Abstract
The molecular and cellular origin of the primary neurons of the inner ear, the vestibular and spiral neurons, is reviewed including how they connect to the specific sensory epithelia and what the molecular nature of their survival is. Primary neurons of the ear depend on a single basic Helix-Loop-Helix (bHLH) protein for their formation, neurogenin 1 (ngn1). An immediate downstream gene is the bHLH gene neuronal differentiation (NeuroD). Targeted null mutations of ngn1 results in absence of primary neuron formation; targeted null mutation of NeuroD results in loss of almost all spiral and many vestibular neurons. NeuroD and a later expressed gene, Brn3a, play a role in pathfinding to and within sensory epithelia. The molecular nature of this pathfinding property is unknown. Reduction of hair cells in ngn1 null mutations suggests a clonal relationship with primary neurons. This relationship may play some role in specifying the identity of hair cells and the primary neurons that connect with them. Primary neuron neurites growth to sensory epithelia is initially independent of trophic factors released from developing sensory epithelia, but becomes rapidly dependent on those factors. Null mutations of specific neurotrophic factors lose distinct primary neuron populations which undergo rapid embryonic cell death.
Keywords: Inner ear, Primary neurons, Development, Molecular origin, Primary neuron survival, Pathfinding
1. Introduction
Development of afferent fibers to the ear of mammals has been studied for over 100 years, starting with the seminal investigations of Retzius [56], Cajal [12], Tello [61], and Lorente de No [40,41]. This work has outlined much of the details of afferent fiber growth using non-experimental tracing techniques. Over the last few years, data have been added that detail those initial observations. Specifically, the molecular basis of primary neuron formation has been clarified [42,43], the topological origin of primary neurons in the otocyst [25] has been identified, neurotrophins and their role in primary neuron survival have been characterized [20], and molecular details of primary neuron navigation [58] are beginning to emerge. While great progress has thus been achieved over the last 100 years of research, several crucial issues remain largely unresolved. For example, it is unclear if and how the topological origin of primary neurons in the otocyst relates to their later connections to specific endorgans, e.g. are primary neurons that delaminate from the saccule projecting to the saccule but not the utricle? It remains equally unclear how the emerging properties of hair cell polarity specific connections are established in the sensory epithelia [45,54].
Embedded in this problem is the question about the molecular basis of nerve fiber guidance and molecular definition of identity of sensory neurons. Simply speaking, we do not know at which point in its developmental history a saccular neuron acquires its identity: inside the otocyst wall, during delamination and migration to its final position, at its final position, after afferents have extended into the hindbrain or after afferents have reached a specific sensory organ. Likewise, we have hardly any clear concept of how this primary neuron identity translates into unique pathfinding properties that allow such a primary neuron to establish its specific peripheral and central connections which are so crucial for its function [45]. Beyond academic interest, such knowledge is essential for any attempt to regrow sensory fibers during regeneration of hair cells to re-establish functional connections.
In this review, I will provide an overview about the current status of primary neuron formation, fiber outgrowth and survival of primary neurons, primarily in the vestibular ganglion. A comparable overview on spiral sensory neurons has recently been published [58].
2. The molecular basis of primary neuron formation: how, where and when are primary neurons generated?
All neurons derive from ectodermal cells which are transformed by a cascade of genes into neuronal precursor cells. Within recent years, several genes have been identified that appear crucial for this switch in phenotype fate. These genes are, because of their apparent capacity to transform ectodermal cells into neurons, referred to as proneural genes. These genes belong to the growing family of basic Helix-Loop-Helix genes (bHLH genes), an ancient protein with a highly conserved DNA binding domain [6]. Proneural bHLH genes form heterodimers with the ubiquitous E-proteins that enable them to bind to DNA and exert their function. These proneural genes have not only the unique capacity to turn ectodermal cells into neurons in gain of function experiments [38], but can also determine cell fate in rather unrelated tissue such as beta-cells of the pancreas [39] or cell fate commitment in the gut [66].
Given this unique capacity, it is therefore not surprising that these genes are regulated through a number of other factors. Some of these factors interfere with the heterodimerization of bHLH genes by binding to the E-proteins. These genes in general inhibit differentiation and are thus referred to as ‘inhibitors of differentiation’ or Id-genes. Others, like the vertebrate hairy and enhancer of split paralogs Hes/Her/Esr, act as classical DNA-binding repressors of proneural gene transcription. The activation of the latter family appears to be regulated by the ubiquitously present delta-notch system, which regulates in neighboring cells the proneural commitment. However, this regulation requires the upregulation of bHLH genes in a limited number of cells to start. Most of the factors that drive this initial upregulation of proneural bHLH genes are still unknown.
In general, the bHLH genes can be divided into three functional groups: true proneural bHLH genes that generate a neural lineage, bHLH genes that drive neural differentiation and bHLH genes that drive the switch from neural to glial cell lineage [6,67].
Loss of function (targeted null mutations of the respective genes) has clarified some of the proneural genes crucial for inner ear primary neuron development (Figs. 1 and 2). The work of Ma et al. [43] showed that inner ear primary neuron formation requires the vertebrate bHLH gene neurogenin 1 (ngn1). Indeed, a follow up study showed that no primary neurons ever from in these mutants [42]. Such ears develop nevertheless fairly normal in their overall form, suggesting that ear formation and development even of many hair cells is largely autonomous of innervation. However, while those hair cells that do form develop morphologically normal in the absence of innervation (except for some minor disorientation), hair cell numbers are to various degrees reduced in ngn1 null mutant mice. Most interestingly, the cochlea is shortened and the saccule almost completely lost. These data suggest an unexpected interaction between proneural cells that form primary neurons and clones that give rise to hair cells, supporting and other inner ear epithelial cells. Several hypotheses have been proposed to account for these data. The simplest explanation would be a clonal relationship of sensory clones with hair cell/supporting cell clones [21,24]. However, other possible interactions can not be excluded [25].
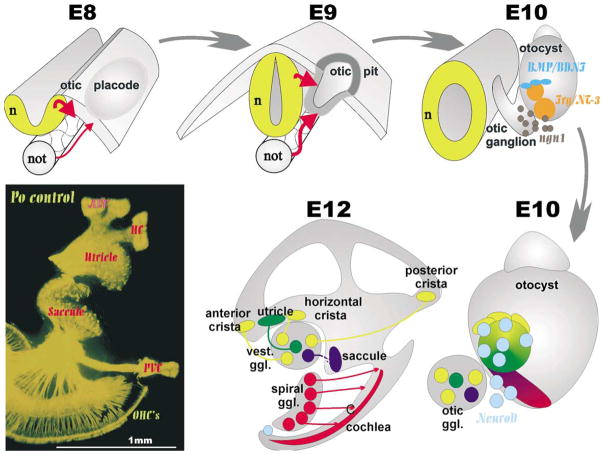
Fig. 1.
This diagram illustrates the origin of primary neurons in a mammalian otocyst. Inductive interactions transform ectoderm lateral to the developing hindbrain into the otic placode that invaginates to form the otocyst. In the otocyst there is upregulation of ngn1 in areas which also express other markers such as BMP-4, lunatic fringe, BDNF and NT-3. Upregulation of ngn1 results in formation of primary neuron precursors which delaminate and migrate to the forming otic ganglion. These primary neuron precursors upregulate another bHLH gene, NeuroD while they delaminate from areas that are close by or appear to become distinct sensory epithelia. Whether or not the vestibular primary neurons that project to a given sensory epithelium are actually delaminating from or nearby the sensory epithelia they later are connected to remains unknown. At birth (lower left) the innervation of the ear is distinct to all endorgans.
