Abstract
H2O2 and mitogen-activated protein kinase (MAPK) cascades play important functions in plant stress responses, but their roles in acclimation response remain unclear. This study examined the functions of H2O2 and MPK1/2 in acclimation-induced cross-tolerance in tomato plants. Mild cold, paraquat, and drought as acclimation stimuli enhanced tolerance to more severe subsequent chilling, photooxidative, and drought stresses. Acclimation-induced cross-tolerance was associated with increased transcript levels of RBOH1 and stress- and defence-related genes, elevated apoplastic H2O2 accumulation, increased activity of NADPH oxidase and antioxidant enzymes, reduced glutathione redox state, and activation of MPK1/2 in tomato. Virus-induced gene silencing of RBOH1, MPK1, and MPK2 or MPK1/2 all compromised acclimation-induced cross-tolerance and associated stress responses. Taken together, these results strongly suggest that acclimation-induced cross-tolerance is largely attributed to RBOH1-dependent H2O2 production at the apoplast, which may subsequently activate MPK1/2 to induce stress responses.
Key words: Cross-tolerance, hydrogen peroxide, mitogen-activated protein kinase, reactive oxygen species, Respiratory burst oxidase homologue 1, signal transduction, Solanum lycopersicum.
Introduction
Plants are often exposed to various unfavourable environmental stresses (i.e. extreme temperatures, drought, salt, fungi, bacteria, and herbicides) throughout their life cycles. To survive against stresses, plants have intricate defence mechanisms to increase their tolerance. Acclimation is the process in which an individual organism adjusts to a gradual change in its environment (such as a change in temperature, humidity, or photoperiod), allowing it to maintain performance across a range of environmental conditions. Recent studies have also revealed that there exists a kind of adaptation mechanism called cross-tolerance, whereby plants tolerant to one stress are often tolerant to a range of other stresses (Pastori and Foyer, 2002; Capiati et al., 2006; Suzuki et al., 2012). Increased anoxia tolerance was reported in Arabidopsis plants in response to heat (Banti et al., 2008), while NaCl and wounding induced resistance to UV-B in barley and salt tolerance in tomato plants (Capiati et al., 2006; Carkirlar et al., 2008). While the capacity to acclimate to novel environments has been well documented in different plant species, very little is still known about the in-depth mechanisms of such acclimation in plants.
Cold acclimation has been most studied in terms of the physiological and molecular mechanisms in plants. At the metabolic level, acclimation induced accumulation of osmolytes, cryoprotectants, and abscisic acid (ABA) and production of compatible solutes (e.g. proline, raffinose, and glycine betaine) to stresses such as low non-freezing temperatures and moderate light (Browse and Xin, 2001). Acclimation is also able to stabilize proteins and cellular structures and to maintain cell turgor by osmotic adjustment and cellular redox balance (Janska et al., 2010). At the molecular level, secondary messengers such as cytosolic Ca2+, nitric oxide (NO), ABA, and reactive oxygen species (ROS) such as hydrogen peroxide (H2O2) are found to be involved in the perception and transduction of low temperature signal to trigger cold acclimation-induced changes in physiological processes (Zhao et al., 2009; Janska et al., 2010; Zhou et al., 2012). Foliar application of these chemicals increased the tolerance to an array of stresses such as drought, salt, and extreme temperature. For example, H2O2 enhanced the transcription of a subset of stress-responsive genes and the antioxidant capacity of cells by increasing the activities of antioxidant enzymes, such as superoxide dismutase (SOD), ascorbate peroxidase (APX), catalase (CAT), and glutathione reductase (GR), and the biosynthesis of non-enzymic antioxidants such as ascorbic acid and glutathione with an increase in the ratio of reduced glutathione (GSH) to oxidized glutathione (GSSG) (Thannickal and Fanburg, 2000; Jiang et al., 2012). Maintaining the redox homeostasis is a prerequisite for the development of tolerance against both biotic and abiotic stresses (Foyer et al., 1997; Mou et al., 2003; Jiang et al., 2012). ROS, especially H2O2 generated by NADPH oxidases encoded by Respiratory Burst Oxidase Homologue (RBOH) genes play important roles in plant responses to biotic and abiotic stresses (Torres et al., 2002; Kwak et al., 2003; Yoshioka et al., 2003; Torres and Dangl, 2005; Marino et al., 2012). In Arabidopsis, there are increased transcript levels of rbohD and rbohE and ROS accumulation in response to infection with virulent Pseudomonas syringae pv. tomato DC3000, and these responses were greatly compromised in rbohD and rbohE mutants (Torres et al., 2002; Kwak et al., 2003). Similarly, silencing RBOHA and RBOHB in Nicotiana benthamiana plants reduced ROS production and compromised resistance to Phytophthora infestans (Yoshioka et al., 2003). Meanwhile, ROS, NO, cytosolic Ca2+, and plant hormones such as ABA and brassinosteroids (BRs) crosstalk in stress responses (Dempsey and Klessig, 1995; Desikan et al., 2004; Wendehenne et al., 2004; Xia et al., 2009; Cui et al., 2011). For example, H2O2 cooperates with NO in plant HR/cell death and abiotic stresses (Wendehenne et al., 2004; Cui et al., 2011) and plays a critical role in BR-induced stress tolerance (Xia et al., 2009). Expression of RBOHs is also regulated by plant hormones such as ABA and BRs. Elevation of ABA and BR levels resulted in increased production of H2O2 via RBOHs together with increased tolerance against a subset of stresses (Xia et al., 2009; Zhang et al., 2009). A major contributor to induced ROS production for RBOHs may act as converging regulators in the orchestration of plant adaptation to environmental stresses (Marino et al., 2012). However, there has been no genetic evidence to show that RBOHs are involved in acclimation-induced cross-tolerance.
The mitogen-activated protein kinase (MAPK) cascade, minimally composed of a MAPK kinase kinase, MAPK kinase, and a MAPK, is one of the major pathways by which extracellular stimuli are transduced into intracellular signals in plant stress responses (Tena et al., 2001; Zhang and Klessig, 2001). For example, wounding induced increased activation of MAPKs and systemic response to insect attack in tomato leaves (Stratmann and Ryan, 1997). For its involvement in plant signal transduction in response to biotic and abiotic stresses, MAPK signalling also interacts with ROS, NO, and ABA signalling pathways (Lu et al., 2002; Samuel and Ellis, 2002; Mittler et al., 2004; Pitzschke et al., 2009). BR- and ABA-induced apoplastic H2O2 could activate MAPKs in plants, leading to enhanced antioxidant defence system in leaves of tomato and maize (Lin et al., 2009; Nie et al., 2013). On the other hand, NO and NADPH oxidase-dependent oxidative bursts are also regulated by MAPK signals in N. benthamiana and tomato plants (Yoshioka et al., 2003; Asai et al., 2008; Nie et al., 2013). It is, therefore, quite plausible that the MAPK cascade is also involved in acclimation-induced cross-responses to multiple stresses.
Previously, Zhou et al. (2012) reported the potential role of H2O2 elevation in cold acclimation in tomato plants. To obtain insights into the signalling events in acclimation-induced abiotic stress cross-tolerance in tomato, the current study investigated whether pretreatment of one type of mild stress induces acclimation to multiple types of subsequent, more severe, stress treatments and if so, how the cross-acclimation is related to apoplastic H2O2 accumulation and expression of RBOH1, the tomato homologue of AtRbohF and NtRbohA, which play a critical role in both stress and adaptation responses in Arabidopsis and tobacco, respectively (Kwak et al., 2003; Yoshioka et al., 2003). This work also examined how cross-acclimation and the associated stress response were affected by silencing of RBOH1 and MPK1 and 2. The results have provided strong evidence that apoplastic H2O2 and RBOH1-mediated MPK1/2 activation plays a critical role in induction of cross-acclimation to abiotic stresses in tomato plants.
Materials and methods
Plant materials, virus-induced gene silencing constructs, and Agrobacterium-mediated virus infection
Tomato (Solanum lycopersicum L. cv. Condine Red) seeds were germinated in a growth medium filled with a mixture of peat and vermiculite (7:3, v/v) in trays in a growth chamber. When the first true leaf was fully expanded, seedlings were transplanted into plastic pots (15cm diameter and 15cm depth, one seedling per pot) containing the same medium and were watered daily with Hoagland’s nutrient solution. The growth conditions were as follows: a 14/10 light/dark cycle, 25/20 °C, and photosynthetic photon flux density (PPFD) 600 μmol m–2 s–1.
