Abstract
Evolution shaped the vertebrate ear into a complicated three-dimensional structure and positioned the sensory epithelia so that they can extract specific aspects of mechanical stimuli to govern vestibular and hearing-related responses of the whole organism. This information is conducted from the ear via specific neuronal connections to distinct areas of the hindbrain for proper processing. During development, the otic placode, a simple sheet of epidermal cells, transforms into a complicated system of ducts and recesses. This placode also generates the mechanoelectrical transducers, the hair cells, and sensory neurons of the vestibular and cochlear (spiral) ganglia of the ear. We argue that ear development can be broken down into dynamic processes that use a number of known and unknown genes to govern the formation of the three-dimensional labyrinth in an interactive fashion. Embedded in this process, but in large part independent of it, is an evolutionary conserved process that induces early the development of the neurosensory component of the ear. We present molecular data suggesting that this later process is, in its basic aspects, related to the mechanosensory cell formation across phyla and is extremely conserved at the molecular level. We suggest that sensory neuron development and maintenance are vertebrate or possibly chordate novelties and present the molecular data to support this notion.
I. Introduction
The evolution of the vertebrate ear has been an enigma ever since man started to think about it. In part, the problems are similar to the evolution of the vertebrate eye and relate to the fact that it is difficult to understand how a complex three-dimensional system that requires coordinated development of morphology (Fekete and Wu, 2002); nonsensory structures such as cupula and tectorial membranes (Goodyear and Richardson, 2002); and sensory structures, including neurons that connect the ear to the brain (Fritzsch et al., 2002), could evolve in a stepwise fashion, with each step being functional and thus providing selective advantage. Although it is tempting to view the processes of morphogenesis and cell fate assignment as distinctly independent phenomena, in actuality they are intertwined and impact each other.
Formation of two- and three-dimensional patterns during morphogenesis may use two types of major mechanisms: morphostatic and morphodynamic (Salazar-Ciudad et al., 2003). In morphostatic mechanisms cell fate assignment (termed herein “induction”) occurs initially, followed by changes in tissue form. In contrast, morphodynamic mechanisms are more complex and simultaneously combine inductive and morphogenetic processes. Generally, morphodynamic mechanisms are likely to appear more often in later development as morphological innovations because they are less likely to disrupt global developmental processes at those stages (Riedl, 1978). Morphostatic mechanisms are usually observed at earlier developmental stages, which would have had more evolutionary time to change from a morphodynamic pattern to a morphostatic mechanism. In later stages of development morphodynamic mechanisms would be more often utilized and would permit already existing complex intermediate phenotypes to produce a wider range of variation and thus respond more easily to selective pressures.
The development of the eye offers an excellent paradigm for developmental mechanisms of sensory organs. In recent years the old notions about multiple parallel evolutions of vertebrate and invertebrate eyes have been reconsidered. In essence, the finding that the transcription factor Pax6 is highly conserved across phyla and is essential for the differentiation of the light-detecting organs in all species studied thus far (Pichaud and Desplan, 2002) has led to a revised perspective of eye evolution. It is now becoming clear that the evolution of developmental transcription factor interactions to generate retina sensors predates the evolution of morphologically distinct eyes and thus represents a highly conserved morphostatic mechanism. The apparent homology at the level of crucial eye transcription factor interactions can now be reconciled with the apparently independent evolution of morphogenetic pathways that generate rather diverse and morphologically distinct eyes in various phyla that are produced using a variety of morphodynamic mechanisms (Pichaud and Desplan, 2002). Basically, the current model of eye evolution suggests two molecularly distinct pathways: one dealing with eye morphogenesis (morphodynamic) and the other with eye histogenesis (morphostatic). Both the morphostatic and morphodynamic processes can be experimentally uncoupled using either teratogens, such as retinoic acid (RA) (Manns and Fritzsch, 1991), or mutations of the Pax6 gene (Pichaud and Desplan, 2002). Thus, eye evolution can provide a useful model for the evolution of ears (Fig. 1).
Figure 1.
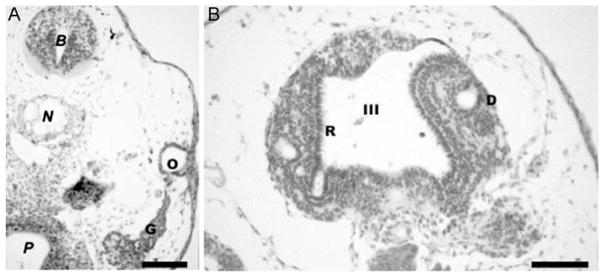
These pictures demonstrate the effect of 5×10−7 M retinoic treatment at stage 13 of Xenopus laevis. Animals were sacrificed at stage 40, embedded in paraffin, and serially sectioned. (A) Note that the ear is reduced to an otocyst vesicle (O) but nevertheless forms numerous ganglion neurons (G) that delaminate from this reduced otocyst. (B) As with the ear, the morphogenesis of the eyes can be completely blocked by RA treatment, leading to the formation of retina receptors (R) facing into the third ventricle (III) of the diencephalons (D). N, notochord; P, Pharynx; B, midbrain. Bar indicates 100 μm.
This chapter builds on this theme and presents the argument that both sensory organs evolved as a combination of two independent processes: an evolutionary conserved morphostatic mechanism involving a set of transcription factors that are essential for neurosensory development and the highly variable morphodynamic processes utilizing sets of transcription factors involved in ear morphogenesis. We base this argument on the evidence suggesting that in both systems these two processes can be experimentally uncoupled (see Chapter 9 by Romand for additional details). The associated factors involved in cell fate assignment in these epithelia and the interrelationship between these two inner ear lineages are also presented. In addition, a brief discussion is presented on the conserved transcription regulatory factors found in the early stages of ear morphogenesis (i.e., the otic placode and the otocyst). This supports the notion of independent evolution of morphogenetic and neurosensory processes. The vertebrate ear has unique structures related to the mechanoelectrical transduction. These structures—the cupula and the otoconia and tectorial membranes, as well as areas of the ear involved in the unique formation of endolymph—are known to be related to genes expressed in the ear (Cowan et al., 2000; El-Amraoui et al., 2001; Goodyear and Richardson, 2002; Hulander et al., 2003; Simmler et al., 2000). These aspects are not dealt with here.
II. Overview of Ideas Related to Ear Evolution
Herein we integrate the concepts of the involvement of morphostatic mechanism(s) in inner ear cell fate assignment and morphodynamic mechanisms prevailing in morphogenesis in the development and evolution of the ear. There is now uniform agreement that the auditory part of the ear evolved from vestibular organs (Fritzsch, 1999; Wever, 1974). For example, it is likely that the auditory organ of the mammalian ear, the cochlea, evolved through the embryonic transformation of parts of the saccule (Fritzsch, 1992; Fritzsch et al., 2002). In contrast to this universally accepted idea of evolutionary transformation of a vestibular organ into an auditory organ, there is no agreement on the evolution of the vestibular part of the ear. Basically, thinking on the evolution of the ear has revolved around two competing ideas.
One idea suggested a transformation of a preexisting lateral-line–like system through invagination into an ear (the octavolateralis hypothesis [Ayers, 1892]). An improved formulation (van Bergeijk, 1966) suggested that the lateral line and the ear develop from a single placode (the acousticolateral placode), share the same sensory receptors (hair cells), and share a common termination in the same central nuclei. Others have proposed that placodes undergo a stepwise refinement from a general placode system to a more specialized lateral line and ear placode system (Noramly and Grainger, 2002; Streit, 2002), but it is still unclear whether a common developmental program exists for the various sensory placodes (Begbie and Graham, 2001b; Groves and Bronner-Fraser, 2000). Still other work has shown that in many slowly developing vertebrates there are clear spatial and temporal differences in the development of lateral line and inner ear placodes, with the inner ear placode developing typically much earlier (Fritzsch et al., 1998a). Moreover, the uncoupling of lateral line from ear development during evolution has resulted in the complete loss of lateral line placodes in terrestrial vertebrates (Fritzsch, 1999; Schlosser, 2002).
A second idea about ear evolution was based on the apparent morphological conservation of hearing organs across phyla, which implied similarities in function (hearing) that are retained in various forms of statocysts (Wever, 1974). Essentially it was assumed that the formation of an “otocyst” was conserved across phyla and its formation predated the evolution of vertebrates. This concept was revived and further refined by comparison between the atrial chambers of tunicate larvae and the vertebrate ear. Other than the general topology, the tunicate atrial chambers bear sensory organs that function as hydrodynamic sensors (Bone and Ryan, 1978; Mackie and Singla, 2003) and express the homolog of the vertebrate Pax2 gene (Favor et al., 1996, Torres and Giraldez, 1998), the Hr-Pax258 gene (Wada et al., 1998). However, the homologous AmphiPax2/5/8 differs in its expression in the nervous system from mammalian Pax2 (Kozmik et al., 1999), and the presumed homology of the atrial chambers of tunicates with vertebrate ears remains in question.
Basically, these ideas revolve around the issue of whether sensory evolution came first followed by morphological evolution (octavolateralis hypothesis) or were both intertwined from the start (statocyst hypothesis). Our overriding concept is that evolution of the neurosensory system predates the evolution of the morphogenetic system that generates the ear and that evolution progresses from simple, via morphostatic processes, to complex as predicated by a variety of morphodynamic mechanisms. An evolutionarily conserved morphostatic mechanism has been proposed for the ear neurosensory components. This concept builds on the novel finding of a molecularly conserved gene that is essential for sensory cell formation across phyla, mammalian atonal homologue 1 (Aton) (Bermingham et al., 1999; Fritzsch et al., 2000; Wang et al., 2002). The basis of this idea is that evolution of mechanosensory transducers is conserved molecularly to establish a transcription factor link to the still unknown mechanosensory transducer channels (Fritzsch and Biesel, 2001). Comparable to the octavolateralis hypothesis, this hypothesis assumes that the evolution of a mechanosensory transducer predates the evolution of a morphologically distinct vertebrate ear but does not require the prior evolution of a lateral line system. It thus circumvents some of the problems associated with the octavolateralis hypothesis while maintaining the basic idea that the evolution of mechanosensory transducers and their developmental molecular basis predates ear morphogenesis (Fritzsch et al., 1998a; Fritzsch et al., 2000). In line with this idea that ear neurosenory cell lineage formation predates morphogenesis are findings in the established outgroup of craniate vertebrates, the lancet Amphioxus. This animal lacks an ear and a lateral line but expresses genes involved in chemosensory and mechanosensory cell formation (Holland et al., 2000; Kozmik et al., 1999; Shimeld and Holland, 2000).
III. Making the Ear: Implementing and Expanding Genes for Ear Morphogenesis
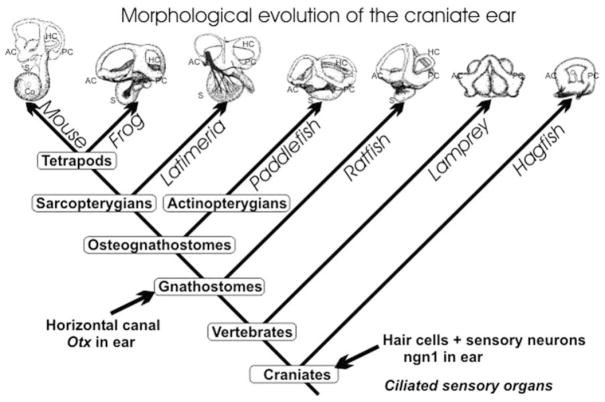
Evolution of the ear is to a large extent evolution of ear morphogenesis (Fig. 2). Logically, one would need to show expression of genes clearly involved in ear morphogenesis and their evolutionary changes to unravel the morphostatic or morphodynamic interactions of genes (Salazar-Ciudad et al., 2003). A number of genes essential for ear formation have been identified, as determined by the ear phenotype (lacking parts of the ear) in null mutant mice (see Chapter 2 by Quint and Steel for further discussion). We concentrate here on the morphostatic mechanisms in ear formation by discussing four genes, forkhead, Gata3, eye absent (Eya), and fibroblast growth factor (Fgf), and their implication for the evolution of a molecular network that governs ear morphogenesis. For more detail on other genes involved in ear morphogenesis and cell fate assignments see the review by Fekete and Wu (2002).
Figure 2.
The morphogenetic evolution of the vertebrate ear is shown. How ciliated mechanosensory cells became associated with this morphogenetic process remains unclear. However, the living vertebrates display a morphocline of increasing complexity in the vestibular system that peaks with the nine distinct sensory receptors in some amphibians. Tetrapods, and possibly the coelacanth Latimeria, have evolved a basilar papilla in addition to the vestibular receptors. The major morphogenetic progress of the vestibular system was the split of the single torus of hagfish into two semicircular canals in lampreys and the formation of a horizontal canal in all gnathostomes ( jawed vertebrates). It is likely that expression of Otx1 was essential for the evolution of the horizontal canal. AC, anterior crista; Co, cochlea; HC, horizontal canal and crista; PC, posterior crista; S, sacculus.
A. Forkhead (Winged Helix) Genes
Overall, forkhead genes are involved in numerous processes, such as patterning, morphogenesis, cell specification, and proliferation (Solomon et al., 2003). In zebrafish, the forkhead/winged helix gene, Foxil, appears to be the first currently known gene expressed in the otic placode and appears to be essential for otocyst formation (Solomon et al., 2003). A forkhead gene expressed in the mammalian ear is BF1, now designated as Foxg1 (Hatini et al., 1999), which is also found in the developing zebrafish ear (Toresson et al., 1998). As indicated by its expression in sensory neurons and hair cells of the ear, the Foxg1 gene appears to play a role in histogenesis (Hatini et al., 1999). Because it is expressed at a later point in the neurosensory development, Foxg1 may also be part of the morphodynamic mechanism. This suggestion is in line with the lack of Foxg1 expression in the epidermis in Amphioxus (Toresson et al., 1998), as well as the lack of any other forkhead genes in the ectoderm (Yu et al., 2002). Certainly Foxil may have an initial role in placodal induction and may be a component of the morphostatic cascade (see Chapter 4 by Brown et al. and Chapter 12 by Riley for further discussion). Additional data is needed to determine if forkhead genes were co-opted into ear formation as part of the morphostatic mechanism and if they also play a role in the morphodynamic cascade associated with later stages of neurosensory cell development.
Null mutant analyses have shown that the putatively homologous Foxil gene in mammals is not essential for ear formation. However, its absence causes ear dysmorphogenesis (Hulander et al., 1998). More recent data suggest that the neonatal dysmorphogenesis is largely due to the lack of pendrin expression in later development (Hulander et al., 2003), which causes endolymphatic hydrops and consequently dysmorphogenesis of the ear. The lack of embryonic dysmorphogenesis suggests either that another forkhead gene is present in the otocyst and provides biological redundancy or that Foxil is not part of the evolutionarily conserved morphostatic mechanism. Probably several more of the over 100 forkhead genes will eventually be found in the developing ear. Unlike the Foxil nulls, preliminary data on Foxg1 null mutant mice suggest severe phenotypic effects on specific sensory neurons and hair cells. In addition, the horizontal crista and its hair cells are absent (Fritzsch, unpublished data). Collectively, the apparent differences spatiotemporal expression and null mutation phenotypes of these two Fox genes suggest some redundancy of forkhead signaling in ear formation and also indicate that forkhead genes have been co-opted into the morphodynamic mechanisms.
B. GATA3
Like the forkhead genes, Gata3 is expressed at the level of the otic placode (George et al., 1994; Lawoko-Kerali et al., 2002) and has been found in the ear of zebrafish and mammals. Its orthologue, pannier, plays a role in sensory organ formation in insects (Sato and Saigo, 2000). Given the early expression of Gata3, it is not at all surprising to see influences on transcription factors regulating morphogenesis and histogenesis of the ear (Lawoko-Kerali et al., 2002). Targeted disruption of GATA3 leads to an arrest of ear development at the otocyst stage (Karis et al., 2001). GATA3 is the only early marker that identifies delaminating spiral sensory neurons (Karis et al., 2001; Lawoko-Kerali et al., 2002), but its role in spiral ganglion cell fate assignment is unclear. Based on pathfinding errors in inner ear efferents associated with the null phenotype (Karis et al., 2001), it is possible that GATA3 is involved in pathfinding and may have a minor role in morphodynamic processes observed later in development.
