Abstract
The purified amino-terminal fragment (ATF) of human urokinase plasminogen activator (residues 1-135), which is not required for activation of plasminogen, binds with high affinity to specific plasma membrane receptors on U937 monocytes. Intact urokinase efficiently competes for 125I-labeled ATF binding; 50% competition occurs with 1 nM urokinase. A large part of receptor-bound urokinase remains on the cell surface for at least 2 hr at 37 degrees C. Differentiation of U937 monocytes into macrophage-like cells specifically increases ATF binding 10- to 20-fold. These results suggest an important role for urokinase in monocyte/macrophage biology: the native enzyme binds to the cells with the amino-terminal domain; the catalytic, carboxyl-terminal domain remains exposed on the cell surface to stimulate localized proteolysis and facilitate cell migration.
Full text
PDF
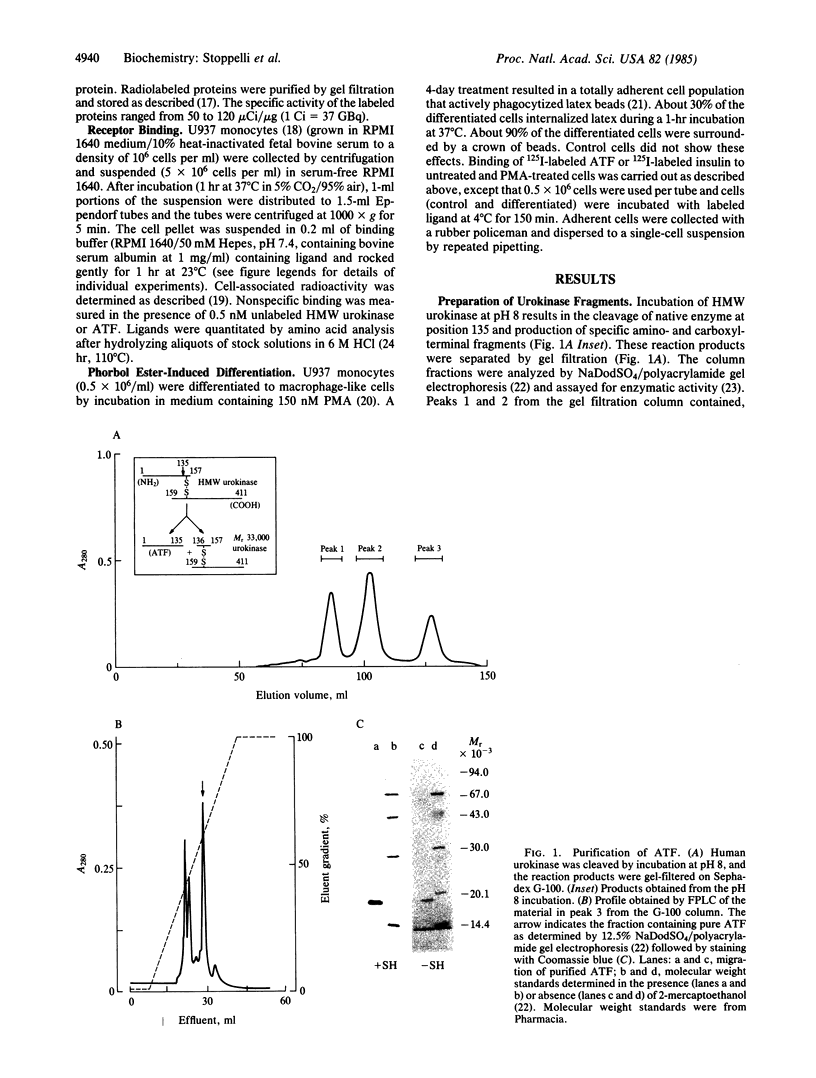
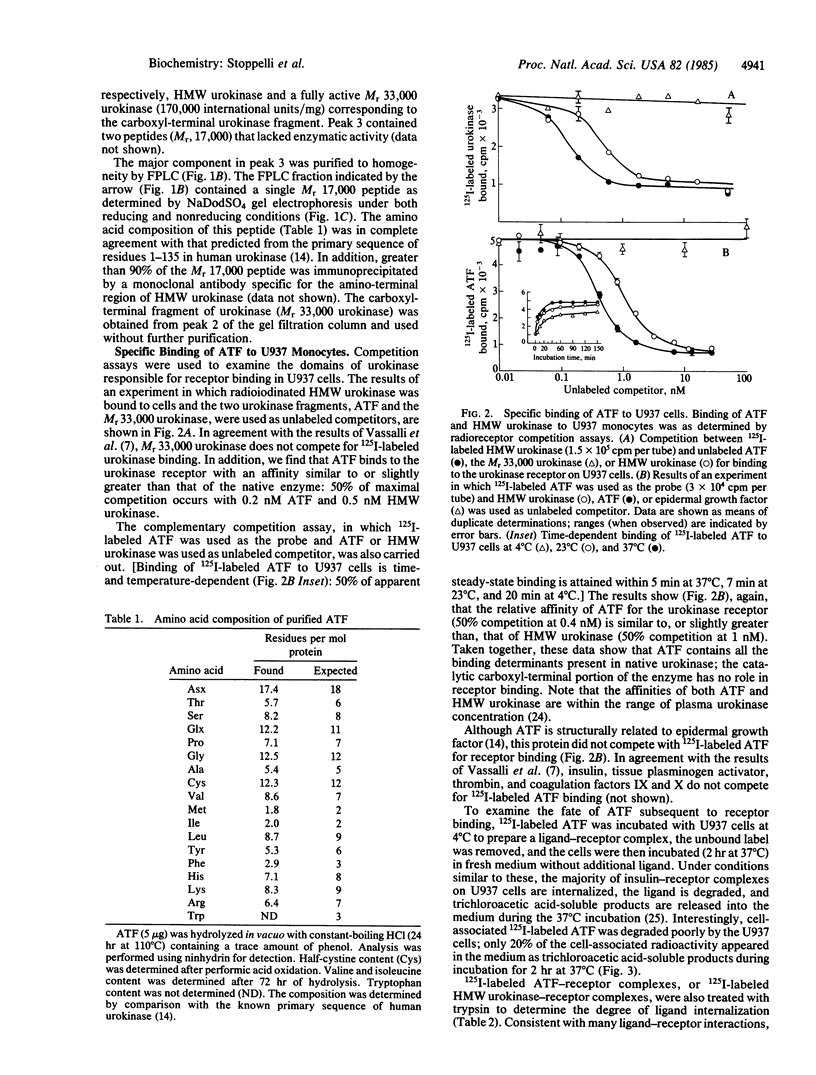
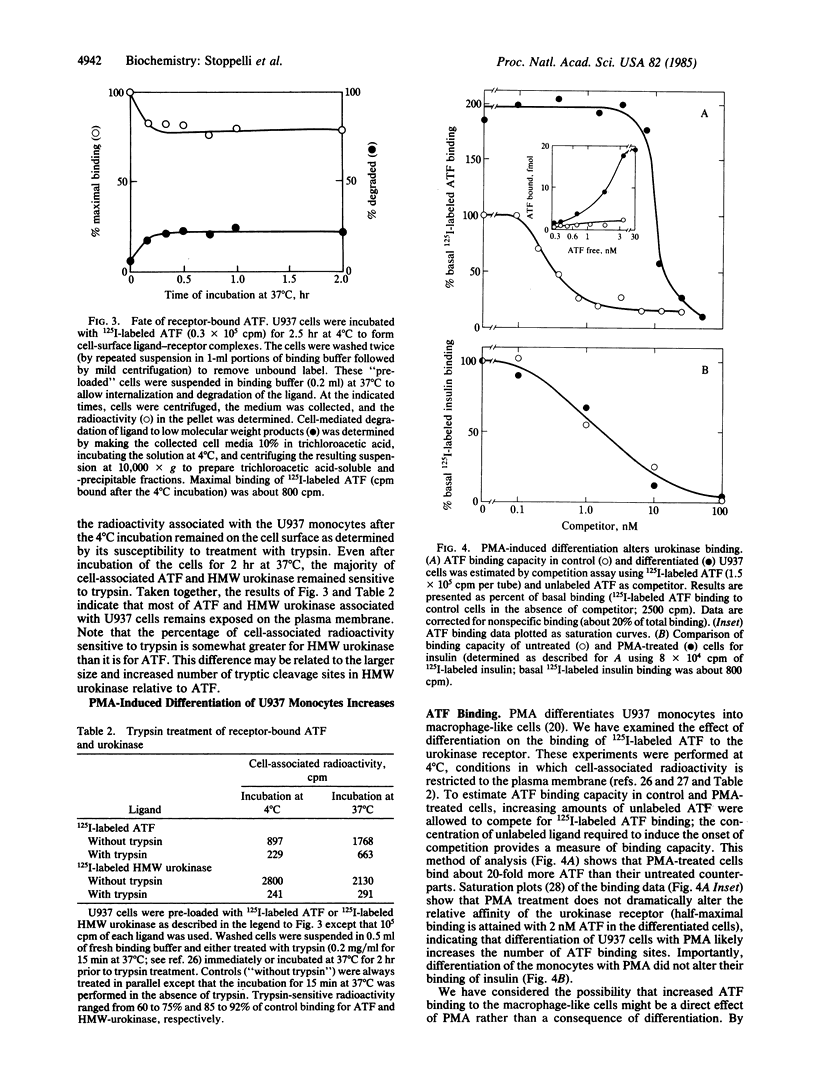

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Assoian R. K., Frolik C. A., Roberts A. B., Miller D. M., Sporn M. B. Transforming growth factor-beta controls receptor levels for epidermal growth factor in NRK fibroblasts. Cell. 1984 Jan;36(1):35–41. doi: 10.1016/0092-8674(84)90071-0. [DOI] [PubMed] [Google Scholar]
- Assoian R. K., Thomas N. E., Kaiser E. T., Tager H. S. [LeuB24]insulin and [AlaB24]insulin: altered structures and cellular processing of B24-substituted insulin analogs. Proc Natl Acad Sci U S A. 1982 Sep;79(17):5147–5151. doi: 10.1073/pnas.79.17.5147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantrell D. A., Smith K. A. Transient expression of interleukin 2 receptors. Consequences for T cell growth. J Exp Med. 1983 Dec 1;158(6):1895–1911. doi: 10.1084/jem.158.6.1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cline M. J., Lehrer R. I. Phagocytosis by human monocytes. Blood. 1968 Sep;32(3):423–435. [PubMed] [Google Scholar]