The tobacco rattle virus (pTRV) virus-induced gene silencing (VIGS) constructs used for silencing of tomato RBOH1 genes was generated by cloning a 311-bp RBOH1 cDNA fragment, which was amplified using the forward (5′-ATACGCGAGCTCAAGAATGGGGTTGATATTGT-3′) and reverse (5′-ATACCGCTCGAGCTCTGACTTATTCCTTAC-3′) primers according to Liu et al. (2002). The amplified fragment was digested with SacI and XhoI and ligated into the same sites of pTRV2. The resulting plasmid was transformed into Agrobacterium tumefaciens GV3101. The pTRV-MPK1, pTRV-MPK2, and cosilencing pTRV-MPK1/2 VIGS constructs were generated as described previously (Kandoth et al., 2007). Agrobacterium-mediated virus infection was performed as previously described (Ekengren et al., 2003). Plants were then kept at 23/21 °C under 125 μmol m–2 s–1 PPFD for 30 d before they were used. Leaflets in the middle of the fifth fully expanded leaves, which showed 20–30% transcript levels of control plants, were used. The expression of MPK1 and MPK2 in pTRV-MPK1, pTRV-MPK2, and pTRV-MPK1/2 plants is shown in Supplementary Fig. S1A (available at JXB online). Each replicate had 12 plants.
Acclimations and tolerance analysis
To investigate acclimation-induced cross-tolerance to abiotic stresses, tomato seedlings at the five-leaf stage were transferred to a growth chamber for pretreatment with cold (8 °C, 400 μmol m–2 s–1 PPFD, 3 d), paraquat (PQ; 10 μM, 25/20 °C, 400 μmol m–2 s–1 PPFD, 2 d), or drought (in growth medium with 20% moisture, 3 d). Control unacclimated plants were maintained in a growth chamber at 25/20 °C and 400 μmol m–2 s–1 PPFD. Then, acclimated and unacclimated plants were exposed to 4 °C and 400 μmol m–2 s–1 PPFD for 3 d for chilling treatment or 50 μM paraquat for 1 d for PQ treatment or were grown in drought medium (moisture less than 15%) for 3 d for drought treatment. There were four replicates and each replicate had 12 plants.
Stress tolerance was determined by analysing gas exchange and chlorophyll fluorescence. The light-saturated CO2 assimilation rate was determined with an infrared gas analyser-based portable photosynthesis system (LI-6400, LI-COR, Lincoln, NE, USA). The air temperature, relative humidity, CO2 concentration, and PPFD used for measurement of Asat were 25 °C, 85%, 380 μmol mol–1, and 1000 μmol m–2 s–1, respectively.
Chlorophyll (Chl) fluorescence was measured using an Imaging-PAM Chlorophyll Fluorometer equipped with a computer-operated PAM-control unit (IMAG-MAXI, Heinz Walz, Effeltrich, Germany). Seedlings were kept in the dark for approximately 30min before measurements were taken. The intensities of the actinic light and saturating light settings were 280 μmol mol–2 s–1 and 2500 μmol mol–2 s–1 photosynthetically active radiation, respectively. The maximum quantum yield of PSII (F v/F m) were measured and calculated as previously described (Zhou et al., 2012).
Analysis of H2O2
Histochemical staining of H2O2 was performed as previously described (Thordal-Christensen et al., 1997), with minor modifications as described previously (Xia et al., 2009). Leaf discs were vacuum infiltrated with 1mg ml–1 3,3′- diaminobenzidine (DAB) in 50mM TRIS-acetate buffer (pH 3.8) and incubated at 25 °C in the dark for 6h. Leaf discs were rinsed in 80% (v/v) ethanol for 10min at 70 °C and mounted in lactic acid/phenol/water (1:1:1, v/v/v), and H2O2 accumulation was detected by an Olympus motorized system microscope (BX61, Olympus, Tokyo, Japan). H2O2 was visualized at the subcellular level using CeCl3 for localization (Zhou et al., 2012). Sections were examined using a transmission electron microscope (H7650, Hitachi, Tokyo, Japan) at an accelerating voltage of 75kV. Electron-dense CeCl3 deposits which are formed in the presence of H2O2 are visible by transmission electron microscopy (Bestwick et al., 1997). H2O2 in leaf tissue was extracted and analysed as previously described (Willekens et al., 1997).
Antioxidant assays
For antioxidant enzyme assays, leaf tissues (0.3g) were ground with a 2ml ice-cold buffer containing 50mM PBS (pH 7.8), 0.2mM EDTA, 2mM AsA, and 2% (w/v) polyvinylpolypyrrolidone. Homogenates were centrifuged at 12 000 g for 20min, and the resulting supernatants were used for the determination of enzyme activity. All steps were performed at 4 °C. An aliquot of the extract was used to determine the protein content, following the method as previously described (Bradford, 1976), using bovine serum albumin as a standard. The activity of CAT, APX, SOD, and GR were measured following the protocol used as previously described (Xia et al., 2009). All spectrophotometric analyses were conducted on a SHIMADZU UV-2410PC spectrophotometer (Shimadzu, Kyoto, Japan). For the measurement of GSH and GSSG, plant leaf tissue (0.3g) was homogenized in 2ml of 2% metaphosphoric acid containing 2mM EDTA and centrifuged at 4 °C for 10min at 14 000 g. Total and oxidized glutathione (GSH+GSSG and GSSG, respectively) levels were determined as previously described (Griffith, 1980).
Isolation of plasma membranes and determination of NADPH oxidase activity
Leaf plasma membranes were isolated using a two-phase aqueous polymer partition system (Xia et al., 2009). The NADPH-dependent 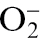 -generating activity in isolated plasma membrane vesicles was determined by following the protocol used as previously described (Zhou et al., 2012).
-generating activity in isolated plasma membrane vesicles was determined by following the protocol used as previously described (Zhou et al., 2012).
Total RNA extraction and gene expression analysis
Total RNA was isolated from tomato leaves using Trizol reagent (Sangon, China), according to the manufacturer’s recommendations. Genomic DNA was removed using a RNeasy Mini Kit (Qiagen, Germany). Total RNA (1 μg) was reverse transcribed using a ReverTra Ace qPCR RT Kit (Toyobo, Osaka, Japan), following the manufacturer’s instructions. Gene-specific quantitative real-time PCR (qRT-PCR) primers were designed based on their cDNA sequences and are listed in Supplemental Table S1. These 10 genes encode MAPKs, defence-regulatory or -related proteinsm and antioxidant enzymes: MPK1 (encoding mitogen-activated protein kinase 1), MPK2 (encoding mitogen-activated protein kinase 2), NPR1 (encoding non-expresser of PR 1), NPR1.1 (encoding non-expresser of PR 1.1), PR1 (encoding pathogenesis-related 1), Fe-SOD (encoding Fe-SOD), Cu/Zn-SOD (encoding Cu/Zn-SOD), cAPX (encoding cytosolic ascorbate peroxidase), CAT1 (encoding catalase 1), and GR1 (encoding glutathione reductase 1). qRT-PCR was performed using the iCycleri Q real-time PCR detection system (Bio-Rad, Hercules, CA, USA). Each reaction (25 μl) consisted of 12.5 μL SYBR Green PCR Master Mix (Takara, Chiga, Japan), 1 μl diluted cDNA, and 0.1 μmol forward and reserve primers. The cycling conditions were as follows: 95 °C for 3min, and 40 cycles of 95 °C for 10 s and 58 °C for 45 s. Tomato Actin was used as an internal control. Relative gene expression was calculated as previously described (Livak and Schmittgen, 2001).
MPK1 and 2 activation assay
Tomato leaves were collected after cross-acclimation, ground in liquid nitrogen, and homogenized in an extraction buffer (100mM HEPES pH 7.5, 5mM EDTA, 5mM EGTA, 10mM DTT, 10mM Na3VO4, 10mM NaF, 50mM β-glycerophosphate, 1mM phenylmethylsulphonyl fluoride, 5 μg/ml antipain, 5 μg/ml aprotinin, 5 μg/ml leupeptin, 10% glycerol, and 7.5% polyvinylpolypyrrolidone). Homogenates were clarified by centrifugation at 16 000 g, extracted proteins were separated by SDS-PAGE, and MPK1 and MPK2 activation was detected by protein blotting using phospho-p44/42 MAPK (ERK1/2, Thr202/Try204) monoclonal antibody (Cell Signalling Technology, Danvers, MA, USA; Faulkner et al., 2013; Nie et al., 2013). ERK1/2 could specifically detect activated MPK1/2 with a molecular mass of 46 kD in tomato plants (Nie et al., 2013).
Statistical analysis
A completely randomized block design with four blocks was applied in each experiment with 10 plants as a replicate. Measurements were replicated four times and randomly arranged in each block. An analysis of variance was carried out according to the general linear model procedure of statistical analysis system (SAS). Differences between treatment means were separated by the Tukey’s test at P < 0.05.
