Abstract
Mesophyll conductance (g m) has been shown to impose significant limitations to net CO2 assimilation (A) in various species during water stress. Net CO2 assimilation is also limited by stomatal conductance to water (g sw), both having been shown to co-vary with leaf hydraulic conductance (K leaf). Lately, several studies have suggested a close functional link between K leaf, g sw, and g m. However, such relationships could only be circumstantial since a recent study has shown that the response of g m to drought could merely be an artefactual consequence of a reduced intercellular CO2 mole fraction (C i). Experiments were conducted on 8-week-old hybrid poplar cuttings to determine the relationship between K leaf, g sw, and g m in clones of contrasting drought tolerance. It was hypothesized that changes in g sw and K leaf in response to drought would not impact on g m over most of its range. The results show that K leaf decreased in concert with g sw as drought proceeded, whereas g m measured at a normalized C i remained relatively constant up to a g sw threshold of ~0.15mol m–2 s–1. This delayed g m response prevented a substantial decline in A at the early stage of the drought, thereby enhancing water use efficiency. Reducing the stomatal limitation of droughted plants by diminishing the ambient CO2 concentration of the air did not modify g m or K leaf. The relationship between gas exchange and leaf hydraulics was similar in both drought-tolerant and drought-sensitive clones despite their contrasting vulnerability to stem cavitation and stomatal response to soil drying. The results support the hypothesis of a partial hydraulic isolation of the mesophyll from the main transpiration pathway.
Key words: Drought, internal CO2 conductance, leaf hydraulic conductance, Populus, stomatal limitation, water use efficiency.
Introduction
Attempts to establish significant relationships between physiological variables relating CO2 assimilation to water transport have been helpful for our understanding of leaf processes under different environmental conditions. For instance, Brodribb and Holbrook (2003, 2004) observed concerted decreases in net photosynthesis (A), stomatal conductance to water (g sw), and leaf hydraulic conductance (K leaf) in tropical tree species during periods of low soil water availability and/or high evaporative demand. These reductions in gas exchange have been considered a way to avoid major cavitation events by maintaining leaf water potential above a critical threshold (Sack and Holbrook, 2006), a mechanism also observed with stem hydraulic conductance (K stem) (Tyree and Sperry, 1988). Further, there is growing evidence of greater diurnal variation in K leaf than in K stem, which suggests that K leaf is more closely coupled to gas exchange (Brodribb and Holbrook, 2004; Sack and Holbrook 2006). Of the different leaf resistance components, xylem cavitation is the most important source of resistance to water transport in highly transpiring plants, whereas the resistance occurring outside the xylem represents about a third of the total resistance to leaf water movement (Sack et al., 2004, 2005). Nonetheless, a decline in water transport outside the xylem generally occurs during drought stress due to a shift from the symplastic–transcellular to the apoplastic water pathways, which also produces a decrease in K leaf and g sw (Pou et al., 2013). Thus, in highly vascularized plants such as hybrid poplar, it may be expected that during drought, leaf xylem cavitation would precede an eventual shift of water flow to the outer xylary water pathways. This in turn would lead to a decrease in K leaf and gas exchange as a way to protect the leaf against excessive cavitation.
When considering how environmental conditions may affect CO2 transport inside leaves, one must take into account the mesophyll conductance (g m) pertaining to the CO2 diffusion path from the substomatal cavities to the chloroplast stroma (Ethier and Livingston, 2004; Evans et al., 2009; Tholen et al., 2012). Although an apparent dynamic regulation of g m in changing microclimates or growth conditions has frequently been reported in recent years (reviewed in Flexas et al., 2008), the physiological basis of such g m responses and how these changes orchestrate themselves with parallel changes in K leaf and g sw remain poorly understood. For example, g m and g sw appear to decline in concert as a result of drought stress (Warren, 2008; Centritto et al., 2009; Barbour et al., 2010). However, some studies dealing mainly with short- to mid-term drought stress show little to no relationship between g m and g sw (Galle et al., 2009; Resco et al., 2009), thereby suggesting a possible threshold drought response of g m in some species (see, for example, Duan et al., 2011).
Because K leaf is a measure of the ease with which liquid water perfuses through the leaf (Sack and Holbrook, 2006), and since part of this pathway (the mesophyll apoplastic–symplastic–transcellular pathway) is shared with CO2 diffusing towards the chloroplast as bicarbonate ion and dissolved gas (Kaldenhoff et al., 2008), one would expect a functional linkage between g m and K leaf, be it in the form of the hydration status of the cell, or even perhaps through convergence of aquaporin-mediated membrane transport (Maurel et al., 2008; Uehlein et al., 2012). Aasamaa et al. (2005) showed that K leaf increases with mesophyll density, a trait known to influence g m (Loreto et al., 1992; Fleck et al., 2010). Moreover, they suggested that membrane-associated traits such as membrane permeability, or simply the frequency of plasmalemmas and tonoplasts in the hydraulic flow path of the mesophyll, were most influential on K leaf. The possibility that membranes also exert strong control over g m is currently a much debated subject (reviewed in Kaldenhoff, 2012). Recently, Ferrio et al. (2012) suggested that the relationship between g m and K leaf would only be noticeable below a certain threshold. Presumably, the decrease of K leaf and g m as a result of water stress would be due to an eventual reduction of plasma membrane H2O/CO2 permeability, which in turn would divert symplastic water transport through the more tortuous apoplastic pathway (see also Ferrio et al., 2009).
Despite the plausibility of the hypothesis of Ferrio et al., recent modelling results by Tholen et al. (2012) offer perhaps a simpler alternative explanation. Indeed, they have theoretically demonstrated that a threshold-like decrease of g m with respect to g sw in response to drought or salinity stress is expected merely from the relative increase in photorespiratory CO2 release ensuing from the eventual reduction of the intercellular CO2 mole fraction (C i) at low enough g sw. Thus, the apparent threshold-like response of g m to drought could merely be an artefactual consequence of a reduction of C i—not a reduction of, say, cell membrane permeability as Ferrio et al. (2012) suggested.
To examine possible functional links between K leaf, g sw, and g m, as well as to verify the possible occurrence of a threshold-like response of g m with respect to the other two leaf variables, the time course of leaf gas exchange was determined in parallel to K leaf and g m in hybrid poplars of contrasting drought tolerance during a short-term drought. It was also determined whether a reduction of stomatal limitation via a low CO2 treatment affects the short-term response of g m after 1 week of reduced irrigation. To avoid any artefactual CO2 effects on g m during measurements, care was taken to conduct the latter under constant C i. It was hypothesized that g m, thus evaluated at a normalized C i, would remain relatively constant and not display a threshold-like response in relation to g sw and K leaf, and that low CO2 treatment at the end of the drought period would increase g sw in the short term, but not g m and possibly not K leaf.
Materials and methods
Plant material and growing conditions
Frozen cuttings of Assiniboine [(Populus×’Walker’:Populus deltoides L.×P.×petrowskiana R.I. Schrod. ex Regel)×male parent unknown] and Okanese [(P.×’Walker’)×P.×petrowskiana], with low and high putative drought tolerance, respectively (Silim et al., 2009), were thawed in water for 24h and sprouted in a mist chamber for ~3 weeks in a 2:1 perlite:Pro-mix growing medium (Premier Horticulture, Rivière-du-Loup, QC, Canada). Plants were transferred to a 2:1 coarse sand:Pro-mix soil in 7 l pots and moved to a greenhouse for ~3 weeks (23/21 °C day/night temperature, 55% relative humidity, 16h photoperiod). When plants reached ~60cm in height, they were transferred to a growth cabinet and acclimated for at least 7 d [22/17 °C day/night temperature, 60% relative humidity, 16h photoperiod, 800 μmol m–2 s–1 photosynthetic photon flux density (PPFD)]. A 20–20–20 complete nutrient solution was applied twice a week for a total of 200 ppm N per week per plant. Experiments described later were carried out in the same growth cabinet under the same growing conditions.
