication
Introduction
For patients with low rectal adenocarcinoma seeking cure, abdominoperineal resection (APR) is usually the procedure of choice. Although chemotherapy and radiation therapy can often shrink rectal cancers and a provide a higher likelihood of sphincter sparing surgery1–5, many patients still require APR to achieve appropriate resection margins. The National Cancer Institute estimates there are approximately 40,000 cases of rectal cancer in the United States each year and approximately one-fifth of these will require APR.6
Even with APR, the cure rate for patients with low rectal cancers is lower than for patients who undergo low anterior resections.7–10 Dissatisfaction with inferior cure rates has led surgeons to consider that a “coned in” dissection during APR may be associated with a high incidence of positive circumferential resection margin and tumor perforation.11,12 For that reason, they argue that wider, extralevator resections should be performed. Proponents of this technique report better results than with standard APR.13,14 Nevertheless, no prospective or randomized trials have tested this hypothesis. Furthermore, the technique involves not just wider resection but also utilization of either a vertical rectus abdominis myocutaneous (VRAM) flap or a gracilis muscle flap to close the wound.15 The addition of a flap closure to an APR has been shown to decrease wound complications but not alter survival.16,17 But the bulk of evidence comes from studies on anal cancer. Rectal cancer patients are different from anal cancer, in that many require chemotherapy after their surgery.18 Wound dehiscence can delay the initiation of chemotherapy. Thus any change in technique that alters the rates of post-operative infections might also change survival.
APR is well recognized as having high morbidity including perineal wound complications, which occur in up to 60% of cases.19–22 Wound dehiscence is a particularly difficult complication since it is unpleasant to the patient and requires expensive wound care. A perineal sinus can persist for months or even years. A number of studies have examined the risk factors for wound complications, but none have had wound dehiscence as the main focus or examined the effect of dehiscence on overall survival.23–25
The aim of this study was to first determine risk factors for post-operative wound dehiscence and to investigate the effect of wound dehiscence on survival.
Materials and Methods
Participants and Study Design
Consecutive adult (age > 18) patients undergoing APR for low rectal adenocarcinoma performed between January 2001 and June 2012 at the Massachusetts General Hospital were identified through a search in the Partners Research Patient Data Registry (RPDR). Low rectal adenocarcinoma was defined as any tumor located less than 1 cm from the puborectalis or less than 5 cm from the anal verge and not considered by the operating surgeon to be a candidate for a sphincter sparing surgery. Patients undergoing APR for reasons other than adenocarcinoma (i.e. squamous cell carcinoma, inflammatory bowel disease) were excluded. This study was reviewed and approved by the Partners Institutional Review Board (Protocol 2012-P-001456/1) with specific waiver of the need for individual patient consent.
Patient files were retrospectively reviewed and pertinent demographic, oncological and surgical information was collected. Study data were collected and managed using REDCap electronic data capture tools hosted at Partners Healthcare.26 REDCap (Research Electronic Data Capture) is a secure, web-based application for data capture for research studies, providing: 1) an intuitive interface for validated data entry; 2) audit trails for tracking data manipulation and export procedures; 3) automated export procedures for data downloads to common statistical packages; and 4) procedures for importing data from external sources. To allow for risk adjustment, a Charlson comorbidity index score was generated for each patient.27 The Charlson comorbidity index predicts the ten-year mortality for a patient and is widely used to risk adjust patient populations.
Outcome Measures
Primary events after surgery included wound dehiscence and survival to death from any cause. Wound dehiscence was defined as separation of the skin at the perineal wound (partial or complete), regardless of the presence of infection. Wounds that were left open after surgery were excluded from the study. Survival information was obtained from the RPDR, which is linked with the Social Security Death Index. Patients with no known death date were censored either at the end of the study period (August 1, 2012) or if records indicated that they left the country.
Secondary outcomes after surgery included urinary retention, urinary tract infection (UTI), and superficial and deep space wound infections. Urinary retention was defined as the need for replacement of a urinary catheter after a failed postoperative voiding trial. Urinary tract infection was defined as a urine culture of > 100,000 colonies/ml urine with no more than two species of organisms. Superficial wound infection was defined as purulent drainage from the superficial incision, pain or tenderness, localized swelling, erythema, or heat. Deep space wound infection was defined as an infection involving any part of the anatomy (for example, organs or spaces) that was opened or manipulated during an operation other than the incision.
Statistical Analysis
Continuous and categorical variables are expressed as mean ± standard deviation and proportions respectively throughout the manuscript with the exception of continuous variables with grossly skewed distributions that are reported as the median with the interquartile range. Bivariate analysis of continuous and categorical variables was performed with logistic regression.