Results
Acclimation-induced cross-tolerance is associated with increased H2O2 accumulation at the apoplast
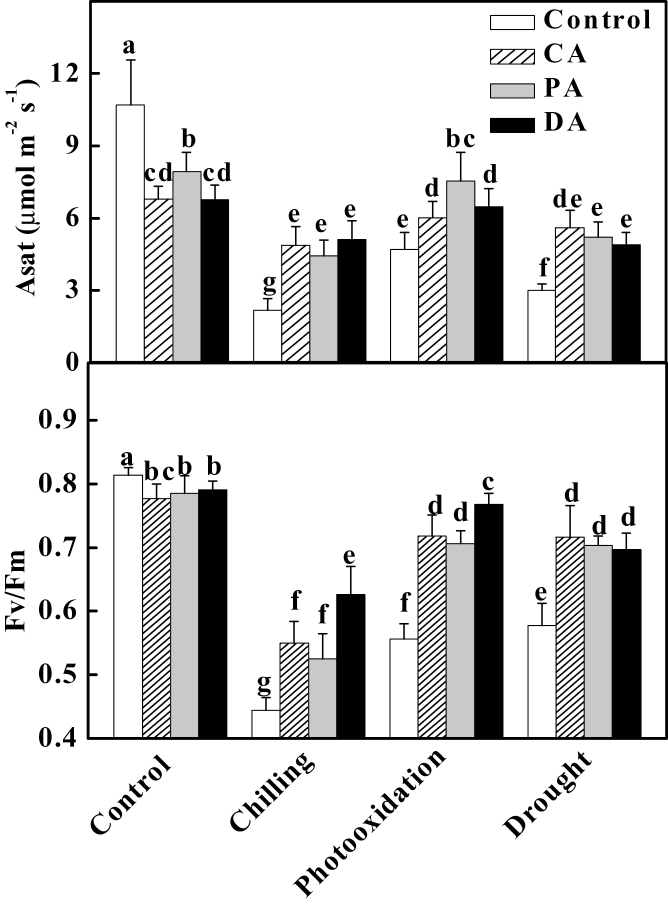
To determine whether cold (CA), drought (DA), and paraquat (PA) acclimation could induce cross-acclimation in tomato, tomato plants were first unacclimated or acclimated to cold at 8 °C, 10 μM PQ, or drought soil moisture at 20% and then exposed to chilling (4 °C) for 3 d, high concentration of PQ (50 μM) for 1 d, and extreme drought (moisture less than 15%) for 3 d. Light-saturated Asat decreased 19–28% while the maximal quantum efficiency of PSII (F v/F m) decreased slightly after these acclimations (Fig. 1). However, Asat of unacclimated plants were decreased by 80, 56, and 72% while F v/F m were reduced 45, 32, and 29% after exposure to chilling, PQ, and extreme drought stresses, respectively (Fig. 1). Importantly, CA, PA, and DA all significantly alleviated the stress-induced decreases in Asat and F v/F m, as indicated from their increases of 28–136% for Asat and 18–29% for F v/F m as compared to unacclimated plants (Fig. 1). All these results suggested that these acclimations could induce cross-tolerance in tomato plants.
Fig. 1.
Effects of cold acclimation (8 °C, 3 d), paraquat acclimation (10 μM, 2 d), and drought acclimation (growth medium with 20% moisture, 3 d) on the light-saturated CO2 assimilation rate (Asat) and the maximum quantum yield of PSII (F v/F m) after chilling (4 °C, 3 d), photooxidation (50 μM paraquat, 1 d), or drought (<15% moisture, 3 d). Leaflets in the middle fifth leaf were used. Data are mean±SD of four biological replicates. Different letters above the bars indicate values that are significantly different (P < 0.05) according to Tukey’s test. Control, no acclimation; CA, cold acclimation; PA, paraquat acclimation; DA, drought acclimation.
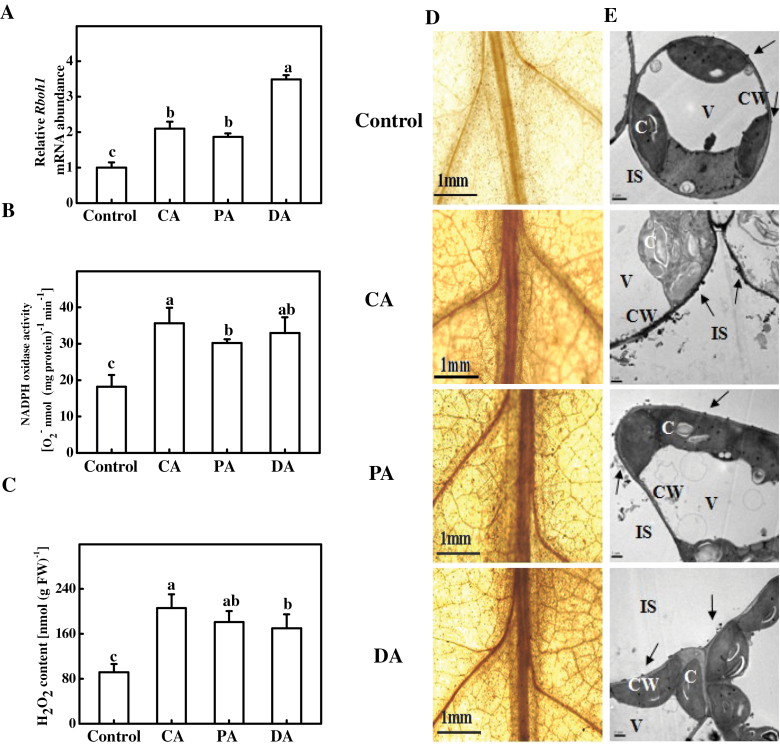
A previous study (Zhou et al., 2012) found that cold acclimation induced H2O2 accumulation in tomato leaves. To determine whether acclimations other than cold acclimation could also induce H2O2 accumulation at the apoplast by triggering NADPH oxidase activity, this study analysed RBOH1 expression, NADPH oxidase activity, and H2O2 accumulation in CA, PA, and DA plants. As shown in Fig. 2, the three types of acclimation all resulted in significant increases in H2O2 accumulation, and the increase was accompanied by significant increases in both RBOH1 expression and plasma membrane NADPH oxidase activity. RBOH1 expression increased by 1.1-fold, 0.9-fold, and 2.5-fold after CA, PA, and DA, respectively. Similarly, NADPH oxidase activity increased by 96, 66, and 81%, and H2O2 concentration increased by 1.3-fold, 98%, and 86% after CA, PA, and DA, respectively (Fig. 2A–C).
Fig. 2.
Cross-acclimation-induced changes in RBOH1 transcript levels (A), NADPH oxidase activity (B), and H2O2 accumulation (C) in tomato leaves after acclimation to cold (8 °C, 3 d), paraquat (10 μM, 2 d), or drought (growth medium with 20% moisture, 3 d). (D) In situ detection of H2O2: leaf segments were loaded with DAB and incubated for 6h and H2O2 accumulation was detected by microscope; bars, 1.0mm. (E) Cytochemical localization of H2O2 in mesophyll cells of leaves, detected with CeCl3 staining and transmission electron microscopy: arrows, CeCl3 precipitates; C, chloroplast; CW, cell wall; IS, intercellular space; V, vacuole. Leaflets in the middle fifth leaf were used. Data are mean±SD of four biological replicates. Different letters above the bars indicate values that are significantly different (P < 0.05) according to Tukey’s test. Control, no acclimation; CA, cold acclimation; PA, paraquat acclimation; DA, drought acclimation.
The in situ detection of H2O2 using DAB staining revealed increased H2O2 accumulation in acclimated leaves, and this was especially apparent in CA plants (Fig. 2D). Using a CeCl3-based procedure, it was found that all these acclimations induced H2O2 accumulation in the cell walls of mesophyll cells that face intercellular spaces (Fig. 2E). These results suggest a potential role of NADPH oxidases in these acclimated plants.
The role of RBOH1 in acclimation-induced cross-tolerance
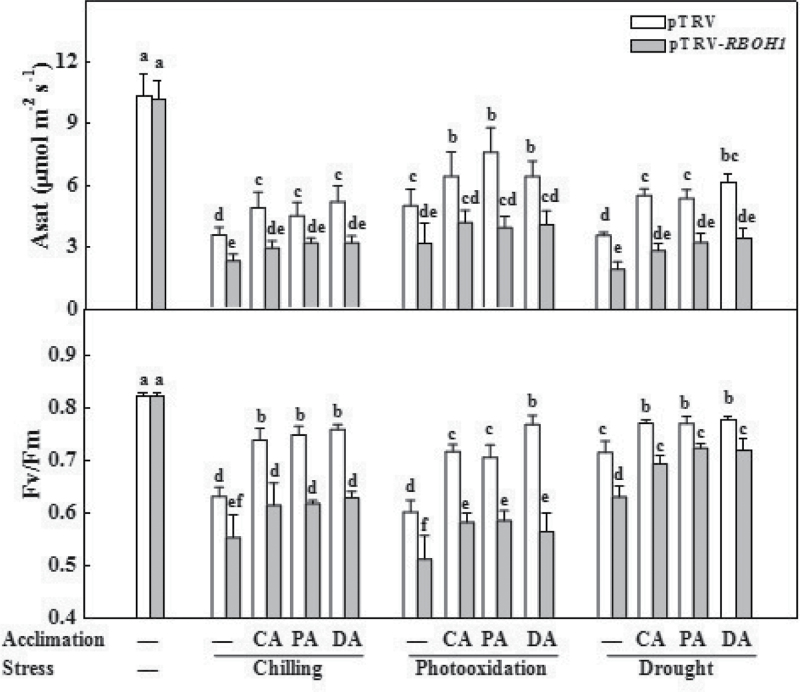
To determine whether acclimation-induced H2O2 accumulation at the apoplast was associated with cross-tolerance, this study compared tolerance against chilling, PQ, and drought stresses in pTRV plants and in RBOH1-silenced plants (pTRV-RBOH1). pTRV-RBOH1 plants showed similar Asat and F v/F m values to those of pTRV plants when they were grown under clement environments (Fig. 3). Chilling, PQ, and drought stresses all resulted in significant decreases in Asat and F v/F m in pTRV plants and pTRV-RBOH1 plants. Meanwhile, pTRV-RBOH1 plants wilted earlier than pTRV plants after exposure to chilling and drought stress and showed more severe leaf bleaching after PQ stress. Importantly, decreases in Asat and F v/F m were largely alleviated by CA, PA, and DA in pTRV plants but not in pTRV-RBOH1 plants. Accordingly, RBOH1 functioned as an important role in acclimation-induced cross-tolerance in tomato plants.
