Abstract
Redundancy and competition between R2R3-MYB activators and repressors on common target genes has been proposed as a fine-tuning mechanism for the regulation of plant secondary metabolism. This hypothesis was tested in white spruce [Picea glauca (Moench) Voss] by investigating the effects of R2R3-MYBs from different subgroups on common targets from distinct metabolic pathways. Comparative analysis of transcript profiling data in spruces overexpressing R2R3-MYBs from loblolly pine (Pinus taeda L.), PtMYB1, PtMYB8, and PtMYB14, defined a set of common genes that display opposite regulation effects. The relationship between the closest MYB homologues and 33 putative target genes was explored by quantitative PCR expression profiling in wild-type P. glauca plants during the diurnal cycle. Significant Spearman’s correlation estimates were consistent with the proposed opposite effect of different R2R3-MYBs on several putative target genes in a time-related and tissue-preferential manner. Expression of sequences coding for 4CL, DHS2, COMT1, SHM4, and a lipase thio/esterase positively correlated with that of PgMYB1 and PgMYB8, but negatively with that of PgMYB14 and PgMYB15. Complementary electrophoretic mobility shift assay (EMSA) and transactivation assay provided experimental evidence that these different R2R3-MYBs are able to bind similar AC cis-elements in the promoter region of Pg4CL and PgDHS2 genes but have opposite effects on their expression. Competitive binding EMSA experiments showed that PgMYB8 competes more strongly than PgMYB15 for the AC-I MYB binding site in the Pg4CL promoter. Together, the results bring a new perspective to the action of R2R3-MYB proteins in the regulation of distinct but interconnecting metabolism pathways.
Key words: Conifers, phenylpropanoid pathway, protein–DNA binding, R2R3-MYB evolution, transcriptional network.
Introduction
During their development, and in response to biotic and abiotic stresses, plants produce thousands of metabolites, which play a wide range of roles in environmental adaptation. The synthesis and accumulation of such products result from the activity of a plethora of enzymes under the control of complex transcriptional networks. For example, the fine regulation of phenylpropanoid biosynthesis is achieved by a combination of transcription factors (TFs) from diverse families such as WRKY, bZIP, MADS-box, bHLH, WD40, and R2R3-MYBs (Zhao and Dixon, 2011). Recent studies of transcriptional regulation of specific aspects of primary and secondary metabolism have begun to define transcriptional networks underlying metabolic responses.
The R2R3-MYBs represent one of the largest families of plant TFs (Kranz et al., 1998; Stracke et al., 2001) and are important regulators of secondary metabolism (Dubos et al., 2010, Feller et al., 2011). Their DNA-binding domain (DBD) is highly conserved among angiosperm and gymnosperm plants (Bedon et al., 2007) but their C-terminus, which typically contains activation and repression domains, is highly variable (Dubos et al., 2010). The R2R3-MYBs have been divided into 22 different subgroups (SGs) based on their DBD and C-termini sequence features, although some of them remain unclassified (Kranz et al., 1998; Stracke et al., 2001). Recent evidence from model plant systems indicates that different MYBs within a subgroup may be functionally redundant. For example, several Arabidopsis SG7 MYBs were shown to regulate the production of flavonol glycosides by acting on similar target genes (Stracke et al., 2007), and the maize ZmMYB31 and ZmMYB42 both regulate CINNAMATE 4-HYDROXYLASE (C4H) (Fornalé et al., 2010). Functional characterizations have also shown that MYBs from different subgroups may act upon overlapping sets of target genes in pathways of flavonoid, monolignol, xylan, or cellulose metabolism (reviewed in Dubos et al., 2010; Zhong et al., 2010).
Many R2R3-MYBs have been described as transcriptional activators of genes from phenylpropanoid, flavonoid, and glucosinolate biosynthetic pathways in plants (Dubos et al., 2010; Zhong et al., 2010; Zhao and Dixon, 2011). Alternatively, a few of them, including members of the SG4 subgroup, have been identified as negative regulators of monolignol and flavonoid biosynthetic pathways (Tamagnone et al., 1998; Jin et al., 2000; Preston et al., 2004; Legay et al., 2007; Fornalé et al., 2010). In Eucalyptus grandiis, two R2R3- MYBs, namely EgMYB1 (Legay et al., 2010) and EgMYB2 (Goicoechea et al., 2005), were shown to act as a repressor and activator of lignin biosynthesis, respectively. The putative interplay of activators and repressors including MYBs acting on common target genes has been proposed as the basis for fine transcriptional regulation of phenylpropanoid metabolism (Vom Endt et al., 2002) and lignin biosynthesis (Zhao and Dixon, 2011). Activation and repression have also been attributed to the action of a single MYB gene (Bhargava et al., 2010). For example, Arabidopsis MYB75, also known as PRODUCTION OF ANTHOCYANIN PIGMENT1 (PAP1), was shown to regulate anthocyanin biosynthesis positively in seedlings (Borevitz et al., 2000) and, more recently, to regulate negatively the expression of monolignol biosynthesis genes and the accumulation of lignin in the inflorescence stems of older plants (Bhargava et al., 2010). Together, these results also suggest that a dual effect may exist when considering different tissues.
Our understanding of functional redundancy and antagonistic effects among R2R3-MYBs has essentially been derived from the studies of flowering plants (i.e. angiosperms) and remains to be extended to other seed-bearing plants. The conifer MYBs Pinus taeda PtMYB1 and PtMYB8 were previously identified as positive regulators of monolignol biosynthesis (Bomal et al., 2008). It was also shown that PtMYB14 and PtMYB15 ectopic overexpression could up-regulate isoprenoid metabolism (Bedon et al., 2010), but these latter two MYBs were suspected to be potential repressors since they harbour the ERF-associated amphilic repression (EAR) motif (Ohta et al., 2001). Here, evidence is presented for antagonistic action among these four MYBs on a set of common target genes in shikimate and monolignol biosynthetic pathways in Picea glauca. Comparative transcript profiling in PtMYB overexpression lines, diurnal variation of gene expression in secondary vascular tissues, transactivation assays, and DNA binding assays were used to identify the putative targets of P. glauca (Pg) R2R3-MYBs. As such, the findings show that several R2R3-MYBs in P. glauca may act upon common targets through activation and repression of expression in two distinct but interconnecting pathways.
Materials and methods
Comparative analysis and functional annotation of microarray data sets
Microarray transcript profiling data were from wild-type and transgenic white spruce [P. glauca (Moench) Voss] overexpressing Pinus taeda PtMYB1, PtMYB8 (Bomal et al., 2008), and PtMYB14 (Bedon et al., 2010). Picea glauca is an established transgenic expression system that is the most homologous available for analysing genes in the Pinaceae. The data sets are available in the public database Array Express (EBI): E-MEXP-3626 for PtMYB1 and PtMYB8 and E-MEXP-3575 for PtMYB14. These data sets were compared by using a fold change of ≥|1.4| [P-value <0.01, false discovery rate (FDR) of 1%] to identify shared sets of up- and down-regulated genes (Supplementary Table S1 available at JXB online). Annotations of cDNA sequences on the microarray were based on Rigault et al. (2011). Assignment to metabolic pathways from the Kyoto Encyclopedia of Genes and Genomes (KEGG) was based on annotations of TAIR homologuess (Supplementary Table S2).
Plant material and diurnal cycle experiment
Wild-type 3-year-old spruce plantlets [P. glauca (Moench) Voss] were transferred to 2 litre pots, and grown for 45 d (mid-April to June) in a greenhouse under a natural photoperiod with weekly irrigation and fertilization [20/20/20 N/P/K (g l–1)]. Two weeks before the diurnal experiment, plants were transferred to a growth chamber (Conviron model PGW36) set to the natural summer solstice photoperiod (16h/8h, day/night) with a photosynthetic photon flux density of 860 μmol photons m–2 s–1 (constant temperature of 23 °C and 70% relative humidity).
For the diurnal cycle experiment, whole secondary xylem (2X) and bark/phloem (2P) tissue samples were collected from the main stem at 3h intervals over a 24h period. A 10cm long stem piece was cut under the first whorl from the top. A scalpel incision was made along the stem, and then 2P tissues were manually peeled off the stem and separated from 2X tissues. Both 2P and 2X tissues were rapidly cut into short pieces, immediately frozen in liquid nitrogen, and stored at –80 °C until further use. Five individual trees were sampled at each time point over the 24h period, and the experiment was performed twice.
RNA extraction, cDNA preparation, and quantitative PCR
Total RNA was isolated as described (Pavy et al., 2008). RNA concentrations were determined using NanoDrop 1000 (Thermo Scientific, Wilmington, DE, USA); RNA integrity used a 2100 Bioanalyzer (Agilent Technologies, Santa Clara, CA, USA). cDNA preparation and quantitative PCR followed Boyle et al. (2009) with some modifications. Briefly, 1 μg of total RNA was reverse transcribed, and a green fluorescent protein (GFP) spike-in (HM151400.1) was added as an internal control. The PCR mixture was a QuantiFast® SYBR® Green PCR kit (QIAGEN, Germantown, MD, USA) or a LightCycler® 480 SYBR Green I Master (Roche, Basel, Switzerland). Gene-specific primers were designed by using Primer 3 software (Supplementary Table S3A at JXB online).
