Abstract
We review the in vivo evidence for afferent fiber guidance to the inner ear sensory epithelia and the central nuclei of termination. Specifically, we highlight our current molecular understanding for the role of hair cells and sensory epithelia in guiding afferents, how disruption of certain signals can alter fiber pathways, even in the presence of normal hair cells, and what role neurotrophins play in fiber guidance of sensory neurons to hair cells. The data suggest that the neurotrophin BDNF is the most important molecule known for inner ear afferent fiber guidance to hair cells in vivo. This suggestion is based on experiments on Ntf3 transgenic mice expressing BDNF under Ntf3 promoter that show deviations of fiber growth in the ear to areas that express BDNF but have no hair cells. However, fiber growth can occur in the absence of BDNF as demonstrated by double mutants for BDNF and Bax. We directly tested the significance of hair cells or sensory epithelia for fiber guidance in mutants that lose hair cells (Pou4f3) or do not form a posterior crista (Fgf10). While these data emphasize the role played by BDNF, normally released from hair cells, there is some limited capacity for directed growth even in the absence of hair cells, BDNF, or sensory epithelia. This directed growth may rely on semaphorins or other matrix proteins because targeted ablation of the sema3 docking site on the sema receptor Npn1 results in targeting errors of fibers even in the presence of hair cells and BDNF. Overall, our data support the notion that targeting of the afferent processes in the ear is molecularly distinct from targeting processes in the central nuclei. This conclusion is derived from data that show no recognizable central projection deviation, even if fibers are massively rerouted in the periphery, as in Ntf3tgBDNF mice in which vestibular fibers project to the cochlea.
1. Introduction
Neurosensory hearing loss is, next to conductive hearing loss, one of the more frequently encountered ailments of the elderly and may afflict as many as 1 in six over the age of 70. Neurosensory hearing loss consists of two distinct but interrelated processes, hair cell loss and loss of sensory neurons. Sensory neuron loss may be a consequence of hair cell loss or may happen through direct neuronal loss in certain neuropathies. Loss of neurons limits the usefulness of cochlear and vestibular implants, currently the only remedy to minimize the devastating personal and social effects neurosensory losses have on affected individuals. The molecular basis of sensory neuron maintenance in the absence of hair cells as well the molecular basis for directed growth of neuronal processes to regenerated hair cells or stimulating electrodes of implants is, next to the regeneration of hair cells, the second most challenging process in inner ear neurosensory development and regeneration. Studying neurosensory development can provide insights into the molecular biology of directed nerve fiber growth in sensory regeneration and thus may contribute to our understanding and clinical application of such information.
Sensory neurons of the ear form through the action of the bHLH gene Neurogenin 1 (Neurog1; formerly Ngn1) in the wall of the developing otocyst. In the absence of Neurog1, no sensory neurons in either the vestibular or cochlear part of the ear ever form (Ma et al., 2000, 1998). Downstream of Neurog1 is another bHLH gene, Neu-rod1 (formerly NeuroD or Beta). This gene mediates certain aspects of pathfinding and migration as well as survival of sensory neurons (Kim et al., 2001; Liu et al., 2000). How Neurog1 and/or Neurod1 activate other downstream genes relevant for connecting developing sensory neurons to specific hair cells of a given sensory epithelium is not fully understood (Fritzsch, 2003a). Numerous data show that hair cells will be innervated by sensory fibers no matter how unusual their position is. Innervation of sensory epithelia will be in a rather specific and highly conserved pattern (Fritzsch et al., 2001) that can be used to identify sensory epithelia across phyla no matter what shape and form (Fritzsch, 2003b). In vitro data indicate that this is so because an unknown factor is released, apparently from hair cells (Bianchi and Cohan, 1991, 1993; Hemond and Morest, 1991). It has been suggested that this substance is not one of the two neurotrophins known to be important for inner ear innervation survival (Fritzsch et al., 2004), BDNF and Ntf3 (formerly NT-3). Specifically, BDNF seems not to play the role of a major attractant of nerve fibers (Bianchi and Cohan, 1993), despite the fact that it is almost exclusively expressed in hair cells of the developing ear (Farinas et al., 2001; Pirvola et al., 1992). However, it cannot be ruled out that other factors generated by supporting cells contribute to the apparent attraction generated by hair cells. This is so because hair cells and supporting cells are clonally related and always occur together in a normal ear (Fekete et al., 1998). Also, supporting cells express the neurotrophin Ntf3 (Farinas et al., 2001; Pirvola et al., 1992).
Beyond the role attributed to hair cells, it is clear that other factors that are not associated with hair cells may play additional roles in overall targeting of fibers across the developing otocyst to specific sensory epithelia (Fritzsch, 2003b; Rubel and Fritzsch, 2002). For example, tissue culture experiments showed that spiral ganglion fibers can extend toward the organ of Corti even if the hair cells have been removed (Sobkowicz, 1992). The initial contact formation will ultimately be refined through a process of pruning and fiber growth (Echteler and Nofsinger, 2000). However, none of these processes have been analyzed in the background of specific neurotrophin mutations to provide information independent of neurotrophin mediated nerve attraction.
Equally important and even less well understood is how the various central projections are determined. It is unknown what molecular mechanisms make cochlear fibers connect selectively to the cochlear nuclei (Rubel and Fritzsch, 2002) and vestibular fibers connect selectively to specific subsets of the vestibular nuclear complex or the cerebellum (Maklad and Fritzsch, 2003a). So far, we know only that the central projection is specifically targeted from earliest fiber growth onward (Maklad and Fritzsch, 2003b), but neonatal refinement of the overall properly targeted projections through activity mediated processes likely plays a role as well (Leake et al., 2002; Rubel and Fritzsch, 2002). Obviously, such specificity of both central and peripheral connections requires that both the peripheral dendrite and the central axon are properly targeted through either similar or complementary mechanisms co-expressed in the same sensory neuron.
Recent years have seen dramatic progress in the understanding of the molecular and cellular basis of connection formation in several sensory systems using loss-of-function (knockout) and transgenic approaches. For example, such analyses have clarified that in the olfactory system there is a close correlation between the type of olfactory receptor formation and the specific projection of such receptor neurons to given glomeruli, their targets in the olfactory bulb (Mombaerts et al., 1996; Zou et al., 2004). While details of the guidance are still unclear, it appears that differential expression of ephrin family members are involved in proper targeting (Cutforth et al., 2003). Ephrin family members also play a major role in the formation of the retino-topic map as revealed by numerous in vitro studies (McLaughlin et al., 2003) and these suggestions were recently confirmed in loss-of-function experiments in vivo (Feldheim et al., 2004). Recent work on the central organization of taste bud projections showed the importance for fiber and target interaction in loss-of-function mutants in this nucleus (Qian et al., 2001). Together these data on nonotic sensory systems highlight the strength of the genetic approach as a tool to molecularly dissect, in vivo, the known or suspected cell and molecular interactions. While progress has been made, it is still a long way before the digital information of the genome can be used in a systems approach to understand the network perturbations caused by mutations and this information can then be used to guide therapeutic intervention (Hood et al., 2004). Such a detailed understanding of the function of individual genes in the context of a developmental network of gene interactions is, in our opinion, an essential prerequisite for any attempt to manipulate fiber growth to generate, for example, more profound connections with cochlear implants.
