Abstract
A common complication associated with aging is the stiffening of skeletal muscles. The purpose of this study was to determine the ability of magnetic resonance elastography (MRE) to study this phenomenon in vivo. Twenty female subjects were included in the study with an age range of 50 to 70 years. Shear modulus was calculated for the tibialis anterior of each subject. There was not a significant relationship between age and shear modulus. However, three subjects had abnormally high values and were among the oldest subjects tested. There was a significant relationship between age and tissue stiffness homogeneity. More research is needed to determine whether the changes seen here are reflective of increased tissue cross-linking or related to reduced muscle quality. However, MRE shows promise as a tool to study aging-related muscle stiffness changes or to evaluate treatments to counteract these changes.
Keywords: advanced glycation end products, biomechanics, aging, MRE
A common complication associated with aging is the stiffening of skeletal muscles. This has been confirmed by several experimental studies (e.g., Gajdosik et al., 2005). There are two primary sources for passive tension in a muscle: the intracellular titin and the extra-cellular collagen matrix (Prado et al. 2005). An increase in muscle stiffness as a result of aging would therefore likely be a result of stiffening of one of these sources of passive tension.
One potential mechanism that may cause increased muscle stiffness is the accumulation of advanced glycation end products (AGEs). It is known that during aging AGEs result in cross-linking of collagen fibers in tissues throughout the body (Avery & Bailey, 2005). These cross-links result in increased tissue stiffness. Negative effects of this stiffening have been seen in numerous tissues, including cardiac muscle (Asif et al., 2000), bone (Vashishth et al., 2001), and articular cartilage (Verzijl et al., 2000). Cross-links have also been shown to accumulate in the skeletal muscle of aged rats (Gosselin et al., 1998).
Stiffening of the collagen matrix of a muscle could explain aging-related increases in muscle stiffness, and an accumulation of AGEs may also explain reduced muscle quality that some authors have seen in aged muscle (e.g., Lynch et al., 1999). It is known that the formation of cross-links makes collagen more resistant to enzyme activity (Schnider & Kohn, 1982). Normally, matrix metalloproteinases act to break down damaged extracellular matrix (Birkedal-Hansen, 1995) as part of the muscle-remodeling process. This process would be impaired as a result of cross-link formation. Advanced glycation end products are also known to up-regulate insulin-like growth) factor-binding protein-related protein-2, which is known to stimulate the production of extracellular matrix (Twigg et al., 2001). Collectively, these consequences will result in the increase in the amount of connective tissue within a muscle and may explain the reduction in muscle quality known to accompany aging.
Magnetic resonance elastography (MRE) is a imaging technique that allows measurement of tissue shear modulus in vivo (Muthupillai et al., 1995). In this technique, a small-amplitude shear wave is induced in a tissue. At the same time, a motion-sensitizing gradient synchronized with the shear wave is applied. From this process, a phase shift can be measured using phase-contrast MRI. The displacement at each voxel can be directly calculated from this phase shift, and the shear wave can be imaged as it moves through the tissue. An inversion technique can then be performed to determine shear modulus from the displacement data.
The purpose of the following study was to test the ability of MRE to determine age-related changes in muscle stiffness, and therefore collagen cross-link concentration, in vivo.
Methods
Twenty female subjects were included in the study with an age range of 50 to 70 years. All subjects were free from any orthopedic, muscular, or neurologic pathology affecting the lower limb. All procedures were reviewed and approved by the Mayo Clinic Institutional Review Board. Scans were performed with the subject lying supine and the foot strapped into a custom-built MR safe jig. The device allowed for fixation of the ankle joint at a given joint angle, and all scans were conducted with the ankle fixed at 20° of plantar flexion. Subjects were instructed to relax all muscles during the scans. The footplate of the jig was fitted with a torque sensor, and ankle torque was recorded throughout the trials to confirm that the subjects remained relaxed.