The transcript level (number of molecules) was calculated using the LRE method (Rutledge and Stewart, 2008) adapted by Boyle et al. (2009). Transcript levels were normalized to the geometric mean of six reference genes: elongation factor 1a (BT102965), cell division cycle 2 (BT106071), ribosomal protein L3A (BT115036), eukaryotic initiation factor 4E (BT112014), ubiquitin-conjugating enzyme (BT109864), and core histone H3 (BT116867). Reference gene variation was analysed with geNorm 3.5 version (Vandesompele et al., 2002).
Statistical analysis of expression data
Spearman’s correlation rank test was used to evaluate statistical dependence between candidate gene and PgMYB expression data. Critical ρ values in a two-tailed test at 0.05 and 0.01 significance were 0.247 and 0.325 for 2X tissue (n=64) and 0.223 and 0.291 for 2P tissue (n=78), respectively (Steele et al., 1980). Due to multiple comparison tests, a q-value was calculated to estimate a minimum FDR (5%) to adjust the significance (P-value) of ρ estimates. Statistical analyses used the R package (Ihaka and Gentleman, 1996).
Isolation of genomic sequences and AC element identification
The sequences upstream of the Pg4CL (BT106671) and PgDHS2 (BT116706) genes were identified using the Universal GenomeWalker™ kit (Clontech, Mountain View, CA, USA) as described by Bedon et al. (2009). For Pg4CL, the complete coding sequence was first obtained by using 5′ rapid amplification of cDNA ends (RACE; SMART RACE cDNA Amplification Kit, Invitrogen, Carlsbad, CA, USA) in order to obtain a full-length cDNA (TA Cloning Kit, Invitrogen). Two overlapping genomic DNA fragments were then obtained with proximal primers 4CL-GSP1 and 4CL-GSP2, and distal primers 4CL-GSP3 and 4CL-GSP4; a single PgDHS2 genomic fragment was obtained with primers DHS2-GSP1 and DHS2-GSP2 (Supplementary Table S3B at JXB online). Amplified Pg4CL (GenBank JN828803) and PgDHS2 (GenBank JN828804) genomic fragments (1885bp and 1353bp, respectively) were cloned using the TA Cloning Kit (Invitrogen) and electroporated into Escherichia coli XL1-Blue cells. Putative AC elements were identified with the Fuzznuc tool (http://mobyle.pasteur.fr/cgi-bin/portal.py; Néron et al., 2009) using canonical AC motif sequences as search key words and in both orientations.
Construction of promoter::GUS reporter and MYB expression vectors
Two overlapping genomic DNA fragments were then obtained with proximal primers 4CL-GSP1 and 4CL-GSP2, and distal primers 4CL-GSP3 and 4CL-GSP4; a single PgDHS2 genomic fragment was obtained with primers DHS2-GSP1 and DHS2-GSP2 (Supplementary Table S3B at JXB online). The digested Pg4CL (XbaI/BamHI) and PgDHS2 (SpeI/BamHI) fragments, 1885bp and 1353bp long, respectively, were inserted into a modified pMJM vector to create promoter::GUS (β-glucuronidase) fusions as described (Bedon et al., 2009). They were then inserted into a modified pCAMBIA2300 vector where the hygromycin resistance gene was replaced by the silencing inhibitor p19 gene (Voinnet et al., 2003). The MYB expression vectors were obtained by PCR amplification of the cDNA coding sequences of PgMYB8 and PgMYB15 as well as PtMYB1, PtMYB8, and PtMYB14. They were transferred into the pCAMBIA2300 expression vector using the Gateway® system (Invitrogen). The Pg/PtMYB coding sequences were flanked by the ubiquitin promoter and the 35S terminator. Both reporter and expression vectors were transferred into Agrobacterium tumefaciens strain AGL1.
Transactivation assay
A transient transactivation assay system was developed using P. glauca somatic embryogenic cells co-transformed with Agrobacterium, based on the stable transformation procedures previously described (Klimaszewska et al., 2004). Briefly, the two Agrobacterium cultures (reporter and expression vectors) were incubated overnight, diluted to an optical density of 1.0, and used for co-cultivation with embryogenic cells (1h at 21 °C). The cells were separated from Agrobacterium by filtration, dispensed onto filter paper discs wetted with liquid medium supplemented with 50 μM acetosyringone, and kept in the dark at 21 °C. Four filter papers (replicate) were used per reporter–expression vector combination. After 6 d of co-culture, histochemical GUS assay was performed, and each GUS-stained filter was photographed separately. Images were analysed using the Assess 2.0 software (St Paul, MN, USA) to obtain a uniform determination (resulting densitometry) of GUS-positive cell clusters representing the maximum number of visible spots.
Recombinant protein purification
For the PgMYB8 expression plasmid, a synthetic DNA segment (GenScript, Piscataway, NJ, USA) was cloned into the pET300/NT-DEST vector (Invitrogen) using the Gateway® technology. The PgMYB14 and PgMYB15 coding sequences were amplified by PCR with NdeI and XhoI primers specific to each gene. Each PCR product was ligated into NdeI and XhoI restriction sites in the pET-30a (+) vector (Novagen) and expressed in E. coli Rosetta strain (Novagen). At 4h after induction with 0.1mM isopropyl-β-d-thiogalactopyranoside, the culture was harvested by centrifugation for 5min at 5000 g. The pellet was resuspended in 50ml of ice-cold MBP buffer [10mM TRIS-HCl, pH 7.5, 30mM NaCl, 1mM EDTA, 1mM phenylmethylsulphonyl fluoride (PMSF), and 1× protease inhibitor cocktail], sonicated for 15min, and centrifuged for 20min at 14 000 g. The recombinant proteins were purified under denaturing conditions based on Haneskog (2006). Protein purification was verified by SDS–PAGE and Coomassie blue staining.
Electrophoretic mobility shift assay (EMSA)
Purified PgMYB8, PgMYB14, and PgMYB15 recombinant proteins were tested for their ability to bind to oligonucleotides from the Pg4CL and PgDHS2 promoters and containing specific AC elements by using EMSA. The 30bp fragments of Pg4CL and PgDHS2 promoters used as probes were as follows: 4CL AC-I, 4CL AC-II, 4CL mAC-I, and 4CL mAC-II; and DHS2 AC-I, DHS2 AC-II, DHS2 mAC-I, and DHS2 mAC-II (Supplementary Table S3D at JXB online). The double-stranded oligonucleotides were end labelled with γ-32P. Recombinant protein (375ng) and labelled probe (0.2ng) were incubated at room temperature for 30min, with 100ng of poly(dI–dC) used as an unspecific competitor in a 20 μl reaction with a final concentration of 20mM TRIS pH 8.0, 10mM NaCl, 2mM EDTA, 2mM dithiothreitol (DTT), and 10% glycerol (v/v). Competitions used unlabelled double-stranded probes (20- or 200-fold excess). Electrophoreses of DNA–protein complexes were on 6% native polyacrylamide gels (1× TRIS-glycine buffer, 35 mA, 4 °C). Migration of radiolabelled probes and complexes was detected on Kodak Biomax XAR film.
DNA binding site competition analysis for the AC-I-containing probe from the Pg4CL promoter was also performed between PgMYB8 and PgMYB15 recombinant proteins. For this, preliminary experiments were performed to optimize probe and protein concentration. The molecular weight of PgMYB8 and PgMYB15 was determined using a 10% denaturing SDS–polyacrylamide gel and pre-stained protein marker (New England BioLabs). Protein concentration was calculated with Quick Start™ Bradford Protein Assay (Bio-Rad). Two types of competition experiments were performed by modifying the stoichiometric ratio between PgMYB8 and PgMYB15: (i) 0.7-, 1.0-, and 1.3-fold amounts of PgMYB8 were used to out-compete a constant amount of PgMYB15; while (ii) 20-, 40-, and 60-fold amounts of PgMYB15 were used to out-compete a constant amount of PgMYB8. To ascertain the competition effect, the concentration of PgMYB8 used in experiment (ii) was 10-fold less than in experiment (i). EMSA conditions were as previously described, except that the AC-I-containing probe concentration was 25- and 10-fold diluted for experiment (i) and (ii), respectively.
Results
Identification and annotation of co-expressed transcripts in spruce plants overexpressing pine MYB genes
Analysis of microarray transcript profiles of transgenic spruce overexpressing the P. taeda genes PtMYB1, PtMYB8 (Bomal et al., 2008), and PtMYB14 (Bedon et al., 2010) identified 202, 715, and 513 misregulated sequences relative to controls, respectively (Fig. 1A; Supplementary Table S1 at JXB online). A total of 70 different sequences were misregulated in all three transgenic backgrounds (Fig. 1A), and nearly all of the transcripts (66 out of 70) showed the same pattern of accumulation in PtMYB1 and PtMYB8 transgenics but had opposite expression in PtMYB14 transgenics (Fig. 1B). A similar trend was observed for sets of misregulated genes obtained from pair-wise comparisons between PtMYB1 and PtMYB8 (55 transcripts), PtMYB1 and PtMYB14 (26 transcripts), and PtMYB8 and PtMYB14 overexpressors (112 transcripts) (Supplementary Fig. S1); an opposite misregulation was largely observed when comparing PtMYB14-overexpressing lines with PtMYB1 or PtMYB8-overexpressing lines, while a similar pattern of accumulation was observed in a PtMYB1 and PtMYB8 overexpressor comparison.