In this review, we will therefore highlight data based on genetic approaches in vivo. However, where appropriate, we will also outline the information gathered by other approaches. We will show that most of the peripheral targeting mechanisms currently known seem to have little effect on the central targeting specificity, indicating that different networks are involved on the dendritic and axonal end of a sensory neuron’s processes.
Overall, we will address the following points:
What is the role of hair cells in guiding afferents?
Can fibers project correctly even in the absence of sensory epithelia?
Can the disruption of certain signals alter fiber pathways even in the presence of normal hair cells?
What is the role of neurotrophins in fiber guidance of sensory neurons?
1.1. The role of hair cells in guiding afferents to and within sensory epithelia
Assessing the role of hair cells in guiding afferents requires elimination of hair cells early in development to investigate whether other mechanisms for guidance exist which do not require hair cells. The effects of loss of some or all hair cells has been studied in the past in tissue culture or using mutants such as the Bronx–Waltzer mutation (Sobkowicz, 1992). These data suggest that at least some pathfinding properties are not mediated by hair cells and also showed the importance of hair cells. In recent years, several additional targeted mutations have become available that allow us to continue this investigation of the relative role played by non-hair cell mediated guidance mechanisms. Abrogation of hair cell formation on fiber extension to the cochlea can now be studied in embryos with two gene mutations, Atoh1 (formerly Math1) and Pou4f3 (formerly Brn3c or Brn3.1). Loss of hair cells can also be achieved later in neonatal development using various mutations such as the Barhl1 homeobox gene (Li et al., 2002) or the Gfi1 zinc finger factor (Hertzano et al., 2004; Wallis et al., 2003) and might present excellent models to study the effect of the absence of hair cells on maintenance of central and peripheral nerve fibers.
Null mutations for Pou4f3 show initial formation of hair cells that fail to differentiate fully and disappear progressively in late neonates and early postnatal mice (Erkman et al., 1996; Hertzano et al., 2004; Vahava et al., 1998; Xiang et al., 1997, 1998). It needs to be stressed that these cochlear hair cells never fully develop their apical specialization (Hertzano et al., 2004) and that most hair cells can not be recognized any more around postnatal day 8 (Xiang et al., 1998). Analysis of the peripheral projection pattern shows almost normal innervation of all cochlear and vestibular organs until approximately 8 days of age (Xiang et al., 2003). In fact, in the apex of the cochlea there is retention of sensory neurons and their innervation to the undifferentiated organ of Corti in the absence of hair cells for at least 6 months (Fig. 1). These data refute the importance of differentiated hair cells for long term maintenance of afferents. However, it cannot be ruled out that even undifferentiated hair cells and supporting cells that form before they degenerate have some attraction for afferents. Indeed, close examination shows that the neurotrophins BDNF and Ntf3 are still expressed in the sensory epithelia but at an apparently reduced level (Xiang et al., 2003). No hair cells could be detected in the cochlea apex of these mutants during the time fibers are extending to the outer hair cells in normal mice and no fibers grow beyond the topographical level equivalent to the inner hair cells in these mutants. These data suggest that growth of fibers to the cochlea is independent of differentiated hair cells, that retention of afferents is independent of hair cells but that growth of fibers to outer hair cells depends on the presence of hair cells. Further analysis is needed to show quantitatively the level of neurotrophin expression using real time PCR (Stankovic and Corfas, 2003) as well as the cellular localization and correlate that directly with the survival of fibers and their specific innervation pattern. Absence of hair cells seems to have little effect on the formation of crudely topographically restricted projection to the apex and the base of the cochlea which seem to develop rather normally in neonates (Fig. 1). Overall, these data are consistent with an analysis showing such topographically restricted projections in cats prior to the onset of hearing (Leake et al., 2002). Whether the topographical refinement of the central projection will be affected in older Pou4f3 null mice has not been investigated yet. Likewise, whether these findings can be extended to the specific vestibular projection to the cerebellum (Maklad and Fritzsch, 2003a) remains to be demonstrated.
Fig. 1.
The Pou domain factor Pou4f3 (Brn3c) is required for hair cell differentiation and maintenance of hair cells past birth. Only few basal turn hair cells can be identified in neonatal animals using Myosin VII immunocytochemistry (a,b). Despite this absence of differentiated hair cells, afferent fibers are targeted to both vestibular and cochlear sensory epithelia (c,d). Such fibers project in a crudely topographical fashion to the cochlear nuclei as revealed by the injection of different fluorescent tracers into the apex and base of the cochlea, respectively (e,f). These data establish that an overall fairly normal peripheral and a crude central projection can develop in the absence of functional hair cells. AC, anterior crista; HC, horizontal crista; HCs, hair cells; IHCs, inner hair cells; OHCs, outer hair cells; PC, posterior crista; VCN, ventral cochlear nucleus. Modified after (Xiang et al., 2003).
We have recently begun to investigate the pattern of innervation in Atoh1 null mice (Bermingham et al., 1999). These mutants never develop recognizable hair cells likely because of a failure to initiate postmitotic differentiation (Chen et al., 2002). Our preliminary data suggest that at least some primordial hair cells form that express a low level of the neurotrophin BDNF and are apparently specifically innervated (Fritzsch et al., submitted). These data indicate that complete elimination, even of hair cell primordia, is needed to exclude the possible attraction of fibers to these primordia. An additional complication arises through the hair celsupporting cell interaction via the delta-notch system (Eddison et al., 2000). The importance of the delta-notch system mediated upregulation of bHLH genes in the CNS development has recently been established through mutational analysis (Hatakeyama et al., 2004). Since hair cell and supporting cell formation is clonally linked (Fekete et al., 1998) and hair cells are involved in the regulation of supporting cell maturation through the delta-notch system (Zine et al., 2001), elimination of hair cell precursors might result in abrogation of entire sensory epithelia. It is, therefore, possible that elimination of the entire formation of sensory patches may be required to investigate the role of other guiding mechanisms to bring fibers to specific sensory epithelia. Alternatively, suspected molecules for guidance such as neurotrophins can be eliminated and the remaining pattern of innervation analyzed in the presence of hair cells but in the absence of such presumptive guidance molecules.