- Cohen S. D., Israel E., Spiess-Meier B., Wainberg M. A. Plasminogen activator is an apparent lymphocyte mitogen. J Immunol. 1981 Apr;126(4):1415–1420. [PubMed] [Google Scholar]
- Early P., Rogers J., Davis M., Calame K., Bond M., Wall R., Hood L. Two mRNAs can be produced from a single immunoglobulin mu gene by alternative RNA processing pathways. Cell. 1980 Jun;20(2):313–319. doi: 10.1016/0092-8674(80)90617-0. [DOI] [PubMed] [Google Scholar]
- Günzler W. A., Steffens G. J., Otting F., Buse G., Flohé L. Structural relationship between human high and low molecular mass urokinase. Hoppe Seylers Z Physiol Chem. 1982 Feb;363(2):133–141. doi: 10.1515/bchm2.1982.363.1.133. [DOI] [PubMed] [Google Scholar]
- Günzler W. A., Steffens G. J., Otting F., Kim S. M., Frankus E., Flohé L. The primary structure of high molecular mass urokinase from human urine. The complete amino acid sequence of the A chain. Hoppe Seylers Z Physiol Chem. 1982 Oct;363(10):1155–1165. doi: 10.1515/bchm2.1982.363.2.1155. [DOI] [PubMed] [Google Scholar]
- King A. C., Cuatrecasas P. Resolution of high and low affinity epidermal growth factor receptors. Inhibition of high affinity component by low temperature, cycloheximide, and phorbol esters. J Biol Chem. 1982 Mar 25;257(6):3053–3060. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Miller C. L., Graziano C. J., Lim R. C. Human monocyte plasminogen activator production: correlation to altered M phi-T lymphocyte interaction. J Immunol. 1982 May;128(5):2194–2200. [PubMed] [Google Scholar]
- Mullins D. E., Rohrlich S. T. The role of proteinases in cellular invasiveness. Biochim Biophys Acta. 1983 Dec 29;695(3-4):177–214. doi: 10.1016/0304-419x(83)90011-2. [DOI] [PubMed] [Google Scholar]
- Nielsen L. S., Hansen J. G., Skriver L., Wilson E. L., Kaltoft K., Zeuthen J., Danø K. Purification of zymogen to plasminogen activator from human glioblastoma cells by affinity chromatography with monoclonal antibody. Biochemistry. 1982 Dec 7;21(25):6410–6415. doi: 10.1021/bi00268a014. [DOI] [PubMed] [Google Scholar]
