Abstract
Numerous studies have shown that Alzheimer’s Disease (AD) pathology begins before the onset of clinical symptoms. Because therapies are likely to be more effective if they are implemented early in the disease progression, it is necessary to identify reliable biomarkers to detect AD pathology in the early stages of the disease, ideally in presymptomatic individuals. Recent research has identified three candidate cerebrospinal fluid (CSF) biomarkers that reflect AD pathology: amyloid beta (Aß42), total tau protein (t-tau), and tau protein phosphorylated at AD-specific epitopes (p-tau). They are useful in supporting the AD diagnosis and have predictive value for AD when patients are in the stage of mild cognitive impairment (MCI). However, their predictive utility in cognitively healthy subjects is still being evaluated. We conducted a review of studies published between 1993 and 2011 and summarized their findings on the role of CSF biomarkers for AD in healthy elderly.
Keywords: Review, Alzheimer’s disease, Cerebrospinal fluid, Biomarkers, Imaging, Atrophy, ApoE, Tau, Amyloid beta, Phosphorylated tau, Normal populations
2. INTRODUCTION
The diagnosis of Alzheimer’s disease (AD) is currently based on clinical criteria, which require a patient to have dementia before a diagnosis can be made [1,2]. The development of sensitive and specific biological markers has prompted the recent recommendation to incorporate biomarkers in research criteria for AD [3] and Mild Cognitive Impairment (MCI) [4]. The early diagnosis and prediction of AD is especially urgent given that the testing of disease-modifying drugs will probably have a greater chance for success when administered in the early stages of AD, before neurodegeneration becomes widespread [2,5].
Biomarkers are objective measures whose presence, concentration, and activity are associated with a disease [6]. An ideal biological marker would detect a fundamental feature of AD; would be reliable, inexpensive, noninvasive and simple to test; and would have specificity greater than 75% and sensitivity greater than 85%. It should reflect a causal path between underlying pathology and clinical symptoms [7,8], and also should reflect therapeutic effects.
AD is characterized by the presence of extracellular plaques (composed of amyloid) and intracellular neurofibrillary tangles (composed of hyperphosphorylated tau protein). Deposition of plaques and tangles is believed to promote neurodegeneration in AD [6,9,10]. It is known that AD pathology begins years before clinical symptoms become evident [11].
Cerebrospinal fluid (CSF), largely produced by the choroid plexus, is found in the ventricular system and subarachnoid spaces surrounding the brain and spinal cord. Because CSF is in direct contact with the brain, its composition is affected by biochemical changes in the brain [6]. CSF amyloid beta 42 (Aß42), total tau (t-tau) and phosphorylated tau (p-tau) proteins have been shown to reflect deposition of amyloid plaques, neuronal death and accumulation of tangles, respectively [12-14]. These markers have been studied separately, in combination with each other, and in conjunction with information from clinical exams, laboratory tests, and brain imaging techniques like magnetic resonance imaging (MRI) and positron emission tomography (PET) in AD patients as well as in MCI and cognitively normal (NL) populations at risk for AD [15-17].
CSF biomarkers have gained an important status in the area of MCI-stage diagnosis of AD as they reflect AD-related pathological changes. However, many questions remain unanswered. One of the major unknowns is how well CSF biomarkers can identify AD pathology and predict future AD symptoms in clinically healthy NL. What is the prevalence and meaning of purportedly AD-related CSF abnormalities in NL subjects? And how well do they correlate in the NL population with imaging markers reflective of structural and functional damage? This review summarizes current studies that have addressed these issues.
Although we will focus on cognitively healthy elderly, it would be difficult to consider the meaning of biomarkers in this group without first discussing how well they discriminate between NL, MCI and AD subjects. To address this question, we analyzed CSF studies that were published in peer-reviewed journals between 1993 and 2011, and where groups had 10 or more subjects. A total of 56 studies fulfilled our criteria and will be reviewed below. We will summarize current knowledge about the relationship between age, Apolipoprotein E (ApoE) genotype and CSF biomarkers among NL elderly. We will present data concerning the prevalence of “abnormal” CSF biomarkers in normal populations and their predictive value for future cognitive decline. Finally, we will discuss the associations between neuroimaging findings and AD CSF markers in cognitively healthy subjects and their relationships with common vascular risk factors.
3. DIAGNOSTIC ACCURACY
3.1 Aß42
The extracellular plaques characteristic of AD are mainly made up of Aß peptides, which are generated by the cleavage of the amyloid precursor protein (APP) and secreted by the cells. Among several forms of Aß, the diagnostic potential of the Aß40 and Aß42 forms has been studied most extensively [18].
The majority of studies have shown that in AD subjects, CSF Aß42 and Aß40 decline markedly over time [2,6,19-22]. It is hypothesized that the abnormally low CSF Aß levels in AD subjects are a reflection of increased deposition of Aß into senile plaques, resulting in reduced CSF Aß concentrations[18,23]. Only a few reports found increased [24] or unchanged [25] Aß42 in AD. Interestingly, increased Aß42 was found only in subjects with early, but not late, stages of AD, allowing authors to hypothesize that Aß42 reduction is preceded by an initial increase in its concentration [24]. Thus, their study group might have consisted of subjects in this particular early period. As for the report of unchanged Aß levels, a small reduction was observed; however, it did not reach statistical significance [25].
Overall, Aß42 shows an inverse relationship with the degree of cognitive impairment, with AD patients having lower levels of Aß42 than MCI and NL [15,26-28,28]. In our meta-analysis of 50 studies, Aß42 levels decreased on average by 39% in AD subjects as compared to controls (Table 1). A previous literature review performed by Blennow showed that at 90% specificity, Aß42 discriminated AD from controls with 85% sensitivity [15].
TABLE 1.
Differences between AD and NL in CSF biomarkers value across different studies.
| First author, year | AD (n) | NL (n) |
AD value as a percentage of NL | ||
|---|---|---|---|---|---|
| Aß42 | T-tau | P-tau | |||
| Andreasen 1999[31] | 53 | 21 | 42.2% | ||
| Andreasen 1999[19] | 407 | 65 | 304.0% | ||
| Andreasen 2001[55] | 43 | 18 | 419.0% | ||
| Arai 1995[84] | 70 | 19 | 443.0% | ||
| Arai 1998[195] | 69 | 17 | 858.0% | ||
| Blennow 1995[41] | 44 | 31 | 283.0% | ||
| Bouwman 2007[96] | 50 | 17 | 54.9% | 200.2% | 158.4% (181) |
| Brettschneider 2006[196] | 73 | 33 | 331.3% | ||
| Buerger 2006[43] | 37 | 10 | 178.6% | 660.5% (231) | |
| Buerger 2009[197] | 17 | 15 | 74.8% | 180.9% | 120.0% (181); 362.5% (231) |
| Caroli 2010[198] | 102 | 114 | 69.6% | 170.9% | |
| Clark 2003[13] | 60 | 73 | 448.0% | ||
| Fellgiebel 2009[199] | 20 | 10 | 218.0% (181) | ||
| Galasko[200] | 82 | 60 | 56.0% | ||
| Ganzer 2003[201] | 105 | 68 | 76.9% | 353.1% | |
| Hampel 2004[46] | 108 | 23 | 186.3% (181); 212.5% (199); 1907.1% (231) | ||
| Hu 2002[202] | 52 | 56 | 226.0% | ||
| Hulstaert 1999[203] | 150 | 100 | 57.0% | 218.0% | |
| Ibach 2005[204] | 76 | 39 | 246.9% | ||
| Ibach 2006[205] | 76 | 39 | 63.3% | 168.1% (181) | |
| Iqbal 2005[204] | 334 | 115 | 64.9% | 181.8% | |
| Itoh 2001[85] | 236 | 95 | 317.0% (199) | ||
| Kanai 1998[206] | 93 | 54 | 45.4% | 225.3% | |
| Kanemaru 2000[207] | 60 | 32 | 49.0% | ||
| Kapaki 2001[37] | 38 | 47 | 51.0% | ||
| Kapaki 2003[208] | 49 | 49 | 49.0% | 360.0% | |
| Kester 2011[100] | 68 | 24 | 65.2% | 150.0% | 138.7% (181) |
| Kurz 1998[209] | 40 | 36 | 442.0% | ||
| Maccioni 2006[210] | 23 | 25 | 74.2% | ||
| Maddalena 2003[52] | 51 | 31 | 57.5% | 192.5% (181) | |
| Maruyama 2001[211] | 19 | 15 | 70.5% | 351.1% | |
| Mattsson 2009[193] | 529 | 304 | 54.8% | 199.6% | 160.7% (181) |
| Motter 1995[54] | 37 | 20 | 61.0% | ||
| Nishimura 1998[212] | 163 | 169 | 227.0% | ||
| Okonkwo 2011[213] | 100 | 114 | 69.7% | 174.5% | 168.0% (181) |
| Pratico 2002 | 28 | 18 | 217.5% | ||
| Riemenschneider 2002[214] | 74 | 40 | 37.0% | 355.0% | |
| Rolstad 2011[215] | 100 | 60 | 68.6% | 170.1% | |
| Rosler 2001[216] | 27 | 49 | 48.0% | 320.0% | |
| Seppala 2011[217] | 56 | 8 | 67.6% | 156.8% | 155.1% (181) |
| Shaw 2009[128] | 100 | 114 | 69.9% | 174.2% | 168.0% (181) |
| Shoji 1998[72] | 55 | 34 | 42.5% | 213.7% | |
| Sjogren 2001[42] | 60 | 17 | 145.0% (181) | ||
| Sjogren 2001[218] | 60 | 32 | 242.0% | ||
| Sunderland 2003[219] | 131 | 72 | 240.0% | ||
| Tapiola 1998[220] | 81 | 33 | 179.0% | ||
| Vanderstichele 2000[221] | 81 | 15 | 75.0% | 178.0% | |
| Vanderstichele 2006[222] | 94 | 60 | 53.7% | 347.5% | 197.8% (181) |
| Vanmechelen 2000[223] | 41 | 17 | 148.0% (181) | ||
Values are given as percentage of value for the control healthy group. For p-tau the numbers in parentheses indicate phosphorylation site studied. Across all studies listed in this table average Aß42 concentration in AD was 60% of that in NL group (28 studies), average p-tau199 concentration was 265% of that in NL (2 studies), p-tau231 was 977% of NL group value (3 studies), p-tau181 was 166% (14 studies) and t-tau 277% (36 studies).
MCI is a clinically heterogenous group with various biomarker profiles. Despite this heterogeneity and the differing levels of risk, patients with MCI are still at increased risk for AD compared to healthy controls: The yearly rates of decline from amnestic MCI to AD were estimated at 12% as compared to 1-2% among normal controls [29]. Interestingly, among non-amnestic MCI, conversion rates to AD still seem to be twice as high as in the cognitively healthy group [30]. An early study showed abnormal Aß and t-tau values in MCI two years before dementia onset [31], leading to the conclusion later supported by others that these markers exhibit abnormal levels in MCI prior to an AD diagnosis [32]. The MCI subjects show average biomarker values which fall between those seen in the NL and AD groups. Our analysis revealed that Aß42 levels were on average 23% lower in MCI than in NL (Table 2).
TABLE 2.
Differences between MCI and NL in CSF biomarkers value across different studies.
| First author, year | MCI (n) |
NL (n) |
MCI values as a percentage of NL | ||
|---|---|---|---|---|---|
| Aß42 | T-tau | P-tau | |||
| Bouwman 2007[96] | 38 | 17 | 70.3% | 178.1% | 137.7% (181) |
| Brys 2009[28,99] | 65 | 21 | 162.9% | 187.3% (231) | |
| Buerger 2005[224] | 59 | 28 | 172.5% (181); 199.2% (199); 864.2% (231) |
||
| Buerger 2009[197] | 17 | 15 | 77.7% | 124.1% | 118.0% (181); 375.0% (231) |
| de Leon 2002[225] | 8 | 10 | 40% | 234% (231) | |
| Fellgiebel 2008[199] | 10 | 10 | 194.2% (181) | ||
| Hansson 2006[226] | 134 | 39 | 66.2% | 175.0% | 125.4% (181) |
| Kester 2011[100] | 62 | 24 | 76.2% | 149.0% | 135.5% (181) |
| Lavados 2005[227] | 37 | 18 | 110.9% | ||
| Maccioni 2006[210] | 45 | 25 | 85.7% | 111.6% (AD epitopes) | |
| Maruyama 2001[211] | 19 | 15 | 95.8% | 290.8% | |
| Mattsson 2009[193] | 750 | 304 | 69.2% | 135.7% | 119.6% (181) |
| Okonkwo 2011[213] | 195 | 114 | 79.4% | 148.7% | 143.6% (181) |
| Pratico 2002[228] | 17 | 18 | 129.7% | ||
| Rolstad 2011[215] | 170 | 60 | 78.8% | 128.1% | |
| Schonknecht 2007[229] | 80 | 24 | 175.9% | 134.6% (181) | |
| Seppala 2011[217] | 57 | 8 | 103.2% | 92.5% | 101.7% (181) |
| Shaw 2009[128] | 196 | 114 | 79.6% | 147.1% | 144.0% (181) |
| Visser 2009[131] | 108 | 89 | 74.5% | 149.6% | 147.6% (181) |
Values are given as percentage of value for the control healthy group. For p-tau the numbers in parentheses indicate phosphorylation site studied. Across all studies listed in this table Aß42 concentration in the MCI group was 77% of that in NL group (13 studies),average p-tau181 concentration was 140% of NL group value (11 studies), p-tau231 was 357% (4 studies), and t-tau 153% (15 studies).
3.2 Tau
Tau is an intracellular protein that contributes to the assembly and stabilization of microtubules in neuronal axons. It was discovered in 1975 in Mark Kirschner’s laboratory at Princeton[33]. The MAPT (microtubule associated protein tau) gene, which encodes tau protein, is located on chromosome 21 and has six isoforms in adult humans [34] (Figure 1). Tau is mildly phosphorylated in the normal adult brain [18]. In AD, tau becomes hyperphosphorylated and loses its ability to assemble and stabilize microtubules [35]. Increase in t-tau concentration reflects the degree of neuronal degeneration and is not specific to AD. For example, a marked increase in t-tau levels has been observed after stroke [36]. Furthermore, ttau increase is accelerated in disorders with extensive and/or rapid neuronal death, such as Creutzfeldt-Jakob disease, as compared to other disorders with limited neuronal degeneration [37].
Figure 1.
Rendering of microtubule associated protein tau [194].
CSF t-tau concentration shows a marked increase in AD [2], reflecting the neuronal damage associated with the disease. All studies in our analysis showed elevated t-tau in AD. This increase averaged 277% in AD as compared to NL (Table 1). A previous meta-analysis of 35 studies found comparable differences, reporting t-tau levels on average 300% higher in AD than in NL. T-tau showed 80% success in discriminating between NL and AD [15,38]. As with Aß42, t-tau levels in MCI subjects are intermediate between NL and AD groups (Table 2). On average t-tau levels were 153% higher in MCI than in NL.
3.3 P-tau
The tangles characteristic of AD are made up of filaments formed from an abnormally phosphorylated form of tau called phospho-tau (ptau) [15]. Tau protein contains more than 80 sites where enzymes (kinases) can attach phosphate groups. These are called phosphorylation sites [39] (Figure 2). Adding phosphate groups in different positions on the amino acid chain alters tau properties. In AD tau becomes excessively phosphorylated. This process compromises tau’s capacity to stabilize microtubules and makes it more resistant to degradation, possibly contributing to the formation of fibrils and tangles [40]. Even though there are multiple phosphorylation sites at serine and threonine residues, the concentration of p-tau in CSF is commonly defined based on its phosphorylation at threonine 181 or threonine 231[2]. P-tau is an important CSF marker used in the detection of AD, and is believed to reflect neurofibrillary pathology. Like t-tau, p-tau concentrations increase in AD. Moreover, the levels of t-tau and p-tau are strongly correlated both in NL and AD[41,42]. This is not the case in Creutzfeldt-Jakob disease [43] or acute stroke[44], where massive increases in CSF t-tau are not accompanied by similar increases in p-tau. P-tau effectively discriminates AD from NL [45]. It is more specific to AD than t-tau, so it allows for better differentiation between Alzheimer’s and frontotemporal or Lewy Body dementia [18,46], hydrocephalus [47], depression [48] or Creutzfeldt-Jakob disease [43]. Our analysis showed that p-tau181 levels were an average of 166% higher in AD than in NL (Table 1). The number of p-tau 231 studies is smaller, but they also show a significant p-tau231 increase in AD as compared to NL subjects (976.71%). In an early study, p-tau231 discriminated AD from healthy controls with a sensitivity of 100% and a specificity of 90.5% [49]. A 2004 review of 11 p-tau studies reported an average sensitivity of 80%, at 92% specificity [15].
Figure 2.
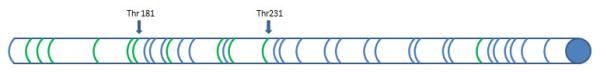
Schematic representation of major tau phosphorylation sites. Green lines represent threonine residues, blue line serine residues.
In a recent meta-analysis of 51 studies, p-tau was classified as a “satisfactory” marker also for discriminating MCI from NL [45]. In our review, as with Aß42 and t-tau, p-tau181 levels for MCI fell in between values for AD and controls, with p-tau181 being on average 140% higher in MCI than in NL (Table 2).
Overall, all three CSF biomarkers have been reported to individually differentiate AD patients from healthy elderly individuals with 80–90% sensitivity and specificity [2]. Both Aß42 and t-tau have been shown to have a good diagnostic accuracy in discriminating NL and cognitively impaired subjects. Since the capacity of Aß and t-tau to discriminate between AD and other dementias may not always be sufficient, adding p-tau181 and p-tau231 improves differential diagnosis [50,51].