How GATA3 interacts with FGFs (Pauley et al., 2003; Pirvola et al., 2000), Nkx (Hadrys et al., 1998), Dlx (Merlo et al., 2002; Solomon and Fritz, 2002), forkheads (Solomon et al., 2003), and other genes involved in early ear histogenesis and morphogenesis (Chang et al., 2002; Fekete and Wu, 2002; Xu et al., 1999) is still uncertain (see Chapter 5 by Bober et al. and Chapter 8 by Wright and Mansour for additional details). However, multiple binding sites for GATA factors in the promoter regions of forkhead genes have been described (David et al., 1999) and are suggestive that GATA3 is part of a morphostatic cascade in the ear.
C. The EYA/SIX/DACH Complex
At early stages in the formation of the embryonic ear, involvement of four genes is observed in the otic vesicle and their presence is conserved across phyla (Noramly and Grainger, 2002). The interaction of these genes has been best documented in Drosophila eye development, where they form an evolutionarily conserved gene network consisting of paired-box (Pax)–eye absent (Eya)–sine oculis (SIX)–dachshund (DACH) genes (Hanson, 2001). This gene network is also observed in zebrafish, chicken, and mouse. A fundamental role may be associated with the process of invagination. For example, this network is observed in involving hypoblast cells at gastrulation and in the forming otic and optic vesicles. It has been suggested that these genes permit cells to migrate without altering their cell fate commitment (Streit, 2002). By altering isoform usage and variation in coexpression patterns, this gene network can be co-opted into a wide variety of different morphogenetic contexts. Thus, these regulatory proteins are usually coexpressed throughout embryogenesis in a wide variety of cell types, tissues, and organs. Their expression in a given tissue does not necessarily imply its homology with other expressing tissues.
In order to understand their individual roles and predict their impact on downstream expression patterns, their protein interactions and functions must be understood. EYA, SIX, and DACH proteins directly interact to form a functional transcription factor. The DNA binding site is contained within SIX proteins, whereas EYA mediates transcriptional transactivation and contains SIX and DACH binding domains. Additional regulatory complexity is provided by DACH, which appears to function as a cofactor by directly interacting with EYA. A conserved expression pattern is found in the otic vesicle where similar isoforms representing these four gene families are found. In the mouse these genes are Pax2, Eya1 and 4, Six1 and 4, and both Dach isoforms (Davis et al., 1999, 2001; Noramly and Grainger, 2002). For ear formation Eya1 and Six1 appear to be individually critical since null mutations in either Eya1 or Six1 do not affect the formation of the otic placode and vesicle, but there is no further morphogenesis (Xu et al., 1997, 1999; Zheng et al., 2003). Biological redundancy is suggested by the formation of the ear in spontaneous and null mutations of the Pax2 gene (Torres and Giraldez, 1998; Xu et al., 1999) and by the absence of any phenotypic changes associated with the loss of the Dach1 gene function (Davis et al., 2001). These data show that this gene network is crucial for ear morphogenesis. Interestingly, the haploid insufficiency observed in human and mouse Eya1 mutations suggests that alterations in gene dosage, protein levels, or function can affect morphogenesis (Abdelhak et al., 1997a,b; Johnson et al., 1999; Vincent et al., 1997).
Similar to other genes implementing ear morphogenesis, these regulatory elements also play a role in histogenesis as indicated by their expression patterns in the developing ear. One example is the Eya4 gene, which is initially expressed in the otic vesicle (Borsani et al., 1999; Wayne et al., 2001). This isoform is present primarily in the upper epithelium of the cochlear duct in a region that develops Reissner’s membrane and the stria vascularis. At E18.5 Eya4 is in areas of the cochlear duct destined to become spiral limbus, organ of Corti, and spiral prominence, with the highest level of expression occurring in the basal turn and in the early external auditory meatus. Diminishing levels of expression are found at later stages in these tissues and in the developing cochlear capsule during the period of ossification from birth to P14. In the vestibular system Eya4 is observed in the developing sensory epithelia. Interestingly, mutating Eya4 results in late onset deafness associated with the DFNA10 locus (Pfister et al., 2002; Wayne et al., 2001).
D. Fibroblast Growth Factors
FGFs are well known for their role in branching morphogenesis. However, they play a pivotal, but not necessarily conserved, role in ear formation and are likely important factors in the morphodynamic mechanisms of ear development (see Chapter 4 by Brown et al. and Chapter 8 by Wright and Mansour for further discussion). A significant morphogenetic variability can be contributed by the large number of isoforms in the Fgf gene family and their four different receptors. In chicken, FGF3 and FGF19 have been suggested for placode induction (Ladher et al., 2000; Vendrell et al., 2000). Other data also suggest a role for FGF2 and FGF8 in chicken ear morphogenesis (Adamska et al., 2001). Zebrafish appears to have an interaction between Fgf3 and Fgf8 that is crucial for ear morphogenesis (Liu et al., 2003; Phillips et al., 2001). In mammals, early effects of Fgf8 have not been studied owing to early embryonic lethality, but Fgf8 is later expressed in the ear (Pirvola et al., 2000, 2002). Fgf19 is not known to play any role in mammalian ear formation and an ear forms in Fgf3 null mutants (Mansour, 1994). Biological redundancy is associated with the FGF3, which signals in parallel with FGF10, FGF7, and FGF22 (Satou et al., 2002) through the Fgfr2b receptor. In FGFR2b null mutants there is a loss of almost all ear morphogenesis (Pirvola et al., 2000). Consistent with the signaling redundancy of FGFs through FGFRs, there appears to be a less severe effect of Fgf10 null mutation on ear morphogenesis (Pauley et al., 2003). Most important is the effect on the vestibular canal growth that is consistent with the FGF’s role in branching morphogenesis (Pauley et al., 2003; Pirvola et al., 2000). Other FGFs and FGF receptors appear to play a role in cellular differentiation of the mouse ear (Colvin et al., 1996; Pirvola et al., 2002). Modeling their molecular interactions (Davidson et al., 2002) will require a much deeper knowledge of the genes, their qualitative and quantitative expression patterns, and their function in ear development.
In summary, all these genes play a role in both morphogenesis and histogenesis of the inner ear, as well as a survival role in the mature system. Their role in the morphostatic and/or morphodynamic mechanisms of ear development must be understood in terms of their spatiotemporal expression patterns before their complete function is ascertained. Dissection of their role in ear development must be approached by using conditional mutant mouse lines to understand the contextual role these regulatory genes are playing. In addition, it is becoming increasingly obvious that ear formation can be negatively affected by numerous genes, and working out their interaction will be paramount for any further understanding of ear development, evolution, and the underlying morphogenetic and histogenetic mechanisms. If taken at face value, the data also suggest limited conservation and possible changes in the role played by apparently orthologous genes across taxa. This raises the specter that the overall network of gene interactions might be conserved but that the interchangeable use of other members (isoforms) of the same gene family can differ among the vertebrate taxa. If true, this would mean that substitution of one isoform for another will alter the context of all other genes expressed in the ear. Thus, this could cause rapid molecular and morphological evolution of certain aspects of ear morphogenesis while keeping the overall formation and histogenesis of the ear largely unchanged, precisely what has been described in ear evolution (see Fig. 2). The task at hand will be to correlate such gene substitutions with major morphological alterations such as the formation of the mammalian cochlea and gnathostome horizontal canal.
IV. Evolution of the Ear: Molecular Origin of Mechanosensory Cells Predates Formation of the Ear
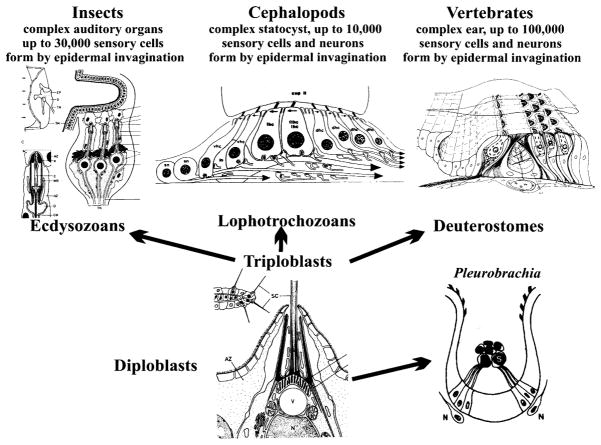
Hair cells are among the few unique vertebrate features not apparently shared with invertebrates (Jorgensen, 1989). Among the distinguishing features of vertebrate hair cells are the asymmetrical apical specializations, which lead to changes in the resting potential proportional to shearing forces acting on a mechanosensory ion channel (Strassmaier and Gillespie, 2002). In addition, up to 500,000 hair cells Corwin, 1981) assemble into a large ( sensory epithelium in a complicated three-dimensional structure, the labyrinth (Lewis et al., 1985). In contrast to most invertebrate sensory systems, vertebrate hair cells are connected to the central nervous system (CNS) via separate neurons grouped into distinct ganglia (Fritzsch, 1988b). Among invertebrates, such sensory cells without axons exist also in some cephalopods (Budelmann, 1992). Among cephalochordates, sensory cells without axons have been described for the lancelet, a likely vertebrate ancestor (Conway Morris, 2000) and ascidians (Burighel et al., 2003). Sensory cells without axons and the generation of a separate set of neurons that connect those sensory cells to the brain might therefore be ancestral features of vertebrates and possibly chordates (Fig. 3) and are shared features of mechanosensory hair cells and taste receptors (Fritzsch et al., 1998a).
Figure 3.
The evolution of sensory cells from simple single cells in the epidermis of diploblastic animals (bottom center) to complex multicellular organs in all three major radiations of triploblasts and in certain diploblasts is shown. Note that among ecdysozoans, insects have complex auditory receptors that consist of sensory cells with axons projecting to the central nervous system. Among lophotrochozoans, cephalopods have equally large statocysts with sensory cells with an axon, as well as sensory cells innervated by specialized sensory neurons. Vertebrates also have complex sensory organs that consist exclusively of sensory hair cells that are innervated by sensory neurons that derive from the same otocyst. bHLH genes that regulate sensory cell development in insects (atonal) and hair cell development in vertebrates (Aton1) can be experimentally interchanged. It remains to be seen, however, whether diploblasts have a conserved atonal-like bHLH gene, which may be the basis of the cell fate commitment toward a mechanosensory cell.
A variety of mechanosensory cell types with axons are present in invertebrates, with the touch receptor of Caenorhabditis and the mechanosensory bristle of Drosophila (Caldwell and Eberl, 2002) representing possible prototypical mechanoelectric transducers comparable to those present in the hypothetical ancestor of all bilaterally symmetrical organisms. Moreover, the numbers of mechanosensory cells in some invertebrates (see Fig. 3) can approach or exceed the numbers reported for inner ear hair cells of vertebrates (Fritzsch and Biesel, 2001). Thus, with respect to neither the overall cellular specialization nor the number of individual cells in a given sensory organ are vertebrates unique among triploblastic organisms.
In the past the reconstruction of hair cell evolution has relied almost exclusively on comparison of adult and developmental features of extant invertebrates and chordates with vertebrates. Using the molecularly characterized developmental mechanisms outlined, we want to establish a plausible scenario for the continuity of interacting genetic networks, as well as for their modification, to achieve the unique vertebrate outcome. To obtain this, we need to compare early development of hair cells in vertebrates with invertebrate sensory cell development and analyze whether homologous genes are used to specify the different sets of cells needed to form a mechanosensory transducer organ in divergent phyla.
V. Developmental Molecular Biology of Hair Cell and Sensory Neuron Formation
Before we can discuss the evolutionary link between hair cell and sensory neuron development in the ear, we need to have a detailed understanding of the molecular basis for the formation of these neurosensory components in the vertebrate ear. Recent years have revealed the molecular basis for their formation using mainly the mouse as a model system. These data suggest the role of the following molecules and their interactions in the formation of these cells.
All neurons in vertebrates are derived from ectodermal cells that are transformed by a cascade of genes into neuronal precursor cells. Several genes have been identified that appear crucial for this switch in fate. These genes are referred to as “proneural genes” because of their apparent capacity to transform ectodermal cells into neurons (Lee, 1997). They all belong to the growing family of basic helix-loop-helix (bHLH) genes that encode an ancient protein family with a highly conserved DNA binding domain (Bertrand et al., 2002). Proneural bHLH proteins form heterodimers with the ubiquitous E-proteins (i.e., the insect daughterless proteins) that enable them to bind to DNA and exert their function. These proteins have not only the unique capacity to turn ectodermal cells into neurons in gain-of-function experiments (Lee, 1997; Ma et al., 1996) but can also determine cell fate in rather unrelated tissues such as pancreas (Liu et al., 2000) or gut (Yang et al., 2001). They can also generate neuron-like cells such as Merkel cells (Bermingham et al., 2001).
Given this unique capacity of the proneural genes, it is obvious that these genes are tightly regulated in their spatiotemporal expression through interaction with a number of other transcript regulatory factors. Some of these factors interfere with the heterodimerization of bHLH proteins by binding to the E-proteins. These genes encode proteins that inhibit differentiation and are thus referred to as “inhibitors of differentiation” or Id genes. Others, such as the vertebrate hairy and enhancer of split paralogs, Hes/Her/Esr, act as classical DNA binding repressors of proneural gene transcription. The activation of the latter family appears to be regulated by the ubiquitously present delta–notch system, which negatively regulates the proneural commitment among neighboring cells. However, this so-called lateral inhibition requires the upregulation of bHLH genes in a limited number of cells to prompt the start of the delta–notch system. Most of the factors that drive this initial upregulation of proneural bHLH genes are still unknown, but the Zic genes are good candidates.
In general, the bHLH genes can be divided into three functional groups: true proneural bHLH genes that generate a neural lineage, bHLH genes that drive neural differentiation, and bHLH genes that drive the switch from neural to glial cell lineage (Bertrand et al., 2002, Zhou and Anderson, 2002).
Loss-of-function (targeted null mutations of the respective genes) experiments have clarified some of the proneural genes crucial for inner ear primary sensory neuron development (Fig. 4). The work of Ma and colleagues (Ma et al., 1998) showed that inner ear primary sensory neuron formation requires the vertebrate bHLH gene neurogenin 1 (Ngn1). Indeed, a follow-up study showed that no primary sensory neurons ever form in these mutants (Ma et al., 2000). Nevertheless, such ears develop fairly normally in their overall histology, suggesting that ear formation and even development of many hair cells is largely autonomous of innervation. Although those hair cells that do form are morphologically normal (except for some minor disorientation), hair cell numbers are reduced to various degrees in Ngn1 null mutant mice. Most interestingly, the cochlea is shortened and the saccule is almost completely lost. In addition, extra rows of hair cells form in the shortened cochlea (Fig. 5). These data suggest a significant interaction between progenitor cells that form primary neurons and progenitor cells that give rise to hair cells, supporting cells, and other inner ear epithelial cells. The simplest explanation would be a clonal relationship between primary sensory clones and hair cells and supporting cells (Fekete and Wu, 2002; Fritzsch et al., 2001). However, other possible interactions cannot be excluded (Fritzsch et al., 2002).
Figure 4.
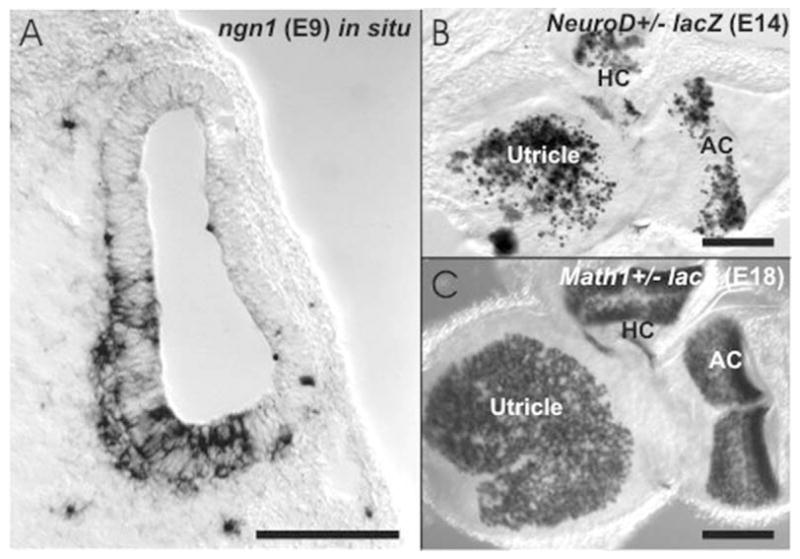
The three known bHLH genes that are essential for neurosensory development in the ear are shown. (A) The cross-sectioned otocyst of a E9 day-old mouse embryo shows the in situ expression of Ngn1 in the ventromedial aspect of the otocyst as well as in delaminating cells. (B) NeuroD is revealed with a LacZ reporter and shows a patchy distribution in many hair cells of the vestibular epithelia. (C) In contrast, Math1 is found in every hair cell of the vestibular and cochlear sensory epithelium at this developmental stage. Bar indicates 100 μm.