Fig. 1.
Comparative analysis of microarray profiles from Pinus taeda PtMYB1, PtMYB8, and PtMYB14 overexpression (OE) in spruce. (A) Numbers of shared and unique misregulated sequences in PtMYB1, PtMYB8, and PtMYB14OE. (B) Expression fold change (FC) of the 70 common misregulated sequences compared with the wild type. Red bars: opposite FC to MYB1-OE. See Table S2 for sequence IDs. (C) KEGG functional categories (%) of the 70 common sequences misexpressed in all three transgenic lines. For details, see Supplementary Table S1 at JXB online.
The set of 66 co-expressed sequences (with opposite expression) common to PtMYB1, PtMYB8, and PtMYB14 transgenics were linked to specific aspects of primary and secondary metabolism (Fig. 1C). The secondary metabolism sequences represented nearly 25% of the total and were linked to flavonoid (10%, PATH ath00941), phenylpropanoid (10%, PATH ath00940), and terpenoid biosynthetic pathways (4.3%, PATH ath00903) (Fig. 1C; Supplementary Table S2 at JXB online). Primary metabolism sequences (15.7% of the total) were distributed among amino acid (11.4%), carbohydrate (2.9%), and lipid (1.4%) metabolism. Sequences assigned to amino acid metabolism were related to cysteine and methionine metabolism (PATH ath00270), as well as phenylalanine, tyrosine, and tryptophan metabolism (PATH ath00400) (Supplementary Table S2). The remaining annotated sequences were assigned to processes such as oxidation–reduction, proteolysis and toxin catabolism (11.5%), cell wall metabolism (4.3%), and stress/defence (8.6%). Around a third of the sequences could not be linked to any known biological process. The sequences obtained from pair-wise comparisons gave similar functional categorizations, although this latter set had slightly higher proportions of sequences among biological processes of cell wall metabolism and stress or defence.
Overall, the sequences assigned to terpenoid and flavonoid pathways, cell wall relaxation, and stress or defence responses were preferentially up-regulated in transgenics overexpressing PtMYB14, consistent with its proposed role in defence (Bedon et al., 2010). Up-regulation of transcripts associated with S-adenosyl methionine, shikimate, and phenylpropanoid biosynthetic pathways was observed in transgenics overexpressing PtMYB1 and PtMYB8 (Supplementary Table S2 at JXB online), consistent with their proposed structural roles including secondary cell wall assembly (Bomal et al., 2008).
Spatio-temporal transcript variation reveals positive and negative correlations among co-expressed genes and potential MYB regulators
Results obtained from the transgenic microarray profiles (Fig. 1; Supplementary Fig. S1 and Tables S1, S2 at JXB online) led to the hypothesis that these different conifer MYBs could target these groups of co-expressed genes in a competitive or antagonistic manner. Aiming to gather data in support of this hypothesis, transcript levels of the candidate target genes were monitored over a diurnal cycle in wild-type spruce trees. A diurnal variation for PgMYBs and co-expressed transcripts was expected based on reports for genes related to phenylpropanoid metabolism (Harmer et al., 2000) and stress (Walley et al., 2007). Reliable transcript accumulation results were obtained for 33 candidate gene sequences by quantitative PCR analysis (Supplementary Table S4). Spearman correlation rank tests were used to identify significant correlations (adjusted P-value ≤0.05, FDR of 5%) between the transcript levels of the candidate genes and those of PgMYB1, PgMYB8, and PgMYB14 as well as PgMYB15, a close homologue of PgMYB14 (Bedon et al., 2010) (Table 1; Supplementary Tables S5, S6).
Table 1.
ρ estimate from Spearman’s correlation rank test between expression data of PgMYB and co-expressed target sequences during a diurnal cycle in wild-type spruce
| Sequence annotation and categorization | Xylem | Bark/phloem | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Name (PN) | GenBank | Path | MYB1 | MYB8 | MYB14 | MYB15 | MYB1 | MYB8 | MYB14 | MYB15 |
| Candidate target | ||||||||||
| SHM4 (49) | BT117395 | AA | 0.32*a | 0.39** | –0.15 | –0.26* | 0.29* | 0.33** | –0.13 | 0.10 |
| MTFR2 (63) | BT119867 | AA | 0.14 | 0.30* | 0.10 | 0.08 | 0.05 | 0.19 | –0.12 | 0.16 |
| MEE58 (54) | BT106824 | AA/SAM | 0.48** | 0.40** | –0.12 | –0.12 | 0.54** | 0.35** | 0.03 | 0.17 |
| ADT2 (17) | BT102177 | AA/SHI | 0.18 | 0.02 | –0.03 | 0.07 | 0.01 | –0.04 | –0.02 | 0.01 |
| CM1 (48) | BT115945 | AA/SHI | 0.39** | 0.40** | –0.09 | –0.25 | 0.35** | 0.45** | –0.02 | 0.09 |
| DHS2 (67) | BT116706 | AA/SHI | 0.43** | 0.52** | –0.17 | –0.35** | 0.43** | 0.61** | 0.04 | –0.04 |
| DFR (10) | BT115327 | FLA | –0.01 | 0.03 | 0.22 | 0.33* | 0.09 | 0.19 | 0.03 | –0.10 |
| CHI (12) | BT101304 | FLA | –0.24 | –0.18 | 0.01 | 0.21 | 0.18 | 0.12 | –0.10 | –0.01 |
| LDOX (16) | BT102953 | FLA | –0.07 | –0.05 | 0.19 | 0.34** | 0.00 | 0.13 | –0.19 | –0.07 |
| TT4/CHS (19) | BT103070 | FLA | 0.09 | 0.04 | 0.31* | 0.39** | 0.22 | 0.13 | 0.17 | 0.07 |
| COMT1 (42) | GQ0043_N14 | PHE | 0.38** | 0.59** | –0.08 | –0.39** | 0.50** | 0.63** | 0.13 | 0.21 |
| BGLU40 (5) | BT101848 | PHE | 0.18 | 0.08 | 0.29* | 0.38** | –0.18 | –0.26 | 0.14 | –0.05 |
| HCT (56) | BT117023 | PHE | 0.38** | 0.52** | 0.00 | –0.04 | 0.45** | 0.41** | 0.03 | 0.09 |
| 4CL2 (61) | BT106671 | PHE | 0.34** | 0.34** | –0.21 | –0.58** | 0.36** | 0.38** | 0.02 | –0.04 |
| PRR2 (68) | BT111350 | PHE | 0.32* | 0.32* | –0.19 | –0.11 | 0.52** | 0.60** | 0.00 | 0.04 |
| CYP76G1 (11) | GQ0205_N10 | TER | 0.13 | 0.12 | 0.33** | 0.42** | 0.40** | 0.36** | 0.14 | 0.06 |
| XTH9 (1) | BT102510 | CW | 0.13 | 0.10 | 0.37** | 0.38** | –0.12 | –0.13 | 0.05 | –0.07 |
| GH17 (38) | BT118546 | CW | 0.02 | 0.10 | 0.05 | 0.08 | 0.15 | 0.21 | 0.09 | 0.20 |
| CYP76C4 (27) | GQ0133_I20 | OX | 0.18 | 0.02 | 0.17 | 0.22 | 0.02 | –0.12 | 0.13 | 0.08 |
| CYP76C5 (28) | BT116913 | OX | 0.22 | 0.28* | 0.04 | 0.15 | 0.00 | 0.13 | 0.12 | 0.03 |
| GSTU18 (33) | BT103013 | TOX | 0.01 | –0.24 | –0.01 | 0.37** | 0.09 | 0.23 | 0.18 | 0.22 |
| Lipase/thio (66) | BT102121 | 0.37** | 0.53** | –0.29* | –0.40** | 0.43** | 0.46** | –0.07 | 0.08 | |
| HRGP (3) | BT117438 | –0.06 | –0.10 | 0.29* | 0.37** | 0.07 | 0.02 | –0.14 | 0.17 | |
| No hit (20) | BT112709 | –0.07 | –0.20 | 0.09 | 0.29* | –0.07 | –0.06 | 0.12 | 0.01 | |
| No hit (22) | BT113910 | –0.13 | –0.08 | 0.12 | 0.49** | 0.20 | 0.16 | 0.19 | 0.25 | |
| DAG (37) | BT115660 | 0.07 | 0.22 | –0.22 | –0.12 | 0.17 | –0.02 | –0.08 | 0.07 | |
| EDGP (40) | BT115833 | –0.05 | 0.08 | 0.24 | 0.20 | 0.21 | 0.31** | 0.08 | –0.10 | |
| PR4 (44) | BT117075 | 0.12 | 0.03 | –0.03 | –0.16 | 0.32** | 0.07 | 0.11 | 0.13 | |
| KT2 (45) | BT116079 | 0.31* | 0.56** | –0.17 | 0.04 | 0.13 | 0.20 | –0.07 | 0.26 | |
| HB3 (51) | BT102955 | 0.15 | 0.04 | 0.21 | 0.13 | 0.60** | 0.47** | 0.28* | 0.00 | |
| LTP (52) | BT103567 | 0.02 | 0.05 | 0.08 | 0.28* | 0.18 | 0.28* | 0.00 | 0.19 | |
| Cys Pase (55) | BT114584 | 0.39** | 0.33** | 0.06 | 0.02 | 0.16 | 0.23 | 0.05 | 0.14 | |
| ATAF1 (58) | BT115770 | 0.18 | 0.20 | 0.13 | 0.11 | 0.17 | 0.07 | 0.16 | 0.10 | |
| MYB TF | ||||||||||
| MYB1 | BT108631 | 1 | 1 | |||||||
| MYB8 | BT108136 | 0.43** | 1 | 0.59** | 1 | |||||
| MYB14 | FJ469917 | 0.03 | 0.00 | 1 | 0.25* | 0.08 | 1 | |||
| MYB15 | FJ469918 | –0.23 | –0.24 | 0.37** | 1 | 0.15 | 0.20 | 0.09 | 1 | |
a ρ estimates in bold were significant at P-values ≤0.05 (*) or ≤0.01(**) in a two-tailed Spearman test. Due to multiple comparison tests, a q-value was calculated to estimate a minimum FDR to adjust the significance (P-value) of ρ estimates.