Finally, using ratios of biomarkers like p-tau/Aß42 and t-tau/Aß42 can also improve diagnostic accuracy [52]. Higher ratio indicates more significant pathology. Maddalena et al. [52] reported a substantial gain in specificity when differentiating NL and AD groups with p-tau/Aß42 as compared to p-tau181 or Aß42 alone. Individuals with higher levels of p-tau181 in combination with lower levels of Aβ42 are more likely to have significant pathology than subjects with either marker alone. Similarly, others found that sensitivity and specificity of p-tau181/Aß42 ratio were significantly higher than those for t-tau [53], p-tau [52], Aß42 [54] or even t-tau/Aß42 ratio [55]. Lastly, there have also been reports of better prediction of decline from NL with p-tau181/Aß42 and t-tau/Aß42 than with any single marker[22],[56].
4. CSF AD BIOMARKERS AND AGE IN NORMAL POPULATION
Age is the strongest risk factor for AD[57]. The prevalence of medial temporal lobe atrophy (characteristic for AD) increases with age [58],[59]. At post-mortem, cognitively healthy subjects have been shown to exhibit AD-like plaques and tangles [60]. In PET imaging, 20-40% of NL elderly show patterns of Pittsburgh compound B (PIB) uptake characteristic of AD [61,62]. PIB is a radiotracer used in PET imaging. It is a derivative of a dye, thioflavin-T, which binds to amyloid aggregates. Since PIB maintains the capacity to bind to amyloid, it is a suitable radiotracer for in vivo amyloid detection with PET [61,62]. Cortical pathology, manifesting as gray matter thinning in AD-affected regions, is also evident among NL individuals, supporting the existence of a pre-clinical phase of dementia [63].
4.1 Aß
Our review of studies describing age - Aß42 relationships in NL elderly gives somewhat conflicting results. Many have found an age-dependent linear decrease in CSF Aß42 concentrations [22,56,64-70]. However, other studies have reported no change [71-74] or an age-dependent increase [75,76]. In a study of 92 NL subjects between 8 and 89 years old, the relationship between Aß and age followed a u-shaped curve, with high Aß40 and Aß42 levels in children, lower levels in adolescents and adults, and an increase in old age[76,77]. Contradictory results were found in another study of 56 subjects, which showed low Aß40 and Aß42 levels in children, with an increase in adulthood and a decline in old age[64]. Several different factors could have contributed to these divergent findings. First, CSF Aß fluctuates 1.5- to 4-fold throughout the day and appears to be time-of-day [78] and sleep-wake cycle dependent[79,80]. Interestingly, diurnal variations in CSF Aß42 concentrations are blunted with age and with brain amyloid accumulation [80]. Second, as opposed to t-tau and p-tau, CSF Aß42 seems to be less stable, exhibiting marked changes depending on CSF storage protocols [81] and on freeze-thaw cycles[82]. Variations in the timing of lumbar puncture or CSF handling could contribute to the lack of consistency in results. In summary, CSF Aß42 may decrease with age, but current data do not support this notion unequivocally. The possibility that the age-Aß42 relationship may not be linear also has to be taken into account. One group observed that brain activity increases Aß42 in the interstitial fluid [83]; this finding implies that Aß concentration may initially increase with age, and after reaching a threshold beyond which clearance mechanisms become dysfunctional, brain deposition results in Aß sequestration and reduction of CSF levels. This hypothesis requires further corroboration.
4.2 Tau
A substantial number of studies have shown consistent negative correlations of various strengths between tau and age [22,42,56,68,72,74,84-88]. Studies that have not found relationships between t-tau and age are in the minority [70,89-91]. Smaller study groups [89,90] or the fact that AD patients were examined may explain their lack of positive findings [91]. Finally, even with larger groups [70], intrinsic differences between populations may result in dissimilar findings.
Existing data suggest that increases in tau concentration are a common finding with increasing age. This is not surprising given that tau reflects neuronal loss and correlates well with brain atrophy, both concomitant with brain aging.
4.3 P-tau
There are a few reports indicating that like t-tau, p-tau concentrations increase with age in NL individuals. This was found for both ptau181 [46,68,69,92] and p-tau231 [46,74,93]. The much less studied p-tau199 did not show association with age in normal controls [85]. Others have argued that p-tau concentrations (in this case p-tau181) are age-independent, [94] further strengthening its importance as a marker specific for AD. In summary, despite some contradictory studies, the majority of the available evidence [46,68,69,74,92,93] suggests that p-tau increases with age in NL. Some reservations remain, since many of the studied cohorts came from memory centers where enrichment with silent AD pathology is expected. It is thus still unclear to what extent increased p-tau levels in elderly controls are correlates of aging or reflect asymptomatic AD. Interestingly, some postulate [95] that phosporylation of tau and formation of neurofibrillary tangles is not toxic per se, but represents a protective cellular response to oxidative stress. According to this hypothesis, increased p-tau accumulation with age (serving as an antioxidant) could reflect adaptive mechanisms [95]. Surpassing the cell’s compensatory capacity could result in increased accumulation and cell death as seen in AD.
A few studies have performed longitudinal CSF examinations in NL. One study showed that Aß42 and t-tau levels, but not p-tau, changed over a span of almost two years in NL, MCI, and AD. However, differences were seen mostly between groups rather than in individuals [96]. In another 1-year study, only t-tau increased significantly in normal controls; no differences were found for Aß42 levels, and no p-tau data were given [97]. Contradicting these findings, others reported that the greatest rate of change over 3 years was observed for Aß42 but not tau or p-tau181 [98]. In a group of NL subjects studied at 2 year intervals, we did not see changes in t-tau, Aß42/Aß40 or p-tau231 between baseline and follow-up [28,99]. The lack of change may be attributed, however, to relatively short observation period in a younger group: the mean age of our cohort was 69 vs. a mean of 75 reported in Jack et al. and Lo et al. In agreement with our findings, Kester et al. did not observe any changes in CSF markers over the span of 2.5 years in normal elderly in their sixties [100], suggesting that longitudinal dynamics in younger cohorts are slower.
Altogether, these data suggest the existence of age-related changes in CSF AD biomarkers: The probability of abnormal findings increases with age. It is tempting to assume that these abnormalities reflect a true pathological process ultimately leading to AD. However, given the lack of longitudinal data documenting the trajectory of individual subjects from normal cognition to AD, this conclusion must await further confirmation. Meanwhile, age-related dynamics of CSF biomarkers have to be taken into consideration while developing diagnostic criteria, since they influence diagnostic accuracy of biomarkers in older groups. As early as 1999, Buerger et al. observed that correct classification rate for t-tau in the young old was significantly higher than in the group of older old [101], suggesting better discriminative power of CSF tau in the younger population. Others found that the difference in CSF biomarkers between young AD patients and controls was greater than the difference between old AD patients and controls [69]. Finally, a recent study found a marked decrease in diagnostic accuracy of single CSF biomarkers in older populations. Specificity was most affected, reaching only 40% for p-tau in the group older than 74 [92]. As a remedy to this problem, the authors propose using a combination of markers, which still showed satisfactory sensitivity and specificity, even though analysis of Alzheimer’s Disease Neuroimaging Initiative (ADNI) data showed that the diagnostic accuracy of the tau/Aß42 ratio was still higher in the younger (<75) than in the older group [102].
5. BIOMARKERS AND APOE IN NORMAL POPULATION
The presence of an ApoE4 allele is the most widely accepted genetic risk factor for late-onset AD [103]. It has been repeatedly shown that the frequency of the ApoE4 allele is significantly higher in AD than in NL [14,64]. The presence of this allele has been associated with lower age of AD onset [104,105]. The risk of developing AD is approximately 15 times greater for E4 homozygotes and 3 times greater for heterozygotes compared to non-carriers [103].
In line with the notion of the ApoE4 allele as a risk factor, multiple studies have shown that ApoE4 carriers, both NL and AD, have more abnormal CSF biomarkers than non-carriers in the same diagnostic group [106-115]. A recent study of ADNI subjects showed that cognitively normal subjects classified as having an AD profile (Aβ42/p-tau181 mixture model AD feature, based on an unsupervised learning approach) were almost 7 times more likely to carry the ApoE4 allele than subjects without the AD signature [116].
Cognitively healthy ApoE4 positive (ApoE4+) subjects have lower Aß42 levels cross-sectionally [107,114,117], greater longitudinal decreases in Aß42 concentrations [118], and show significant elevations in Aß40/Aß42 ratios [119] compared with non-carriers. Others reported an interesting age-by-genotype interaction where ApoE4+ subjects exhibited a sharp decline in Aß42 concentration beginning in their 60s, while ApoE4− subjects showed significantly less change[73]. Some observed that Aß42 levels were comparable between ApoE4+ AD patients and ApoE4+ NL [120].
Findings regarding t-tau are somewhat less consistent. Some studies showed higher CSF t-tau levels in ApoE4 carriers [74,107,121], while others did not find a significant relationship between tau levels and ApoE4 status[91,108,114]. We observed that cognitively intact ApoE4+ individuals had higher t-tau and p-tau231 levels than their peers. Furthermore, p-tau231 concentration increased with age in both genotype groups, but this increase was steeper in ApoE4+ subjects [74]. Increased p-tau181 levels in NL ApoE4 carriers have also been confirmed by others [107]. Longitudinally, two reports analyzing ADNI data did not find significant differences in the rate of change in biomarkers between NL carriers and non-carriers [98,122]. However, the follow-up periods (12 and 35 months, respectively) might not have been long enough to observe a significant change.
ApoE2 appears to be associated with lower risk of AD [123]: some found that ApoE2 carriers had higher levels of Aß, lower levels of p-tau 181, and marginally lower levels of t-tau [124]. The mechanisms of protective ApoE2 effects are not fully understood. Subjects carrying this allele seem to have less brain atrophy [125]. In post-mortem studies, ApoE2 carriers without dementia had increased levels of postsynaptic density protein 95 [126]. Animal studies indicate that the presence of the ApoE2 allele improves excitatory synaptic transmission impaired in the presence of ApoE4 [127], suggesting that one possible mechanism of ApoE2 protection may rely on its effects on synaptic function.
In summary, ApoE genotype seems to affect CSF biomarker levels in cognitively healthy subjects, consistent with the hypothesis that an ApoE4+ genotype confers a greater risk for AD (Table 3). Conversely, ApoE2 carriers exhibit biomarker profiles indicative of less AD pathology.
TABLE 3.
CSF biomarkers and ApoE in normal population, a summary of findings.
| First author, year | ApoE4+ (n) | ApoE4− (n) | Results | |
|---|---|---|---|---|
| De Meyer 2010 [116] | 8 | 65 | individuals with AD-like CSF profile were seven times more likely to carry an ApoE4 allele. |
|
| Fagan 2000 [119] | 12 | 13 | higher Aß40/Aß42 in ApoE4+ than ApoE4− subjects | |
| Fjell 2010 [115] | 21 | 37 | lower Aß42 in ApoE4+ than ApoE4− subjects a trend towards higher t-tau and p-tau in ApoE4+ subjects |
|
| Glodzik-Sobanska 2009 [74] |
30 | 48 | higher t-tau in ApoE4+ than ApoE4− subjects higher p-tau231 in ApoE4+ than ApoE4− subjects a steeper increase in p-tau 231 with age in ApoE4+ subjects |
|
| Golombowski 1997 [138] | 3 | 9 | higher t-tau in ApoE4+ than ApoE4− subjects | |
| Herukka 2007 [106] | 62 | 76 | lower Aß42 in ApoE4+ than ApoE4− subjects higher t-tau in ApoE4+ than ApoE4− subjects higher p-tau in ApoE4+ than ApoE4− subjects |
|
| Kester 2009 [107] | 63 | 111 | lower Aß42 in ApoE4+ than ApoE4− subjects higher t-tau in ApoE4+ than ApoE4− subjects a trend towards higher p-tau in ApoE4+ subjects |
|
| Lo 2011 [98] | 61 | 168 | ApoE4 status did not modify the rate of Aß42 change. | |
| Mulder 2010 [113] | 3 | 8 | ApoE4+ group had a higher proportion of individuals with AD-like CSF profile |
|
| Peskind 2006 [73] | 184 total, n in groups not given |
ApoE4+ subjects showed sharp decline in Aß42 in their 60s, while ApoE4− showed significantly less change. |
||
| Popp 2010 [112] | 64 | 216 | lower Aß42 in ApoE4+ than ApoE4− subjects no difference in p-tau181 |
|
| Prince 2004 [117] | 32 | 86 | lower Aß42 in ApoE4+ than ApoE4− subjects | |
| Smach 2008 [110] | 9 | 44 | lower Aß42 in ApoE4+ than ApoE4− subjects in tau | no difference |
| Stenset 2006 [109] | 77 | 46 | lower Aß42 in ApoE4+ than ApoE4− subjects | |
| Stomrud 2010 [118] | 10 | 27 | ApoE4+ subjects have greater longitudinal decreases in Aß42 and higher t-tau at follow-up |
|
| Sunderland 2004 [108] | 57 | 85 | lower Aß42 in ApoE4+ than ApoE4− subjects in tau | no difference |
| Tapiola 2000 [111] | 13 | 25 | lower Aß42 in ApoE4+ than ApoE4− subjects higher t-tau in ApoE4+ than ApoE4− subjects |
|
| Vemuri 2010 [114] | 27 | 82 | lower Aß42 in ApoE4+ than ApoE4− subjects in tau | no difference |
| Vemuri 2010a [122] | 22 | 70 | ApoE4 status did not modify the annual rate of Aß42 or t-tau change. | |
6. PREVALENCE of a CSF- AD “SIGNATURE” in NORMAL SUBJECTS
The issue of a threshold for “abnormality” for CSF biomarkers is a difficult one, especially in clinically healthy subjects. There are several approaches to define what is abnormal. First, abnormality may be defined based on comparison between the NL and AD groups. Replicated values best differentiating two clinical categories may be used as a threshold [128]. Second, abnormality can be defined based on the distribution of values within a certain population, where subjects with values exceeding, for example, 2 standard deviations (SD) below or above the mean can be considered “abnormal”. Finally, possibly the most valid, but also the most difficult to obtain, would be a definition based on longitudinal observation of clinical progression in a group starting as healthy and declining to AD at follow-up evaluations.
A recent paper reported that 38% of normal subjects over age 60 had signs of “latent AD,” defined as abnormal Aβ42 and t-tau. 5% had abnormal Aβ42 and normal tau, while 12% had normal Aβ42 and elevated tau [129]. Cutoff values were defined as 2 SD outside the mean of a normal group below the age of 50. In an earlier paper from the same group, 16% of controls had an elevated tau/Aß42 ratio (defined as 2 SD outside the mean for the group of 60 and younger). The group with the elevated ratio was older (mean age 71 vs. 49 in the low tau/Aß42 group), had increased frequency of ApoE4, and were more likely to decline to MCI during follow-up of up to 42 months [56]. Shaw et al. developed a “CSF biomarker signature” for AD based on ROC analyses of autopsy-confirmed AD cases compared with controls. Application of this method generated cutoff values that classified 38% of a normal ADNI cohort (mean age of 76) as having a low Aß42 level and 34% as having an abnormal t-tau/Aß42 ratio [128]. De Meyer et al. identified 36% of cognitively normal ADNI subjects as having an AD profile (the authors used an unsupervised learning approach to create Aβ42/p-tau181 mixture model) [116].
In our study of 115 NL subjects (mean age 63 years), we dichotomized CSF biomarker values into “high” and “low” groups based on previously published cutoff values for discrimination between AD and normal. Thirty percent of our subjects were classified as having high t-tau, 21% as having high p-tau231, and more than 40% as having a low Aβ42/ Aβ40 ratio [130].
The prevalence of an abnormal CSF signature seems to be even higher among normal subjects who are at risk for AD. Subjective cognitive impairment (the presence of a memory complaint in the absence of objective impairment on neuropsychological tests) is such a risk factor. In the DESCRIPTA study, the CSF AD profile (calculated as: Aβ42/(240+[1·18*T-tau])<1) was present in 52% of subjects with subjective cognitive impairment and in 31% of healthy controls [131].
In conclusion, a substantial number of healthy subjects over age 60 (25-40%) has CSF biomarker concentrations in ranges that can be considered abnormal, regardless of the definition used. At least a portion of this population is likely at risk for AD, but a considerable number of them may never develop the disease. One possible explanation for the lack of disease expression in this latter group is cognitive reserve, where certain characteristics of the brain or life experience delay the expression of AD despite the presence of brain pathology. The question of how to classify this group of healthy individuals with abnormal biomarker concentrations will be even more pressing should preventative therapies become available, and the decision about whether to treat subjects with an “AD CSF profile” will depend on the risk related to such therapies.
It is conceivable that the large number of individuals with abnormal biomarker levels indicates that the abnormalities are trait- rather than state-dependent. The fact that differences in metabolic brain activity are already observed in young ApoE4+ individuals[132] may also speak in favor of this explanation. In this case, different markers of imminent decline would be needed to make a decision about if and when to initiate therapy. The profile of anomalous biomarkers is also worth mentioning. Although a large group of subjects have both Aß42 and t-tau outside the normal range, a significant number of healthy individuals express either Aß42 or t-tau anomaly. Different pathways leading to neurodegeneration would be a tempting though unverified conclusion, since the course and the outcome of these abnormalities are yet poorly defined. There is insufficient data showing when these biomarkers become abnormal and in which order. It is not known which biomarker is the first to show changes in the pre-clinical disease progression. Even though a widely discussed work by Jack et al. [133] proposes Aß42 as the first in the temporal ordering of biomarkers, this hypothesis still requires confirmation with longitudinal data [134].
7. PREDICTION of DECLINE FROM NL
Most studies investigating the predictive potential of CSF biomarkers identified Aß42 [135-137] or tau/Aß42 ratios [22,56,138] as the best predictors of cognitive decline in NL.
In a study of 55 women followed for 8 years, baseline Aß42 correlated negatively with change in MMSE score [135]. A 3-year longitudinal study of 85-year-old demonstrated that individuals with lower CSF Aß42 were more likely to develop dementia at follow-up. Aß40 levels did not differ between groups, and there were no significant differences between ApoE4 carriers and non-carriers [137]. Others reported that low levels of Aß42 at baseline were associated with development of subjective memory impairment, lower MMSE score, and inability to live in regular housing 3 years later [136]. A later study by the same group showed that cognitive decline at 4-year follow-up was associated with a greater longitudinal decrease in Aß42 and a greater increase in p-tau 181 [118].