Fig. 3.
Changes in light-saturated CO2 assimilation rate (Asat) and maximum quantum yield of PSII (F v/F m) in response to chilling (4 °C, 3 d), paraquat (50 μM, 1 d), and drought (<15% moisture, 3d) in RBOH1-silenced plants after cross-acclimation pretreatment. Leaflets in the middle fifth leaf were used. Data are mean±SD of four biological replicates. Different letters above the bars indicate values that are significantly different (P < 0.05) according to Tukey’s test. —, no acclimation; CA, cold acclimation; PA, paraquat acclimation; DA, drought acclimation.
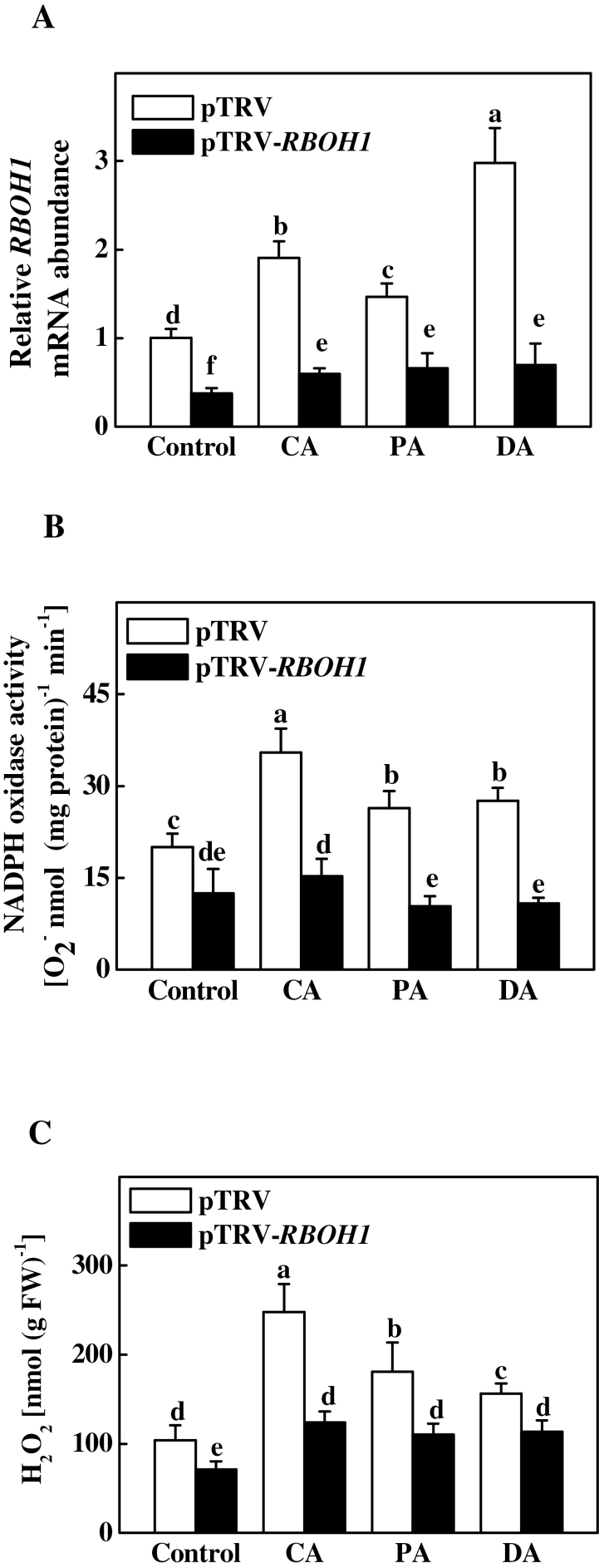
RBOH1 transcript levels, NADPH oxidase activity, and H2O2 accumulation were significantly lower in pTRV-RBOH1 plants as compared to pTRV plants under normal conditions (Fig 4, Supplementary Fig. S2). Similarly to that observed in normal plants, CA, PA, and DA resulted in significant increases in RBOH1 transcript levels, NADPH oxidase activity, and H2O2 accumulation in the leaves of pTRV plants. Such increases in RBOH1 transcript levels, NADPH oxidase activity, and H2O2 accumulation, however, were not observed in pTRV-RBOH1 plants after CA, PA, and DA (Fig. 4, Supplementary Fig. S2), suggesting that RBOH1 is necessary for CA-, PA-, and DA-induced increases in NADPH oxidase activity and H2O2 content.
Fig. 4.
Changes in RBOH1 transcript levels (A), NADPH oxidase activity (B), and H2O2 accumulation (C) in RBOH1-silenced tomato plants after acclimation to cold (8 °C, 3 d), paraquat (10 μM, 2 d), or drought (20% moisture, 3 d). Leaflets in the middle fifth leaf were used. Data are mean±SD of four biological replicates. Different letters above the bars indicate values that are significantly different (P < 0.05) according to Tukey’s test. Control, no acclimation; CA, cold acclimation; PA, paraquat acclimation; DA, drought acclimation; FW, fresh weight.
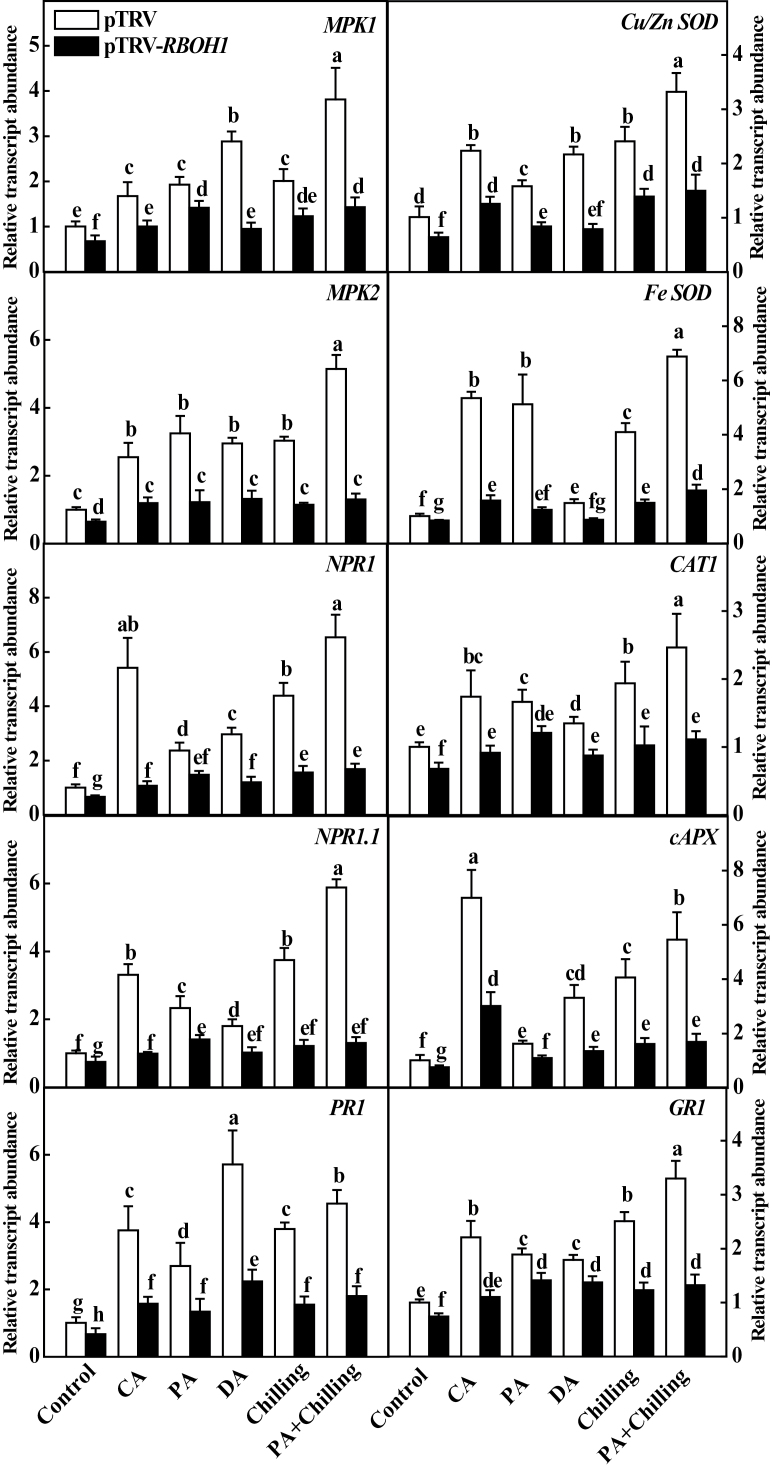
To get insight into the mechanism for cross-acclimation-induced H2O2 accumulation and enhanced stress tolerance, this study examined changes in the transcript levels of 10 stress-responsive and defence-related genes in VIGS plants in response to different acclimation and chilling stresses (Fig. 5). These genes analysed are MPK1, MPK2, NPR1, NPR1.1, PR1, Fe-SOD, Cu/Zn-SOD, cAPX, CAT1, and GR1. Silencing of RBOH1 resulted in significant decreases in the transcript levels of all these genes (Fig. 5). In contrast, all acclimation treatments induced increased transcript levels of these genes, ranging from 2- to 8-fold in pTRV plants. Importantly, silencing of RBOH1 compromised CA-, PA-, DA-, and chilling-induced upregulation of all 10 genes (Fig. 5). Similarly, chilling also induced transcription of these stress-responsive and defence-related genes, and the increases were more significant in PA plants (Fig. 5). However, PA- and chilling-induced transcription was again compromised in pTRV-RBOH1 plants.