Genes positively correlated with PgMYB1/PgMYB8 and negatively correlated with PgMYB14/PgMYB15 are underlined.
Complete expression data sets from the diurnal cycle experiment and used for Spearman’s correlation rank tests are presented in Supplementary Table S4 at JXB online. PN, probe number. Path: CW, cell wall; PHE, phenylpropanoid; FLA, flavonoid; TER, terpenoid; AA, amino acid; SHI, shikimate; SAM, S-adenosylmethionine; OX, oxidation–reduction; TOX, toxin catabolism.
Complete correlation tests are presented in Supplementary Table S5 (xylem) and Table S6 (bark/phloem).
The transcripts of five genes correlated positively (adjusted P-value ≤0.034, q-value ≤0.05) with those of PgMYB1 and PgMYB8, and negatively with those of PgMYB15 and, to a lesser extent, PgMYB14 (see Table 1). These genes were annotated as coding for 4-coumarate CoA ligase (4CL), 3-deoxy-7-phosphoheptulonate synthase (DHS2), caffeic acid O-3-methyltransferase 1 (COMT1), serine hydroxymethyltransferase 4 (SHM4), and a lipase/thioesterase (Lipase/thio). This transcript profiling experiment was carried out in actively growing secondary tissues of xylem and in bark with phloem. The opposite correlations were observed only in the xylem and not in the bark/phloem (Table 1). As they were obtained from a group of plants of diverse genetic backgrounds and from several sampling time points, the correlations may be relatively robust.
Transcripts from several other sequences gave significant positive correlations (ρ estimates) (Table 1) that were consistent with observations in the transgenic spruce plants, but nearly all of the significant correlations were uniquely observed with those of PgMYB1 and PgMYB8, or uniquely with PgMYB15 and PgMYB14. The transcript levels of PgMYB1 and PgMYB8 were positively correlated in both xylem and bark/phloem, with sequences assigned to phenylpropanoid (HCT and PRR2), shikimate (CM1), and S-adenosylmethionine (MEE58) metabolism, and with sequences not assigned to a particular metabolic process, such as KT2 for xylem and CYP76G1 and HB3 for bark/phloem tissues (Table 1). The observed negative correlations with transcripts of PgMYB1 and PgMYB8 were very weak (non-significant) in both tissue types. In contrast, the positive correlations with transcripts of PgMYB15 and PgMYB14 (to a lesser extent) were only observed in xylem and not in the bark/phloem tissues. These sequences were related to flavonoid (DFR, LDOX, and TT4/CHS), terpenoid (CYP76G1), phenylpropanoid (BGLU40), and cell wall (XTH9) metabolism, as well as toxin catabolism (GST18).
Analyses of transcript accumulation data were expanded to include 10 additional PgMYBs, putatively linked to secondary cell wall deposition and stress response (Bomal et al., 2008; Bedon et al., 2010). Transcript accumulation of the target genes that significantly correlated with PgMYB1, PgMYB8, PgMYB14, and PgMYB15 also correlated with several other PgMYB genes (Supplementary Tables S5, S6 at JXB online): significant correlation estimates were obtained between expression data of PgMYB1/PgMYB8 and those of PgMYB2, PgMYB4, PgMYB16, and PgMYB17 in xylem and bark/phloem tissues, while expression data of PgMYB14/PgMYB15 correlated with those of PgMYB13 in xylem tissues, and PgMYB3, PgMYB5, and PgMYB13 in bark/phloem tissues.
Transactivation assays reveal opposite effects of selected spruce and pine MYBs on candidate target promoters
The transactivation and repression of promoters from two of the candidate target genes (Pg4CL and PgDHS2) were tested for the four different MYBs hypothesized to have opposite effects on expression (see Materials and methods). Upstream flanking sequences were isolated for Pg4CL (1885bp) and PgDHS2 (1353bp), and assayed functionally for gene expression activity by co-transformation in P. glauca cell cultures. Strong evidence for transactivation of the Pg4CL and PgDHS2 promoters was obtained for the spruce gene PgMYB8 and for the pine genes PtMYB1 and PtMYB8 (Table 2). For each of the combinations tested, the GUS activity stain increased from 3- to ≥10-fold, compared with the controls transformed with the promoter construct alone. In contrast, co-transformation with either the spruce gene PgMYB15 or the pine gene PtMYB14 decreased the GUS staining to ≤20% of the level observed in the controls, representing at least a 5-fold decrease (Table 2).
Table 2.
Densitometry analysis of GUS staining in transactivation assays for MYB–promoter interactions
| TF vector | GSa | PgDHS2pro | Pg4CLpro | ||||
|---|---|---|---|---|---|---|---|
| No TFb | TFc | Ratio TF/no TF | No TF | TF | Ratio TF/no TF | ||
| PgMYB8 | Spot | 24 | 223 | 9.29 | 26 | 308 | 11.85 |
| Density | 3254 | 34 590 | 10.63 | 3574 | 45 939 | 12.85 | |
| PgMYB15 | Spot | 24 | 2 | 0.08 | 26 | 0 | 0.00 |
| Density | 2291 | 266 | 0.12 | 3574 | 0 | 0.00 | |
| PtMYB1 | Spot | 113 | 373 | 3.30 | 16 | 85 | 5.31 |
| Density | 17 298 | 63 295 | 3.66 | 2424 | 14 279 | 5.89 | |
| PtMYB8 | Spot | 72 | 191 | 2.65 | 28 | 156 | 5.57 |
| Density | 9672 | 31 545 | 3.26 | 3347 | 18 595 | 5.56 | |
| PtMYB14 | Spot | 168 | 36 | 0.21 | 75 | 11 | 0.15 |
| Density | 24347 | 4858 | 0.20 | 11258 | 1397 | 0.12 | |
a GUS staining (GS), number of blue spots or total density across all four filters (replicates) (see Materials and methods).
b No TF, empty vector control.
c TF, MYB expression vector.
PgMYB8 and PgMYB15 bind to and compete for AC cis-elements present in both Pg4CL and PgDHS2 promoters
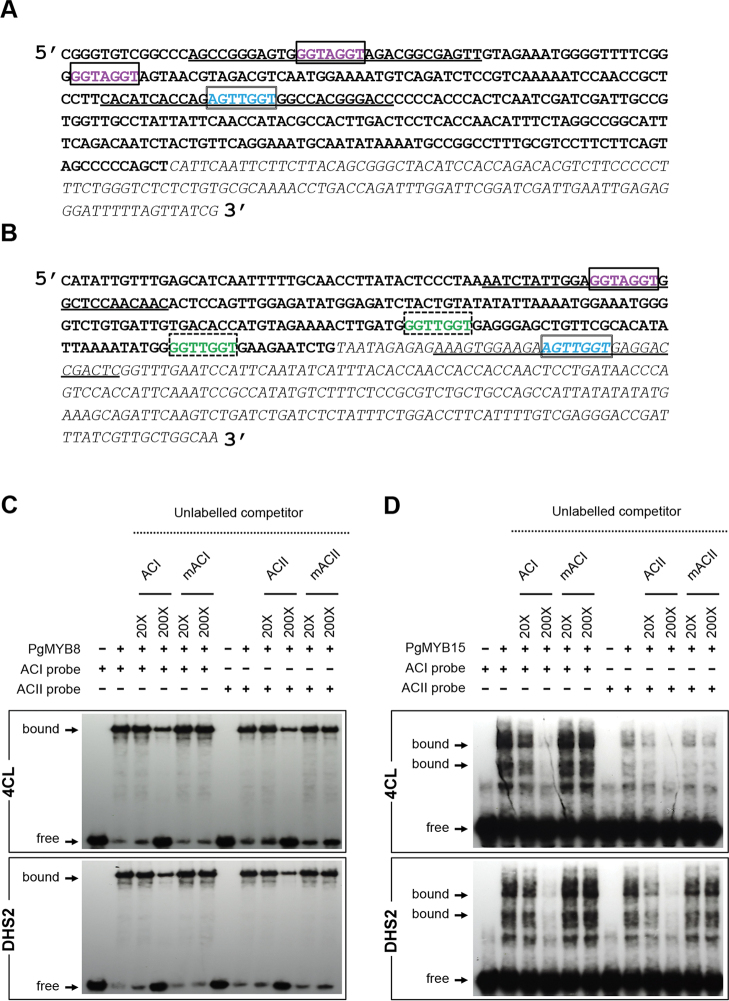
Promoters of several genes of the monolignol and shikimate biosynthetic pathways have been shown to contain AC elements (Patzlaff et al., 2003a , b; Raes et al., 2003; Chen et al., 2006; Prouse and Campbell, 2012). Both AC-I (ACCTACC) and AC-II (ACCAACC/T) sequences were found to be present in the upstream flanking and 5′-untranslated region (UTR) sequences of the Pg4CL and PgDHS2 spruce genes (Fig. 2A, B). An AC-I sequence matching the canonical ACCTACC was found in one and two copies in 5′ upstream flanking sequences of PgDHS2 and Pg4CL genes, respectively; one putative AC-I box from each promoter was targeted for binding assays (Fig. 2A, B). For AC-II, one putative element was located in the 5′ upstream region of the Pg4CL gene (ACCAACT), whereas two copies of a different sequence (ACCAACC) were 5′ upstream of the PgDHS2 gene, and one copy of ACCAACT was in the 5′ UTR (Fig. 2A, B). Aiming to compare the interaction of different MYBs and an AC-II element associated with each gene directly, the conserved sequence (ACCAACT) was targeted in the oligonucleotides despite the location of one of them in a 5′ UTR. To examine whether PgMYB8, and PgMYB15 were able to bind to these putative cis-elements, 30bp oligonucleotide probes (containing AC-I or AC-II elements and flanking regions of either Pg4CL or PgDHS2 promoters; Fig. 2C, D) were used in EMSAs.
