Abstract
Background
New research criteria for preclinical Alzheimer’s disease (AD)have been proposed by the National Institute on Aging and Alzheimer’s Association. They include stages for cognitively normal individuals with abnormal amyloid markers (stage 1), abnormal amyloid and injury markers (stage 2) and abnormal amyloid and injury markers and subtle cognitive changes (stage 3). We investigated the occurrence and long-term outcome of these stages.
Methods
Cerebrospinal fluidamyloid-β1–42 and tau levels and a memory composite score were used to classify 311 cognitively normal(Clinical Dementia Rating [CDR]=0) research participants ≥65 years as normal (both markers normal), preclinical AD stage 1–3, or Suspected Non-Alzheimer Pathophysiology (SNAP, abnormal injury marker without abnormal amyloid marker). Outcome measures were progression to CDR≥0·5 symptomatic AD and mortality up to 15 years after baseline (average=4 years).
Findings
129 (41·5%) of participants were normal, 47 (15%)were in stage 1, 36 (12%) in stage 2, 13 (4%)in stage 3, 72 (23%) had SNAP, and 14 (4·5%) remained unclassified. The proportion of preclinical AD (stage 1–3) in our cohort was higher in individuals older than 72 years and in APOE-ε4 carriers. The 5-year progression rate to CDR≥0·5 symptomatic AD was 2% for normal participants, 11% for stage 1, 26% for stage 2, 56% for stage 3, and 5% for SNAP. Compared with normal individuals, participants with preclinical AD had an increased risk of death (HR=6·2, p=0·0396).
Interpretation
Preclinical AD is common in cognitively normal elderly and strongly associated with future cognitive decline and mortality. Preclinical AD thus should be an important target for therapeutic interventions.
Introduction
Alzheimer’s disease (AD) starts with a preclinical phase in which AD neuropathology begins to accumulate but cognitive performance is normal.1–3 Now that biomarkers for AD have become available, it is possible to identify preclinical AD in vivo in cognitively normal individuals.4 Information regarding the occurrence and outcome of preclinical AD is crucial for the understanding of AD pathophysiology and design of secondary prevention trials. New research criteria for preclinical AD have been proposed by the Preclinical Working Group of the National Institute on Aging and Alzheimer's Association (NIA-AA).5 The aim of this study was to determine the prevalence and long-term outcome of preclinical AD according to these criteria in a cohort of cognitively normal individuals.
The NIA-AA criteria for preclinical AD proposed ordered stages for cognitively normal individuals with abnormal amyloid markers (stage 1), abnormal amyloid and injury markers (stage 2) and abnormal amyloid and injury markers and subtle cognitive changes (stage 3).5 A recent study using structural and amyloid imaging markers to stage individuals found that the short-term (1 year) progression rate to Mild Cognitive Impairment (MCI) or dementia increased with advancing preclinical AD stage.6
We determined the proportion and long-term cognitive and mortality outcome of the NIA-AA preclinical AD stages in a large cohort of cognitively normal research participants using cerebrospinal fluid (CSF) markers to define the preclinical AD stages. CSF amyloid-β (Aβ)1–42 was used as a marker of amyloid, and CSF tau was used as a marker of neuronal injury. We also tested whether the proportion and cognitive outcome of preclinical AD were influenced by age or a polipoprotein E (APOE) genotype.
Methods
Research participants
Participants were cognitively normal community-dwelling volunteers enrolled between 1998 and 2011 in longitudinal studies of memory and aging at the Knight Alzheimer’s Disease Research Center (KADRC)of the Washington University School of Medicine (WUSM)in St. Louis. Details about recruitment and assessment methods for these participants have been published.7 The study is based on continuous enrollment. Participants were living independently in the community at study entry and were evaluated annually unless prevented by death, illness, refusal, or relocation from St Louis. As participants in our study agree to longitudinal study, including multiple neuroimaging procedures and serial lumbar punctures (LPs), they are unlikely to be representative of the general population, nor were they selected at random from the population. However, our sample is closely similar to other research samples of cognitively normal older adults and early symptomatic AD.8 Additional information about our cohort is provided in Supplemental Text 1. Participants in the current study (n=311) were selected from the larger KADRC cohort based on the following criteria: a) completion of baseline cognitive and CSF evaluation, b) baseline Clinical Dementia Rating (CDR) score of 0, c) ≥65 years of age at the time of LP, d) at least one annual clinical follow-up assessment, and e) good general health. The Human Research Protection Office at WUSM approved the studies, including Healthy Aging and Senile Dementia, P01-AG03991; Alzheimer’s Disease Research Center, P50-AG05681; Antecedent biomarkers for AD: The Adult Children Study, P01-AG026276, and written informed consent was obtained from all participants.
Clinical and cognitive assessment
Cognitive assessment was performed annually and included assignment of CDR and CDR-sum of boxes (CDR-SB),9 Mini-Mental State Examination (MMSE) and a psychometric test battery.10 The CDR is a global dementia staging system determining the presence or absence of dementia and, when present, its severity. The global CDR stages are 0, indicating cognitive normality, and 0.5, 1, 2, and 3, indicating very mild impairment or very mild dementia, mild, moderate, and severe dementia, respectively.11 The CDR-SB is a more quantitative representation of the global CDR, derived directly from the individual ratings in six cognitive and functional domains, or “boxes” (i.e., memory, orientation, judgment and problem solving, community affairs, home and hobbies, and personal care), that are used to generate the global CDR. The CDR-SB is the total score of all the separate boxes (range 0–18, with 0 as the best score). Participants with a CDR of 0 typically have all box scores scored as 0; however, a global CDR of 0 can also be assigned in the presence of one box score of 0.5 in a non-memory domain. In our research cohort, the clinical diagnosis of AD in individuals who are CDR 0·5 or greater is based on criteria from the NINCDS-ADRDA,12 in accordance with standard protocols.7 In individuals with CDR 0·5, AD was diagnosed if memory and at least one other domain received a score ≥0·5 and the clinician felt the cognitive impairments to be due to AD (probable AD), thus referred to as CDR 0.5 symptomatic AD. The rate of postmortem confirmation of the clinical AD diagnosis from the KADRC is 93%.7 Baseline CDR score and diagnosis were based on the cognitive assessment closest to the time of the LP (mean interval, 2·6 months (SD 2·1)).
The NIA-AA criteria for preclinical AD do not operationalize the “subtle cognitive changes” needed for stage 3. Since episodic memory is usually affected earliest in AD,13,14 we used an episodic memory composite score as the measure of cognition to define stage 3. The composite measure was based on factor analyses10 and included the sum of the three free recall trials from the Buschke Selective Reminding Test15 total scores from the “easy” and “hard” trials of Associate Learning from the Wechsler Memory Scale-Revised(WMS-R16), and the total number of correctly recalled units from the WMS-R Logical Memory immediate recall test. Raw scores from each test were converted to z-scores using robust cognitively normal comparison groups from the KADRC who enrolled as cognitively normal and who never progressed to a dementia diagnosis during follow-up.17 Scores of the 3 tests were averaged together to create a composite episodic memory score and were converted again to a z-score within the population. The cut-off at the lowest 10th percentile of the distribution in our sample(<−1·25) was applied to indicate memory impairment.