In multivariable analysis of risk factors for wound dehiscence, logistic regression was used. The model was constructed using a stepwise procedure (backwards Wald) with a P value of 0.2 to enter and 0.05 to eliminate variables. All variables were considered for inclusion in the model. For the survival analysis, comparison between patients who developed wound dehiscence and those that did not was performed using the log-rank test. To control for differences in patient characteristics, a Cox Proportional hazard model was used. Again, a stepwise procedure (backwards Wald) was used with a P value of 0.2 to enter and 0.05 to eliminate variables. Age, Charlson score and tumor stage were forced into the model as all are known predictors of mortality. All other variables (with the exclusion of individual comorbidities as they would be collinear with the Charlson score and pathology data as it would be collinear with tumor stage) were considered for inclusion in the model. SAS statistical software (version 9.2; SAS Institutes Inc., Cary, NC, USA) was used for all analysis. All tests were two sided with an alpha level of 0.05.
Results
Patient Characteristics
249 patients underwent APR for rectal adenocarcinoma during the study period. The mean age was 62.6 ± 14.4 years and 63.8% were male (Table 1). Most of the patients were Caucasian (89.1%) and over a quarter were obese (26.9%). The patients were mostly healthy with a mean Charlson score of 3.74 ± 2.4. In terms of oncologic history, 14.4% underwent resection for recurrence and 13.1% had metastases at the time of surgery. Over two-thirds underwent preoperative chemotherapy (71.8%) and/or preoperative radiation (74.7%). For peri-operative variables, 8.8% of the procedures were laparoscopically assisted and the mean operative time was 268.9 ± 137.9 minutes (Table 2). 44.6% of patients underwent concurrent omental flap and/or omentopexy and 3.6% had a VRAM flap repair of the perineal wound.
Table 1.
Baseline Characteristics
| Total = 249 | |
|---|---|
|
Demographic
| |
| Age, mean, y ± SD | 62.6 ± 14.4 |
| Male | 159 (63.8) |
| Caucasian | 222 (89.1) |
| BMI, mean, kg/m2 ± SD | 27.9 ± 5.6 |
| Obese | 67 (26.9) |
|
Comorbities
| |
| COPD | 15 (6.0) |
| CHF | 6 (2.4) |
| MI | 21 (8.4) |
| HTN | 94 (37.7) |
| DM | 38 (15.2) |
| HCT, mean, % ± SD | 37.9 ± 4.4 |
| Renal Disease | 9 (3.6) |
| Hx of Other Cancer | 21 (8.4) |
| Charlson score, mean ± SD | 3.74 ± 2.4 |
| IBD | 9 (3.6) |
| Smoking | 48 (19.3) |
| Alcohol Abuse | 21 (8.4) |
|
Oncologic History
| |
| Resection for recurrence | 36 (14.4) |
| CEA, median, mcg/L [IQR] | 3 [1.5–7.8] |
| Metastatic Disease | 36 (14.4) |
| Neoadjuvant Chemotherapy | 179 (71.8) |
| Neoadjuvant Radiation | 186 (74.7) |
| Tumor Stage | |
| I | 19 (7.6) |
| II | 80 (32.1) |
| III | 115 (46.2) |
| IV | 36 (14.4) |
Data are expressed as n (%) unless otherwise specified.
BMI, Body Mass Index; COPD, Chronic Obstructive Pulmonary Disease; CHF, Congestive Heart Failure; MI, Myocardial Infarction; HTN, Hypertension; DM, Diabetes Mellitus; HCT, Hematocrit; IBD, Inflammatory Bowel Disease; CEA, Carcinoembryonic Antigen; IQR, Inter Quartile Range.
Table 2.
Peri-operative Characteristics
| Total = 249 | |
|---|---|
| Ureteral Tubes | 34 (13.6) |
| Lap Assisted | 22 (8.8) |
| Length of Operation, mean, min ± SD | 268.9 ± 137.9 |
| IORT | 27 (10.8) |
| VRAM Flap | 9 (3.6) |
| Omental Flap/Omentopexy | 111 (44.6) |
| Ureteral Injury | 3 (1.2) |
Data are expressed as n (%) unless otherwise specified.
IORT, Intraoperative Radiation Therapy; VRAM, Vertical Rectus Myocutaneous.
Surgical Outcomes
Overall outcomes are displayed in Table 3. Notably, urinary retention (20.1%), blood transfusion (18.9%) and UTI (13.6%) were seen in greater than ten percent of cases. 30.9% of patients experienced a wound complication. Incidence of superficial perineal wound infection was 10.8% and only 4.4% of patients had a deep space infection. 69 patients (27.7%) experienced wound dehiscence. For those that suffered dehiscence, the median time to heal was 117 days and the range was from 7 to 1096 days.
Table 3.
Post-operative Surgical Outcomes
| Total =249 | |
|---|---|
|
In-Hospital
| |
| LOS, median, d [IQR] | 7 [5–9] |
| ICU | 21 (8.4) |
| Return to OR | 8 (3.2) |
| Pneumonia | 11 (4.4) |
| Reintubation | 2 (0.8) |
| Blood transfusion | 47 (18.9) |
| Urinary Retention | 50 (20.1) |
| UTI | 34 (13.6) |
|
Wound
| |
| Any Wound Issue | 77 (30.9) |
| Superficial Infection | 27 (10.8) |
| Deep Space Infection | 11 (4.4) |
| Dehiscence | 69 (27.7) |
| Time to Dehiscence Healing, median, d [IQR] a | 117 [85–245] |
Data are expressed as n (%) unless otherwise specified.