1.2. Fibers project correctly even in the absence of sensory epithelia
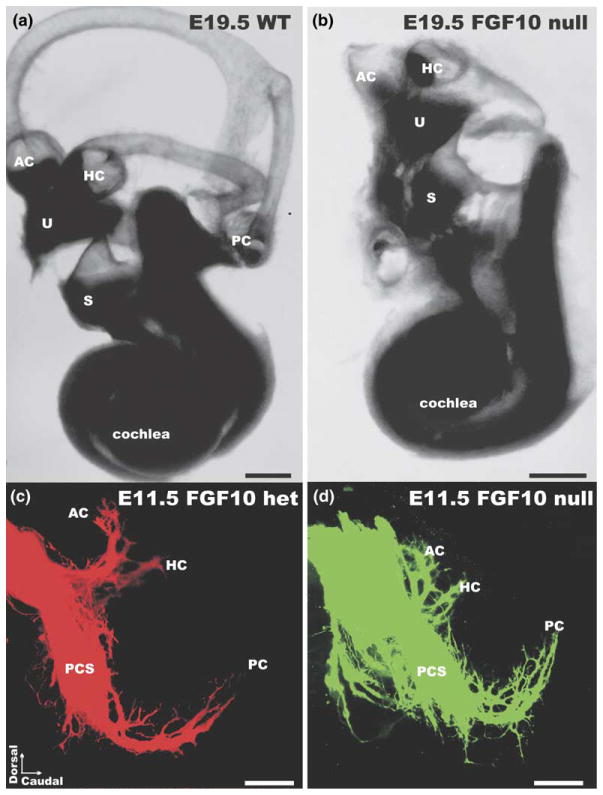
Fibroblast growth factors have been shown to be essential for ear formation (Pirvola et al., 2002; Wright and Mansour, 2003a) and may also play a role in patterning of innervation (Brumwell et al., 2000; Wright and Mansour, 2003b). We recently showed that FGF10 is essential for semicircular canal formation but is also necessary for the formation of the posterior canal crista (Pauley et al., 2003). Interestingly, while no hair cell or sensory primordia of the posterior crista seem to form, there is, although reduced, an initial formation of posterior crista-specific sensory neurons. Those neurons project toward the absent posterior crista (Fig. 2) but disappear, likely because of failure to receive neurotrophic support in the absence of any target, within two days (Pauley et al., 2003). These data suggest that initial pathfinding properties may not be related to sensory epithelia and their attraction for fibers. In addition, other factors may play a role such as the topology of sensory neuron formation in the otocyst wall, allowing neurons that delaminate from specific areas to project crudely correctly at least to the areas they delaminated from (Carney and Silver, 1983; Fritzsch et al., 2002). Alternatively, sensory neurons may leave their dendrites behind as trailing processes thus requiring only limited near target guidance of fibers toward and within sensory epithelia (Fritzsch, 2003a). These preliminary assessments require further confirmation by the demonstration that no molecules known to be associated with sensory epithelia formation are ever expressed in the area of future posterior crista in Fgf10 null mutant mice. It also needs to be shown that posterior crista sensory neurons derive from distinctly different areas than the sensory epithelia, a possibility recently suggested (Raft et al., 2004).
Fig. 2.
Fgf10 null mutant mice do not develop semicircular canals and have no posterior canal crista (a,b). Such animals do not show a projection of afferent or efferent fibers to the posterior region of the ear either in late embryonic stages. However, at early embryonic stages posterior crista sensory neurons apparently form and project toward the area of the posterior crista almost like in wildtype or heterorzygotic littermates (c,d). Within two days such projections are lost, presumably because of lack of neurotrophic support. These data support the idea that some pathfinding properties of vestibular afferents reside outside sensory epithelia. AC, anterior cristae; HC, horizontal cristae; PCS, posterior crista sensory neurons; PC, posterior crista; S, saccule. Modified after (Pauley et al., 2003).
1.3. Disruption of certain signals results in axonal targeting defects despite otherwise normal hair cells
The data on Fgf10 null mutants clearly suggest that some pathfinding information derives from outside the sensory epithelia. In other sensory systems, pathfinding decisions may be intrinsically specified by their receptors, as is the case for odorant receptors in the olfactory system (Zou et al., 2004). Alternatively, projections are guided and refined by graded distributions of specific guidance cues and their receptors, such as ephrin ligands and their cognate receptors in the visual system (O’Leary and Wilkinson, 1999) or semaphorins and their receptors in both the peripheral and central nervous system (Pasterkamp and Verhaagen, 2001). Ephrin receptors and ligands are distributed in the developing ear (Bianchi and Liu, 1999) but no alterations in projection patterns have been found in the only mutant analyzed thus far, the EphB2 null mutant mouse (Cowan et al., 2000), possibly because of redundancy of ephrin ligand signaling through several ephrin receptors. Conditional mutations that eliminate several ephrin ligands and/or receptors simultaneously will be needed to address this complex problem. Such approaches are now possible using the growing availability of ear specific Cre transgenic mice combined with increasingly available floxed ephrin ligands and receptors.
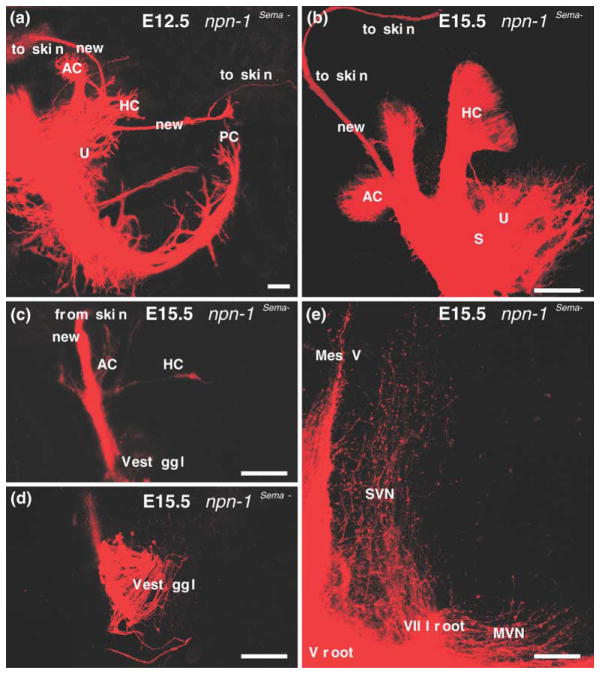
Comparable to the ephrin ligands and their receptors, intriguing patterns of expression of some members of the semaphorin family of guidance cues and their receptors, neuropilin 1 and neuropilin 2, have been described in the ear (Miyazaki et al., 1999; Murakami et al., 2001). Null mutations of some of the ligands (Sema3a) and both neuropilin receptors exist (Cloutier et al., 2002). The Npn-1 null mice exhibit an early embryonic lethal phenotype, prior to ear innervation. Thus, studying the effect of the semaphorin receptor Npn-1 during ear innervation development required the targeted elimination of a single semaphorin docking site in Npn-1 or the conditional elimination of semaphorin ligands and/or receptors only in the ear with either approach being likely to generate viable mutations. The former was recently done by altering the endogenous Sema3a docking site of the Npn-1 receptor such that the receptor could not bind to semaphorin ligands, and the pattern of inner ear innervation was studied (Gu et al., 2003). These Npn-1Sema- mice exhibited profound reorganization of fibers into unusual trajectories around the ear. For example, projections to the posterior crista were found to pass along the anterior side of the otocyst as an extension of fibers targeted for the utricle (Fig. 3). Other fibers were found to overshoot sensory epithelia and terminate instead in the skin above the ear (Fig. 3). A somewhat similar, transient phenotype was reported for some Pou4f1 (Brn3a) null mutant mice (Huang et al., 2001), but it is unknown whether this Pou domain factor regulates Npn-1 or plexin expression in sensory neurons.
Fig. 3.
Mice with a targeted replacement of the Sema3a docking site at the neuropilin1 receptor (Npn-1Sema- knock-in mice) show distinct defects in the inner ear innervation. Specifically, in these mutants, fibers may not stop near hair cells or sensory epithelia but continue to grow until they reach the skin above the ear (a,b). Injections of a lipophilic tracer into the skin above the ear will label the trigeminal system but also fibers that show side-branches to the vestibular endorgans as they pass through the ear (c). In addition, these fibers can be traced to vestibular ganglion cells (d) and can be shown to project centrally like vestibular fibers into the vestibular nuclei rather than like trigeminal fibers. These data suggest that Sema3a is at least one of the stop signals at or near sensory epithelia that directs fibers to hair cells. The data also show that at least during embryonic development specification of central projections of vestibular neurons does not depend on being connected to hair cells. AC, anterior cristae; HC, horizontal crista; MesV, nucleus mesencephalicus V; MVN, medial vestibular nucleus; PC, posterior crista; S, saccule; SVN, superior vestibular nucleus; U, utricle. Modified after (Gu et al., 2003).