Fig. 2.
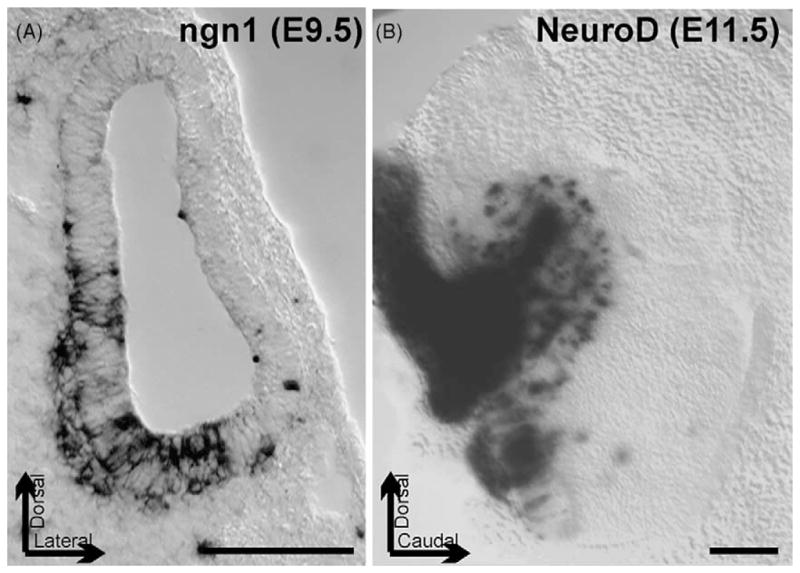
These images show the expression of the bHLH gene ngn1 in a 9.5-day-old mouse embryo otocyst as revealed by in situ hybridization, and of NeuroD in the developing cochlear and vestibular primary neurons of a 11.5-day-old mouse embryo using a LacZ reporter. Note that ngn1 is expressed inside the otocyst wall as well as in delaminating sensory neurons. NeuroD expression is both in the delaminated differentiating primary neurons that form the otic (vestibulocochlear) ganglion (left) and in cells in the otocyst wall. Bar indicates 100 μm, arrows indicate position of the preparations.
Other data have shown that hair cells require the bHLH gene Mammalian atonal homologue 1 (Math1) for their differentiation [5]. However, Math1, the bHLH gene responsible for hair cell differentiation, does not function as a true proneural gene to establish a neural lineage in other parts of the mammalian brain [6]. In contrast to most vertebrates, in mammals Math1 functions only to select progenitor cells from a pool of already specified neuroepithelial stem cells [6]. In this context, recent data suggest that the gene that establishes the hair cell lineage generation is still unknown [14]. Confirming and extending this conclusion are recent data on organ of Corti tissue culture that show expression of MyoVIIa, MyoV, α9AchR, fimbrin, Brn3c and other markers independent of Math1 [57]. This conclusion is also supported by the precocious expression of the neurotrophin BDNF in the cochlea in what appears to be hair cell progenitors prior to the upregulation of Math1 [14,20]. Preliminary data of our group suggest the BDNF is even expressed in hair cell progenitors in Math1 null mutants. Either ngn1 and/or an as yet unspecified proneural gene could play the role to induce neuroepithelial commitment to the parts of the otocyst that will give rise to the Math1 mediated hair cell differentiation.
As a consequence of the absence of primary neuron formation in ngn1 null mutants, the ear develops completely isolated from the brain as afferents do not form and neither efferents nor autonomic fibers appear to reach the ear in these animals [42]. In fact, it appears that branchial motoneurons, including the branchial arch derived inner ear efferent neurons, reroute into cranial nerves that form in the absence of neural crest derived primary neurons such as the facial nerve [42]. At least for these fibers it is thus clear that the vestibular and cochlear afferents are an essential pathway to grow along and their absence can not be substituted by other information to reach the ear.
The next step in primary neuron differentiation is apparently mediated by the bHLH genes of the NeuroD family [35,38]. As is the case in other true pro-neuronal genes, ngn1 is only transiently upregulated in primary neuron precursors (Fig. 2). As primary neuron precursors delaminate from the otocyst wall, the sensory neuroblasts downregulate ngn1 and upregulate NeuroD [35,39,42]. Based on data in the CNS, the presence of either ngn1 or NeuroD could play a role for the continued proliferation of these neuroblasts through interference with the cell cycle [6]. In the ear, however, NeuroD appears to be essential for the maturation of the primary neurons as many apoptotic cells were found in NeuroD null mutants [35,39]. Later embryonic death of primary neurons is mediated by neurotrophins [26]. However, the known absence of neurotrophin receptors in NeuroD null mutants [35,39] is not easily related to the early onset of neuronal death in NeuroD null mutants prior to effects reported for neurotrophin null mutants and suggests that additional mechanisms may be involved.
In addition, NeuroD appears to affect delamination of neuroblasts from the otocyst [39] and causes aberrant migration of surviving vestibular neurons [35]. The remaining neurons have disorganized projections which resemble in their phenotype somewhat the vestibular projection defects known for the Pou domain factor mutation Brn3a [33]. Whether all the migration and projection defects of the NeuroD mutations are only related to the fact that NeuroD is upstream of Brn3a remains to be seen in transgenic animals in which Brn3a is expressed under NeuroD promoter control thus eliminating possible regulations of NeuroD other than Brn3a.
The differential effect of NeuroD in the cochlea primary neurons (almost completely lost) and vestibular primary neurons (many more survive, but are dislocated and project aberrantly) may relate to the known fact that specific neuronal fate acquisition require interaction with homeobox genes such as Pax genes (Pax2 is expressed in the ear; [36]). In contrast to the vestibular sensory neurons, cochlear (spiral) primary neurons express GATA3 [34,36]. GATA factors such as pannier and presumably its vertebrate homologue GATA3 as well [6,34] can interact with bHLH dimers for transcriptional regulation and may be directly involved in specific aspects of cochlear and vestibular phenotype fate determination via regulation of bHLH gene transcription. Unfortunately, most GATA3 null mutants are very early lethal before hair cell differentiation is achieved. Nevertheless it is important to note that a human GATA3 mutation exists and causes deafness [64].
Primary neuron primordia can be identified either as delaminating cells [13] emigrating through the basal lamina surrounding the otocyst or as cells that express specific markers such as ngn1 [43], NeuroD [35,39], neurotrophins [20] or other genes [21]. Such delaminating cells are first apparent shortly after the otocyst closes in mice (E9.5) or even before the otocyst is completely formed in chicken [1]. Later, primary neuron primordia express many other genes such as GATA3 [34,36]; Brn3a [33] and others such as neurotrophin receptors [20]. Indeed, those genes apparently are sequentially upregulated but whether other intermediates exist and how they interact is still unclear. Logically, taking a transgenic knock-in strategy by replacing specific genes such as NeuroD with Brn3a or trkC may help to understand what additional regulation these genes perform other than the proposed upregulation of downstream genes (ngn1 > NeuroD > Brn3a > trkC).