Only for patients who experienced perineal wound dehiscence. Measured from date of identification of dehiscence to date of healing.
LOS, Length of Stay; IQR, Inter Quartile Range; ICU, Intensive Care Unit; OR, Operating Room; UTI, Urinary Tract Infection.
In a bivariate analysis of risk factors for wound dehiscence, frequency of dehiscence was higher among patients with: increased BMI (OR 1.06; 95% CI 1.01–1.11; P=0.01), chronic obstructive pulmonary disease (COPD) (OR 3.2; 95% CI 1.1–9.3; P=0.03), history of other malignant neoplasm (OR 2.2; 95% CI 1.1–4.5; P=0.03) and operative time greater than the median (239 min) (OR 2.3; 95% CI 1.3–4.0; P=0.01 (Table 4). In females only, frequency of dehiscence was greater in those undergoing concomitant posterior vaginectomy (OR 2.2; 95% CI 1.2–8.9; P=0.02),
Table 4.
Bivariate and Multivariable Analysis of Risk Factors for Wound Dehiscence
| Demographic | Bivariate
|
Multivariable
|
|||
|---|---|---|---|---|---|
| Dehiscence | OR (95% CI) | P | OR (95% CI) | P | |
| Age > 65 years | 31/105 (29.5%) | 1.2 (0.7–2.0) | 0.61 | ||
| No | 39/144 (27.1%) | ||||
| Male | 43/159 (27.1%) | 0.8 (0.5–1.5) | 0.56 | ||
| Female | 27/90 (30%) | ||||
| Caucasian | 63/222 (28.4%) | 1.5 (0.6–3.8) | 0.42 | ||
| Non-Caucasian | 7/27 (25.9%) | ||||
| BMI, mean, kg/m2 ± SD | 27.2 ± 4.8 29.2 ± 7.3 |
1.06 (1.01–1.11) | 0.01 | 1.09 (1.03–1.15) | 0.002 |
| Comorbidities | |||||
| COPD | 8/15 (53.3%) | 3.2 (1.1–9.3) | 0.03 | 5.8 (1.9–18.0) | 0.002 |
| No | 61/233 (26.2%) | ||||
| CHF | 2/5 (40%) | 1.7 (0.3–10.7) | 0.54 | ||
| No | 67/243 (27.6%) | ||||
| MI | 8/21 (38.1%) | 1.7 (0.7–4.2) | 0.28 | ||
| No | 61/227 (26.9%) | ||||
| HTN | 27/93 (29%) | 1.1 (0.6–1.9) | 0.74 | ||
| No | 42/155 (27.1%) | ||||
| DM | 12/38 (31.6%) | 1.3 (0.6–2.6) | 0.57 | ||
| No | 57/210 (27.1%) | ||||
| HCT < 30 | 2/14 (13.3%) | 0.4 (0.1–1.7) | 0.21 | ||
| No | 67/233 (28.8%) | ||||
| Renal Disease | 3/9 (33.3%) | 1.3 (0.3–5.4) | 0.71 | ||
| No | 66/239 (27.6%) | ||||
| Hx of Other Cancer | 17/40 (42.5%) | 2.2 (1.1–4.5) | 0.03 | 3.1 (1.5–6.6) | 0.01 |
| No | 52/208 (25%) | ||||
| Charlson, mean ± SD | 3.61 ± 2.3 4.1 ± 2.7 |
1.1 (1.0–1.2) | 0.10 | NS | |
| IBD | 5/9 (55.6%) | 3.4 (0.9–13.1) | 0.07 | 6.6 (1.4–32.5) | 0.02 |
| No | 64/239 (26.8%) | ||||
| Smoking | 15/48 (31.2%) | 1.2 (0.6–2.4) | 0.55 | ||
| No | 54/200 (27%) | ||||
| Alcohol Abuse | 9/20 (45%) | 2.3 (0.9–5.8) | 0.08 | NS | |
| No | 60/227 (26.4%) | ||||
| Oncologic | |||||
| Recurrence | 9/20 (45%) | 1.9 (0.9–4.0) | 0.09 | 2.8 (1.2–6.3) | 0.01 |
| No | 60/227 (26.4%) | ||||
| CEA > 3 mcg/L | 22/102 (21.6%) | 0.6 (0.3–1.0) | 0.07 | NS | |
| No | 47/146 (32.2%) | ||||
| Neoadjuvant Chemo | 52/178 (29.2%) | 1.3 (0.7–2.4) | 0.43 | ||
| No | 17/70 (24.3%) | ||||
| Neoadjuvant Radiation | 54/186 (29%) | 1.3 (0.7–2.5) | 0.46 | ||
| No | 15/62 (24.2%) | ||||
| Stage | 0.12 | NS | |||
| I | 3/19 (15.8%) | REF | |||
| II | 22/80 (27.5%) | 1.9 (0.5–7.3) | |||
| III | 39/114 (34.2%) | 2.8 (0.8–10.1) | |||
| IV | 6/36 (16.7%) | 1.1 (0.2–4.8) | |||
| Peri-operative | |||||
| Lap Assisted | 5/22 (22.7%) | 0.7 (0.3–2.1) | 0.57 | ||