Fig. 2.
Analysis of PgMYB8 and PgMYB15 binding to AC elements present in Pg4CL and PgDHS2 promoters in spruce. (A, B) Upstream flanking and 5′ UTR sequences (450bp) of the spruce (A) Pg4CL and (B) PgDHS2 genes. For both Pg4CL and PgDHS2 sequences, underlined nucleotides correspond to the 30bp probes (containing either an AC-I or AC-II element) designed for EMSA with PgMYB8, PgMYB14, and PgMYB15 recombinant proteins. The sequences shown represent the regions in which AC elements were identified in the 4CL promoter (1885bp) and DHS2 promoter (1353bp). Nucleotide representations are as follows: bold in upstream regions; italics in 5′ UTR sequences; AC-I sequences are boxed and AC-II sequences have a double box (ACCAACT) or a dashed box (ACCAACC). (C, D) EMSA testing of the binding of PgMYB8 (C) and PgMYB15 (D) recombinant protein to the labelled AC-I- and AC-II-containing probe [30bp fragments from Pg4CL (top) and PgDHS2 (bottom) promoters; see Fig. 3 for details]. Competition analyses used unlabelled probes containing AC-I, ACII, or mutated AC elements (mAC). Electrophoretic shifts (bound) and free DNA probes are indicated by arrows. (This figure is available in colour at JXB online.)
PgMYB8 and PgMYB15 recombinant proteins were able to bind specifically the AC-I element present in the promoter region of Pg4CL and PgDHS2 genes. A clear mobility shift was obtained for the AC-I-containing probe in the presence of PgMYB8 (Fig. 2C) as well as PgMYB15 (Fig. 2D). Unlabelled DNA oligonucleotides containing AC-I cis-elements in increasing concentrations competed with the binding of PgMYB8 and PgMYB15, while those containing a mutated AC element did not. The EMSA performed with the AC-II elements from both of the promoters indicated a clear mobility shift for PgMYB8 (Fig. 2C). In contrast, weak affinity for the AC-II element in the DHS2 probe was observed with PgMYB15 (Fig. 2D). For PgMYB8 and PgMYB15, competition with unlabelled AC-II probe altered the binding profile, while a AC-II mutated probe did not compete (Fig. 2C, D). No evidence for mobility shift was seen with a Pg4CL AC-II-containing probe and recombinant protein PgMYB15 (Fig. 2D).
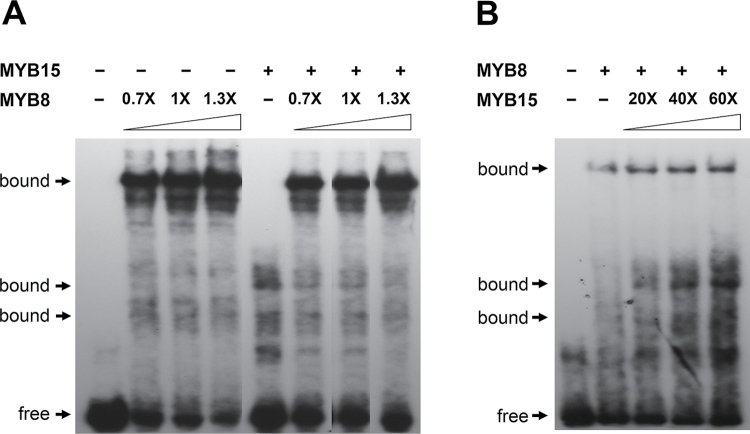
DNA binding site competition analysis for the AC-I-containing probe from Pg4CL indicated that PgMYB8 recombinant protein had a very strong affinity for the AC-I element compared with PgMYB15 (Fig. 3). PgMYB8 effectively out-competed PgMYB15 at a stoichiometric ratio of 0.7 (PgMYB8 to PgMYB15) (Fig. 3A), while a 60-fold amount of PgMYB15 was inefficient to out-compete a constant amount of PgMYB8 (Fig. 3B).
Fig. 3.
DNA binding competition analysis between PgMYB8 and PgMYB15 for an AC-I-containing promoter fragment from Pg4CL. EMSA using MYB8 and MYB15 recombinant proteins and labelled oligonucleotides from Pg4CL with a variable amount of (A) PgMYB8 protein or (B) PgMYB15 protein. Increasing stoichiometric ratios of MYB recombinant protein are indicated. Electrophoretic shifts (bound) and free DNA probes are indicated by arrows. Exposure showing the best resolution of the specific TF–DNA complex was used for each panel.
Discussion
Results from the present study identified a set of target genes encoding enzymes of primary and secondary metabolism that are proposed to be differentially regulated by the two types of R2R3-MYBs in P. glauca. Several of the pathways are interconnected, and flux into each one of them may vary during development or in response to environmental cues. Evidence has been provided that regulation of these genes may involve complex transcriptional control by different MYBs with opposite effects, as well as MYBs with overlapping functions.
Discovery of putative common target genes for conifer MYBs belonging to different subgroups
This report presents comparative analyses of two pairs of functionally redundant conifer R2R3-MYBs from pine (Pt) and spruce (Pg), namely Pt/PgMYB1 and Pt/PgMYB8 of subgroups SG8 and SG13 (Bedon et al., 2007), and Pt/PgMYB14 and Pt/PgMYB15 of subgroup SG4 (Bedon et al., 2010), which were shown to impact different aspects of primary and secondary metabolism. On the one hand, Pt/PgMYB1 and Pt/PgMYB8 were linked to positive control of shikimate and monolignol biosynthetic genes and were proposed to be members of a transcriptional network similar to the SND1 cascade in spruce and pine (Bomal et al., 2008), which regulates secondary cell wall formation in Arabidopsis (Zhong et al., 2007, 2010). On the other hand, Pt/PgMYB14 and Pt/PgMYB15 were identified as putative positive regulators of isoprenoid and flavonoid metabolism, and linked to the regulation of defence responses in spruce and pine (Bedon et al., 2010).
In the present study, a comparative analysis of transcript profiles from constitutive overexpression of PtMYB1, PtMYB8, and PtMYB14 in spruce indicated that MYBs from different subgroups (SG8, SG13, and SG4) may impact a common set of genes. The transcript profiling results also pointed to activation or repression (i.e. up-/down-regulation) depending on the MYB that is overexpressed. Specifically, genes that were co-expressed in PtMYB1- and PtMYB8-overexpressing lines (e.g. up-regulation of shikimate and monolignol genes and down-regulation of flavonoid genes) had opposite expression in PtMYB14-overexpressing lines (Fig. 1). The fact that 95% of the common sequences displayed an opposite expression profile between PtMYB1/PtMYB8 and PtMYB14 (Fig. 1) is unlikely to appear by chance. In addition, pair-wise comparisons between PtMYB1 and PtMYB14, or PtMYB8 and PtMYB14 gave opposite expression profiles for 95% and 89% of the genes, respectively (Supplementary Fig. S1 at JXB online).