Gas exchange and fluorescence measurement
Gas exchange measurements were carried out when plants had reached a leaf plastochron index (LPI) of at least 12 (Larson and Isebrands, 1971). Plants were measured between 9:00h and 12:00h with a LI-6400XT equipped with a 6400–40 Leaf Chamber Fluorometer (LCF, LI-COR Biosciences, Lincoln, NE, USA). Leaves were initially acclimated under 800 μmol m–2 s–1 PPFD, 25.0±0.2 °C leaf temperature, 380 μmol mol–1 leaf chamber air CO2 (C a), 17.5±0.5 mmol mol–1 water vapour mole fraction, and 250 μmol s–1 air flow rate. Net CO2 assimilation rate at ambient conditions (A Ca=380), stomatal conductance to water vapour (g sw), and intercellular CO2 mole fraction (C i) were recorded once gas exchange variables reached steady state. Photochemical efficiency of photosystem II (ΦPSII) was estimated by recording steady-state fluorescence (F s) and maximal fluorescence under a saturating flash (F m′) of ~10 000 μmol m–2 s–1 using:
| (1) |
Mesophyll conductance measurements and calibration
The variable J method (Harley et al., 1992) was used to estimate g m using:
| (2) |
where J f is the photochemical electron transport rate estimated from chlorophyll fluorescence, Γ* is the chloroplastic CO2 photocompensation point, and R d is the non-photorespiratory mitochondrial respiration in the light, which was estimated from the intersection point of two detailed A–C i curves performed at 500 and 150 μmol m–2 s–1 PPFD. For these ‘Laisk method’ measurements of R d, a larger 2×3cm leaf chamber and an air flow rate of 500 μmol s–1 were used to minimize chamber leaks (LI-COR, 2012). Since the intersection point of the A–C i curves of the Laisk method can only give an apparent intercellular CO2 photocompensation point (C i*), this value is usually converted to its chloroplastic Γ* equivalent assuming (von Caemmerer et al., 1994; Ethier and Livingston, 2004):
| (3) |
However, this relationship assumes that the great majority of the gross intracellular CO2 flux crossing the chloroplast envelope also experiences the diffusional resistance due to the cell wall and plasmalemma (r wp). As explained in detail in Tholen et al. (2012), this is very unlikely, especially around Γ* where the net outgoing flux R d crossing the wall/plasmalemma is typically only ~20% of the intracellular flux entering the chloroplast. Under such conditions, Equation 3 may be re-written as:
| (4) |
where r ch is the diffusional resistance due to the chloroplast envelope and stroma. Given that current estimates put r ch ≥ r wp in non-sclerophylls (Uehlein et al., 2008; Tholen and Zhu, 2011; Tholen et al., 2012), it is clear that C i* is probably greater than Γ*, rather than smaller as Equation 3 has it (see Tholen et al., 2012 for further discussion). Given such uncertainties, it was chosen here to estimate Γ* in hybrid poplar from previous in vitro determinations of the specificity factor (S c/o) of Rubisco purified from other woody deciduous species (S c/o=100–104; see Balaguer et al., 1996; Bota et al., 2002), which on average corresponded to a Γ* of ~38 μmol mol–1 at 21% O2. This Γ* value appears in accordance with Equation 4 as it is below the average C i* value found here for the poplar clones (40.3 μmol mol–1); it also compares very well with the popular tobacco Γ* values commonly used to model photosynthesis and estimate g m (see von Caemmerer et al., 1994; Bernacchi et al., 2002). Furthermore, Galmès et al. (2006) have shown the unreliability of in vivo Γ* estimates under water stress conditions, whereas leaf respiration—in particular dark leaf respiration (R n)—appeared less affected by drought. Consequently, the Laisk method was only used to estimate R d and establish a relationship with R n under well-watered conditions, and then this relationship was used to estimate R d from R n measurements in all subsequent experiments (see next section).
Calibrations of the relationship between the photochemical electron transport rate estimated from fluorescence measurements (J f) and gas exchange (J CO2) were carried out as described by Hassiotou et al. (2009). Briefly, CO2 response curves were carried out under non-photorespiratory conditions (1% O2) at a PPFD of 1000 μmol m–2 s–1 by progressively increasing C a from 300 μmol mol–1 up to saturating CO2 (between 800 and 1000 μmol mol–1), which were followed by photosynthetic light responses curves, decreasing PPFD from 1500 to 500 μmol m–2 s–1 (Fig. 1). This allowed the calculation of J f as:
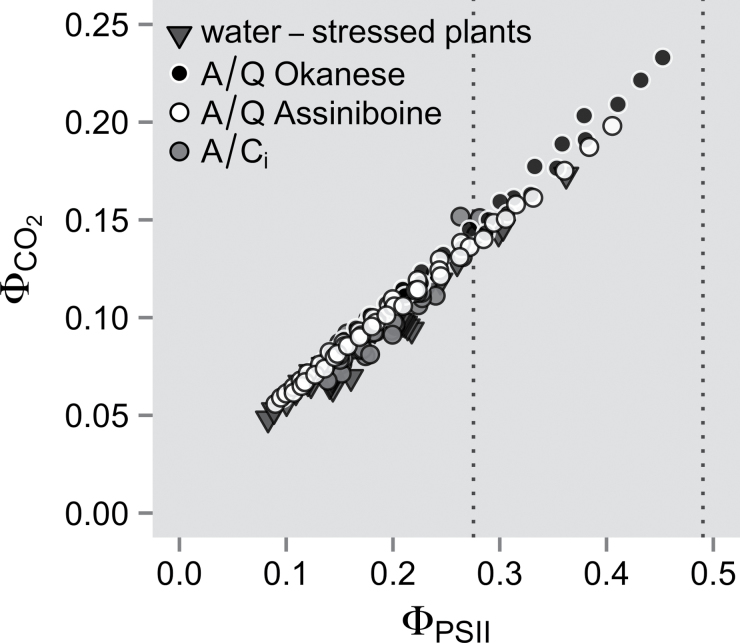
Fig. 1.
Relationship between the photochemical electron transport rate estimated from fluorescence measurements (J f) and gas exchange (J CO2) under 1% O2. Photosynthetic response curves to light (A/Q) and CO2 (A/C i) were carried out on three plants per clone, and the slope (s) and intercept of the relationship between ΦCO2 and ΦPSII were used to calibrate J f (see Equation 5). A/C i data were pooled together for clarity. Water-stressed plants were also measured and were in a similar range to well-watered plants. The dotted lines indicate the range of ΦPSII values observed during the short-term soil drying experiments (see Fig. 3). A/Q Okanese, ΦCO2=0.012+0.480×ΦPSII; A/Q Assiniboine, ΦCO2=0.016+0.450×ΦPSII; A/C i, ΦCO2=0.003+0.496×ΦPSII; water-stressed plants, ΦCO2=0.012+0.433×ΦPSII.
| (5) |
where s is the slope and c the intercept of the relationship of ΦPSII and ΦCO2 at 1% O2, ΦCO2 being calculated as:
| (6) |
Calibrations carried out on water-stressed plants resulted in similar regressions; hence, data from both well-watered and stressed plants were pooled in the final (Fig. 1).
Nevertheless, such calibration of J f under non-photorespiratory conditions did not always produce acceptable g m values (see Gilbert et al., 2012 for a detailed discussion of this issue), as many were negative or too high, particularly when the plants were under water stress. Consequently, an alternative method, in the authors’ view more likely to produce more reliable estimates of s under 21% O2, was evaluated. It is based on the premise that at 21% O2, g m estimates should in theory vary little over a range of C i values falling around the RuBP-limited A–C i curve region where ΦPSII is found to be constant (Tholen and Zhu, 2011). It was confirmed that this theoretically based assumption held for poplar using g m measurements obtained with the standard isotopic method of Evans et al. (1986) (GE and SP, unpublished; see also Supplementary Fig. S1 available at JXB online). Thus, for this alternative J f calibration method, detailed A–C i curve analysis around the aforementioned C i region of interest combined with chlorophyll fluorescence measurements under 21% O2 was used. s and g m were then fitted over the C i range of interest using the RuBP-limited photosynthesis equation given in Ethier et al. (2006) (see their equation 3) in order to minimize the sum of squares of errors. This method hence solves s (and so J f) and g m simultaneously using the measured A–C i, ΦPSII, Γ*, and R d as inputs.
Net CO2 assimilation and leaf transpiration rates measured with the LI-6400XT over the aforementioned C a range were corrected for leaks following the method of Flexas et al. (2007a). Briefly, this consisted of subtracting the apparent CO2 assimilation rate of a dead leaf (immersed in boiling water for 5min) estimated at the corresponding leaf chamber CO2 concentration and air flow rate, taking care to allow the leaf to become fully equilibrated with the surrounding water vapour concentration of the chamber (set to 17.5 mmol mol–1). For H2O leak corrections, the apparent transpiration rate of the empty leaf chamber recorded at 17.5±0.5 mmol mol–1 water vapour concentration and appropriate air flow rate was subtracted from measured leaf transpiration rates.
Post-calibration estimation of R d from dark respiration measurements
Because during the short-term drought experiments the water potential of each leaf used for gas exchange needed to be subsequently rapidly measured with a pressure chamber to determine leaf hydraulic conductance (see ‘Leaf hydraulic conductance’ below), it was necessary to use a rapid proxy method to estimate R d immediately following the leaf water potential (Ψleaf) determination. For this, the leaf petiole was placed in a water-filled beaker and recut under water before clamping the leaf to a LI-6400 equipped with a 2×3cm leaf chamber, then covering it with a dark cloth to measure its R n continuously for ~20min. To convert from R n to R d, the following relationship previously established on the plants used for J f calibrations was used:
| (7) |
Dark respiration was measured at a leaf chamber C a set equal to the room C a to avoid CO2 leakage effects. It was established that pressurization of leaves prior to the determination of dark respiration rates across a range of Ψleaf (–0.4 to –1MPa) had no impact on R n compared with that of unpressurized leaves (data not shown).
Short-term drought
Gas exchange of three plants per clone was repeatedly measured over a 12 d period of soil drying, for a total of nine measuring days. Plants were first measured under well-watered conditions, and the irrigation was then reduced on the following days to achieve a wide range of g sw values. Measurements were carried on the same newly mature leaf (LPI=5 on day 1) throughout the experiment. Soil water potential (Ψsoil) was monitored on each measurement day using custom-made tensiometers and a digital reader (Tensimeter, Soil Moisture Equipment, Santa Barbara, CA, USA).