| No | 63/225 (28.4%) | ||||
| Operation Time > 239 min | 44/122 (36.1%) | 2.3 (1.3–4.0) | 0.005 | NS | |
| No | 25/126 (19.8%) | ||||
| IORT | 8/26 (30.8%) | 1.2 (0.5–2.8) | 0.74 | ||
| No | 61/220 (27.7%) | ||||
| VRAM Flap | 4/9 (44.4%) | 2.1 (0.6–8.2) | 0.27 | ||
| No | 65/239 (27.2%) | ||||
| Omental Flap/Omentopexy | 33/108 (30.6%) | 1.3 (0.7–2.3) | 0.37 | ||
| No | 35/138 (25.4%) | ||||
| Posterior vaginectomya | 11/22 (50%) | 2.2 (1.2–8.9) | 0.02 | ||
| No | 16/68 (23.5%) | ||||
| RO resection | 61/225 (27.1%) | 0.7 (0.3–1.7) | 0.44 | ||
| No | 8/23 (34.8%) | ||||
| Node positive | 23/77 (29.9%) | 1.2 (0.6–2.1) | 0.63 | ||
| No | 46/171 (26.9%) | ||||
| CRM < 5mm | 45/158 (28.5%) | 1.1 (0.6–1.9) | 0.76 | ||
Numbers in bold are variables with P< 0.2 and are included for consideration in the multivariable analysis.
Frequency calculated from only the female population.
CI, Confidence Interval; BMI, Body Mass Index; COPD, Chronic Obstructive Pulmonary Disease; CHF, Congestive Heart Failure; MI, Myocardial Infarction; HTN, Hypertension; DM, Diabetes Mellitus; HCT, Hematocrit; NS, Not Significant; IBD, Inflammatory Bowel Disease; CEA, Carcinoembryonic Antigen; IORT, Intraoperative Radiation Therapy; VRAM, Vertical Rectus Myocutaneous; CRM, Circumferential Resection Margin.
It was interesting that there were no significant differences between the two groups from an oncological perspective. The frequency of dehiscence did not differ between patients who received neoadjuvant chemo/radiotherapy and those who did not and there were no significant differences in frequency of metastatic disease or stage. There was no difference in dehiscence frequency between those receiving Intraoperative Radiation Therapy (IORT) and those that did not. Finally, dehiscence frequency was similar between those with R0 resection, Circumferential Resection Margin (CRM) < 5mm, positive nodes and those without.
In the multivariable model, increasing BMI (OR 1.09; 95% CI 1.03–1.15; P=0.002), COPD (OR 5.8; 95% CI 1.9–18.0; P=0.002), history of other malignant neoplasm (OR 3.1; 95% CI 1.5–6.6; P=0.01), inflammatory bowel disease (IBD) (OR 6.6; 95% CI 1.4–32.5; P=0.02), and APR for recurrent rectal cancer (OR 2.8; 95% CI 1.2–6.3; P=0.02) were all associated with increased odds of wound dehiscence (Table 4).
Oncologic Outcomes and Survival
Over the study period there were 70 deaths (28.1%). For patients who did not die, median follow-up time was 45.3 (range 1.2–129.5) months. In the survival analysis, patients who experienced post-operative wound dehiscence had lower mean survival compared to those that did not (66.6 months vs. 76.6 months; log-rank P=0.01) (Figure 1). Five-year survival in the postoperative dehiscence group was 47.3%, markedly lower than the 66.3% five-year survival in the non dehiscence group. In the multivariable Cox proportional hazard model, perineal wound dehiscence was associated with significantly increased hazard of death (HR: 1.7; 95% CI: 1.1–2.8; P=0.02) (Table 5).
Figure 1.
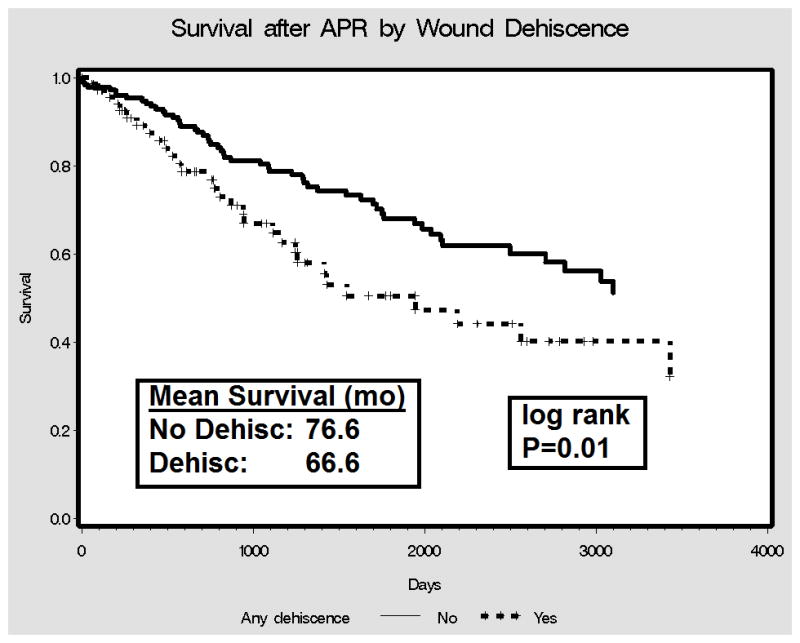
Survival After APR by Perineal Wound Dehiscence
Table 5.