It has been shown that constitutive overexpression of TFs may cause pleiotropic effects (Zhang, 2003). In this context, the constitutive accumulation of PtMYBs in transgenic spruce may have sequestrated components of the transcriptional machinery away from cis-regulatory DNA elements by competing with cognate TFs (Gill and Ptashne, 1988). It was observed that the spatio-temporal transcript profiles of PgMYB genes and some of the candidate target genes were poorly correlated during a diurnal cycle, whatever the tissues tested (Table 1), indicating that pleiotropic effects may have affected some genes. Nevertheless, the RNA transcript profiling results were consistent with the proposed opposition effect, in a time-related and tissue-preferential manner, for several sequences in non-transgenic spruce plantlets (Table 1; Supplementary Tables S4, S5 at JXB online). Together, the expression profiles from the transgenics and the diurnal profile suggested that different conifer MYBs (from SG8, SG13, and SG4 subgroups) could directly target genes coding for key enzymes from interconnected pathways of amino acid (DHS2 and SHM4) and phenylpropanoid (4CL and COMT1) metabolism, specifically in differentiating secondary xylem. Another explanation for the identification of a set of common misregulated genes could be linked to indirect effects of PtMYB overexpression, such as non-specific binding to cis-elements by highly conserved DBDs, as observed among conifer MYBs (Bedon et al., 2007). As suggested in Arabidopsis (Jin et al., 2000), structural genes with similar cis-regulatory motifs may be strongly influenced by such a dosage-linked effect and may become coordinately regulated due to high levels of specific regulators. This is likely to appear in gain-of-function experiments where abnormally high levels of MYBs are induced by overexpression. In the transgenic spruce used here, PtMYB1, PtMYB8, and PtMYB14 transcript levels represented 33-, 40-, and 300-fold increases compared with their spruce endogenous counterparts PgMYB1, PgMYB8, and PgMYB14, respectively (Bomal et al., 2008; Bedon et al., 2010). However, these latter PgMYB transcripts showed a 3- to 5-fold variation in non-transgenic plants during the diurnal cycle. Under such circumstances, unspecific binding due to high levels of MYBs would be unexpected.
Competition between MYBs from different subgroups for the regulation of shikimate and phenylpropanoid biosynthesis genes in conifers
It has been shown here that conifer R2R3-MYBs from different subgroups could have direct and opposite effects on the regulation of two pivotal genes encoding enzymes of the shikimate and monolignol biosynthetic pathways: DHS2 (3-deoxy-d-arabino-heptulosonate 7-phosphate synthase), which provides precursors for phenylpropanoid and flavonoid metabolism (Amthor, 2003), and 4CL (4-coumarate:CoA ligase), which converts 4-coumaric acid and other substituted cinnamic acids for use in the biosynthesis of phenolic compounds and the production of flavonoids and monolignols (Ehlting et al., 1999; Amthor, 2003). Transactivation assays and EMSAs provided two lines of evidence, in addition to expression data that support this conclusion. First, transactivation assays revealed strong activation of the Pg4CL and PgDHS2 promoters for the spruce gene PgMYB8 of SG13 and a repression effect of PgMYB15 (SG4) on both promoters. The closest pine homologues (PtMYB1, PtMYB8, and PtMYB14) showed similar activation and repression effects. Secondly, PgMYB8 (SG13) as well as PgMYB14 and PgMYB15 (SG4) recombinant proteins are able to bind the same cis-element (AC-I) in the promoter region of these two genes. In Arabidopsis, typical AC cis-elements, also called MYB recognition elements (MREs) (Feldbrügge et al., 1997), were found in the promoters of the majority of shikimate, flavonoid, and monolignol biosynthetic genes (Raes et al., 2003; Hartmann et al., 2005; Chen et al., 2006; Prouse and Campbell, 2012).
Two types of AC elements, AC-I (ACCTACC) and AC-II (ACCAACC/T), were identified in the promoter region of both the PgDHS2 and Pg4CL genes in spruce. EMSA analyses revealed that PgMYB8 (SG13), PgMYB14 (SG4), and PgMYB15 (SG4) recombinant proteins are able to bind the AC-I element in both of the promoter environments tested, suggesting a lack of MYB subgroup specificity for this element. Conversely, the PgMYB8 recombinant protein showed a strong affinity for the AC-II element while PgMYB14 and PgMYB15 showed no or weak affinity, respectively. The position of AC-II elements varied between the two genes (either 5′ upstream flanking or in the putative 5′ UTR) but only had a small impact on the binding results. The extended affinity of conifer MYB for different AC elements (AC-I, AC-II, and AC-III) has been previously reported for PtMYB1 (Patzlaff et al., 2003a , b) and PtMYB4 (Gómez-Maldonado et al., 2004; Morse et al., 2009). Interestingly, recent work in Pinus pinaster (Pp) has also shown that PpMYB8 could bind an AC-II element in the promoter region of the Prephenate AminoTransferase (PAT) gene from the arogenate pathway (Craven-Bartle et al., 2013). Thus, the affinity of spruce PgMYB8, PgMYB14, and PgMYB15 for AC elements in promoters of other genes of shikimate, arogenate, and phenylalanine metabolism should be further explored and extended to PgMYB from different subgroups.
The idea that a single target gene may be under the control of functionally redundant MYBs from the same subgroup is supported by increasing evidence (see Dubos et al., 2010; Zhong et al., 2010). In Arabidopsis, AtMYB58 and AtMYB63 (both of the SG3 subgroup) directly activated phenylpropanoid biosynthetic genes from PAL to CAD (Zhou et al., 2009). Similarly, AtMYB11, AtMYB12, and AtMYB111 (all of the SG7 subgroup) activated the transcription of target genes from CHALCONE SYNTHASE (CHS) to FLAVONOL SYNTHASE (FLS) genes from flavonoid biosynthetic pathways (Stracke et al., 2007). Redundancy in repression has also been observed for Antirrhinum AmMYB308 and AmMYB330 for the genes between C4H and CAD from the phenylpropanoid and monolignol biosynthetic pathways (Tamagnone et al., 1998). In the conifers spruce and pine, PgMYB1, PgMYB8, and PgMYB14/PgMYB15 are phylogenetically distinct but share conserved DBDs (Bedon et al., 2007, 2010). Therefore, their ability to compete for cis-elements via conserved DBDs is not unexpected. The affinity for AC-I cis-elements observed for PgMYB8 and PgMYB14/PgMYB15 is congruent with this explanation and might be the basis of competition among MYBs on the same target. However, DNA binding site competition analysis revealed a strong propensity of PgMYB8 recombinant protein to bind the AC-I element when competing with PgMYB15 (Fig. 3). Although other methods (such as quantitative assay in yeast or surface plasmon resonance) should be used to ascertain this latter result fully, it suggests that particular tissular and/or environmental (nutrition, stress) conditions may be needed for PgMYB15 to compete more strongly with PgMYB8. A signature motif similar to the basic helix–loop–helix (bHLH) interaction site has been identified in PgMYB14 and PgMYB15 peptide sequences (Bedon et al., 2010). The bHLH motif may entail interactions with other proteins, such as bHLH and WD40, which have been identified as important MYB partners in flavonoid pathways in different plant species (Vom Endt et al., 2002; Ramsay and Glover, 2005). In addition, the conifer MYBs from the present study vary in their C-termini, which typically harbour the activation and repression domains (Dubos et al., 2010). According to Bedon et al. (2010), PgMYB14 and PgMYB15 harbour the C2 repressor motif containing the core EAR motif defined by Kranz et al. (1998). Interestingly, the E. grandiis EgMYB1 sequence was shown to be very close to Pt/Pg MYB14/15 (Bedon et al., 2007, 2010) and was shown to act as a repressor of lignin biosynthesis (Legay et al., 2010). The differential regulatory effect of these PgMYBs on the same target is likely to be due to the presence of activation or repression domains, but complementary functional studies are needed to demonstrate whether PgMYB14 and PgMYB15 may act as active or passive repressors.
MYBs as competing activators and repressors within a single transcriptional network for the control of primary and secondary metabolism
Several R2R3-MYBs have been described as regulators of specific aspects of primary or secondary plant metabolism, such as flavonoid, anthocyanin, monolignol, benzenoid, isoprenoid, shikimate, or glucosinolate biosynthesis (see Dubos et al., 2010; Feller et al., 2011). Their role has generally been associated with one single pathway, with an activation or repression function within the transcriptional network. However, studies in maize and Arabidopsis pointed out the dual activation/repression effect of R2R3-MYBs on genes from different pathways that compete for carbon flux. Zea mays ZmMYB31 was overexpressed in Arabidopsis and shown to down-regulate several genes involved in monolignol biosynthesis but also to up-regulate some flavonoid biosynthesis genes (Fornalé et al., 2010). The authors proposed that ZmMYB31 might play an important role in carbon partitioning between these pathways. Similarly, Arabidopsis AtMYB75 was shown to regulate anthocyanin biosynthesis positively in early development and to regulate the accumulation of lignin negatively in older plants (Bhargava et al., 2010). A similar dual effect on monolignol and anthocyanin biosynthetic pathways was revealed in the atmyb32 mutant that had an increased level of COMT1 transcripts and decreased amounts of DFR and ANS transcripts compared with the wild type (Preston et al., 2004).
Evolution of R2R3-MYBs and plant transcriptional networks
The present study identified conifer MYBs that could act on different aspects of primary and secondary metabolism by modulating the expression of key target genes from interconnecting pathways (Fig. 4). These MYBs belong to different subgroups and have opposite effects on two pivotal genes in the shikimate and monolignol biosynthetic pathways. As proposed in Fig. 4, PgMYB15 could thus impact carbon flux dedicated to monolignol synthesis by competing negatively for promoter binding sites with PgMYB8. This repression could simultanously affect gene expression of SHM4 (carbohydrate metabolism level), DHS2 (shikimate level), as well as 4CL and COMT (monolignol level). Due to its repressive action, PgMYB15 could indirectly stimulate flavonoid and/or anthocyanin metabolism. In addition, the co-expression patterns of several other MYBs tested in the system used here indicate that regulation in these pathways may be extended to include other MYBs as well (see Supplementary Tables S5, S6 at JXB online).