Fig. 5.
Relative expression of stress-responsive and defence-related genes in response to acclimation and chilling in RBOH1-silenced plants. Leaflets in the middle fifth leaf were used. Data are mean±SD of four biological replicates. Different letters above the bars indicate values that are significantly different (P < 0.05) according to Tukey’s test. Control, no acclimation; CA, cold acclimation; PA, paraquat acclimation; DA, drought acclimation.
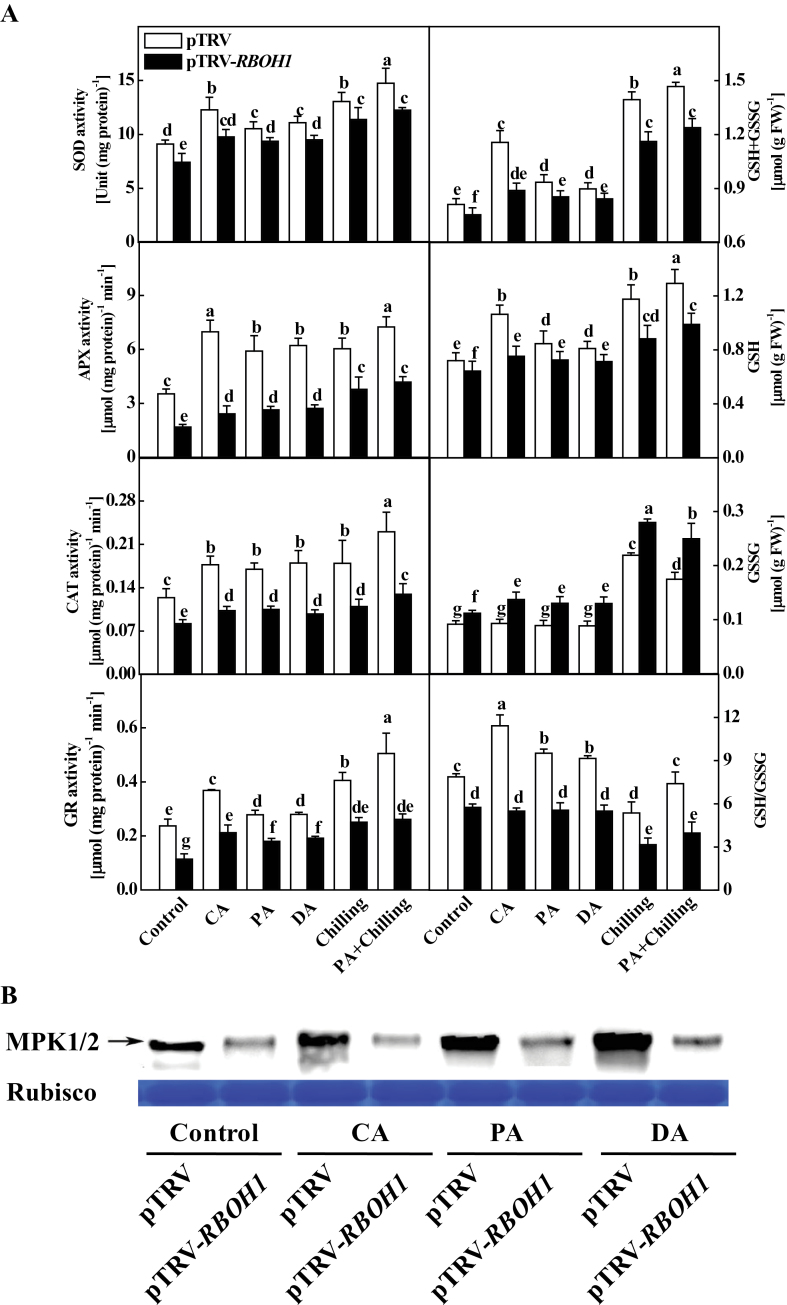
This study then analysed changes in the activities of antioxidant enzymes (SOD, APX, CAT, and GR) and glutathione levels (GSH and GSSG) in pTRV and pTRV-RBOH1 plants after different acclimation and chilling treatments. Similarly to the changes in their transcript levels, silencing of RBOH1 resulted in significant decreases in the activities of all these antioxidant enzymes (Fig. 6A). On the other hand, silencing of RBOH1 resulted in significant decreases in GSH content and increased GSSG content, leading to a substantial reduction in both overall GSH+GSSG content and GSH/GSSG ratio (Fig. 6A). In contrast, chilling induced increased GSH and GSSG accumulation and decreased GSH/GSSG ratios. Interestingly, CA, PA, and DA all significantly induced GSH accumulation and reduced GSSG content in pTRV plants, causing sharp increases in total GSH+GSSG and GSH/GSSG ratio (Fig. 6A). Importantly, silencing of RBOH1 abolished acclimation- and chill-induced increases in GSH content but busted GSSG accumulation, leading to significant decreases in GSH/GSSG ratios (Fig. 6A).
Fig. 6.
Changes in antioxidant enzyme activities, glutathione homeostasis, and MPK1/2 activation in response to acclimation and chilling in RBOH1-silenced plants. (A) Changes in antioxidant enzyme activities and glutathione homeostasis: leaflets in the middle fifth leaf were used; data are mean±SD of four biological replicates; different letters above the bars indicate values that are significantly different (P < 0.05) according to Tukey’s test. (B) Changes in MPK1/2 activation: control, no acclimation; CA, cold acclimation; PA, paraquat acclimation; DA, drought acclimation; FW, fresh weight.
In agreement with the increase in transcript levels of MPK1 and MPK2, MPK1/2 activation was also induced in pTRV plants after CA, PA, and DA. Silencing of RBOH1 led to a 70% reduction in MPK1/2 activation when compared to that of pTRV plants. Significantly, CA, PA, and DA treatments all failed to induce MPK1/2 activation in pTRV-RBOH1 plants (Fig. 6B), suggesting that H2O2 at the apoplast is essential for activation of MPK1/2.