Fagan et al. reported that high tau/Aß42 ratio and p-tau/Aß42 ratio both predicted decline from NL, with hazard ratios (HR) of 5.21 and 4.39, respectively [22]. High values were defined as the top 15% of all values, and the follow-up period was 3-4 years. A subsequent study from the same group confirmed this finding in a much bigger cohort with a follow-up period of 6 years. The HR for high (highest tertile) tau/Aß42 was 5.76 [138]. In line with this finding, Li et al. also reported that cognitively healthy subjects with elevated CSF tau/Aß42 ratio (defined as 2 SD outside the mean for the group aged 60 and younger), were more likely to decline to MCI during follow-up of up to 42 months [56].
We observed that higher baseline p-tau231 levels predicted lower delayed recall score 2 years later. Subjects whose performance on the delayed recall test did not improve over time (lack of learning effects) also showed greater longitudinal reduction in MTL gray matter. No differences were seen for total tau or the Aß42/Aß40 ratio [139]. Interestingly, Desikan et al. found that among 107 NL subjects, followed for 3 years, baseline low Aß42 predicted decline only in subjects who also had high p-tau levels [140], emphasizing the importance of both markers in reflecting amyloid and neurofibrillary pathology.
Although these data suggest the potential usefulness of CSF biomarkers to predict future decline in NL populations (Table 4), the evidence gathered thus far is still unsatisfactory. Most importantly, data on individual trajectories or even their estimates are still missing. Because observation time has been limited, there is still not enough information to precisely predict outcome in younger subjects (in their fifties and sixties). Ongoing longitudinal efforts are needed in order to bridge this gap.
TABLE 4.
Prediction of decline from NL with CSF biomarkers, a summary of findings.
| First author, year | decliners (n) |
non decliners (n) |
Outcome measure | Predictor |
|---|---|---|---|---|
| Craig-Schapiro 2010 [138] |
26 | 148 | conversion from CDR=0 to CDR<0. |
CSF YKL*-40/ Aß42 ratio predicted conversion |
| Desikan 2012 [140] | 107 NL, n in groups not given |
change in CDR or ADAS-Cog | low Aß42 predicted decline only in subjects who also had high p-tau |
|
| Fagan 2007 [22] | 13 | 48 | conversion from CDR=0 to CDR<0. |
t-tau/ Aß42 and p-tau181/Aß42 predicted decline |
| Glodzik 2011 [138] | 20 | 37 | reduced memory performance | p-tau231 predicted decline in delayed recall performance. |
| Gustafson 2006 [135] | 6 | 45 | conversion to dementia or decline in MMSE |
lower Aß42 levels associated with greater decline |
| Li 2007 [56] | 4 | 43 | conversion to MCI. | t-tau/Aß42 predicted conversion |
| Skoog 2003 [137] | 7 | 35 | conversion to dementia. | low Aß42 predicted conversion to dementia; Aß40 did not |
| Stomrud 2007 [138] | 15 | 42 | decline in MMSE, development of subjective memory impairment affecting quality of life. |
low baseline Aß42 predicted decline at 3-year follow-up. |
| Visser 2009 [131] | 149 NL, n in groups not given |
change in memory or cognition composite score, MMSE, or conversion to AD |
low Aß42 was best predictor of decline | |
YKL-40: human cartilage glycoprotein-39
8. CSF BIOMARKERS AND IMAGING IN NORMAL POPULATION
Cerebral atrophy, as measured by structural MRI, indicates brain volume reductions possibly due to loss of dendrites, synapses and neurons [141]. Although cerebral atrophy is not specific to AD, certain brain regions are more affected than others, giving a characteristic AD profile (for review [142]). Atrophy can therefore be considered a valid biomarker of neurodegeneration [143-145]. This is especially true for the medial temporal lobe (MTL). Hippocampal atrophy predicts decline from NL to AD, and structural MTL changes can be detected before clinical symptoms are fully developed [142,146,147] (Figure 3).
Figure 3.
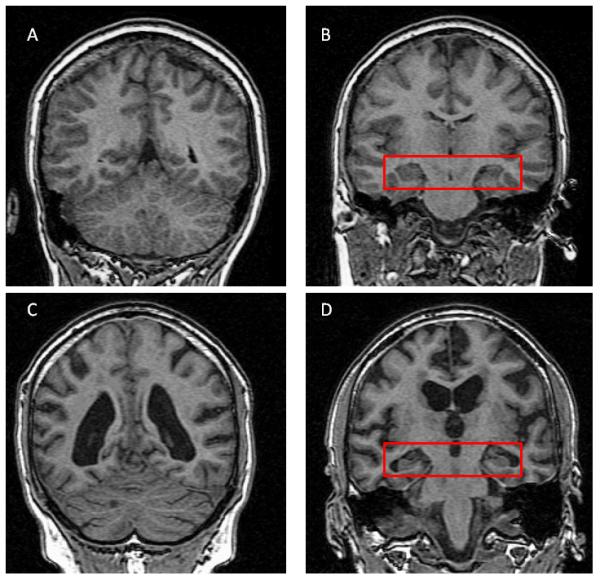
MRI of healthy normal brain (A,B) and a brain of a patient with AD (C,D). Please note ventricular enlargement (C) as compared to ventricles on (A). Red box on B (Healthy) and D (AD) contains both hippocampi. Please note enlargement of CSF spaces and shrinkage of hippocampal gray matter on (D).
Positron Emission Tomography (PET) with 18F-Fluorodeoxyglucose (FDG) and Pittsburgh Compound B (PiB) tracers have also been extensively studied in AD and MCI subjects. FDG-PET measures brain glucose metabolism and has been validated as a marker of the synaptic dysfunction associated with AD compared to age-matched controls [148,149]. Changes in glucose metabolism, reflecting synaptic loss, have been found to occur in preclinical AD [150-153]. A characteristic pattern of parieto-temporal and posterior cingulate hypometabolism is a widely accepted feature of AD [154]. The application of region-of-interest based approaches to study the structures of the MTL also reveals metabolic reduction in hippocampus and entorhinal cortex [154]. Furthermore, hypometabolism in these regions can predict cognitive decline in NL individuals [150,155].
PiB-PET has been shown to be a valid biomarker for AD-associated amyloid plaque load [156-159] (Figure 4). On PiB-PET, the AD profile consists of cortical PiB binding in the precuneus/posterior cingulate and frontal cortex, as well as the lateral temporal and parietal cortex, and more variably, the striatum and occipital cortex. The sensorimotor and medial temporal cortices are less affected [157]. Below we present evidence for association between CSF and imaging markers of AD in cognitively healthy groups. We believe that evidence of such association will further corroborate the validity of CSF markers as early indices of AD pathology, particularly if their presence is related to atrophy or dysfunction in AD-specific brain regions.
Figure 4.
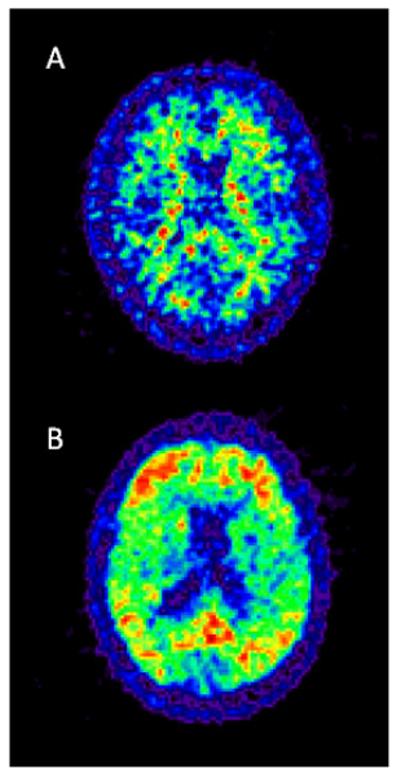
PiB-PET of a healthy control (A) and AD patient (B). Please note non-specific accumulation of the tracer in white matter in healthy subjects (A) as opposed to cortical deposition in AD (B).
Both MRI and CSF biomarkers are considered to reflect AD pathology, but there have been relatively few studies about the association between the two in the NL population [14]. Neuropathological studies where imaging was performed prior to death showed that neurofibrillary pathology was a stronger correlate of atrophy than Aß deposition [160,161]. Previously, associations have been reported between cross-sectional CSF biomarkers and brain atrophy measures across all diagnostic categories (NL, MCI, AD) [14,162] and in AD only [14]. Similarly, there were reports of associations between baseline CSF biomarkers and whole brain [163], temporal [164] or hippocampal atrophy rates [165] in AD subjects, but not NL individuals. It is likely that the relatively small number of NL subjects in these studies can explain the negative findings.
In recent years, a growing number of studies encompassing larger groups of NL indicate a relationship between CSF markers and structural or functional brain changes. Our own studies showed, that higher p-tau231 at baseline (split at the median) was related to increased MTL atrophy rate over the span of two years [139]. Additionally, we reported that at cross-section, high p-tau231 was associated with less gray matter in the right MTL (entorhinal cortex) and the left inferior temporal gyrus. Subjects with high tau (above the median for the group) had less gray matter (GM) in the right hippocampus, left precuneus, anterior cingulate, and insula than the low t-tau group [130]. These are regions implicated in AD pathology. We did not observe relationships between CSF Aß and GM measures in AD-specific brain regions. Showing some similarity with our results, others found that p-tau181 was significantly associated with hippocampal radial distance (the distance from the medial core to points at the hippocampal surface) in normal controls. In addition, across all diagnostic groups, phosphorylated tau showed the strongest association, among other CSF biomarkers, with hippocampal atrophy [162]. Another group reported that high p-tau181 was related to the thinning of the entorhinal cortex [166]. In the same study, low Aß42 was associated with thinning of the medial orbital frontal cortex. High p-tau181 and low Aß42 were defined based on cutoffs proposed by Shaw et al., derived from autopsy-confirmed CSF data of control and AD subjects [128]. In another study, both higher p-tau181 and lower concentrations of CSF Aß were associated with increased rates of ventricular enlargement. Furthermore, lower Aß was associated with cortical thinning in many AD-related brain regions at cross-section [167].
Contrary to these findings, others have reported that CSF Aß42 levels, but not p-tau levels, are associated with brain atrophy in NL both at cross-section and longitudinally. Fagan et al. found that CSF Aß42 levels were positively correlated with whole-brain volume in non-demented individuals [168]. These results were further developed by Schott et al., who showed that NL with low Aß42 (as defined by Shaw et al. [128]) had significantly higher whole brain atrophy rates than their peers with high CSF Aß42 [169]. Dickerson et al. used an structural AD signature created based on the thickness of 9 AD-related cortical regions. Normal subjects with average thickness below 1 standard deviation for the normative group were classified as having high risk for AD. Subjects in this group had lower CSF Aß42 concentrations than the remainder of the study sample [170]. Others observed that Aß42 correlated with changes in multiple brain regions (not including the MTL), only when Aß42 reached a certain threshold [115]. Some found that ventricular volume was negatively associated with CSF Aβ only in ApoE ε4 positive NL subjects [171]. A small study found that NL subjects with low Aß42 concentrations (based on the cutoff proposed by Fagan et al. [168]) had cortical thinning in many areas, including AD-specific regions [172]. Interestingly, one study showed that low Aß status was associated with entorhinal cortex atrophy rate only in subjects with high p-tau181 concentrations. A similar relationship was also observed for regions affected later in the disease process [63]. Once again, high ptau181 was defined as in Shaw et al. [128]. The authors suggested that the presence of p-tau could represent a critical link between Aß deposition and volume loss. Alternatively, these data could also imply that amyloid accumulation may be a necessary but insufficient indicator (or cause) of AD– related neurodegeneration.
Very few studies have directly examined the associations between FDG uptake or PiB accumulation and CSF biomarkers in cognitively healthy controls. We observed significant negative associations between p-tau231 and glucose metabolism in the middle occipital gyrus, parhippocampal gyrus and thalamus, as well as p-tau231/Aß42 ratio and glucose metabolism in the parhippocampal gyrus and thalamus [173]. Petrie et al. described hypometabolism in the posterior cingulate, precuneus, and parahippocampal regions in conjunction with both high tau and p-tau 181 concentrations. Lower CSF Aß42 concentrations were associated with hypometabolism only in the inferior temporal cortex [174].
Fagan et al. reported an association between low CSF Aß42 and high PiB uptake in a mixed group of NL and cognitively impaired subjects [23]. These findings were subsequently replicated in a large group of healthy controls where a robust inverse relationship between cortical PiB binding and CSF Aß42 was observed. P-tau181 also correlated linearly with cortical amyloid [175].
By and large, a picture emerges where all three AD CSF biomarkers are correlated with different aspects of imaging (Figure 5). A growing body of data challenges the older view that only tau markers are related to structural brain changes, although they still seem to be better associated with atrophy. Furthermore, tau markers also appear to be more consistently related to the atrophy and metabolism of the MTL, whereas CSF Aß42 correlates better with global structural indices. The finding that both CSF Aß42 and tau markers correlated with imaging is in agreement with a recent analysis of ADNI data where the authors showed that CSF Aβ had effects on brain structure that were independent of tau, even though these effects were significantly reduced after controlling for CSF tau [176]. Overall, CSF and imaging markers are reasonably correlated in NL subjects, indicating that even in this stage they reflect a common process or that there may be a causal relationship between them.
Figure 5.
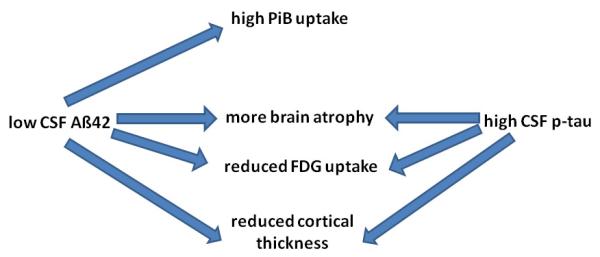
Schematic representation of relationships between CSF Aß42 and p-tau and imaging findings. Review criteria: We searched PubMed for English-language articles on Alzheimer’s Disease using the keywords “Alzheimer,” “amyloid,” “biomarker,” “CSF,” “MRI,” “PET,” “tau,” and several keywords related to each of the above. We also identified papers from the references in the papers retrieved in the original searches.
9. CSF AD BIOMARKERS AND VASCULAR RISK FACTORS IN NL SUBJECTS
Vascular risk factors have been proven to increase the risk for AD. It is not yet established whether conditions related to increased cardiovascular risk also influence AD CSF markers in NL, making them “more pathological”. Available data yielded inconsistent results: Many large neuropathological studies did not show any increase in amyloid or neurofibrillary pathology with increased vascular brain damage [177], while smaller reports [178] and animal data [179,180] suggest that vascular disease and ischemia can intensify AD pathology.
In line with neuropathological data, we also did not observe differences in biomarkers levels between groups of NL subjects with and without hypertension [130]. Others demonstrated that the association between hypertension, t-tau and p-tau181 was modified by the presence of the ApoE genotype. Hypertension had the strongest association with t-tau and p-tau181 levels in ApoE4 homozygotes. Hypertension was not associated with Aß42 [181].
Some have proposed that insulin resistance could play a role in AD pathogenesis [182]. Several observations have driven this hypothesis: 1) impairment of brain glucose metabolism increases the risk of dementia, 2) diabetes (impaired peripheral glucose metabolism) is a risk factor for AD. In line with this theory it was found that CSF Aß42 was inversely associated with CSF to plasma glucose ratio. This relationship was observed only in ApoE4+ subjects. No significant relationship was found between glucose levels and t-tau or p-tau [183]. Others did not find any relationship between HbA1C or fasting glucose levels and t-tau, p-tau, or Aß42 [184].
White matter lesions (WML) manifesting as hyperintensive changes on T2-weighted MRI images are due to small vessel disease [185]. Scandinavian researchers observed that WML are associated with low CSF Aß42 [109]. A subsequent study from the same group also indicated an interaction with ApoE4 genotype. This study divided memory clinic subjects with various degrees of subjective or objective cognitive impairment into three groups based on WML load. They found that the odds ratio of having low Aß42 was significantly higher in the presence of ApoE4 only in the group with high WML load[186]. Contrary to these findings, another study failed to reveal an association between WML and any of the CSF AD biomarkers [187].
Regarding other risk factors, it was observed that hypercholesterolemia increased the probability of low CSF Aß42 levels [109]. Others reported that lower CSF Aß42 as well as higher t-tau and t-tau/ Aß42 were associated with lower body mass index. Somewhat counter-intuitively, the prevalence of “abnormal” biomarkers was higher in normal weight compared to overweight individuals [188]. A possible explanation points to systemic energy metabolism dysfunction and sarcopenia as early indicators of AD, rather than obesity later in life as a potential risk factor for AD-type dementias. This view was confirmed in a paper using an ADNI cohort, where the authors found that more abnormal CSF biomarkers were related to lower BMI. However, this was true only across all diagnostic groups (NL, MCI, AD) [189].
Finally, emerging evidence indicates that some strategies like physical exercise aimed at counteracting negative effects of aging and vascular risk are related to favorable CSF biomarker profiles. Liang et al. reported that individuals who engaged more in physical exercise have lower t-tau, ptau181, and higher Aß42 levels than their sedentary peers [190]. Another group observed that in NL adults high intensity physical activity attenuated the changes in CSF Aß42 induced by a diet high in saturated fat/high glycemic index [191].
The question of whether vascular risk factors contribute to the expression of AD-related pathology or diminish brain reserve, facilitating the clinical manifestation of an already pre-existing AD process, still remains a matter of debate. We believe, however, that the data presented above suggest the possibility of an association between vascular risk factors and amplification of “AD-like” pathology in CSF. This line of thinking is strengthened by work indicating beneficial effects of interventions. Moreover, the relationship between CSF markers and vascular pathology is very likely modified by the ApoE genotype, which is itself related to vascular risk. Further work is needed to reconcile the negative histopathology findings and clinical/CSF data.
10. ETHICAL IMPLICATIONS
The recent developments in biomarker research give rise to an important ethical issue. It has been suggested that the use of biomarkers to identify prodromal AD in NL individuals will not be justifiable until disease-modifying treatments become available. In particular, it has been argued that the use of biomarkers to predict AD in asymptomatic people is unwarranted in the absence of registered drugs with distinct disease-modifying effects[2,192].