Fig. 4.
Proposed action of MYBs in the regulatory network controlling primary and secondary metabolism in conifers. An antagonistic action of PgMYB8 (activation) and PgMYB15 (repression) on interconnecting metabolic pathways is underlined (in bold): DHS2, 4CL, SHM4, and COMT are identified (boxed) as target genes under the control of both MYBs. Dashed lines indicate putative indirect effects of PgMYB15 on flavonoid and anthocyanin metabolic pathways. The network is also thought to involve PgMYB1 and PgMYB14, among others, which have not yet been tested in promoter binding assays but produce similar results as PgMYB8 (activation) and PgMYB15 (repression) in co-transformation assays, respectively. Genes that may fall under the antagonistic action of other MYBs are in parentheses. PHE, phenylalanine; TYR, tyrosine; TRP, tryptophan. (This figure is available in colour at JXB online.)
Evidence was previously obtained of partial redundancy among two R2R3-MYBs from P. taeda, PtMYB1 and PtMYB8, involved in monolignol regulation, and it was proposed that spruce and pine MYB8 could cross-regulate other R2R3-MYBs (Bomal et al., 2008), but comparative data have been lacking. A genome-wide phylogenetic investigation of plants indicated that most families of TFs have expanded very significantly in flowering plants compared with Bryophyta/Lycopodiophyta, typically doubling in size around the time of their radiation (Lang et al., 2010). Gymnosperms were not included in the study of Lang et al. (2010) but, as a representative of the gymnosperm lineage, P. glauca was estimated to have fewer MYBs than angiosperms: a total of 120 R2R3-MYBs, representing 0.43% of the catalogue of 27 720 unique expressed sequences (Rigault et al., 2011), compared with 0.96% of the annotated genes for both Arabidopsis and poplar. However, at least one subfamily of R2R3-MYBs (e.g. the SG4 subgroup) was shown to be larger in conifers than in most angiosperms (Bedon et al., 2010). More thorough investigations of the MYB family evolution in conifers to confirm their divergence with angiosperms may now be undertaken with the recent availability of the P. glauca genome (Birol et al., 2013) and the P. abies genome (Nystedt et al., 2013), although tracing their evolution will need a broader analysis. In turn, functional relationships between MYBs are likely to be closely linked to gene family evolution. The present findings in P. glauca R2R3-MYBs indicate that functional redundancy and antagonistic effects among R2R3-MYBs may be more widespread among seed-bearing plant taxa than previously shown.
In conclusion, it is proposed that MYBs from the present study are part of a transcriptional network covering complementary aspects of primary and secondary metabolism in conifer trees, as illustrated in Fig. 4. Within this network, MYBs may act as competing activators and/or repressors of key target genes from interconnecting biosynthetic pathways. This dual mode of action of MYB proteins may confer a way for plants to switch their metabolism in response to environmental cues, resulting in phenotypic plasticity. Gene duplication is considered as an important source of material for evolutionary novelties (Lynch and Conery, 2000) and has been evoked as an important mechanism leading to new functions for R2R3-MYB genes (Grotewold, 2005; Feller et al., 2011). Monophyletic gene family amplification, suggested for conifer R2R3-MYB genes from subgroup SG4 (Bedon et al., 2010), could be the basis of functional compensation needed to preserve the stoichiometric relationship between activators and repressors within a transcriptional network.
Supplementary data
Supplementary data are available at JXB online.
Figure S1. Comparative analysis of microarray data associated with Pinus taeda MYB overexpression in spruce.
Table S1. Cross-comparison between 9K microarray expression data obtained from wild type (WT) versus PtMYB1, PtMYB8, and PtMYB14 overexpressors.
Table S2. Predicted annotations and KEGG functional categorization of co-expressed sequences common to PtMYB1, PtMYB8, and PtMYB14 overexpressors.
Table S3. Primer sequences used for quantitative PCR analysis, molecular cloning, and EMSAs.
Table S4. Transcript accumulation of PgMYB, and putative target genes in secondary xylem and bark/phloem tissues during a diurnal cycle of 3-year-old spruce.
Table S5. Spearman’s rank test correlation coefficients for pair-wise comparison between expression data of PgMYB and putative target genes in secondary xylem tissue of 3-year-old spruce.
Table S6. Spearman’s rank test correlation coefficients for pair-wise comparison between expression data of PgMYB and putative target genes in bark/phloem tissue of 3-year-old spruce.
Table S7. Densitometry data of GUS staining in transactivation assays for MYB–promoter interactions.
Acknowledgements
The authors are grateful to Denis Lachance, Marie-Josée Morency, and Gervais Pelletier for technical assistance with transactivation assays and Pamela Cheers for editorial work. Funding was received from Genome Canada and Génome Québec to JM and AS for the Arborea project and from the NSERC to JM.
References
- Amthor J. 2003. Efficiency of lignin biosynthesis: a quantitative analysis. Annals of Botany 91, 673–695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedon F, Levasseur C, Grima-Pettenati J, Séguin A, MacKay J. 2009. Sequence analysis and functional characterization of the promoter of the Picea glauca Cinnamyl Alcohol Dehydrogenase gene in transgenic white spruce plants. Plant Cell Reports 28, 787–800 [DOI] [PubMed] [Google Scholar]
- Bedon F, Grima-Pettenati J, MacKay J. 2007. Conifer R2R3-MYB transcription factors: sequence analyses and gene expression in wood-forming tissues of white spruce (Picea glauca). BMC Plant Biology 7, 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedon F, Bomal C, Caron S, et al. 2010. Subgroup 4 R2R3-MYBs in conifer trees: gene family expansion and contribution to the isoprenoid- and flavonoid-oriented responses. Journal of Experimental Botany 61, 3847–3864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhargava A, Mansfield SD, Hall HC, Douglas CJ, Ellis BE. 2010. MYB75 functions in regulation of secondary cell wall formation in the Arabidopsis inflorescence stem. Plant Physiology 154, 1428–1438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birol I, Raymond A, Jackman SD, et al. 2013. Assembling the 20 Gb white spruce (Picea glauca) genome from whole-genome shotgun sequencing data. Bioinformatics 29, 1492–1497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bomal C, Bedon F, Caron S, et al. 2008. Involvement of Pinus taeda MYB1 and MYB8 in phenylpropanoid metabolism and secondary cell wall biogenesis: a comparative in planta analysis. Journal of Experimental Botany 59, 3925–3939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borevitz JO, Xia Y, Blount J, Dixon RA, Lamb C. 2000. Activation tagging identifies a conserved MYB regulator of phenylpropanoid biosynthesis. The Plant Cell 12, 2383–2394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle B, Dallaire N, MacKay J. 2009. Evaluation of the impact of single nucleotide polymorphisms and primer mismatches on quantitative PCR. BMC Biotechnology 9, 75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craven-Bartle B, Pascual MB, Cánovas F, Ávila C. 2013. A Myb transcription factor regulates genes of the phenylalanine pathway in maritime pine. The Plant Journal 74, 755–766 [DOI] [PubMed] [Google Scholar]
- Chen Y, Zhang X, Wu W, Chen Z, Gu H, Qu L-J. 2006. Overexpression of the wounding-responsive gene AtMYB15 activates the shikimate pathway in Arabidopsis. Journal of International Plant Biology 48, 1084–1095 [Google Scholar]
- Dubos C, Le Gourrierec J, Baudry A, et al. 2008. MYBL2 is a new regulator of flavonoid biosynthesis in Arabidopsis thaliana . The Plant Journal 55, 940–953 [DOI] [PubMed] [Google Scholar]
- Dubos C, Stracke R, Grotewold E, Weisshaar B, Martin C, Lepiniec L. 2010. MYB transcription factors in Arabidopsis. Trends in Plant Science 15, 573–581 [DOI] [PubMed] [Google Scholar]
- Ehlting J, Büttner D, Wang Q, Douglas CJ, Somssich IE, Kombrink E. 1999. Three 4-coumarate:coenzyme A ligases in Arabidopsis thaliana represent two evolutionarily divergent classes in angiosperms. The Plant Journal 19, 9–20 [DOI] [PubMed] [Google Scholar]
- Feldbrügge M, Sprenger M, Hahlbrock K, Weisshaar B. 1997. PcMYB1, a novel plant protein containing a DNA-binding domain with one MYB repeat, interacts in vivo with a light-regulatory promoter unit. The Plant Journal 11, 1079–1093 [DOI] [PubMed] [Google Scholar]
- Feller A, Machemer K, Braun EL, Grotewold E. 2011. Evolutionary and comparative analysis of MYB and bHLH plant transcription factors. The Plant Journal 66, 94–116 [DOI] [PubMed] [Google Scholar]
- Fornalé S, Shi X, Chai C, et al. 2010. ZmMYB31 directly represses maize lignin genes and redirects the phenylpropanoid metabolic flux. The Plant Journal 64, 633–644 [DOI] [PubMed] [Google Scholar]