Our primary outcome measure was the proportion of preclinical AD stages in our cohortas defined by CSF markers. Secondary outcome measures were cognitive decline on the CDR-SB and MMSE, progression to CDR 0·5 symptomatic AD at the latest available follow-up before drop-out (6% [n=20] died, 12·5% [n=39] lost to follow-up when still alive) or before a CDR rating ≥1, and mortality. “CDR 0·5 symptomatic AD” differs from MCI as defined in the criteria of “MCI due to AD” or “prodromal AD” (Albert et al 2011, Dubois et al 2007),although it is very similar. As part of the assessment to define whether a participant is cognitively impaired with or without symptomatic AD, a thorough, informant- and participant-based interview is completed. Cognitive assessment of the participant, evaluating recent and long-term memory, executive function, reasoning, language, and visual spatial function is also performed. The final CDR rating and diagnosis is based on both current and historical cognitive performance. However, in contrast to MCI, an absolute cut-off score on a specific cognitive test is not used as the basis to define the presence of cognitive impairment. Moreover, CDR 0·5 symptomatic AD requires clinical change in two cognitive domains, while for MCI, cognitive complaints are sufficient to meet the criteria (Supplemental Table 1).
Neuropathological examination
In a subset of participants (n=14) neuropathologic examination was performed using established protocols.20 The AD neuropathologic changes were rated using the NIA-AA guidelines.21 Other criteria were applied where appropriate.
CSF markers
CSF samples (20–25 mL) were collected at 8 AM after overnight fasting. LPs (L4/L5) were performed by a trained neurologist using a 22-gauge a traumatic Sprottespinal needle. Samples were gently inverted to avoid possible gradient effects, briefly centrifuged at low speed, and aliquoted (0·5 mL) into polypropylene tubes prior to freezing at −84°C. Samples were analyzed for total tau (t-tau), phosphorylated tau 181 (p-tau 181), and Aβ1–42 by enzyme-linked immunosorbant assay (INNOTEST; Innogenetics, Ghent, Belgium).
CSF markers were dichotomized (normal/abnormal) by defining a cut-off that could best differentiate CDR 0 participants from an independent cohort with CDR 0·5 symptomatic AD at baseline (Supplemental Table 2), based on the You den index (sensitivity+specificity-1).The resultant optimum cut-offs for abnormal were <459 for Aβ1–42, >339 for t-tau, and >67 for p-tau 181 (all pg/mL).
APOE genotyping
TaqMan assays (Applied Biosystems, Foster City, CA) for both ABI#C_3084793_20 and ABI#C_904973_10 were used for APOE genotyping, as described.22
Classification in the NIA-AA preclinical AD stages
At baseline, participants were classified as normal if both episodic memory and CSF markers were normal, in stage 1 if only Aβ1–42 was abnormal, in stage 2 if Aβ1–42 and t-tau or p-tau 181 were abnormal, and in stage 3 if additionally the participant’s performance was below the memory test threshold (Table 1). Participants were classified in the Suspected Non-Alzheimer Pathophysiology (SNAP) group if they had abnormal t-tau or p-tau 181 in the presence of normal Aβ1–42, regardless of episodic memory performance23 (Table 1). Participants who did not fit within one of the groups were included in the un classified group. Given the uncertainty of classification of this latter group, these individuals were excluded from our main analyses.
Table 1.
Glossary of preclinical AD stages and symptomatic AD
| Normal group | CDR 01, amyloid (−), neural injury (−), subtle cognitive decline (−) |
|---|---|
| Preclinical AD stage 1 | CDR 0, amyloid (+)2, neural injury (−), subtle cognitive decline (−) |
| Preclinical AD stage 2 | CDR 0, amyloid (+), neural injury (+)3, subtle cognitive decline (−) |
| Preclinical AD stage 3 | CDR 0, amyloid (+), neural injury (+), subtle cognitive decline (+)4 |
| SNAP group5 | CDR 0, amyloid (−), neural injury (+), subtle cognitive decline (−/+) |
| Unclassified group | CDR 0, amyloid (−/+), neural injury (−), subtle cognitive decline (+) |
| Symptomatic AD | CDR>06, memory and at least one other domain received a score of ≥0·5 and the clinician felt the cognitive impairments to be due to AD (probable AD), no reference to biomarkers |
CDR 0, Clinical Dementia Rating=0, no dementia
Amyloid, CSF Aβ42 (+) <459pg/mL
Neural injury, CSF tau (+) >339pg/mL or ptau 181 (+) >67pg/mL
Subtle cognitive decline, episodic memory composite score (+) in the lowest 10th percentile
SNAP, Suspected Non-Alzheimer Pathophysiology
CDR>0, CDR 0·5= very mild dementia, CDR 1=mild dementia, CDR 2=moderate dementia, CDR 3=severe dementia
Statistical analyses
Baseline differences between the stages were analyzed using ANOVA for continuous variables and Fisher’s exact tests and logistic regression models for categorical variables. Missing data of cognitive tests at follow-up were modeled with mixed models. We first performed an omnibus test for joint significance of the stage variables and only proceeded with subgroup analyses if this overall test was statistically significant. We performed competing-risks survival analyses using Fine & Gray sub distribution hazards models (SHR)24 to investigate the predictive accuracy of the preclinical AD stages for progression to CDR≥0·5 symptomatic AD during the available follow-up period, with normal individuals as a reference group, uncorrected and corrected for baseline age, gender, education, and APOE genotype. Unlike standard Cox hazards models that usually treat mortality as censoring, these models considered mortality as a competing event that can impede progression to symptomatic AD. Standard Cox proportional hazards models (HR) were utilized to assess the predictive capacity of preclinical AD stages for mortality during the available follow-up period, in both unadjusted and adjusted analyses. The relationship between the stages and rate of change in CDR-SB and MMSE over time were assessed with general linear mixed models including linear time effects,25 adjusted for baseline age, gender, education, and APOE genotype. Analyses included baseline score and all available follow-up scores. The final models were specified with a random intercept and slope, as these models provided the best measures on Akaike's information criterion26 for analyzing the corresponding clinical/cognitive measures as compared to models with other covariance structures. Adjusted plots were created by plotting predicted curves/slopes for each of the five AD stage groups within each combination of APOE-ε4 and gender with age (72.6 years) and education (15.5 years) fixed at the sample means. Only plots for APOE-ε4 negative females were shown as this is the most populous group out of the four combinations from the two dichotomous factors, APOE-ε4 and gender. Effects of preclinical AD stages (either on risk of converting to a higher CDR or on rate of change in MMSE), however, remain the same regardless of the combinations of APOE-ε4 and gender. Subdistribution hazards models were implemented using the STCRREG command in STATA 12 (StataCorp. College Station, TX). All other statistical analyses were performed with SPSS version 19·0 (Chicago, IL), with significance set at p<0·05.
Role of the funding source
The sponsors of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding authors had full access to all data in the study and had final responsibility for the decision to submit for publication.
Results
Baseline demographics
Baseline demographics of the total sample and separate stages are listed in Table 2. One hundred and twenty-nine (41·5%) participants were classified as normal, 47 (15%) in stage 1, 36 (12%) in stage 2, 13 (4%) in stage 3, and 72 (23%) in the SNAP group. Fourteen (4·5%) participants remained unclassified.Supplemental Table 3illustrates the distribution across the stages. MMSE and memory scores were lower in stage 3 than the other groups. The proportion of preclinical AD (stage 1–3) was higher in individuals older than 72 years(=median age of sample) compared to younger individuals(36·5 vs. 26%, p=0·0442) and in APOE-ε4 carriers compared to non-carriers (47 vs. 23%, p<0·0001;Supplemental Table 4).
Table 2.