We have also analyzed the central termination of such fibers in the skin in the Npn-1Sema- knock-in mice by applying lipophilic dyes to the fibers in the skin at embryonic day 15 mutants. The findings show that these fibers form very few collaterals to vestibular sensory organs, derive from vestibular ganglia and terminate centrally in vestibular nuclei (Fig. 3). Most importantly, these data suggest that Sema3a mediated signaling via Npn-1 provides a stop signal at or near the sensory epithelia. The absence of this stop signal, even in the presence of otherwise normal hair cells, leads to an overshooting of fibers which extend outside of the ear and into the skin. A more detailed analysis of mice lacking the various semaphorin ligands, and the plexin and neuropilin receptors needs to be performed to fully understand the effects thus far described in the Npn-1Sema- knock-in mice (Gu et al., 2003). Floxed alleles for Npn-1 and Npn-2, when combined with ear or hair cell specific Cre expressing mice such as the recently available Pax2-Cre (Ohyama and Groves, 2004) or a hair cell specific Cre, such as the Prestin-Cre (Tian et al., 2004), provided a proper time of Cre upregulation is achieved, would allow for organ specific or hair cell specific dissection of the function of each receptor, alone or in combination. Together such future directions could help to clarify the apparently significant role of this large family of ligands and receptors in the formation of the ear innervation pattern.
1.4. The role of neurotrophins in fiber guidance of sensory neurons
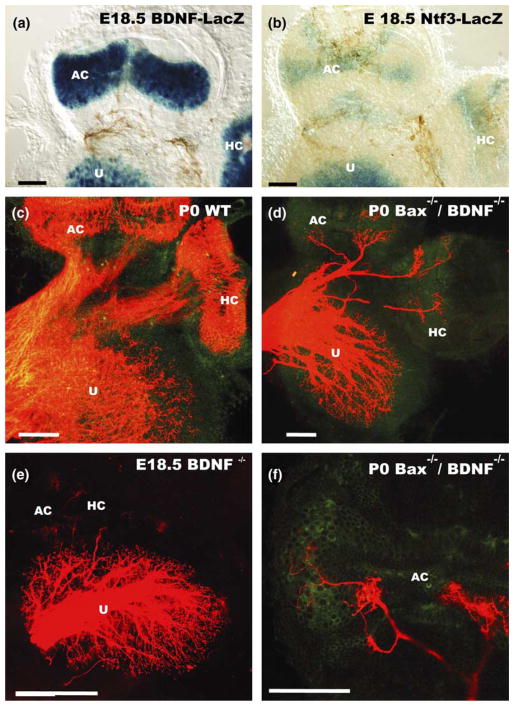
Only two neurotrophins and their cognant high affinity receptors are necessary for the maintenance of all inner ear innervation, BDNF with Ntrk2 and Ntf3 (formerly NT-3) with Ntrk3 (Fritzsch et al., 2004; for review). No sensory neurons survive in the absence of both neurotrophins (Ernfors et al., 1995; Liebl et al., 1997) or both neurotrophin receptors (Fritzsch et al., 1995; Silos-Santiago et al., 1997). In the ear, in single receptor or ligand null mutants, the neurotrophin ligands show complex alterations in their spatiotemporal pattern of expression (Farinas et al., 2001) that appears to be directly related to the spatially restricted loss of sensory neurons (Fritzsch et al., 1997, 2004). For example, loss of BDNF results in absence of all crista innervation except for an occasional fiber (Fig. 4). In contrast, gravistatic organs and the cochlea show only reduced innervation and a limited loss of spiral ganglion neurons (Bianchi et al., 1996; Fritzsch et al., 1997). Ntf3 null mutants show a loss of 85% of spiral ganglion cells with complete loss of all basal turn sensory neurons (Farinas et al., 1994; Fritzsch et al., 1997).
Fig. 4.
The neurotrophins BDNF (a) and Ntf3 (b) are both expressed in late embryonic canal cristae. However, only hair cells are positive for BDNF (a) while Ntf3 is expressed in the stroma of the crista as well as in adjacent dark cells near the crista (b). In wildtype mice afferent fibers are targeted to cristae as well as the utricle and innervate densely hair cells (c). In mutants, in which BDNF has been eliminated, hardly any fiber projects to canal cristae (e). However, breeding BDNF null mice into a Bax null background results in survival of some neurons and growth of some neurites in the absence of BDNF (d,f). These fibers extend toward cristae (d) but innervate predominantly areas of Ntf3 expression with only an occasional fiber extending to hair cells (f). These data suggest that BDNF is not only a major survival factor but also helps direct growth of fibers to hair cells. Nevertheless, the residual ability of fibers to grow toward hair cells suggests that some additional attractive substances may be released from hair cells. AC, anterior crista; HC, horizontal crista; U, utricle. Modified after (Hellard et al., 2004).
Recently, we directly tested the prediction that hair cells can have some attraction for sensory fibers independent of BDNF by combining BDNF null mutation with the Bax mutation. Bax null mice do not show neuronal cell death even in the absence of BDNF. Our analysis shows a more profound innervation of the anterior and horizontal crista, but not of the posterior crista (Hellard et al., 2004). Close examination shows that many, but not all fibers, are targeted to the cristae and seem to innervate hair cells (Fig. 4). However, several fibers were found outside the cristae and those inside the cristae tended to be near the base where the Ntf3 expression was found (Figs. 4 and 6). Consistent with the data on the transgenic mice, these data suggest that hair cells do exert some very limited attraction on nerve fibers that is not mediated by BDNF.
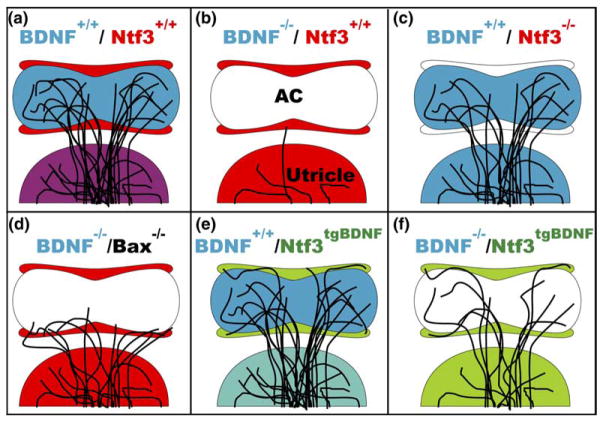
Fig. 6.