In addition, to get a better picture of the time course of A, g sw, g m, and K leaf, continuous measurements of gas exchange and plant and soil water potentials were performed on one plant per clone during 5 d of soil drying. Gas exchange was monitored during daytime with a LI-6400XT equipped with a 2×3cm adaptor chamber for a PAM-2000 chlorophyll fluorometer probe (Heinz Walz GmbH, Effeltrich, Germany), set at a flash intensity above 6000 μmol m–2 s–1. Through Equation 7, R d was assumed to follow the observed decreasing trend of R n as drought proceeded, dropping by ~40–50% after 5 d (from an estimated average initial R d of ~1.1 μmol m–2 s–1). Soil water potential was measured using one tensiometer connected to a datalogger (CR7, Campbell Scientific, Logan, UT, USA). A leaf psychrometer (L-51, Wescor, Logan, UT, USA) was attached on an adjacent leaf to the one used for gas exchange to measure Ψleaf, while another was attached to an adjacent dark covered leaf to measure Ψstem. Psychrometer chambers were lightly lined with petroleum jelly to establish a good contact with the leaves (Savage et al., 1983; Campbell and McInnes, 1999). Destructive Ψleaf and Ψstem measurements using a pressure chamber were carried out during the time course to correct the psychrometer readings according to a relationship between spot scale (psychrometer) and leaf scale (pressure chamber) water potential measurements established a priori (Ψleaf=1.39 Ψpsychrometer, n=96, R 2=0.78; Ψstem=0.45 Ψpsychrometer, n=113, R 2=0.54). To maintain a good contact between the psychrometers and the leaves, the psychrometers were removed daily, inspected, and cleaned if necessary, before resetting them to the leaves. Furthermore, the dark cover for Ψstem was removed daily after the final gas exchange measurements, ~4–5h before lights off.
Reduction of drought-induced stomatal limitation using low C a
Reducing air [CO2] to very low concentrations around drought- or salt-stressed leaves leads to the opening of stomata to levels that result in a significant reduction of diffusional limitations (Centritto et al., 2003; Bunce, 2007). This technique was thus used on water-stressed leaves to decrease stomatal limitation and monitor changes in A, g m, and leaf hydraulics.
Prior to soil drying and subsequent low C a treatment, gas exchange of one leaf (LPI 5–7) was measured under well-watered conditions. Every other day, gas exchange parameters of the same leaf were measured to monitor changes in g sw and to adjust irrigation based on a g sw reduction of ~66% from the well-watered conditions. Soil water content (15cm TDR probe and Cable tester 1502C, Tektronix, Beaverton, OR, USA) and Ψsoil were monitored daily to adjust irrigation between gas exchange measurements. Well-watered plants were also monitored as comparison.
After 7 d of reduced irrigation, the low C a treatment was initiated. Gas exchange was measured first under ambient C a. The plant was then inserted in an ~13 l cylindrical cuvette designed to control the CO2 and H2O mole fractions around the plant. Between four and seven leaves, including the previously measured leaf, could be inserted in this large plant cuvette, while the upper and lower parts of the plant (~2–4 leaves each) remained under ambient growth chamber atmosphere (see Supplementary Fig. S2 at JXB online for a full description of the cuvette). The target leaf was clamped with the LI-6400XT LCF chamber inside the 13 l plant cuvette and both were sealed. The large plant cuvette operated as an open system, with an inflow of ~12 l min–1. The plant cuvette CO2 mole fraction was decreased to ~75 μmol mol–1 and the H2O mole fraction was maintained at ~17 mmol mol–1 by circulating the cuvette air through a H2O scrubbing Drierite™ column in series with a CO2 scrubbing column. A LI-840 CO2/H2O Analyzer (LI-COR Biosciences, Lincoln, NE, USA) was used to monitor the plant cuvette gas concentrations. Leaf temperature was maintained at 25±0.2 °C in the leaf chamber. Air temperature in the plant cuvette was ~26 °C, ~1 °C higher than the ambient air temperature outside the cuvette.
After g sw had peaked and stabilized, the CO2 mole fraction was increased in the leaf chamber to reach a C i of ~275 μmol mol–1 (see chlorophyll fluorescence calibration in the Results section) and C a in the large plant cuvette was increased in parallel to match the C a established in the leaf chamber. Once leaf gas exchange had reached steady state, three consecutive measurements were taken, each at least 2min apart.
Leaf hydraulic conductance
For both the short-term drought and the low C a experiment, K leaf was estimated using the evaporative flux method:
| (8) |
where E is leaf transpiration measured using the LI-6400XT, and Ψstem (stem water potential; measured on a dark-adapted leaf) and Ψleaf are measured using a Scholander-type pressure chamber (Model 610, PMS instruments, Albany, OR, USA) or the leaf psychrometers.
Vulnerability curves
Vulnerability curves were carried out using a low-pressure flow meter (LPFM; Sperry et al., 1988). Shoots were cut from plants in the growth chamber, put in a plastic bag, and brought to the lab. After an equilibration period of at least 5min, water potential was measured on a leaf. Stems were then cut under water, and stem segments of ~7cm long were prepared based on previous work by Harvey and van den Driessche (1997). Initial stem hydraulic conductivity (K i) was determined under a pressure head of 4.7 kPa by collecting water flowing out of the segment into a 10ml pipette and measuring the increasing water column in the pipette with a pressure transducer connected to a datalogger (CR23X, Campbell Scientific). When steady state was reached, stem segments were flushed at a pressure of 120 kPa for 7min to remove emboli before measuring maximum hydraulic conductivity (K max). Both flushes and conductivity measurements were carried out using a KCl (0.1M) solution filtered at 0.2 μm (Cai and Tyree, 2010). To reach more negative Ψstem values, shoots were left to dry in the lab and stem segments were measured upon reaching a desired value. Percentage loss conductivity (PLC) was calculated as:
| (9) |
The vulnerability curves were constructed by plotting PLC against Ψstem, and then fitting a Weibull function:
| (10) |
where b and c are fitting constants. Fitting was carried out using the nls function in R 3.0.0 (R Core Team, 2013).
Statistical analysis
Statistical analyses were carried out with R 3.0.0. Mixed models were used to determine the hierarchical structure (i.e. plants within a measurement cycle) using the nlme procedure (Pinheiro et al., 2013). Initial rates of leaf gas exchange were compared between clones to examine clonal differences. For the low C a experiment, final measurements were analysed using a two-level factorial design (clone×low C a treatment).
Results
Calibration of the chlorophyll fluorescence method for estimating the photochemical electron transport rates
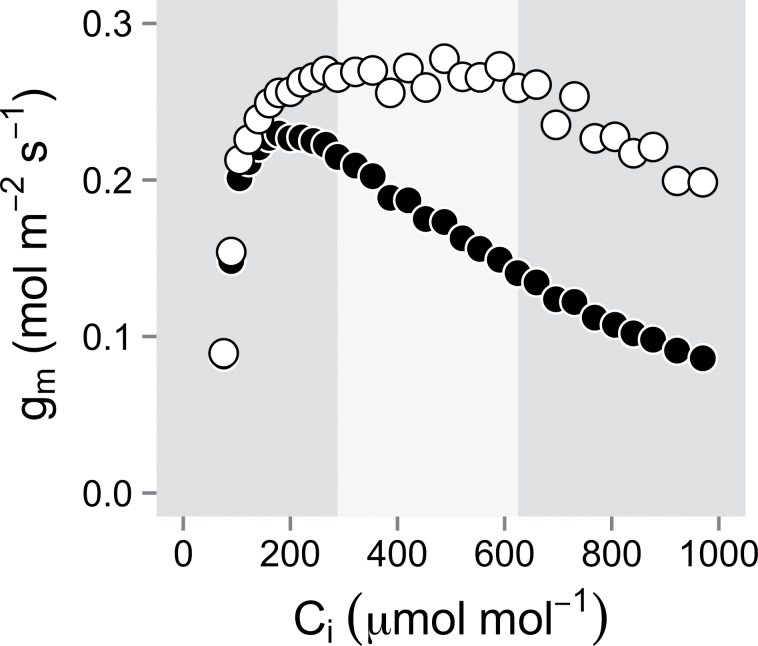
Estimates of g m based on the calibration of J f under 1% O2 exhibited a positively skewed shape when plotted against C i, with a narrow maximum at ~200 μmol mol–1 (Fig. 2). However, using the proposed alternative calibration method at 21% O2, g m estimates increased slightly and were more stable for several hundreds of ppm above a C i of 200 μmol mol–1 (Fig. 2). Thus, in order to improve the estimates of g m as C i decreases under soil drying conditions, C a was adjusted in the leaf chamber to reach a normalized C i of 275 μmol mol–1 (hereafter named C i=275), a value consistently identified as the minimal C i required to reach a stable and maximal g m. Using a normalized C i value resulted in <5% of g m estimates being discarded (i.e. negative or too high values) compared with 15% when measuring at ambient C a (380 μmol mol–1). Overall, there was a slight difference in s values between clones (see Fig. 1) and the proposed calibration method yielded s estimates that were ~5% lower than those found under 1% O2 (Fig. 2).
Fig. 2.
Apparent response of mesophyll conductance (g m) to intercellular CO2 mole fraction (C i) using the 1% O2 calibration method (filled circles) and the proposed 21% O2 A/C i calibration method (open circles) on one Okanese leaf. The light coloured area represents the RuBP-limited range where ΦPSII is found to be constant. Estimates of s under 1% and 21% O2 were 0.4799 and 0.4572, respectively.