Sample characteristics by preclinical AD stage at baseline
| All N=311 |
Normal group N=129 |
Stage 1 N=47 |
Stage 2 N=36 |
Stage 3 N=13 |
SNAP group N=72 |
Unclassified N=14 |
|
|---|---|---|---|---|---|---|---|
| Age | 72·9 (6·0) | 706 (4·6)1,2,3,S,U | 73·8 (6·9) N,3,U | 74·4 (5·8) N,3,U | 78·1 (4·9) N,1,2,S | 73·6 (5·8) N,3,U | 78·3 (6·7) N,1,2,S |
| Female, n (%) | 171 (55%) | 73 (57) | 23 (49) | 23 (64) 3 | 4 (31)2 | 43 (60) | 5 (36) |
| Education, y | 15·4 (2·9) | 15·2 (2·6) 1 | 16·9 (3·2) N2,3,S,U | 15·3 (2·7) 1 | 14·3 (2·7)1 | 15·3 (3·1)1 | 14·4 (2·9)1 |
| Race, n (%)* | |||||||
| Caucasian | 294 (92%) | 118 (92) | 38 (81) | 33 (100) | 13 (100) | 70 (98) | 12 (86) |
| African American | 22 (7%) | 10 (7) | 9 (19) | - | - | 1 (1) | 2 (14) |
| Indian | 1 (0·5%) | 1 (1) | - | - | - | - | - |
| AIAN | 1 (0·5%) | - | - | - | - | 1 (1) | - |
| APOE-ε4+, n (%) | 106 (34%) | 32 (25)1,2,3 | 22 (47)N | 18 (50)N | 9 (69)N,S,U | 22 (31)3 | 3 (21)3 |
| MMSE** | 28·9 (1·3) | 292 (1·0)1,3,U | 28·6 (1·4) N,3 | 28·9 (1.3)3 | 27·5 (2·2) N,1,2,S | 29·1 (1·1)3,U | 28·3 (1·6)N,S |
| CDR-SB | 0·03 (0·1) | 0·03 (0·1) | 0·04 (0·1) | 0·03 (0·1) | 0·08 (0·2) | 0·02 (0·1) | 0·04 (0·1) |
| Episodic memory, z-score | 0·0 (1·0) | 0·20 (0·8)3,U | 0·23 (0·9)3,U | 0·01 (0.9)3,U | −2·05 (0·4)N,1,2,S | 0·14 (1·0)3,U | −1·45 (0·2)N,1,2,S |
| Aβ1–42, pg/mL | 610 (259) | 712 (207)1,2,3,S | 355 (75)N,S,U | 350 (71)N,S,U | 321 (63)N,S,U | 770 (237)N,1,2,S | 641 (288)1,2,3,S |
| T-tau, pg/mL | 328 (188) | 222 (59)2,3,S | 210 (66)2,3,S | 552 (243)N,1,S,U | 592 (150)N,1,S,U | 460 (153)N,1,2,3,U | 202 (63)2,3,S |
| P-tau181, pg/mL | 61 (30) | 43 (10)2,3,S | 43 (12)2,3,S | 93 (36)N,1,U | 95 (20)N,1,U | 85 (25)N,1,U | 36 (98)2,3,S |
| Follow-up, y (range) | 3·9 (1–15) | 4·2 (1–13) | 4·0 (1–11) | 3·2 (1–13) | 2·0 (1–6) | 3·0 (1–15) | 4.2 (1–8) |
| Progression to CDR≥0·5 | 32 (10%) | 2 (2%)1,2,3,U | 6 (13%)N,3 | 9 (25%)N,S | 7 (54%)N,1,S | 4 (6%)2,3,U | 4 (29%)N,S |
| symptomatic AD, n | |||||||
| Mortality, n | 20 (6%) | 2 (2%)1,3 | 5 (11%)N | 3 (8%) | 4 (31%)N,S | 5 (7%)3 | 1 (7%) |
Results are mean (SD), median (range), or number (%). Preclinical AD stages are defined by CSF markers based on optimal Youden cut-offs: Abnormal CSF Aβ1–42 <459 pg/mL, t-tau >339 pg/mL, p-tau >67 pg/mL. Episodic memory is a composite score of the Associate Learning Test, Logical Memory Test, and Selective Reminding Test, with a cut-off at the lowest 10th percentile:−1·25 SD. AD=Alzheimer’s disease, SNAP=Suspected Non-Alzheimer Pathophysiology, AIAN=American Indians and Alaska Natives,APOE=apolipoprotein E, MMSE=Mini Mental State Examination(range 0–30, with 30 as the best score), CDR=Clinical Dementia Rating Scale (range 0–3, with 0 as the best score), CDR-SB=Clinical Dementia Rating scale Sum of Boxes(range 0–18, with 0 as the best score), Aβ=beta amyloid, t-tau=total tau, p-tau=phosphorylated tau.
Fisher’s exact test p-value=0·007.
The MMSE score was missing for 1 participant at baseline.
p<0·05 compared to the normal group,
p<0·05 compared to stage 1,
p<0·05 compared to stage 2,
p<0·05 compared to stage 3,
p<0·05 compared to the SNAP group,
p<0·05 compared to the unclassified group. See Supplemental Table 6 for exact p-values.
Prediction of progression to symptomatic AD
The number of participants available at 5 years follow-up was 110 (35%) and at 10 years 14(5%). After a median follow-up of 3·9 (range 1–15) years, 2(2%) participants in the normal group progressed to CDR≥0·5 symptomatic AD, 6 (13%) in stage 1, 9 (25%) in stage 2, 7 (54%) in stage 3, 4 (6%) in the SNAP group, and 4 (29%) in the unclassified group (Table 3). Of the 32 progressors, 22(69%) had CDR 0·5 symptomatic AD as diagnosis at last follow-up, 6(19%) CDR 1 symptomatic AD, and 4 (12%) CDR 2 symptomatic AD.
Table 3.
Prediction of preclinical AD stages for CDR≥0·5 symptomatic AD and mortality
| Progression to CDR≥0·5 symptomatic AD |
Mortality risk |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| 5-year progression rate |
Uncorrected SHR (95% CI) |
Difference compared to other groups |
Corrected SHR (95% CI) |
Difference compared to other groups |
Uncorrected HR (95% CI) |
Difference compared to other groups |
Corrected HR (95% CI) |
Difference compared to other groups |
|
| Normal group | 2% | Reference | 1: p=0·0160 | Reference | 1: p=0·0791 | Reference | 1: p=0·0363 | Reference | 1: p=0·1897 |
| 2: p=0·0002 | 2: p=0·0004 | 2: p=0·0449 | 2: p=0·0781 | ||||||
| 3: p<0·0001 | 3: p=0·0001 | 3: p=0·0001 | 3: p=0·0010 | ||||||
| S: p=0·1986 | S: p=0·2670 | S: p=0·0968 | S: p=0·0710 | ||||||
| Stage 1 | 11% | 7·0 | 2: p=0·0606 | 4·6 | 2: p=0·0536 | 5·9 | 2: p=0·9233 | 3·7 | 2: p=0·5903 |
| (1·4–34·1) | 3: p=0·0004 | (0·8–25·6) | 3: p=0·0021 | (1·1–31·0) | 3: p=0·0149 | (0·5–26·4) | 3: p=0·0128 | ||
| S: p=0·1977 | S: p=0·3646 | S: p=0·5807 | S: p=0·6365 | ||||||
| Stage 2 | 26% | 18·1 | 1: p=0·0606 | 14·3 | 1: p=0·0536 | 6·3 | 1: p=0·9233 | 6·0 | 1: p=0·5903 |
| (3·9–83·1) | 3: p=0·0656 | (3·3–61·9) | 3: p=0·1587 | (1·0–38·3) | 3: p=0·0364 | (0·8–44·2) | 3: p=0·0403 | ||
| S: p=0·0047 | S: p=0·0053 | S: p=0·5673 | S: p=0·8560 | ||||||
| Stage 3 | 56% | 49·2 | 1: p=0·0004 | 33·8 | 1: p=0·0021 | 31.5 | 1: p=0·0149 | 31.5 | 1: p=0·0128 |
| (10·1–240·4) | 2: p=0·0656 | (6·1–186·7) | 2: p=0·1587 | (5·6–176·0) | 2: p=0·0364 | (4·1–244·6) | 2: p=0·0403 | ||
| S: p<0·0001 | S: p=0·0004 | S: p=0·0048 | S: p=0·0195 | ||||||
| SNAP group | 5% | 3·1 | 1: p=0·1977 | 2·5 | 1: p=0·3646 | 4·1 | 1: p=0·5807 | 5·2 | 1: p=0·6365 |
| (0·6–17·6) | 2: p=0·0047 | (0·5–12·6) | 2: p=0·0053 | (0·8–21·5) | 2: p=0·5673 | (0·9–30·9) | 2: p=0·8560 | ||
| 3: p<0·0001 | 3: p=0·0004 | 3: p=0·0048 | 3: p=0·0195 | ||||||
Results are 5-year progression rate (cumulative incidence rate) to CDR≥0·5 symptomatic AD and associated sub hazard ratio (SHR, 95% CI) for progression to CDR≥0·5 symptomatic AD calculated using a Fine & Gray sub distribution hazards model, and hazard ratio (HR, 95% CI) for mortality calculated using Cox regression analyses. Analyses are shown as both uncorrected and corrected for baseline age, gender, education, and APOE genotype. AD=Alzheimer’s disease, CDR=Clinical Dementia Rating scale (range 0–3, with 0 as the best score), SNAP=Suspected Non-Alzheimer Pathophysiology, SHR=Subhazard Ratio, HR=Hazard Ratio. N=normal group, 1=stage 1, 2=stage 2, 3=stage 3, S=SNAP group.