3. Topological origin of primary sensory neurons
Experimental analysis suggests that all primary neurons derive from the ventral quadrant of the developing ear [63]. This early work has now been extensively confirmed using several of the above outlined markers [21,25]. Specifically, it has been suggested that a large area of the ventro-lateral otocyst gives rise to primary neurons in mice (Figs. 1 and 2) and this area may be somewhat more medial in chicken [1]. Based on several markers, these areas are at least partially overlapping with the future sensory epithelia [25]. Despite this recently achieved insight, our understanding of overlapping expression of genes in the developing ear is still rudimentary and is perhaps based on less than 10% of the relevant genes. Nevertheless, it is already obvious that various degrees of overlapping expression as well as abutting expression territories exist, that form the basis for the boundary model of sensory organ formation [21]. BMP-4 expression in the developing sensory epithelia [21] may be directly related to the expression of Math1 and ngn1 in a gradient fashion comparable to the spinal cord [31]. It is possible that the upregulation of BMP-4 could force the ngn1 expression to its perimeter while allowing Math1 upregulation in the center of the BMP-4 expression domain. Detailed double in situ analysis of ngn1 and BMP-4 as well as of Math1 and BMP-4 are needed to support or refute this suggestion.
The possibility for unique identities of early primary neuron precursors is underscored by differential expression of several genes, even in early delaminating cells [36] and the fact that only most cochlear but many fewer vestibular neurons die in NeuroD null mutants [35]. Overall, the already known diversity of gene expression indicates that the various areas of the otocyst could provide unique identities for delaminating precursors based on overlapping and discrete expression of transcription factors. Despite this interesting start, it remains to be seen how differential origin in the otocyst relates to differential gene expression and, ultimately, differential projection of primary neurons to specific sensory epithelia of the ear and specific areas of the brain [45]. Ultimately, it is possible, given sufficient nested expression of various transcription factors in the ear, that primary neuron precursors acquire a unique cell fate assignment in the ear in analogy to that of sensory and motoneurons in the CNS [11,31,55]. If these initial data in the ear can be confirmed and extended by future work, development of distinct peripheral and central projections would be a consequence of such molecularly acquired cell fates already in the otocyst.
In this context it is important to realize that proliferation, delamination, migration to the final position and development of central and peripheral projections is a prolonged phase in mammals and birds that lasts for several days [58,59]. As previously pointed out by Carney and Silver [13] and recently confirmed by Farinas et al. [20] and Fritzsch et al. [25], delaminating cells (which are likely neuronal precursors based on NeuroD expression) apparently migrate away from the otocyst along fibers of more differentiated neurons that project toward the future sensory epithelia. Indeed, it appears that spatio-temporal distinct and changing populations of primary neuron precursors specifically extend along the fibers that reach toward the future sensory epithelium. Thus, it is possible that fate acquisition, as specified through the gene expression mosaic in the otocyst, results in restricted areas of primary neuron delamination with specific fates.
Such primary neurons subsequently may project back to the area they delaminated from using other delaminating cells as substrate to extend their peripheral processes (Fig. 3). Such a scenario would allow primary neurons to be randomly distributed in the sensory ganglia and to project nevertheless specifically to the ear and the brain, using distinct and different guidance principle to navigate to their various targets. In fact, despite earlier suggestions to the contrary, recent data clearly show that most primary neurons to distinct sensory epithelia are mixed (Fig. 4) in their distribution rather than completely sorted [44]. Among the ear sensory epithelia, the cochlea is an exception with its fairly highly organized peripheral and central projection [41]. But even here, topologically mismatched primary neurons do exist (Fig. 4). In contrast, the distribution of primary neurons in the vestibular ganglion is more random and the peripheral, exclusive projection to distinct endorgans contrasts with the highly overlapping central representation of individual sensory epithelia [44,45]. Indeed, tracing of early primary neurons show that some may already have an axon to the brain before they delaminate (Fig. 3).
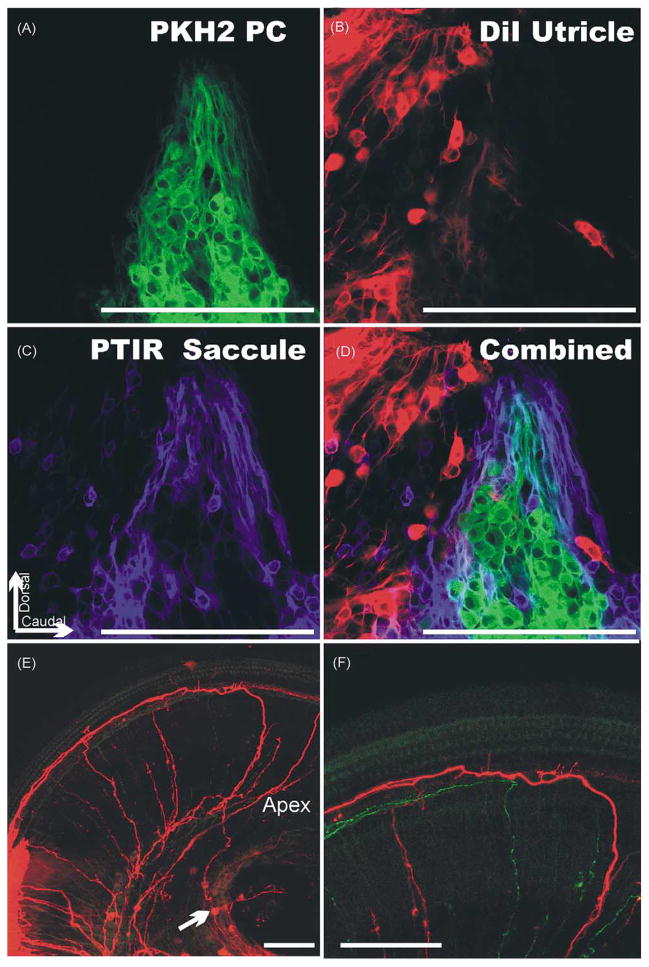
Fig. 3.
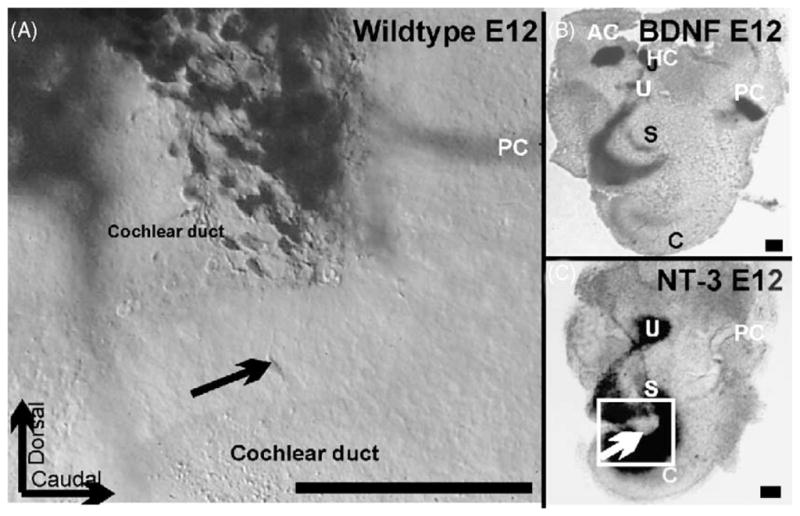
These images show the distribution of spiral primary neurons near and in the forming cochlear duct of an E12 embryo as shown by dextran amine filling from the brainstem (A) and the expression of BDNF (B) and NT-3 (C) at E12 as shown by a LacZ reporter that reveals the distribution of gene expression specific for those two genes. Arrows in A and C indicate a comparable area of the cochlear duct which shows delaminating NT-3 positive precursors that apparently can be filled from the brainstem. Bar indicates 100 μm.