Bivariate and Multivariable Cox Proportional Hazard Analyses of Mortality.
| Overall Mortality | Number of events/Number of patients | Bivariate
|
Multivariable
|
||
|---|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | ||
| Perineal Wound Dehiscence | 30/69 | 1.7 (1.1–2.7) | 0.02 | 1.7 (1.1–2.8) | 0.024 |
| No | 54/180 | ||||
| Age in years | N/A | 1.04 (1.03–1.06) | <0.0001 | 1.04 (1.02–1.07) | 0.001 |
| Male | 52/159 | 0.9 (0.5–1.3) | 0.5 | ||
| Female | 32/90 | ||||
| Caucasian | 77/222 | 1.0 (0.5–2.2) | 0.96 | ||
| Non Caucasian | 7/27 | ||||
| BMI | |||||
| Obese (>30) | 20/67 | 0.7 (0.4–1.2) | 0.17 | NS | |
| Non-obese (<=30) | 64/181 | ||||
| Charlson Score | N/A | 1.2 (1.1–1.3) | <0.0001 | 1.1 (1.0–1.2) | 0.174 |
| Smoking | 16/48 | 0.9 (0.5–1.6) | 0.81 | ||
| No | 68/200 | ||||
| Resection for Recurrence | 13/35 | 1.3 (0.7–2.3) | 0.41 | ||
| No | 71/213 | ||||
| Neoadjuvant Chemo | 51/178 | 0.6 (0.4–1.0) | 0.06 | NS | |
| No | 33/70 | ||||
| Neoadjuvant Radiation | 54/186 | 0.7 (0.4–1.1) | 0.07 | NS | |
| No | 30/62 | ||||
| Stage | 0.001 | <0.0001 | |||
| I | 6/19 | 1a | 1a | ||
| II | 25/79 | 1.2 (0.5–3.0) | 1.4 (0.5–3.4) | ||
| III | 35/114 | 1.6 (0.7–3.8) | 2.2 (0.9–5.6) | ||
| IV | 18/36 | 3.8 (1.5–9.7) | 8.2 (3.1–21.6) | ||
Referent group.
HR, Hazard ratio. BMI, Body Mass Index.
Numbers in bold are variables with P< 0.2 and are included for consideration in the multivariable analysis.
Discussion
Post operative wound dehiscence, though unpleasant and costly, has traditionally been thought to have no effect on long term survival. In this study of 249 patients undergoing APR for rectal adenocarcinoma increased BMI, history of other malignant neoplasm, COPD, IBD, and operating for recurrent disease were all associated with increased odds of post-operative perineal wound dehiscence. In turn, dehiscence was shown to have a significant association with increased mortality in a multivariable analysis.
This study has a number of strengths and weaknesses. The association between dehiscence and decreased survival is a novel one. In the bivariate analysis, the two groups were similar in terms of both preoperative and perioperative oncologic factors. With similar rates of metastasis at presentation, positive margins, positive nodes, IORT, and posterior vaginectomy in women, similar survival outcomes would be expected. Instead, a marked difference in survival was found. This study attempted to control, via statistical analysis, for potential confounders such as age, increased comorbidity and tumor stage, so that the occurrence of dehiscence was not simply a proxy for poor health. Unfortunately, this study was unable to identify very many modifiable risk factors. Besides advocating weight loss, there is little that clinicians can do to prevent wound dehiscence and its associated decrease in survival.
Several limitations deserve mention in this study. First, although this study used multivariable regression for both the occurrence of wound dehiscence and the subsequent effect on survival to reduce the biases inherent in observational studies, there could be hidden biases since our study only controlled for the observed variables. Second, this study’s cohort was drawn from a single, tertiary care center with experienced colorectal surgeons. It may not accurately represent the universe of patients undergoing APR.