There is currently no way to definitively predict who will or will not develop AD, but a patient informed of abnormal biomarker levels might interpret those results as an indication that they will develop the disease. Even when a very reliable early screen is developed, it will likely not attain 100% accuracy. There will therefore be a risk of error, meaning that an individual might be falsely told that they are at high risk for developing AD. The repercussions of such false information could be severe.
However, the prediction of AD in asymptomatic individuals also offers potential benefits, such as the opportunity for individuals to take advantage of existing drug treatments or take other measures to maintain a good quality of life as long as possible. Research and ethical analysis will be necessary to evaluate the risks and benefits of disclosing abnormal biomarker results to asymptomatic individuals.
11. CONCLUSIONS
Our analysis indicates that t-tau, p-tau, and Aß42 are valuable as biomarkers of AD. At present, their strength relies mostly in supporting neurodegenerative etiology in research criteria for MCI and AD [3,4], and their reasonable capacity to predict the conversion from MCI to AD [1,193]. A combination of biomarkers seems to be more useful in prediction than a single analyte.
In cognitively intact subjects, biomarkers reflect major risk factors for AD, such as age and genotype. They correlate with structural and functional brain changes believed to be related to AD. CSF biomarkers may also be of value for predicting cognitive deterioration in this group. However, despite an immense amount of work done so far, their predictive value in NL populations is still uncertain and awaits confirmation in studies offering long follow-up periods and repeated CSF samplings in the same individuals. Growing evidence indicates that conditions other than the “pure AD” process (i.e. vascular risk, lifestyle modifications) may influence biomarker concentrations. This opens new avenues of research into risk assessment and risk modification.
ACKNOWLEDGEMENTS
This study was supported by the following grants: NIH-NIA AG12101, AG022374 and HL-111724-01.
Abbreviations
- AD
Alzheimer’s Disease
- APP
Amyloid Precursor Protein
- APPs
Secreted Amyloid Precursor Protein
- CBF
Cerebral Blood Flow
- CDR
Clinical Dementia Rating
- CSF
Cerebrospinal Fluid
- FDG-PET
Positron Emission Tomography with Fluodeoxyglucose
- GM
gray matter
- MCI
Mild Cognitive Impairment
- MMSE
Mini Mental State Examination
- NL
Normal Controls
- PIB
Pittsburgh Compound B
- ADNI
Alzheimer’s Disease Neuroimaging Initiative
13. REFERENCES
- [1].Allan CL, Sexton CE, Welchew D, Ebmeier KP. Imaging and biomarkers for Alzheimer’s disease. Maturitas. 2010;65:138–142. doi: 10.1016/j.maturitas.2009.12.006. [DOI] [PubMed] [Google Scholar]
- [2].Blennow K, Hampel H, Weiner M, Zetterberg H. Cerebrospinal fluid and plasma biomarkers in Alzheimer disease. Nat Rev Neurol. 2010;6:131–144. doi: 10.1038/nrneurol.2010.4. [DOI] [PubMed] [Google Scholar]
- [3].McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR, Jr., Kawas C, Klunk WE, Koroshetz WJ, Manly JJ, Mayeux R, Mohs RC, Morris JC, Rossor MN, Scheltens P, Carillo MC, Thies B, Weintraub S, Phelps CH. The diagnosis of dementia due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s & Dementia. 2011;7:263–269. doi: 10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Albert MS, DeKosky ST, Dickson D, Dubois B, Feldman HH, Fox NC, Gamst A, Holtzman DM, Jagust WJ, Petersen RC, Snyder PJ, Carillo MC, Thies B, Phelps CH. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s & Dementia. 2011:270–279. doi: 10.1016/j.jalz.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Garcia-Alloza M, Subramanian M, Thyssen D, Borrelli LA, Das P, Golde TE, Fauq A, Hyman BT, Bacskai BJ. Existing plaques and neuritic abnormalities in APP:PS1 mice are not affected by administration of the gamma-secretase inhibitor LY-411575. Mol Neurodegener. 2009;6:4–19. doi: 10.1186/1750-1326-4-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Anoop A, Singh PK, Jacob RS, Maji SK. CSF Biomarkers for Alzheimer’s Disease Diagnosis. Int J Alzheimers Dis. 2010;2010:1–12. doi: 10.4061/2010/606802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Borroni B, Premi E, Di Luca M, Padovani A. Combined Biomarkers for Early Alzheimer Disease Diagnosis. Current Medicinal Chemistry. 2007;14:1171–1178. doi: 10.2174/092986707780598005. [DOI] [PubMed] [Google Scholar]
- [8].Consensus Working Group Consensus report of the Working Group on: “Molecular and Biochemical Markers of Alzheimer’s Disease”. The Ronald and Nancy Reagan Research Institute of the Alzheimer’s Association and the National Institute on Aging. Neurobiology of Aging. 1998;19:109–116. [PubMed] [Google Scholar]
- [9].Goedert M, Spillantini MG. A century of Alzheimer’s disease. Science. 2006;314:777–781. doi: 10.1126/science.1132814. [DOI] [PubMed] [Google Scholar]
- [10].Spires-Jones TL, Stoothoff WH, de CA, Jones PB, Hyman BT. Tau pathophysiology in neurodegeneration: a tangled issue. Trends Neurosci. 2009;32:150–159. doi: 10.1016/j.tins.2008.11.007. [DOI] [PubMed] [Google Scholar]
- [11].Bennett DASJA, Arvanitakis Z, Kelly JF, Aggarwal NT, Shah RC, Wilson RS. Neuropathology of older persons without cognitive impairment from two community-based studies. Neurol. 2006;66:1837–1844. doi: 10.1212/01.wnl.0000219668.47116.e6. [DOI] [PubMed] [Google Scholar]
- [12].Blennow K, Hampel H. CSF markers for incipient Alzheimer’s disease. The Lancet Neurology. 2003;2:605–613. doi: 10.1016/s1474-4422(03)00530-1. [DOI] [PubMed] [Google Scholar]
- [13].Clark CM, Karlawish JH. Alzheimer disease: current concepts and emerging diagnostic and therapeutic strategies. Ann Intern Med. 2003;138:400–410. doi: 10.7326/0003-4819-138-5-200303040-00010. [DOI] [PubMed] [Google Scholar]
- [14].Schoonenboom NS, van der Flier WM, Blankenstein MA, Bouwman FH, van Kamp GJ, Barkhof F, Scheltens P. CSF and MRI markers independently contribute to the diagnosis of Alzheimer’s disease. Neurobiol Aging. 2008;29:669–675. doi: 10.1016/j.neurobiolaging.2006.11.018. [DOI] [PubMed] [Google Scholar]
- [15].Blennow K. CSF biomarkers for mild cognitive impairment. J Intern Med. 2004;256:224–234. doi: 10.1111/j.1365-2796.2004.01368.x. [DOI] [PubMed] [Google Scholar]
- [16].de Leon MJ, De Santi S, Zinkowski R, Mehta PD, Pratico D, Segal S, Rusinek H, Li J, Tsui W, Saint Louis LA, Clark CM, Tarshish C, Li Y, Lair L, Javier E, Rich K, Lesbre P, Mosconi L, Reisberg B, Sadowski M, DeBernadis JF, Kerkman DJ, Hampel H, Wahlund LO, Davies P. Longitudinal CSF and MRI biomarkers improve the diagnosis of mild cognitive impairment. Neurobiology of Aging. 2006;27:394–401. doi: 10.1016/j.neurobiolaging.2005.07.003. [DOI] [PubMed] [Google Scholar]
- [17].de Leon MJ, De Santi S, Zinkowski R, Mehta PD, Pratico D, Segal S, Clark C, Kerkman D, DeBernardis J, Li J, Lair L, Reisberg B, Tsui W, Rusinek H. MRI and CSF studies in the early diagnosis of Alzheimer’s disease. J Intern Med. 2004;256:205–223. doi: 10.1111/j.1365-2796.2004.01381.x. [DOI] [PubMed] [Google Scholar]
- [18].Formichi P, Battisti C, Radi E, Federico A. Cerebrospinal fluid tau, A beta, and phosphorylated tau protein for the diagnosis of Alzheimer’s disease. J Cell Physiol. 2006;208:39–46. doi: 10.1002/jcp.20602. [DOI] [PubMed] [Google Scholar]
- [19].Andreasen N, Minthon L, Vanmechelen E. Cerebrospinal fluid tau and Abeta42 as predictors of development of Alzheimer’s disease in patients with mild cognitive impairment. Neuroscience Letters. 1999;273:5–8. doi: 10.1016/s0304-3940(99)00617-5. [DOI] [PubMed] [Google Scholar]
- [20].Hampel H, Mitchell A, Blennow K, Frank R, Brettschneider S, Weller L, Moller HJ. Core biological marker candidates of Alzheimer’s disease – perspectives for diagnosis, prediction of outcome and reflection of biological activity. J Neurol Transm. 2004;111:247–272. doi: 10.1007/s00702-003-0065-z. [DOI] [PubMed] [Google Scholar]
- [21].Lewczuk P, Esselmann H, Otto M, Maler JM, Henkel AW, Henkel MK, Eikenberg O, Antz C, Krause WR, Reulbach U, Kornhuber J, Wiltfang J. Neurochemical diagnosis of Alzheimer’s dementia by CSF A[beta]42, A[beta]42/A[beta]40 ratio and total tau. Neurobiology of Aging. 2004;25:273–281. doi: 10.1016/S0197-4580(03)00086-1. [DOI] [PubMed] [Google Scholar]
- [22].Fagan AM, Roe CM, Xiong C, Mintun M, Morris JC, Holtzman DM. Cerebrospinal Fluid tau/beta-Amyloid42 Ratio as a Prediction of Cognitive Decline in Nondemented Older Adults. Arch Neurol. 2007;64:343–349. doi: 10.1001/archneur.64.3.noc60123. [DOI] [PubMed] [Google Scholar]
- [23].Fagan AM, Mintun MA, Mach RH, Lee S, Dence CS, Shah AR, LaRossa GN, Spinner ML, Klunk WE, Mathis C, DeKosky ST, Morris JC, Holtzman DM. Inverse relation between in vivo amyloid imaging load and cerebrospinal fluid. Ann Neurol. 2006;59:512–519. doi: 10.1002/ana.20730. [DOI] [PubMed] [Google Scholar]
- [24].Jensen M, Schroder J, Blomberg M, Engvall B, Pantel J, Ida N, Basun H, Wahlund LO, Werle E, Jauss Cerebrospinal fluid A beta42 is increased early in sporadic Alzheimer’s disease and declines with disease progression. Ann Neurol. 1999;45:504–511. doi: 10.1002/1531-8249(199904)45:4<504::aid-ana12>3.0.co;2-9. et a. [DOI] [PubMed] [Google Scholar]
- [25].Csernansky JG, Miller JP, McKeel D, Morris JC. Relationships among cerebrospinal fluid biomarkers in dementia of the Alzheimer type. Alzheimer Dis Assoc Disord. 2002;16:144–149. doi: 10.1097/00002093-200207000-00003. [DOI] [PubMed] [Google Scholar]
- [26].Vemuri P, Wiste HJ, Weigand SD, Shaw LM, Trojanowski JQ, Weiner MW, Knopman DS, Petersen RC, Jack CRJ, on behalf of the Alzheimer’s Diasese Neuroimaging Initiative MRI and CSF biomarkers in normal, MCI, and AD subjects: Diagnostic discrimination and cognitive correlations. Neurol. 2009;73:287–293. doi: 10.1212/WNL.0b013e3181af79e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Shoji M, Matsubara E, Murakami T, Manabe Y, Abe K, Kanai M, Ikeda M, Tomidokoro Y, Shizuka M, Watanabe M. Cerebrospinal fluid tau in dementia disorders: a large scale multicenter study by a Japanese study group. Neurobiology of Aging. 2002;23:363–370. doi: 10.1016/s0197-4580(01)00309-8. [DOI] [PubMed] [Google Scholar]
- [28].Brys M, Pirraglia E, Rich K, Rolstad S, Mosconi L, Switalski R, Glodzik-Sobanska L, De Santi S, Zinkowski R, Mehta D, Pratico D, Saint Louis LA, Wallin A, Blennow K, de Leon MJ. Prediction and longitudinal study of CSF biomarkers in mild cognitive impairment. Neurobiology of Aging. 2009;30:682–690. doi: 10.1016/j.neurobiolaging.2007.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol. 1999;56:303–308. doi: 10.1001/archneur.56.3.303. [DOI] [PubMed] [Google Scholar]
- [30].Jungwirth S, Zehetmayer S, Hinterberger M, Tragl KH, Fischer P. The validity of amnestic MCI and non-amnestic MCI at age 75 in the prediction of Alzheimer’s dementia and vascular dementia. Int Psychogeriat. 2012;24:959–966. doi: 10.1017/S1041610211002870. [DOI] [PubMed] [Google Scholar]
- [31].Andreasen N, Hesse C, Davidsson P, Minthon L, Wallin A, Winblad B, Vanderstichele H, Vanmechelen E, Blennow K. Cerebrospinal fluid beta-amyloid(1-42) in Alzheimer disease: differences between early- and late-onset Alzheimer disease and stability during the course of disease. Arch Neurol. 1999:673–680. doi: 10.1001/archneur.56.6.673. [DOI] [PubMed] [Google Scholar]
- [32].Andreasen N, Blennow K. CSF biomarkers for mild cognitive impairment and early Alzheimer’s disease. Clinical Neurology and Neurosurgery. 2005;107:165–173. doi: 10.1016/j.clineuro.2004.10.011. [DOI] [PubMed] [Google Scholar]
- [33].Weingarten MD, Lockwood AH, Hwo SY, Kirschner MW. A protein factor essential for microtubule assembly. Proc Natl Acad Sci U S A. 1975;72:1858–1862. doi: 10.1073/pnas.72.5.1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Billingsley ML, Kincaid RL. Regulated phosphorylation and dephosphorylation of tau protein: effects on microtubule interaction, intracellular trafficking and neurodegeneration. Biochem J. 1997;323(Pt 3):577–591. doi: 10.1042/bj3230577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Mandelkow EM, Mandelkow E. Tau in Alzheimer’s disease. Trends Cell Biol. 1998;8:425–427. doi: 10.1016/s0962-8924(98)01368-3. [DOI] [PubMed] [Google Scholar]
- [36].Hesse C, Rosengren L, Andreasen N, Davidsson P, Vanderstichele H, Vanmechelen E, Blennow K. Transient increase in total tau but not phospho-tau in human cerebrospinal fluid after acute stroke. Neuroscience Letters. 2001;297:187–90. doi: 10.1016/s0304-3940(00)01697-9. [DOI] [PubMed] [Google Scholar]
- [37].Kapaki E, Kilidireas K, Paraskevas GP, Michalopoulou M, Patsouris E. Highly increased CSF tau protein and decreased beta-amyloid (1-42) in sporadic CJD: a discrimination from Alzheimer’s disease? J Neurol Neurosurg Psychiatry. 2001;71:401–403. doi: 10.1136/jnnp.71.3.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Blennow K. Cerebrospinal Fluid Protein Biomarkers for Alzheimer’s Disease. Neurotherapeutics. 2004;1:213–225. doi: 10.1602/neurorx.1.2.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Martin L, Latypova X, Terrro F. Post-translational modifications of tau protein: Implications for Alzheimer’s disease. Neurochemistry International. 2011;58:458–471. doi: 10.1016/j.neuint.2010.12.023. [DOI] [PubMed] [Google Scholar]
- [40].Johnson GVW, Jenkins SM. Tau protein in normal and Alzheimer’s disease brain. Journal of Alzheimer’s Disease. 1999;1:307–328. doi: 10.3233/jad-1999-14-511. [DOI] [PubMed] [Google Scholar]
- [41].Blennow K, Wallin A, Agren H, Spenger C, Siegfried J, Vanmechelen E. Tau protein in cerebrospinal fluid: a biochemical marker for axonal degeneration in Alzheimer disease? Molecular & Chemical Neuropathology. 1995;26:231–245. doi: 10.1007/BF02815140. [DOI] [PubMed] [Google Scholar]
- [42].Sjogren M, Davidsson P, Tullberg M, Minthon L, Wallin A, Wikkelso C, et al. Both total and phosphorylated tau are increased in Alzheimer’s disease. J Neurol Neurosurg Psychiatry. 2001;70:624–30. doi: 10.1136/jnnp.70.5.624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Buerger K, Otto M, Teipel SJ, Zinkowski R, Blennow K, DeBernardis J, Kerkman D, Schroder J, Schonknecht P, Cepek L. Dissociation between CSF total tau and tau protein phosphorylated at threonine 231 in Creutzfeldt-Jakob disease. Neurobiology of Aging. 2006;27:10–15. doi: 10.1016/j.neurobiolaging.2004.12.003. [DOI] [PubMed] [Google Scholar]
- [44].Hesse C, Rosengren L, Vanmechelen E, Vanderstichele H, Jensen C, Davidsson P, Blennow K. Cerebrospinal fluid markers for Alzheimer’s disease evaluated after acute ischemic stroke. J Alzheimers Dis. 2000;2:199–206. doi: 10.3233/jad-2000-23-402. [DOI] [PubMed] [Google Scholar]
- [45].Mitchell AJ. CSF phosphorylated tau in the diagnosis and prognosis of mild cognitive impairment and Alzheimer’s disease: a meta-analysis of 51 studies. J Neurol Neurosurg Psychiatry. 2009;80:966–975. doi: 10.1136/jnnp.2008.167791. [DOI] [PubMed] [Google Scholar]
- [46].Hampel H, Buerger K, Zinkowski R, Teipel SJ, Goernitz A, Andreasen N, Sjoegren M, DeBernardis J, Kerkman D, Ishiguro K, Ohno H, Vanmechelen E, Vanderstichele H, McCulloch C, Moller HJ, Davies P, Blennow K. Measurement of phosphorylated tau epitopes in the differential diagnosis of Alzheimer disease: a comparative cerebrospinal fluid study. Arch of General Psychiatry. 2004;61:95–102. doi: 10.1001/archpsyc.61.1.95. [DOI] [PubMed] [Google Scholar]