Figure 5.
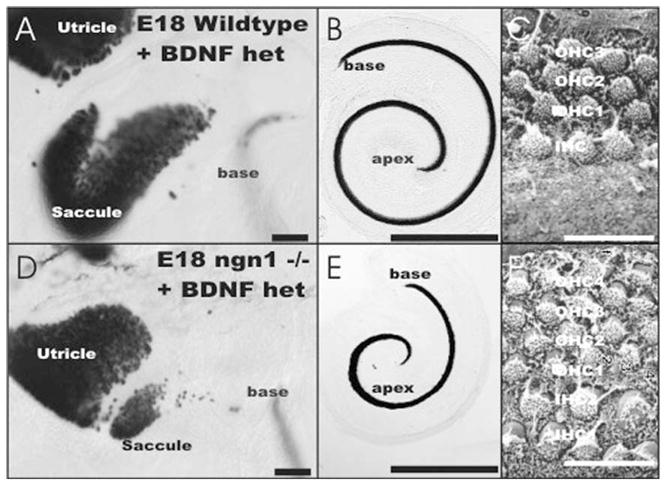
The effects of a null mutation of Ngn1 on the development of hair cells is shown. Using a BDNF LacZ reporter to label hair cells (A, B, D, E) obvious differences appear. The saccule (A, D) is almost completely lost in Ngn1 null mutant mice. The cochlea (B, E) is shortened and shows only a single turn. In addition to being shorter, the upper middle turn is wider and shows up to six rather than the usual four rows of hair cells (C, F). Bar indicates 100 μm (A, D), 1 mm (B, E), and 10 μm (C, F).
Other data have shown that hair cells require the expression of the bHLH gene Math1(mouse Aton1) for their formation (Bermingham et al., 1999). However, Math1, the bHLH gene responsible for hair cell differentiation, does not function as a true proneural gene to establish a neural lineage in the mammalian brain (Bertrand et al., 2002). In contrast to how it functions in most vertebrates, in mammals Aton functions only to select progenitor cells from a pool of already specified neuroepithelial stem cells (Bertrand et al., 2002). In this context data suggest that the gene that establishes the hair cell lineage is still unknown (Chen et al., 2002). Confirming and extending this conclusion are data on organ of Corti tissue culture showing that Math1 is not required for the initial expression of MyoVIIa, MyoVI, α9AchR, fimbrin, Brn3c, and other hair cell markers (Rivolta et al., 2002). This conclusion is also supported by the precocious expression of the neurotrophin BDNF in the cochlea, prior to the upregulation of Math1, in what appears to be hair cell progenitors (Chen et al., 2002; Farinas et al., 2001). Preliminary data of our group suggest that BDNF is even expressed in hair cell progenitors in Math1 null mutants. Interestingly, some hair cells apparently require Math1 to drive BDNF expression, whereas other hair cells express BDNF even in the undifferentiated hair cell precursors that form in Math1 null mutants. However, expression of BDNF is in some but not all undifferentiated precursors of Math1 null mutants. This suggests that other genes also regulate BDNF expression in hair cell precursors and supports the conclusion of the Math1–LacZ analysis that hair cell precursor formation does not require Math1.
Either Ngn1 and/or an as yet unspecified proneural gene could play the role of inducing neuroepithelial commitment in the parts of the otocyst that give rise to Math1-mediated hair cell differentiation. As a consequence of the absence of primary sensory neuron formation in Ngn1 null mutants, the ear develops completely isolated from direct brainstem connections because afferents do not form and neither efferents nor autonomic fibers appear to reach the ear in these animals (Ma et al., 2000).
Absence of proneural genes in insects leads to the complete collapse of sensory organ formation (Caldwell and Eberl, 2002). We therefore analyzed double null mutants for both Ngn1 and Math1. If these were the only proneural bHLH genes that were independently expressed in the ear, one would expect severe consequences for ear morphology. However, our preliminary results on a single double-null mutant that also expresses BDNF–LacZ show that absence of both Ngn1 and Math1 is compatible with ear formation (Fig. 6). Either a third as yet undescribed bHLH gene is present in the mammalian ear or the morphogenesis of the ear is fully independent of the neurogenesis of sensory neurons and the formation of hair cells. The fact that RA treatment can block entirely the morphogenesis but still allow neurogenesis of the ear placode to proceed (Fritzsch et al., 1998a) would be compatible with this latter suggestion. Further studies on this subject are clearly warranted.
Figure 6.
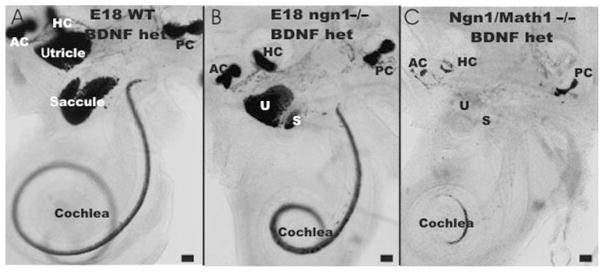
The effect of loss of both Ngn1 and Math1 on ear development and development of hair cell precursors is shown in three littermates. Note the absence of all sensory neurons and the reduction in size of the saccule and the cochlea in Ngn1 null mice (A, B). Combining Ngn1 null with Math1 null (C) is a simple combination of the Ngn1 phenotype (reduction in cochlea and saccule) with the Math1 null phenotype (absence of BDNF–LacZ-positive cells in the utricle, saccule, and basal turn of the cochlea). In contrast, the apex and the three canal cristae show BDNF–LacZ-positive cells even in the double-null mutants. Bar indicates 100 μm.
The next step in primary sensory neuron differentiation is apparently mediated by the bHLH genes of the NeuroD family (Kim et al., 2001; Lee, 1997). As is the case in other true proneural genes, Ngn1 is only transiently upregulated in primary neuron precursors. As primary sensory neuron precursors delaminate from the otocyst wall, the primary sensory neuroblasts downregulate Ngn1 and upregulate NeuroD (Kim et al., 2001; Liu et al., 2000; Ma et al., 2000). Based on data in the CNS, the presence of either Ngn1 or NeuroD (alternate symbol for Neurod1) seems to be involved in the continued proliferation of neuroblasts through interference with the cell cycle (Bertrand et al., 2002). Indeed, factors involved in regulating proliferation are only now becoming apparent in other developing systems such as muscle and olfactory epithelium (Wu et al., 2003). How many of these regulatory elements are utilized in the ear remains speculative at the moment.
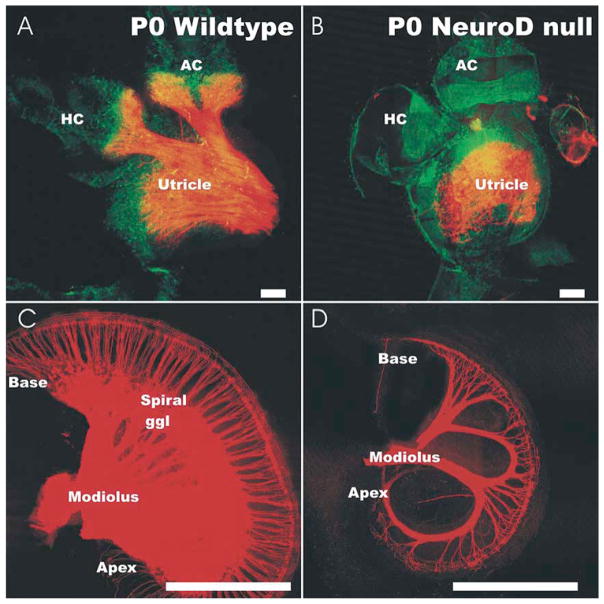
It appears that NeuroD affects delamination of neuroblasts from the otocyst (Liu et al., 2000) and causes aberrant migration of surviving vestibular neurons in NeuroD null mutants (Kim et al., 2001). In NeuroD null mice the surviving neurons form disorganized projections to the cochlea and the utricle (Fig. 7). These resemble the vestibular projection defects known from mutation in the Pou domain factor, Pou4f1 (also designated as Brn3a) (Huang et al., 2001). It remains to be seen whether all the migration and projection defects in the NeuroD mutant can be attributed to the fact that NeuroD is upstream of Brn3a.
Figure 7.
The effect of a NeuroD null mutation on the pattern of innervation of the cochlea and vestibular system is revealed with Dil tracing and confocal microscopy. All vestibular and cochlear epithelia show hair cells, but some hair cells have no innervation. In the vestibular system, the utricle shows a somewhat disorganized innervation in the NeuroD mutant (B) but may lack all innervation of canal cristae (A, B). The cochlea shows a reduction in size and a loss of most spiral sensory neurons (C, D). The remaining neurons expand their peripheral processes to innervate most inner hair cells and a few outer hair cells. Bar indicates 1 mm (C, D) and 100 μm (A, B).
NeuroD null mutation differential affects the cochlear primary neurons (almost completely lost) more than the vestibular primary neurons (many survive, but they are dislocated and project aberrantly). This may relate to the fact that the acquisition of specific neuronal fates requires interaction with homeobox genes such as Pax genes (Lawoko-Kerali et al., 2002). In contrast to the vestibular primary sensory neurons, cochlea (spiral) primary sensory neurons express Gata3 (Karis et al., 2001; Lawoko-Kerali et al., 2002). GATA factors, including pannier and presumably its vertebrate homologue GATA3 (Bertrand et al., 2002; Karis et al., 2001), can interact with bHLH dimers for transcriptional regulation and may be directly involved in specific aspects of cochlear and vestibular fate determination via regulation of bHLH gene transcription. Unfortunately, most Gata3 null mutants result in embryonic lethality before hair cell differentiation is achieved. Nevertheless, it is important to note that a human GATA3 mutation exists and causes deafness (Van Esch et al., 2000).
In summary, the genes that regulate formation of the neurosensory aspects of ear development are beginning to emerge and their function has been tested in specific null mutant mice. Although the beginning of this line of research has provided dramatic breakthroughs in our understanding of this process, numerous open questions remain before this research can be extended to guide aspects of neurosensory regeneration that would benefit humans with neurosensory hearing loss.
A. Invertebrate Sensory Cell Developmental Factors
Drosophila sensory organ development requires a set of genes that have been shown to be conserved in vertebrates. The early specification of position is influenced by a number of genes such as zinc finger and TGFβ-related genes (Caldwell and Eberl, 2002). Homologues of these genes exist in vertebrates and their role in ear development is considerable (Brigande et al., 2000; Cantos et al., 2000; Fekete and Wu, 2002; Karis et al., 2001). These genes may regulate expression of various bHLH genes such as achaete/scute in external sensilla and atonal in chordotonal organs. In addition, in vertebrates Bmp4 and its antagonists play a role in patterning of prepatterning gene expression (Bally-Cuif and Hammerschmidt, 2003). Such genes are crucial for overall development of sensory elements in both vertebrates and invertebrates by setting up proneuronal clusters and by controlling their cell cycle.
Further development of these proneuronal clusters requires a set of intrinsic signaling genes that apparently act in a stereotyped fashion, are highly conserved across evolution, but have increased in the number of genes between unicellular and multicellular organisms (Venter et al., 2001). One cell, the sensory organ precursor cell, strongly expresses a bHLH gene. This bHLH gene expression upregulates Delta gene expression. DELTA downregulates prosensory bHLH gene expression in adjacent cells through its receptor, NOTCH. In fact, Delta/Notch expression can cause irreversible loss of neurogenic capacity (Morrison et al., 2000), thus being able to fine-tune the pattern of proneuronal clusters. This process leads to the selection of clusters or single proneuronal cells in a rich variety of patterns (Chan and Jan, 1999). Acting in parallel, other factors, such as NUMB and PROSPERO, help to reinforce cellular commitment (Sen et al., 2003).
Most interesting is the high degree of conservation of the cellular function of the atonal/Math1 genes across phyla. Whereas minor differences such as the loss of proneural function in the vertebrate Math1 gene are intriguing, the common function in mechanosensory transducing cells appears to be conserved. Indeed, the conservation goes so far that the Math1 transgenes can rescue the atonal mutant phenotype in the fly (Ben-Arie et al., 2000). In contrast to many other transcription factors, atonal transgenes in Math1 null mutant mice rescue hair cell differentiation (Wang et al., 2002), thus suggesting an extraordinary conservation of function, if embedded in the right context. However, Math1 is a powerful differentiation factor but not a true proneural gene in mammals. It remains to be shown whether other bHLH genes can rescue hair cell differentiation if expressed under Math1 promoter control. Such partial rescue of function has been demonstrated in Mash1/Ngn2 transgenic mice (Cau et al., 2002) and need to be tested for Math1.
Beyond the similarity in developmental regulatory genes, other unique hair cell–specific markers such as the unconventional myosin VIIa are also present in the fly scolopidial attachment (Caldwell et al., 2002). It has been suggested that this high degree of conservation of transcriptional factors across phyla relates to the evolution of the mechanoelectrical transducer system (Fritzsch et al., 2000) that might be common in great detail across phyla (Walker et al., 2000). However, the simple fact that this mechanoelectrical transducer channel has not yet been identified leaves this argument open to future scrutiny.
B. Vertebrate Hair Cell Development
Relying in part on the distribution of Delta and Notch, several investigators (Adam et al., 1998; Eddison et al., 2000) have proposed homology between cells in the insect sensilla and those of the vertebrate ear sensory epithelium. However, this idea does not take the full complement of gene complexity of vertebrates and insects into account (Fritzsch et al., 2000). Briefly, it appears that the mouse atonal homologue 1 gene Math1, a proneuronal bHLH gene, is necessary for hair cell formation (Bermingham et al., 1999). Yet a mammalian atonal paralogue gene, Ngn1, is essential for sensory neuron development (Ma et al., 1998, 2000). Neurogenin-like genes have been identified in insects and the lancelet (Holland et al., 2000). These data could simply indicate that gene multiplication has already in urbilaterians generated various bHLH genes (Dehal et al., 2002). Those genes are now assigned to different functions in the various existing metazoan lines (see Fig. 3). Also, continued further increase in bHLH genes exists in various metazoans, thus complicating an evolutionary analysis.
Unfortunately, current analysis of the bHLH genes and their regulators does not indicate expression outside the CNS in the lancelet, except in presumed chemosensory cells (Holland et al., 2000). Interestingly, Ngn1 is also essential for olfactory sensory neuron formation (Wu et al., 2003). Assuming conservation of bHLH function, this suggests that the many sensory organs in the skin of the lancelet may be directed in their development by an as yet undetected proneuronal bHLH gene(s). Finding this gene(s) is essential to establish molecular continuity between chordate and vertebrates in sensory cell development. Alternatively, in light of atonal involvement in internal proprioreceptors (Wang et al., 2002), it is possible that the ear is related to an as yet unidentified proprioreceptor in the common bilaterian ancestor, lost in the lancelet.
The sequencing of the second potential vertebrate outgroup among chordates, the tunicate Ciona (sea squirt), indicates the presence of a number of bHLH genes (Dehal et al., 2002). However, in the absence of more detailed information about their expression, in particular in the atrium (Imai et al., 2002), no clear conclusion about conservation across chordates can be drawn for these genes. The presence of sensory cells in the atrium, if combined with in situ hybridization for the Math1 and Ngn1 orthologues in tunicates, could potentially clarify the cellular and molecular origin of the vertebrate ear. However, the forkhead Eya and Fgf genes, crucial for ear formation and morphogenesis, either are not expressed outside the CNS in tunicates (Imai et al., 2002) or their expression in the atrium is not known. It is entirely possible that both tunicates and lancelet have diverged so far from the common ancestral pattern through regressive evolution, an event well known in the mechanosensory system of the ear and the lateral line (Fritzsch, 1988a; Fritzsch and Wake, 1988), that they will not reveal the sensory neuronal origin of the vertebrate ear.