Other studies have examined wound healing after APR. A high risk of wound infection after APR has been well described.19–21,24,25,28 The wound complication rate in this study (30.8%) is comparable to those found by Bullard et al (41%), Artioukh et al (26%) and Chessin et al (37.2%).19–20,28 Similarly, the rate of dehiscence in this study (27.7%) parallels the findings of Bullard et al (24%), Artioukh et al (22.5%) and Butler et al (22.5%).19,21,28 The perineal wound is particular vulnerable to breakdown due to factors such as pre-operative radiation, tension, and its location in a “dirty” area. Multiple risk factors for wound complications have been identified. There is a consensus that neoadjuvant chemoradiation is a major risk factor for poor wound outcomes in the perineum after APR.23,28,29 It is interesting that this study found only a non-significant trend toward increased dehiscence in patients who underwent chemoradiation therapy. This is perhaps because a high proportion of our patients underwent neoadjuvant chemoradiation. Similarly, obesity and smoking are associated with increased wound complications after APR.25,30 Our analysis supported an association with obesity but not smoking. Overall, a strength of this study is the examination of the long term survival effects of wound dehiscence which have not been previously examined.
The known risk of perineal wound issues has generated many techniques for closing the perineal wound. In his original manuscript, Miles described leaving the wound open, packed and allowed to heal by secondary intention.31 While this approach might avoid some complications of primary closure, it is a great discomfort and an inconvenience for the patient. The use of omental pedicle flaps has been well described, though with conflicting results. Studies have reported decreased risk of dehiscence32 and improved wound healing.33,34 Other studies have documented no benefit.35,36 The findings in this study support no difference in wound dehiscence with or without an omental flap. More recently, muscle flap closure has shown great promise. Lefevre et. al. demonstrated decreased morbidity and reduced healing time with the use of a VRAM flap in patients undergoing APR for anal cancer.16
In considering the association between dehiscence and survival, one possible explanation is that perineal wound dehiscence would delay postoperative chemotherapy. Since postoperative chemotherapy is the standard of care to prevent local and distant recurrence, any delay could have a negative effect on survival.18, 37
We believe this study brings to light the importance of wound dehiscence after APR for rectal adenocarcinoma with significant implications for clinicians. Perineal wound dehiscence will only become more of an issue as surgeons move toward more aggressive, extralevator APR.13,38 We question whether the survival benefit seen in extralevator APR is truly from the wider resection, or instead from the fact that most of these patients receive flap coverage of their perineal wound with subsequent decreased wound complications and a shorter interval to postoperative chemotherapy. In future trials, the use of flap coverage is an important factor that needs to be taken into account. Further prospective or randomized controlled studies utilizing muscle flap closure will hopefully elucidate the best practice for minimizing wound dehiscence. Included in these studies should be detailed information about the effect of postoperative chemotherapy. This is a question unable to be answered from this study. For now, we recommend increased surveillance and careful wound care for high risk patients as identified in this study.
Conclusion
The adjusted risk of death was 1.7 times higher in patients who experienced dehiscence than in those who did not. Attention to perineal wound closure with consideration of flap creation should at least be given to patients with a history of malignant neoplasm, those with IBD, those with rectal cancer recurrence, and females undergoing posterior vaginectomy. Pre-operative weight loss should also reduce dehiscence risk.
Acknowledgments
Support: Dr. Hawkins is supported by a grant from the Brigham & Women’s Center for Surgery and Public Health Arthur Tracy Cabot Fellowship and the NIH NHLBI T32 (HL007734) Harvard/Longwood Vascular Surgery Training Program.
The authors thank Maria J. Schaumeier, MD of the Harvard Center for Surgery and Public Health for her review of the manuscript.
Footnotes
Conflicts of Interest: None
Author contribution:
Study conception and design: Hawkins, Bordeianou
Acquisition of data: Hawkins, Bordeianou
Analysis and interpretation of data: Hawkins, Bordeianou
Drafting of Manuscript: Hawkins, Berger, Shellito, Sylla, Bordeianou
Critical revision: Hawkins, Berger, Shellito, Sylla, Bordeianou
Presented as a podium presentation at the 2013 Annual Scientific Meeting of the American Society of Colon & Rectal Surgeons, Phoenix, AZ, April 27–May 1, 2013.
