Abstract
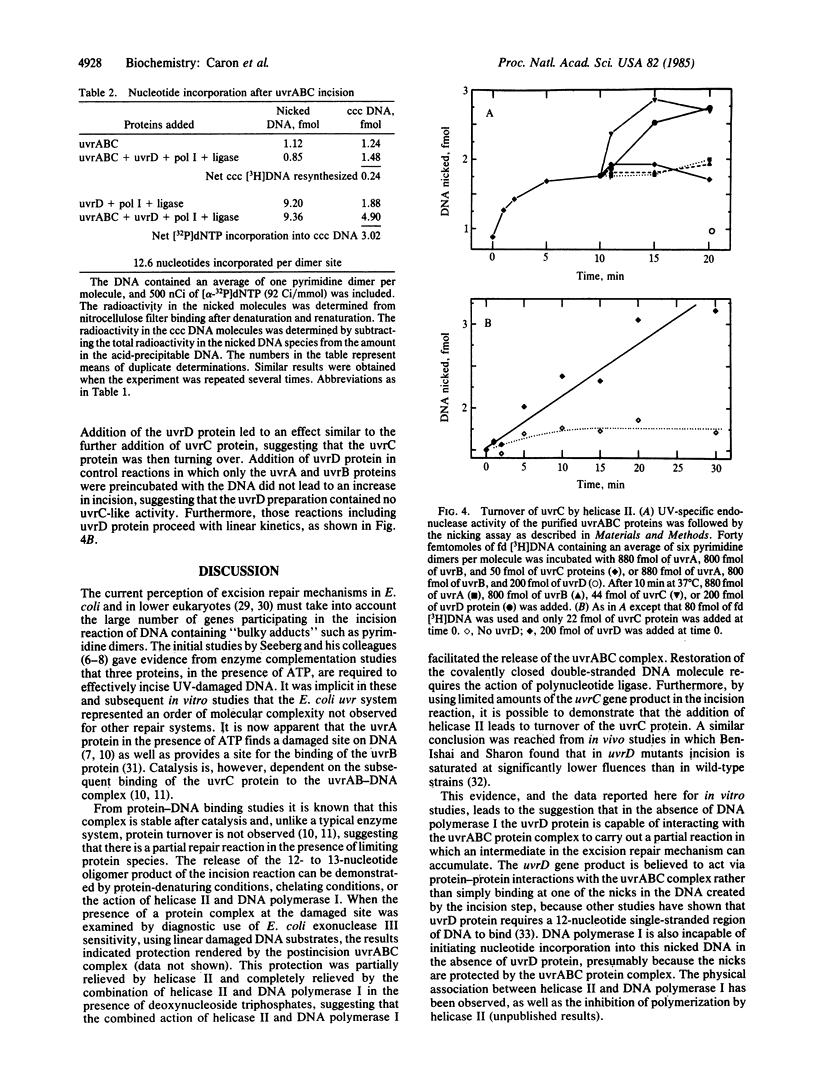
The bimodal-incision nature of the reaction of UV-irradiated DNA catalyzed by the Escherichia coli uvrABC protein complex potentially leads to excision of a 12- to 13-nucleotide-long damaged fragment. However, the oligonucleotide fragment containing the UV-induced pyrimidine dimer is not released under nondenaturing in vitro reaction conditions. Also, the uvrABC proteins are stably bound to the incised DNA and do not turn over after the incision event. In this communication it is shown that release of the damaged fragment from the parental uvrABC-incised DNA is dependent upon either chelating conditions or the simultaneous addition of the uvrD gene product (helicase II) and the polA gene product (DNA polymerase I) when polymerization of deoxynucleoside triphosphate substrates is concomitantly catalyzed. The product of this multiprotein-catalyzed series of reactions serves as a substrate for polynucleotide ligase, resulting in the restoration of the integrity of the strands of DNA. The addition of the uvrD protein to the incised DNA-uvrABC complex also results in turnover of the uvrC protein. It is suggested that the repair processes of incision, excision, resynthesis, and ligation are coordinately catalyzed by a complex of proteins in a "repairosome" configuration.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abdel-Monem M., Chanal M. C., Hoffmann-Berling H. DNA unwinding enzyme II of Escherichia coli. 1. Purification and characterization of the ATPase activity. Eur J Biochem. 1977 Sep 15;79(1):33–38. doi: 10.1111/j.1432-1033.1977.tb11780.x. [DOI] [PubMed] [Google Scholar]
- Arai K., Kornberg A. Unique primed start of phage phi X174 DNA replication and mobility of the primosome in a direction opposite chain synthesis. Proc Natl Acad Sci U S A. 1981 Jan;78(1):69–73. doi: 10.1073/pnas.78.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chase J. W., Masker W. E., Murphy J. B. Pyrimidine dimer excision in Escherichia coli strains deficient in exonucleases V and VII and in the 5' leads to 3' exonuclease of DNA polymerase I. J Bacteriol. 1979 Jan;137(1):234–242. doi: 10.1128/jb.137.1.234-242.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demple B., Linn S. DNA N-glycosylases and UV repair. Nature. 1980 Sep 18;287(5779):203–208. doi: 10.1038/287203a0. [DOI] [PubMed] [Google Scholar]
- Geisow M. J., Mas-Oliva J. Receptors, channels and whole-body NMR in Mexico. Nature. 1981 Dec 3;294(5840):400–402. doi: 10.1038/294400a0. [DOI] [PubMed] [Google Scholar]
- Grafstrom R. H., Park L., Grossman L. Enzymatic repair of pyrimidine dimer-containing DNA. A 5' dimer DNA glycosylase: 3'-apyrimidinic endonuclease mechanism from Micrococcus luteus. J Biol Chem. 1982 Nov 25;257(22):13465–13474. [PubMed] [Google Scholar]
- Grossman L., Riazuddin S., Haseltine W. A., Lindan C. Nucleotide excision repair of damaged DNA. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 2):947–955. doi: 10.1101/sqb.1979.043.01.104. [DOI] [PubMed] [Google Scholar]