Role of MAPK1 and 2 activation in acclimation-induced cross-tolerance
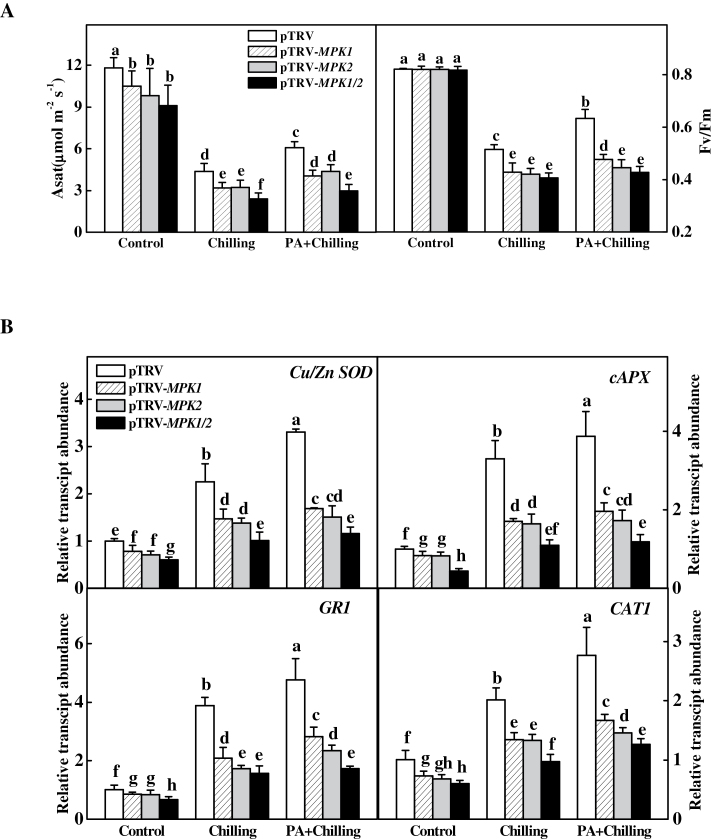
To determine the role of MPK1 and 2 in H2O2-mediated cross-tolerance, this study compared PQ-induced tolerance in plants silenced with MPK1 (pTRV-MPK1), MPK2 (pTRV-MPK2), and MPK1/2 co-silenced (pTRV-MPK1/2) plants with that of pTRV plants for their differences in Asat and F v/F m after chilling at 4 °C. pTRV-MPK1, pTRV-MPK2, and pTRV-MPK1/2 plants grew weaker than pTRV plants, and Asat values of those plants were also lower than that of pTRV plants, but F v/F m values were similar to that of pTRV plants when they were grown under the normal environment (Fig. 7A, Supplementary S1B). However, pTRV-MPK1, pTRV-MPK2, and pTRV-MPK1/2 plants showed lower Asat and F v/F m values than pTRV plants after exposure to a chilling at 4 °C for 3 d. Importantly, silencing of MPK1, MPK2, and MPK1/2 all compromised PA-induced chilling tolerance, as indicated by decreases in both Asat and F v/F m.
Fig. 7.
Changes in light-saturated CO2 assimilation rate (Asat) and maximum quantum yield of PSII (F v/F m) (A) and expression of antioxidant-related genes (B) in response to chilling stress after paraquat acclimation in MPK1, MPK2-silenced, and MPK1/2 co-silenced plants. Leaflets in the middle fifth leaf were used. Data are mean±SD of four biological replicates. Different letters above the bars indicate values that are significantly different (P < 0.05) according to Tukey’s test. Control, no acclimation; PA, paraquat acclimation.
This study then examined the transcript levels of antioxidant-related genes in PQ-acclimated pTRV-MPK1, pTRV-MPK2, and pTRV-MPK1/2 plants after chilling. Silencing of MPK1 and MPK2, or MPK1/2 all resulted in decreased transcript levels of Cu/Zn-SOD, cAPX, GR1, and CAT1 in the normal environment, and blocked CA- and PA-induced increases in the transcript levels (Fig. 7B). Furthermore, cosilencing of MPK1 and 2 resulted in substantially lower transcript levels of these genes than silencing of either MPK1 or MPK2 (Fig. 7B). All these results suggested that MPK1 and MPK2 are necessary components for acclimation-induced stress tolerance.
Discussion
There have been many reports about acclimation-induced stress tolerance in plants. For example, wounding acclimation enhanced salt tolerance in tomato while heat and salt acclimation increased stress response against anoxia in Arabidopsis and UV in barley (Capiati et al., 2006; Banti et al., 2008; Carkirlar et al., 2008). The current study found that cold acclimation not only increased tolerance to chilling but also to drought and photooxidative stress, and it is also true for other acclimation, suggesting that pretreatment of different types of mild abiotic stresses could induce a common effect on tolerance to a spectrum of stresses.
This work found that RBOH1-dependent H2O2 production plays a critical role in acclimation-induced stress tolerance in tomato plants. ROS, especially H2O2, produced at the apoplast play an indispensable role in signal recognition and transduction in plant growth, development, and stress response. Hormones such as ABA and BRs could trigger apoplastic H2O2 generation while exogenous H2O2 significantly increases tolerance against chilling, paraquat, and high light in plants such as potato and cucumber (Wu et al., 1995; Kwak et al., 2003; Xia et al., 2009). Meanwhile, cold-acclimation-induced cold tolerance is associated with increased H2O2 accumulation, and loss of function of RBOHs resulted in reduced tolerance against biotic and abiotic stresses (Dat et al., 2003; Zhou et al., 2012). The current study found that not only CA but also DA and PA induced RBOH1 transcription, NADPH oxidase activity, and H2O2 accumulation at the apoplast. In tomato, RBOH1 is involved in responsiveness to wounding (Sagi et al., 2004). The current study found that silencing of RBOH1 compromised acclimation-induced tolerance, transcription of stress-responsive and defence-related genes, and activation of MPK1/2. Taken together, these findings indicate that H2O2 is a universal signalling molecule in acclimation and that RBOH1-dependent H2O2 generation plays a critical role in acclimation-induced cross-tolerance in tomato plants.
The responses of plants to acclimation and stress are frequently associated with changes in the cellular redox state (Foyer and Noctor, 2005). The current study found that acclimation leads to increases in both the activity of antioxidant enzymes and GSH accumulation and, ultimately, to an increase in the GSH/GSSG ratio while silencing of RBOH1 abolished induction of such changes (Fig. 6). H2O2 could influence the expression of stress-responsive and defence-related genes and the activity of antioxidant enzymes, GSH biosynthesis, and defence responses in plants (Desikan et al., 2001; Vandenabeele et al., 2003; Xia et al., 2009). In agreement with these studies, these acclimations induced significant changes in the antioxidant-related gene transcript levels, enzyme activities, and redox homeostasis, and this effect was highly dependent on accumulation of RBOH1-induced H2O2 after acclimation in this study (Figs. 5 and 6). Importantly, acclimated plants exhibited higher GSH/GSSG ratios than those of unacclimated plants during acclimation and chilling. Glutathione homeostasis could influence plant metabolism and stress response by modifying the activity of redox-sensitive enzymes such as Rubisco activase through reduction/oxidation of disulphide bridges/sulphydryl groups and glutathionylation of sulphydryl groups (Szalai et al., 2009; Jiang et al., 2012). Accordingly, acclimation-induced changes in glutathione homeostasis may partially contribute to increased CO2 assimilation in acclimated plants (Fig. 6A). There is also evidence that the glutathione redox state is involved in the regulation of the transcription and stability of defence-related genes and proteins (Baena-Gonzalez and Aro, 2002; Mou et al., 2003). Interestingly, there were significant increases in the transcript levels of NPR1 and PR1, which are essential regulators for the onset of systemic acquired resistance in acclimated plants, and the increases were again abolished in RBOH1-silenced plants (Fig. 5). Recently, this study group reported that BRs enhance tolerance against chilling/PQ stresses and CO2 assimilation by H2O2-dependent change of the glutathione redox state (Xia et al., 2009; Jiang et al., 2012). Therefore, acclimation-induced stress tolerance appears to involve a conserved stress-responsive and defence-related mechanism that is activated by the RBOHs-H2O2-GSH/GSSG-dependent signalling pathway. It will be of great interest to study whether these acclimations could induce resistance against biotic stress.
MAPK cascades are known to mediate the transduction of environmental and developmental signals into intracellular responses (Mizoguchi et al., 1996; Teige et al., 2004). Tomato MPK1/2 are orthologues of Arabidopsis MPK6, which is involved in plant responses to pathogens (Menke et al., 2004; Schikora et al., 2011). Several studies revealed that MPK1/2 function in host-specific Avr Pto-dependent resistance to the bacterial pathogen P. syringae (Ekengren et al., 2003; Pedley and Martin, 2004) and in Mi-1-mediated resistance to aphids and herbivorous insects in tomato plants (Li et al., 2006; Kandoth et al., 2007). The current study observed that pTRV-MPK1, pTRV-MPK2, and pTRV-MPK1/2 plants showed decreased tolerance against chilling, extending an earlier observation that SlMPK1 and SlMPK2 are not only involved in plant pest resistance but also in plant tolerance to abiotic stresses (Nie et al., 2013). Significantly, all acclimations increased MPK1/2 activation while silencing of MPK1 and MPK2 abolished PA-induced transcription in antioxidant genes and chilling tolerance, as observed in BR-induced stress response and MPK1/2 activation (Fig. 7; Nie et al., 2013). All these results indicate that MPK1/2 play an important role in acclimation-induced stress response.
Studies have revealed that there is an interesting relationship between NADPH oxidase-produced ROS and MAPK activation in plants exposed to various stresses or stimuli (Yoshioka et al., 2003; Pitzschke and Hirt, 2006; Nie et al., 2013). Several plant hormones such as ABA and BRs as well as ABA- and BR-induced H2O2 are known to activate MAPKs, which are involved in ABA- and BR-induced antioxidant defence responses (Zhang et al., 2006; Lin et al., 2009). There are also evidences that MAPKs are involved in regulation of RBOHs in plants (Yoshioka et al., 2003; Asai et al., 2008; Zhang et al., 2010). The current study found that acclimation induced H2O2 accumulation and activation of MPK1/2, while silencing of RBOH1 resulted in reduced MPK1/2 activation and abolished acclimation-induced activation of MPK1/2, suggesting that NADPH oxidase-produced H2O2 regulated MPK1/2 activation during acclimation (Fig. 6B). Very recently, (Nie et al., 2013) found that there exists a positive feedback circuit between RBOH1 and MPK1/2 in BRs signalling cascade. It is, therefore, plausible that H2O2-activated MAPKs are also important in maintaining H2O2 generation by NADPH oxidase during acclimation.