Fig. 4.
Three differently fluorescing lipophilic tracers were used to selectively label the vestibular primary neurons that project to the posterior vertical crista (PC; A), the utricle (B) and the saccule (C). The combination of all three shows there is segregation of PC primary neurons into a single group. Nevertheless, other primary neurons projecting to other endorgans are interspersed among them (D). Note also the complete absence of any double labeling (D) suggesting that each primary neuron projects to only one endorgan. The cochlear labeling (E, F) shows labeling of an apical spiral primary neuron (arrow in E) that projects first about 200 μm apical, runs in a radial bundle to the cochlea where it forms collaterals to inner hair cells (F) and extends for 500 μm towards the base where the fiber was labeled by the DiI injection (E). Bar indicates 100 μm.
The literature is filled with suggestions that cell fate commitment occurs after either central or peripheral contacts are established [30,50,51]. It is difficult to see how cell fate specification of primary neurons during neurite extension or after neurites have reached the central and peripheral target could provide the means for such a sophisticated distinct patterning process of dendritic and axonal projections, in particular since both branches of a given inner ear neuron seem to grow almost simultaneously and little reorganization of either peripheral or central projections has been experimentally revealed [27,37,45]. In fact, the actual cell distribution in the ganglion is very difficult if not impossible to reconcile with notions of cell fate acquisition in the final position. It appears thus more logical to assume that initial fiber growth of both the central and peripheral fibers is highly organized and guided by not yet in detail determined mechanisms. To be able to navigate requires, however, that neuronal fate identity should have been established prior to peripheral and central neurite growth. Indeed, some primary neurons have already extended their central processes before they delaminate [10], while others have reached the sensory epithelium they later innervate before they have migrated to their final position in the vestibular sensory ganglion. However, it remains unresolved how detailed a projection pattern will be specified by these means and how many other navigational cues play a role in the development of ear innervation.
In this context, among the patterns of innervation revealed by detailed tracing experiments, the posterior crista innervation is unique in that it seems to form a rather coherent, tightly packed population of the inferior vestibular ganglion, surrounded by saccular primary neurons [44]. Labeling the inner ear afferents form the brain as early as embryonic Day 11 shows a cluster of primary neurons distributed along the posterior crista nerve. One day later these primary neurons have migrated outside the forming otic capsule in the space between the brain and the ear. Together with saccular sensory neurons, they form the inferior vestibular ganglion.
These data suggest that primary neurons destined to project to the posterior crista are formed somewhere along the trajectory of the nerve to the posterior crista. However, it remains unclear whether they share a clonal relationship with the posterior crista or are independently derived from the otocyst. Absence of any apparent major reduction of hair cells in the posterior crista of ngn1 null mutant mice tends to support the latter suggestion.
The ear shares many aspects of development with the lateral line system of hair cells distributed in patches over the body and head of fishes and many amphibians [16]. In the lateral line system, distinct placodes give rise to distinct populations of primary neurons [30,50] that are connected to discrete, spatially well segregated lines of neuromasts which project into discrete central representations [2,23]. In analogy to this system, we propose that distinct areas of the developing otocyst wall can be viewed as ‘primary neuron forming placodes’. As with the lateral line placodes, which extend or migrate along the head and body wall as the animal grows, these distinct primary neuron primordia of the ear may progressively segregate as the otocyst enlarges. It remains thus entirely possible that many afferent fibers of all developing sensory epithelia stay in continuous contact with the sensory primordia as these segregate and become dispersed in the ever growing ear. Indeed, tracing from the brain shows fibers to the hair cell primordia as revealed by several markers as early as embryonic Day 10 [25].
However, alternative views have been voiced and it has been suggested that primary neurons do not derive from the same sites of the otic vesicle they later innervate [49]. Indeed, in the lateral line system it is clear that primary neurons for ampullary electroreceptors belong only to the anteroventral and anterodorsal branch of the lateral line nerves [23]. Nevertheless, it appears that a small postotic group of ampullary electroreceptors is innervated by nerve fibers derived from the preotic group of primary neurons [50]. This indicates that some reorganization of peripheral projections is possible in the lateral line system and a larger effect along the same line is to be expected in the ear, where spatial constraints are not as prevalent as in the developing lateral line system. It also needs to be pointed out that the view presented above for the ear has to be compared with entire lines, not with individual neuromasts.
Comparable to the unresolved problem of how distinct connections between neuromasts and their primary neurons are established, there is hardly any data available on how distinct vestibular or cochlear afferents target their respective hair cells. In analogy to the lateral line system [30] it appears unlikely that this is accomplished through a strict lineage relationship where only progenitors of specific sensory neurons/hair cell clones become innervated. Such issues may be solvable in the mechanosensory lateral line system of salamanders were only two afferents innervate a given neuromast and both fibers contribute to two discrete fascicles of each main lateral line nerve projection into the brain [22,23] whereas the more complex lateral line of fishes and the inner ear might proof to be to difficult to provide unequivocal insights.
Indeed, the numerous criss-crossing fibers found even in non-experimental animals in the ear underneath the utricle and anterior horizontal crista seems to indicate that peripheral target finding is less than straightforward. In fact, the many anastomoses described between different sensory epithelia [65] indicate that such alternate targeting may be fairly common in the ear. Again, detailed double or even triple labeling experiments of fibers coming from given sensory epithelia are needed to reveal how closely spaced those afferents are growing. Such data are necessary to fully delineate the magnitude of precision targeting as compared to randomly wandering fibers. Using clonal analysis following microinjection should help reveal how much a role clonal relationships play in the overall targeting of primary neurons to specific sensory epithelia, if any.
In conclusion, it seems likely that sensory cell fate determination starts as early as in the otocyst. Such cell fate determination may, through unknown mechanisms, determine first where those primary neuron precursors migrate to and, secondly, where there peripheral and central projections extend to. Clearly, NeuroD and Brn3a are important players for this pathfinding [33,35], but next to nothing is known about their molecular partners outside the sensory neurons. In the simplest scenario, primary neurons project their dendrites back to the area where the cell bodies originated from, but have to navigate subsequently to target specific hair cells within a given endorgan. However, this is likely not the only way peripheral fiber projections are established as evidenced by numerous anastomoses between sensory epithelia. Given the multitude of pathfinding molecules that have been characterized over the last several years [32,62] it seems logical that other mechanisms come into play in the ear as well and mechanisms may be modifications of a common theme specific for a given sensory modality (i.e. cochlea, gravistatic and angular acceleration endorgans).