- Haseltine W. A., Gordon L. K., Lindan C. P., Grafstrom R. H., Shaper N. L., Grossman L. Cleavage of pyrimidine dimers in specific DNA sequences by a pyrimidine dimer DNA-glycosylase of M. luteus. Nature. 1980 Jun 26;285(5767):634–641. doi: 10.1038/285634a0. [DOI] [PubMed] [Google Scholar]
- Hickson I. D., Arthur H. M., Bramhill D., Emmerson P. T. The E. coli uvrD gene product is DNA helicase II. Mol Gen Genet. 1983;190(2):265–270. doi: 10.1007/BF00330649. [DOI] [PubMed] [Google Scholar]
- Kuemmerle N. B., Ley R. D., Masker W. E. The effect of mutations in the uvrD cistron of Escherichia coli on repair resynthesis. Mutat Res. 1982 Jun;94(2):285–297. doi: 10.1016/0027-5107(82)90292-5. [DOI] [PubMed] [Google Scholar]
- Kuemmerle N. B., Masker W. E. Effect of the uvrD mutation on excision repair. J Bacteriol. 1980 May;142(2):535–546. doi: 10.1128/jb.142.2.535-546.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn B., Abdel-Monem M., Krell H., Hoffmann-Berling H. Evidence for two mechanisms for DNA unwinding catalyzed by DNA helicases. J Biol Chem. 1979 Nov 25;254(22):11343–11350. [PubMed] [Google Scholar]
- Kumura K., Sekiguchi M. Identification of the uvrD gene product of Escherichia coli as DNA helicase II and its induction by DNA-damaging agents. J Biol Chem. 1984 Feb 10;259(3):1560–1565. [PubMed] [Google Scholar]
- Lehman I. R., Chien J. R. Persistence of deoxyribonucleic acid polymerase I and its 5'--3' exonuclease activity in PolA mutants of Escherichia coli K12. J Biol Chem. 1973 Nov 25;248(22):7717–7723. [PubMed] [Google Scholar]
- Little J. W., Mount D. W. The SOS regulatory system of Escherichia coli. Cell. 1982 May;29(1):11–22. doi: 10.1016/0092-8674(82)90085-x. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- McHenry C., Kornberg A. DNA polymerase III holoenzyme of Escherichia coli. Purification and resolution into subunits. J Biol Chem. 1977 Sep 25;252(18):6478–6484. [PubMed] [Google Scholar]
- O'Farrell P. H., Kutter E., Nakanishi M. A restriction map of the bacteriophage T4 genome. Mol Gen Genet. 1980;179(2):421–435. doi: 10.1007/BF00425473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oeda K., Horiuchi T., Sekiguchi M. The uvrD gene of E. coli encodes a DNA-dependent ATPase. Nature. 1982 Jul 1;298(5869):98–100. doi: 10.1038/298098a0. [DOI] [PubMed] [Google Scholar]
- Radany E. H., Friedberg E. C. A pyrimidine dimer-DNA glycosylase activity associated with the v gene product of bacterophage T4. Nature. 1980 Jul 10;286(5769):182–185. doi: 10.1038/286182a0. [DOI] [PubMed] [Google Scholar]
- Reynolds R. J., Friedberg E. C. Molecular mechanisms of pyrimidine dimer excision in Saccharomyces cerevisiae: incision of ultraviolet-irradiated deoxyribonucleic acid in vivo. J Bacteriol. 1981 May;146(2):692–704. doi: 10.1128/jb.146.2.692-704.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sancar A., Rupp W. D. A novel repair enzyme: UVRABC excision nuclease of Escherichia coli cuts a DNA strand on both sides of the damaged region. Cell. 1983 May;33(1):249–260. doi: 10.1016/0092-8674(83)90354-9. [DOI] [PubMed] [Google Scholar]