- Gill G, Ptashne M. 1988. Negative effect of the transcriptional activator GAL4. Nature 334, 721–724 [DOI] [PubMed] [Google Scholar]
- Goicoechea M, Lacombe E, Legay S, et al. 2005. EgMYB2, a new transcriptional activator from Eucalyptus xylem, regulates secondary cell wall formation and lignin biosynthesis. The Plant Journal 43, 553–567 [DOI] [PubMed] [Google Scholar]
- Gómez-Maldonado J, Ávila C, Torre F, Canas R, Cánovas FM, Campbell MM. 2004. Functional interactions between a glutamine synthetase promoter and MYB proteins. The Plant Journal 39, 513–526 [DOI] [PubMed] [Google Scholar]
- Grotewold E. 2005. Plant metabolic diversity: a regulatory perspective. Trends in Plant Science 10, 57–62 [DOI] [PubMed] [Google Scholar]
- Haneskog L. 2006. Preparation of denatured protein samples from cell paste or cell extract. Cold Spring Harbor Protocols 10.1101/pdb.prot4266 [DOI] [PubMed] [Google Scholar]
- Harmer SL, Hogenesch JB, Straume M, Chang H-S, Han B, Zhu T, Wang X, Kreps JA, Kay SA. 2000. Orchestrated transcription of key pathways in Arabidopsis by the circadian clock. Science 290, 2110–2113 [DOI] [PubMed] [Google Scholar]
- Hartmann U, Sagasser M, Mehrtens F, Stracke R, Weisshaar B. 2005. Differential combinatorial interactions of cis-acting elements recognized by R2R3-MYB, BZIP, and BHLH factors control light-responsive and tissue-specific activation of phenylpropanoid biosynthesis genes. Plant Molecular Biology 57, 155–171 [DOI] [PubMed] [Google Scholar]
- Ihaka R, Gentleman R. 1996. R: a language for data analysis and graphics. Journal of Computational and Graphical Statistics 5, 299–314 [Google Scholar]
- Jin H, Cominelli E, Bailey P, Parr A, Mehrtens F, Jones J, Tonelli C, Weisshaar B, Martin C. 2000. Transcriptional repression by AtMYB4 controls production of UV-protecting sunscreens in Arabidopsis . The EMBO Journal 19, 6150–6161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klimaszewska K, Rutledge RG, Séguin A. 2004. Genetic transformation of conifers utilizing somatic embryogenesis. Methods in Molecular Biology 286, 151–164 [DOI] [PubMed] [Google Scholar]
- Kranz HD, Denekamp M, Greco R, et al. 1998. Towards functional characterization of the members of the R2R3-MYB gene family from Arabidopsis thaliana . The Plant Journal 16, 263–276 [DOI] [PubMed] [Google Scholar]
- Lang D, Weiche B, Timmerhaus G, Richardt S, Riano-Pachon DM, Correa LGG, Reski R, Mueller-Roeber B, Rensing SA. 2010. Genome-wide phylogenetic comparative analysis of plant transcriptional regulation: a timeline of loss, gain, expansion, and correlation with complexity. Genome Biology and Evolution 2, 488–503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legay S, Lacombe E, Goicoechea M, Brière C, Séguin A, MacKay J, Grima-Pettenati J. 2007. Molecular characterization of EgMYB1, a putative transcriptional repressor of the lignin biosynthetic pathway. Plant Science 173, 542–549 [Google Scholar]
- Legay S, Sivadon P, Blervacq A-S, et al. 2010. Egmyb1, an R2R3-MYB transcription factor from eucalyptus, negatively regulates secondary cell wall formation in transgenic arabidopsis and poplar. New Phytologist 188, 774–786 [DOI] [PubMed] [Google Scholar]
- Lynch M, Conery JS. 2000. The evolutionary fate and consequences of duplicate genes. Science 290, 1151–1155 [DOI] [PubMed] [Google Scholar]
- Morse AM, Whetten RW, Dubos C, Campbell MM. 2009. Post-transcriptional modification of an R2R3-MYB transcription factor by a MAP kinase during xylem development. New Phytologist 183, 1001–1013 [DOI] [PubMed] [Google Scholar]
- Néron B, Ménager H, Maufrais C, Joly N, Maupetit J, Letort S, Carrere S, Tuffery P, Letondal C. 2009. Mobyle: a new full web bioinformatics framework. Bioinformatics 25, 3005–3011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nystedt N, Street NR, Wetterbom A, et al. 2013. The Norway spruce genome and conifer genome evolution. Nature 497, 579–584 [DOI] [PubMed] [Google Scholar]
- Ohta M, Matsui K, Hiratsu K, Shinshi H, Ohme-Takagi M. 2001. Repression domains of class II ERF transcriptional repressors share an essential motif for active repression. The Plant Cell 13, 1959–1968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patzlaff A, McInnis S, Courtenay A, et al. 2003a. Characterisation of a pine MYB that regulates lignification. The Plant Journal 36, 743–754 [DOI] [PubMed] [Google Scholar]
- Patzlaff A, Newman LJ, Dubos C, Whetten RW, Smith C, McInnis S, Bevan MW, Sederoff RR, Campbell MM. 2003b. Characterisation of PtMYB1, an R2R3-MYB from pine xylem. Plant Molecular Biology 53, 597–608 [DOI] [PubMed] [Google Scholar]
- Pavy N, Boyle B, Nelson C, et al. 2008. Identification of conserved core xylem gene sets: conifer cDNA microarray development, transcript profiling and computational analyses. New Phytologist 180, 766–786 [DOI] [PubMed] [Google Scholar]
- Preston J, Wheeler J, Heazlewood J, Li SF, Parish RW. 2004. AtMYB32 is required for normal pollen development in Arabidopsis thaliana . The Plant Journal 40, 979–995 [DOI] [PubMed] [Google Scholar]
- Prouse MB, Campbell MM. 2012. The interaction between MYB proteins and their target DNA binding sites. Biochimica et Biophysica Acta 1819, 67–77 [DOI] [PubMed] [Google Scholar]
- Raes J, Rohde A, Christensen JH, van der Peer Y, Boerjan W. 2003. Genome-wide characterization of the lignification toolbox in Arabidopsis. Plant Physiology 133, 1051–1071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsay NA, Glover BJ. 2005. MYB–bHLH–WD40 protein complex and the evolution of cellular diversity. Trends in Plant Science 10, 63–70 [DOI] [PubMed] [Google Scholar]
- Rigault P, Boyle B, Lepage P, Cooke JEK, Bousquet J, MacKay J. 2011. A white spruce gene catalog for conifer genome analyses. Plant Physiology 157, 14–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutledge RG, Stewart D. 2008. A kinetic-based sigmoidal model for the polymerase chain reaction and its application to high-capacity absolute quantitative real-time PCR. BMC Biotechnology 8, 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steele RGD, Torrie JH, Dickey DA. 1980. Principles and procedures of statistics: a biometrical approach. New York: McGraw-Hill [Google Scholar]
- Stracke R, Weber M, Weisshaar B. 2001. The R2R3-MYB gene family in Arabidopsis thaliana . Current Opinion in Plant Biology 4, 447–456 [DOI] [PubMed] [Google Scholar]
- Stracke R, Ishihara H, Huep G, Barsch A, Mehrtens F, Niehaus K, Weisshaar B. 2007. Differential regulation of closely related R2R3-MYB transcription factors controls flavonol accumulation in different parts of the Arabidopsis thaliana seedling. The Plant Journal 50, 660–677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamagnone L, Merida A, Parr A, Mackay S, Culianez-Macia FA, Roberts K, Martin C. 1998. The AmMYB308 and AmMYB330 transcription factors from Antirrhinum regulate phenylpropanoid and lignin biosynthesis in transgenic tobacco. The Plant Cell 10, 135–154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, Speleman F. 2002. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biology 3, research0034.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voinnet O, Rivas S, Mestre P, Baulcombe D. 2003. An enhanced transient expression system in plants based on suppression of gene silencing by the p19 protein of tomato bushy stunt virus. The Plant Journal 33, 949–956 [DOI] [PubMed] [Google Scholar]
- Vom Endt D, Kijne JW, Memelink J. 2002. Transcription factors controlling plant secondary metabolism: what regulates the regulators? Phytochemistry 61, 107–114 [DOI] [PubMed] [Google Scholar]
- Walley JW, Coughlan S, Hudson ME, Covington MF, Kaspi R, Banu G, Harmer SL, Dehesh K. 2007. Mechanical stress induces biotic and abiotic stress responses via a novel cis-element. PLoS Genetics 3, e172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang JZ. 2003. Overexpression analysis of plant transcription factors. Current Opinion in Plant Biology 6, 430–440 [DOI] [PubMed] [Google Scholar]
- Zhao Q, Dixon RA. 2011. Transcriptional networks for lignin biosynthesis: more complex than we thought? Trends in Plant Science 16, 227–233 [DOI] [PubMed] [Google Scholar]
- Zhong R, Richardson EA, Ye Z-H. 2007. The MYB46 transcription factor is a direct target of SND1 and regulates secondary wall biosynthesis in Arabidopsis. The Plant Cell 19, 2776–2792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong R, Lee C, Ye Z-H. 2010. Evolutionary conservation of the transcriptional network regulating secondary cell wall biosynthesis. Trends in Plant Science 15, 625–632 [DOI] [PubMed] [Google Scholar]
- Zhou J, Lee C, Zhong R, Ye Z-H. 2009. MYB58 and MYB63 are transcriptional activators of the lignin biosynthetic pathway during secondary cell wall formation in Arabidopsis . The Plant Cell 21, 248–266 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.