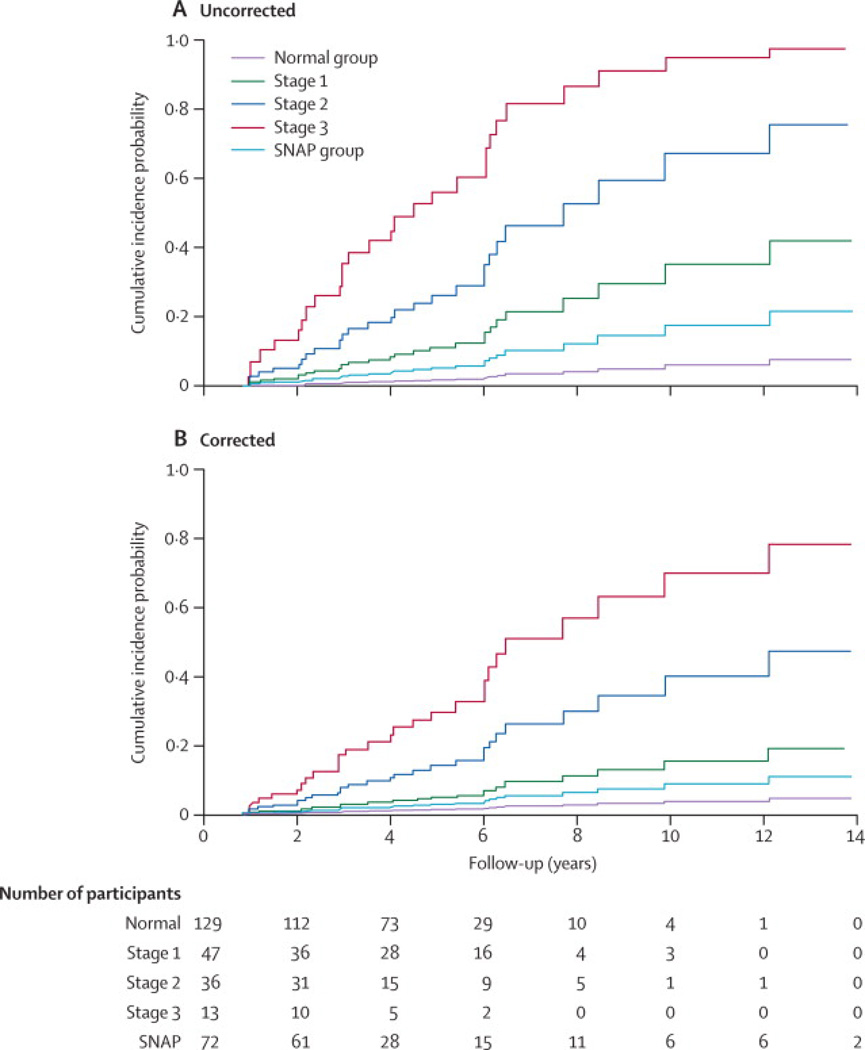
Survival analyses showed that, taking into account mortality, participants in each preclinical AD stage had a higher risk of progression to CDR≥0·5 symptomatic AD than normal participants (stage 1 SHR=70, 95%CI 1·4–34·1, p=0·0160; stage 2 SHR=18·1, 95%CI 3·9–83·1, p=0·0002; stage 3 SHR=49·2, 95%CI 10·1–240·4, p<0·0001; Table 3,Figure 1A). Preclinical AD stages also differed from each other, with more severe stages associated with higher risk of progression to symptomatic AD, although the difference between stages 2 and 3 did not reach statistical significance (p=0·0656).The progression rate of participants in the SNAP group did not differ from that of normal individuals. After correction for covariates, results remained essentially the same, except that progression in stage 1 was no longer different from that of normal individuals (SHR=4·6, 95%CI 0·8–25·6, p=0·0791), which was mainly driven by the correction forage. The estimated 5-year progression (cumulative incidence) rate to CDR≥0·5 symptomatic AD was 2% for normal participants, 11% for participants in stage 1, 26% in stage 2, 56% in stage 3, and 5% in the SNAP group. The risk of progression was not different between older (>72 years)and younger individuals with preclinical AD (SHR=2·0, 95% CI 0·7– 5·5; p=0·1856)or between APOE-ε4 carriers and non-carriers with preclinical AD (SHR= 1·1, 95% CI 0·5–2·6, p=0·7605; Supplemental Table 4).
Figure 1. Cumulative incidence for progression to CDR≥0.5 symptomatic AD by preclinical AD stage.
Graphs show the CDR≥0·5 symptomatic AD cumulative incidence for each preclinical AD stage, uncorrected for covariates (A) and corrected for age, gender, education, and APOE genotype (B). The black line represents participants in the normal group; light blue, stage 1; dark blue, stage 2; red, stage 3; and grey, SNAP. AD=Alzheimer’s disease, APOE=apolipoprotein E, CDR=Clinical Dementia Rating scale (range 0–3, with 0 as the best score), SNAP=Suspected Non-Alzheimer Pathophysiology.
Prediction of mortality and autopsy diagnosis
Twenty (6%) participants died at follow-up (Table 2). Compared with normal individuals, participants with preclinical AD (stage 1–3) had an increased risk of death (HR=6·2, 95% CI 1·1–35·0, p=0·0396),adjusting for covariates, which increased with advancing stage (Table 3). The risk of death in the SNAP group tended to differ from that of normal individuals (HR=5·2, 95%CI 0·9–30·9, p=0·0710). Of the 9 participants with preclinical AD who came to autopsy, 8 received a neuropathologic diagnosis of AD with intermediate to high neuropathologic AD change and 1 of AD with low neuropathologic AD change (Table 4).In the SNAP cases, 3 of the 4 that came to autopsy had modest AD pathology and were rated as ‘low’ level neuropathologic AD change according to NIA-AA criteria, which makes it unlikely that this pathology explains the cognitive impairments. Of these 3 individuals with SNAP, 2 were in Thal Phase 1–2 and 1 was in Thal Phase 3 but this individual had a low neurofibrillary tangle score and vascular co morbidity.17 All participants with SNAP had a neuritic plaque score of 0. Other coexisting pathologies in these individuals were modest and were thought unlikely to have contributed significantly to the cognitive status. It should be noted that the time to death after baseline LP ranged from 2 to 11 years for all participants. Therefore, it is plausible that AD pathology would have accumulated by the time of autopsy.