Based on insights gained from other systems, it seems logical to explore the function of the ephrin ligands and receptors, known to be expressed in the developing ear [9]. However, no serious defect on fiber outgrowth has been reported thus far [18]. Other factors are the large members of cell adhesion molecules, some of which are expressed in intricate patterns in the developing ear [19]. However, no experimental studies exist that would support their function in fiber guidance in vivo. The semaphorins and their receptors, the neuropilins and plexins, are playing a growing role in neuronal development and regeneration [15,52]. Plexins and semaphorins have been described in the developing ear [46,48], but their potential function has not yet been explored in the existing null mutant mice, in particular the plexin null mutant mice [15]. Studying those ligands and receptors in more detail and analyzing the potential pathfinding problems in null mutant mice should help deepen our understanding of the molecular mechanisms for fiber guidance in the developing ear. A more detailed summary of the known and suspected guidance principles in the cochlea was recently published and the reader is referred to that review [58].
Last, but not least, recent data suggest that glia–axon interaction may play a role in proper pathfinding in the lateral line system [29]. By logical extension, such a role could also be played by glial cells in the developing ear, in particular for the outgrowth of fibers toward the brain [4]. Such issues could be studied in erbB receptor mutant mice which are known to eliminate glial cells [47]. Indeed, preliminary data on erbB2 null mutant mice strongly support this notion (Morris and Fritzsch, unpublished data).
4. Timing of primary neuron formation
Detailed informations on the ‘birthdate’ of primary neurons have been generated for chicken and mammals using 3H-thymidine technique. In mice, the data suggest that there are several days of continuous proliferation that provides the total number of primary neurons for a given epithelium. For example, primary neurons for the cochlea become postmitotic between E11 and E16 whereas those for the vestibular ganglion become postmitotic between E10 and E14 [59]. In this context it remains unclear what becomes of delaminating precursors that leave the utricle and saccule as late as E16 [25]. If they do not form sensory neurons, what else do they become?
Primary neuron terminal mitosis starts as early as 10 days [59] in agreement with the earliest expression of ngn1 reported in the ear (E9.5; [43]). However, it is unclear how many of the primary neuron precursors that delaminate from the otocyst undergo further divisions. Judging from the distribution of mitotic spindles in these cells outside the otocyst, proliferation is extensive [3]. Detailed pulse-chase experiments are necessary to understand what is going on with these apparently proliferating precursors. Ideally, clonal analysis needs to be performed on these precursors to establish the size and distribution of the primary neuron clone. Such work is now feasible using the newly developed approach of viral injection into the otocyst of mice (Brigande and Fekete, personal communication).
5. Survival and development of connections with sensory epithelia: a race against time?
Above I have outlined some data and more questions concerning the spatial and temporal origin of inner ear primary neurons and its possible role in establishing primary neuron identity. Here I will outline how crucially critical the fast establishment of primary neuron identity is in order to establish connections with specific sensory epithelia for survival of primary sensory neurons. I like to argue that primary neuron survival is an integrated part of the formation of primary neuron specific connections in the ear and elsewhere and thus can reveal details of the patterning process of the differentiating ear.
The idea that neurotrophins guide fibers to the sensory epithelia goes back to the work of Cajal [12]. Later, tissue culture experiments have clearly demonstrated the existence of diffusible substances that seem to help direct fiber growth to the ear and to support the survival of primary neurons [7]. Within the last 10 years, some of these factors have been identified [53] and their effects characterized in targeted null mutations [26]. Indeed, the spatio-temporal expression domains of the two neurotrophins essential for the ear, BDNF and NT-3, seem to translate into a spatio-temporal loss of primary neurons in specific mutants [20] that can be rescued by transgenic expression of one neurotrophin under the promoter control of the other neurotrophin [17].
Soon after delamination is initiated, primary neurons express neurotrophins (Fig. 3). These neurotrophins expressed in a given primary neuron are identical with the neurotrophins expressed in the area of the otocyst they are delaminating from [25]. However, expanding the labeling to the majority, if not all primary neurons delaminating from the ear using the marker NeuroD, suggests that not all primary neurons that delaminate express neurotrophins and primary neurons delaminate from an area that is larger than that delineated by neurotrophins (Figs. 2 and 3). Indeed, it needs to be shown that the delaminating BDNF or NT-3 positive precursors are in fact neuronal precursors and are NeuroD positive. The data at hand are suggestive for this [25,35,39] but proof requires in situ hybridization for NeuroD in conjunction with BDNF or NT-3 lacZ markers. Alternatively, BDNF and/or NT-3 positive delaminating cells should be further traced in NeuroD null mutants. At least those delaminating cells that are non-neuronal would hardly be affected by the absence of NeuroD.
Detailed analysis of NT-3 and BDNF lacZ positive cells in combination with immunocytochemistry shows that as soon as these cells start to express neuronal markers such as tubulin or trk receptors, they lose the neurotrophin expression [25]. These data also suggest that fibers project toward sensory epithelia right between the delaminating neurotrophin positive precursors. Detailed analysis of peripheral projections in neurotrophin null mutants suggests that the initial projection is topologically rather normal (Fig. 5), albeit reduced in number [8,20]. Within only 2–3 days, fibers and subsequently primary neurons disappear in a specific pattern in single neurotrophin null mutations [26,28] and all die in double null mutations [60].
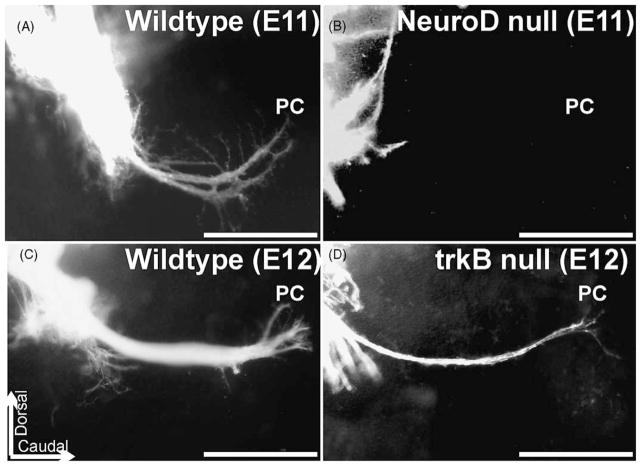
Fig. 5.
The effects of NeuroD null mutation (top) and of trkB mutation (bottom) are compared on afferent growth to the posterior crista (PC). DiI tracing of fibers is shown in whole mounted ears. Note that many NeuroD null mice show no or disorganized outgrowth of fibers to the PC as early as E11 (A, B). In contrast, some fibers extend to the PC in trkB (or BDNF, data not shown) null mutations until E12 (C, D). Bar indicates 100 μm.
These data suggest that the initial fiber growth is as independent of neurotrophins as the delamination and migration of primary neurons and may indeed use similar molecular aspects. However, subsequent to this early, neurotrophin-independent outgrowth, there is a critical period of neurotrophin dependency that will result in elimination of all connections that reach areas normally or experimentally deprived of the ear specific neurotrophins. Such aberrantly growing fibers have been reported in the developing [40] but not in the adult ear. Indeed, the partially overlapping expression of neurotrophins reported in the developing mammalian ear [53] seems to be directly translated into specific loss of primary neurons [20].