This schematic illustrates (a) the normal areas of expression of BDNF and Ntf3 in the utricle, hair cells of the anterior canal crista, and cells surrounding the sensory area of the crista, and the normal pattern of innervation of the crista. (b–f) The variations in expression pattern of neurotrophins and resultant patterns of crista innervation are shown. BDNF is required for neuron growth and survival (b) whereas Ntf3 is not (c). When Bax is also knocked out in the BDNF null mutant, fibers survive and project to areas of Ntf3 expression (d). When Ntf3 is replaced with BDNF, fibers innervate the sensory cells as usual, but also project to non-sensory, BDNF-expressing areas, demonstrating a positive attraction of fibers to BDNF even when expressed in an abnormal location (e). Combining the transgenic mouse in which Ntf3 is replaced with BDNF with BDNF null mutant shows that fibers can innervate hair cells that do not express BDNF, suggesting that other hair cell or supporting cell factors are involved in fiber attraction, but are also attracted to BDNF expressing non-sensory patches (f). White areas are areas in which neither BDNF or Ntf3 are expressed. Red represents Ntf3 expression. Blue represents BDNF expression. Purple represents normal overlap of BDNF and Ntf3 expression in hair cells and supporting cells, respectively. Green represents BDNF expression in place of Ntf3 expression and teal represents an overlap of normal BDNF expression in hair cells with BDNF expressed under the Ntf3 promoter in supporting cells. AC, anterioventral crista.
From this analysis, one might predict a functional equivalence of either neurotrophin mediated by the apparently redundant expression of both neurotrophin receptors in all inner ear sensory neurons. (Farinas et al., 2001) Indeed, this co-expression of both receptors allowed direct testing of this prediction by studying the remaining sensory neurons to the cochlea of transgenic mice expressing BDNF under Ntf3 promoter control (Coppola et al., 2001) or of Ntf3 under BDNF promoter control (Agerman et al., 2003). In Ntf3tgBDNF (BDNF under Ntf3 promoter control) mutants, BDNF is expressed instead of Ntf3 such that there is no Ntf3 expression, and BDNF is expressed in both its normal location and in the Ntf3 location. The opposite is true for Ntf3tgBDNF mutants. Both studies agree that, at least in the cochlea, there is functional equivalence of these neurotrophins for sensory neuron survival.
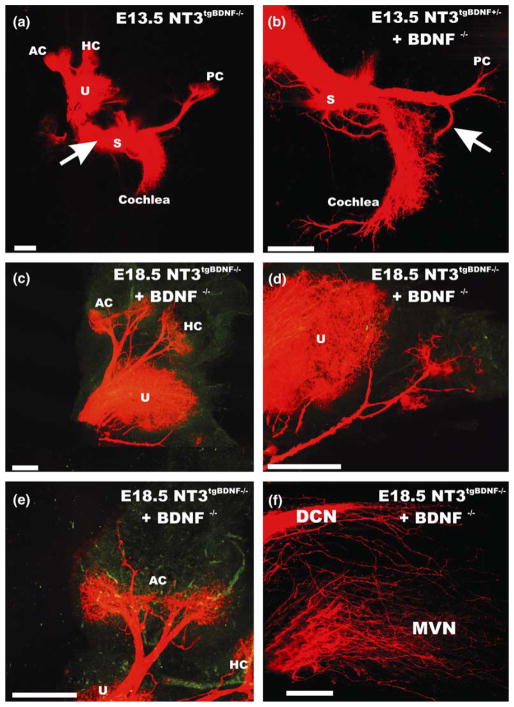
Using these transgenic mice, we showed that premature expression of BDNF under Ntf3 promoter control leads to massive rerouting of vestibular fibers to the basal turn of the cochlea (Fig. 5). Ntf3 is only expressed late in development in the crista of the semicircular canals and is around the sensory cristae rather than in supporting cells of the cristae (Fig. 4). Importantly, in the Ntf3tgBDNF mutants, fibers to the posterior crista stall on their way to the crista and turn around to innervate the basal turn of the cochlea (Fig. 5). This effect depends directly on the lack of early BDNF expression in the posterior crista and is most profound in the combined Ntf3tgBDNF/BDNF null mutants described below. Most interesting are the innervation defects in anterior and horizontal crista. Simple misexpression of BDNF under Ntf3 promoter control results in fibers innervating areas outside the sensory epithelia, suggesting that BDNF acts as short range attraction for fibers even in the presence of hair cells (Figs. 5 and 6). More recently, we combined the Ntf3tgBDNF mice with the BDNF null mutation, to test the effect of misexpression of BDNF on vestibular fiber pathfinding (Tessarollo et al., 2004). In these mice, there is no Ntf3 expression and BDNF is only expressed in the Ntf3 pattern and not in its normal pattern. Eliminating BDNF expression under its own promoter control, and therefore in hair cells, showed profound rescue of fibers to the cristae organs (Fig. 5). These fibers target hair cells inside the cristae organs as well as the BDNF expressing epithelial cells outside the cristae organs (Figs. 5 and 6) suggesting that factors other than BDNF can cause a very short range attraction to hair cells, provided neurons survive in BDNF null mutants and have fibers near the crista epithelia. This suggestion is supported by the complete rerouting of posterior cristae fibers into the basal turn of the cochlea in Ntf3tgBDNF mice combined with BDNF null mutation, presumably because of the limited and delayed upregulation of BDNF around the posterior crista epithelium that can not compete with the more extensive and earlier expression of BDNF under Ntf3 promoter control in the basal turn of the cochlea. Moreover, the presence of fibers outside sensory epithelia in areas that express BDNF in the transgenic misexpressors suggest that BDNF can override the attraction of hair cells, even if hair cells do express BDNF.
Fig. 5.
The effect of misexpression of BDNF under the Ntf3 promoter control on vestibular fiber pathfinding is shown without (a) or with a combined elimination of BDNF (b–f). Replacing of Ntf3 with BDNF results in rerouting of saccular and posterior crista afferents even in the presence of normal BDNF expression (a). However, combining the transgenic expression of BDNF under Ntf3 promoter control with absence of BDNF results in more profound rerouting of fibers to the basal turn of the cochlea (b) as well as rescue of fibers to the anterior and horizontal crista (d,e). Despite the fact that no neurotrophin is expressed in hair cells in these animals, fibers preferentially innervate hair cells (e) but also project outside the sensory epithelia to areas of expression of Ntf3 (c–e). These data suggest that attractors other than BDNF must exist in hair cells but also suggest that such attractors can be overridden by BDNF which by itself can attract fibers outside the sensory epithelia. Central projections from the cochlea (f) show fibers not only to the cochlear nuclei but also to vestibular nuclei. These data suggest that the central projection is regulated molecularly distinct from the peripheral projection. AC, anterior crista; DCN, dorsal cochlear nucleus; HC, horizontal crista; MVN, medial vestibular nucleus; PC, posterior crista; U, utricle. Modified after (Tessarollo et al., 2004).
We also analyzed the central projection of the vestibular fibers that enter the sensory epithelia but do not, at least in the vast majority, innervate cochlear hair cells. Different colored lipophilic tracers injected into the posterior crista and the basal turn of the cochlear directly show that many of these fibers terminate not in the auditory nuclei but rather in he nearby vestibular nuclei (Tessarollo et al., 2004). These data suggest that the central projection of vestibular fibers is not dependent on the target but rather reflects properties that are distinct for the central projection independent of their peripheral connection. Moreover, these data fully agree with our data on the Npn-1Sema-knock-in mice and together argue that guidance of central fiber projection is molecularly distinct form peripheral dendrite to hair cell targeting mechanisms. This implies that each end of the neurite growth process has to develop its own, unique, yet closely coupled molecular targeting machinery to accomplish proper navigation independent of, and yet crucially dependent upon, each other.