Table 4.
Clinical, biomarker and neuropathologic features of participants who died (n=20)
| Case # | Stage | Age (baseli ne) |
Gender | Education (years) |
MMS E baselin e /last |
CSF Aβ1–42 (pg/mL) |
CSF Tau (pg/mL) |
Cause of death |
Time to death (years after baseline) |
Last in-person clinical diagnosis (years after baseline) |
Last in person CDR |
Clinical diagnosis at expiration1 |
CDR at expiration1 |
Primary NP diagnosis |
Secondary NP diagnosis |
Other NP diagnosis |
NIA-AA* |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Normal | 74 | Female | 12 | 28 / 27 | 465 | 250 | Severe burns |
14 | Uncertain dementia (11) |
0·5 | - | - | No autopsy | - | ||
| 2 | Normal | 70 | Male | 12 | 29 / 29 | 812 | 261 | Severe burns |
2 | No dementia (1) | 0 | - | - | Pending | - | ||
| 3 | Stage 1 | 78 | Female | 12 | 30 / 27 | 433 | 333 | Myocardial infarction |
11 | Symptomatic AD (10) |
0·5 | Primary Vascular dementia |
1 | AD | Intermediate | ||
| 4 | Stage 1 | 81 | Female | 18 | 30 / 22 | 264 | 246 | Inanition | 9 | Symptomatic AD (6) |
2 | Symptomatic AD |
3 | AD | TDP-MTL | High | |
| 5 | Stage 1 | 78 | Female | 18 | 28 / 26 | 315 | 251 | Inanition | 9 | No dementia (5) | 0 | Symptomatic | 3 | AD | HS | Intermediate | |
| 6 | Stage 1 | 90 | Female | 20 | 30 / 27 | 272 | 182 | Congestive heart failure | 5 | No dementia (3) | 0 | No dementia | 0 | AD | SVD | Low | |
| 7 | Stage 1 | 91 | Female | 18 | 28 / 27 | 383 | 242 | Inanition | 4 | Uncertain | 0·5 | Uncertain | 0.5 | AD | SVD | Intermediate | |
| 8 | Stage 2 | 80 | Female | 16 | 29 / 28 | 457 | 543 | Bronchopne umonia | 6 | Uncertain dementia (3) |
0·5 | Symptomatic AD |
0.5 | AD | SVD | AGD, SDH | Intermediate |
| 9 | Stage 2 | 85 | Male | 8 | 29 / 28 | 411 | 582 | Unknown | 4 | CDR 0 no dementia (3) |
0 | - | - | No autopsy | - | ||
| 10 | Stage 2 | 82 | Female | 12 | 30 / 29 | 267 | 490 | Unknown | 3 | No dementia (2) | 0 | - | - | No autopsy | - | ||
| 11 | Stage 3 | 88 | Male | 12 | 28 / 27 | 303 | 575 | Congestive heart failure |
2 | Symptomatic AD(2) |
0·5 | Symptomatic AD |
0.5 | AD | Intermediate | ||
| 12 | Stage 3 | 79 | Female | 16 | 28 / 27 | 283 | 393 | Multiple organ failure |
4 | No dementia (2) | 0 | No dementia | 0 | AD | DLB | High | |
| 13 | Stage 3 | 71 | Female | 12 | 29 / 24 | 318 | 548 | Ovarian cancer |
5 | Symptomatic AD(4) |
0·5 | Symptomatic AD |
1 | AD | SVD | High | |
| 14 | Stage 3 | 78 | Male | 16 | 28 / 28 | 410 | 463 | Motor vehicle accident |
1 | No dementia (1) | 0 | - | - | No autopsy | - | ||
| 15 | SNAP | 90 | Male | 19 | 26 / 25 | 682 | 520 | Respiratory failure |
2 | No dementia (1) | 0 | Symptomatic AD |
0.5 | AD | AGD | Low | |
| 16 | SNAP | 90 | Female | 12 | 29 / 30 | 786 | 739 | Unknown | 3 | No dementia (3) | 0 | No dementia | 0 | AD | SVD | Astrocytoma | Low |
| 17 | SNAP | 86 | Female | 18 | 28 / 28 | 738 | 495 | Atheroscler otic heart disease |
5 | Symptomatic AD (4), with CVD |
1 | Symptomatic AD |
1 | SVD | NFT-MTL | Not | |
| 18 | SNAP | 75 | Female | 14 | 28 / 30 | 1109 | 624 | Metastatic carcinoma |
4 | Uncertain dementia (3) |
0·5 | Uncertain dementia |
0.5 | AD | Low | ||
| 19 | SNAP | 84 | Male | 12 | 29 / 27 | 486 | 364 | Respiratory failure |
3 | No dementia (2) | 1 | - | - | No autopsy | - | ||
| 20 | Unclas sified |
76 | Female | 12 | 29 / 25 | 670 | 156 | Mitral valve stenosis |
9 | Symptomatic AD (8) |
1 | Symptomatic AD |
2 | AD | SVD | LB | Low |
Data are characteristics for each participant who died (n=20). Neuropathological examination was performed in a subgroup of these participants (n=14). Aβ=beta amyloid, AD=Alzheimer’s disease, AGD=argyrophilic grain disease, CDR=Clinical Dementia Rating scale, CSF=cerebrospinal fluid, CVD= cardiovascular disease contributing to cognitive impairment, DLB=dementia with Lewy bodies, HS=hippocampal sclerosis, LB=Lewy bodies unspecified, NFT-MTL=neurofibrillary tangles in medial temporal lobe, NP=neuropathology, p-tau=phosphorylated tau, SDH=subdural hematoma, SNAP=Suspected Non-Alzheimer Pathophysiology, SVD= small vessel disease with infarcts/microinfarcts, TDP-MTL=TDP-43-proteinopathy in medial temporal lobe, t-tau=total tau.
NIA-AA criteria: AD neuropathologic change: not, low, intermediate, or high. Only intermediate or high neuropathologic change is considered sufficient to account for dementia.
Expiration: clinical diagnosis and CDR assigned after participant death, based on review of historical clinical records and interview with informant as to cognitive abilities of the participant just prior to their death.
Prediction of annual clinical and cognitive decline
Increase in CDR-SB was faster in each preclinical stage compared to the normal group (stage 1 p=0·0289, stage 2 p<0·0001, stage 3 p=0·0009), faster in stages 2 and 3 compared to the SNAP group (stage 2 p=0·0021 and stage 3 p=0·0048), and faster in stage 3 compared to stage 1 (p=0·0373; Table 5, Supplemental Figure 1). Participants in stages 2 and 3 showed faster decline on the MMSE than participants in the normal group (stage 2 p=0·0021, stage 3 p=0·0233) and in the SNAP group(stage 2 p=0·0047, stage 3 p=0·0279; Table 5, Supplemental Figure 1).Individual cognitive trajectories on the CDR-SB and MMSE are presented in Supplemental Figure 2.
Table 5.