Similar to data for other developmental genes that are highly conserved across phyla, these data suggest that essential components of a common genetic network play major roles in both invertebrate and vertebrate sensory cell formation. In fact, null mutants of Atonal (ato) or Math1 cause the absence of specific mechanosensory cells in insects and vertebrates, respectively, and thus show that these bHLH genes constitute a crucial node for the overall cellular development network, hence its conservation across phyla. Although we have at least one important universal developmental gene for a subset of insect and vertebrate mechanoreceptors, a number of issues still need to be resolved. For example, which other bHLH genes (if any) are also able to substitute for atonal (assuming that many bHLH genes can be interchanged to varying degrees) as a consequence of the properties of their conserved DNA binding domain.
VI. Evolution of Sensory Neurons: Heterochronic Alteration of HLH Gene Regulation
One novel feature of the ear, compared to insect and worm mechanosensory cells, is the existence of sensory neurons connecting the hair cells to the brain. It is likely that sensory neurons formed after an ancestral bHLH gene duplication that allowed separate assignment of fate to Ngn1 (sensory neurons) and Math1 (hair cell) expressing cells by retaining Math1 for the sensory (hair) cell and recruiting neurogenin for the sensory neuron. It appears that neurogenin and atonal coexisted in invertebrates. In the lancelet neurogenins are not recruited for peripheral nervous system development (Holland et al., 2000). The role of neurogenins and atonal in salp development is still unknown (Dehal et al., 2002), but paralogues of bHLH genes exist. In mammals, neurogenin expression exists in the olfactory system formation, where it is downstream to achaete-scute complex homolog-like 1 (Ascl1) (Calof et al., 2002). It is thus possible to have a sequential expression of several bHLH genes in a developing cluster of prosensory cells. We presume that such sequential expression in mechanosensory precursors was transformed in evolution into separate expression in distinct clones. This separate expression will ultimately lead to the formation of different cell types in the vertebrate mechanosensory precursors, sensory neurons, hair cells, and supporting cells.
Transforming proneuronal clusters, which give rise to ciliated sensory neurons in the bilaterian ancestor of insects and vertebrates, into cell clusters that give rise to both hair cells and sensory neurons in vertebrates could have been accomplished by recruiting an existing neurogenin gene to govern the divergent sensory neuron development in the vertebrate lineage. We propose that another round of division of sensory neuron precursor cells gave rise to the hair cell (retaining Aton) and sensory neuron (recruiting Ngn1). Such a scenario suggests that both hair cells and sensory neurons together are homologous to ciliated sensory neurons of insects. It also suggests a clonal relationship between vertebrate hair cells and sensory neurons.
Interestingly, sensory neurons emigrate from the area of the future sensory epithelia (Farinas et al., 2001, Fritzsch et al., 2002) much like the equally Ngn1-dependent dorsal root ganglion cell precursors delaminate from the spinal cord and hindbrain (Ma et al., 1999). It is possible that this happens for a similar reason, escaping the antineuronal effect of BMP4 expressed in the dorsal part of the spinal cord (Gowan et al., 2001) and the developing sensory epithelia (Morsli et al., 1998). It seems reasonable to assume, by analogy to insects, that some of the pathfinding properties for these sensory neurons are coextensive with the program that leads to the formation of sensory organs (Ghysen and Dambly-Chandiere, 2000). A set of novel downstream genes seems to have evolved, in part related to further specifying neuronal connectivity (Gu et al., 2003; Pauley et al., 2003; Xiang et al., 2003). It is conceivable that the function of Ngn1, which is expressed in cells of neural crest descent, will lead to different sensory projections than when the same gene is expressed in cells of otocyst descent.
The early formation of sensory neurons in inner ear development suggests a significant reorganization of the developmental pathways to implement this evolutionary novelty (Ma et al., 1998). In fact, existing data suggest that Ngn1 is expressed at least one day prior to Math1 (Ma et al., 1998; Zine et al., 2001). Moreover, the behavior of sensory neurons (being formed early and migrating away) is reminiscent of the formation of a glial cell in insect sensilla development (Reddy and Rodrigues, 1999). It is possible that the insect sensilla development was modified in ancestral vertebrates by the early upregulation of the newly recruited Ngn1 in the common precursor of sensory neurons, hair cells, and supporting cells.
VII. Guidance of Afferent Fibers: The Role of Hair Cells and Other Mechanisms Revisited
Primary neuron primordia can be identified either as delaminating cells (Carney and Silver, 1983; Farinas et al., 2001; Fritzsch et al., 2002) emigrating through the basal lamina surrounding the otocyst or as cells that express specific markers such as Ngn1 (Ma et al., 1998), NeuroD (Kim et al., 2001; Liu et al., 2000), neurotrophins (Farinas et al., 2001), or other genes (Fekete and Wu, 2002). Such delaminating cells are first apparent shortly after the otocyst closes in mouse (E9.5) or even before the otocyst is completely formed in chicken (Adam et al., 1998). Later, primary neuron primordia express many other genes such as Gata3 (Karis et al., 2001; Lawoko-Kerali et al., 2002); Brn3a (Huang et al., 2001); and others such as neurotrophin receptors (Farinas et al., 2001), Shh (Riccomagno et al., 2002), and Fgfs (Pauley et al., 2003).
The possibility of unique identities for early primary sensory neuron precursors is underscored by differential expression of several genes, even in early delaminating cells (Lawoko-Kerali et al., 2002), and the fact that in Neurod1 null mutants most cochlear neurons die in contrast to the loss of many fewer vestibular neurons (see Fig. 7) (Kim et al., 2001). Overall, the already known diversity of gene expression indicates that the various areas of the otocyst could provide unique identities to delaminating precursors based on overlapping and discrete regions of transcription factor expression. Despite this interesting start, it remains to be seen how differential areas of origin in the otocyst relate to differential gene expression and, ultimately, differential projection of primary neurons to specific sensory epithelia of the ear and specific areas of the brain (Maklad and Fritzsch, 2002). Ultimately it is possible, given sufficient nested expression patterns of various transcription factors within the ear, that primary neuron precursors acquire a unique cell fate assignment in the ear by analogy to that of neural-crest–derived primary sensory neurons and motoneurons in the CNS (Brunet and Pattyn, 2002; Gowan et al., 2001; Qian et al., 2001). If these initial data in the ear can be confirmed and extended by future work, development of distinct peripheral and central projections would be a consequence of such molecularly acquired cell fates already predetermined in the otocyst.
In this context it is important to realize that proliferation, delamination, migration to the final position, and development of central and peripheral projections is a prolonged phase in mammals and birds that lasts for several days (Rubel and Fritzsch, 2002; Ruben, 1967). As previously pointed out by Carney and Silver (1983) and recently confirmed (Farinas et al., 2001; Fritzsch et al., 2002), delaminating cells (which are likely neuronal precursors based on Neurod1 expression) apparently migrate away from the otocyst along fibers of more differentiated neurons that project toward the future sensory epithelia. Indeed, it appears that spatiotemporally distinct populations of primary sensory neuron precursors specifically extend along the existing neuronal fibers that reach toward the future primary sensory epithelium. Thus, it is possible that fate acquisition, as specified through the gene expression mosaic in the otocyst, results in restricted areas of primary sensory neuron delamination with specific and predetermined fates.
Primary sensory neurons with acquired identities may subsequently project back to the area from which they delaminated using other delaminating cells as substrate to extend their peripheral processes. Such a scenario would allow primary sensory neurons to be randomly distributed in the ganglia and to project nevertheless specifically to the ear and the brain, using distinct and different guidance cues to navigate to their various targets. In fact, recent data clearly show that most primary neurons projecting to distinct sensory epithelia are mixed in their distribution rather than completely sorted within the ganglion (Maklad and Fritzsch, 1999). Among the ear sensory epithelia, the cochlea of mammals is an exception with its highly organized peripheral and central projection (Lorente de No, 1933). But even here, topologically mismatched primary sensory neurons can comingle (Fritzsch, 2003). In contrast, the distribution of primary sensory neurons in vestibular ganglion is more random and the peripheral, exclusive projection to distinct endorgans contrasts with the highly overlapping topology of the central auditory nuclear representation of individual sensory epithelia (Maklad and Fritzsch, 1999, 2002). Indeed, tracing of early primary neurons shows that some may already have extended an axon toward the brain before they delaminate from the otic epithelium.
The literature is filled with suggestions that cell fate commitment occurs after either central or peripheral contacts are established (Gompel et al., 2001; Northcutt et al., 1995). Alternatively, it seems possible that primary sensory neuron cell fate determination starts in the otocyst prior to delamination. Such cell fate determination may, through unknown mechanisms, determine first where those primary sensory neuron precursors migrate to and, second, where their peripheral and central projections extend. Clearly, NeuroD and Brn3a are important players for this pathfinding (Huang et al., 2001; Kim et al., 2001), but next to nothing is known about their downstream molecular partners outside the primary sensory neurons. In the simplest scenario, primary sensory neurons project their dendrites back to the area from which the cell bodies originated but must subsequently navigate to target endorgan-specific hair cells. However, this is unlikely to be the only way peripheral fiber projections are established as evidenced by numerous anastomoses between sensory epithelia. Given the multitude of pathfinding molecules that have been characterized (Huang et al., 2002; Tessier-Lavigne and Goodman, 1996), it seems likely that multiple mechanisms come into play in the ear as well. Such mechanisms may be modifications of a common theme specific for a given primary sensory modality (i.e., cochlea and gravistatic and angular acceleration endorgans).
Based on insights gained from other systems, it seems logical to explore the function of the ephrin ligands and receptors, known to be expressed in the developing ear (Bianchi and Liu, 1999). However, no serious defect on fiber outgrowth has been reported thus far in the relevant null mutant mouse lines (Cowan et al., 2000). Other factors associated with guidance are the members of cell adhesion molecules, some of which are expressed in intricate patterns in the developing ear (Davies and Holley, 2002). Again, no experimental studies exist that support their function in fiber guidance in vivo. The semaphorins and their receptors, the neuropilins and plexins, are well known for their roles in neuronal pathfinding and regeneration (Cloutier et al., 2002; Pasterkamp and Verhaagen, 2001). Plexins and semaphorins have been described in the developing ear (Miyazaki et al., 1999; Murakami et al., 2001), but their potential function has not yet been explored using existing null mutant mice, in particular the plexin null line (Cloutier et al., 2002). Recent work on semaphorin receptor mutants indicates that these receptors may play a crucial role in guiding afferents (Gu et al., 2003). Given that many semaphorins signal via just two receptors, a large degree of redundancy can be expected and thus understanding the functions of these receptors and their ligands in the ear may take some time (Suto et al., 2003). As with the FGFs, both qualitative and quantitive expression patterns may further complicate this issue.
Last but not least, data suggest that glia–axon interactions may play a role in proper pathfinding in the lateral line system (Gilmour et al., 2002). By logical extension, such a role could also be played by glial cells in the developing ear, in particular for the outgrowth of fibers toward the brain (Begbie and Graham, 2001a). Such issues could be studied in erbB receptor mutant mice, which are known to eliminate glial cells (Morris et al., 1999). Indeed, preliminary data on Erbb2 null mutant mice strongly support this notion (Morris and Fritzsch, unpublished data).
A. The Role of Hair Cells in Fiber Guidance and Survival
Numerous observations suggest that hair cells attract fibers to innervate them (Bianchi and Cohan, 1991, 1993), and some neurotrophic secretion might in part mediate these attractions (Cajal, 1919). One way of exploring neurotrophin effects in the ear would be to eliminate neurotrophins in the target of the inner ear afferents, the hair cells, by eliminating hair cells or preventing their differentiation. This is expected to eliminate expression of BDNF because in older embryos BDNF is expressed exclusively in hair cells within the ear (see Figs. 5 and 6). In addition, if no hair cells form, it is likely that supporting cells may not form normally because both are apparently linked in their differentiation via reciprocal interactions mediated through the delta–notch regulatory system (Zine et al., 2001). Recent investigations into two mutations that result in undifferentiated hair cells provide no indication for losses of sensory neurons resulting from neurotrophin deficiency. However, there are examples of sensory neuron loss that appear to be caused by abnormally low expression of the trk receptors in other mutants (Huang et al., 2001; Kim et al., 2001; Liu et al., 2000).
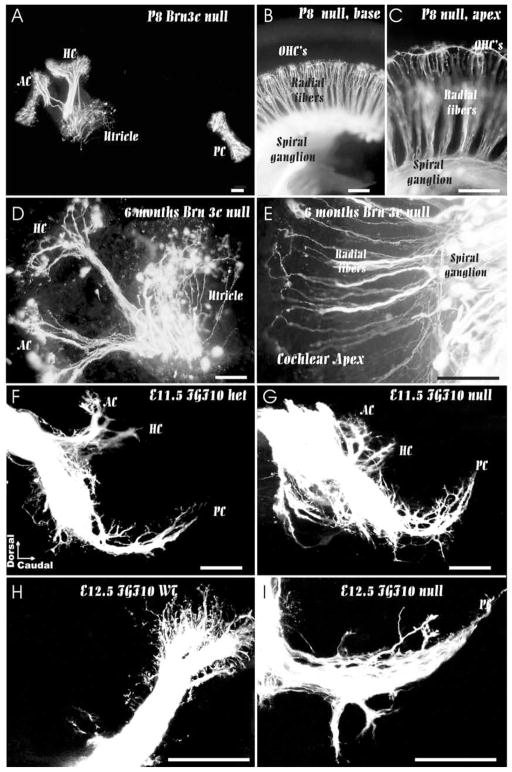
Mice lacking the Pou-domain–containing transcription factor, Brn3c, develop only a limited complement of morphologically undifferentiated hair cells, which can be identified as hair cells by hair-cell–specific molecular marker expression (Xiang et al., 1998). Closer examination of afferent innervation (Fig. 8) showed no correlation of fiber loss with failure to form differentiated hair cells (Xiang et al., 2003). Specifically, a robust sensory innervation persists through embryogenesis into early neonatal life. The survival throughout embryogenesis is apparently mediated by the limited expression of two neurotrophins, BDNF and NT-3, in undifferentiated sensory epithelia as revealed with in situ hybridization studies. Even several-month–old animals had a considerable innervation of the apical turn of the cochlea. However, this long-term retention of cochlear innervation is likely not mediated by neurotrophins because these are downregulated in neonatal animals (Wheeler et al., 1994).
Figure 8.
These images show that directed fiber growth and even long term retention of fibers is possible without differentiated hair cells. In Brn3c null mutants, fibers grow to all sensory epithelia (A–C) and some are retained even 6 month old mice in the absence of hair cells (D, E). In FGF10 null mutant mice, there is no formation of a posterior crista (PC) but fibers grow toward this part of the ear at embryonic day 11 (F, G). However, in the absence of a posterior crista, fibers can not expand to innervate a sensory epithelium (H, I) and eventually disappear. AC, anterior crista; HC, horizontal crista; OHC’s, outer hair cells; PC, posterior crista. Bar indicates 100 μm.
It also seemed possible that examination of mutants lacking the bHLH transcription factor Math1 would make it possible to test the possible functions of guidance and survival factors released from hair cells on the guidance and survival of sensory afferent neurons. Analysis shows that Math1 is required for hair cell differentiation and probably acts upstream of Brn3c (Bermingham et al., 1999; Fritzsch et al., 2000). Surprisingly, there is very little effect of this mutation on the initial fiber growth of sensory neurons (Fritzsch et al., 2003). However, older embryos show a severe reduction of afferents that does not correspond to the pattern of loss observed in neurotrophin null mutations. Closer examination of the expression of BDNF using the BDNF–LacZ reporter showed that even in Math1 null mutants some undifferentiated hair cell precursors form and express BDNF (see Fig. 6). Thus, at least in some hair cell precursors BDNF expression does not require Math1-mediated hair cell differentiation. Even complete elimination of hair cells as in the posterior crista of FGF10 null mutant mice (Pauley et al., 2003) is compatible with directed fiber growth (see Fig. 8).