2. Uncited reference
Acknowledgments
This work was supported by a grant from NIDC (RO1 DC005590; BF) and NASA (NAG2-1595; BF). We thank Dr. D. Ginty for comments on an earlier draft.
References
- Agerman K, Hjerling-Leffler J, Blanchard MP, Scarfone E, Canlon B, Nosrat C, Ernfors P. BDNF gene replacement reveals multiple mechanisms for establishing neurotrophin specificity during sensory nervous system development. Development. 2003;130:1479–1491. doi: 10.1242/dev.00378. [DOI] [PubMed] [Google Scholar]
- Bermingham NA, Hassan BA, Price SD, Vollrath MA, Ben-Arie N, Eatock RA, Bellen HJ, Lysakowski A, Zoghbi HY. Math1: an essential gene for the generation of inner ear hair cells. Science. 1999;284:1837–1841. doi: 10.1126/science.284.5421.1837. [DOI] [PubMed] [Google Scholar]
- Bianchi LM, Cohan CS. Developmental regulation of a neurite-promoting factor influencing statoacoustic neurons. Brain Res Dev Brain Res. 1991;64:167–174. doi: 10.1016/0165-3806(91)90221-4. [DOI] [PubMed] [Google Scholar]
- Bianchi LM, Cohan CS. Effects of the neurotrophins and CNTF on developing statoacoustic neurons: comparison with an otocyst-derived factor. Dev Biol. 1993;159:353–365. doi: 10.1006/dbio.1993.1247. [DOI] [PubMed] [Google Scholar]
- Bianchi LM, Liu H. Comparison of ephrin-A ligand and EphA receptor distribution in the developing inner ear. Anat Rec. 1999;254:127–134. doi: 10.1002/(SICI)1097-0185(19990101)254:1<127::AID-AR16>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Bianchi LM, Conover JC, Fritzsch B, DeChiara T, Lindsay RM, Yancopoulos GD. Degeneration of vestibular neurons in late embryogenesis of both heterozygous and homozygous BDNF null mutant mice. Development. 1996;122:1965–1973. doi: 10.1242/dev.122.6.1965. [DOI] [PubMed] [Google Scholar]
- Brumwell CL, Hossain WA, Morest DK, Bernd P. Role for basic fibroblast growth factor (FGF-2) in tyrosine kinase (TrkB) expression in the early development and innervation of the auditory receptor: in vitro and in situ studies. Exp Neurol. 2000;162:121–145. doi: 10.1006/exnr.2000.7317. [DOI] [PubMed] [Google Scholar]
- Carney PR, Silver J. Studies on cell migration and axon guidance in the developing distal auditory system of the mouse. J Comp Neurol. 1983;215:359–369. doi: 10.1002/cne.902150402. [DOI] [PubMed] [Google Scholar]
- Chen P, Johnson JE, Zoghbi HY, Segil N. The role of Math1 in inner ear development: uncoupling the establishment of the sensory primordium from hair cell fate determination. Development. 2002;129:2495–2505. doi: 10.1242/dev.129.10.2495. [DOI] [PubMed] [Google Scholar]
- Cloutier JF, Giger RJ, Koentges G, Dulac C, Kolodkin AL, Ginty DD. Neuropilin-2 mediates axonal fasciculation, zonal segregation, but not axonal convergence, of primary accessory olfactory neurons. Neuron. 2002;33:877–892. doi: 10.1016/s0896-6273(02)00635-9. [DOI] [PubMed] [Google Scholar]
- Coppola V, Kucera J, Palko ME, Martinez-De Velasco J, Lyons WE, Fritzsch B, Tessarollo L. Dissection of NT3 functions in vivo by gene replacement strategy. Development. 2001;128:4315–4327. doi: 10.1242/dev.128.21.4315. [DOI] [PubMed] [Google Scholar]
- Cowan CA, Yokoyama N, Bianchi LM, Henkemeyer M, Fritzsch B. EphB2 guides axons at the midline and is necessary for normal vestibular function. Neuron. 2000;26:417–430. doi: 10.1016/s0896-6273(00)81174-5. [DOI] [PubMed] [Google Scholar]
- Cutforth T, Moring L, Mendelsohn M, Nemes A, Shah NM, Kim MM, Frisen J, Axel R. Axonal ephrin-As and odorant receptors: coordinate determination of the olfactory sensory map. Cell. 2003;114:311–322. doi: 10.1016/s0092-8674(03)00568-3. [DOI] [PubMed] [Google Scholar]
- Echteler SM, Nofsinger YC. Development of ganglion cell topography in the postnatal cochlea. J Comp Neurol. 2000;425:436–446. [PubMed] [Google Scholar]
- Eddison M, Le Roux I, Lewis J. Notch signaling in the development of the inner ear: lessons from Drosophila. Proc Natl Acad Sci USA. 2000;97:11692–11699. doi: 10.1073/pnas.97.22.11692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erkman L, McEvilly RJ, Luo L, Ryan AK, Hooshmand F, O’Connell SM, Keithley EM, Rapaport DH, Ryan AF, Rosenfeld MG. Role of transcription factors Brn-3.1 and Brn-3.2 in auditory and visual system development. Nature. 1996;381:603–606. doi: 10.1038/381603a0. [DOI] [PubMed] [Google Scholar]
- Ernfors P, Van De Water T, Loring J, Jaenisch R. Complementary roles of BDNF and NT-3 in vestibular and auditory development. Neuron. 1995;14:1153–1164. doi: 10.1016/0896-6273(95)90263-5. [DOI] [PubMed] [Google Scholar]
- Farinas I, Jones KR, Backus C, Wang XY, Reichardt LF. Severe sensory and sympathetic deficits in mice lacking neurotrophin-3. Nature. 1994;369:658–661. doi: 10.1038/369658a0. [DOI] [PubMed] [Google Scholar]
- Farinas I, Jones KR, Tessarollo L, Vigers AJ, Huang E, Kirstein M, de Caprona DC, Coppola V, Backus C, Reichardt LF, Fritzsch B. Spatial shaping of cochlear innervation by temporally regulated neurotrophin expression. J Neurosci. 2001;21:6170–6180. doi: 10.1523/JNEUROSCI.21-16-06170.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fekete DM, Muthukumar S, Karagogeos D. Hair cells and supporting cells share a common progenitor in the avian inner ear. J Neurosci. 1998;18:7811–7821. doi: 10.1523/JNEUROSCI.18-19-07811.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldheim DA, Nakamoto M, Osterfield M, Gale NW, DeChiara TM, Rohatgi R, Yancopoulos GD, Flanagan JG. Loss-of-function analysis of EphA receptors in retinotectal mapping. J Neurosci. 2004;24:2542–2550. doi: 10.1523/JNEUROSCI.0239-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritzsch B. Development of inner ear afferent connections: forming primary neurons and connecting them to the developing sensory epithelia. Brain Res Bull. 2003a;60:423–433. doi: 10.1016/s0361-9230(03)00048-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritzsch B. The ear of Latimeria chalumnae revisited. Zoology. 2003b;106:243–248. doi: 10.1078/0944-2006-00120. [DOI] [PubMed] [Google Scholar]