Cross-tolerance could be generated by signalling cross-talk by means of shared components that interrelate the signalling cascades triggered by each type of stress, and, as a result, one type of stress can activate responses that lead to tolerance to other types of stresses (Capiati et al., 2006). It is not surprising that other signalling molecules such as NO and Ca2+ could also be involved in acclimation and cross-tolerance. In tomato, CDPK1, a Ca2+-dependent protein kinase, participates in responses to wounding and salt stress (Capiati et al., 2006). More recently, it has been found that nitrate reductase-dependent NO generation participates in cold-induced chilling tolerance (Zhao et al., 2009). ROS such as H2O2 are also involved in regulation of the generation or the levels of these signals, which may contribute to the critical role of ROS in acclimation and subsequent cross-tolerance.
In conclusion, this work demonstrated that mild cold, PQ, or drought pretreatment can result in enhanced tolerance to multiple abiotic stresses by triggering a significant increase in endogenous H2O2 level at the apoplast, which is associated with upregulation of RBOH1 transcription. The elevated H2O2 induced transcription of a subset of stress- and defence-related genes, activity of antioxidant enzymes, reduced cellular redox status, and activation of MPK1/2. Silencing of RBOH1 and MPK1/2 both compromised acclimation-induced stress tolerance and associated changes in gene transcription. All these findings support the involvement of a RBOH1-dependent activation of MPK1/2 in acclimation-induced cross-tolerance in plants.
Supplementary material
Supplementary data are available at JXB online.
Supplementary Fig. S1. Relative mRNA abundance of MPK1 and MPK2 and phenotypes in VIGS plants.
Supplementary Fig. S2. Cross-acclimation-induced ROS accumulation in pTRV and pTRV-RBOH1 plants.
Supplementary Table S1. Primers for qRT-PCR.
Acknowledgements
This work was supported by the National Basic Research Program of China (2009CB119000) and the National Natural Science Foundation of China (31372109, 30972033). The authors are grateful to Prof CH Foyer for her critical reading of the draft.
References
- Asai S, Ohta K, Yoshioka H. 2008. MAPK signaling regulates nitric oxide and NADPH oxidase-dependent oxidative bursts in Nicotiana benthamiana . The Plant Cell 20, 1390–1406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baena-Gonzalez E, Aro EM. 2002. Biogenesis, assembly and turnover of photosystem II units. Philosophical Transactions of the Royal Society B 357, 1451–1460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banti V, Loreti E, Novi G, Santaniello A, Alpi A, Perata P. 2008. Heat acclimation and cross-tolerance against anoxia in Arabidopsis . Plant, Cell and Environment 31, 1029–1037 [DOI] [PubMed] [Google Scholar]
- Bestwick CS, Brown IR, Bennett MH, Mansfield JW. 1997. Localization of hydrogen peroxide accumulation during the hypersensitive reaction of lettuce cells to Pseudomonas syringae pv phaseolicola . The Plant Cell 9, 209–221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford MM. 1976. Rapid and sensitive method for quantitation of microgram quantities of protein utilizing principle of protein-dye binding. Analytical Biochemistry 72, 248–254 [DOI] [PubMed] [Google Scholar]
- Browse J, Xin ZG. 2001. Temperature sensing and cold acclimation. Current Opinion in Plant Biology 4, 241–246 [DOI] [PubMed] [Google Scholar]
- Capiati DA, Pais SM, Tellez-Inon MT. 2006. Wounding increases salt tolerance in tomato plants: evidence on the participation of calmodulin-like activities in cross-tolerance signalling. Journal of Experimental Botany 57, 2391–2400 [DOI] [PubMed] [Google Scholar]
- Carkirlar H, Cicek N, Fedina I, Georgieva K, Dogru A, Velitchkova M. 2008. NaCl induced cross-acclimation to UV-B radiation in four barley (Hordeum vulgare L.) cultivars. Acta Physiologiae Plantarum 30, 561–567 [Google Scholar]
- Cui JX, Zhou YH, Ding JG, Xia XJ, Shi K, Chen SC, Asami T, Chen Z, Yu JQ. 2011. Role of nitric oxide in hydrogen peroxide-dependent induction of abiotic stress tolerance by brassinosteroids in cucumber. Plant, Cell and Environment 34, 347–358 [DOI] [PubMed] [Google Scholar]
- Dat JF, Pellinen R, Beeckman T, Van de, Cotte B, Langebartels C, Kangasjarvi J, Inze D, Van, Breusegem F. 2003. Changes in hydrogen peroxide homeostasis trigger an active cell death process in tobacco. The Plant Journal 33, 621–632 [DOI] [PubMed] [Google Scholar]
- Dempsey DA, Klessig DF. 1995. Signals in plant disease resistance. Bulletin de l’Institut Pasteur 93, 167–186 [Google Scholar]
- Desikan R, Cheung M, Clarke A, Golding S, Sagi M, Fluhr R, Rock C, Hancock J, Neill S. 2004. Hydrogen peroxide is a common signal for darkness- and ABA-induced stomatal closure in Pisum sativum . Functional Plant Biology 31, 913–920 [DOI] [PubMed] [Google Scholar]
- Desikan R, Mackerness SAH, Hancock JT, Neill SJ. 2001. Regulation of the Arabidopsis transcriptome by oxidative stress. Plant Physiology 127, 159–172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekengren SK, Liu YL, Schiff M, Dinesh-Kumar SP, Martin GB. 2003. Two MAPK cascades, NPR1, and TGA transcription factors play a role in Pto-mediated disease resistance in tomato. The Plant Journal 36, 905–917 [DOI] [PubMed] [Google Scholar]
- Faulkner C, Petutschnig E, Benitez-Alfonso Y, Beck M, Robatzek S, Lipka V, Maule AJ. 2013. LYM2-dependent chitin perception limits molecular flux via plasmodesmata. Proceedings of the National Academy of Sciences, USA 110, 9166–9170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foyer CH, LopezDelgado H, Dat JF, Scott IM. 1997. Hydrogen peroxide- and glutathione-associated mechanisms of acclimatory stress tolerance and signalling. Physiologia Plantarum 100, 241–254 [Google Scholar]
- Foyer CH, Noctor G. 2005. Redox homeostasis and antioxidant signaling: a metabolic interface between stress perception and physiological responses. The Plant Cell 17, 1866–1875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith OW. 1980. Determination of glutathione and glutathione disulfide using glutathione-reductase and 2-vinylpyridine. Analytical Biochemistry 106, 207–212 [DOI] [PubMed] [Google Scholar]
- Janska A, Marsik P, Zelenkova S, Ovesna J. 2010. Cold stress and acclimation—what is important for metabolic adjustment? Plant Biology 12, 395–405 [DOI] [PubMed] [Google Scholar]
- Jiang YP, Cheng F, Zhou YH, Xia XJ, Mao WH, Shi K, Chen ZX, Yu JQ. 2012. Cellular glutathione redox homeostasis plays an important role in the brassinosteroid-induced increase in CO2 assimilation in Cucumis sativus . New Phytologist 194, 932–943 [DOI] [PubMed] [Google Scholar]
- Kandoth PK, Ranf S, Pancholi SS, Jayanty S, Walla MD, Miller W, Howe GA, Lincoln DE, Stratmann JW. 2007. Tomato MAPKs LeMPK1, LeMPK2, and LeMPK3 function in the systemin-mediated defense response against herbivorous insects. Proceedings of the National Academy of Sciences, USA 104, 12205–12210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwak JM, Mori IC, Pei ZM, Leonhardt N, Torres MA, Dangl JL, Bloom RE, Bodde S, Jones JDG, Schroeder JI. 2003. NADPH oxidase AtrbohD and AtrbohF genes function in ROS-dependent ABA signaling in Arabidopsis . EMBO Journal 22, 2623–2633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Xie QG, Smith-Becker J, Navarre DA, Kaloshian I. 2006. Mi-1-mediated aphid resistance involves salicylic acid and mitogen-activated protein kinase signaling cascades. Molecular Plant–Microbe Interactions 19, 655–664 [DOI] [PubMed] [Google Scholar]
- Lin F, Ding HD, Wang JX, Zhang H, Zhang AY, Zhang Y, Tan MP, Dong W, Jiang MY. 2009. Positive feedback regulation of maize NADPH oxidase by mitogen-activated protein kinase cascade in abscisic acid signalling. Journal of Experimental Botany 60, 3221–3238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Schiff M, Dinesh-Kumar SP. 2002. Virus-induced gene silencing in tomato. The Plant Journal 31, 777–786 [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2–ΔΔCT method. Methods 25, 402–408 [DOI] [PubMed] [Google Scholar]