Gas exchange responses to short-term drought
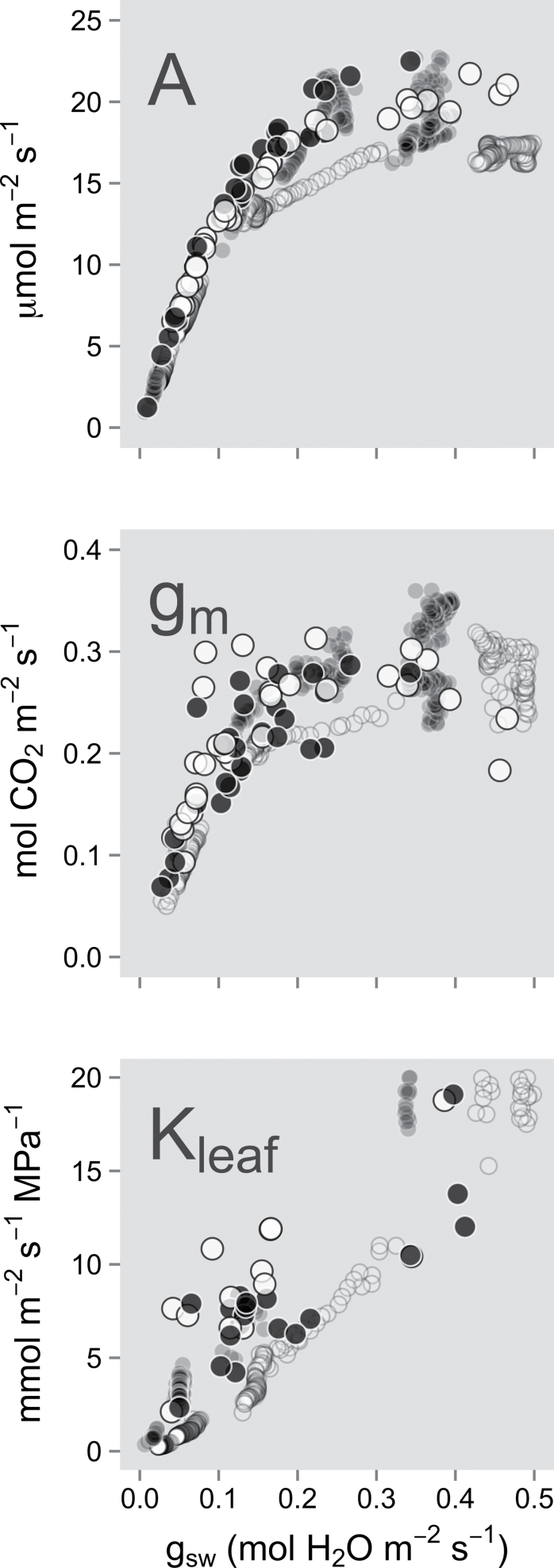
Drought-sensitive Assiniboine and drought-tolerant Okanese had, under well-watered conditions, a similar photosynthesis rate under ambient C a (A Ca=380; F 1,17=2.11, P=0.16), and a similar normalized g m_Ci=275 (F 1,16=2.93, P=0.11), although g sw was slightly higher in Assiniboine (F 1,17=3.62, P=0.07; Fig. 3). Twelve days of reduced irrigation gradually decreased photosynthesis (A Ca=380), g sw, transpiration (E), K leaf, and leaf and soil water potential (Ψleaf and Ψsoil) to very low values (e.g. a 90% decrease in g sw; see Figs 3 and 4). The decline in Ψleaf, E, and K leaf was concomitant with g sw, while the decline in A lagged behind K leaf. Normalized g m_Ci=275 remained constant over most of the drought-induced g sw range, and decreased only when g sw fell below a threshold of ~0.15mol m–2 s–1 (i.e. 68% and 56% of the maximum g sw for Assiniboine and Okanese, respectively). However, because too low or near zero g sw led to difficult if not impossible g m fits, g m_Ci=275 values below 0.05mol m–2 s–1 were not included in the analysis as scatter increased tremendously below that threshold, even using the proposed calibration method.
Fig. 3.
Changes in photosynthesis (A), mesophyll conductance (g m), and leaf hydraulic conductance (K leaf) in relation to stomatal conductance to water (g sw) during a short-term, moderate water stress. Three plants per clone were measured over 12 d of reduced irrigation, using the same leaf per plant. Assiniboine (drought sensitive; open circles), Okanese (drought tolerant; filled circles). Data from a separate 5 day soil drying experiment are also shown (translucent circles; one plant per clone; same colour code).
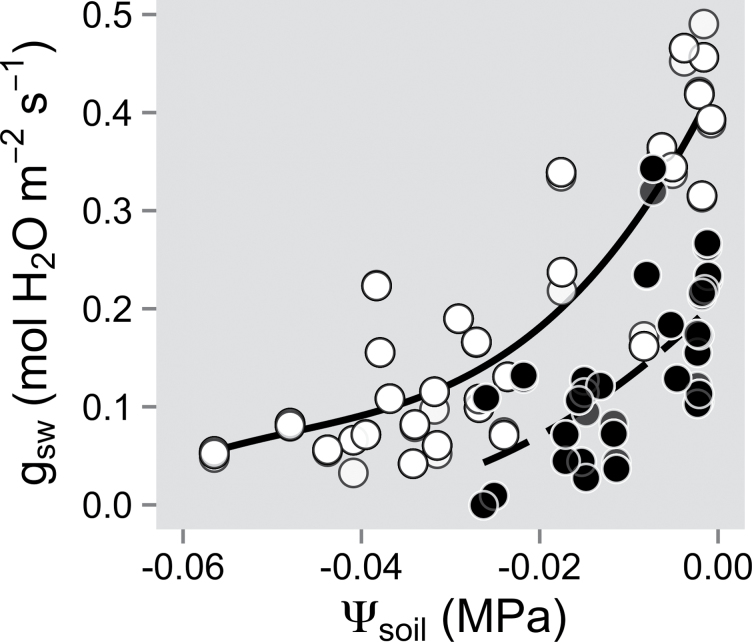
Fig. 4.
Responses of stomatal conductance (g sw) to decreasing soil water potential (Ψsoil) during the short-term drought experiment. A second-order polynomial curve was fitted for each clone [Assiniboine (open circles), R 2=0.79; and Okanese (filled circles), R 2=0.39; P < 0.0001].
Although the drought response of A Ca=380, K leaf, and g m_Ci=275 appeared similar for both clones when plotted against g sw, for any given Ψsoil, g sw of Okanese was always lower than that of Assiniboine. Hence, for Okanese, the minimal g sw was observed at a Ψsoil of –0.03MPa, compared with –0.06MPa for Assiniboine (Fig. 4). Assiniboine reached higher g sw values under well-watered conditions, but this did not translate into an increase in A Ca=380, and both clones exhibited a similar decline in A Ca=380 at common g sw. Similar gas exchange versus leaf hydraulic relationships to the 12 d reduced irrigation experiment were obtained over 5 d of water depletion during which A Ca=380, g sw, K leaf, and g m (C i=225–275 μmol mol–1) were recorded continuously. Once again, g sw and K leaf decreased in concert, the decline in A Ca=380 lagged behind the two previous variables, and g m remained constant up to a g sw threshold of ~0.15mol m–2 s–1 (Fig. 3; see also Supplementary Fig. S3 at JXB online).
Lowering C a to decrease drought-induced stomatal limitation
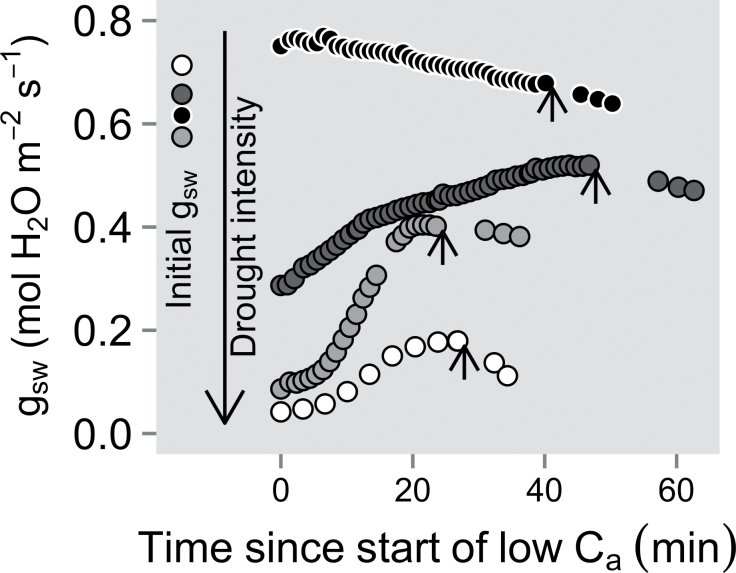
After 7 d of reduced irrigation, g sw was decreased by 75% on average in Assiniboine and 69% in Okanese. In both clones, lowering C a around leaves resulted in a 3- to 4-fold increase in g sw and E, which translated into a significantly more negative Ψleaf (Table 1). When C a was reduced, g sw reached a new steady state within 20–40min for water-stressed plants, and after 50–75min in well-watered plants (Fig. 5). Furthermore, g sw rapidly declined after a return to a higher C a in severely stressed plants (white dots in Fig. 5), while well-watered plants showed a more progressive return towards their initial g sw level. In contrast to g sw and Ψleaf, no significant changes in K leaf, A Ci=275, and g m_Ci=275 were observed under low C a. There were no clonal differences in gas exchange responses to low CO2 treatment under reduced irrigation, although Assiniboine exhibited higher K leaf than Okanese (Table 1).
Table 1.