Together the data show that at the time of this publication none of the attempts to eliminate neurotrophin expression in the ear through mutation of essential transcription factors has made it possible to test the proposal of Ramon and Cajal (Cajal, 1919) that hair cells secrete neurotrophic substance(s) that attract sensory afferents. Unfortunately, the finding of neurotrophin expression in delaminating sensory neurons has made the interpretation of neurotrophin effects even more complex. Clearly, the limited expression of BDNF in the undifferentiated hair cell precursors of Math1 null mutant mice is apparently enough to support many afferents throughout embryonic life. Consequently, we cannot exclude a biologically significant effect of the limited expression of neurotrophins within delaminating sensory neurons. Thus none of the mutations described has critically tested an exclusive role for hair cells in attraction and maintenance of inner ear sensory neurons.
VIII. Survival of Afferents: Evolving a Novel Mechanism and Expanding it to Fit the Increasing Complexity of Ear Development
One of the major novelties in vertebrate development, the formation of a neurotrophin-mediated cell survival loop, appears to be unique to vertebrates, with perhaps some genes of unclear function present in invertebrates (Hallbook, 1999; Hallbook et al., 1998). Interestingly, some Mollusca, but not insects, have separate sensory cells and sensory neurons (Budelmann, 1992). Thus, neurotrophin-mediated cell survival may have evolved in conjunction with the bHLH gene recruitment to distinct sensory neuron formation. It is also possible that the neurotrophins and their receptors may have evolved during the ancestral vertebrate gene duplication from primordial proteins (Hallbook, 1999, Jaaro et al., 2001).
Two neurotrophic factors, BDNF and NT-3, and their high-affinity receptors, trkB and trkC, have been identified by in situ hybridization and other molecular techniques in the ear (Farinas et al., 2001; Fritzsch et al., 1999; Pirvola et al., 1992; Wheeler et al., 1994). Targeted mutations of each of these neurotrophins and receptors have clarified their relative contributions to the survival of different sensory neurons in the ear (Fritzsch et al., 1999). These data have shown that there is a dramatic loss of 85% of cochlear sensory (spiral ganglion) neurons in Ntf3 null mutants (Farinas et al., 1994, 2001; Fritzsch et al., 1997) and 80–85% of vestibular neurons in the Bdnf null mutant (Bianchi et al., 1996; Ernfors et al., 1995; Jones et al., 1994; Schimmang et al., 1995). Somewhat similar effects have been described in neurotrophin receptor null mutants (Fritzsch et al., 1995, 2001b). Detailed counting has shown that neuronal loss happens within 2–3 days after the fibers have first extended toward the sensory epithelia (Bianchi et al., 1996; Farinas et al., 2001). Together these data suggest that vestibular and cochlear sensory neurons have distinct but complementary neurotrophin requirements (Ernfors et al., 1995).
The relative distribution of neurotrophins with more prominent expression of BDNF in the vestibular system and of NT-3 in the cochlea does support the evidence for complementary roles of these neurotrophins in the vestibular and cochlear sensory epithelia, respectively (Farinas et al., 2001; Pirvola et al., 1992). In the context of the hypothesized role of neurotrophin function in numerical matching of pre- and postsynaptic targets, it needs to be pointed out that in the ear there is no uniform relationship between afferents and hair cells, which can vary from 30–1 (convergence on a single inner hair cell) to 1–30 (divergence on outer hair cells and some vestibular fibers). It remains questionable how a single neurotrophin, such as BDNF, distributed fairly uniform in all hair cells, should be able to mediate these differences. Clearly, quantitative data on specific amounts of BDNF expressed in different types of hair cells are needed to evaluate this aspect of ear innervation.
Whereas specific null mutations have shown significant effects on sensory neuron survival projecting to distinct endorgans of the ear, it remains unclear whether more neurotrophins or other neurotrophic factors might add to the survival of inner ear sensory neurons. However, double mutant mice, which lack both the neurotrophin receptors Ntrk2 and Ntrk3 or both neurotrophins Bdnf and Ntf3, have no surviving sensory neurons in the inner ear at birth (Ernfors et al., 1995; Liebl et al., 1997; Silos-Santiago et al., 1997). This dramatic effect of double mutations on ear innervation therefore puts to rest any speculations about additional neurotrophins and neurotrophin receptor requirements. These data on double mutants also show that even if other ligands and receptors are present their function for the development of the inner ear sensory neurons is not critical compared with BDNF/NT-3 and their receptors trkB/trkC, similar to the ubiquitous p75 neurotrophin receptor.
Specific losses of distinct cochlear and vestibular afferents occur in single neurotrophin and neurotrophin receptor null mutant mice, and these losses are related to a highly dynamic pattern of expression of neurotrophins but not of neurotrophin receptors in the ear (Farinas et al., 2001; Pirvola et al., 1992). Both during development and in the adult sensory neurons there appears to be a uniform expression of both trkB and trkC on all of the sensory neurons in the ear (Farinas et al., 2001; Fritzsch et al., 1999).
In addition, primary sensory neurons express neurotrophins soon after delamination is initiated from the placodal epithelium (Farinas et al., 2001). Furthermore, a given primary sensory neuron expresses the same neurotrophin, which is present in the area of the otocyst from which it delaminated (Fritzsch et al., 2002). Comparison of BDNF and NT-3–LacZ-positive cells with delaminating neurons marked by NeuroD–LacZ also suggests that the delaminating BDNF or NT-3-positive precursors are in fact NeuroD-expressing neuronal precursors (Fritzsch et al., 2002; Kim et al., 2001; Liu et al., 2000), but proof will require colabeling for NeuroD and each of the neurotrophins.
These data indicate that initial fiber growth occurs normally in the absence of neurotrophins. Indeed, initial fiber growth may use the same molecular cues recognized by the delaminating primary sensory neuron precursors. However, there is subsequently a critical period of neurotrophin dependency that will result in elimination of all connections that reach areas normally or experimentally deprived of specific neurotrophins. Such aberrantly growing fibers have been reported in the developing, but not in the adult, ear (Lorente de No, 1926). The partially overlapping expression of neurotrophins reported in the developing mammalian ear (Farinas et al., 2001; Pirvola et al., 1992) seems to translate into a spatiotemporal loss of primary sensory neurons in specific mutants (Farinas et al., 2001). Particularly, in the cochlea there is a delayed upregulation of BDNF expression in the basal turn, leaving all basal turn neurons solely dependent on NT-3 during a brief but critical period of embryogenesis. Thus, if NT-3 is absent, there is a progressive loss of spiral neurons, especially in the basal turn, where BDNF is not present to compensate for the absence of NT-3. Overall, sensory neuron loss will occur in an embryo with a targeted mutation in Bdnf or Ntf3 only where the other is not present to compensate for its absence.
This suggestion has led to the prediction that in the ear, NT-3 and BDNF are functionally equivalent and can be substituted for each other without compromising the survival and development of sensory neurons. Consistent with this prediction, data show that the topological loss of sensory neurons in the basal turn in the Ntf3 mutant can be rescued by transgenic expression of Bdnf under the control of Ntf3 gene regulatory elements (Coppola et al., 2001). Likewise, the corresponding transgenic animal in which the Ntf3 coding region is inserted into the Bdnf gene is equally effective in rescuing the BDNF phenotype in the cochlea, but not in the vestibular system (Agerman et al., 2003).
Overall, these data support a role for a very early onset of elimination of exuberant or unconnected afferents and the primary sensory neurons that generate these axons. This verification of proper connections occurs immediately after the fibers have reached and started to invade their target organs (as early as E11 in the canal epithelia and E13 in the basal turn of the cochlea). The most interesting and striking effect of the single neurotrophin null mutation is the dependence of the basal turn cochlear neurons on NT-3 (Fig. 9). This is because BDNF shows a delayed expression in the basal turn. Clearly, BDNF can not only compensate for NT-3 and rescue the basal turn neurons (Coppola et al., 2001) but can also attract vestibular fibers from the nearby nerve to the posterior crista to innervate the basal turn instead. It is conceivable that evolutionary pressures have resulted in the delayed expression of BDNF in the basal turn because of the need to avoid misrouting of vestibular afferents. Misrouting of vestibular fibers to cochlear hair cells, assuming they maintain their normal connections in the CNS, will result in auditory information interfering with perceptions of position and motion.
Figure 9.
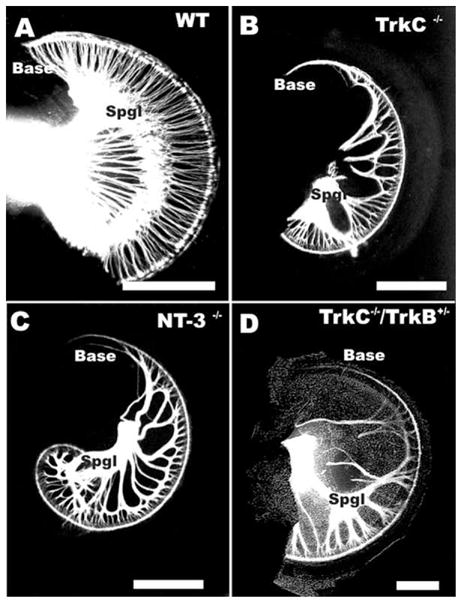
The effects of neurotrophin and neurotrophin receptor null mutations on the pattern of cochlear innervation is shown. Note the severe reduction in density of cochlear innervation in a pair of neurotrophin ligand (NT-3) and neurotrophin receptor(trkC) null mutants (B, C). In addition, there is a complete loss of all sensory neurons in the basal tip (Base) of either neurotrophin or neurotrophin receptor null mutant (compare A with B, C). A Ntf3 null mutation combined with Ntrk3 heterozygosity (designated as TrkC−/−/TrkB+/−) results in further loss of innervation and a patchy distribution of the remaining sensory neurons (D). Note that afferents from middle turn spiral sensory neurons (Spgl) expand along inner hair cells (shown as a DIC image) to reach the base. Bar indicates 1 mm.
Interestingly, neurotrophins are apparently downregulated in neonates (Fritzsch et al., 1999; Wheeler et al., 1994), and they appear to be largely lost in adults, despite the fact that their trk receptors are still expressed in the sensory neurons (Fritzsch et al., 1999). This has led to the suggestion that other neurotrophic factors may play a role in the neonatal death of sensory neurons (Echteler and Nofsinger, 2000; Hashino et al., 1999), a suggestion that requires further experimental verification in mutants with conditional targeting of neurotrophin genes.
IX. Splitting Hair Cell and Neuron Populations: Coevolving Sensory Epithelia and Their Innervation
In general, neurosensory evolution of the ear is based on the multiplication of existing sensory patches and their innervation (Fritzsch and Wake, 1988; Fritzsch et al., 2002). These modifications are likely followed by functional diversification through creation of modified, unique acellular covering structures (Goodyear and Richardson, 2002) that allow transduction of a previously unexplored property of the mechanical energy that reaches the ear. Clearly, formation of separate epithelia can occur within the same compartment of the ear and such compartments, like the lagenar recess, can be retained in the absence of sensory patch formation (Fritzsch and Wake, 1988). Nevertheless, it appears reasonable to assume that position within a given otic recess will influence hair cell differentiation. It seems possible that the forming cochlear duct is providing a morphodynamic influence on the differentiation of cochlear hair cells. Such tests require transplantation of hair cells from one sensory patch (i.e., the horizontal crista) into another patch (i.e., the cochlea), an experiment that has never been carried out.
The simplest ear to be found in extant vertebrates is the hagfish ear. This ear has only three sensory epithelia: one common macula and two crista organs in a single canal (Fritzsch, 2001a, b; Lewis et al., 1985). The largest number of sensory patches in the ear is found in certain species of limbless amphibians (see Fig. 2), which have nine different sensory patches: three canal cristae, utricle, saccule, lagena, neglected papilla, basilar papilla, and amphibian papilla (Fritzsch and Wake, 1988; Sarasin and Sarasin, 1892). Descriptive developmental evidence has long suggested that the evolution of multiple sensory epithelia comes about through developmental splitting of a single sensory anlage (Fritzsch and Wake, 1998; Norris, 1892). Moreover, it appears that organs such as the lagena may have evolved independently three times in vertebrates, each time by splitting off from the saccule (Fritzsch, 1992). In contrast, other organs such as the basilar papilla may have evolved only once, namely during the segregation of the lagena from the saccule in the tetrapod ancestors (Fritzsch, 1987).
Such segregation and functional diversification are best documented for the neglected/amphibian papilla system. It appears that location of each of the two sensory patches to either the utricle or the saccule and association with the perilymphatic sound conduction system may determine the future function as a sound pressure receiver or as an additional vestibular receptor (Brichta and Goldberg, 1998; Fritzsch and Wake, 1988). Another well-documented example is the utricle in some bony fishes (e.g., herring) that forms three distinct sensory patches. Although two of these patches retain their ancestral function as gravistatic receptors, one patch has acquired a novel function as a sound pressure receiver by association with the perilymphatic sound conduction system (Fritzsch, 2001a; Lewis et al., 1985).
Clearly, forming a sensory epithelium that can access a novel sensory stimulus requires transformation of existing mechanoelectrical transducers through morphological alterations to tap into this novel mechanical energy source. Sound pressure reception is not accomplished by the mere formation of a novel sensory epithelium that can be dedicated to perceive this energy. However, it is reasonable to assume that once such an uncommitted receptor is available, changes in the ear morphology using some of the genes outlined may achieve changes upon which further refinement can act in the slow process of selection of appropriate function-based modification. It is conceivable that such alterations will take place as soon as a new receptor forms simply because of the invariable alteration of FGF and BMP interaction that comes with that change of the formation of a new sensory epithelium. Indeed, comparing just BMP4 expression in chicken and mice (Morsli et al., 1998; Wu and Oh, 1996) shows differences in the expression patterns that need to be further explored by comparing the expression of BMP4 with those of FGF ligands and receptors (Pirvola et al., 2002).
It is noteworthy that incomplete separation of normally segregated sensory patches has been reported in mutations with altered morphologies (Fritzsch et al., 2001a; Pauley et al., 2003). This implies that morphogenesis and segregation of sensory patches are linked, but not necessary causal. One alternative explanation is simply that reduced morphogenesis limits the capacity of the sensory patches to segregate.
X. Summary and Conclusions: Evolving Developmental Mechanisms
We have seen the beginning of a process that will untangle the evolution of developmental genetic networks that combines mechanoelectrical cellular conservation via evolutionary “preserved” morphostatic pathways with novel morphogenetic features upon which specific aspects of mechanical stimulation are maintained. This apparent contradiction of extreme conservation in the molecular governance of mechanoelectrical transducer cell formation across phyla (Wang et al., 2002) contrasts sharply with the apparent evolution of unique genes that are expressed exclusively in the vertebrate ear to govern development of vertebrate-specific acellular structures of the ear (El-Amraoui et al., 2001). Between these two extremes the genes for ear morphogenesis are represented mostly by developmental modules specifically modified to suit aspects of ear development. Within the context of these modified morphogenic modules are the “ancient” genetic pathways for the ear-specific neuronal elements. Overall, ear evolution resembles the networks of interactions and their alterations known from complex ecosystems. Any alteration in frequency of expression of a single aspect can have significant effects on the system as a whole. Keeping the mechanotransducer sensory element constant allows evolution to optimize aspects of ear morphogenesis to maximize acquisition of specific signals. Evolution of the vertebrate ear can thus be best understood as an iterative developmental transformation toward optimized signal transmission.
Such aspects of ear evolution are particularly clear when comparing the morphocline of hagfish, lamprey, and jawed vertebrate ears (see Fig. 2). Hagfish have a single canal with two epithelia that bear no cupula. Thus, hagfish will see similar rotational movements in the plane of the single torus with opposing activities in either sensory epithelium. Lampreys have evolved a two-canal system that allows extraction of two distinct angular movements at an angle of about 90° to each other in each ear. Jawed vertebrates have evolved a third canal that allows them to extract selectively horizontal angular movements, a stimulus that needs computational work to be extracted from the single or two-canal labyrinth of jawless vertebrates. Clearly, optimizing the extraction of angular stimuli in three orthogonal planes is not a matter of optimizing the mechanoelectrical transducer but rather of optimizing morphogenesis of the ear. It is therefore expected that diversity in morphogenetically important transcription factors will be equivalent to that of ear morphogenesis, whereas transcription factors involved in hair cell formation should be much less variable among vertebrates.
Acknowledgments
This work was supported by grants from NIDCD (R01 DC005590; BF; R01 DC04279; KWB) and NASA (NRA 01-OBPR-06). We wish to express our gratitude to Drs. D. Nichols, H. Zoghbi, Q. Ma, and J. Lee for providing material and intellectual stimuli.