Annual rate of change in CDR-SB and MMSE according to preclinical AD stage
| Stages | Slope CDR-SB | P-value slope | Difference compared to other groups |
Slope MMSE | P-value slope | Difference compared to other groups |
|---|---|---|---|---|---|---|
| Normal group | 0·03 (0·03) | P=0·2784 | 1: p=0·0289 | −0·01 (0·03) | P=06955 | 1: p=0·2324 |
| 2: p<0·0001 | 2: p=0·0021 | |||||
| 3: p=0·0009 | 3: p=0·0233 | |||||
| S: p=0·3870 | S: p=09857 | |||||
| Stage 1 | 0·15 (0·04) | P=0·0014 | 2: p=0·0638 | −0·09 (0·05) | P=0·0966 | 2: p=0·0655 |
| 3: p=0·0373 | 3: p=0·1019 | |||||
| S: p=0·2096 | S: p=02925 | |||||
| Stage 2 | 0·28 (0·05) | P<0·0001 | 1: p=0·0638 | −0·24 (0·06) | P=0·0003 | 1: p=0·0655 |
| 3: p=03848 | 3: p=0·6227 | |||||
| S: p=0·0021 | S: p=0·0047 | |||||
| Stage 3 | 0·37 (0·10) | P=0·0002 | 1: p=0·0373 | −0·31 (0·12) | P=0·0145 | 1: p=0·1019 |
| 2: p=03848 | 2: p=0·6227 | |||||
| S: p=0·0048 | S: p=0·0279 | |||||
| SNAP group | 0·07 (0·04) | P=0·0685 | 1: p=0·2096 | −0·01 (0·04) | P=0·7578 | 1: p=0·2925 |
| 2: p=0·0021 | 2: p=0·0047 | |||||
| 3: p=0·0048 | 3: p=0·0279 |
Data are slopes (SE) corrected for age, gender, education, and APOE genotype and comparison to other groups. AD=Alzheimer’s disease, CDR-SB=Clinical Dementia Rating scale Sum of Boxes(range 0–18, with 0 as the best score), MMSE=Mini Mental State Examination(range 0–30, with 30 as the best score), SNAP=Suspected Non-Alzheimer Pathophysiology. N=normal group, 1=stage 1, 2=stage 2, 3=stage 3, S=SNAP group.
Discussion
The main findings of our study are that preclinical AD can be defined by CSF markers, is common in individuals over the age of 65 and is associated with an increased risk for cognitive decline, progression to CDR≥0·5 symptomatic AD, and mortality on a group level. The proportion of preclinical AD of 31% in our cohort is consistent with previous clinicopathological studies1–3 and the population-based Mayo Clinic Study of Aging (MCSA) utilizing imaging measures.23 The validity of our biomarker-based diagnosis of preclinical AD was further supported by the observation that 8 out of 9 participants with preclinical AD who came to autopsy had intermediate to high AD neuropathologic change. The distribution across the preclinical AD stages was also similar to that reported in the MCSA.23
Individuals with preclinical AD exhibited a faster progression rate to CDR≥0·5 symptomatic AD, as supported by faster decline on continuous measures for function and global cognition. Importantly, progression rates differed between the preclinical AD stages, demonstrating that stage 1,2, and 3 reflect different and progressive disease severities. The MCSA also showed an increased rate of decline with advancing stage, although only 1-year follow-up data were reported.6
Preclinical AD was associated with a higher mortality risk, which also increased with advancing stage. To our knowledge, no other studies have examined mortality risk in preclinical AD, but our findings are consistent with clinical studies in individuals with incident or very mild AD dementia.27,28 There is no clear explanation for increased mortality risk. It could be that risk factors for AD are also associated with other life-threatening diseases. Alternatively, AD-related cognitive impairments could increase mortality risk because they may hamper diagnosis and management of other diseases or increase the risk for accidents.29,30 Increased mortality risk may also have resulted from AD pathology, which may compromise the physiological response to other illnesses.31 However, in order to understand the relation between preclinical AD and mortality, further research is needed.
We found that the proportion of preclinical AD was almost doubled in older individuals and in APOE-ε4 carriers, which is in line with previous studies.32 However, neither age (younger [<72] vs. older [≥72] ) nor APOE genotype predicted rate of decline, although small sample sizes for these sub analyses reduced statistical power. While APOE-ε4 is often found to be a good predictor for cognitive decline in unselected populations, the lack of its prognostic utility in individuals with AD pathology is consistent with previous studies.33
Almost 20% of our participants had SNAP (abnormal tau but normal Aβ1–42), in line with the MCSA study.23 Cognitive decline in the SNAP group was similar to that in normal individuals, although mortality tended to be increased (HR=5·2, 95%CI p=0·0710). Autopsy data showed no to low AD pathology change suggesting that these individuals may have other diseases (Table 4).
The selection of cut-offs is critical for the staging of NIA-AA stages.6 We used the You den index to define the CSF cut-offs. These values were slightly lower than those previously used in a similar cohort (Aβ1–42<500 pg/mL, t-tau>440 pg/mL, and p-tau>78 pg/mL)34. Use of the previous, more liberal Aβ1–42 cut-offs would lead to a slightly higher proportion of preclinical AD (40%),but the progression to symptomatic AD remained the same (Supplemental Table 6A). Our cognitive cut-off at the 10th percentile was in line with that used in the MCSA.6,23
Also the choice of cognitive tests may impact the NIA-AA staging and outcome. We defined subtle cognitive changes as low scores on a memory composite test. If subtle cognitive change was defined as a low score in any cognitive domain (episodic memory, semantic memory, working memory, or visuospatial score, as described in Johnson et al17), the number of individuals in stage 3 and the unclassified group would increase. Progression rates to symptomatic AD in these groups, however, would be lower (Supplemental Table 6B). While we used a composite score of 3 memory tests based on factor analyses, the use of a specific memory test could have led to different results.
Participants in stage 3 differed from those with MCI or early dementia in that they had a CDR score of 0, and therefore no change in cognitive function and no interference in activities of daily living. Still, some of them may have met psychometric criteria of MCI. A previous study in AD autosomal-dominant mutation carriers has shown that individuals with preclinical AD indeed may show impairments on tests with clinically normal performance, a condition referred to as “asymptomatic pre-MCI”.35
Fourteen participants remained unclassified and their outcome has not been investigated. They showed an increased risk for progression to symptomatic AD but not for mortality compared to the normal group (Supplemental Table 7). Although amyloid pathology may be present in these individuals (Table 4), future studies are needed to clarify their characteristics and outcome.
Our result sarestrikingly consistent to those recently reported in the MCSA,6,23 although there were important differences in study design. The MCSA used imaging markers for staging individuals and cognitive tests to define clinical diagnosis rather than the CDR. Furthermore, the biomarker cut-offs were defined as those yielding 90% sensitivity for diagnosing AD dementia from a separate AD cohort and a global cognitive test score was used to define subtle cognitive change. The similarity in findings among the studies could suggest that CSF and imaging markers have a similar ability in identifying individuals with preclinical AD and predicting clinical outcome. However, this does not imply that CSF and imaging makers are equivalent markers. Head-to-head comparison may yield a different conclusion.
Our study has several limitations. The number of participants who progressed to symptomatic AD in each stage was somewhat small, and results should therefore be carefully interpreted. Furthermore, AD clinical diagnosis at follow-up was only neuropathologically validated in only a small subset of participants. This may have led to misclassification, although the rate of postmortem confirmation of AD diagnosis at the ADRC is high (93%).7 Like the MCSA, participants were mainly Caucasian and highly educated, and findings may not apply to individuals with other backgrounds. Although we included cognitively normal individuals (CDR=0), some of them had a CDR-SB score of 0.5 (one score of 0.5 in a non-memory domain)and could be considered suspicious (n=18). However, analyses without these subjects revealed similar results (Supplemental Table 6C). The major strengths of this study included the large sample size of well-characterized participants and the relatively long follow-up period of up to 15 years (average=4 years).