- Lu C, Han MH, Guevara-Garcia A, Fedoroff NV. 2002. Mitogen-activated protein kinase signaling in postgermination arrest of development by abscisic acid. Proceedings of the National Academy of Sciences, USA 99, 15812–15817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marino D, Dunand C, Puppo A, Pauly N. 2012. A burst of plant NADPH oxidases. Trends in Plant Science 17, 9–15 [DOI] [PubMed] [Google Scholar]
- Menke FLH, van, Pelt JA, Pieterse CMJ, Klessig DF. 2004. Silencing of the mitogen-activated protein kinase MPK6 compromises disease resistance in Arabidopsis . The Plant Cell 16, 897–907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittler R, Vanderauwera S, Gollery M, Van, Breusegem F. 2004. Reactive oxygen gene network of plants. Trends in Plant Science 9, 490–498 [DOI] [PubMed] [Google Scholar]
- Mizoguchi T, Irie K, Hirayama T, Hayashida N, YamaguchiShinozaki K, Matsumoto K, Shinozaki K. 1996. A gene encoding a mitogen-activated protein kinase kinase kinase is induced simultaneously with genes for a mitogen-activated protein kinase and an S6 ribosomal protein kinase by touch, cold, and water stress in Arabidopsis thaliana . Proceedings of the National Academy of Sciences, USA 93, 765–769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mou Z, Fan WH, Dong XN. 2003. Inducers of plant systemic acquired resistance regulate NPR1 function through redox changes. Cell 113, 935–944 [DOI] [PubMed] [Google Scholar]
- Nie WF, Wang MM, Xia XJ, Zhou YH, Shi K, Chen ZX, Yu JQ. 2013. Silencing of tomato RBOH1 and MPK2 abolishes brassinosteroid-induced H2O2 generation and stress tolerance. Plant, Cell and Environment 36, 789–803 [DOI] [PubMed] [Google Scholar]
- Pastori GM, Foyer CH. 2002. Common components, networks, and pathways of cross-tolerance to stress. The central role of ‘redox’ and abscisic acid-mediated controls. Plant Physiology 129, 460–468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedley KF, Martin GB. 2004. Identification of MAPKs and their possible MAPK kinase activators involved in the Pto-mediated defense response of tomato. Journal of Biological Chemistry 279, 49229–49235 [DOI] [PubMed] [Google Scholar]
- Pitzschke A, Hirt H. 2006. Mitogen-activated protein kinases and reactive oxygen species signaling in plants. Plant Physiology 141, 351–356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitzschke A, Schikora A, Hirt H. 2009. MAPK cascade signalling networks in plant defence. Current Opinion in Plant Biology 12, 421–426 [DOI] [PubMed] [Google Scholar]
- Sagi M, Davydov O, Orazova S, Yesbergenova Z, Ophir R, Stratmann JW, Fluhr R. 2004. Plant respiratory burst oxidase homologs impinge on wound responsiveness and development in Lycopersicon esculentum . The Plant Cell 16, 616–628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuel MA, Ellis BE. 2002. Double jeopardy: both overexpression and suppression of a redox-activated plant mitogen-activated protein kinase render tobacco plants ozone sensitive. The Plant Cell 14, 2059–2069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schikora A, Schenk ST, Stein E, Molitor A, Zuccaro A, Kogel KH. 2011. N-Acyl-homoserine lactone confers resistance toward biotrophic and hemibiotrophic pathogens via altered activation of AtMPK6. Plant Physiology 157, 1407–1418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stratmann JW, Ryan CA. 1997. Myelin basic protein kinase activity in tomato leaves is induced systemically by wounding and increases in response to systemin and oligosaccharide elicitors. Proceedings of the National Academy of Sciences, USA 94, 11085–11089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki N, Koussevitzky S, Mittler R, Miller G. 2012. ROS and redox signalling in the response of plants to abiotic stress. Plant, Cell and Environment 35, 259–270 [DOI] [PubMed] [Google Scholar]
- Szalai G, Kellos T, Galiba G, Kocsy G. 2009. Glutathione as an antioxidant and regulatory molecule in plants under abiotic stress conditions. Journal of Plant Growth Regulation 28, 66–80 [Google Scholar]
- Teige M, Scheikl E, Eulgem T, Doczi F, Ichimura K, Shinozaki K, Dangl JL, Hirt H. 2004. The MKK2 pathway mediates cold and salt stress signaling in Arabidopsis . Molecular Cell 15, 141–152 [DOI] [PubMed] [Google Scholar]
- Tena G, Asai T, Chiu WL, Sheen J. 2001. Plant mitogen-activated protein kinase signaling cascades. Current Opinion in Plant Biology 4, 392–400 [DOI] [PubMed] [Google Scholar]
- Thannickal VJ, Fanburg BL. 2000. Reactive oxygen species in cell signaling. American Journal of Physiology. Lung Cellular and Molecular Physiology 279, L1005–L1028 [DOI] [PubMed] [Google Scholar]
- Thordal-Christensen H, Zhang ZG, Wei YD, Collinge DB. 1997. Subcellular localization of H2O2 in plants. H2O2 accumulation in papillae and hypersensitive response during the barley-powdery mildew interaction. The Plant Journal 11, 1187–1194 [Google Scholar]
- Torres MA, Dangl JL. 2005. Functions of the respiratory burst oxidase in biotic interactions, abiotic stress and development. Current Opinion in Plant Biology 8, 397–403 [DOI] [PubMed] [Google Scholar]
- Torres MA, Dangl JL, Jones JDG. 2002. Arabidopsis gp91phox homologues AtrbohD and AtrbohF are required for accumulation of reactive oxygen intermediates in the plant defense response. Proceedings of the National Academy of Sciences, USA 99, 517–522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenabeele S, Van Der, Kelen K, Dat J, et al. 2003. A comprehensive analysis of hydrogen peroxide-induced gene expression in tobacco. Proceedings of the National Academy of Sciences, USA 100, 16113–16118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendehenne D, Durner J, Klessig DF. 2004. Nitric oxide: a new player in plant signalling and defence responses. Current Opinion in Plant Biology 7, 449–455 [DOI] [PubMed] [Google Scholar]
- Willekens H, Chamnongpol S, Davey M, Schraudner M, Langebartels C, VanMontagu M, Inze D, VanCamp W. 1997. Catalase is a sink for H2O2 and is indispensable for stress defence in C-3 plants. EMBO Journal 16, 4806–4816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu GS, Shortt BJ, Lawrence EB, Levine EB, Fitzsimmons KC, Shah DM. 1995. Disease resistance conferred by expression of a gene encoding H2O2-generating glucose-oxidase in transgenic potato plants. The Plant Cell 7, 1357–1368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia XJ, Wang YJ, Zhou YH, Tao Y, Mao WH, Shi K, Asami T, Chen Z, Yu JQ. 2009. Reactive oxygen species are involved in brassinosteroid-induced stress tolerance in cucumber. Plant Physiology 150, 801–814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshioka H, Numata N, Nakajima K, Katou S, Kawakita K, Rowland O, Jones JDG, Doke N. 2003. Nicotiana benthamiana gp91phox homologs NbrbohA and NbrbohB participate in H2O2 accumulation and resistance to Phytophthora infestans . The Plant Cell 15, 706–718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang A, Zhang J, Ye NH, Cao JM, Tan MP, Zhang JH, Jiang MY. 2010. ZmMPK5 is required for the NADPH oxidase-mediated self-propagation of apoplastic H2O2 in brassinosteroid-induced antioxidant defence in leaves of maize. Journal of Experimental Botany 61, 4399–4411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang AY, Jiang MY, Zhang JH, Tan MP, Hu XL. 2006. Mitogen-activated protein kinase is involved in abscisic acid-induced antioxidant defense and acts downstream of reactive oxygen species production in leaves of maize plants. Plant Physiology 141, 475–487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang SQ, Klessig DF. 2001. MAPK cascades in plant defense signaling. Trends in Plant Science 6, 520–527 [DOI] [PubMed] [Google Scholar]
- Zhang YM, Tan JL, Guo ZF, Lu SY, He SJ, Shu W, Zhou BY. 2009. Increased abscisic acid levels in transgenic tobacco over-expressing 9-cis-epoxycarotenoid dioxygenase influence H2O2 and NO production and antioxidant defences. Plant, Cell and Environment 32, 509–519 [DOI] [PubMed] [Google Scholar]
- Zhao MG, Chen L, Zhang LL, Zhang WH. 2009. Nitric reductase-dependent nitric oxide production is involved in cold acclimation and freezing tolerance in Arabidopsis . Plant Physiology 151, 755–767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Wang J, Shi K, Xia XJ, Zhou YH, Yu JQ. 2012. Hydrogen peroxide is involved in the cold acclimation-induced chilling tolerance of tomato plants. Plant Physiology and Biochemistry 60, 141–149 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.