References
- 1.Garcia-Aguilar J, Hernandez de Anda E, Sirivongs P, Lee SH, Madoff RD, Rothenberger DA. A pathologic complete response to preoperative chemoradiation is associated with lower local recurrence and improved survival in rectal cancer patients treated by mesorectal excision. Dis Colon Rectum. 2003;46:298–304. doi: 10.1007/s10350-004-6545-x. [DOI] [PubMed] [Google Scholar]
- 2.Chen ET, Mohiuddin M, Brodovsky H, Fishbein G, Marks G. Downstaging of advanced rectal cancer following combined preoperative chemotherapy and high dose radiation. Int J Radiat Oncol Biol Phys. 1994;30:169–175. doi: 10.1016/0360-3016(94)90532-0. [DOI] [PubMed] [Google Scholar]
- 3.Minsky BD, Cohen AM, Enker WE, Paty P. Sphincter preservation with preoperative radiation therapy and coloanal anastomosis. Int J Radiat Oncol Biol Phys. 1995;31:553–559. doi: 10.1016/0360-3016(94)00375-U. [DOI] [PubMed] [Google Scholar]
- 4.Theodoropoulos G, Wise WE, Padmanabhan A, et al. T-level downstaging and complete pathologic response after preoperative chemoradiation for advanced rectal cancer result in decreased recurrence and improved disease-free survival. Dis Colon Rectum. 2002;45:895–903. doi: 10.1007/s10350-004-6325-7. [DOI] [PubMed] [Google Scholar]
- 5.Moore HG, Gittleman AE, Minsky BD, et al. Rate of pathologic complete response with increased interval between preoperative combined modality therapy and rectal cancer resection. Dis Colon Rectum. 2004;47:279–286. doi: 10.1007/s10350-003-0062-1. [DOI] [PubMed] [Google Scholar]
- 6.Tilney HS, Heriot AG, Purkayastha S, et al. A national perspective on the decline of abdominoperineal resection for rectal cancer. Ann Surg. 2008;247:77–84. doi: 10.1097/SLA.0b013e31816076c3. [DOI] [PubMed] [Google Scholar]
- 7.Nagtegaal ID, van de Velde CJ, Marijnen CA, et al. Low rectal cancer: A call for a change of approach in abdominoperineal resection. J Clin Oncol. 2005;23:9257–9264. doi: 10.1200/JCO.2005.02.9231. [DOI] [PubMed] [Google Scholar]
- 8.West NP, Finan PJ, Anderin C, Lindholm J, Holm T, Quirke P. Evidence of the oncologic superiority of cylindrical abdominoperineal excision for low rectal cancer. J Clin Oncol. 2008;26:3517–3522. doi: 10.1200/JCO.2007.14.5961. [DOI] [PubMed] [Google Scholar]
- 9.Weiser MR, Quah HM, Shia J, et al. Sphincter preservation in low rectal cancer is facilitated by preoperative chemoradiation and intersphincteric dissection. Ann Surg. 2009;249:236–242. doi: 10.1097/SLA.0b013e318195e17c. [DOI] [PubMed] [Google Scholar]
- 10.Silberfein EJ, Kattepogu KM, Hu CY, et al. Long-term survival and recurrence outcomes following surgery for distal rectal cancer. Ann Surg Oncol. 2010;17:2863–2869. doi: 10.1245/s10434-010-1119-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tekkis PP, Heriot AG, Smith J, et al. Comparison of circumferential margin involvement between restorative and nonrestorative resections for rectal cancer. Colorectal Dis. 2005;7:369–374. doi: 10.1111/j.1463-1318.2005.00767.x. [DOI] [PubMed] [Google Scholar]
- 12.Anderin C, Martling A, Hellborg H, Holm T. A population-based study on outcome in relation to the type of resection in low rectal cancer. Dis Colon Rectum. 2010;53:753–760. doi: 10.1007/DCR.0b013e3181cf7e27. [DOI] [PubMed] [Google Scholar]
- 13.Stelzner S, Koehler C, Stelzer J, Sims A, Witzigmann H. Extended abdominoperineal excision vs. standard abdominoperineal excision in rectal cancer--a systematic overview. Int J Colorectal Dis. 2011;26:1227–1240. doi: 10.1007/s00384-011-1235-3. [DOI] [PubMed] [Google Scholar]
- 14.Stelzner S, Hellmich G, Schubert C, Puffer E, Haroske G, Witzigmann H. Short-term outcome of extra-levator abdominoperineal excision for rectal cancer. Int J Colorectal Dis. 2011;26:919–925. doi: 10.1007/s00384-011-1157-0. [DOI] [PubMed] [Google Scholar]
- 15.Holm T, Ljung A, Haggmark T, Jurell G, Lagergren J. Extended abdominoperineal resection with gluteus maximus flap reconstruction of the pelvic floor for rectal cancer. Br J Surg. 2007;94:232–238. doi: 10.1002/bjs.5489. [DOI] [PubMed] [Google Scholar]
- 16.Lefevre JH, Parc Y, Kerneis S, et al. Abdomino-perineal resection for anal cancer: Impact of a vertical rectus abdominis myocutaneus flap on survival, recurrence, morbidity, and wound healing. Ann Surg. 2009;250:707–711. doi: 10.1097/SLA.0b013e3181bce334. [DOI] [PubMed] [Google Scholar]
- 17.Sunesen KG, Buntzen S, Tei T, Lindegaard JC, Norgaard M, Laurberg S. Perineal healing and survival after anal cancer salvage surgery: 10-year experience with primary perineal reconstruction using the vertical rectus abdominis myocutaneous (VRAM) flap. Ann Surg Oncol. 2009;16:68–77. doi: 10.1245/s10434-008-0208-4. [DOI] [PubMed] [Google Scholar]