Vibration was applied by a small bar tapper to the distal end of the tibialis anterior muscle belly. The tapper was oriented parallel to the long axis of the leg and secured tightly in place with a Velcro band. The tapper was pneumatically powered and comprised two components. The active component was an acoustic speaker operating at 100 Hz. The passive component was made up of a cylindrical chamber with one end being a flexible drum connected to a rigid bar tapper and the other end connected to the active component via a long rubber hose. The resulting vibration produced shear waves with amplitudes on the order of micrometers.
A gradient echo sequence was used to record a series of axial scout images of the shank. From these images, a sagittal plane was selected that passed through the center of the tibialis anterior, and MRE scans were performed in this plane (Figure 1). The scans produce an image file and a motion file (Figure 2). Two additional files are created through processing: a phase difference signal to noise plot and a modulus map (Figure 2). Before processing, the data were phase unwrapped and a directional filter was applied (Manduca et al., 2003). The phase of each pixel was determined by extracting the harmonic component at 100 Hz. Shear modulus was calculated at each pixel by determining the phase gradient for a small window around each point (Manduca et al., 2001), converting the phase gradient into a spatial frequency, and using the following equation.
where G = shear modulus, ρ = tissue density (assumed to be 1000 kg/m3), f = vibration frequency, and K = spatial frequency.
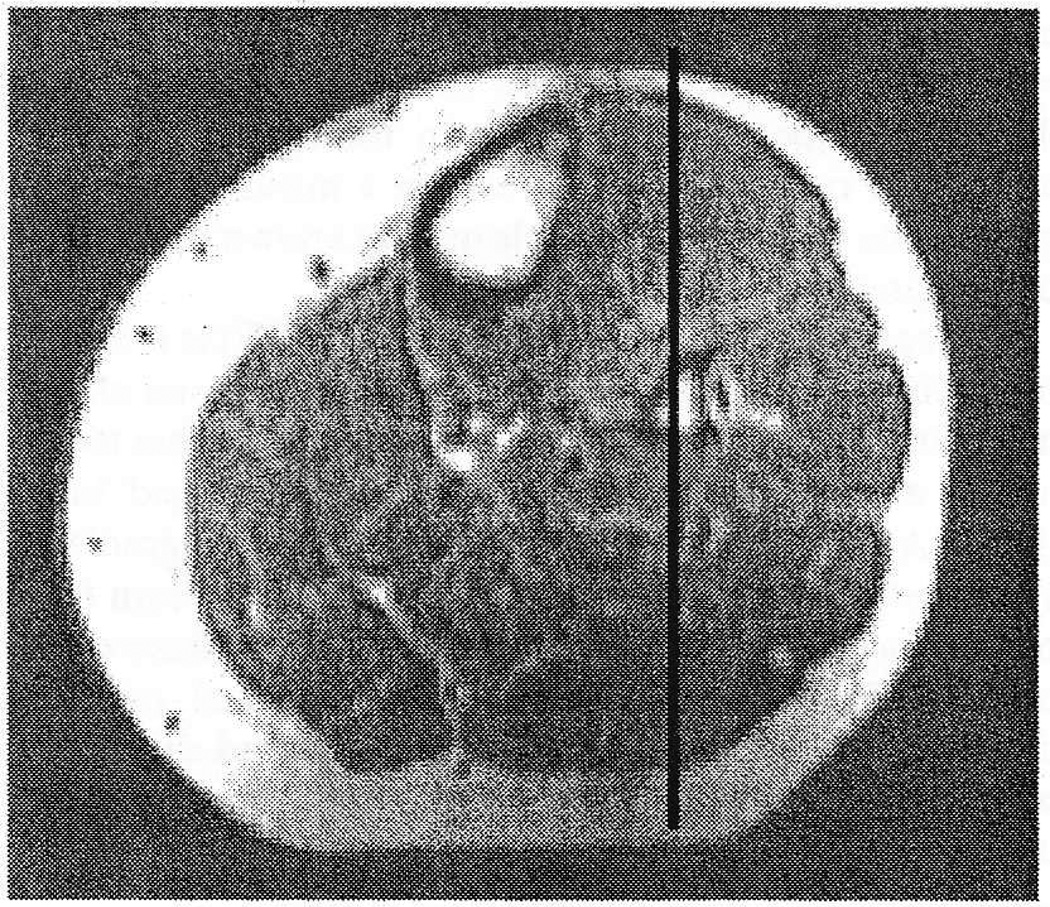
Figure 1.