- [47].Kapaki EN, Paraskevas GP, Tzerakis NG, Sfagos C, Seretis A, Kararizou E, Vassilopoulos D. Cerebrospinal fluid tau, phospho-tau181 and beta-amyloid1-42 in idiopathic normal pressure hydrocephalus: a discrimination from Alzheimer’s disease. Eur J Neurol. 2007;14:168–173. doi: 10.1111/j.1468-1331.2006.01593.x. [DOI] [PubMed] [Google Scholar]
- [48].Buerger K, Zinkowski R, Teipel SJ, Arai H, DeBernardis J, Kerkman D, McCulloch C, Padberg F, Faltraco F, Goernitz A, Tapiola T, Rapoport SI, Pirttila T, Moller HJ, Hampel H. Differentiation of Geriatric Major Depression from Alzheimer’s disease with CSF tau protein phosphorylated at Threonine 231. Am J Psychiat. 2003;160:376–379. doi: 10.1176/appi.ajp.160.2.376. [DOI] [PubMed] [Google Scholar]
- [49].Buerger K, Zinkowski R, Teipel SJ, Tapiola T, Arai H, Blennow K, Andreasen N, Hofmann-Kiefer K, DeBernardis J, Kerkman D, McCulloch C, Kohnken R, Padberg F, Pirttila T, Schapiro M, Rapoport S, Moller HJ, Davies P, Hampel H. Differential diagnosis of Alzheimer disease with cerebrospinal fluid levels of tau protein phosphorylated at Threonine 231. Arch Neurol. 2002;59:1267–1272. doi: 10.1001/archneur.59.8.1267. [DOI] [PubMed] [Google Scholar]
- [50].Hampel H, Teipel SJ. Total and Phosphorylated Tau Proteins: Evaluation as Core Biomarker Candidates in Frontotemporal Dementia. Dement Geriatr Cogn Disord. 2004;17:350–354. doi: 10.1159/000077170. [DOI] [PubMed] [Google Scholar]
- [51].Blennow K, Vanmechelen E. CSF markers for pathogenic processes in Alzheimer’s disease: diagnostic implications and use in clinical neurochemistry. Brain Research Bulletin. 2003;61:235–242. doi: 10.1016/s0361-9230(03)00086-8. [DOI] [PubMed] [Google Scholar]
- [52].Maddalena A, Papassotiropoulos A, Muller-Tillmanns B, Jung HH, Hegi T, Nitsch RM, Hock C. Biochemical diagnosis of Alzheimer disease by measuring the cerebrospinal fluid ratio of phosphorylated tau protein to beta-amyloid peptide42. Arch Neurol. 2003;60:1202–1206. doi: 10.1001/archneur.60.9.1202. [DOI] [PubMed] [Google Scholar]
- [53].Vandermeeren M, Mercken M, Vanmechelen E, Six J, Van d V, Martin JJ, Cras P. Detection of tau proteins in normal and Alzheimer’s disease cerebrospinal fluid with a sensitive sandwich enzyme-linked immunosorbent assay. J Neurochem. 1993;61:1828–1834. doi: 10.1111/j.1471-4159.1993.tb09823.x. [DOI] [PubMed] [Google Scholar]
- [54].Motter R, Vigo-Pelfrey C, Kholodenko D, Barbour R, Johnson-Wood K, Galasko D, Chang L, Miller B, Clark C, Green R, Olson D, Southwick P, Wolfert R, Munroe B, Liederburg I, Seubert P, Schenk D. Reduction of B-Amyloid peptide42 in the cerebrospinal fluid of patients with Alzheimer’s disease. Ann Neurol. 1995;38:643–648. doi: 10.1002/ana.410380413. [DOI] [PubMed] [Google Scholar]
- [55].Andreasen N, Minthon L, Davidsson P, Vanmechelen E, Vanderstichele H, Winblad B, Blennow K. Evaluation of CSF-tau and CSF-Abeta42 as diagnostic markers for alzheimer disease in clinical practice. Arch Neurol. 2001;58:373–379. doi: 10.1001/archneur.58.3.373. [DOI] [PubMed] [Google Scholar]
- [56].Li G, Sokal I, Quinn JF, Leverenz JB, Brodey M, Schellenberg GD, Kaye JA, Raskind MA, Zhang J, Peskind ER, Montine TJ. CSF tau/A{beta}42 ratio for increased risk of mild cognitive impairment: A follow-up study. Neurol. 2007;69:631–639. doi: 10.1212/01.wnl.0000267428.62582.aa. [DOI] [PubMed] [Google Scholar]
- [57].Bachman DL, Wolf MD, Linn R, Knoefel JE, Cobb J, Belanger BS, D’Agostino RB, White LR. Incidence of dementia and probable Alzheimer’s disease in a general population: the Framingham Study. Neurol. 1993;43:515–519. doi: 10.1212/wnl.43.3_part_1.515. [DOI] [PubMed] [Google Scholar]
- [58].de Leon MJ, George AE, Golomb J, Tarshish C, Convit A, Kluger A, De Santi S, McRae T, Ferris SH, Reisberg B, Ince C, Rusinek H, Bobinski M, Quinn B, Miller DC, Wisniewski HM. Frequency of hippocampal formation atrophy in normal aging and Alzheimer’s disease. Neurobiology of Aging. 1997;18:1–11. doi: 10.1016/s0197-4580(96)00213-8. [DOI] [PubMed] [Google Scholar]
- [59].de Leon MJ, McRae T, Tsai JR, George AE, Marcus DL, Freedman M, Wolf AP, McEwen B. Abnormal cortisol response in Alzheimer’s disease linked to hippocampal atrophy. Lancet. 1988;2:391–392. doi: 10.1016/s0140-6736(88)92855-3. [DOI] [PubMed] [Google Scholar]
- [60].Knopman DS, Parisi JE, Salviati A, Floriach-Robert M, Boeve BF, Ivnik RJ, Smith GE, Dickson DW, Johnson KA, Petersen LE, McDonald W, Braak H, Petersen RC. Neuropathology of cognitively normal elderly. Journal of Neuropathol and Exper. 2003;62:1087–1095. doi: 10.1093/jnen/62.11.1087. [DOI] [PubMed] [Google Scholar]
- [61].Reiman EM, Chen K, Liu X, Bandy D, Yu M, Lee W, Ayutyanont N, Keppler J, Reeder SA, Langbaum JB, Alexander GE, Klunk WE, Mathis CA, Price JC, Aizenstein HJ, DeKosky ST, Caselli RJ. Fibrillar amyloid-beta burden in cognitively normal people at 3 levels of genetic risk for Alzheimer’s disease. Proc Natl Acad Sci USA. 2009;106:6820–6825. doi: 10.1073/pnas.0900345106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Klunk WE, Mathis CA. Amyloid Imaging and (What is “Normal”?) Aging. In: Jagust W, D’Esposito M, editors. Imaging the Aging Brain. Oxford University Press; Oxford: 2009. [Google Scholar]
- [63].Desikan RS, McEvoy LK, Thompson WK, Holland D, Roddey JC, Blennow K, Aisen PS, Brewer JB, Hyman BT, Dale AM. Amyloid-beta associated volume loss occurs only in the presence of phospho-tau. Ann Neurol. 2011;70:657–661. doi: 10.1002/ana.22509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Fukuyama R, Mizuno T, Mori S, Nakajima K, Fushiki S, Yanagisawa K. Age-dependent change in the levels of Abeta40 and Abeta42 in cerebrospinal fluid from control subjects, and a decrease in the ratio of Abeta42 to Abeta40 level in cerebrospinal fluid from Alzheimer’s disease patients. European Neurology. 2000:155–160. doi: 10.1159/000008156. [DOI] [PubMed] [Google Scholar]
- [65].Kunicki S, Richardson J, Mehta PD, Kim KS, Zorychta E. The effects of age, apolipoprotein E phenotype and gender on the concentration of amyloid-beta (A beta) 40, A beta 4242, apolipoprotein E and transthyretin in human cerebrospinal fluid. Clinical Biochemistry. 1998;31:409–415. doi: 10.1016/s0009-9120(98)00027-7. [DOI] [PubMed] [Google Scholar]
- [66].Burkhard PR, Fournier R, Mermillod B, Krause KH, Bouras C, Irminger I. Cerebrospinal fluid tau and Abeta42 concentrations in healthy subjects: delineation of reference intervals and their limitations. Clin Chem Lab Med. 2004;42:396–407. doi: 10.1515/CCLM.2004.071. [DOI] [PubMed] [Google Scholar]
- [67].Nakamura T, Shoji M, Harigaya Y, Watanabe M, Hosoda K, Cheung TT, Shaffer LM, Golde TE, Younkin LH, Younkin SG. Amyloid beta protein levels in cerebrospinal fluid are elevated in early-onset Alzheimer’s disease. Ann Neurol. 1994;36:903–911. doi: 10.1002/ana.410360616. [DOI] [PubMed] [Google Scholar]
- [68].Kester MI, van der Vlies AE, Blankenstein MA, Pijnenburg YA, Van Elk EJ, Scheltens P, van der Flier WM. CSF biomarkers predict rate of cognitive decline in Alzheimer disease. Neurol. 2009;73:1353–1358. doi: 10.1212/WNL.0b013e3181bd8271. [DOI] [PubMed] [Google Scholar]
- [69].Bouwman FH, Schoonenboom NS, Verwey NA, Van Elk EJ, Kok A, Blankenstein MA, Scheltens P, van de. CSF biomarker levels in early and late onset Alzheimer’s disease. Neurobiology of Aging. 2009;30:1895–1901. doi: 10.1016/j.neurobiolaging.2008.02.007. [DOI] [PubMed] [Google Scholar]
- [70].Burkhard PR, Fournier R, Mermillod B, Krauze KH, Bouras C, Irminger I. Cerebrospinal fluid tau and Abeta42 concentrations in healthy subjects: delineation of reference intervals and their limitations. Clin Chem Lab Med. 2004;42:396–407. doi: 10.1515/CCLM.2004.071. [DOI] [PubMed] [Google Scholar]
- [71].Southwick PC, Yamagata SK, Echols CL, Higson GJ, Neynaber SA, Parson RE, Munroe WA. Assessment of amyloid b protein in cerebrospinal fluid as an aid in the diagnostic of Alzheimer’s disease. J Neurochem. 1996;66:259–265. doi: 10.1046/j.1471-4159.1996.66010259.x. [DOI] [PubMed] [Google Scholar]
- [72].Shoji M, Matsubara E, Kanai M, Watanabe M, Nakamura T, Tomidokoro Y, Shizuka M, Wakabayashi K, Igeta Y, Ikeda Y, Mizushima K, Amari M, Ishiguro K, Kawarabayashi T, Harigaya Y, Okamoto K, Hirai S. Combination assay of CSF Tau, A[beta]1-40 and A[beta]1-42(43) as a biochemical marker of Alzheimer’s disease. Journal of the Neurological Sciences. 1998;158:134–140. doi: 10.1016/s0022-510x(98)00122-1. [DOI] [PubMed] [Google Scholar]
- [73].Peskind ER, Li G, Shofer J, Quinn JF, Kaye JA, Clark CM, Farlow MR, DeCarli C, Raskind MA, Schellenberg GD, Lee VMY, Galasko DR. Age and Apolipoprotein E4 Allele Effects on Cerebrospinal Fluid beta-Amyloid 42 in Adults With Normal Cognition. Arch Neurol. 2006;63:936–939. doi: 10.1001/archneur.63.7.936. [DOI] [PubMed] [Google Scholar]
- [74].Glodzik-Sobanska L, Pirraglia E, Brys M, De Santi S, Mosconi L, Rich KE, Switalski R, Saint Louis LA, Sadowski M, Martiniuk F, Mehta PD, Pratico D, Zinkowski R, Blennow K, de Leon MJ. The effects of normal aging and ApoE genotype on the levels of CSF biomarkers for Alzheimer’s disease. Neurobiology Aging. 2009;30:672–681. doi: 10.1016/j.neurobiolaging.2007.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].van Gool WA, Schenk DB, Bolhuis PA. Concentrations of amyloid-fl protein in cerebrospinal fluid increase with age in patients free from neurodegenerative disease. Neurosci Lett. 1994;172:122–124. doi: 10.1016/0304-3940(94)90677-7. [DOI] [PubMed] [Google Scholar]
- [76].Shoji M, Kanai M, Matsubara E, Tomidokoro Y, Shizuka M, Ikeda Y, Ikeda M, Harigaya Y, Okamoto K, Hirai S. The levels of cerebrospinal fluid Abeta40 and Abeta42(43) are regulated age-dependently. Neurobiology of Aging. 2001:209–215. doi: 10.1016/s0197-4580(00)00229-3. [DOI] [PubMed] [Google Scholar]
- [77].Shoji M, Kanai M. Cerebrospinal fluid Abeta40 and Abeta42: Natural course and clinical usefulness. J Alzheimers Dis. 2001;3:313–321. [PubMed] [Google Scholar]
- [78].Bateman RJ, Wen G, Morris JC, Holtzman DM. Fluctuations of CSF amyloid-{beta} levels: Implications for a diagnostic and therapeutic biomarker. Neurol. 2007;68:666–669. doi: 10.1212/01.wnl.0000256043.50901.e3. [DOI] [PubMed] [Google Scholar]
- [79].Kang JE, Lim MM, Bateman RJ, Lee JJ, Smyth LP, Cirrito JR, Fujiki N, Nishino S, Holtzman DM. Amyloid-beta dynamics are regulated by orexin and the sleep-wake cycle. Science. 2009;326:1005–1007. doi: 10.1126/science.1180962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Huang Y, Potter R, Sigurdson W, Santacruz A, Shih S, Ju YE, Kasten T, Morris JC, Mintun M, Duntley S, Bateman RJ. Effects of age and amyloid deposition on abeta dynamics in the human central nervous system. Arch Neurol. 2012;69:51–58. doi: 10.1001/archneurol.2011.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Kaiser E, Schonknecht P, Thomann PA, Hunt A, Schroder J. Influence of delayed CSF storage on concentrations of phospho-tau protein (181), total tau protein and beta-amyloid (1-42) Neurosci Lett. 2007;417:193–195. doi: 10.1016/j.neulet.2007.02.045. [DOI] [PubMed] [Google Scholar]
- [82].Schoonenboom NS, Mulder C, Vanderstichele H, Van Elk EJ, Kok A, van Kamp GJ, Scheltens P, Blankenstein MA. Effects of processing and storage conditions on amyloid beta (1-42) and tau concentrations in cerebrospinal fluid: implications for use in clinical practice. Clin Chem. 2005;51:189–195. doi: 10.1373/clinchem.2004.039735. [DOI] [PubMed] [Google Scholar]
- [83].Cirrito RJ, Yamada AK, Finn MB, Sloviter SR, Bales RK, May CP, Schoepp DD, Paul MS, Holtzman MD. Synaptic Activity Regulates Interstitial Fluid Amyloid-B Levels in Vivo. Neuron. 2005;48:913–922. doi: 10.1016/j.neuron.2005.10.028. [DOI] [PubMed] [Google Scholar]
- [84].Arai H, Terajima M, Miura M, Higuchi S, Muramatsu T, Machida N, Seiki H, Takase S, Clark CM, Lee VMY, Trojanowski JQ, Sasaki H. Tau in cerebrospinal fluid: A potential diagnostic marker in Alzheimer’s disease. Ann Neurol. 1995;38:649–652. doi: 10.1002/ana.410380414. [DOI] [PubMed] [Google Scholar]
- [85].Itoh N, Arai H, Urakami H, Ishiguro K, Ohno H, Hampel H, Buerger K, Wiltfang J, Otto M, Kretzschmar HA, Moeller HJ, Imagawa M, Kohno H, Nakashima K, Kuzuhara S, Sasaski H, Imahori H. Large-scale, multicenter study of cerebrospinal fluid tau protein phosphorylated at serine 199 for the antemortem diagnosis of Alzheimer’s disease. Ann Neurol. 2001;50:150–156. doi: 10.1002/ana.1054. [DOI] [PubMed] [Google Scholar]
- [86].Blomberg M, Jensen M, Basun H, Lannfelt L, Wahlund LO. Cerebrospinal fluid tau levels increase with age in healthy individuals. Dementia and Geriatric Cognitive Disorders. 2001;12:127–132. doi: 10.1159/000051246. [DOI] [PubMed] [Google Scholar]
- [87].Briani C, Ruggero S, Naccarato M, Cagnin A, Ricchieri GL, Pasqui L, Pizzolato G, Battistin L. Combined analysis of CSF +ƒA42 peptide and tau protein and serum antibodies to glycosaminoglycans in Alzheimer’s disease: preliminary data. J Neural Transm. 2002;V109:393–398. doi: 10.1007/s007020200031. [DOI] [PubMed] [Google Scholar]
- [88].Rosler N, Wichart I, Jellinger KA. Total tau protein immunoreactivity in lumbar cerebrospinal fluid of patients with Alzheimer’s disease. J Neurol Neurosurg Psychiatry. 1996;60:237–238. doi: 10.1136/jnnp.60.2.237-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Andreasen N, Vanmechelen E, Van d V, Davidsson P, Hesse C, Tarvonen S, Raiha I, Sourander L, Winblad B, Blennow K. Cerebrospinal fluid tau protein as a biochemical marker for Alzheimer’s disease: a community based follow up study. Journal of Neurology, Neurosurgery & Psychiatry. 1998;64:298–305. doi: 10.1136/jnnp.64.3.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Lewczuk P, Esselmann H, Bibl M, Beck G, Maler JM, Otto M, Kornhuber J, Wiltfang J. Tau Protein Phosphorylated at Threonine 181 in CSF as a Neurochemical Biomarker in Alzheimer’s Disease. J Mol Neurosci. 2004;23:115–122. doi: 10.1385/JMN:23:1-2:115. [DOI] [PubMed] [Google Scholar]
- [91].Huey ED, Mirza N, Putnam KT, Soares H, Csako G, Levy JA, Copenhaver B, Cohen RM, Sunderland T. Stability of CSF beta-amyloid(1-42) and tau levels by APOE genotype in Alzheimer patients. Dement Geriatr Cogn Disord. 2006;22:48–53. doi: 10.1159/000093261. [DOI] [PubMed] [Google Scholar]
- [92].Mattsson N, Rosen E, Hansson O, Andreasen N, Parnetti L, Jonsson M, Herukka SK, van der Flier WM, Blankenstein MA, Ewers M, Rich K, Kaiser E, Verbeek MM, Olde RM, Tsolaki M, Mulugeta E, Aarsland D, Visser PJ, Schroder J, Marcusson J, de LM, Hampel H, Scheltens P, Wallin A, Eriksdotter-Jonhagen M, Minthon L, Winblad B, Blennow K, Zetterberg H. Age and diagnostic performance of Alzheimer disease CSF biomarkers. Neurol. 2012;78:468–476. doi: 10.1212/WNL.0b013e3182477eed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Ewers M, Buerger K, Teipel SJ, Scheltens P, Schroder J, Zinkowski RP, Bouwman FH, Schonknecht P, Schoonenboom NS, Andreasen N, Wallin A, DeBernardis JF, Kerkman DJ, Heindl B, Blennow K, Hampel H. Multicenter assessment of CSF-phosphorylated tau for the prediction of conversion of MCI. Neurol. 2007;69:2205–2212. doi: 10.1212/01.wnl.0000286944.22262.ff. [DOI] [PubMed] [Google Scholar]