- Fritzsch B, Farinas I, Reichardt LF. Lack of neurotrophin-3 causes losses of both classes of spiral ganglion neurons in the cochlea in a region-specific fashion. J Neurosci. 1997;17:6213–6225. doi: 10.1523/JNEUROSCI.17-16-06213.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritzsch B, Signore M, Simeone A. Otx1 null mutant mice show partial segregation of sensory epithelia comparable to lamprey ears. Dev Genes Evol. 2001;211:388–396. doi: 10.1007/s004270100166. [DOI] [PubMed] [Google Scholar]
- Fritzsch B, Silos-Santiago I, Bianchi LM, Farinas I. The role of neurotrophic factors in regulating the development of inner ear innervation. Trends Neurosci. 1997;20:159–164. doi: 10.1016/s0166-2236(96)01007-7. [DOI] [PubMed] [Google Scholar]
- Fritzsch B, Tessarollo L, Coppola V, Reichardt LF. Neurotrophins in the ear: their roles in sensory neuron survival and fiber guidance. Prog Brain Res. 2004;146:265–278. doi: 10.1016/S0079-6123(03)46017-2. [DOI] [PubMed] [Google Scholar]
- Fritzsch B, Silos-Santiago I, Smeyne R, Fagan AM, Barbacid M. Reduction and loss of inner ear innervation in trkB and trkC receptor knockout mice: A whole mount DiI and scanning electron microscopic analysis. Audit Neurosci. 1995;1:401–417. [Google Scholar]
- Fritzsch B, Beisel KW, Jones K, Farinas I, Maklad A, Lee J, Reichardt LF. Development and evolution of inner ear sensory epithelia and their innervation. J Neurobiol. 2002;53:143–156. doi: 10.1002/neu.10098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu C, Rodriguez ER, Reimert DV, Shu T, Fritzsch B, Richards LJ, Kolodkin AL, Ginty DD. Neuropilin-1 conveys semaphorin and VEGF signaling during neural and cardiovascular development. Dev Cell. 2003;5:45–57. doi: 10.1016/s1534-5807(03)00169-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatakeyama J, Bessho Y, Katoh K, Ookawara S, Fujioka M, Guillemot F, Kageyama R. Hes genes regulate size, shape and histogenesis of the nervous system by control of the timing of neural stem cell differentiation. Development. 2004 doi: 10.1242/dev.01436. [DOI] [PubMed] [Google Scholar]
- Hellard D, Brosenitsch T, Fritzsch B, Katz DM. Cranial sensory neuron development in the absence of brain-derived neurotrophic factor (BDNF) in BDNF/Bax double null mice. Dev Biol. 2004;275:34–43. doi: 10.1016/j.ydbio.2004.07.021. [DOI] [PubMed] [Google Scholar]
- Hemond SG, Morest DK. Formation of the cochlea in the chicken embryo: sequence of innervation and localization of basal lamina-associated molecules. Brain Res Dev Brain Res. 1991;61:87–96. doi: 10.1016/0165-3806(91)90117-2. [DOI] [PubMed] [Google Scholar]
- Hertzano R, Montcouquiol M, Rashi-Elkeles S, Elkon R, Yucel R, Frankel WN, Rechavi G, Moroy T, Friedman TB, Kelley MW, Avraham KB. Transcription profiling of inner ears from Pou4f3ddl/ddl identifies Gfi1 as a target of the Pou4f3 deafness gene. Hum Mol Genet. 2004;13:2143–2153. doi: 10.1093/hmg/ddh218. [DOI] [PubMed] [Google Scholar]
- Hood L, Heath JR, Phelps ME, Lin B. Systems biology and new technologies enable predictive and preventative medicine. Science. 2004;306:640–643. doi: 10.1126/science.1104635. [DOI] [PubMed] [Google Scholar]
- Huang EJ, Liu W, Fritzsch B, Bianchi LM, Reichardt LF, Xiang M. Brn3a is a transcriptional regulator of soma size, target field innervation and axon pathfinding of inner ear sensory neurons. Development. 2001;128:2421–2432. doi: 10.1242/dev.128.13.2421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim WY, Fritzsch B, Serls A, Bakel LA, Huang EJ, Reichardt LF, Barth DS, Lee JE. NeuroD-null mice are deaf due to a severe loss of the inner ear sensory neurons during development. Development. 2001;128:417–4126. doi: 10.1242/dev.128.3.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leake PA, Snyder RL, Hradek GT. Postnatal refinement of auditory nerve projections to the cochlear nucleus in cats. J Comp Neurol. 2002;448:6–27. doi: 10.1002/cne.10176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Price SM, Cahill H, Ryugo DK, Shen MM, Xiang M. Hearing loss caused by progressive degeneration of cochlear hair cells in mice deficient for the Barhl1 homeobox gene. Development. 2002;129:3523–3532. doi: 10.1242/dev.129.14.3523. [DOI] [PubMed] [Google Scholar]
- Liebl DJ, Tessarollo L, Palko ME, Parada LF. Absence of sensory neurons before target innervation in brain-derived neurotrophic factor-, neurotrophin3-, and trkC-deficient embryonic mice. J Neurosci. 1997;17:9113–9127. doi: 10.1523/JNEUROSCI.17-23-09113.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M, Pereira FA, Price SD, Chu MJ, Shope C, Himes D, Eatock RA, Brownell WE, Lysakowski A, Tsai MJ. Essential role of BETA2/NeuroD1 in development of the vestibular and auditory systems. Gene Dev. 2000;14:2839–2854. doi: 10.1101/gad.840500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Q, Anderson DJ, Fritzsch B. Neurogenin 1 null mutant ears develop fewer, morphologically normal hair cells in smaller sensory epithelia devoid of innervation. J Assoc Res Otolaryngol. 2000;1:129–143. doi: 10.1007/s101620010017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Q, Chen Z, del Barco Barrantes I, de la Pompa JL, Anderson DJ. Neurogenin1 is essential for the determination of neuronal precursors for proximal cranial sensory ganglia. Neuron. 1998;20:469–482. doi: 10.1016/s0896-6273(00)80988-5. [DOI] [PubMed] [Google Scholar]
- Maklad A, Fritzsch B. Partial segregation of posterior crista and saccular fibers to the nodulus and uvula of the cerebellum in mice, and its development. Brain Res Dev Brain Res. 2003a;140:223–236. doi: 10.1016/s0165-3806(02)00609-0. [DOI] [PubMed] [Google Scholar]
- Maklad A, Fritzsch B. Development of vestibular afferent projections into the hindbrain and their central targets. Brain Res Bull. 2003b;60:497–510. doi: 10.1016/s0361-9230(03)00054-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin T, Hindges R, O’Leary DD. Regulation of axial patterning of the retina and its topographic mapping in the brain. Curr Opin Neurobiol. 2003;13:57–69. doi: 10.1016/s0959-4388(03)00014-x. [DOI] [PubMed] [Google Scholar]
- Miyazaki N, Furuyama T, Takeda N, Inoue T, Kubo T, Inagaki S. Expression of mouse semaphorin H mRNA in the inner ear of mouse fetuses. Neurosci Lett. 1999;261:127–129. doi: 10.1016/s0304-3940(98)00988-4. [DOI] [PubMed] [Google Scholar]