These data support a role for a very early onset of elimination of inappropriately or unconnected afferents and their sensory neurons. This verification of proper connection for survival sets in immediately after fibers have normally reached and start to invade their target organs (as early as E11 in the canal epithelia, E12 in the basal turn of the cochlea). These early survival effects do not preclude a subsequent or even concomitant role in fiber guidance, by neurotrophic or neurotropic means. Indeed, in transgenic rescues in which BDNF is expressed under NT-3 promoter control, more afferents seem to grow to the cochlea basal turn to innervate outer hair cells at greater density. In addition, some afferents seem to get lured underneath the basilar membrane without ever reaching the hair cells of the organ of Corti [17]. It is conceivable that such ‘disoriented fiber growth’ is a consequence of conflict between other pathfinding molecules and neurotrophin signaling. Clearly, such transgenic animals have to be studied in more detail to reveal the nature of these unusual pathfinding properties and to relate them with spatiotemporal patterns of neurotrophin misexpression in these transgenic animals.
6. Summary and conclusion
The most significant progress in understanding the development of the primary neurons over the last 10 years has been the clarification of the role of bHLH genes in primary neuron formation, migration and projection development and of neurotrophins and their receptors in survival of sensory neurons. Unfortunately, we still have not completely characterized the spatio-temporal expression of many transcription factors known or suspected to influence bHLH gene expression or to provide information about the cell fate identity of sensory neurons. As emphasized in a recent review [58], we do not understand how such clear binary decision as between the type 1 and type 2 spiral primary neurons of the cochlea is achieved at a molecular level. The possibility of identity acquisition prior to delamination has been raised. While supported by the current evidence, such data are too inconclusive to provide a solid base for this idea. As with primary neuron cell death in NeuroD null mutants prior to the time neurons die after neurotrophin depletion, or with the possible second functional role of neurotrophins as molecular guidance, multiple effects are likely to determine the fate of delaminating neuroblasts both inside and outside the otocyst. Full characterization of all molecules involved using microarrays and in situ hybridization are important next steps. Combining these techniques with analysis of existing mutants such as ngn1 or NeuroD null mutants, will provide much of the needed data to clarify some of the issues raised in this review.
Acknowledgments
This work was supported by grants form NIH (RO1 DC005590; 2PO1 DC00215) and NASA (NAG2-1353). I wish to express my gratitude to numerous collaborators who helped me with this review and the collection of the data that form the basis for this review: Dr. K.W. Beisel, L.F. Reichardt, I. Farinas, I. Silos-Santiago, J. Lee, E. Huang, D. Nichols, N. Bermingham, U. Pirvola, H. Zoghbi, and Q. Ma. I wish to express my gratitude to Drs. D. Fekete and M. Rivolta for critically reviewing this paper.
References
- 1.Adam J, Myat A, Le Roux I, Eddison M, Henrique D, Ish-Horowicz D, Lewis J. Cell fate choices and the expression of Notch, Delta and Serrate homologues in the chick inner ear: parallels with Drosophila sense-organ development. Development. 1998;125:4645–4654. doi: 10.1242/dev.125.23.4645. [DOI] [PubMed] [Google Scholar]
- 2.Alexandre D, Ghysen A. Somatotopy of the lateral line projection in larval zebrafish. Proc Natl Acad Sci USA. 1999;96:7558–7562. doi: 10.1073/pnas.96.13.7558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Altman J, Bayer SA. Development of the cranial nerve ganglia and related nuclei in the rat. Adv Anat Embryol Cell Biol. 1982;74:1–90. doi: 10.1007/978-3-642-68479-1. [DOI] [PubMed] [Google Scholar]
- 4.Begbie J, Graham A. Integration between the epibranchial placodes and the hindbrain. Science. 2001;294:595–598. doi: 10.1126/science.1062028. [DOI] [PubMed] [Google Scholar]
- 5.Bermingham NA, Hassan BA, Price SD, Vollrath MA, Ben-Arie N, Eatock RA, Bellen HJ, Lysakowski A, Zoghbi HY. Math1: an essential gene for the generation of inner ear hair cells. Science. 1999;284:1837–1841. doi: 10.1126/science.284.5421.1837. [DOI] [PubMed] [Google Scholar]
- 6.Bertrand N, Castro DS, Guillemot F. Proneural genes and the specification of neural cell types. Nat Rev Neurosci. 2002;3:517–530. doi: 10.1038/nrn874. [DOI] [PubMed] [Google Scholar]
- 7.Bianchi LM, Cohan CS. Developmental regulation of a neurite-promoting factor influencing statoacoustic neurons. Brain Res Dev Brain Res. 1991;64:167–174. doi: 10.1016/0165-3806(91)90221-4. [DOI] [PubMed] [Google Scholar]
- 8.Bianchi LM, Conover JC, Fritzsch B, DeChiara T, Lindsay RM, Yancopoulos GD. Degeneration of vestibular neurons in late embryogenesis of both heterozygous and homozygous BDNF null mutant mice. Development. 1996;122:1965–1973. doi: 10.1242/dev.122.6.1965. [DOI] [PubMed] [Google Scholar]
- 9.Bianchi LM, Liu H. Comparison of ephrin-A ligand and EphA receptor distribution in the developing inner ear. Anat Rec. 1999;254:127–134. doi: 10.1002/(SICI)1097-0185(19990101)254:1<127::AID-AR16>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 10.Bruce LL, Kingsley J, Nichols DH, Fritzsch B. The development of vestibulocochlear efferents and cochlear afferents in mice. Int J Dev Neurosci. 1997;15:671–692. doi: 10.1016/s0736-5748(96)00120-7. [DOI] [PubMed] [Google Scholar]
- 11.Brunet JF, Pattyn A. Phox2 genes—from patterning to connectivity. Curr Opin Genet Dev. 2002;12:435–440. doi: 10.1016/s0959-437x(02)00322-2. [DOI] [PubMed] [Google Scholar]
- 12.Cajal SR. Accion neurotropica de los epitelios. Trab del Lab de Invest Biol. 1919;17:1–153. [Google Scholar]
- 13.Carney PR, Silver J. Studies on cell migration and axon guidance in the developing distal auditory system of the mouse. J Comp Neurol. 1983;215:359–369. doi: 10.1002/cne.902150402. [DOI] [PubMed] [Google Scholar]
