Abstract
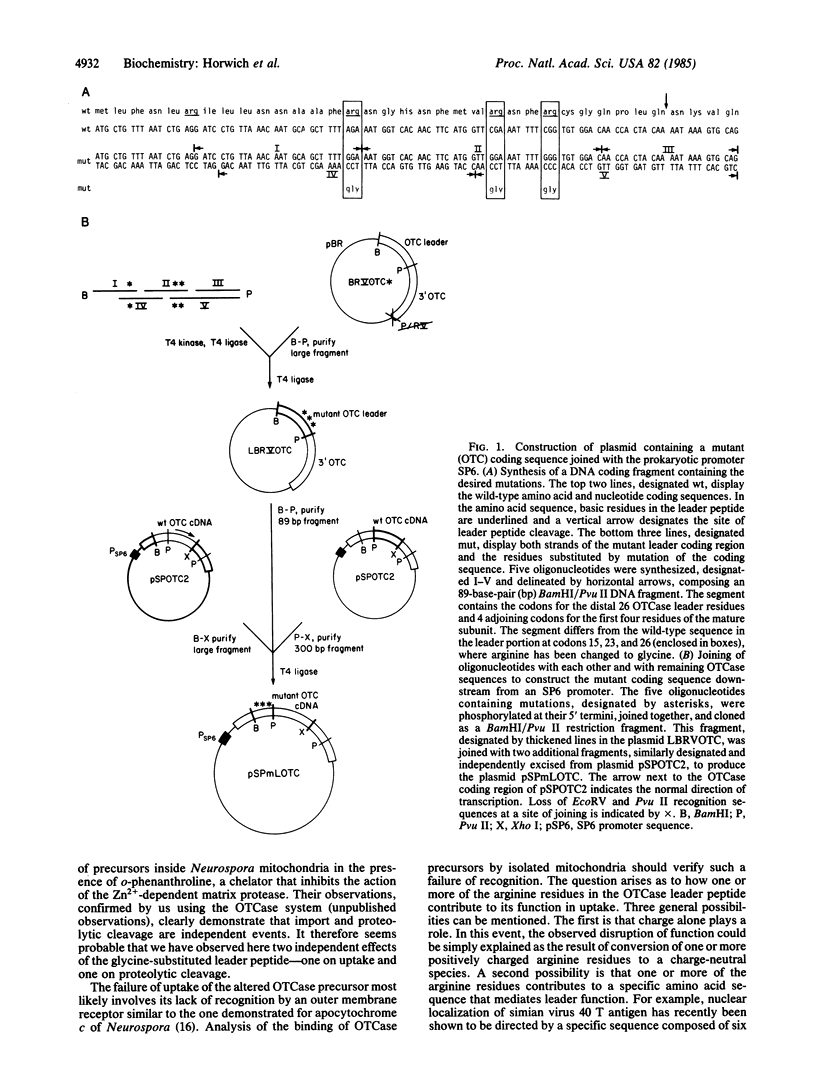
Most mitochondrial proteins are encoded in the nucleus and translated in the cytoplasm as larger precursors containing NH2-terminal "leader" peptides, which are strikingly basic in overall amino acid composition. Recent experiments indicate that these leader peptides are both necessary and sufficient to direct post-translational recognition and import of precursors by mitochondria. In this report, we demonstrate a critical role for one or more of the basic arginine residues in the leader peptide of the subunit precursor for the human mitochondrial matrix enzyme, ornithine transcarbamoylase (ornithine carbamoyltransferase, carbamoylphosphate: L-ornithine carbamoyltransferase, EC 2.1.3.3). The distal three of four basic residues, all arginines, in the leader peptide of ornithine transcarbamoylase were replaced at once with charge-neutral glycine residues. The altered ornithine transcarbamoylase precursor failed to be taken up by intact mitochondria in vitro. Moreover, it also failed to be proteolytically cleaved upon incubation with a mitochondrial matrix fraction containing the Zn2+-dependent protease, which normally cleaves the leader peptide.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Conboy J. G., Fenton W. A., Rosenberg L. E. Processing of pre-ornithine transcarbamylase requires a zinc-dependent protease localized to the mitochondrial matrix. Biochem Biophys Res Commun. 1982 Mar 15;105(1):1–7. doi: 10.1016/s0006-291x(82)80002-8. [DOI] [PubMed] [Google Scholar]
- Conboy J. G., Rosenberg L. E. Posttranslational uptake and processing of in vitro synthesized ornithine transcarbamoylase precursor by isolated rat liver mitochondria. Proc Natl Acad Sci U S A. 1981 May;78(5):3073–3077. doi: 10.1073/pnas.78.5.3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenton W. A., Hack A. M., Helfgott D., Rosenberg L. E. Biogenesis of the mitochondrial enzyme methylmalonyl-CoA mutase. Synthesis and processing of a precursor in a cell-free system and in cultured cells. J Biol Chem. 1984 May 25;259(10):6616–6621. [PubMed] [Google Scholar]
- Hay R., Böhni P., Gasser S. How mitochondria import proteins. Biochim Biophys Acta. 1984 Jan 27;779(1):65–87. doi: 10.1016/0304-4157(84)90004-2. [DOI] [PubMed] [Google Scholar]
- Hennig B., Koehler H., Neupert W. Receptor sites involved in posttranslational transport of apocytochrome c into mitochondria: specificity, affinity, and number of sites. Proc Natl Acad Sci U S A. 1983 Aug;80(16):4963–4967. doi: 10.1073/pnas.80.16.4963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwich A. L., Fenton W. A., Firgaira F. A., Fox J. E., Kolansky D., Mellman I. S., Rosenberg L. E. Expression of amplified DNA sequences for ornithine transcarbamylase in HeLa cells: arginine residues may be required for mitochondrial import of enzyme precursor. J Cell Biol. 1985 May;100(5):1515–1521. doi: 10.1083/jcb.100.5.1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwich A. L., Fenton W. A., Williams K. R., Kalousek F., Kraus J. P., Doolittle R. F., Konigsberg W., Rosenberg L. E. Structure and expression of a complementary DNA for the nuclear coded precursor of human mitochondrial ornithine transcarbamylase. Science. 1984 Jun 8;224(4653):1068–1074. doi: 10.1126/science.6372096. [DOI] [PubMed] [Google Scholar]