- [94].Scheurich A, Urban PP, Koch-Khoury N, Fellgiebel A. CSF phospho-tau is independent of age, cognitive status and gender of neurological patients. J Neurol. 2010;257:609–614. doi: 10.1007/s00415-009-5382-1. [DOI] [PubMed] [Google Scholar]
- [95].Castellani R, Nunomura A, Lee H, Perry G, Smith MA. Phosphorylated Tau: toxic, protective, or none of the above. Journal of Alzheimer’s Disease. 2008;14:377–383. doi: 10.3233/jad-2008-14404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Bouwman FH, van der Flier WM, Schoonenboom NS, Van Elk EJ, Kok A, Rijmen F, Blankenstein MA, Scheltens P. Longitudinal changes of CSF biomarkers in memory clinic patients. Neurol. 2007;69:1006–1011. doi: 10.1212/01.wnl.0000271375.37131.04. [DOI] [PubMed] [Google Scholar]
- [97].Jack CR, Jr., Vemuri P, Viste HJ, Weigand SD, Aisen PS, Trojanowski JQ, Shaw LM, Bernstein MA, Petersen RC, Weiner MW, Knopman DS, for the Alzheimer’s Diasease Neuroimaging Initiative Evidence for Ordering of Alzheimer Disease Biomarkers. Arch Neurol. 2011;68:1526–1535. doi: 10.1001/archneurol.2011.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Lo RY, Hubbard AE, Shaw LM, Trojanowski JQ, Petersen RC, Aisen PS, Weiner MW, Jagust WJ. Longitudinal changes of biomarkers in cognitive decline. Arch Neurol. 2011;68:1257–1265. doi: 10.1001/archneurol.2011.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Brys M, Pirraglia E, Rich K, Rolstad S, Mosconi L, Switalski R, Glodzik-Sobanska L, De SS, Zinkowski R, Mehta P, Pratico D, Saint Louis LA, Wallin A, Blennow K, de Leon MJ. Prediction and longitudinal study of CSF biomarkers in mild cognitive impairment. Neurobiol Aging. 2009;30:682–690. doi: 10.1016/j.neurobiolaging.2007.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Kester MI, Scheffer PG, Koel-Simmelink MJ, Twaalfhoven H, Verwey NA, Veerhuis R, Twisk JW, Bouwman FH, Blankenstein MA, Scheltens P, Teunissen C, van der Flier WM. Serial CSF sampling in Alzheimer’s disease: specific versus non-specific markers. Neurobiol Aging. 2011 doi: 10.1016/j.neurobiolaging.2011.05.013. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- [101].Buerger nee BK, Padberg F, Nolde T, Teipel SJ, Stubner S, Haslinger A, Schwarz MJ, Sunderland T, Arai H, Rapoport SI, Moller HJ, Hampel H. Cerebrospinal fluid tau protein shows a better discrimination in young old (<70 years) than in old old patients with Alzheimer’s disease compared with controls. Neuroscience Letters. 1999;277:21–24. doi: 10.1016/s0304-3940(99)00845-9. [DOI] [PubMed] [Google Scholar]
- [102].Schmand B, Eikelenboom P, van Gool WA. Value of Neuropsychological Tests, Neuroimaging, and Biomarkers for Diagnosing Alzheimer’s Disease in Younger and Older Age Cohorts. J Am Geriatr Soc. 2011;59:1705–1710. doi: 10.1111/j.1532-5415.2011.03539.x. [DOI] [PubMed] [Google Scholar]
- [103].Farrer LA, Cupples LA, Kukull WA, Volicer L, Wells JM, Kurz A, Green RC, Chui H. Risk of Alzheimer disease is associated with parental age among apolipoprotein Epsilon 4 heterozygotes. Alzheimer’s Research. 1997;3:83–91. [Google Scholar]
- [104].Poirier J, Davignon J, Bouthillier D, Kogan S, Bertrand P, Gauthier S. Apolipoprotein E polymorphism and Alzheimer’s disease. Lancet. 1993;342:697–699. doi: 10.1016/0140-6736(93)91705-q. [DOI] [PubMed] [Google Scholar]
- [105].Blennow K, de Leon MJ, Zetterberg H. Alzheimer’s disease. Lancet Neurology. 2006;368:387–403. doi: 10.1016/S0140-6736(06)69113-7. [DOI] [PubMed] [Google Scholar]
- [106].Herukka SK, Helisalmi S, Hallikainen M, Tervo S, Soininen H, Pirttila T. CSF Abeta42, Tau and phosphorylated Tau, APOE epsilon4 allele and MCI type in progressive MCI. Neurobiol Aging. 2007;28:507–514. doi: 10.1016/j.neurobiolaging.2006.02.001. [DOI] [PubMed] [Google Scholar]
- [107].Kester MI, Blankenstein MA, Bouwman FH, Van Elk EJ, Scheltens P, van der Flier WM. CSF Biomarkers in Alzheimer’s Disease and Controls: Associations with APOE Genotype are Modified by Age. Journal of Alzheimer’s Disease. 2009;16:601–607. doi: 10.3233/JAD-2009-0999. [DOI] [PubMed] [Google Scholar]
- [108].Sunderland T, Mirza N, Putnam KT, Linker G, Bhupali D, Durham R, Soeares H, Kimmel L, Friedman D, Bergerson J, Casko G, Levy JA, Bartko JJ, Cohen RM. Cerebrospinal fluid beta-amyloid1-42 and tau in control subjects at risk for Alzheimer’s disease: the effect of APOE epsilon4 allele. Biol Psychiatry. 2004;56:670–676. doi: 10.1016/j.biopsych.2004.07.021. [DOI] [PubMed] [Google Scholar]
- [109].Stenset V, Johnsen L, Kocot D, Negaard A, Skinningsrud A, Gulbrandsen P, Wallin A, Fladby T. Associations between white matter lesions, cerebrovascular risk factors, and low CSF Abeta42. Neurol. 2006;67:830–833. doi: 10.1212/01.wnl.0000234030.77831.5a. [DOI] [PubMed] [Google Scholar]
- [110].Smach MA, Charfeddine B, Lammouchi T, Harrabi I, Ben OL, Dridi H, Bennamou S, Limem K. CSF beta-amyloid 1-42 and tau in Tunisian patients with Alzheimer’s disease: the effect of APOE epsilon4 allele. Neurosci Lett. 2008;440:145–149. doi: 10.1016/j.neulet.2008.05.076. [DOI] [PubMed] [Google Scholar]
- [111].Tapiola T, Pirttila T, Mehta PD, Alafuzofff I, Lehtovirta M, Soininen H. Relationship between apoE genotype and CSF beta-amyloid (1-42) and tau in patients with probable and definite Alzheimer’s disease. Neurobiology of Aging. 2000;21:735–740. doi: 10.1016/s0197-4580(00)00164-0. [DOI] [PubMed] [Google Scholar]
- [112].Popp J, Lewczuk P, Frommann I, Kolsch H, Kornhuber J, Maier W, Jessen F. Cerebrospinal fluid markers for Alzheimer’s disease over the lifespan: effects of age and the APOEepsilon4 genotype. J Alzheimers Dis. 2010;22:459–468. doi: 10.3233/JAD-2010-100561. [DOI] [PubMed] [Google Scholar]
- [113].Mulder SD, van der Flier WM, Verheijen JH, Mulder C, Scheltens P, Blankenstein MA, Hack CE, Veerhuis R. BACE1 activity in cerebrospinal fluid and its relation to markers of AD pathology. J Alzheimers Dis. 2010;20:253–260. doi: 10.3233/JAD-2010-1367. [DOI] [PubMed] [Google Scholar]
- [114].Vemuri P, Wiste HJ, Weigand SD, Knopman DS, Shaw LM, Trojanowski JQ, Aisen PS, Weiner M, Petersen RC, Jack CR., Jr. Effect of apolipoprotein E on biomarkers of amyloid load and neuronal pathology in Alzheimer disease. Ann Neurol. 2010;67:308–316. doi: 10.1002/ana.21953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Fjell AM, Walhovd KB, Fennema-Notestine C, McEvoy LK, Hagler DJ, Holland D, Blennow K, Brewer JB, Dale AM. Brain atrophy in healthy aging is related to CSF levels of Abeta1-42. Cereb Cortex. 2010;20:2069–2079. doi: 10.1093/cercor/bhp279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].De Meyer G, Shapiro F, Vanderstichele H, Vanmechelen E, Engelborghs S, De Deyn PP, Coart E, Hansson O, Minthon L, Zetterberg H, Blennow K, Shaw L, Trojanowski JQ. Diagnosis-independent Alzheimer disease biomarker signature in cognitively normal elderly people. Arch Neurol. 2010;67:949–956. doi: 10.1001/archneurol.2010.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Prince JA, Zetterberg H, Andreasen N, Marcusson J, Blennow K. APOE epsilon4 allele is associated with reduced cerebrospinal fluid levels of Abeta42. Neurol. 2004;62:2116–2118. doi: 10.1212/01.wnl.0000128088.08695.05. [DOI] [PubMed] [Google Scholar]
- [118].Stomrud E, Hansson O, Zetterberg H, Blennow K, Minthon L, Londos E. Correlation of longitudinal cerebrospinal fluid biomarkers with cognitive decline in healthy older adults. Arch Neurol. 2010;67:217–223. doi: 10.1001/archneurol.2009.316. [DOI] [PubMed] [Google Scholar]
- [119].Fagan AM, Younkin LH, Morris JC, Fryer JD, Cole TG, Younkin SG, Holtzman DM. Differences in the Abeta40/Abeta42 ratio associated with cerebrospinal fluid lipoproteins as a function of apolipoprotein E genotype. Ann Neurol. 2000;48:201–210. [PubMed] [Google Scholar]
- [120].Mehta PD, Pirttila T, Mehta SP, Sersen EA, Aisen PS, Wisniewski HM. Plasma and cerebrospinal fluid levels of amyloid beta proteins 1-40 and 1-42 in Alzheimer disease. Arch Neurol. 2000;57:100–105. doi: 10.1001/archneur.57.1.100. [DOI] [PubMed] [Google Scholar]
- [121].Golombowski S, Muller-Spahn F, Romig H, Mendla K, Hock C. Dependence of cerebrospinal fluid Tau protein levels on apolipoprotein E4 allele frequency in patients with Alzheimer’s disease. Neuroscience Letters. 1997;225:213–215. doi: 10.1016/s0304-3940(97)00228-0. [DOI] [PubMed] [Google Scholar]
- [122].Vemuri P, Wiste HJ, Weigand SD, Knopman DS, Trojanowski JQ, Shaw LM, Bernstein MA, Aisen PS, Weiner M, Petersen RC, Jack CR., Jr. Serial MRI and CSF biomarkers in normal aging, MCI, and AD. Neurol. 2010;75:143–151. doi: 10.1212/WNL.0b013e3181e7ca82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Corder EH, Saunders AM, Risch NJ, Strittmatter WJ, Schmechel DE. Protective effect of apolipoprotein E type 2 allele for late onset Alzheimer disease. Nature and Genetics. 1994:180–184. doi: 10.1038/ng0694-180. et.al. [DOI] [PubMed] [Google Scholar]
- [124].Chiang GC, Insel PS, Tosun D, Schuff N, Truran-Sacrey D, Raptentsetsang ST, Jack CR, Jr., Aisen PS, Petersen RC, Weiner MW. Hippocampal atrophy rates and CSF biomarkers in elderly APOE2 normal subjects. Neurol. 2010;75:1976–1981. doi: 10.1212/WNL.0b013e3181ffe4d1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Nicoll JAR, Savva GM, Stewart J, Matthews FE, Bryne C, Ince P, on behalf of the Medical Research Council Cognitive Function and Ageing Study Association between APOE genotype, neuropathology and dementia in the older population of England and Wales. Neuropathology and Applied Neurobiology. 2011;37:285–294. doi: 10.1111/j.1365-2990.2010.01130.x. [DOI] [PubMed] [Google Scholar]
- [126].Love S, Siew LK, Dawbarn D, Wilcock GK, Ben-Shlomo Y, Allen SJ. Premorbid effects of APOE on synaptic proteins in human temporal neocortex. Neurobiology of Aging. 2012;27:797–803. doi: 10.1016/j.neurobiolaging.2005.04.008. [DOI] [PubMed] [Google Scholar]
- [127].Klein RC, Mace BE, Moore SD, Sullivan PM. Progressive los of synaptic integrity in human apolipoprotein E4 targeted replacement mice nad attenuation by apolipoprotein E2. Neurosci. 2010;171:1265–1272. doi: 10.1016/j.neuroscience.2010.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Shaw LM, Vanderstichele H, Knapik-Czajka M, Clark CM, Aisen PS, Petersen RC, Blennow K, Soares H, Simon A, Lewczuk P, Dean R, Siemers E, Potter W, Lee VM, Trojanowski JQ. Cerebrospinal fluid biomarker signature in Alzheimer’s disease neuroimaging initiative subjects. Ann Neurol. 2009;65:403–413. doi: 10.1002/ana.21610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Montine TJ, Peskind ER, Quinn JF, Wilson AM, Montine KS, Galasko D. Increased cerebrospinal fluid F2-isoprostanes are associated with aging and latent Alzheimer’s disease as identified by biomarkers. Neuromolecular Med. 2011;13:37–43. doi: 10.1007/s12017-010-8126-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Glodzik L, Mosconi L, Tsui W, De SS, Zinkowski R, Pirraglia E, Rich KE, McHugh P, Li Y, Williams S, Ali F, Zetterberg H, Blennow K, Mehta P, de Leon MJ. Alzheimer’s disease markers, hypertension, and gray matter damage in normal elderly. Neurobiol Aging. 2011 doi: 10.1016/j.neurobiolaging.2011.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Visser PJ, Verhey F, Knol DL, Scheltens P, Wahlund LO, Freund-Lev Yi, Tsolak M, Minthon L, Wallin AK, Hampel H, Bürger K, Pirttila T, Soininen H, Rikkert MO, Verbeek MM, Spiru L, Blennow K. Prevalence and prognostic value of CSF markers of Alzheimer’s disease pathology in patients with subjective cognitive impairment or mild cognitive impairment in the DESCRIPA study: a prospective cohort study. Lancet Neurol. 2009;8:619–27. doi: 10.1016/S1474-4422(09)70139-5. [DOI] [PubMed] [Google Scholar]
- [132].Reiman EM, Chen K, Alexander GE, Caselli RJ, Bandy D, Osborne D, Saunders AM, Hardy J. Functional brain abnormalities in young adults at genetic risk for late-onset Alzheimer’s dementia. Proc Natl Acad Sci U S A. 2004;101:284–289. doi: 10.1073/pnas.2635903100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Jack CR, Jr., Knopman DS, Jagust WJ, Shaw LM, Aisen PS, Weiner MW, Petersen RC, Trojanowski JQ. Hypothetical model of dynamic biomarkers of the Alzheimer’s pathological cascade. Lancet Neurol. 2010;8:119–128. doi: 10.1016/S1474-4422(09)70299-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Glodzik L, Galvin JE, Pirraglia E, de Leon MJ. Ordering of Alzheimer Disease Biomarkers. Letter. Arch Neurol. 2012 doi: 10.1001/archneurol.2011.2906. in press. [DOI] [PubMed] [Google Scholar]
- [135].Gustafson DR, Skoog I, Rosengren L, Zetterberg H, Blennow K. Cerebrospinal fluid beta-amyloid 1-42 concentration may predict cognitive decline in older women. J Neurol Neurosurg Psychiatry. 2007;78:461–464. doi: 10.1136/jnnp.2006.100529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Stomrud E, Hansson O, Blennow K, Minthon L, Londos E. Cerebrospinal Fluid Biomarkers Predict Decline in Subjective Cognitive Function over 3 Years in Healthy Elderly. Dementia and Geriatric Cognitive Disorders. 2007;24:118–124. doi: 10.1159/000105017. [DOI] [PubMed] [Google Scholar]
- [137].Skoog I, Davidsson P, Aevarsson +, Vanderstichele H, Vanmechelen E, Blennow K. Cerebrospinal Fluid Beta-Amyloid 42 Is Reduced before the Onset of Sporadic Dementia: A Population-Based Study in 85-Year-Olds. Dementia and Geriatric Cognitive Disorders. 2003;15:169–176. doi: 10.1159/000068478. [DOI] [PubMed] [Google Scholar]
- [138].Craig-Schapiro R, Perrin RJ, Roe CM, Xiong C, Carter D, Cairns NJ, Mintun MA, Peskind ER, Li G, Galasko DR, Clark CM, Quinn JF, D’Angio G, Malone JP, Townsend RR, Morris JC, Fagan AM, Holtzman DM. YKL-40: A Novel Prognostic Fluid Biomarker for Preclinical Alzheimer’s Disease. Biological Psychiatry. 2010;68:903–912. doi: 10.1016/j.biopsych.2010.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Glodzik L, De SS, Tsui WH, Mosconi L, Zinkowski R, Pirraglia E, Wang HY, Li Y, Rich KE, Zetterberg H, Blennow K, Mehta P, de Leon MJ. Phosphorylated tau 231, memory decline and medial temporal atrophy in normal elders. Neurobiol Aging. 2011;32:2131–2141. doi: 10.1016/j.neurobiolaging.2009.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Desikan R, McEvoy LK, Thompson WK, Holland D, Brewer JB, Aisen PS, Sperling RA, Dale AM, for the Alzheimer’s Disease Neuroimaging Initiative Amyloid--Associated Clinical Decline Occurs Only in the Presence of Elevated P-tau. Arch Neurol. 2012 doi: 10.1001/archneurol.2011.3354. published online 04/23/2012, doi:10.1001/archneurol.2011.3354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Freeman SH, Kandel R, Cruz L, Rozkalne A, Newell K, Frosch MP, Hedley-Whyte ET, Locascio JJ, Lipsitz LA, Hyman BT. Preservation of neuronal number despite age-related cortical brain atrophy in elderly subjects without Alzheimer disease. J Neuropathol Exp Neurol. 2008;67:1205–1212. doi: 10.1097/NEN.0b013e31818fc72f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Glodzik-Sobanska L, Rusinek H, Mosconi L, Li Y, Zhan J, De Santi S, Convit A, Rich KE, Brys M, de Leon MJ. The role of quantitative structural imaging in the early diagnosis of Alzheimer’s disease. Neuroimaging Clinics of North America. 2005;15:803–826. doi: 10.1016/j.nic.2005.09.004. [DOI] [PubMed] [Google Scholar]
- [143].Bobinski M, de Leon MJ, Wegiel J, De Santi S, Convit A, Saint Louis LA, Rusinek H, Wisniewski HM. The histological validation of post mortem magnetic resonance imaging-determined hippocampal volume in Alzheimer’s disease. Neurosci. 2000;95:721–725. doi: 10.1016/s0306-4522(99)00476-5. [DOI] [PubMed] [Google Scholar]