Sagittal image of the shank showing location of the scan plane.
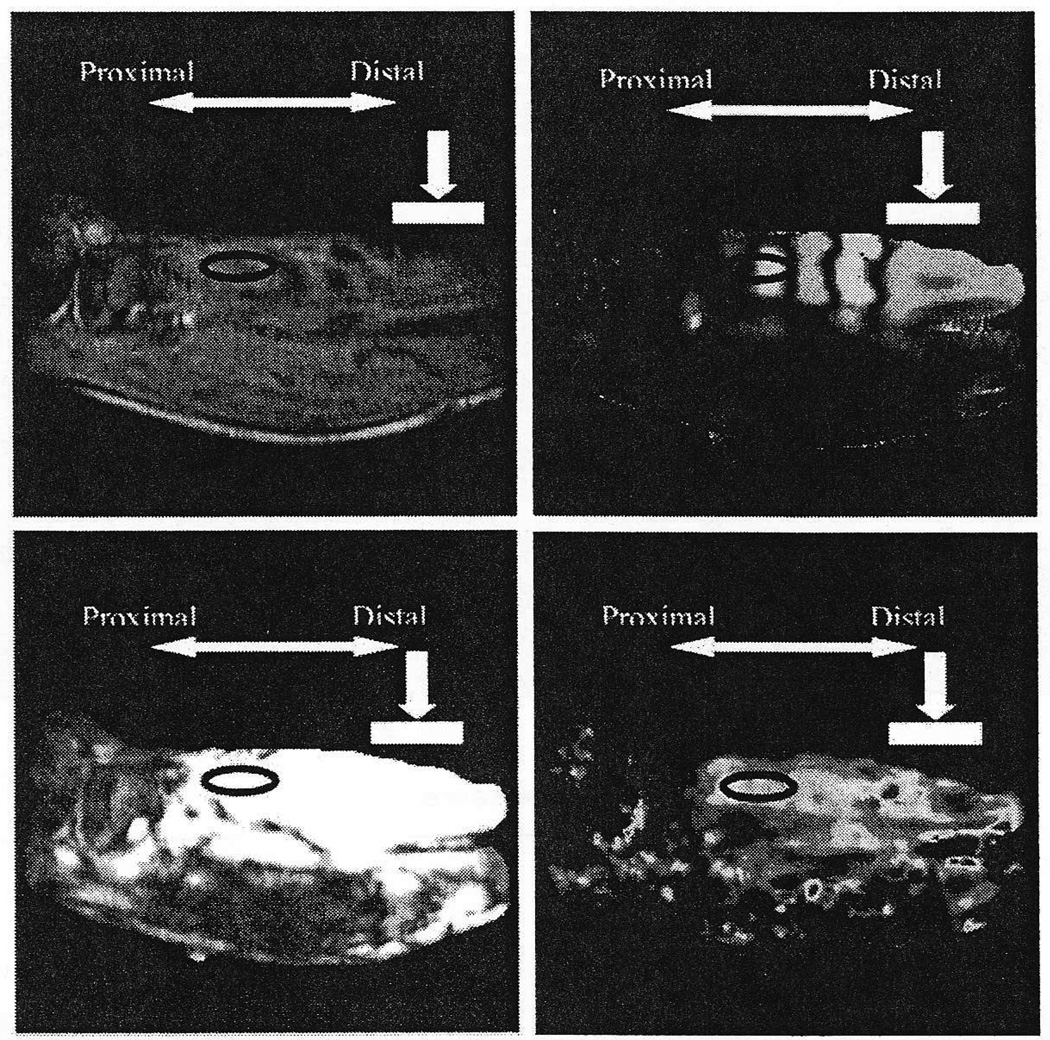
Figure 2.