Effect of low air CO2 concentration around leaves on gas exchange and leaf hydraulics of water-stressed [foliar transpiration (E) <3 mmol m–2 s–1] hybrid poplar clones contrasting in drought tolerance
| Clone (drought tolerance) | C a | A Ci=275 (μmol m–2 s–1)a | g m_Ci=275 (mol m–2 s–1) | g sw (mol m–2 s–1) | E (mmol m–2 s–1) | K leaf (mmol m–2 s–1 MPa–1) | Ψleaf (MPa) |
|---|---|---|---|---|---|---|---|
| Okanese | Ambient | 18.7 (2.4)b | 0.224 (0.042) | 0.115 (0.053) | 1.65 (0.63) | 6.43 (2.1) | –0.87 (0.07) |
| (tolerant) | Low | 20.9 (2.4) | 0.270 (0.036) | 0.325 (0.053) | 4.08 (0.63) | 9.28 (2.0) | –0.97 (0.07) |
| Assiniboine | Ambient | 21.7 (2.3) | 0.285 (0.037) | 0.119 (0.051) | 1.64 (0.59) | 12.2 (2.2) | –0.76 (0.07) |
| (sensitive) | Low | 15.0 (2.6) | 0.204 (0.040) | 0.206 (0.058) | 3.07 (0.68) | 14.5 (2.9) | –1.06 (0.08) |
| P-valuesc | Clone | 0.69 | 0.96 | 0.22 | 0.34 | 0.04 | 0.66 |
| C a | 0.35 | 0.59 | 0.007 | 0.007 | 0.21 | 0.01 | |
| Clone×C a | 0.06 | 0.10 | 0.23 | 0.44 | 0.90 | 0.21 |
a Photosynthesis (A Ci=275) and mesophyll conductance (g m_Ci=275) measured at a normalized C i of 275 μmol mol–1, stomatal conductance to water (g sw), transpiration (E), leaf hydraulic conductance (K leaf), and leaf water potential (Ψleaf).
b Standard error in parentheses.
c Numerator d.f. = 1; denominator d.f. = 20 (A Ci=275, g sw, Ψleaf, E), 17 (g m_Ci=275), 11 (K leaf).
Fig. 5.
Time courses of stomatal conductance (g sw) when the upper foliage of well-watered and water-stressed Assiniboine plants was exposed to a reduction in ambient CO2 concentration (C a ~75 μmol mol–1). Different levels of water stress were examined, expressed here as the percentage of g sw relative to their initial well-watered state (g sw_ini) evaluated 7 d prior to the low C a treatment (black circles, well-watered, where g sw increased compared with g sw_ini; dark grey circles, 44% of g sw_ini; light grey circles, 15% of g sw_ini; and white circles, 6% of g sw_ini). The short arrows indicate when C a was increased from ~75 μmol mol–1 to a CO2 concentration resulting in a C i of ~275 μmol mol–1. The data points to the right of the arrows were measured at that C i, showing the high sensitivity of severely stressed plants to the return to near ambient C a.
Vulnerability curves
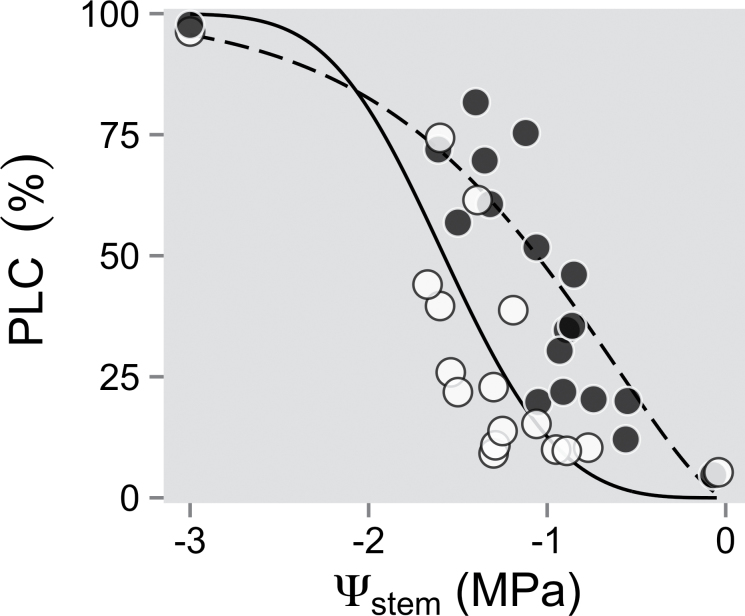
Since each clone exhibited a similar A versus g sw response to drought, as well as a similar response to low C a exposure, vulnerability to cavitation was measured to determine further the clonal differences in water stress response. Drought-tolerant Okanese exhibited a higher reduction in K stem over the observed Ψstem range compared with Assiniboine (Fig. 6). For instance, the PLC was up to 50% in Okanese upon reaching a Ψstem of –1MPa, while Assiniboine had ~15% PLC at the same potential. However, PLC increased rapidly after –1.2MPa Ψstem in Assiniboine to reach similar PLC values to Okanese near –1.5MPa (Fig. 6). Nevertheless, values of Ψleaf observed throughout the experiment were on average between –0.6MPa and –0.9MPa (first and third quartiles) for both clones, and Ψstem ranged between –0.3MPa and –0.6MPa. Thus, both clones usually did not reach a PLC of 50% but suffered only ~25% loss of conductivity under the moderate drought conditions of this study.
Fig. 6.
Stem vulnerability curve to cavitation (PLC: percentage loss conductivity) in drought-tolerant Okanese (filled circles) and drought-sensitive Assiniboine (open circles) hybrid poplar clones.
Discussion
Methodological considerations on the estimation of g m
Potential methodological errors and the underlying assumptions necessary in the estimation of g m using the variable J method were discussed initially by Harley et al. (1992), and subsequently elaborated upon by several other authors (e.g. Warren 2008; Pons et al., 2009; Gilbert et al., 2012). Theoretically, positively skewed g m versus C i curves like those observed in this study when following the standard 1% O2 J f calibration protocol (see also Flexas et al., 2007b; Hassiotou et al., 2009; Gilbert et al., 2012) are predicted to result from small overestimations of J f, and/or possibly from underestimations of Γ*, or of R d (see Harley et al., 1992; Gilbert et al., 2012). However, Tholen et al. (2012) demonstrated that even if all relevant parameters in the variable J method equation were exact, g m would still be expected to decrease apparently sharply when the photorespiratory CO2 flux rises at low C i. One way to alleviate this artefactual CO2 sensitivity problem is by measuring g m at a normalized C i, preferably around the g m versus C i curve region where g m is expected to peak (i.e. the narrow C i range where estimates of g m are expected to be least affected by small errors of J f, etc.; see Fig. 2). However, even after taking this precaution, it was noted that ~5% of the initial g m estimates for leaves subjected to water stress were deemed unreliable. One possibility that could explain this poor model performance is that the 1% O2 calibration method incompletely accounts for alternative electron sinks under 21% O2; especially under water stress (Makino et al., 2002; Kitao et al., 2003; Laisk et al., 2006). In an attempt to address this possibility, we tested an alternative J f calibration method performed under 21% O2, and which solves s and g m concurrently from the CO2 response of photosynthesis and ΦPSII in the RuBP-limited A–C i curve region where photorespiration is expected to be low enough to exert little influence on g m (see Fig. 2, and further details in the Materials and methods). From the present assessment, this alternative J f calibration procedure yielded slightly lower photochemical electron transport rates than the 1% O2 calibration method, which resulted in ~20% higher g m estimates, but did not improve the rejection of data under water stress conditions. Nevertheless, it is believed that calibrating ΦCO2 under 21% O2 should better account for potential alternative electron sinks under normal atmospheric conditions while greatly simplifying the calibration procedure.
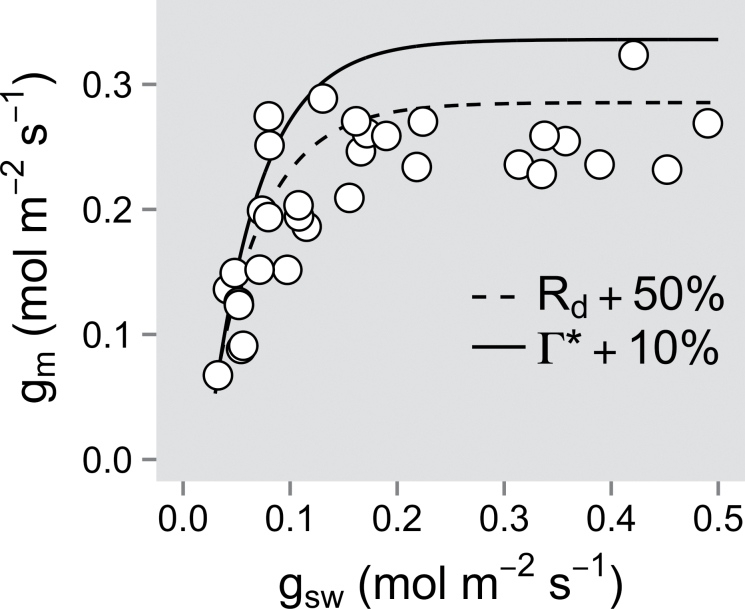
To evaluate possible g m inaccuracies introduced by errors in the estimation of Γ* and R d, an analysis of the sensitivity of g m to changes in Γ* (±10%) and R d (±50%) was performed. As already pointed out by others (e.g. Harley et al., 1992; Pons et al., 2009), g m was significantly more sensitive to potential errors in Γ* in comparison with R d (see Fig.7)—in the present simulations, a 120% increase in R d was necessary to obtain a g m value corresponding to a +10% change in Γ*. However, because the estimation of g m was restricted to a narrow C i range neighbouring 275 μmol mol–1, such errors in Γ* and/or R d are not expected to change the conclusions regarding the delayed threshold response of g m with respect to g sw (see Fig. 7).
Fig. 7.
Sensitivity of mesophyll conductance (g m) estimates to a 10% increase in chloroplastic CO2 photocompensation point (Γ*, solid line) or a 50% increase in non-photorespiratory mitochondrial respiration in the light (R d, dashed line). Asymptotic fits of the simulated data were used to represent the observed variations. Data from Assiniboine in Fig. 3 were used (original data: open circles).