While we consider this study preliminary and hypothesis generating, our findings have several important implications. First, they demonstrate that preclinical AD is common. It can be diagnosed by CSF markers as shown by neuropathological validation. The strong association with future cognitive decline and mortality makes preclinical AD an important target for therapeutic intervention. Second, they demonstrate that the proposed NIA-AA staging of preclinical AD indeed reflects different disease stages given differences in rate of progression. Third, the study has implications for the design of secondary prevention trials. Age and APOE genotype may be useful for prescreening of individuals for biomarker assessment, and trials may stratify individuals based on their preclinical AD stage since their proportion and prognosis differ. The rate of cognitive decline was relatively low compared to individuals with MCI or dementia. This means that trials with preclinical AD need larger sample sizes or a longer follow-up in order to find effects on the current cognitive outcome measures. Furthermore, mortality should be considered as an end-point in trials. Fourth, both occurrence and outcome of preclinical AD are dependent on tests and CSF cut-offs used, which highlights the need for standardization.
Together, our data demonstrate the prognostic utility of the proposed NIA-AA stages for preclinical AD in the prediction of future cognitive decline and mortality, supporting the potential use of NIA-AA criteria in clinical studies as well as for AD trials. Future research is needed on longitudinal biomarker changes over time and on causes of the increased mortality risk in preclinical AD.
Research in Context
Systematic review
Studies were identified by searches of PubMed up to April 2013 with the search terms “preclinical”, “Alzheimer’s disease”, “cerebrospinal fluid”, “amyloid”, “tau”, “NIA-AA”, “cognition”, “autopsy”, and “mortality”. We included studies assessing preclinical Alzheimer’s disease and its outcome. We assessed papers on amyloid-β (Aβ)1–42 and tau in cerebrospinal fluid (CSF) with cognitive decline and Alzheimer’s disease (AD) type dementia as primary outcome measures. Systematic review of the literature showed that AD pathology starts long before clinical symptoms appear, and that CSFAβ1–42 and tau are well established biomarkers for the disease. A recent study on staging of preclinical AD according to the National Institute on Aging and Alzheimer's Association (NIA-AA) criteria using imaging markers found that short-term cognitive decline increased with advancing preclinical AD stage. No study has been performed on the NIA-AA staging of preclinical AD using CSF markers and its long-term cognitive outcome. Moreover, no study has investigated mortality risk in preclinical AD.
Interpretation
This study shows that preclinical AD is common in individuals over the age of 65 and can be identified by Aβ1–42 and tau in cerebrospinal fluid, as shown by neuropathological validation. Our findings demonstrate that the proposed NIA-AA staging of preclinical AD indeed reflects different disease stages, given differences in rate of progression to symptomatic AD and in decline on continuous measures for function and global cognition. Furthermore, preclinical AD is associated with a higher mortality risk, which also increases with advancing stage. The strong association with future cognitive decline and mortality makes preclinical AD an important target for therapeutic intervention.
Supplementary Material
Acknowledgments
We gratefully acknowledge the contributions of Ms. S. Sathyan, Ms. A. Shah, Mr. M. Amos, Ms. E. Householder, our lumbar puncture physicians, the Clinical, Psychometrics, Neuropathology, and Genetic Cores of the Knight Alzheimer’s Disease Research Center, and our dedicated research participants.
Funding: Internationale Stichting Alzheimer Onderzoek (ISAO; S.J.B.V.), the Center for Translational Molecular Medicine (www.ctmm.nl) project LeARN (grant 02N-01), the EU/EFPIA Innovative Medicines Initiative Joint Undertaking (EMIF grant n° 115372, P.J.V., S.J.B.V.),P50 AG05681 (J.C.M.), P01 AG03991 (J.C.M.), P01 AG026276 (J.C.M.), and P30 NS057105 (D.M.H.) from the National Institute of Aging of the National Institutes of Health, and the Charles and Joanne Knight Alzheimer Research Initiative (J.C.M).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author contributions
S.J.B.V., P.J.V., D.M.H. and A.M.F. designed the study.
S.J.B.V. analyzed the data with assistance from C.X. and M.S.J.
S.J.B.V. interpreted the data and wrote the manuscript with assistance from P.J.V and A.M.F.
J.H. oversaw the cognitive data collection and interpretation.
E.A.G. provided the dataset.
N.J.C. oversaw the neuropathological studies.
J.C.M. oversaw the clinical studies.
A.M.F. oversaw the CSF studies.
All authors critically reviewed the manuscript for intellectual content and approved the final draft.
Conflicts of interest
Ms. Stephanie Vos receives research support from the Center for Translational Molecular Medicine, project LeARN (grant 02N-01) and received funds from Internationale Stichting Alzheimer Onderzoek (ISAO) to perform this study. No conflict of interest exists.
Dr. Xiong reports no disclosures.
Dr. Visser has served as an advisory board member of Bristol-Myers Squibb. He receives/received research grants from Bristol-Myers Squibb, the European Union’s 6th and 7th Framework programme, Life Sciences, Genomics and Biotechnology for Health and Innovative Medicines Initiative Joint Undertaking under grant agreement n° 115372, resources of which are composed of financial contribution from the European Union's 7th Framework programme and EFPIA companies (PJV), Diagenic, Norway, and Innogenetics, Belgium. No conflict of interest exists.
Mr. Mateusz Jasielec reports no disclosures.
Dr. Hassenstab reports no disclosures.
Dr. Grant reports no disclosures.
Dr. Cairns reports no disclosures.
Dr. Morris has participated or is currently participating in clinical trials of anti-dementia drugs sponsored by the following companies: Janssen Immunotherapy, and Pfizer. Dr. Morris has served as a consultant for the following companies: Eisai, Esteve, Janssen Alzheimer Immunotherapy Program, Glaxo-Smith-Kline, Novartis, and Pfizer. He receives research support from Eli Lilly/Avid Radiopharmaceuticals and is funded by NIH grants # P50AG005681; P01AG003991; P01AG026276 and U19AG032438. Neither Dr. Morris nor his family owns stock or has equity interest (outside of mutual funds or other externally directed accounts) in any pharmaceutical or biotechnology company. No conflict of interest exists. Dr. Holtzman reports consulting for Bristol-Myers-Squibb, AstraZeneca, and Genentech, and is on the scientific advisory board of C2N Diagnostics. His research grant support is from the NIH, Ellison Medical Foundation, Cure Alzheimer’s Fund, Pfizer, Astra Zeneca, C2N Diagnostics and Integrated Diagnostics. No conflict of interest exists.
Dr. Fagan serves as an advisory board member for Roche and Eli Lilly. No conflict of interest exists.