References
- Abdelhak S, Kalatzis V, Heilig R, Compain S, Samson D, et al. Clustering of mutations responsible for branchio-oto-renal (BOR) syndrome in the eyes absent homologous region (eyaHR) of EYA1. Hum Mol Genet. 1997a;6:2247–2255. doi: 10.1093/hmg/6.13.2247. [DOI] [PubMed] [Google Scholar]
- Abdelhak S, Kalatzis V, Heilig R, Compain S, Samson D, et al. A human homologue of the Drosophila eyes absent gene underlies branchio-oto-renal (BOR) syndrome and identifies a novel gene family. Nat Genet. 1997b;15:157–164. doi: 10.1038/ng0297-157. [DOI] [PubMed] [Google Scholar]
- Adam J, Myat A, Le Roux I, Eddison M, Henrique D, et al. Cell fate choices and the expression of Notch, Delta and Serrate homologues in the chick inner ear: Parallels with Drosophila sense-organ development. Development. 1998;125:4645–4654. doi: 10.1242/dev.125.23.4645. [DOI] [PubMed] [Google Scholar]
- Adamska M, Herbrand H, Adamski M, Kruger M, Braun T, Bober E. FGFs control the patterning of the inner ear but are not able to induce the full ear program. Mech Dev. 2001;109:303–313. doi: 10.1016/s0925-4773(01)00550-0. [DOI] [PubMed] [Google Scholar]
- Agerman K, Hjerling-Leffler J, Blanchard MP, Scarfone E, Canlon B, et al. BDNF gene replacement reveals multiple mechanisms for establishing neurotrophin specificity during sensory nervous system development. Development. 2003;130:1479–1491. doi: 10.1242/dev.00378. [DOI] [PubMed] [Google Scholar]
- Ayers H. Vertebrate cephalogenesis. J Morphol. 1892;6:1–360. [Google Scholar]
- Bally-Cuif L, Hammerschmidt M. Induction and patterning of neuronal development, and its connection to cell cycle control. Curr Opin Neurobiol. 2003;13:16–25. doi: 10.1016/s0959-4388(03)00015-1. [DOI] [PubMed] [Google Scholar]
- Begbie J, Graham A. Integration between the epibranchial placodes and the hindbrain. Science. 2001a;294:595–598. doi: 10.1126/science.1062028. [DOI] [PubMed] [Google Scholar]
- Begbie J, Graham A. The ectodermal placodes: A dysfunctional family. Philos Trans R Soc Lond B Biol Sci. 2001b;356:1655–1660. doi: 10.1098/rstb.2001.0920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Arie N, Hassan BA, Bermingham NA, Malicki DM, Armstrong D, et al. Functional conservation of atonal and Math1 in the CNS and PNS. Development. 2000;127:1039–1048. doi: 10.1242/dev.127.5.1039. [DOI] [PubMed] [Google Scholar]
- Bermingham NA, Hassan BA, Price SD, Vollrath MA, Ben-Arie N, et al. Math1: An essential gene for the generation of inner ear hair cells. Science. 1999;284:1837–1841. doi: 10.1126/science.284.5421.1837. [DOI] [PubMed] [Google Scholar]
- Bermingham NA, Hassan BA, Wang VY, Fernandez M, Banfi S, et al. Proprioceptor pathway development is dependent on Math1. Neuron. 2001;30:411–422. doi: 10.1016/s0896-6273(01)00305-1. [DOI] [PubMed] [Google Scholar]
- Bertrand N, Castro DS, Guillemot F. Proneural genes and the specification of neural cell types. Nat Rev Neuro Sci. 2002;3:517–530. doi: 10.1038/nrn874. [DOI] [PubMed] [Google Scholar]
- Bianchi LM, Cohan CS. Developmental regulation of a neurite-promoting factor influencing statoacoustic neurons. Brain Res Dev Brain Res. 1991;64:167–174. doi: 10.1016/0165-3806(91)90221-4. [DOI] [PubMed] [Google Scholar]
- Bianchi LM, Cohan CS. Effects of the neurotrophins and CNTF on developing statoacoustic neurons: Comparison with an otocyst-derived factor. Dev Biol. 1993;159:353–365. doi: 10.1006/dbio.1993.1247. [DOI] [PubMed] [Google Scholar]
- Bianchi LM, Conover JC, Fritzsch B, DeChiara T, Lindsay RM, Yancopoulos GD. Degeneration of vestibular neurons in late embryogenesis of both heterozygous and homozygous BDNF null mutant mice. Development. 1996;122:1965–1973. doi: 10.1242/dev.122.6.1965. [DOI] [PubMed] [Google Scholar]
- Bianchi LM, Liu H. Comparison of ephrin-A ligand and EphA receptor distribution in the developing inner ear. Anat Rec. 1999;254:127–134. doi: 10.1002/(SICI)1097-0185(19990101)254:1<127::AID-AR16>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Bone Q, Ryan KP. Cupular sense organs in Ciona (Tunicata: Ascidiacea) J Zool (London) 1978;186:417–429. [Google Scholar]
- Borsani G, DeGrandi A, Ballabio A, Bulfone A, Bernard L, et al. EYA4, a novel vertebrate gene related to Drosophila eyes absent. Hum Mol Genet. 1999;8:11–23. doi: 10.1093/hmg/8.1.11. [DOI] [PubMed] [Google Scholar]
- Brichta AM, Goldberg JM. The papilla neglecta of turtles: A detector of head rotations with unique sensory coding properties. J Neurosci. 1998;18:4314–4324. doi: 10.1523/JNEUROSCI.18-11-04314.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brigande JV, Kiernan AE, Gao X, Iten LE, Fekete DM. Molecular genetics of pattern formation in the inner ear: Do compartment boundaries play a role? Proc Natl Acad Sci USA. 2000;97:11700–11706. doi: 10.1073/pnas.97.22.11700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunet JF, Pattyn A. Phox2 genes—from patterning to connectivity. Curr Opin Genet Dev. 2002;12:435–440. doi: 10.1016/s0959-437x(02)00322-2. [DOI] [PubMed] [Google Scholar]
- Budelmann BU. Hearing in nonarthropod invertebrates. In: Popper AN, editor. The Evolutionary Biology of Hearing. Springer Verlag; New York: 1992. pp. 141–156. [Google Scholar]
- Burighel P, Lane NJ, Fabio G, Stefano T, Zaniolo G, Carnevali MD, Manni L. Novel, secondary sensory cell organ in ascidians: In search of the ancestor of the vertebrate lateral line. J Comp Neurol. 2003;461:236–249. doi: 10.1002/cne.10666. [DOI] [PubMed] [Google Scholar]
- Cajal SR. Accion neurotropica de los epitelios. Trab del Lab de Invest Biol. 1919;17:1–153. [Google Scholar]
- Caldwell JC, Eberl DF. Towards a molecular understanding of Drosophila hearing. J Neurobiol. 2002;53:172–189. doi: 10.1002/neu.10126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calof AL, Bonnin A, Crocker C, Kawauchi S, Murray RC, et al. Progenitor cells of the olfactory receptor neuron lineage. Microsc Res Tech. 2002;58:176–188. doi: 10.1002/jemt.10147. [DOI] [PubMed] [Google Scholar]
- Cantos R, Cole LK, Acampora D, Simeone A, Wu DK. Patterning of the mammalian cochlea. Proc Natl Acad Sci USA. 2000;97:11707–11713. doi: 10.1073/pnas.97.22.11707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carney PR, Silver J. Studies on cell migration and axon guidance in the developing distal auditory system of the mouse. J Comp Neurol. 1983;215:359–369. doi: 10.1002/cne.902150402. [DOI] [PubMed] [Google Scholar]
- Cau E, Casarosa S, Guillemot F. Mash1 and Ngn1 control distinct steps of determination and differentiation in the olfactory sensory neuron lineage. Development. 2002;129:1871–1880. doi: 10.1242/dev.129.8.1871. [DOI] [PubMed] [Google Scholar]
- Chan YM, Jan YN. Conservation of neurogenic genes and mechanisms. Curr Opin Neurobiol. 1999;9:582–588. doi: 10.1016/S0959-4388(99)00017-3. [DOI] [PubMed] [Google Scholar]
- Chang W, ten Dijke P, Wu DK. BMP pathways are involved in otic capsule formation and epithelial-mesenchymal signaling in the developing chicken inner ear. Dev Biol. 2002;251:380–394. doi: 10.1006/dbio.2002.0822. [DOI] [PubMed] [Google Scholar]
- Chen P, Johnson JE, Zoghbi HY, Segil N. The role of Math1 in inner ear development: Uncoupling the establishment of the sensory primordium from hair cell fate determination. Development. 2002;129:2495–2505. doi: 10.1242/dev.129.10.2495. [DOI] [PubMed] [Google Scholar]
- Cloutier JF, Giger RJ, Koentges G, Dulac C, Kolodkin AL, Ginty DD. Neuropilin-2 mediates axonal fasciculation, zonal segregation, but not axonal convergence, of primary accessory olfactory neurons. Neuron. 2002;33:877–892. doi: 10.1016/s0896-6273(02)00635-9. [DOI] [PubMed] [Google Scholar]
- Colvin JS, Bohne BA, Harding GW, McEwen DG, Ornitz DM. Skeletal overgrowth and deafness in mice lacking fibroblast growth factor receptor 3. Nat Genet. 1996;12:390–397. doi: 10.1038/ng0496-390. [DOI] [PubMed] [Google Scholar]
- Conway Morris S. The Cambrian “explosion”: Slow-fuse or megatonnage? Proc Natl Acad Sci USA. 2000;97:4426–4429. doi: 10.1073/pnas.97.9.4426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppola V, Kucera J, Palko ME, Martinez-De Velasco J, Lyons WE, et al. Dissection of NT3 functions in vivo by gene replacement strategy. Development. 2001;128:4315–4327. doi: 10.1242/dev.128.21.4315. [DOI] [PubMed] [Google Scholar]
- Corwin JT. Postembryonic production and aging of inner ear hair cells in sharks. J Comp Neurol. 1981;201:541–553. doi: 10.1002/cne.902010406. [DOI] [PubMed] [Google Scholar]
- Cowan CA, Yokoyama N, Bianchi LM, Henkemeyer M, Fritzsch B. EphB2 guides axons at the midline and is necessary for normal vestibular function. Neuron. 2000;26:417–430. doi: 10.1016/s0896-6273(00)81174-5. [DOI] [PubMed] [Google Scholar]
- David ES, Luke NH, Livingston BT. Characterization of a gene encoding a developmentally regulated winged helix transcription factor of the sea urchin Strongylocentrotus purpuratus. Gene. 1999;236:97–105. doi: 10.1016/s0378-1119(99)00248-6. [DOI] [PubMed] [Google Scholar]
- Davidson EH, Rast JP, Oliveri P, Ransick A, Calestani C, et al. A genomic regulatory network for development. Science. 2002;295:1669–1678. doi: 10.1126/science.1069883. [DOI] [PubMed] [Google Scholar]
- Davies D, Holley MC. Differential expression of alpha3 and alpha6 integrins in the developing mouse inner ear. J Comp Neurol. 2002;445:122–132. doi: 10.1002/cne.10161. [DOI] [PubMed] [Google Scholar]
- Davis RJ, Shen W, Heanue TA, Mardon G. Mouse Dach, a homologue of Drosophila dachshund, is expressed in the developing retina, brain and limbs. Dev Genes Evol. 1999;209:526–536. doi: 10.1007/s004270050285. [DOI] [PubMed] [Google Scholar]
- Davis RJ, Shen W, Sandler YI, Heanue TA, Mardon G. Characterization of mouse Dach2, a homologue of Drosophila dachshund. Mech Dev. 2001;102:169–179. doi: 10.1016/s0925-4773(01)00307-0. [DOI] [PubMed] [Google Scholar]
- Dehal P, Satou Y, Campbell RK, Chapman J, Degnan B, et al. The draft genome of Ciona intestinalis: Insights into chordate and vertebrate origins. Science. 2002;298:2157–2167. doi: 10.1126/science.1080049. [DOI] [PubMed] [Google Scholar]
- Echteler SM, Nofsinger YC. Development of ganglion cell topography in the postnatal cochlea. J Comp Neurol. 2000;425:436–446. [PubMed] [Google Scholar]
- Eddison M, Le Roux I, Lewis J. Notch signaling in the development of the inner ear: Lessons from Drosophila. Proc Natl Acad Sci USA. 2000;97:11692–11699. doi: 10.1073/pnas.97.22.11692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Amraoui A, Cohen-Salmon M, Petit C, Simmler MC. Spatiotemporal expression of otogelin in the developing and adult mouse inner ear. Hear Res. 2001;158:151–159. doi: 10.1016/s0378-5955(01)00312-4. [DOI] [PubMed] [Google Scholar]
- Ernfors P, Van De Water T, Loring J, Jaenisch R. Complementary roles of BDNF and NT-3 in vestibular and auditory development. Neuron. 1995;14:1153–1164. doi: 10.1016/0896-6273(95)90263-5. [DOI] [PubMed] [Google Scholar]
- Farinas I, Jones KR, Backus C, Wang XY, Reichardt LF. Severe sensory and sympathetic deficits in mice lacking neurotrophin-3. Nature. 1994;369:658–661. doi: 10.1038/369658a0. [DOI] [PubMed] [Google Scholar]
- Farinas I, Jones KR, Tessarollo L, Vigers AJ, Huang E, et al. Spatial shaping of cochlear innervation by temporally regulated neurotrophin expression. J NeuroSci. 2001;21:6170–6180. doi: 10.1523/JNEUROSCI.21-16-06170.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favor J, Sandulache R, Neuhauser-Klaus A, Pretsch W, Chatterjee B, et al. The mouse Pax2(1Neu) mutation is identical to a human PAX2 mutation in a family with renal-coloboma syndrome and results in developmental defects of the brain, ear, eye, and kidney. Proc Natl Acad Sci USA. 1996;93:13870–13875. doi: 10.1073/pnas.93.24.13870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fekete DM, Wu DK. Revisiting cell fate specification in the inner ear. Curr Opin Neurobiol. 2002;12:35–42. doi: 10.1016/s0959-4388(02)00287-8. [DOI] [PubMed] [Google Scholar]
- Fritzsch B. The inner ear of the coelacanth fish Latimeria has tetrapod affinities. Nature. 1987;327:153–154. doi: 10.1038/327153a0. [DOI] [PubMed] [Google Scholar]
- Fritzsch B. The amphibian octavo-lateralis system and its regressive and progressive evolution. Acta Biol Hung. 1988a;39:305–322. [PubMed] [Google Scholar]
- Fritzsch B. The lateral-line and inner-ear afferents in larval and adult urodeles. Brain Behav Evol. 1988b;31:325–348. doi: 10.1159/000116599. [DOI] [PubMed] [Google Scholar]
- Fritzsch B. The water-to-land transition: Evolution of the tetrapod basilar papilla, middle ear and auditory nuclei. In: Popper AN, editor. The Evolutionary Biology of Hearing. Springer Verlag; New York: 1992. pp. 351–375. [Google Scholar]
- Fritzsch B. Hearing in two worlds: Theoretical and realistic adaptive changes of the aquatic and terrestrial ear for sound reception. In: Fay RR, Popper AN, editors. Springer Handbook of Auditory Research Comparative Hearing: Fish and Amphibians. Springer-Verlag; New York: 1999. pp. 15–42. [Google Scholar]
- Fritzsch B. The morphology and function of fish ears. In: Ostrander G, editor. The Laboratory Fish. Academic Press; Exeter: 2001a. pp. 250–259. [Google Scholar]
- Fritzsch B. The cellular organization of the fish ear. In: Ostrander G, editor. The Laboratory Fish. Academic Press; Exeter: 2001b. pp. 480–487. [Google Scholar]
- Fritzsch B. Development of inner ear afferent connections: Forming primary neurons and connecting them to the developing sensory epithelia. Brain Res Bull. 2003;60:423–433. doi: 10.1016/s0361-9230(03)00048-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritzsch B, Barald K, Lomax M. Early embryology of the vertebrate ear. In: Fay RR, editor. Springer Handbook of Auditory Research. Vol XII. Development of the Auditory System. Springer Verlag; New York: 1998a. pp. 80–145. [Google Scholar]