- [144].Frisoni GB, Fox NC, Jack CR, Jr., Scheltens P, Thompson PM. The clinical use of structural MRI in Alzheimer disease. Nat Rev Neurol. 2010;6:67–77. doi: 10.1038/nrneurol.2009.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Zarow C, Vinters HV, Ellis WG, Weiner MW, Mungas D, White L, Chui HC. Correlates of Hippocampal Neuron Number in Alzheimer’s Disease and Ischemic Vascular Dementia. Ann Neurol. 2005;57:896–903. doi: 10.1002/ana.20503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].de Leon MJ, Convit A, De Santi S, Bobinski M, George AE, Wisniewski HM, Rusinek H, Carroll R, Saint Louis LA. Contribution of structural neuroimaging to the early diagnosis of Alzheimer’s disease. Int Psychogeriat. 1997;9:183–190. doi: 10.1017/s1041610297004900. [DOI] [PubMed] [Google Scholar]
- [147].Rusinek H, De Santi S, Frid D, Tsui W, Tarshish C, Convit A, de Leon MJ. Regional brain atrophy rate predicts future cognitive decline: 6-year longitudinal MR imaging study of normal aging. Radiology. 2003;229:691–696. doi: 10.1148/radiol.2293021299. [DOI] [PubMed] [Google Scholar]
- [148].Hoffman JM, Welsh-Bohmer KA, Hanson M, Crain B, Hulette C, Earl N, Coleman RE. FDG PET imaging in patients with pathologically verified dementia. J Nucl Med. 2000;41:1920–1928. [PubMed] [Google Scholar]
- [149].Jagust W, Reed B, Mungas D, Ellis W, DeCarli C. What does fluorodeoxyglucose PET imaging add to a clinical diagnosis of dementia? Neurol. 2007;69:871–877. doi: 10.1212/01.wnl.0000269790.05105.16. [DOI] [PubMed] [Google Scholar]
- [150].de Leon MJ, Convit A, Wolf OT, Tarshish CY, De Santi S, Rusinek H, Tsui W, Kandil E, Scherer AJ, Roche A, Imossi A, Thorn E, Bobinski M, Caraos C, Lesbre P, Schlyer D, Poirier J, Reisberg B, Fowler J. Prediction of cognitive decline in normal elderly subjects with 2-[18F]fluoro-2-deoxy-D-glucose/positron-emission tomography (FDG/PET) Proc Natl Acad Sci USA. 2001;98:10966–10971. doi: 10.1073/pnas.191044198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].Jagust WJ, Gitcho A, Sun F, Kuczynski B, Mungas D, Haan M. Brain imaging evidence of preclinical Alzheimer’s disease in normal aging. Ann Neurol. 2006;59:673–681. doi: 10.1002/ana.20799. [DOI] [PubMed] [Google Scholar]
- [152].Mosconi L, Pupi A, de Leon MJ. Brain glucose hypometabolism and oxidative stress in preclinical Alzheimer’s Disease. Ann NY Acad Sci. 2009;1147:180–195. doi: 10.1196/annals.1427.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [153].Reiman EM, Caselli RJ, Yun LS, Chen K, Bandy D, Minoshima S, Thibodeau SN, Osborne D. Preclinical evidence of Alzheimer’s disease in persons homozygous for the E4 allele for apolipoprotein E. The New England Journal of Medicine. 1996;334:752–758. doi: 10.1056/NEJM199603213341202. [DOI] [PubMed] [Google Scholar]
- [154].Mosconi L. Brain glucose metabolism in the early and specific diagnosis of Alzheimer’s disease. Eur J Nucl Med. 2005;32:486–510. doi: 10.1007/s00259-005-1762-7. [DOI] [PubMed] [Google Scholar]
- [155].Mosconi L, De Santi S, Li J, Tsui WH, Li Y, Boppana M, Rusinek H, de Leon MJ. Hippocampal hypometabolism predicts cognitive decline from normal aging. Neurobiology of Aging. 2008;29:676–692. doi: 10.1016/j.neurobiolaging.2006.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [156].Klunk WE, Engler H, Nordberg A, Yanming W, Blomqvist G, Holt DP, Bergstrom M, Savitcheva I, Huang GF, Estrada S, Ausen B, Debnath ML, Barletta J, Price JC, Sandell J, Lopresti BJ, Wall A, Koivisto P, Antoni, Mathis CA, Langstrom B. Imaging brain amyloid in Alzheimer’s disease with Pittsburgh Compound-B. Ann Neurol. 2004;55:306–319. doi: 10.1002/ana.20009. [DOI] [PubMed] [Google Scholar]
- [157].Rowe CC, Ng S, Ackermann U, Gong SJ, Pike K, Savage G, Cowie TF, Dickinson KL, Maruff P, Darby D, Smith C, Woodward M, Merory J, Tochon-Danguy H, O’Keefe G, Klunk WE, Mathis CA, Price JC, Masters CL, Villemagne VL. Imaging beta-amyloid burden in aging and dementia. Neurol. 2007;68:1718–1725. doi: 10.1212/01.wnl.0000261919.22630.ea. [DOI] [PubMed] [Google Scholar]
- [158].Edison P, Archer HA, Hinz R, Hammers A, Pavese N, Tai YF, Hotton G, Cutler D, Fox N, Kennedy A, Rossor M, Brooks DJ. Amyloid, hypometabolism, and cognition in Alzheimer disease: An [11C]PIB and [18F]FDG PET study. Neurol. 2007;68:501–508. doi: 10.1212/01.wnl.0000244749.20056.d4. [DOI] [PubMed] [Google Scholar]
- [159].Ikonomovic MD, Klunk WE, Abrahamson EE, Mathis CA, Price JC, Tsopelas ND, Lopresti BJ, Ziolko S, Bi W, Paljug WR, Debnath ML, Hope CE, Isanski BA, Hamilton RL, DeKosky ST. Post-mortem correlates of in vivo PiB-PET amyloid imaging in a typical case of Alzheimer’s disease. Brain. 2008;131:1630–1645. doi: 10.1093/brain/awn016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [160].Josephs KA, Whitwell JL, Ahmed Z, Shiung MM, Weigand SD, Knopman DS, Boeve BF, Parisi JE, Petersen RC, Dickson DW, Jack CRJr. Beta-Amyloid Burden Is Not Associated with Rates of Brain Atrophy. Ann Neurol. 2008;63:204–212. doi: 10.1002/ana.21223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [161].Silbert LC, Quinn JF, Moore MM, Corbridge E, Ball MJ, Murdoch G, Sexton G, Kaye JA. Changes in premorbid brain volume predict Alzheimer’s disease pathology. Neurol. 2003;61:487–492. doi: 10.1212/01.wnl.0000079053.77227.14. [DOI] [PubMed] [Google Scholar]
- [162].Apostolova LG, Hwang KS, Andrawis JP, Green AE, Babakchanian S, Morra JH, Cummings JL, Toga AW, Trojanowski JQ, Shaw LM, Jack CR, Jr., Petersen RC, Aisen PS, Jagust WJ, Koeppe RA, Mathis CA, Weiner MW, Thompson PM. 3D PIB and CSF biomarker associations with hippocampal atrophy in ADNI subjects. Neurobiol Aging. 2010;31:1284–1303. doi: 10.1016/j.neurobiolaging.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [163].Sluimer J, Bouwman FH, Vrenken H, Blankenstein MA, Barkhof F, van der Flier WM, Scheltens P. Whole-brain atrophy rate and CSF biomarker levels in MCI and AD: A longitudinal study. Neurobiol Aging. 2008;31:758–764. doi: 10.1016/j.neurobiolaging.2008.06.016. [DOI] [PubMed] [Google Scholar]
- [164].Loew AD, Yanowsky I, Parikshak N, Hua X, Lee S, Toga AW, Jack CRJ, Bernstein MA, Briston PJ, Gunter JL, Ward CP, Borowski BJ, Shaw LM, Trojanowski JQ, Fleisher AS, Harvey D, Kornak J, Schuff N, Alexander GE, Weiner MW, Thompson PM, the Alzheimer’s Disease Neuroimaging Initiative Alzheimer’s Disease Neuroimaging Initiative: A one-year follow up study using tensor-based morphometry correlating degenerative rates, biomarkers and cognition. Neuroimage. 2009;45:645–655. doi: 10.1016/j.neuroimage.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [165].Henneman WJ, Vrenken H, Barnes J, Sluimer IC, Verwey NA, Blankenstein MA, Klein M, Fox NC, Scheltens P, Barkhof F, van der Flier WM. Baseline CSF p-tau levels independently predict progression of hippocampal atrophy in Alzheimer disease. Neurol. 2009;73:935–940. doi: 10.1212/WNL.0b013e3181b879ac. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [166].Desikan RS, Sabuncu MR, Schmansky NJ, Reuter M, Cabral HJ, Hess CP, Weiner MW, Biffi A, Anderson CD, Rosand J, Salat DH, Kemper TL, Dale AM, Sperling RA, Fischl B, for the Alzheimer’s Diasease Neuroimaging Initiative Selective Disruption of the Cerebral Neocortex in Alzheimer’s Disease. PLoS One. 2010;5:e12853. doi: 10.1371/journal.pone.0012853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [167].Tosun D, Schuff N, Truran-Sacrey D, Shaw LM, Trojanowski JQ, Aisen P, Peterson R, Weiner MW. Relations between brain tissue loss, CSF biomarkers, and the ApoE genetic profile: a longitudinal MRI study. Neurobiol Aging. 2010;31:1340–1354. doi: 10.1016/j.neurobiolaging.2010.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [168].Fagan AM, Head D, Shah AR, Marcus D, Mintun M, Morris JC, Holtzman DM. Decreased Cerebrospinal Fluid A42 Correlates with Brain Atrophy in Cognitively Normal Elderly. Ann Neurol. 2009;65:176–183. doi: 10.1002/ana.21559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [169].Schott JM, Bartlett JW, Barnes J, Leung KK, Ourselin S, Fox NC. Reduced sample sizes for atrophy outcomes in Alzheimer’s disease trials: baseline adjustment. Neurobiol Aging. 2010;31:1452–1462. doi: 10.1016/j.neurobiolaging.2010.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [170].Dickerson BC, Wolk DA. MRI cortical thickness biomarker predicts AD-like CSF and cognitive decline in normal adults. Neurol. 2012;78:84–90. doi: 10.1212/WNL.0b013e31823efc6c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [171].Ott BR, Cohen RA, Gongvatana A, Okonkwo OC, Johanson CE, Stopa EG, Donahue JE, Silverberg GD, Alzheimer’s Disease Neuroimaging Initiative Brain ventricular volume and cerebrospinal fluid biomarkers of Alzheimer’s disease. J Alzheimers Dis. 2010;20:647–657. doi: 10.3233/JAD-2010-1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [172].Fortea J, Sala-Llonch R, Bartres-Faz D, Llado A, Sole-Padulles C, Bosch B, Antonell A, Olives J, Sanchez-Valle R, Molinuevo JL, Rami L. Cognitively Preserved Subjects with Transitional Cerebrospinal Fluid ß-Amyloid 1-42 Values Have Thicker Cortex in Alzheimer’s Disease Vulnerable Areas. Biological Psychiatry. 2011;70:183–190. doi: 10.1016/j.biopsych.2011.02.017. [DOI] [PubMed] [Google Scholar]
- [173].Mosconi L, De Santi S, Brys M, Tsui WH, Pirraglia E, Glodzik-Sobanska L, Rich KE, Switalski R, Mehta PD, Pratico D, Zinkowski R, Blennow K, de Leon MJ. Hypometabolism and altered CSF markers in normal ApoE E4 carriers with subjective memory complaints. Biol Psychiatry. 2008;63:609–618. doi: 10.1016/j.biopsych.2007.05.030. PMC236268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [174].Petrie EC, Cross DJ, Galasko D, Schellenberg GD, Raskind MA, Peskind ER, Minoshima S. Preclinical Evidence of Alzheimer Changes. Convergent Cerebrospinal Fluid Biomarker and FluorodeoxyglucosePositron Emission Tomography Findings. Arch Neurol. 2009;66:632–637. doi: 10.1001/archneurol.2009.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [175].Fagan AM, Mintun MA, Shah AR, Aldea P, Roe CM, Mach RH, Marcus D, Morris JC, Holtzman DM. Cerebrospinal fluid tau and ptau(181) increase with cortical amyloid deposition in cognitively normal individuals: implications for future clinical trials of Alzheimer’s disease. EMBO Mol Med. 2009;1:371–380. doi: 10.1002/emmm.200900048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [176].Han DS, Gruhl J, Beckett L, Dodge HH, Stricker NH, Farias S, Mungas D. Beta amyloid, tau, neuroimaging, and cognition: sequence modeling of biomarkers for Alzheimer’s Disease. Brain Imaging and Behavior. 2012 doi: 10.1007/s11682-012-9177-0. DOI 10.1007/s11682-012-9177-0- [DOI] [PMC free article] [PubMed] [Google Scholar]
- [177].Schneider JA, Aggarwal NT, Barnes L, Boyle P, Bennett DA. The neuropathology of older persons with and without dementia from community versus clinic cohorts. J Alzheimer’s Dis. 2009;18:691–701. doi: 10.3233/JAD-2009-1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [178].Sparks DL, Scheff SW, Liu H, Landers TM, Coyne CM, Hunsaker JCI. Increased incidence of neurofibrillary tangles (NFT) in non-demented individuals with hypertension. Journal of the Neurological Sciences. 1995;131:162–169. doi: 10.1016/0022-510x(95)00105-b. [DOI] [PubMed] [Google Scholar]
- [179].Sun X, He G, Qing H, Zhou W, Dobie F, Cai F, Staufenbiel M, Huang LE, Song W. Hypoxia facilitates Alzheimer’s disease pathogenesis by up-regulating BACE1 gene expression. PNAS. 2006;103:18727–18732. doi: 10.1073/pnas.0606298103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [180].Koike MA, Green KN, Blurton-Jones M, LaFerla FM. Oligemic hypoperfusion differentially affects tau and amyloid-beta. Am J Pathol. 2010;177:300–310. doi: 10.2353/ajpath.2010.090750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [181].Kester MI, van der Flier WM, Mandic G, Blankenstein MA, Scheltens P, Muller M. Joint effect of hypertension and APOE genotype on CSF biomarkers for Alzheimer’s disease. J Alzheimer’s Dis. 2010;20:1083–1090. doi: 10.3233/JAD-2010-091198. [DOI] [PubMed] [Google Scholar]
- [182].Craft S, Watson GS. Insulin and neurodegenerative disease: shared and specific mechanisms. Lancet Neurol. 2004;3:169–178. doi: 10.1016/S1474-4422(04)00681-7. [DOI] [PubMed] [Google Scholar]
- [183].Dumurgier J, Paquet C, Peoc’h K, Lapalus P, Mouton-Liger F, Benisty S, Chasseigneaux S, Chabriat H, Hugon J. CSF Abeta1-42 Levels and Glucose Metabolism in Alzheimer’s Disease>. J Alzheimers Dis. 2011;27:845–851. doi: 10.3233/JAD-2011-111007. [DOI] [PubMed] [Google Scholar]
- [184].Exalto LG, van der Flier WM, Scheltens P, Biessels GJ. Glycemia and levels of cerebrospinal fluid amyloid and tau in patients attending a memory clinic. J Am Geriatr Soc. 2010;58:1318–1321. doi: 10.1111/j.1532-5415.2010.02854.x. [DOI] [PubMed] [Google Scholar]
- [185].Fazekas F, Kleinert R, Offenbacher H, Schmidt R, Kleinert G, Payer F, Radner H, Lechner H. Pathologic correlates of incidental MRI white matter signal hypertensities. Neurol. 1993;43:1683–1689. doi: 10.1212/wnl.43.9.1683. [DOI] [PubMed] [Google Scholar]
- [186].Stenset V, Hofoss D, Johnsen L, Berstad AE, Negaard A, Skinningsrud A, Gjerstad L, Fladby T. White matter lesion load increases the risk of low CSF Abeta42 in apolipoprotein E-varepsilon4 carriers attending a memory clinic. J Neuroimaging. 2011;21:e78–e82. doi: 10.1111/j.1552-6569.2009.00444.x. [DOI] [PubMed] [Google Scholar]
- [187].Jonsson M, Zetterberg H, van Straaten E, Lind K, Syversen S, Edman A, Blennow K, Rosengren L, Pantoni L, Inzitari D, Wallin A. Cerebrospinal fluid biomarkers of white matter lesions – cross-sectional results from the LADIS study. European Journal of Neurology. 2010;17:377–382. doi: 10.1111/j.1468-1331.2009.02808.x. [DOI] [PubMed] [Google Scholar]
- [188].Vidoni ED, Townley RA, Honea RA, Burns JM, for the Alzheimer’s Diasease Neuroimaging Initiative Alzheimer disease biomarkers are associated with body mass index. Neurol. 2011;77:1913–1920. doi: 10.1212/WNL.0b013e318238eec1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [189].Ewers M, Schmitz S, Hansson O, Walsh C, Fitzpatrick A, Bennett D, Minthon L, Trojanowski JQ, Shaw LM, Faluyi YO, Vellas B, Dubois B, Blennow K, Buerger K, Teipel SJ, Weiner M, Hampel H. Body mass index is associated with biological CSF markers of core brain pathology of Alzheimer’s disease. Neurobiol Aging. 2011 doi: 10.1016/j.neurobiolaging.2011.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [190].Liang KY, Mintun MA, Fagan AM, Goate AM, Bugg JM, Holtzman DM, Morris JC, Head D. Exercise and Alzheimer’s Disease biomarkers in cognitively normal older adults. Ann Neurol. 2010;68:311–318. doi: 10.1002/ana.22096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [191].Baker LD, Bayer-Carter JL, Skinner J, Montine TJ, Cholerton BA, Callaghan M, Walter BK, Tsai E, Postupna N, Lampe J, Craft S. High-intensity physical activity modulates diet effects on cerebrospinal amyloid levels in normal aging and mild cognitive impairment. Journal of Alzheimer’s Disease. 2011;28:1–10. doi: 10.3233/JAD-2011-111076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [192].Kolata G. Tests Detect Alzheimer’s Risks, but Should Patients Be Told? 2010. p. A1.