References
- 1.Price J, Morris JC. Tangles and plaques in non demented aging and "preclinical" Alzheimer's disease. Ann Neurol. 1999;45:358–368. doi: 10.1002/1531-8249(199903)45:3<358::aid-ana12>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 2.Hulette C, Welsh-Bohmer K, Murray M, Saunders A, Mash D, Mcintyre L. Neuropathological and neuropsychological changes in "normal" aging: evidence for preclinical Alzheimer disease in cognitively normal individuals. J Neuropathol Exp Neurol. 1998;57:1168–1274. doi: 10.1097/00005072-199812000-00009. [DOI] [PubMed] [Google Scholar]
- 3.Braak H, Braak E. Frequency of stages of Alzheimer-related lesions in different age categories. Neurobiol Aging. 1997;18:351–357. doi: 10.1016/s0197-4580(97)00056-0. [DOI] [PubMed] [Google Scholar]
- 4.Bateman RJ, Xiong C, Benzinger TL, et al. Clincial and biomarker changes in dominantly inherited Alzheimer’s disease. N Engl J Med. 2012;367:795–804. doi: 10.1056/NEJMoa1202753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sperling RA, Aisen PS, Beckett LA, et al. Toward defining the preclinical stages of Alzheimer’s disease: Recommendations from the National Institute on Aging and the Alzheimer’s Association workgroup. Alzheimer’s & Dementia. 2011;7:280–292. doi: 10.1016/j.jalz.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Knopman DS, Jack CR, Wiste HJ, et al. Short-term clinical outcomes for stages of NIA-AA preclinical Alzheimer disease. Neurology. 2012;78:1576–1582. doi: 10.1212/WNL.0b013e3182563bbe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berg L, McKeel DW, Miller JP, et al. Clinicopathologic studies in cognitively healthy aging and Alzheimer’s disease: relation of histologic markers to dementia severity, age, sex, and apoE genotype. Arch Neurol. 1998;55:326–335. doi: 10.1001/archneur.55.3.326. [DOI] [PubMed] [Google Scholar]
- 8.Villareal DT, Grant E, Miller JP, Strorandt M, McKeel DW, Morris JC. Clinical outcomes of possible versus probable Alzheimer’s disease. Neurology. 2003;61:661–7. doi: 10.1212/wnl.61.5.661. [DOI] [PubMed] [Google Scholar]
- 9.Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993;43:2412–2414. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- 10.Johnson DK, Storandt M, Morris JC, Langford ZD, Galvin JE. Cognitive profiles in dementia: Alzheimer disease versus nondemented aging. Neurology. 2008;71:1783–1889. doi: 10.1212/01.wnl.0000335972.35970.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Berg L, Miller JP, Baty J, Rubin EH, Morris JC, Figiel G. Mild senile dementia of the Alzheimer type. 4. Evaluation of intervention. Ann Neurol. 1992;31:242–249. doi: 10.1002/ana.410310303. [DOI] [PubMed] [Google Scholar]
- 12.McKhann G, Drachman D, Folstein M, Katzman R, Price D, EM S. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work-Group under the auspices of the Department of Health and Human Services Task Force on Alzheimer's disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 13.Storandt M, Grant EA, Miller JP, Morris JC. Longitudinal course and neuropathologic outcomes in original vs revised MCI and in pre-MCI. Neurology. 2006;67:467–473. doi: 10.1212/01.wnl.0000228231.26111.6e. [DOI] [PubMed] [Google Scholar]
- 14.Bondi MW, Jak AJ, Delano-Wood L, Jacobson MW, Delis DC, Salmon DP. Neuropsychological contributions to the early identification of Alzheimer’s disease. Neuropsychol Rev. 2008;18:73–90. doi: 10.1007/s11065-008-9054-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grober E, Buschke H, Crystal H, Bang S. Screening for dementia by memory testing. Neurology. 1988;38:900–993. doi: 10.1212/wnl.38.6.900. [DOI] [PubMed] [Google Scholar]
- 16.Wechsler D. Manual: Wechsler Memory Scale-Revised. San Antonio, TX: Psychological Corporation; 1987. [Google Scholar]
- 17.Johnson DK, Storandt M, Morris JC, Galvin JE. Longitudinal study of the transition from healthy aging to Alzheimer's disease. Arch Neurol. 2009;66:1254–1559. doi: 10.1001/archneurol.2009.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Albert MS, DeKosky ST, Dickson D, Dubois B, Feldman HH, Fox NC, et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: Recommendations from the National Institute on Aging and Alzheimer’s Association workgroup. Alzheimers Dement. 2011;7:270–279. doi: 10.1016/j.jalz.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dubois B, Feldman HH, Jacova C, Dekosky ST, Barberger-Gateau P, Cummings J, et al. Research criteria for the diagnosis of Alzheimer's disease: revising the NINCDS-ADRDA criteria. Lancet Neurol. 2007;6:734–746. doi: 10.1016/S1474-4422(07)70178-3. [DOI] [PubMed] [Google Scholar]
- 20.Cairns NJ, Taylor-Reinwald L, Morris JC the Alzheimer'sDisease Neuroimaging Initiative. Autopsy consent, brain collection, and standardized neuropathologic assessment of ADNI participants: the essential role of the neuropathology core. Alzheimer’s Dement. 2010;6:274–379. doi: 10.1016/j.jalz.2010.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Montine TJ, Phelps CH, Beach TG, et al. National Institute on Aging-Alzheimer's Association guidelines for the neuropathologic assessment of Alzheimer's disease: a practical approach. Acta Neuropathol. 2012;123:1–11. doi: 10.1007/s00401-011-0910-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Castellano JM, Kim J, Stewart FR. Human apoeE isoforms differentially regulate brain amyloid-ß peptide clearance. Sci Transl Med. 2011 doi: 10.1126/scitranslmed.3002156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jack CR, Knopman DS, Weigand SD, et al. An operational approach to National Institute on Aging-Alzheimer’s Association criteria for preclinical Alzheimer disease. Ann Neurol. 2012;71:765–775. doi: 10.1002/ana.22628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fine J, Gray R. A proportional hazards model for the sub distribution of a competing risk. J Amer Statist Assoc. 1999;94:496–509. [Google Scholar]
- 25.Diggle PJ, Heagerty P, Liang K-Y, Zeger SL. Analysis of Longitudinal Data. 2nd ed. New York: Oxford University Press; 2002. [Google Scholar]
- 26.Akaike Hirotugu. A new look at the statistical model identification. IEEE Transactions on Automatic Control. 1974;19:716–823. [Google Scholar]
- 27.Lönnroos E, Kyyrönen P, Bell JS, van der Cammen TJ, Hartikainen S. Risk of death among persons with Alzheimer’s disease: a national register-based nested case-control study. J Alzheimers Dis. 2013;33:157–164. doi: 10.3233/JAD-2012-120808. [DOI] [PubMed] [Google Scholar]
- 28.Wallin AK, Blennow K, Zetterberg H, Londos E, Minthon L, Hansson O. CSF biomarkers predict a more malignant outcome in Alzheimer disease. Neurology. 2010;74:1531–1547. doi: 10.1212/WNL.0b013e3181dd4dd8. [DOI] [PubMed] [Google Scholar]
- 29.Lopponen M, Raiha I, Isoaho R, Vahlberg T, Puolijoki H, Kivela SL. Dementia associates with under medication of cardiovascular diseases in the elderly: a population-based study. Dement Geriatr Cogn Disord. 2006;22:132–141. doi: 10.1159/000093739. [DOI] [PubMed] [Google Scholar]
- 30.Wadley VG, Okonkwo O, Crowe M, et al. Mild cognitive impairment and everyday function: an investigation of driving performance. J Geriatr Psychiatry Neurol. 2009;22:87–94. doi: 10.1177/0891988708328215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Simic G, Stanic G, Mladinov M, Jovanov-Milosevic N, Kostovic I, Hof PR. Does Alzheimer’s disease begin in the brainstem? Neuropathol Appl Neurobiol. 2009;35:532–654. doi: 10.1111/j.1365-2990.2009.01038.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Morris JC, Roe CM, Xiong C, et al. APOE predicts amyloid-beta but not tau Alzheimer pathology in cognitively normal aging. Ann Neurol. 2010;67:122–131. doi: 10.1002/ana.21843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Desikan RS, McEvoy LK, Holland D, et al. Apolipoprotein E ε4 does not modulate amyloid-β-associated neurodegeneration in preclinical Alzheimer disease. Am J Neuroradiol. 2013;34:505–510. doi: 10.3174/ajnr.A3267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tarawneh R, D’Angelo G, Macy E, et al. Visinin-like protein 1: Diagnostic and prognostic biomarker in Alzheimer's disease. Ann Neurol. 2011;70:274–285. doi: 10.1002/ana.22448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Acosta-Baena N, Sepulveda-Falla D, Lopera-Gomez GM, et al. Pre-dementia clinical stages in presenilin 1 E280A familial early-onset Alzheimer’s disease: a retrospective cohort study. Lancet Neurol. 2011;10:213–220. doi: 10.1016/S1474-4422(10)70323-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.