Threshold response of g m to drought
Drought stress usually decreases A, g sw, and K leaf concomitantly (Brodribb and Holbrook, 2006; Brodribb et al., 2007; Warren, 2008; Galle et al., 2009; Barbour et al., 2010). Drought stress has also been shown to decrease g m monotonically with respect to A and g sw (Flexas et al., 2007b, 2008; Warren, 2008; Perez-Martin et al., 2009), although Flexas et al. (2002) reported a lower rate of g m decrease at g sw >0.15mol H2O m–2 s–1 in grapevine. However, these declines in g m may only be circumstantial since a recent study has shown that g m is expected to decrease apparently sharply in the absence of any change in intraleaf diffusional resistance component, once the photorespiratory rate of a water-stressed leaf rises significantly due to stomatal closure and ensuing reduction in C i (Tholen et al., 2012). Measuring g m at a normalized C i alleviates this effect, which allowed detection of a threshold response of g m with respect to g sw (~0.15mol H2O m–2 s–1), thereby confirming the observations of Ferrio et al. (2012). This recent study demonstrated a strong linear relationship between g m and K lamina (hydraulic conductance of the leaf lamina), but only when K lamina fell below 8 mmol H2O m–2 s–1 MPa–1 (~70% of maximum value) as a result of water stress. Above this threshold point, corresponding to g m values ≥0.2mol m–2 s–1, the relationship did not hold, as the effective water path length influencing K lamina reached a constant baseline value. Hence, the g m–K lamina sensitivity region corresponded to the point where the water path length between the xylem and the evaporative surface increased significantly above this baseline value.
The small difference in g m threshold between the study of Ferrio et al. and the present study could arise from the different species and their respective hydraulic connectivity, but also because a constant C i was used for g m measurement with the variable J method (Harley et al., 1992). Using a normalized C i improved g m estimates, as they reached similar values between clones. Nonetheless, in both studies, g sw responded rapidly to early signs of water stress, and g m followed afterwards. Concomitantly, ΦPSII, although not an ideal proxy for g m, has shown a delay in decrease as Ψleaf declined, beginning its descent often past a g sw reduction of 50% or even 80% (Brodribb and Holbrook, 2003), as observed in the present study (Fig. 3). Hydraulic compartmentalization of the mesophyll might have contributed to this delayed response of g m. Two factors are important when considering compartmentalization: the linkage between the xylem and the epidermis, and the degree of uncoupling of the mesophyll cell to the hydraulic apparatus (Zwieniecki et al., 2007). Thus, stomata, sensing early signs of water stress through small changes in Ψleaf, could have started to close while more negative Ψleaf values (i.e. more severe stress) may have been needed to initiate a response in mesophyll cells or chloroplasts, leading to a decrease in g m. Zwieniecki et al. (2007) explained that such hydraulic compartmentalization would allow mesophyll cells to be buffered against short-term changes in leaf water status, a beneficial characteristic to C-assimilating cells. Also, the bulk of mesophyll cells have been shown to be rather unresponsive to exogeneously applied abscisic acid (ABA) (Shatil-Cohen et al., 2011), which reinforces the idea that mesophyll cells are somewhat isolated from the main hydraulic path linking the xylem conduits and their bundle sheath extensions to the epidermis and stomata. Such structural considerations may act in conjunction with membrane-associated mechanisms such as gating of aquaporin channels and ensuing reduction of the symplastic water transport (Cochard et al., 2007; Almeida-Rodriguez et al., 2010; Pou et al., 2013), which might eventually divert more water to the more tortuous apoplastic pathway (Ferrio et al., 2012). The above model appears to fit well the hydraulic response of the hybrid poplar leaves despite the fact that it makes little mention of xylem cavitation. Certainly, the present results show that Ψstem did not reach low enough values to induce substantial stem cavitation (see also Secchi and Zwieniecki, 2010), but this does not necessarily mean that significant leaf cavitation could not have occurred. Indeed, Sack et al. (2004, 2005) reported that the xylem-related component of total leaf resistance dominates in highly transpiring plants such as hybrid poplar, and recently Johnson et al. (2012) demonstrated that in some species leaf xylem cavitation may account for most of the decrease in K leaf under drought.
Exposure to low C a decouples g s and g m
Centritto et al. (2003) proposed a method to separate diffusional limitations from non-diffusional limitations in salt-stressed plants: they lowered C a to force stomata to open, thus reducing stomatal limitation upon returning to ambient C a. Surprisingly, the low C a treatment appeared to reduce the mesophyll limitation just as rapidly as the stomatal limitation. However, the C i values at which Centritto et al. (2003) evaluated g m after pre-conditioning the stressed plants to low C a was appreciably higher than before the low C a treatment. Thus, because g m was not evaluated at a normalized C i in this study, it is likely that the conclusions of Centritto et al. (2003) about the rapid response of g m to salt stress were confounded by the low C i photorespiratory artefacts described in Tholen et al. (2012). In the present case, C i was controlled close to 275 μmol mol–1 throughout and no significant change in g m in response to low C a pre-conditioning was detected.
Apart from its obvious consequences on gas exchange, low C a treatment has major consequences for the leaf water status. As in the present study, Bunce (2007) reported a decrease (more negative) in Ψleaf using low C a on a variety of water-stressed herbaceous and woody plants. Interestingly, the hybrid poplar plants used here reached a common Ψleaf value (approximately –0.92MPa to –1.01MPa) under low C a regardless of drought intensity, which suggests that the degree of stomatal opening could have been modulated to keep Ψleaf above a catastrophic cavitation level (see also the results of Tardieu and Simonneau, 1998). If leaf xylem cavitation and concomitant decrease in Ψleaf were responsible for the decline in K leaf under drought, K leaf would have been expected to decrease more as a result of the low C a treatment since Ψleaf was further reduced (see Table 1 compared with Supplementary Fig. S4 at JXB online). However, it did not, and similar observations by Bunce (2007) led him to question the dominant role of leaf cavitation in drought-induced K leaf decline. One recent alternative explanation is a possible dual regulation of g sw and K leaf by ABA under soil drying conditions (Pantin et al., 2013). Indeed, in the present study, water-stressed plants exhibited greater stomatal sensitivity to changes in C a than well-watered plants (Fig. 5), a trait normally associated with the presence of higher levels of ABA (Raschke, 1975; Bunce, 2007).
Cochard et al. (2007) reported that aquaporin expression in the leaf vein bundle sheaths could be a major control point on leaf water status, observing a substantial drop in Ψleaf when aquaporin activity was inhibited. Shatil-Cohen et al. (2011) have also shown that either drought or exogenous application of ABA triggered a reduction of the permeability coefficient (and thus K leaf) of bundle sheath cells, but not that of the mesophyll cells. As previously mentioned, this suggests a partial hydraulic isolation of mesophyll cells from the xylem–bundle sheath–stomata pathway, thereby possibly delaying ABA delivery. Since certain aquaporins have been reported to have a CO2 channel role (Otto et al., 2010; Uehlein et al., 2012), it may be that g m is less sensitive to drought stress than g sw or K leaf because regulation of mesophyll aquaporins is expected to take place at a more advanced drought stress due to hydraulic compartmentalization.
Delayed g m response to drought favours water use efficiency in hybrid poplar
One aim of the present study was to compare two clones of contrasting drought tolerance in order to determine if they differ in their g sw, K leaf, and g m relationships. Both drought-tolerant Okanese and drought-sensitive Assiniboine exhibited a similar g m threshold response to drought (Fig. 3), potentially suggesting a partial hydraulic isolation of the mesophyll cells. This in turn allowed a delay in the decline of A, thereby favouring a higher water use efficiency (WUE) during the early stages of drought. However, those clones differed in their vulnerability to stem cavitation and in the Ψsoil at which their respective minimal g sw was reached (Fig. 4), as observed in other hybrid poplar studies (Silim et al., 2009; Arango-Velez et al., 2011; Larcheveque et al., 2011). Although the vulnerability curves in the present study showed that Okanese allowed more cavitation to occur at similar Ψstem values than Assiniboine (Fig. 6), both clones modulated g sw to maintain Ψstem at levels preventing conductivity to drop below ~25%, a feature previously observed for those same clones under severe drought stress (Arango-Velez et al., 2011). While Okanese maintained similar A Ca=380 and g m to Assiniboine at a given g sw, it adopted a more conservative water use strategy by lowering g sw more than Assiniboine under increasing soil water depletion (see also Arango-Velez et al., 2011). Hence, Okanese exhibited a higher intrinsic water use efficiency (WUEi) than Assiniboine at comparable Ψsoil (it also displayed a higher g m/g sw ratio; data not shown). Such maintenance of high g m/g sw ratios has recently been identified as an important trait to improve WUE in cultivated plants (Barbour et al., 2010; Flexas et al., 2013).
Conclusion
When subjecting young hybrid poplar clones of contrasting drought tolerance to a short period of water stress, K leaf and g sw decreased monotonically in concert, while g m remained constant over most of its range, causing a delay in the decline of A. Only when g sw fell below an approximate threshold of 0.15mol m–2 s–1, ~33% (Assiniboine) to 40% (Okanese) of maximum g sw, was a decrease in g m observed (and this even when measured at a normalized C i to remove the photorespiratory bias modelled by Tholen et al., 2012), in accordance with the recent results of Ferrio et al. (2012). The present results thus support the recent suggestion that the bulk mesophyll is partially isolated (i.e. buffered) from the major transpiration pathway delivering ABA to the stomata (Shatil-Cohen et al., 2011; Pantin et al., 2013). The delayed photosynthetic decline mediated by such a g m threshold response resulted in enhancing WUE in the early stage of drought.
Supplementary data
Supplementary data are available at JXB online.