- 18.NIH consensus conference. Adjuvant therapy for patients with colon and rectal cancer. JAMA. 1990;264:1444–1450. [PubMed] [Google Scholar]
- 19.Artioukh DY, Smith RA, Gokul K. Risk factors for impaired healing of the perineal wound after abdominoperineal resection of rectum for carcinoma. Colorectal Dis. 2007;9:362–367. doi: 10.1111/j.1463-1318.2006.01159.x. [DOI] [PubMed] [Google Scholar]
- 20.Chessin DB, Hartley J, Cohen AM, et al. Rectus flap reconstruction decreases perineal wound complications after pelvic chemoradiation and surgery: A cohort study. Ann Surg Oncol. 2005;12:104–110. doi: 10.1245/ASO.2005.03.100. [DOI] [PubMed] [Google Scholar]
- 21.Butler CE, Gundeslioglu AO, Rodriguez-Bigas MA. Outcomes of immediate vertical rectus abdominis myocutaneous flap reconstruction for irradiated abdominoperineal resection defects. J Am Coll Surg. 2008;206:694–703. doi: 10.1016/j.jamcollsurg.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 22.Nelson RA, Butler CE. Surgical outcomes of VRAM versus thigh flaps for immediate reconstruction of pelvic and perineal cancer resection defects. Plast Reconstr Surg. 2009;123:175–183. doi: 10.1097/PRS.0b013e3181904df7. [DOI] [PubMed] [Google Scholar]
- 23.Farid H, O’Connell TX. Methods to decrease the morbidity of abdominoperineal resection. Am Surg. 1995;61:1061–1064. [PubMed] [Google Scholar]
- 24.Luna-Perez P, Rodriguez-Ramirez S, Vega J, Sandoval E, Labastida S. Morbidity and mortality following abdominoperineal resection for low rectal adenocarcinoma. Rev Invest Clin. 2001;53:388–395. [PubMed] [Google Scholar]
- 25.Christian CK, Kwaan MR, Betensky RA, Breen EM, Zinner MJ, Bleday R. Risk factors for perineal wound complications following abdominoperineal resection. Dis Colon Rectum. 2005;48:43–48. doi: 10.1007/s10350-004-0855-x. [DOI] [PubMed] [Google Scholar]
- 26.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 28.Bullard KM, Trudel JL, Baxter NN, Rothenberger DA. Primary perineal wound closure after preoperative radiotherapy and abdominoperineal resection has a high incidence of wound failure. Dis Colon Rectum. 2005;48:438–443. doi: 10.1007/s10350-004-0827-1. [DOI] [PubMed] [Google Scholar]
- 29.Nissan A, Guillem JG, Paty PB, et al. Abdominoperineal resection for rectal cancer at a specialty center. Dis Colon Rectum. 2001;44:27–35. doi: 10.1007/BF02234816. discussion 35–6. [DOI] [PubMed] [Google Scholar]
- 30.Wiatrek RL, Thomas JS, Papaconstantinou HT. Perineal wound complications after abdominoperineal resection. Clin Colon Rectal Surg. 2008;21:76–85. doi: 10.1055/s-2008-1055325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miles W. A method of of perfoming abdominoperineal resection for adenocarcinoma of the low rectum. Lancet. 1908;2:1812. [Google Scholar]
- 32.Hay JM, Fingerhut A, Paquet JC, Flamant Y. Management of the pelvic space with or without omentoplasty after abdominoperineal resection for carcinoma of the rectum: A prospective multicenter study. the french association for surgical research. Eur J Surg. 1997;163:199–206. [PubMed] [Google Scholar]
- 33.Page CP, Carlton PK, Jr, Becker DW. Closure of the pelvic and perineal wounds after removal of the rectum and anus. Dis Colon Rectum. 1980;23:2–9. doi: 10.1007/BF02587192. [DOI] [PubMed] [Google Scholar]
- 34.Moreaux J, Horiot A, Barrat F, Mabille J. Obliteration of the pelvic space with pedicled omentum after excision of the rectum for cancer. Am J Surg. 1984;148:640–644. doi: 10.1016/0002-9610(84)90342-8. [DOI] [PubMed] [Google Scholar]
- 35.O’Leary DP. Use of the greater omentum in colorectal surgery. Dis Colon Rectum. 1999;42:533–539. doi: 10.1007/BF02234183. [DOI] [PubMed] [Google Scholar]
- 36.van der Wal BC, Cleffken BI, Gulec B, Kaufman HS, Choti MA. Results of salvage abdominoperineal resection for recurrent anal carcinoma following combined chemoradiation therapy. J Gastrointest Surg. 2001;5:383–387. doi: 10.1016/s1091-255x(01)80066-4. [DOI] [PubMed] [Google Scholar]
- 37.Biagi JJ, Raphael MJ, Mackillop WJ, Kong W, King WD, Booth CM. Association Between Time to Initiation of Adjuvant Chemotherapy and Survival in Colorectal Cancer: A Systematic Review and Meta-analysis. JAMA. 2011;305:2335–2342. doi: 10.1001/jama.2011.749. [DOI] [PubMed] [Google Scholar]
- 38.Foster JD, Pathak S, Smart NJ, et al. Reconstruction of the perineum following extralevator abdominoperineal excision for carcinoma of the lower rectum: A systematic review. Colorectal Dis. 2012;14:1052–1059. doi: 10.1111/j.1463-1318.2012.03169.x. [DOI] [PubMed] [Google Scholar]