Typical output from a MRE scan. The white bar indicates tapper location. The black oval indicates the region where modulus was calculated. Top left: image file. Top right: motion file. Bottom left: phase difference signal to noise plot. The white region indicated a SNR of 10 or above. Bottom right: modulus map.
Only the modulus of a region of interest was used to characterize the tibialis anterior (Figure 2). The image file is used to identify the boundaries of the tibialis anterior. The region of interest was centered in the muscle in the anterior-posterior direction. The superior-inferior location of this region was selected based on balancing two opposing criteria. First, it was desirable to place the region proximally because the area near the tapper has higher noise as a result of longitudinal waves. However, as the region is moved proximally away from the vibration source, the magnitude of the signal is diminished. To ensure the region was placed such that appropriate signal remained, a phase difference signal-to-noise ratio was calculated by using measurements from the back-ground to estimate noise. The region was placed as proximally as possible while remaining in the area where the signal-to-noise ratio was above 10.
The magnitude of the shear modulus within the muscle was calculated as the mean value for each voxel within the region of interest. The standard deviation of the moduli within this region was used as a measure of tissue homogeneity. Linear regression analysis was used to determine whether there was a significant relationship between age and shear modulus and between age and tissue homogeneity.
Results
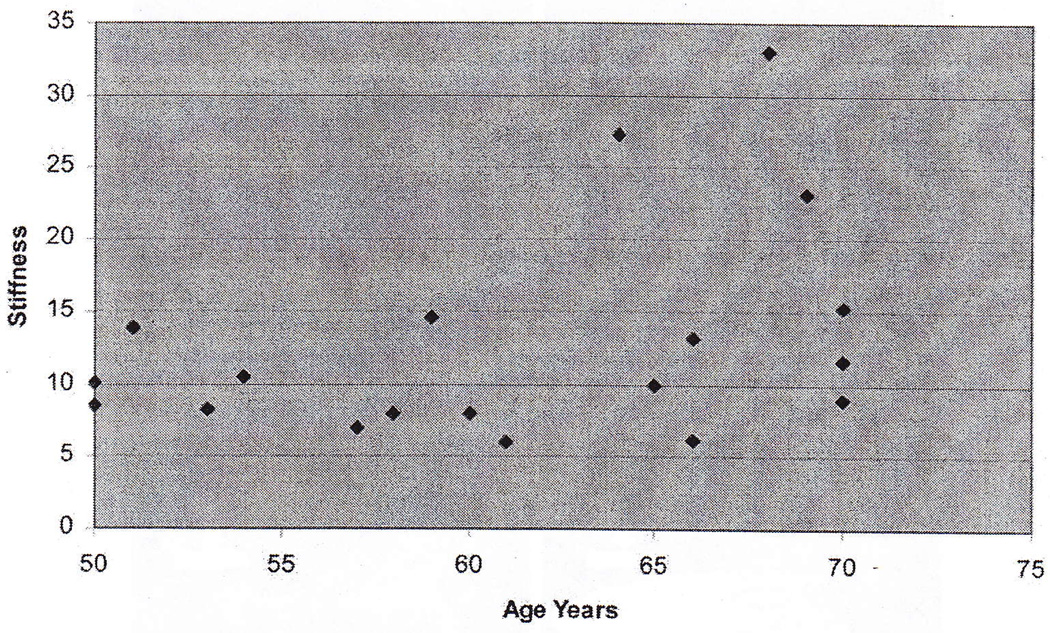
There was not a significant relationship between age and shear modulus (Figure 3). The mean modulus was 12.8 ± 7.4 kPa. However, three of the subjects appeared to be outliers. These three subjects had values for modulus that were 1.3, 2.0, and 2.8 times the interquartile range above the third quartile. The mean modulus for these thee subjects was 27.8 ± 5.0 kPa. They had a mean age of 67 ± 2.6 years. The mean modulus of the remaining 16 subjects was 10.0 ± 2.9 kPa. They had a mean age of 60 ± 7.2 years.