- Ossowski L., Quigley J. P., Kellerman G. M., Reich E. Fibrinolysis associated with oncogenic transformation. Requirement of plasminogen for correlated changes in cellular morphology, colony formation in agar, and cell migration. J Exp Med. 1973 Nov 1;138(5):1056–1064. doi: 10.1084/jem.138.5.1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ossowski L., Reich E. Antibodies to plasminogen activator inhibit human tumor metastasis. Cell. 1983 Dec;35(3 Pt 2):611–619. doi: 10.1016/0092-8674(83)90093-4. [DOI] [PubMed] [Google Scholar]
- PLOUG J., KJELDGAARD N. O. Urokinase an activator of plasminogen from human urine. I. Isolation and properties. Biochim Biophys Acta. 1957 May;24(2):278–282. doi: 10.1016/0006-3002(57)90194-4. [DOI] [PubMed] [Google Scholar]
- Ralph P., Williams N., Moore M. A., Litcofsky P. B. Induction of antibody-dependent and nonspecific tumor killing in human monocytic leukemia cells by nonlymphocyte factors and phorbol ester. Cell Immunol. 1982 Aug;71(2):215–223. doi: 10.1016/0008-8749(82)90257-x. [DOI] [PubMed] [Google Scholar]
- Robert A., Grunberger G., Carpenter J. L., Dayer J. M., Orci L., Gorden P. The insulin receptor of a human monocyte-like cell line: characterization and function. Endocrinology. 1984 Jan;114(1):247–253. doi: 10.1210/endo-114-1-247. [DOI] [PubMed] [Google Scholar]
- Rodbard D. Mathematics of hormone-receptor interaction. I. Basic principles. Adv Exp Med Biol. 1973;36(0):289–326. doi: 10.1007/978-1-4684-3237-4_14. [DOI] [PubMed] [Google Scholar]
- Salerno G., Verde P., Nolli M. L., Corti A., Szöts H., Meo T., Johnson J., Bullock S., Cassani G., Blasi F. Monoclonal antibodies to human urokinase identify the single-chain pro-urokinase precursor. Proc Natl Acad Sci U S A. 1984 Jan;81(1):110–114. doi: 10.1073/pnas.81.1.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savage C. R., Jr, Cohen S. Epidermal growth factor and a new derivative. Rapid isolation procedures and biological and chemical characterization. J Biol Chem. 1972 Dec 10;247(23):7609–7611. [PubMed] [Google Scholar]