- Horwich A. L., Kalousek F., Mellman I., Rosenberg L. E. A leader peptide is sufficient to direct mitochondrial import of a chimeric protein. EMBO J. 1985 May;4(5):1129–1135. doi: 10.1002/j.1460-2075.1985.tb03750.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurt E. C., Pesold-Hurt B., Schatz G. The cleavable prepiece of an imported mitochondrial protein is sufficient to direct cytosolic dihydrofolate reductase into the mitochondrial matrix. FEBS Lett. 1984 Dec 10;178(2):306–310. doi: 10.1016/0014-5793(84)80622-5. [DOI] [PubMed] [Google Scholar]
- Kalderon D., Roberts B. L., Richardson W. D., Smith A. E. A short amino acid sequence able to specify nuclear location. Cell. 1984 Dec;39(3 Pt 2):499–509. doi: 10.1016/0092-8674(84)90457-4. [DOI] [PubMed] [Google Scholar]
- Kraus J. P., Hodges P. E., Williamson C. L., Horwich A. L., Kalousek F., Williams K. R., Rosenberg L. E. A cDNA clone for the precursor of rat mitochondrial ornithine transcarbamylase: comparison of rat and human leader sequences and conservation of catalytic sites. Nucleic Acids Res. 1985 Feb 11;13(3):943–952. doi: 10.1093/nar/13.3.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieg P. A., Melton D. A. Functional messenger RNAs are produced by SP6 in vitro transcription of cloned cDNAs. Nucleic Acids Res. 1984 Sep 25;12(18):7057–7070. doi: 10.1093/nar/12.18.7057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maccecchini M. L., Rudin Y., Blobel G., Schatz G. Import of proteins into mitochondria: precursor forms of the extramitochondrially made F1-ATPase subunits in yeast. Proc Natl Acad Sci U S A. 1979 Jan;76(1):343–347. doi: 10.1073/pnas.76.1.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori M., Miura S., Tatibana M., Cohen P. P. Characterization of a protease apparently involved in processing of pre-ornithine transcarbamylase of rat liver. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7044–7048. doi: 10.1073/pnas.77.12.7044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takiguchi M., Miura S., Mori M., Tatibana M., Nagata S., Kaziro Y. Molecular cloning and nucleotide sequence of cDNA for rat ornithine carbamoyltransferase precursor. Proc Natl Acad Sci U S A. 1984 Dec;81(23):7412–7416. doi: 10.1073/pnas.81.23.7412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Broeck G., Timko M. P., Kausch A. P., Cashmore A. R., Van Montagu M., Herrera-Estrella L. Targeting of a foreign protein to chloroplasts by fusion to the transit peptide from the small subunit of ribulose 1,5-bisphosphate carboxylase. 1985 Jan 31-Feb 6Nature. 313(6001):358–363. doi: 10.1038/313358a0. [DOI] [PubMed] [Google Scholar]
- Walter P., Gilmore R., Blobel G. Protein translocation across the endoplasmic reticulum. Cell. 1984 Aug;38(1):5–8. doi: 10.1016/0092-8674(84)90520-8. [DOI] [PubMed] [Google Scholar]
- Zwizinski C., Neupert W. Precursor proteins are transported into mitochondria in the absence of proteolytic cleavage of the additional sequences. J Biol Chem. 1983 Nov 10;258(21):13340–13346. [PubMed] [Google Scholar]