Figure S1. Apparent sensitivity of mesophyll conductance (g m) to intercellular CO2 mole fraction (C i): comparison of the responses observed under three different g m estimation methods.
Figure S2. Schematic of the large plant cuvette used in the low C a experiment.
Figure S3. Continuous measurements of photosynthesis (A), mesophyll conductance (g m), stomatal conductance to water (g sw), and leaf hydraulic conductance (K leaf) during 5 d of water depletion.
Figure S4. Relationship between leaf hydraulic conductance (K leaf) and leaf water potential (Ψleaf) during 5 d of water depletion.
Acknowledgements
We are grateful to Bill Schroeder from Agriculture and Agri-Food Canada, Indian Head, SK, for providing us with hybrid poplar cuttings. We thank two anonymous reviewers for their useful comments. This work was supported by a National Science and Engineering Research Council of Canada (NSERC) Discovery grant to SP, and NSERC and Fonds québécois de recherche–Nature et technologies (FQRNT) graduate scholarships to GTR.
References
- Aasamaa K, Niinemets U, Sober A. 2005. Leaf hydraulic conductance in relation to anatomical and functional traits during Populus tremula leaf ontogeny. Tree Physiology 25, 1409–1418 [DOI] [PubMed] [Google Scholar]
- Almeida-Rodriguez AM, Cooke JE, Yeh F, Zwiazek JJ. 2010. Functional characterization of drought-responsive aquaporins in Populus balsamifera and Populus simonii×balsamifera clones with different drought resistance strategies. Physiologia Plantarum 140, 321–333 [DOI] [PubMed] [Google Scholar]
- Arango-Velez A, Zwiazek JJ, Thomas BR, Tyree MT. 2011. Stomatal factors and vulnerability of stem xylem to cavitation in poplars. Physiologia Plantarum 143, 154–165 [DOI] [PubMed] [Google Scholar]
- Balaguer L, Affi D, Dizengremel P, Dreyer E. 1996. Specificity factor of ribulose bisphosphate carboxylase/oxygenase of Quercus robur . Plant Physiology and Biochemistry 34, 879–883 [Google Scholar]
- Barbour MM, Warren CR, Farquhar GD, Forrester G, Brown H. 2010. Variability in mesophyll conductance between barley genotypes, and effects on transpiration efficiency and carbon isotope discrimination. Plant, Cell and Environment 33, 1176–1185 [DOI] [PubMed] [Google Scholar]
- Bernacchi CJ, Portis AR, Nakano H, von Caemmerer S, Long SP. 2002. Temperature response of mesophyll conductance. Implications for the determination of Rubisco enzyme kinetics and for limitations to photosynthesis in vivo . Plant Physiology 130, 1992–1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bota J, Flexas J, Keys AJ, Loveland J, Parry MAJ, Medrano H. 2002. CO2/O2 specificity factor of ribulose-1,5-bisphosphate carboxylase/oxygenase in grapevines (Vitis vinifera L.): first in vitro determination and comparison to in vivo estimations. Vitis 41, 163–168 [Google Scholar]
- Brodribb TJ, Feild TS, Jordan GJ. 2007. Leaf maximum photosynthetic rate and venation are linked by hydraulics. Plant Physiology 144, 1890–1898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodribb TJ, Holbrook NM. 2003. Stomatal closure during leaf dehydration, correlation with other leaf physiological traits. Plant Physiology 132, 2166–2173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodribb TJ, Holbrook NM. 2004. Diurnal depression of leaf hydraulic conductance in a tropical tree species. Plant, Cell and Environment 27, 820–827 [Google Scholar]
- Brodribb TJ, Holbrook NM. 2006. Declining hydraulic efficiency as transpiring leaves desiccate: two types of response. Plant, Cell and Environment 29, 2205–2215 [DOI] [PubMed] [Google Scholar]
- Bunce JA. 2007. Low carbon dioxide concentrations can reverse stomatal closure during water stress. Physiologia Plantarum 130, 552–559 [Google Scholar]
- Cai J, Tyree MT. 2010. The impact of vessel size on vulnerability curves: data and models for within-species variability in saplings of aspen, Populus tremuloides Michx. Plant, Cell and Environment 33, 1059–1069 [DOI] [PubMed] [Google Scholar]
- Campbell CS, McInnes KJ. 1999. Response of in situ leaf psychrometer to cuticle removal by abrasion. Agronomy Journal 91, 859–862 [Google Scholar]
- Centritto M, Lauteri M, Monteverdi MC, Serraj R. 2009. Leaf gas exchange, carbon isotope discrimination, and grain yield in contrasting rice genotypes subjected to water deficits during the reproductive stage. Journal of Experimental Botany 60, 2325–2339 [DOI] [PubMed] [Google Scholar]
- Centritto M, Loreto F, Chartzoulakis K. 2003. The use of low [CO2] to estimate diffusional and non-diffusional limitations of photosynthetic capacity of salt-stressed olive saplings. Plant, Cell and Environment 26, 585–594 [Google Scholar]
- Cochard H, Venisse JS, Barigah TS, Brunel N, Herbette S, Guilliot A, Tyree MT, Sakr S. 2007. Putative role of aquaporins in variable hydraulic conductance of leaves in response to light. Plant Physiology 143, 122–133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan B, Ran F, Zhang X, Zhang Y. 2011. Long-term acclimation of mesophyll conductance, carbon isotope discrimination and growth in two contrasting Picea asperata populations exposed to drought and enhanced UV-B radiation for three years. Agricultural and Forest Meteorology 151, 116–126 [Google Scholar]
- Ethier GJ, Livingston NJ. 2004. On the need to incorporate sensitivity to CO2 transfer conductance into the Farquhar–von Caemmerer–Berry leaf photosynthesis model. Plant, Cell and Environment 27, 137–153 [Google Scholar]
- Ethier GJ, Livingston NJ, Harrison DL, Black TA, Moran JA. 2006. Low stomatal and internal conductance to CO2 versus Rubisco deactivation as determinants of the photosynthetic decline of ageing evergreen leaves. Plant, Cell and Environment 29, 2168–2184 [DOI] [PubMed] [Google Scholar]
- Evans JR, Kaldenhoff R, Genty B, Terashima I. 2009. Resistances along the CO2 diffusion pathway inside leaves. Journal of Experimental Botany 60, 2235–2248 [DOI] [PubMed] [Google Scholar]
- Evans JR, Sharkey TD, Berry JA, Farquhar GD. 1986. Carbon isotope discrimination measured concurrently with gas exchange to investigate CO2 diffusion in leaves of higher plants. Australian Journal of Plant Physiology 13, 281–292 [Google Scholar]
- Ferrio JP, Cuntz M, Offermann C, Siegwolf R, Saurer M, Gessler A. 2009. Effect of water availability on leaf water isotopic enrichment in beech seedlings shows limitations of current fractionation models. Plant, Cell and Environment 32, 1285–1296 [DOI] [PubMed] [Google Scholar]
- Ferrio JP, Pou A, Florez-Sarasa I, Gessler A, Kodama N, Flexas J, Ribas-Carbo M. 2012. The Peclet effect on leaf water enrichment correlates with leaf hydraulic conductance and mesophyll conductance for CO2 . Plant, Cell and Environment 35, 611–625 [DOI] [PubMed] [Google Scholar]
- Fleck I, Peña-Rojas K, Aranda X. 2010. Mesophyll conductance to CO2 and leaf morphological characteristics under drought stress during Quercus ilex L. resprouting. Annals of Forest Science 67, 308–308 [Google Scholar]
- Flexas J, Bota J, Escalona JM, Sampol B, Medrano H. 2002. Effects of drought on photosynthesis in grapevines under field conditions: an evaluation of stomatal and mesophyll limitations. Functional Plant Biology 29, 461–471 [DOI] [PubMed] [Google Scholar]
- Flexas J, Diaz-Espejo A, Berry J A, Cifre J, Galmes J, Kaldenhoff R, Medrano H, Ribas-Carbo M. 2007a. Analysis of leakage in IRGA’s leaf chambers of open gas exchange systems: quantification and its effects in photosynthesis parameterization. Journal of Experimental Botany 58, 1533–1543 [DOI] [PubMed] [Google Scholar]
- Flexas J, Diaz-Espejo A, Galmes J, Kaldenhoff R, Medrano H, Ribas-Carbo M. 2007b. Rapid variations of mesophyll conductance in response to changes in CO2 concentration around leaves. Plant, Cell and Environment 30, 1284–1298 [DOI] [PubMed] [Google Scholar]
- Flexas J, Niinemets U, Galle A, et al. 2013. Diffusional conductances to CO2 as a target for increasing photosynthesis and photosynthetic water-use efficiency. Photosynthesis Research. 117, 45–59 [DOI] [PubMed] [Google Scholar]
- Flexas J, Ribas-Carbo M, Diaz-Espejo A, Galmes J, Medrano H. 2008. Mesophyll conductance to CO2: current knowledge and future prospects. Plant, Cell and Environment 31, 602–621 [DOI] [PubMed] [Google Scholar]
- Galle A, Florez-Sarasa I, Tomas M, Pou A, Medrano H, Ribas-Carbo M, Flexas J. 2009. The role of mesophyll conductance during water stress and recovery in tobacco (Nicotiana sylvestris): acclimation or limitation? Journal of Experimental Botany 60, 2407–2418 [DOI] [PubMed] [Google Scholar]
- Galmès J, Medrano H, Flexas J. 2006. Acclimation of Rubisco specificity factor to drought in tobacco: discrepancies between in vitro and in vivo estimations. Journal of Experimental Botany 57, 3659–3667 [DOI] [PubMed] [Google Scholar]