- [193].Mattsson N, Zetterberg H, Hansson O, Andreasen N, Parnetti L, Jonsson M, Herukka SK, van der Flier WM, Blankenstein MA, Ewers M, Rich K, Kaiser E, Verbeek M, Tsolaki M, Mlugeta E, Rosen E, Aarsland D, Visser PJ, Schroder J, Marcusson J, de Leon MJ, Hampel H, Scheltens P, Pirttila T, Wallin A, Joha, n ME, Minth L, Winbl B, Blenn K. CSF Biomarkers and Incipient Alzheimer Disease in Patients With Mild Cognitive Impairment. JAMA: the Journal of the American Medical Association. 2009;302:385–393. doi: 10.1001/jama.2009.1064. [DOI] [PubMed] [Google Scholar]
- [194].Kluft C. HRT effects on inflammatory markers: is chronic inflammation a contra-indication for HRT? International Congress Series. 2002;1229:103–108. [Google Scholar]
- [195].Arai H, Satoh-Nakagawa T, Higuchi M, Morikawa Y, Miura M, Kawakami H, Seki H, Takase S, Sasaki H. No increase in cerebrospinal fluid tau protein levels in patients with vascular dementia. Neuroscience Letters. 1998;256:174–176. doi: 10.1016/s0304-3940(98)00781-2. [DOI] [PubMed] [Google Scholar]
- [196].Brettschneider J, Petzold A, Sussmuth SD, Ludolph AC, Tumani H. Axonal damage markers in cerebrospinal fluid are increased in ALS. Neurol. 2006;66:852–856. doi: 10.1212/01.wnl.0000203120.85850.54. [DOI] [PubMed] [Google Scholar]
- [197].Buerger K, Frisoni G, Uspenskaya O, Ewers M, Zetterberg H, Geroldi C, Binetti G, Johannsen P, Rossini PM, Wahlund LO, Vellas B, Blennow K, Hampel H. Validation of Alzheimer’s disease CSF and plasma biological markers: the multicentre reliability study of the pilot European Alzheimer’s Disease Neuroimaging Initiative (E-ADNI) Exp Gerontol. 2009;44:579–585. doi: 10.1016/j.exger.2009.06.003. [DOI] [PubMed] [Google Scholar]
- [198].Caroli A, Frisoni GB. The dynamics of Alzheimer’s disease biomarkers in the Alzheimer’s Disease Neuroimaging Initiative cohort. Neurobiol Aging. 2010;31:1263–1274. doi: 10.1016/j.neurobiolaging.2010.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [199].Fellgiebel A, Kojro E, Muller MJ, Scheurich A, Schmidt LG, Fahrenholz F. CSF APPs alpha and phosphorylated tau protein levels in mild cognitive impairment and dementia of Alzheimer’s type. J Geriatr Psychiatry Neurol. 2009;22:3–9. doi: 10.1177/0891988708327810. [DOI] [PubMed] [Google Scholar]
- [200].Galasko D, Chang L, Motter R, Clark CM, Kaye J, Knopman D, Thomas R, Kholodenko D, Schenk D, Lieberburg I, Miller B, Green R, Basherad R, Kertiles L, Boss MA, Seubert P. High cerebrospinal fluid tau and low amyloid beta42 levels in the clinical diagnosis of Alzheimer disease and relation to apolipoprotein E genotype. Arch Neurol. 1998;55:937–945. doi: 10.1001/archneur.55.7.937. [DOI] [PubMed] [Google Scholar]
- [201].Ganzer S, Arlt S, Schoder V, Buhmann C, Mandelkow EM, Finckh U, Beisiegel U, Naber D, Muller-Thomsen T. CSF-tau, CSF-Abeta1-42, ApoE-genotype and clinical parameters in the diagnosis of Alzheimer’s disease: combination of CSF-tau and MMSE yields highest sensitivity and specificity. J Neural Transm. 2003;110:1149–1160. doi: 10.1007/s00702-003-0017-7. [DOI] [PubMed] [Google Scholar]
- [202].Hu YY, He SS, Wang X, Duan QH, Grundke-Iqbal I, Iqbal K, Wang J. Levels of nonphosphorylated and phosphorylated tau in cerebrospinal fluid of Alzheimer’s disease patients : an ultrasensitive bienzyme-substrate-recycle enzyme-linked immunosorbent assay. American Journal of Pathology. 2002;160:1269–1278. doi: 10.1016/S0002-9440(10)62554-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [203].Hulstaert F, Blennow K, Ivanoiu A, Schoonderwaldt HC, Riemenschneider M, De Deyn PP, Bancher C, Cras P, Wiltfang J, Mehta PD, Iqbal K, Pottel H, Vanmechelen E, Vanderstichele H. Improved discrimination of AD patients using b-amyloid (1-42) and tau levels in CSF. Neurol. 1999;52:1555–1562. doi: 10.1212/wnl.52.8.1555. [DOI] [PubMed] [Google Scholar]
- [204].Iqbal K, Alonso AC, Chen S, Chohan O, El-Akkad E, Gong G-X, Khatoon S, Li B, Liu F, Rahman A, Tanimukai H, Grundke-Iqbal I. Tau pathology in Alzheimer disease and other taupathies. Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease. 2005;1739:198–210. doi: 10.1016/j.bbadis.2004.09.008. [DOI] [PubMed] [Google Scholar]
- [205].Ibach B, Binder H, Dragon M, Poljansky S, Haen E, Schmitz E, Koch H, Putzhammer A, Kluenemann H, Wieland W, Hajak G. Cerebrospinal fluid tau and beta-amyloid in Alzheimer patients, disease controls and an age-matched random sample. Neurobiol Aging. 2006;27:1202–1211. doi: 10.1016/j.neurobiolaging.2005.06.005. [DOI] [PubMed] [Google Scholar]
- [206].Kanai M, Matsubara E, Isoe K, Urakami K, Nakashima K, Arai H, Sasaki H, Abe K, Iwatsubo T, Kosaka T, Watanabe M, Tomidokoro Y, Shizuka M, Mizushima K, Nakamura T, Igeta Y, Ikeda Y, Amari M, Kawarabayashi T, Ishiguro K, Harigaya Y, Wakabayashi K, Okamoto K, Hirai S, Shoji M. Longitudinal study of cerebrospinal fluid levels of tau, A beta1-40, and A beta1-42(43) in Alzheimer’s disease: a study in Japan. Ann Neurol. 1998:17–26. doi: 10.1002/ana.410440108. [DOI] [PubMed] [Google Scholar]
- [207].Kanemaru K, Kameda N, Yamanouchi H. Decreased CSF amyloid beta42 and normal tau levels in dementia with Lewy bodies. Neurol. 2000;54:1875–1876. doi: 10.1212/wnl.54.9.1875. [DOI] [PubMed] [Google Scholar]
- [208].Kapaki E, Paraskevas GP, Zalonis I, Zournas C. CSF tau protein and beta-amyloid (1-42) in Alzheimer’s disease diagnosis: discrimination from normal ageing and other dementias in the Greek population. European Journal Of Neurology: The Official Journal Of The European Federation Of Neurological Societies. 2003;10:119–128. doi: 10.1046/j.1468-1331.2003.00562.x. [DOI] [PubMed] [Google Scholar]
- [209].Kurz A, Riemenschneider M, Buch K, Willoch F, Bartenstein P, Muller U, Guder W. Tau protein in cerebrospinal fluid is significantly increased at the earliest clinical stage of Alzheimer disease. Alzheimer Dis Assoc Disord. 1998;12:372–377. doi: 10.1097/00002093-199812000-00020. [DOI] [PubMed] [Google Scholar]
- [210].Maccioni RB, Lavados M, Guillon M, Mujica C, Bosch R, Farias G, Fuentes P. Anomalously phosphorylated tau and Abeta fragments in the CSF correlates with cognitive impairment in MCI subjects. Neurobiol Aging. 2006;27:237–244. doi: 10.1016/j.neurobiolaging.2005.01.011. [DOI] [PubMed] [Google Scholar]
- [211].Maruyama M, Arai H, Sugita M, Tanji H, Higuchi M, Okamura N, Matsui T, Higuchi S, Matsushita S, Yoshida Cerebrospinal fluid amyloid beta(1-42) levels in the mild cognitive impairment stage of Alzheimer’s disease. Exp Neurol. 2001;172:433–436. doi: 10.1006/exnr.2001.7814. et a. [DOI] [PubMed] [Google Scholar]
- [212].Nishimura T, Takeda M, Nakamura Y, Yosbida Y, Arai H, Sasaki H, Shouji M, Hirai S, Khise K, Tanaka K, Hamamoto M, Yamamoto H, Matsubayashi T, Urakami K, Adachi Y, Nakashima K, Toji H, Nakamura S, Yoshida H. Basic and clinical studies on the measurement of tau protein in cerebrospinal fluid as a biological marker for Alzheimer’s disease and related disorders: multicenter study in Japan. Methods & Findings in Experimental & Clinical Pharmacology. 1998;20:227–235. [PubMed] [Google Scholar]
- [213].Okonkwo OC, Mielke MM, Griffith HR, Moghekar AR, O’Brien RJ, Shaw LM, Trojanowski JQ, Albert MS. Cerebrospinal fluid profiles and prospective course and outcome in patients with amnestic mild cognitive impairment. Arch Neurol. 2011;68:113–119. doi: 10.1001/archneurol.2010.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [214].Riemenschneider M, Wagenpfeil S, Diehl J, Lautenschlager N, Theml T, Heldmann B, Drzezga A, Jahn T, Forstl H, Kurz Tau and Abeta42 protein in CSF of patients with frontotemporal degeneration. Neurol. 2002;58:1622–1628. doi: 10.1212/wnl.58.11.1622. et a. [DOI] [PubMed] [Google Scholar]
- [215].Rolstad S, Berg AI, Bjerke M, Blennow K, Johansson B, Zetterberg H, Wallin A. Amyloid-beta is associated with cognitive impairment in healthy elderly and subjective cognitive impairment. J Alzheimers Dis. 2011;26:135–142. doi: 10.3233/JAD-2011-110038. [DOI] [PubMed] [Google Scholar]
- [216].Rosler N, Wichart I, Jellinger KA. Clinical significance of neurobiochemical profiles in the lumbar cerebrospinal fluid of Alzheimer’s disease patients. J Neural Transm. 2001;108:231–246. doi: 10.1007/s007020170091. [DOI] [PubMed] [Google Scholar]
- [217].Seppala TT, Koivisto AM, Hartikainen P, Helisalmi S, Soininen H, Herukka SK. Longitudinal changes of CSF biomarkers in Alzheimer’s disease. J Alzheimers Dis. 2011;25:583–594. doi: 10.3233/JAD-2011-101911. [DOI] [PubMed] [Google Scholar]
- [218].Sjogren M, Minthon L, Davidsson P, Granerus A-K, Clarberg A, Vanderstichele H, Vanmechelen E, Wallin A, Blennow K. CSF levels of tau, beta-amyloid(1-42) and GAP-43 in frontotemporal dementia, other types of dementia and normal aging. J Neural Transm. 2000;107:563–579. doi: 10.1007/s007020070079. [DOI] [PubMed] [Google Scholar]
- [219].Sunderland T, Linker G, Mirza N, Putnam KT, Friedman DL, Kimmel LH, Bergeson J, Manetti GJ, Zimmermann M, Tang B, Bartko JJ, Cohen RM. Decreased {beta}-Amyloid1-42 and Increased Tau Levels in Cerebrospinal Fluid of Patients With Alzheimer Disease. JAMA: the Journal of the American Medical Association. 2003;289:2094. doi: 10.1001/jama.289.16.2094. [DOI] [PubMed] [Google Scholar]
- [220].Tapiola T, Lehtovirta M, Ramberg J, Helisalmi S, Linnaranta K, Riekkinen P, Sr., Soininen H. CSF tau is related to apolipoprotein E genotype in early Alzheimer’s disease. Neurol. 1998;50:169–174. doi: 10.1212/wnl.50.1.169. [DOI] [PubMed] [Google Scholar]
- [221].Vanderstichele H, Van KE, Hesse C, Davidsson P, Buyse MA, Andreasen N, Minthon L, Wallin A, Blennow K, Vanmechelen E. Standardization of measurement of beta-amyloid(1-42) in cerebrospinal fluid and plasma. Amyloid. 2000;7:245–258. doi: 10.3109/13506120009146438. [DOI] [PubMed] [Google Scholar]
- [222].Vanderstichele H, De Vreese K, Blennow K, Andreasen N, Sindic CJ, Ivanoiu A, Hampel H, Buerger K, Parnetti L, Lanari A, Padovani A, DiLuca M, Blaser M, Olsson AO, Pottel H, Hulstaert F, Vanmechelen E. Analytical performance and clinical utility of the INNOTEST PHOSPHO-TAU(181P) assay for discrimination between Alzheimer’s disease and dementia with Lewy bodies. Clin Chem Lab Med. 2006;44:1472–1480. doi: 10.1515/CCLM.2006.258. [DOI] [PubMed] [Google Scholar]
- [223].Vanmechelen E, Vanderstichele H, Davidsson P, Van Kerschaver E, Van Der Perre B, Sjogren M, Andreasen N, Blennow K. Quantification of tau phosphorylated at threonine 181 in human cerebrospinal fluid: a sandwich ELISA with a synthetic phosphopeptide for standardization. Neuroscience Letters. 2000;285:49–52. doi: 10.1016/s0304-3940(00)01036-3. [DOI] [PubMed] [Google Scholar]
- [224].Buerger K, Ewers M, Andreasen N, Zinkowski R, Ishiguro K, Vanmechelen E, Teipel SJ, Graz C, Blennow K, Hampel H. Phosphorylated tau predicts rate of cognitive decline in MCI subjects: A comparative CSF study. Neurol. 2005;65:1502–1503. doi: 10.1212/01.wnl.0000183284.92920.f2. [DOI] [PubMed] [Google Scholar]
- [225].de Leon MJ, Segal CY, Tarshish CY, De Santi S, Zinkowski R, Mehta PD, Convit A, Caraos C, Rusinek H, Tsui W, Saint-Louis LA, DeBernardis J, Kerkman D, Qadri F, Gary A, Lesbre P, Wisniewski H, Poirier J, Davies P. Longitudinal CSF tau load increases in mild cognitive impairment. Neuroscience Letters. 2002;333:183–186. doi: 10.1016/s0304-3940(02)01038-8. [DOI] [PubMed] [Google Scholar]
- [226].Hansson O, Zetterberg H, Buchhave P, Londos E, Blennow K, Minthon L. Association between CSF biomarkers and incipient Alzheimer’s disease in patients with mild cognitive impairment: a follow-up study. The Lancet Neurology. 2006;5:228–234. doi: 10.1016/S1474-4422(06)70355-6. [DOI] [PubMed] [Google Scholar]
- [227].Lavados M, Farias G, Rothhammer F, Guillon M, Mujica MC, Maccioni C, Maccioni RB. ApoE alleles and tau markers in patients with different levels of cognitive impairment. Arch Med Res. 2005;36:474–479. doi: 10.1016/j.arcmed.2005.03.036. [DOI] [PubMed] [Google Scholar]
- [228].Pratico D, Clark CM, Liun F, Lee VY, Trojanowski JQ. Increase of brain oxidative stress in mild cognitive impairment: a possible predictor of Alzheimer disease. Arch Neurol. 2002;59:972–976. doi: 10.1001/archneur.59.6.972. [DOI] [PubMed] [Google Scholar]
- [229].Schonknecht P, Pantel J, Kaiser E, Thomann P, Schroder J. Increased tau protein differentiates mild cognitive impairment from geriatric depression and predicts conversion to dementia. Neurosci Lett. 2007;416:39–42. doi: 10.1016/j.neulet.2007.01.070. [DOI] [PubMed] [Google Scholar]