- Seeberg E. Multiprotein interactions in strand cleavage of DNA damaged by UV and chemicals. Prog Nucleic Acid Res Mol Biol. 1981;26:217–226. doi: 10.1016/s0079-6603(08)60406-7. [DOI] [PubMed] [Google Scholar]
- Seeberg E. Reconstitution of an Escherichia coli repair endonuclease activity from the separated uvrA+ and uvrB+/uvrC+ gene products. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2569–2573. doi: 10.1073/pnas.75.6.2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeberg E., Steinum A. L. Purification and properties of the uvrA protein from Escherichia coli. Proc Natl Acad Sci U S A. 1982 Feb;79(4):988–992. doi: 10.1073/pnas.79.4.988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stellwagen N. C. Anomalous electrophoresis of deoxyribonucleic acid restriction fragments on polyacrylamide gels. Biochemistry. 1983 Dec 20;22(26):6186–6193. doi: 10.1021/bi00295a023. [DOI] [PubMed] [Google Scholar]
- Tait R. C., Harris A. L., Smith D. W. DNA repair in Escherichia coli mutants deficient in DNA polymerases I, II and-or 3. Proc Natl Acad Sci U S A. 1974 Mar;71(3):675–679. doi: 10.1073/pnas.71.3.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taucher-Scholz G., Hoffmann-Berling H. Identification of the gene for DNA helicase II of Escherichia coli. Eur J Biochem. 1983 Dec 15;137(3):573–580. doi: 10.1111/j.1432-1033.1983.tb07864.x. [DOI] [PubMed] [Google Scholar]
- Wilcox D. R., Prakash L. Incision and postincision steps of pyrimidine dimer removal in excision-defective mutants of Saccharomyces cerevisiae. J Bacteriol. 1981 Nov;148(2):618–623. doi: 10.1128/jb.148.2.618-623.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung A. T., Mattes W. B., Oh E. Y., Grossman L. Enzymatic properties of purified Escherichia coli uvrABC proteins. Proc Natl Acad Sci U S A. 1983 Oct;80(20):6157–6161. doi: 10.1073/pnas.80.20.6157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youngs D. A., Smith K. C. Genetic control of multiple pathways of post-replicational repair in uvrB strains of Escherichia coli K-12. J Bacteriol. 1976 Jan;125(1):102–110. doi: 10.1128/jb.125.1.102-110.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youngs D. A., Smith K. C. The involvement of polynucleotide ligase in the repair of UV-induced DNA damage in Escherichia coli K-12 cells. Mol Gen Genet. 1977 Mar 28;152(1):37–41. doi: 10.1007/BF00264937. [DOI] [PubMed] [Google Scholar]
- Youngs D. A., Van der Schueren E., Smith K. C. Separate branches of the uvr gene-dependent excision repair process in ultraviolet-irradiated Escherichia coli K-12 cells; their dependence upon growth medium and the polA, recA, recB, and exrA genes. J Bacteriol. 1974 Feb;117(2):717–725. doi: 10.1128/jb.117.2.717-725.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]