- Mombaerts P, Wang F, Dulac C, Chao SK, Nemes A, Mendelsohn M, Edmondson J, Axel R. Visualizing an olfactory sensory map. Cell. 1996;87:675–686. doi: 10.1016/s0092-8674(00)81387-2. [DOI] [PubMed] [Google Scholar]
- Murakami Y, Suto F, Shimizu M, Shinoda T, Kameyama T, Fujisawa H. Differential expression of plexin-A subfamily members in the mouse nervous system. Dev Dynam. 2001;220:246–258. doi: 10.1002/1097-0177(20010301)220:3<246::AID-DVDY1112>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- O’Leary DD, Wilkinson DG. Eph receptors and ephrins in neural development. Curr Opin Neurobiol. 1999;9:65–73. doi: 10.1016/s0959-4388(99)80008-7. [DOI] [PubMed] [Google Scholar]
- Ohyama T, Groves AK. Generation of Pax2-Cre mice by modification of a Pax2 bacterial artificial chromosome. Genesis. 2004;38:195–199. doi: 10.1002/gene.20017. [DOI] [PubMed] [Google Scholar]
- Pasterkamp RJ, Verhaagen J. Emerging roles for semaphorins in neural regeneration. Brain Res Brain Res Rev. 2001;35:36–54. doi: 10.1016/s0165-0173(00)00050-3. [DOI] [PubMed] [Google Scholar]
- Pauley S, Wright TJ, Pirvola U, Ornitz D, Beisel K, Fritzsch B. Expression and function of FGF10 in mammalian inner ear development. Dev Dynam. 2003;227:203–215. doi: 10.1002/dvdy.10297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirvola U, Ylikoski J, Palgi J, Lehtonen E, Arumae U, Saarma M. Brain-derived neurotrophic factor and neurotrophin-3 mRNAs in the peripheral target fields of developing inner ear ganglia. Proc Natl Acad Sci USA. 1992;89:9915–9919. doi: 10.1073/pnas.89.20.9915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirvola U, Ylikoski J, Trokovic R, Hebert J, McConnell S, Partanen J. FGFR1 is required for the development of the auditory sensory epithelium. Neuron. 2002;35:671. doi: 10.1016/s0896-6273(02)00824-3. [DOI] [PubMed] [Google Scholar]
- Qian Y, Fritzsch B, Shirasawa S, Chen CL, Choi Y, Ma Q. Formation of brainstem (nor)adrenergic centers and first-order relay visceral sensory neurons is dependent on homeodomain protein Rnx/Tlx3. Gene Dev. 2001;15:2533–2545. doi: 10.1101/gad.921501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raft S, Nowotschin S, Liao J, Morrow BE. Suppression of neural fate and control of inner ear morphogenesis by Tbx1. Development. 2004;131:1801–1812. doi: 10.1242/dev.01067. [DOI] [PubMed] [Google Scholar]
- Rubel EW, Fritzsch B. Auditory system development: primary auditory neurons and their targets. Annu Rev Neurosci. 2002;25:51–101. doi: 10.1146/annurev.neuro.25.112701.142849. [DOI] [PubMed] [Google Scholar]
- Silos-Santiago I, Fagan AM, Garber M, Fritzsch B, Barbacid M. Severe sensory deficits but normal CNS development in newborn mice lacking TrkB and TrkC tyrosine protein kinase receptors. Eur J Neurosci. 1997;9:2045–2056. doi: 10.1111/j.1460-9568.1997.tb01372.x. [DOI] [PubMed] [Google Scholar]
- Sobkowicz HM. The development of innervaton in the organ of Corti. In: Romand R, editor. Development of Auditory and Vestibular Systems. Vol. 2. Elsevier; Amsterdam: 1992. pp. 59–100. [Google Scholar]
- Stankovic KM, Corfas G. Real-time quantitative RT-PCR for low-abundance transcripts in the inner ear: analysis of neurotrophic factor expression. Hearing Res. 2003;185:97–108. doi: 10.1016/s0378-5955(03)00298-3. [DOI] [PubMed] [Google Scholar]
- Tessarollo L, Coppola V, Fritzsch B. NT-3 replacement with brain-derived neurotrophic factor redirects vestibular nerve fibers to the cochlea. J Neurosci. 2004;24:2575–2584. doi: 10.1523/JNEUROSCI.5514-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian Y, Li M, Fritzsch B, Zuo J. Creation of a transgenic mouse for hair-cell gene targeting by using a modified bacterial artificial chromosome containing Prestin. DevDynam. 2004;231:199–203. doi: 10.1002/dvdy.20106. [DOI] [PubMed] [Google Scholar]
- Vahava O, Morell R, Lynch ED, Weiss S, Kagan ME, Ahituv N, Morrow JE, Lee MK, Skvorak AB, Morton CC, Blumenfeld A, Frydman M, Friedman TB, King MC, Avraham KB. Mutation in transcription factor POU4F3 associated with inherited progressive hearing loss in humans. Science. 1998;279:1950–1954. doi: 10.1126/science.279.5358.1950. [DOI] [PubMed] [Google Scholar]
- Wallis D, Hamblen M, Zhou Y, Venken KJ, Schumacher A, Grimes HL, Zoghbi HY, Orkin SH, Bellen HJ. The zinc finger transcription factor Gfi1, implicated in lymphomagenesis, is required for inner ear hair cell differentiation and survival. Development. 2003;130:221–232. doi: 10.1242/dev.00190. [DOI] [PubMed] [Google Scholar]
- Wright TJ, Mansour SL. FGF signaling in ear development and innervation. Curr Top Dev Biol. 2003a;57:225–259. doi: 10.1016/s0070-2153(03)57008-9. [DOI] [PubMed] [Google Scholar]
- Wright TJ, Mansour SL. Fgf3 and Fgf10 are required for mouse otic placode induction. Development. 2003b;130:3379–3390. doi: 10.1242/dev.00555. [DOI] [PubMed] [Google Scholar]
- Xiang M, Gao WQ, Hasson T, Shin JJ. Requirement for Brn-3c in maturation and survival, but not in fate determination of inner ear hair cells. Development. 1998;125:3935–3946. doi: 10.1242/dev.125.20.3935. [DOI] [PubMed] [Google Scholar]
- Xiang M, Maklad A, Pirvola U, Fritzsch B. Brn3c null mutant mice show long-term, incomplete retention of some afferent inner ear innervation. BMC Neurosci. 2003;4:2. doi: 10.1186/1471-2202-4-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang M, Gan L, Li D, Chen ZY, Zhou L, O’Malley BW, Jr, Klein W, Nathans J. Essential role of POU-domain factor Brn-3c in auditory and vestibular hair cell development. Proc Natl Acad Sci USA. 1997;94:9445–9450. doi: 10.1073/pnas.94.17.9445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zine A, Aubert A, Qiu J, Therianos S, Guillemot F, Kageyama R, de Ribaupierre F. Hes1 and Hes5 activities are required for the normal development of the hair cells in the mammalian inner ear. J Neurosci. 2001;21:4712–4720. doi: 10.1523/JNEUROSCI.21-13-04712.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou DJ, Feinstein P, Rivers AL, Mathews GA, Kim A, Greer CA, Mombaerts P, Firestein S. Postnatal refinement of peripheral olfactory projections. Science. 2004;304:1976–1979. doi: 10.1126/science.1093468. [DOI] [PubMed] [Google Scholar]