- 14.Chen P, Johnson JE, Zoghbi HY, Segil N. The role of Math1 in inner ear development: uncoupling the establishment of the sensory primordium from hair cell fate determination. Development. 2002;129:2495–2505. doi: 10.1242/dev.129.10.2495. [DOI] [PubMed] [Google Scholar]
- 15.Cloutier JF, Giger RJ, Koentges G, Dulac C, Kolodkin AL, Ginty DD. Neuropilin-2 mediates axonal fasciculation, zonal segregation, but not axonal convergence, of primary accessory olfactory neurons. Neuron. 2002;33:877–892. doi: 10.1016/s0896-6273(02)00635-9. [DOI] [PubMed] [Google Scholar]
- 16.Coombs S, Görner P, Münz H. The Mechanosensory Lateral Line: Neurobiology and Evolution. xvii. Springer; New York: 1989. p. 724. [Google Scholar]
- 17.Coppola V, Kucera J, Palko ME, Martinez-De Velasco J, Lyons WE, Fritzsch B, Tessarollo L. Dissection of NT3 functions in vivo by gene replacement strategy. Development. 2001;128:4315–4327. doi: 10.1242/dev.128.21.4315. [DOI] [PubMed] [Google Scholar]
- 18.Cowan CA, Yokoyama N, Bianchi LM, Henkemeyer M, Fritzsch B. EphB2 guides axons at the midline and is necessary for normal vestibular function. Neuron. 2000;26:417–430. doi: 10.1016/s0896-6273(00)81174-5. [DOI] [PubMed] [Google Scholar]
- 19.Davies D, Holley MC. Differential expression of alpha3 and alpha6 integrins in the developing mouse inner ear. J Comp Neurol. 2002;445:122–132. doi: 10.1002/cne.10161. [DOI] [PubMed] [Google Scholar]
- 20.Farinas I, Jones KR, Tessarollo L, Vigers AJ, Huang E, Kirstein M, de Caprona DC, Coppola V, Backus C, Reichardt LF, Fritzsch B. Spatial shaping of cochlear innervation by temporally regulated neurotrophin expression. J Neurosci. 2001;21:6170–6180. doi: 10.1523/JNEUROSCI.21-16-06170.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fekete DM, Wu DK. Revisiting cell fate specification in the inner ear. Curr Opin Neurobiol. 2002;12:35–42. doi: 10.1016/s0959-4388(02)00287-8. [DOI] [PubMed] [Google Scholar]
- 22.Fritzsch B. The pattern of lateral-line afferents in urodeles. A horseradish-peroxidase study. Cell Tiss Res. 1981;218:581–594. doi: 10.1007/BF00210117. [DOI] [PubMed] [Google Scholar]
- 23.Fritzsch B. The lateral-line and inner-ear afferents in larval and adult urodeles. Brain Behav E. 1988;31:325–348. doi: 10.1159/000116599. [DOI] [PubMed] [Google Scholar]
- 24.Fritzsch B, Beisel KW. Evolution and development of the vertebrate ear. Brain Res Bull. 2001;55:711–721. doi: 10.1016/s0361-9230(01)00558-5. [DOI] [PubMed] [Google Scholar]
- 25.Fritzsch B, Beisel KW, Jones K, Farinas I, Maklad A, Lee J, Reichardt LF. Development and evolution of inner ear sensory epithelia and their innervation. J Neurobiol. 2002;53:143–156. doi: 10.1002/neu.10098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fritzsch B, Pirvola U, Ylikoski J. Making and breaking the innervation of the ear: neurotrophic support during ear development and its clinical implications. Cell Tiss Res. 1999;295:369–382. doi: 10.1007/s004410051244. [DOI] [PubMed] [Google Scholar]
- 27.Fritzsch B, Silos-Santiago I, Smeyne R, Fagan AM, Barbacid M. Reduction and loss of inner ear innervation in trkB and trkC receptor knockout mice: a whole mount DiI and scanning electron microscopic analysis. Audit Neurosci. 1995;1:401–417. [Google Scholar]
- 28.Fritzsch B, Silos-Santiago I, Bianchi LM, Farinas I. The role of neurotrophic factors in regulating the development of inner ear innervation. Trends Neurosci. 1997;20:159–164. doi: 10.1016/s0166-2236(96)01007-7. [DOI] [PubMed] [Google Scholar]
- 29.Gilmour DT, Maischein HM, Nusslein-Volhard C. Migration and function of a glial subtype in the vertebrate peripheral nervous system. Neuron. 2002;34:577–588. doi: 10.1016/s0896-6273(02)00683-9. [DOI] [PubMed] [Google Scholar]
- 30.Gompel N, Dambly-Chaudiere C, Ghysen A. Neuronal differences prefigure somatotopy in the zebrafish lateral line. Development. 2001;128:387–393. doi: 10.1242/dev.128.3.387. [DOI] [PubMed] [Google Scholar]
- 31.Gowan K, Helms AW, Hunsaker TL, Collisson T, Ebert PJ, Odom R, Johnson JE. Crossinhibitory activities of Ngn1 and Math1 allow specification of distinct dorsal interneurons. Neuron. 2001;31:219–232. doi: 10.1016/s0896-6273(01)00367-1. [DOI] [PubMed] [Google Scholar]
- 32.Huang X, Cheng HJ, Tessier-Lavigne M, Jin Y. MAX-1, a novel PH/MyTH4/FERM domain cytoplasmic protein implicated in netrin-mediated axon repulsion. Neuron. 2002;34:563–576. doi: 10.1016/s0896-6273(02)00672-4. [DOI] [PubMed] [Google Scholar]
- 33.Huang EJ, Liu W, Fritzsch B, Bianchi LM, Reichardt LF, Xiang M. Brn3a is a transcriptional regulator of soma size, target field innervation and axon pathfinding of inner ear sensory neurons. Development. 2001;128:2421–2432. doi: 10.1242/dev.128.13.2421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Karis A, Pata I, van Doorninck JH, Grosveld F, de Zeeuw CI, de Caprona D, Fritzsch B. Transcription factor GATA-3 alters pathway selection of olivocochlear neurons and affects morphogenesis of the ear. J Comp Neurol. 2001;429:615–630. doi: 10.1002/1096-9861(20010122)429:4<615::aid-cne8>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 35.Kim WY, Fritzsch B, Serls A, Bakel LA, Huang EJ, Reichardt LF, Barth DS, Lee JE. NeuroD-null mice are deaf due to a severe loss of the inner ear sensory neurons during development. Development. 2001;128:417–426. doi: 10.1242/dev.128.3.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lawoko-Kerali G, Rivolta MN, Holley M. Expression of the transcription factors GATA3 and Pax2 during development of the mammalian inner ear. J Comp Neurol. 2002;442:378–391. doi: 10.1002/cne.10088. [DOI] [PubMed] [Google Scholar]
- 37.Leake PA, Snyder RL, Hradek GT. Postnatal refinement of auditory nerve projections to the cochlear nucleus in cats. J Comp Neurol. 2002;448:6–27. doi: 10.1002/cne.10176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee JE. Basic helix-loop-helix genes in neural development. Curr Opin Neurobiol. 1997;7:13–20. doi: 10.1016/s0959-4388(97)80115-8. [DOI] [PubMed] [Google Scholar]
- 39.Liu M, Pereira FA, Price SD, Chu MJ, Shope C, Himes D, Eatock RA, Brownell WE, Lysakowski A, Tsai MJ. Essential role of BETA2/NeuroD1 in development of the vestibular and auditory systems. Genes Dev. 2000;14:2839–2854. doi: 10.1101/gad.840500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lorente de No R. Etudes sur l’anatomie et la physiologie du labyrinthe de l’oreille et du VIII nerf. II. Quelques donnees au sujet de l’anatomie des organes sensoriels du labyrinthe. Trav Lab Rech Biol Univ Madrid. 1926;24:53–153. [Google Scholar]