- Gilbert ME, Pou A, Zwieniecki MA, Holbrook NM. 2012. On measuring the response of mesophyll conductance to carbon dioxide with the variable J method. Journal of Experimental Botany 63, 413–425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harley PC, Loreto F, Di M, Giorgio, Sharkey TD. 1992. Theoretical considerations when estimating the mesophyll conductance to CO2 flux by analysis of the response of photosynthesis to CO2 . Plant Physiology 98, 1429–1436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey HP, van den Driessche R. 1997. Nutrition, xylem cavitation and drought resistance in hybrid poplar. Tree Physiology 17, 647–654 [DOI] [PubMed] [Google Scholar]
- Hassiotou F, Ludwig M, Renton M, Veneklaas EJ, Evans JR. 2009. Influence of leaf dry mass per area, CO2, and irradiance on mesophyll conductance in sclerophylls. Journal of Experimental Botany 60, 2303–2314 [DOI] [PubMed] [Google Scholar]
- Johnson DM, McCulloh KA, Woodruff DR, Meinzer FC. 2012. Evidence for xylem embolism as a primary factor in dehydration-induced declines in leaf hydraulic conductance. Plant, Cell and Environment 35, 760–769 [DOI] [PubMed] [Google Scholar]
- Kaldenhoff R. 2012. Mechanisms underlying CO2 diffusion in leaves. Current Opinion in Plant Biology 15, 276–281 [DOI] [PubMed] [Google Scholar]
- Kaldenhoff R, Ribas-Carbo M, Sans JF, Lovisolo C, Heckwolf M, Uehlein N. 2008. Aquaporins and plant water balance. Plant, Cell and Environment 31, 658–666 [DOI] [PubMed] [Google Scholar]
- Kitao M, Lei TT, Koike T, Tobita H, Maruyama Y. 2003. Higher electron transport rate observed at low intercellular CO2 concentration in long-term drought-acclimated leaves of Japanese mountain birch (Betula ermanii). Physiologia Plantarum 118, 406–413 [Google Scholar]
- Laisk A, Eichelmann H, Oja V, Rasulov B, Ramma H. 2006. Photosystem II cycle and alternative electron flow in leaves. Plant and Cell Physiology 47, 972–983 [DOI] [PubMed] [Google Scholar]
- Larcheveque M, Maurel M, Desrochers A, Larocque GR. 2011. How does drought tolerance compare between two improved hybrids of balsam poplar and an unimproved native species? Tree Physiology 31, 240–249 [DOI] [PubMed] [Google Scholar]
- Larson PR, Isebrands JG. 1971. The plastochron index as applied to developmental studies of cottonwood. Canadian Journal of Forest Research 1, 1–11 [Google Scholar]
- LI-COR Biosciences 2012. LI-6400 XT user manual. Lincoln, NE: LI-COR Biosciences [Google Scholar]
- Loreto F, Harley PC, Di, Marco G, Sharkey TD. 1992. Estimation of mesophyll conductance to CO2 flux by three different methods. Plant Physiology 98, 1437–1443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makino A, Miyake C, Yokota A. 2002. Physiological functions of the water–water cycle (Mehler reaction) and the cyclic electron flow around PSI in rice leaves. Plant and Cell Physiology 43, 1017–1026 [DOI] [PubMed] [Google Scholar]
- Maurel C, Verdoucq L, Luu DT, Santoni V. 2008. Plant aquaporins: membrane channels with multiple integrated functions. Annual Review of Plant Biology 59, 595–624 [DOI] [PubMed] [Google Scholar]
- Otto B, Uehlein N, Sdorra S, et al. 2010. Aquaporin tetramer composition modifies the function of tobacco aquaporins. Journal of Biological Chemistry 285, 31253–31260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantin F, Monnet F, Jannaud D, Costa JM, Renaud J, Muller B, Simonneau T, Genty B. 2013. The dual effect of abscisic acid on stomata. New Phytologist 197, 65–72 [DOI] [PubMed] [Google Scholar]
- Perez-Martin A, Flexas J, Ribas-Carbo M, Bota J, Tomas M, Infante JM, Diaz-Espejo A. 2009. Interactive effects of soil water deficit and air vapour pressure deficit on mesophyll conductance to CO2 in Vitis vinifera and Olea europaea . Journal of Experimental Botany 60, 2391–2405 [DOI] [PubMed] [Google Scholar]
- Pinheiro J, Bates D, DebRoy S, Sarkar D, R Core Team 2013. nlme: linear and nonlinear mixed effects models R package version 3.1–109
- Pons TL, Flexas J, von Caemmerer S, Evans JR, Genty B, Ribas-Carbo M, Brugnoli E. 2009. Estimating mesophyll conductance to CO2: methodology, potential errors, and recommendations. Journal of Experimental Botany 60, 2217–2234 [DOI] [PubMed] [Google Scholar]
- Pou A, Medrano H, Flexas J, Tyerman SD. 2013. A putative role for TIP and PIP aquaporins in dynamics of leaf hydraulic and stomatal conductances in grapevine under water stress and re-watering. Plant, Cell and Environment 36, 828–843 [DOI] [PubMed] [Google Scholar]
- Raschke K. 1975. Simultaneous requirement of carbon dioxide and abscisic acid for stomatal closing in Xanthium strumarium L. Planta 125, 243–259 [DOI] [PubMed] [Google Scholar]
- R Core Team 2013. R: a language and environment for statistical computing R Foundation for Statistical Computing, Vienna, Austria. http://www.R-project.org/
- Resco V, Ewers BE, Sun W, Huxman TE, Weltzin JF, Williams DG. 2009. Drought-induced hydraulic limitations constrain leaf gas exchange recovery after precipitation pulses in the C3 woody legume, Prosopis velutina . New Phytologist 181, 672–682 [DOI] [PubMed] [Google Scholar]
- Sack L, Holbrook NM. 2006. Leaf hydraulics. Annual Review of Plant Biology 57, 361–381 [DOI] [PubMed] [Google Scholar]
- Sack L, Streeter CM, Holbrook NM. 2004. Hydraulic analysis of water flow through leaves of sugar maple and red oak. Plant Physiology 134, 1824–1833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sack L, Tyree MT, Holbrook NM. 2005. Leaf hydraulic architecture correlates with regeneration irradiance in tropical rainforest trees. New Phytologist 167, 403–413 [DOI] [PubMed] [Google Scholar]
- Savage MJ, Wiebe HH, Cass A. 1983. In situ field measurement of leaf water potential using thermocouple psychrometers. Plant Physiology 73, 609–613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Secchi F, Zwieniecki MA. 2010. Patterns of PIP gene expression in Populus trichocarpa during recovery from xylem embolism suggest a major role for the PIP1 aquaporin subfamily as moderators of refilling process. Plant, Cell and Environment 33, 1285–1297 [DOI] [PubMed] [Google Scholar]
- Shatil-Cohen A, Attia Z, Moshelion M. 2011. Bundle-sheath cell regulation of xylem–mesophyll water transport via aquaporins under drought stress: a target of xylem-borne ABA? The Plant Journal 67, 72–80 [DOI] [PubMed] [Google Scholar]
- Silim S, Nash R, Reynard D, White B, Schroeder W. 2009. Leaf gas exchange and water potential responses to drought in nine poplar (Populus spp.) clones with contrasting drought tolerance. Trees-Structure and Function 23, 959–969 [Google Scholar]
- Sperry JS, Donnelly JR, Tyree MT. 1988. A method for measuring hydraulic conductivity and embolism in xylem. Plant, Cell and Environment 11, 35–40 [Google Scholar]
- Tardieu F, Simonneau T. 1998. Variability among species of stomatal control under fluctuating soil water status and evaporative demand: modelling isohydric and anisohydric behaviours. Journal of Experimental Botany 49, 419–432 [Google Scholar]
- Tholen D, Ethier G, Genty B, Pepin S, Zhu XG. 2012. Variable mesophyll conductance revisited: theoretical background and experimental implications. Plant, Cell and Environment 35, 2087–2103 [DOI] [PubMed] [Google Scholar]
- Tholen D, Zhu XG. 2011. The mechanistic basis of internal conductance: a theoretical analysis of mesophyll cell photosynthesis and CO2 diffusion. Plant Physiology 156, 90–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyree MT, Sperry JS. 1988. Do woody plants operate near the point of catastrophic xylem dysfunction caused by dynamic water stress? Answers from a model. Plant Physiology 88, 574–580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uehlein N, Otto B, Hanson DT, Fischer M, McDowell N, Kaldenhoff R. 2008. Function of Nicotiana tabacum aquaporins as chloroplast gas pores challenges the concept of membrane CO2 permeability. The Plant Cell 20, 648–657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uehlein N, Sperling H, Heckwolf M, Kaldenhoff R. 2012. The Arabidopsis aquaporin PIP1;2 rules cellular CO2 uptake. Plant, Cell and Environment 35, 1077–1083 [DOI] [PubMed] [Google Scholar]
- von Caemmerer S, Evans JR, Hudson GS, Andrews TJ. 1994. The kinetics of ribulose-1,5-bisphosphate carboxylase/oxygenase in vivo inferred from measurements of photosynthesis in leaves of transgenic tobacco. Planta 195, 88–97 [Google Scholar]
- Warren CR. 2008. Soil water deficits decrease the internal conductance to CO2 transfer but atmospheric water deficits do not. Journal of Experimental Botany 59, 327–334 [DOI] [PubMed] [Google Scholar]
- Zwieniecki MA, Brodribb TJ, Holbrook NM. 2007. Hydraulic design of leaves: insights from rehydration kinetics. Plant, Cell and Environment 30, 910–921 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.