- Fritzsch B, Barbacid M, Silos-Santiago I. Nerve dependency of developing and mature sensory receptor cells. Ann NY Acad Sci. 1998b;855:14–27. doi: 10.1111/j.1749-6632.1998.tb10543.x. [DOI] [PubMed] [Google Scholar]
- Fritzsch B, Beisel K. Development and maintenance of ear innervation and function: Lessons from mutations in mouse and man. Am J Hum Genet. 1998;63:1263–1270. doi: 10.1086/302126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritzsch B, Beisel KW. Evolution and development of the vertebrate ear. Brain Res Bull. 2001;55:711–721. doi: 10.1016/s0361-9230(01)00558-5. [DOI] [PubMed] [Google Scholar]
- Fritzsch B, Beisel KW, Bermingham NA. Developmental evolutionary biology of the vertebrate ear: Conserving mechanoelectric transduction and developmental pathways in diverging morphologies. Neuroreport. 2000;11:R35–44. doi: 10.1097/00001756-200011270-00013. [DOI] [PubMed] [Google Scholar]
- Fritzsch B, Beisel KW, Jones K, Farinas I, Maklad A, et al. Development and evolution of inner ear sensory epithelia and their innervation. J Neurobiol. 2002;53:143–156. doi: 10.1002/neu.10098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritzsch B, Farinas I, Reichardt LF. Lack of neurotrophin 3 causes losses of both classes of spiral ganglion neurons in the cochlea in a region-specific fashion. J Neurosci. 1997;17:6213–6225. doi: 10.1523/JNEUROSCI.17-16-06213.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritzsch B, Nichols DH, Bermingham NA, Zoghbi HY, Wang VY, et al. Afferent projections in Math1 null mutants follow the limited expression of the neurotrophin BDNF in undifferentiated hair cell precursors. Development. 2003 (submitted for publication) [Google Scholar]
- Fritzsch B, Pirvola U, Ylikoski J. Making and breaking the innervation of the ear: Neurotrophic support during ear development and its clinical implications. Cell Tissue Res. 1999;295:369–382. doi: 10.1007/s004410051244. [DOI] [PubMed] [Google Scholar]
- Fritzsch B, Signore M, Simeone A. Otx1 null mutant mice show partial segregation of sensory epithelia comparable to lamprey ears. Dev Genes Evol. 2001a;211:388–396. doi: 10.1007/s004270100166. [DOI] [PubMed] [Google Scholar]
- Fritzsch B, Silos-Santiago I, Farinas I, Jones K. Neurotrophins and neurotrophin receptors involved in supporting afferent inner ear innervation. In: Mocchetti I, editor. The Neurotrophins. Salzburger and Graham; 2001b. [Google Scholar]
- Fritzsch B, Silos-Santiago I, Smeyne R, Fagan AM, Barbacid M. Reduction and loss of inner ear innervation in trkB and trkC receptor knockout mice: A whole mount DiI and scanning electron microscopic analysis. Audit Neurosci. 1995;1:401–417. [Google Scholar]
- Fritzsch B, Wake MH. The inner ear of gymnophione amphibians and its nerve supply: A comparative study of regressive events in a complex sensory system. Zoomorphol. 1988;108:210–217. [Google Scholar]
- George KM, Leonard MW, Roth ME, Lieuw KH, Kioussis D, et al. Embryonic expression and cloning of the murine GATA-3 gene. Development. 1994;120:2673–2686. doi: 10.1242/dev.120.9.2673. [DOI] [PubMed] [Google Scholar]
- Ghysen A, Dambly-Chaudiere C. A genetic programme for neuronal connectivity. Trends Genet. 2000;16:221–226. doi: 10.1016/s0168-9525(99)01969-1. [DOI] [PubMed] [Google Scholar]
- Gilmour DT, Maischein HM, Nusslein-Volhard C. Migration and function of a glial subtype in the vertebrate peripheral nervous system. Neuron. 2002;34:577–588. doi: 10.1016/s0896-6273(02)00683-9. [DOI] [PubMed] [Google Scholar]
- Gompel N, Dambly-Chaudiere C, Ghysen A. Neuronal differences prefigure somatotopy in the zebrafish lateral line. Development. 2001;128:387–393. doi: 10.1242/dev.128.3.387. [DOI] [PubMed] [Google Scholar]
- Goodyear RJ, Richardson GP. Extracellular matrices associated with the apical surfaces of sensory epithelia in the inner ear: Molecular and structural diversity. J Neurobiol. 2002;53:212–227. doi: 10.1002/neu.10097. [DOI] [PubMed] [Google Scholar]
- Gowan K, Helms AW, Hunsaker TL, Collisson T, Ebert PJ, et al. Cross-inhibitory activities of Ngn1 and Math1 allow specification of distinct dorsal interneurons. Neuron. 2001;31:219–232. doi: 10.1016/s0896-6273(01)00367-1. [DOI] [PubMed] [Google Scholar]
- Groves AK, Bronner-Fraser M. Competence, specification and commitment in otic placode induction. Development. 2000;127:3489–3499. doi: 10.1242/dev.127.16.3489. [DOI] [PubMed] [Google Scholar]
- Gu C, Reimert DC, Shu T, Fritzsch B, Richards LJ, et al. Neuropilin-1 integrates Semaphorin and VEGF signaling during neural and cardiovascular development. Developmental Cell. 2003;5:45–67. doi: 10.1016/s1534-5807(03)00169-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadrys T, Braun T, Rinkwitz-Brandt S, Arnold HH, Bober E. Nkx5-1 controls semicircular canal formation in the mouse inner ear. Development. 1998;125:33–39. doi: 10.1242/dev.125.1.33. [DOI] [PubMed] [Google Scholar]
- Hallbook F. Evolution of the vertebrate neurotrophin and Trk receptor gene families. Curr Opin Neurobiol. 1999;9:616–621. doi: 10.1016/S0959-4388(99)00011-2. [DOI] [PubMed] [Google Scholar]
- Hallbook F, Lundin LG, Kullander K. Lampetra fluviatilis neurotrophin homolog, descendant of a neurotrophin ancestor, discloses the early molecular evolution of neurotrophins in the vertebrate subphylum. J NeuroSci. 1998;18:8700–8711. doi: 10.1523/JNEUROSCI.18-21-08700.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson IM. Mammalian homologues of the Drosophila eye specification genes. Semin Cell Dev Biol. 2001;12:475–484. doi: 10.1006/scdb.2001.0271. [DOI] [PubMed] [Google Scholar]
- Hashino E, Dolnick RY, Cohan CS. Developing vestibular ganglion neurons switch trophic sensitivity from BDNF to GDNF after target innervation. J Neurobiol. 1999;38:414–427. doi: 10.1002/(sici)1097-4695(19990215)38:3<414::aid-neu9>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- Hatini V, Ye X, Balas G, Lai E. Dynamics of placodal lineage development revealed by targeted transgene expression. Dev Dyn. 1999;215:332–343. doi: 10.1002/(SICI)1097-0177(199908)215:4<332::AID-AJA5>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Holland LZ, Schubert M, Holland ND, Neuman T. Evolutionary conservation of the presumptive neural plate markers AmphiSox1/2/3 and AmphiNeurogenin in the invertebrate chordate amphioxus. Dev Biol. 2000;226:18–33. doi: 10.1006/dbio.2000.9810. [DOI] [PubMed] [Google Scholar]
- Huang EJ, Liu W, Fritzsch B, Bianchi LM, Reichardt LF, Xiang M. Brn3a is a transcriptional regulator of soma size, target field innervation and axon pathfinding of inner ear sensory neurons. Development. 2001;128:2421–2432. doi: 10.1242/dev.128.13.2421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X, Cheng HJ, Tessier-Lavigne M, Jin Y. MAX-1, a novel PH/MyTH4/FERM domain cytoplasmic protein implicated in netrin-mediated axon repulsion. Neuron. 2002;34:563–576. doi: 10.1016/s0896-6273(02)00672-4. [DOI] [PubMed] [Google Scholar]
- Hulander M, Kiernan AE, Blomqvist SR, Carlsson P, Samuelsson EJ, et al. Lack of pendrin expression leads to deafness and expansion of the endolymphatic compartment in inner ears of Foxil null mutant mice. Development. 2003;130:2013–2025. doi: 10.1242/dev.00376. [DOI] [PubMed] [Google Scholar]
- Hulander M, Wurst W, Carlsson P, Enerback S. The winged helix transcription factor Fkh10 is required for normal development of the inner ear. Nat Genet. 1998;20:374–376. doi: 10.1038/3850. [DOI] [PubMed] [Google Scholar]
- Imai KS, Satoh N, Satou Y. Region specific gene expressions in the central nervous system of the ascidian embryo. Gene Expr Patterns. 2002;2:319–321. doi: 10.1016/s0925-4773(02)00383-0. [DOI] [PubMed] [Google Scholar]
- Jaaro H, Beck G, Conticello SG, Fainzilber M. Evolving better brains: A need for neurotrophins? Trends NeuroSci. 2001;24:79–85. doi: 10.1016/s0166-2236(00)01690-8. [DOI] [PubMed] [Google Scholar]
- Johnson KR, Cook SA, Erway LC, Matthews AN, Sanford LP, et al. Inner ear and kidney anomalies caused by IAP insertion in an intron of the Eyal gene in a mouse model of BOR syndrome. Hum Mol Genet. 1999;8:645–653. doi: 10.1093/hmg/8.4.645. [DOI] [PubMed] [Google Scholar]
- Jones KR, Farinas I, Backus C, Reichardt LF. Targeted disruption of the BDNF gene perturbs brain and sensory neuron development but not motor neuron development. Cell. 1994;76:989–999. doi: 10.1016/0092-8674(94)90377-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorgensen JM. Evolution of octavolateralis sensory cells. In: Muenz, editor. The Mechanosensory Lateral Line. Neurobiology and Evolution. Springer Verlag; New York: 1989. pp. 99–115. [Google Scholar]
- Karis A, Pata I, van Doorninck JH, Grosveld F, de Zeeuw CI, et al. Transcription factor GATA-3 alters pathway selection of olivocochlear neurons and affects morphogenesis of the ear. J Comp Neurol. 2001;429:615–630. doi: 10.1002/1096-9861(20010122)429:4<615::aid-cne8>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Kim WY, Fritzsch B, Serls A, Bakel LA, Huang EJ, et al. NeuroD-null mice are deaf due to a severe loss of the inner ear sensory neurons during development. Development. 2001;128:417–426. doi: 10.1242/dev.128.3.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozmik Z, Holland ND, Kalousova A, Paces J, Schubert M, Holland LZ. Characterization of an amphioxus paired box gene, AmphiPax2/5/8: Developmental expression patterns in optic support cells, nephridium, thyroid-like structures and pharyngeal gill slits, but not in the midbrain-hindbrain boundary region. Development. 1999;126:1295–1304. doi: 10.1242/dev.126.6.1295. [DOI] [PubMed] [Google Scholar]
- Ladher RK, Anakwe KU, Gurney AL, Schoenwolf GC, Francis-West PH. Identification of synergistic signals initiating inner ear development. Science. 2000;290:1965–1967. doi: 10.1126/science.290.5498.1965. [DOI] [PubMed] [Google Scholar]
- Lawoko-Kerali G, Rivolta MN, Holley M. Expression of the transcription factors GATA3 and Pax2 during development of the mammalian inner ear. J Comp Neurol. 2002;442:378–391. doi: 10.1002/cne.10088. [DOI] [PubMed] [Google Scholar]
- Lee JE. Basic helix-loop-helix genes in neural development. Curr Opin Neurobiol. 1997;7:13–20. doi: 10.1016/s0959-4388(97)80115-8. [DOI] [PubMed] [Google Scholar]
- Lewis ER, Leverenz EL, Bialek WS. The Vertebrate Inner Ear. CRC Press; Boca Raton: 1985. [Google Scholar]
- Liebl DJ, Tessarollo L, Palko ME, Parada LF. Absence of sensory neurons before target innervation in brain-derived neurotrophic factor-, neurotrophin3-, and trkC-deficient embryonic mice. J NeuroSci. 1997;17:9113–9127. doi: 10.1523/JNEUROSCI.17-23-09113.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D, Chu H, Maves L, Yan YL, Morcos PA, et al. Fgf3 and Fgf8 dependent and independent transcription factors are required for otic placode specification. Development. 2003;130:2213–2224. doi: 10.1242/dev.00445. [DOI] [PubMed] [Google Scholar]
- Liu M, Pereira FA, Price SD, Chu MJ, Shope C, et al. Essential role of BETA2/NeuroD1 in development of the vestibular and auditory systems. Genes Dev. 2000;14:2839–2854. doi: 10.1101/gad.840500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorente de No R. Etudes sur l’anatomie et la physiologie du labyrinthe de l’oreille et du VIII nerf. II Quelques donnees au sujet de l’anatomie des organes sensoriels du labyrinthe. Trav Lab Rech Biol Univ Madrid. 1926;24:53–153. [Google Scholar]
- Lorente de No R. Anatomy of the eighth nerve: The central projections of the nerve endings of the internal ear. Laryngoscope. 1933;43:1–38. doi: 10.1097/00005537-199605000-00004. [DOI] [PubMed] [Google Scholar]
- Ma Q, Anderson DJ, Fritzsch B. Neurogenin 1 null mutant ears develop fewer, morphologically normal hair cells in smaller sensory epithelia devoid of innervation. J Assoc Res Otolaryngol. 2000;1:129–143. doi: 10.1007/s101620010017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Q, Chen Z, del Barco Barrantes I, de la Pompa JL, Anderson DJ. Neurogenin1 is essential for the determination of neuronal precursors for proximal cranial sensory ganglia. Neuron. 1998;20:469–482. doi: 10.1016/s0896-6273(00)80988-5. [DOI] [PubMed] [Google Scholar]
- Ma Q, Fode C, Guillemot F, Anderson DJ. Neurogenin1 and neurogenin2 control two distinct waves of neurogenesis in developing dorsal root ganglia. Genes Dev. 1999;13:1717–1728. doi: 10.1101/gad.13.13.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Q, Kintner C, Anderson DJ. Identification of neurogenin, a vertebrate neuronal determination gene. Cell. 1996;87:43–52. doi: 10.1016/s0092-8674(00)81321-5. [DOI] [PubMed] [Google Scholar]
- Mackie GO, Singla CL. The capsular organ of Chelyosoma productum (Ascidiacea: Corellidae): A new tunicate hydrodynamic sense organ. Brain Behav Evol. 2003;61:45–58. doi: 10.1159/000068878. [DOI] [PubMed] [Google Scholar]
- Maklad A, Fritzsch B. Incomplete segregation of endorgan-specific vestibular ganglion cells in mice and rats. J Vestib Res. 1999;9:387–399. [PubMed] [Google Scholar]
- Maklad A, Fritzsch B. The developmental segregation of posterior crista and saccular vestibular fibers in mice: A carbocyanine tracer study using confocal microscopy. Dev Brain Res. 2002;135:1–17. doi: 10.1016/s0165-3806(01)00327-3. [DOI] [PubMed] [Google Scholar]
- Manns M, Fritzsch B. The eye in the brain: Retinoic acid effects morphogenesis of the eye and pathway selection of axons but not the differentiation of the retina in Xenopus laevis. Neurosci Lett. 1991;127:150–154. doi: 10.1016/0304-3940(91)90782-o. [DOI] [PubMed] [Google Scholar]
- Mansour SL. Targeted disruption of int-2 (fgf-3) causes developmental defects in the tail and inner ear. Mol Reprod Dev. 1994;39:62–67. doi: 10.1002/mrd.1080390111. discussion 67–68. [DOI] [PubMed] [Google Scholar]
- Merlo GR, Paleari L, Mantero S, Zerega B, Adamska M, et al. The Dlx5 homeobox gene is essential for vestibular morphogenesis in the mouse embryo through a BMP4-mediated pathway. Dev Biol. 2002;248:157–169. doi: 10.1006/dbio.2002.0713. [DOI] [PubMed] [Google Scholar]