- 41.Lorente de No R. Anatomy of the eighth nerve: the central projections of the nerve endings of the internal ear. Laryngoscope. 1933;43:1–38. [Google Scholar]
- 42.Ma Q, Anderson DJ, Fritzsch B. Neurogenin 1 null mutant ears develop fewer, morphologically normal hair cells in smaller sensory epithelia devoid of innervation. J Assoc Res Otolaryngol. 2000;1:129–143. doi: 10.1007/s101620010017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ma Q, Chen Z, del Barco Barrantes I, de la Pompa JL, Anderson DJ. Neurogenin1 is essential for the determination of neuronal precursors for proximal cranial sensory ganglia. Neuron. 1998;20:469–482. doi: 10.1016/s0896-6273(00)80988-5. [DOI] [PubMed] [Google Scholar]
- 44.Maklad A, Fritzsch B. Incomplete segregation of endorgan-specific vestibular ganglion cells in mice and rats. J Vestib Res. 1999;9:387–399. [PubMed] [Google Scholar]
- 45.Maklad A, Fritzsch B. The developmental segregation of posterior crista and saccular vestibular fibers in mice: a carbocyanine tracer study using confocal microscopy. Dev Brain Res. 2002;135:1–17. doi: 10.1016/s0165-3806(01)00327-3. [DOI] [PubMed] [Google Scholar]
- 46.Miyazaki N, Furuyama T, Takeda N, Inoue T, Kubo T, Inagaki S. Expression of mouse semaphorin H mRNA in the inner ear of mouse fetuses. Neurosci Lett. 1999;261:127–129. doi: 10.1016/s0304-3940(98)00988-4. [DOI] [PubMed] [Google Scholar]
- 47.Morris JK, Lin W, Hauser C, Marchuk Y, Getman D, Lee KF. Rescue of the cardiac defect in ErbB2 mutant mice reveals essential roles of ErbB2 in peripheral nervous system development. Neuron. 1999;23:273–283. doi: 10.1016/s0896-6273(00)80779-5. [DOI] [PubMed] [Google Scholar]
- 48.Murakami Y, Suto F, Shimizu M, Shinoda T, Kameyama T, Fujisawa H. Differential expression of plexin-A subfamily members in the mouse nervous system. Dev Dyn. 2001;220:246–258. doi: 10.1002/1097-0177(20010301)220:3<246::AID-DVDY1112>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 49.Noden DM, Van de Water TR. Genetic analyses of mammalian ear development. Trends Neurosci. 1992;15:235–237. doi: 10.1016/0166-2236(92)90056-e. [DOI] [PubMed] [Google Scholar]
- 50.Northcutt RG, Brandle K, Fritzsch B. Electroreceptors and mechanosensory lateral line organs arise from single placodes in axolotls. Dev Biol. 1995;168:358–373. doi: 10.1006/dbio.1995.1086. [DOI] [PubMed] [Google Scholar]
- 51.Northcutt RG, Catania KC, Criley BB. Development of lateral line organs in the axolotl. J Comp Neurol. 1994;340:480–514. doi: 10.1002/cne.903400404. [DOI] [PubMed] [Google Scholar]
- 52.Pasterkamp RJ, Verhaagen J. Emerging roles for semaphorins in neural regeneration. Brain Res Brain Res Rev. 2001;35:36–54. doi: 10.1016/s0165-0173(00)00050-3. [DOI] [PubMed] [Google Scholar]
- 53.Pirvola U, Ylikoski J, Palgi J, Lehtonen E, Arumae U, Saarma M. Brain-derived neurotrophic factor and neurotrophin 3 mRNAs in the peripheral target fields of developing inner ear ganglia. Proc Natl Acad Sci USA. 1992;89:9915–9919. doi: 10.1073/pnas.89.20.9915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Purcell IM, Perachio AA. Peripheral patterns of terminal innervation of vestibular primary afferent neurons projecting to the vestibulocerebellum in the gerbil. J Comp Neurol. 2001;433:48–61. doi: 10.1002/cne.1124. [DOI] [PubMed] [Google Scholar]
- 55.Qian Y, Fritzsch B, Shirasawa S, Chen CL, Choi Y, Ma Q. Formation of brainstem (nor)adrenergic centers and first-order relay visceral sensory neurons is dependent on homeodomain protein Rnx/Tlx3. Genes Dev. 2001;15:2533–2545. doi: 10.1101/gad.921501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Retzius G. Zur Entwicklung der Zellen des Ganglion spirale acustici und zur Endigungsweise des Gehoernerven bei den Saeugetieren. Biologische Untersuchungen. 1893;7:52–57. [Google Scholar]
- 57.Rivolta MN, Halsall A, Johnson CM, Tones MA, Holley MC. Transcript profiling of functionally related groups of genes during conditional differentiation of a Mammalian cochlear hair cell line. Genome Res. 2002;12:1091–1099. doi: 10.1101/gr.225602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rubel EW, Fritzsch B. Auditory system development: primary auditory neurons and their targets. Annu Rev Neurosci. 2002;25:51–101. doi: 10.1146/annurev.neuro.25.112701.142849. [DOI] [PubMed] [Google Scholar]
- 59.Ruben RJ. Development of the inner ear of the mouse: a radioautographic study of terminal mitoses. Acta Otolaryngol. 1967;220(Suppl):221–244. [PubMed] [Google Scholar]
- 60.Silos-Santiago I, Fagan AM, Garber M, Fritzsch B, Barbacid M. Severe sensory deficits but normal CNS development in newborn mice lacking TrkB and TrkC tyrosine protein kinase receptors. Eur J Neurosci. 1997;9:2045–2056. doi: 10.1111/j.1460-9568.1997.tb01372.x. [DOI] [PubMed] [Google Scholar]
- 61.Tello JF. Le reticule des cellules ciliees du labyrinth chez la souris et son independance des terminaisons nerveuses de la huitieme paire. Trab del Lab de Invest Biol. 1931;27:151–186. [Google Scholar]
- 62.Tessier-Lavigne M, Goodman CS. The molecular biology of axon guidance. Science. 1996;274:1123–1133. doi: 10.1126/science.274.5290.1123. [DOI] [PubMed] [Google Scholar]
- 63.Van de Water TR. Embryogenesis of the inner ear: “in vitro studies”. In: Romand R, editor. Development of auditory and vestibular systems. Academic Press; New York: 1983. pp. 337–374. [Google Scholar]
- 64.Van Esch H, Groenen P, Nesbit MA, Schuffenhauer S, Lichtner P, Vanderlinden G, Harding B, Beetz R, Bilous RW, Holdaway I, Shaw NJ, Fryns JP, Van de Ven W, Thakker RV, Devriendt K. GATA3 haplo-insufficiency causes human HDR syndrome. Nature. 2000;406:419–422. doi: 10.1038/35019088. [DOI] [PubMed] [Google Scholar]
- 65.Werner CF. Das Gehoerorgan der Wirbeltiere und des Menschen. Thieme; Leipzig: 1960. p. 309. [Google Scholar]
- 66.Yang Q, Bermingham NA, Finegold MJ, Zoghbi HY. Requirement of Math1 for secretory cell lineage commitment in the mouse intestine. Science. 2001;294:2155–2158. doi: 10.1126/science.1065718. [DOI] [PubMed] [Google Scholar]
- 67.Zhou Q, Anderson DJ. The bHLH transcription factors OLIG2 and OLIG1 couple neuronal and glial subtype specification. Cell. 2002;109:61–73. doi: 10.1016/s0092-8674(02)00677-3. [DOI] [PubMed] [Google Scholar]