- Sporn M. B., Todaro G. J. Autocrine secretion and malignant transformation of cells. N Engl J Med. 1980 Oct 9;303(15):878–880. doi: 10.1056/NEJM198010093031511. [DOI] [PubMed] [Google Scholar]
- Sundström C., Nilsson K. Establishment and characterization of a human histiocytic lymphoma cell line (U-937). Int J Cancer. 1976 May 15;17(5):565–577. doi: 10.1002/ijc.2910170504. [DOI] [PubMed] [Google Scholar]
- Unkeless J. C., Gordon S., Reich E. Secretion of plasminogen activator by stimulated macrophages. J Exp Med. 1974 Apr 1;139(4):834–850. doi: 10.1084/jem.139.4.834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassalli J. D., Baccino D., Belin D. A cellular binding site for the Mr 55,000 form of the human plasminogen activator, urokinase. J Cell Biol. 1985 Jan;100(1):86–92. doi: 10.1083/jcb.100.1.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassalli J. D., Dayer J. M., Wohlwend A., Belin D. Concomitant secretion of prourokinase and of a plasminogen activator-specific inhibitor by cultured human monocytes-macrophages. J Exp Med. 1984 Jun 1;159(6):1653–1668. doi: 10.1084/jem.159.6.1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verde P., Stoppelli M. P., Galeffi P., Di Nocera P., Blasi F. Identification and primary sequence of an unspliced human urokinase poly(A)+ RNA. Proc Natl Acad Sci U S A. 1984 Aug;81(15):4727–4731. doi: 10.1073/pnas.81.15.4727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wun T. C., Ossowski L., Reich E. A proenzyme form of human urokinase. J Biol Chem. 1982 Jun 25;257(12):7262–7268. [PubMed] [Google Scholar]
- Wun T. C., Schleuning W. D., Reich E. Isolation and characterization of urokinase from human plasma. J Biol Chem. 1982 Mar 25;257(6):3276–3283. [PubMed] [Google Scholar]