- Miyazaki N, Furuyama T, Takeda N, Inoue T, Kubo T, Inagaki S. Expression of mouse semaphorin H mRNA in the inner ear of mouse fetuses. Neurosci Lett. 1999;261:127–129. doi: 10.1016/s0304-3940(98)00988-4. [DOI] [PubMed] [Google Scholar]
- Morris JK, Lin W, Hauser C, Marchuk Y, Getman D, Lee KF. Rescue of the cardiac defect in ErbB2 mutant mice reveals essential roles of ErbB2 in peripheral nervous system development. Neuron. 1999;23:273–283. doi: 10.1016/s0896-6273(00)80779-5. [DOI] [PubMed] [Google Scholar]
- Morrison SJ, Perez SE, Qiao Z, Verdi JM, Hicks C, et al. Transient notch activation initiates an irreversible switch from neurogenesis to gliogenesis by neural crest stem cells. Cell. 2000;101:499–510. doi: 10.1016/s0092-8674(00)80860-0. [DOI] [PubMed] [Google Scholar]
- Morsli H, Choo D, Ryan A, Johnson R, Wu DK. Development of the mouse inner ear and origin of its sensory organs. J NeuroSci. 1998;18:3327–3335. doi: 10.1523/JNEUROSCI.18-09-03327.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami Y, Suto F, Shimizu M, Shinoda T, Kameyama T, Fujisawa H. Differential expression of plexin-A subfamily members in the mouse nervous system. Dev Dyn. 2001;220:246–258. doi: 10.1002/1097-0177(20010301)220:3<246::AID-DVDY1112>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Noramly S, Grainger RM. Determination of the embryonic inner ear. J Neurobiol. 2002;53:100–128. doi: 10.1002/neu.10131. [DOI] [PubMed] [Google Scholar]
- Norris HW. Studies on the development of the ear in Amblystoma I Development of the auditory vesicle. J Morphol. 1892;7:23–34. [Google Scholar]
- Northcutt RG, Brandle K, Fritzsch B. Electroreceptors and mechanosensory lateral line organs arise from single placodes in axolotls. Dev Biol. 1995;168:358–373. doi: 10.1006/dbio.1995.1086. [DOI] [PubMed] [Google Scholar]
- Pasterkamp RJ, Verhaagen J. Emerging roles for semaphorins in neural regeneration. Brain Res Brain Res Rev. 2001;35:36–54. doi: 10.1016/s0165-0173(00)00050-3. [DOI] [PubMed] [Google Scholar]
- Pauley S, Wright TJ, Pirvola U, Ornitz D, Beisel K, Fritzsch B. Expression and function of FGF10 in mammalian inner ear development. Dev Dyn. 2003;227:203–215. doi: 10.1002/dvdy.10297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfister M, Toth T, Thiele H, Haack B, Blin N, et al. A 4-bp insertion in the eya-homologous region (eyaHR) of EYA4 causes hearing impairment in a Hungarian family linked to DFNA10. Mol Med. 2002;8:607–611. [PMC free article] [PubMed] [Google Scholar]
- Phillips BT, Bolding K, Riley BB. Zebrafish fgf3 and fgf8 encode redundant functions required for otic placode induction. Dev Biol. 2001;235:351–365. doi: 10.1006/dbio.2001.0297. [DOI] [PubMed] [Google Scholar]
- Pichaud F, Desplan C. Pax genes and eye organogenesis. Curr Opin Genet Dev. 2002;12:430–434. doi: 10.1016/s0959-437x(02)00321-0. [DOI] [PubMed] [Google Scholar]
- Pirvola U, Spencer-Dene B, Xing-Qun L, Kettunen P, Thesleff I, et al. FGF/FGFR-2(IIIb) signaling is essential for inner ear morphogenesis. J NeuroSci. 2000;20:6125–6134. doi: 10.1523/JNEUROSCI.20-16-06125.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirvola U, Ylikoski J, Palgi J, Lehtonen E, Arumae U, Saarma M. Brain-derived neurotrophic factor and neurotrophin 3 mRNAs in the peripheral target fields of developing inner ear ganglia. Proc Natl Acad Sci USA. 1992;89:9915–9919. doi: 10.1073/pnas.89.20.9915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirvola U, Ylikoski J, Trokovic R, Hebert J, McConnell S, Partanen J. FGFR1 is required for the development of the auditory sensory epithelium. Neuron. 2002;35:671. doi: 10.1016/s0896-6273(02)00824-3. [DOI] [PubMed] [Google Scholar]
- Qian Y, Fritzsch B, Shirasawa S, Chen CL, Choi Y, Ma Q. Formation of brainstem (nor)adrenergic centers and first-order relay visceral sensory neurons is dependent on homeodomain protein Rnx/Tlx3. Genes Dev. 2001;15:2533–2545. doi: 10.1101/gad.921501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy GV, Rodrigues V. A glial cell arises from an additional division within the mechanosensory lineage during development of the microchaete on the Drosophila notum. Development. 1999;126:4617–4622. doi: 10.1242/dev.126.20.4617. [DOI] [PubMed] [Google Scholar]
- Riccomagno MM, Martinu L, Mulheisen M, Wu DK, Epstein DJ. Specification of the mammalian cochlea is dependent on sonic hedgehog. Genes Dev. 2002;16:2365–2378. doi: 10.1101/gad.1013302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riedl R. Order in Living Systems: A Systems Analysis of Evolution. Wiley; New York: 1978. [Google Scholar]
- Rivolta MN, Halsall A, Johnson CM, Tones MA, Holley MC. Transcript profiling of functionally related groups of genes during conditional differentiation of a mammalian cochlear hair cell line. Genome Res. 2002;12:1091–1099. doi: 10.1101/gr.225602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubel EW, Fritzsch B. Auditory system development: Primary auditory neurons and their targets. Annu Rev NeuroSci. 2002;25:51–101. doi: 10.1146/annurev.neuro.25.112701.142849. [DOI] [PubMed] [Google Scholar]
- Ruben RJ. Development of the inner ear of the mouse: A radioautographic study of terminal mitoses. Acta Otolaryngol Suppl. 1967;220:221–244. [PubMed] [Google Scholar]
- Salazar-Ciudad I, Jernvall J, Newman SA. Mechanisms of pattern formation in development and evolution. Development. 2003;130:2027–2037. doi: 10.1242/dev.00425. [DOI] [PubMed] [Google Scholar]
- Sarasin P, Sarasin F. Über das Gehörorgan der Caeciliiden. Anat Anz. 1892;7:812–815. [Google Scholar]
- Sato M, Saigo K. Involvement of pannier and u-shaped in regulation of decapentaplegic-dependent wingless expression in developing Drosophila notum. Mech Dev. 2000;93:127–138. doi: 10.1016/s0925-4773(00)00282-3. [DOI] [PubMed] [Google Scholar]
- Satou Y, Imai KS, Satoh N. Fgf genes in the basal chordate Ciona intestinalis. Dev Genes Evol. 2002;212:432–438. doi: 10.1007/s00427-002-0266-8. [DOI] [PubMed] [Google Scholar]
- Schimmang T, Minichiello L, Vazquez E, San Jose I, Giraldez F, et al. Developing inner ear sensory neurons require TrkB and TrkC receptors for innervation of their peripheral targets. Development. 1995;121:3381–3389. doi: 10.1242/dev.121.10.3381. [DOI] [PubMed] [Google Scholar]
- Schlosser G. Development and evolution of lateral line placodes in amphibians. I Development. Zoology. 2002;105:119–146. doi: 10.1078/0944-2006-00058. [DOI] [PubMed] [Google Scholar]
- Sen A, Reddy GV, Rodrigues V. Combinatorial expression of Prospero, Seven-up, and Elav identifies progenitor cell types during sense-organ differentiation in the Drosophila antenna. Dev Biol. 2003;254:79–92. doi: 10.1016/s0012-1606(02)00021-0. [DOI] [PubMed] [Google Scholar]
- Shimeld SM, Holland PW. Vertebrate innovations. Proc Natl Acad Sci USA. 2000;97:4449–4452. doi: 10.1073/pnas.97.9.4449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silos-Santiago I, Fagan AM, Garber M, Fritzsch B, Barbacid M. Severe sensory deficits but normal CNS development in newborn mice lacking TrkB and TrkC tyrosine protein kinase receptors. Eur J NeuroSci. 1997;9:2045–2056. doi: 10.1111/j.1460-9568.1997.tb01372.x. [DOI] [PubMed] [Google Scholar]
- Simmler MC, Cohen-Salmon M, El-Amraoui A, Guillaud L, Benichou JC, et al. Targeted disruption of otog results in deafness and severe imbalance. Nat Genet. 2000;24:139–143. doi: 10.1038/72793. [DOI] [PubMed] [Google Scholar]
- Solomon KS, Fritz A. Concerted action of two dlx paralogs in sensory placode formation. Development. 2002;129:3127–3136. doi: 10.1242/dev.129.13.3127. [DOI] [PubMed] [Google Scholar]
- Solomon KS, Kudoh T, Dawid IB, Fritz A. Zebrafish foxil mediates otic placode formation and jaw development. Development. 2003;130:929–940. doi: 10.1242/dev.00308. [DOI] [PubMed] [Google Scholar]
- Strassmaier M, Gillespie PG. The hair cell’s transduction channel. Curr Opin Neurobiol. 2002;12:380–386. doi: 10.1016/s0959-4388(02)00344-6. [DOI] [PubMed] [Google Scholar]
- Streit A. Extensive cell movements accompany formation of the otic placode. Dev Biol. 2002;249:237–254. doi: 10.1006/dbio.2002.0739. [DOI] [PubMed] [Google Scholar]
- Suto F, Murakami Y, Nakamura F, Goshima Y, Fujisawa H. Identification and characterization of a novel mouse plexin, plexin-A4. Mech Dev. 2003;120:385–396. doi: 10.1016/s0925-4773(02)00421-5. [DOI] [PubMed] [Google Scholar]
- Tessier-Lavigne M, Goodman CS. The molecular biology of axon guidance. Science. 1996;274:1123–1133. doi: 10.1126/science.274.5290.1123. [DOI] [PubMed] [Google Scholar]
- Toresson H, Martinez-Barbera JP, Bardsley A, Caubit X, Krauss S. Conservation of BF-1 expression in amphioxus and zebrafish suggests evolutionary ancestry of anterior cell types that contribute to the vertebrate telencephalon. Dev Genes Evol. 1998;208:431–439. doi: 10.1007/s004270050200. [DOI] [PubMed] [Google Scholar]
- Torres M, Giraldez F. The development of the vertebrate inner ear. Mech Dev. 1998;71:5–21. doi: 10.1016/s0925-4773(97)00155-x. [DOI] [PubMed] [Google Scholar]
- van Bergeijk WA. Evolution of the sense of hearing in vertebrates. Am Zoologist. 1966;6:371–377. doi: 10.1093/icb/6.3.371. [DOI] [PubMed] [Google Scholar]
- Van Esch H, Groenen P, Nesbit MA, Schuffenhauer S, Lichtner P, et al. GATA3 haplo-insufficiency causes human HDR syndrome. Nature. 2000;406:419–422. doi: 10.1038/35019088. [DOI] [PubMed] [Google Scholar]
- Vendrell V, Carnicero E, Giraldez F, Alonso MT, Schimmang T. Induction of inner ear fate by FGF3. Development. 2000;127:2011–2019. doi: 10.1242/dev.127.10.2011. [DOI] [PubMed] [Google Scholar]
- Venter JC, Adams MD, Myers EW, Li PW, Mural RJ, et al. The sequence of the human genome. Science. 2001;291:1304–1351. doi: 10.1126/science.1058040. [DOI] [PubMed] [Google Scholar]
- Vincent C, Kalatzis V, Abdelhak S, Chaib H, Compain S, et al. BOR and BO syndromes are allelic defects of EYA1. Eur J Hum Genet. 1997;5:242–246. [PubMed] [Google Scholar]
- Wada H, Saiga H, Satoh N, Holland PW. Tripartite organization of the ancestral chordate brain and the antiquity of placodes: Insights from ascidian Pax-2/5/8, Hox and Otx genes. Development. 1998;125:1113–1122. doi: 10.1242/dev.125.6.1113. [DOI] [PubMed] [Google Scholar]
- Walker RG, Willingham AT, Zuker CS. A Drosophila mechanosensory transduction channel. Science. 2000;287:2229–2234. doi: 10.1126/science.287.5461.2229. [DOI] [PubMed] [Google Scholar]
- Wang VY, Hassan BA, Bellen HJ, Zoghbi HY. Drosophila atonal fully rescues the phenotype of Math1 null mice: New functions evolve in new cellular contexts. Curr Biol. 2002;12:1611–1616. doi: 10.1016/s0960-9822(02)01144-2. [DOI] [PubMed] [Google Scholar]
- Wayne S, Robertson NG, DeClau F, Chen N, Verhoeven K, et al. Mutations in the transcriptional activator EYA4 cause late-onset deafness at the DFNA10 locus. Hum Mol Genet. 2001;10:195–200. doi: 10.1093/hmg/10.3.195. [DOI] [PubMed] [Google Scholar]
- Wever EG. The evolution of vertebrate hearing. In: Neff WD, editor. Auditory System, Vol. V/1, Handbook of Sensory Physiology. Springer Verlag; Berlin: 1974. pp. 423–454. [Google Scholar]
- Wheeler EF, Bothwell M, Schecterson LC, von Bartheld CS. Expression of BDNF and NT-3 mRNA in hair cells of the organ of Corti: Quantitative analysis in developing rats. Hear Res. 1994;73:46–56. doi: 10.1016/0378-5955(94)90281-x. [DOI] [PubMed] [Google Scholar]
- Wu DK, Oh SH. Sensory organ generation in the chick inner ear. J NeuroSci. 1996;16:6454–6462. doi: 10.1523/JNEUROSCI.16-20-06454.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu HH, Ivkovic S, Murray RC, Jaramillo S, Lyons KM, et al. Autoregulation of neurogenesis by GDF11. Neuron. 2003;37:197–207. doi: 10.1016/s0896-6273(02)01172-8. [DOI] [PubMed] [Google Scholar]
- Xiang M, Gao WQ, Hasson T, Shin JJ. Requirement for Brn-3c in maturation and survival, but not in fate determination of inner ear hair cells. Development. 1998;125:3935–3946. doi: 10.1242/dev.125.20.3935. [DOI] [PubMed] [Google Scholar]
- Xiang M, Maklad A, Pirvola U, Fritzsch B. Brn3c null mutant mice show long-term, incomplete retention of some afferent inner ear innervation. BMC Neurosci. 2003 Jan 30; doi: 10.1186/1471-2202-4-2. (e-pub) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu PX, Adams J, Peters H, Brown MC, Heaney S, Maas R. Eya1-deficient mice lack ears and kidneys and show abnormal apoptosis of organ primordia. Nat Genet. 1999;23:113–117. doi: 10.1038/12722. [DOI] [PubMed] [Google Scholar]
- Xu PX, Woo I, Her H, Beier DR, Maas RL. Mouse Eya homologues of the Drosophila eyes absent gene require Pax6 for expression in lens and nasal placode. Development. 1997;124:219–231. doi: 10.1242/dev.124.1.219. [DOI] [PubMed] [Google Scholar]
- Yang Q, Bermingham NA, Finegold MJ, Zoghbi HY. Requirement of Math1 for secretory cell lineage commitment in the mouse intestine. Science. 2001;294:2155–2158. doi: 10.1126/science.1065718. [DOI] [PubMed] [Google Scholar]
- Yu JK, Holland ND, Holland LZ. An amphioxus winged helix/forkhead gene, AmphiFoxD: Insights into vertebrate neural crest evolution. Dev Dyn. 2002;225:289–297. doi: 10.1002/dvdy.10173. [DOI] [PubMed] [Google Scholar]
- Zheng WL, Huang L, Wei ZB, Silvius D, Tang B, Xu PX. The role of six1 in mammalian auditory system development. Development. 2003;130:3989–4000. doi: 10.1242/dev.00628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Q, Anderson DJ. The bHLH transcription factors OLIG2 and OLIG1 couple neuronal and glial subtype specification. Cell. 2002;109:61–73. doi: 10.1016/s0092-8674(02)00677-3. [DOI] [PubMed] [Google Scholar]
- Zine A, Aubert A, Qiu J, Therianos S, Guillemot F, Kageyama R, de Ribaupierre F. Hes1 and Hes5 activities are required for the normal development of the hair cells in the mammalian inner ear. J NeuroSci. 2001;21:4712–4720. doi: 10.1523/JNEUROSCI.21-13-04712.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]