Figure 3.
Shear modulus vs. age.
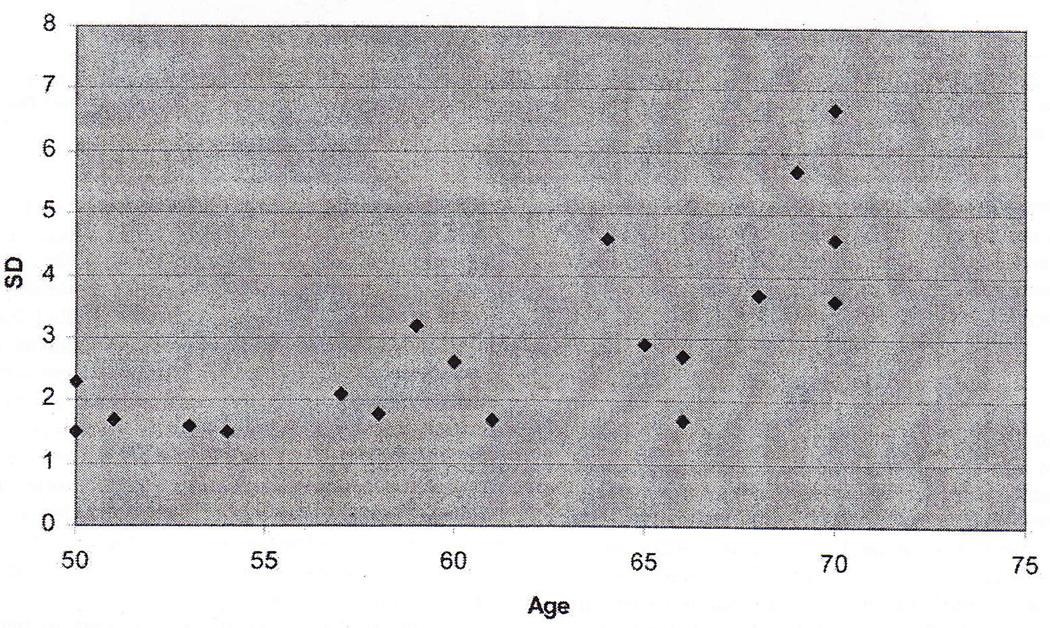
There was a significant relationship (p < .05) between age and the standard deviation of the modulus (Figure 4). The R2 value for a linear fit to the data was 0.53. However, from visual inspection of the data, it appears the relationship may in fact be flat until approximately age 55.
Figure 4.
Standard deviation of shear modulus vs. age.
Discussion
The purpose of this study was to determine whether MRE is sensitive enough to detect age-related changes in muscle stiffness. While there was not a significant relationship between age and shear modulus, MRE was able to identify individuals who were outside of the normal range of muscle stiffness. There was a significant relationship between age and tissue homogeneity, as represented by the standard deviation of shear modulus, potentially indicating that this is a more sensitive measure for evaluating age-related changes in muscle.
The lack of a significant relationship between age and shear modulus is not entirely surprising. A number of other cofactors are likely to contribute to muscle stiffness, most notably physical activity levels (Gosselin et al., 1998). Controlling for physical activity would likely strengthen the relationship between age and stiffness; In addition, the youngest subject in which abnormal stiffness was detected was 64 years old. If older subjects were included in the study, a clearer relationship may have been seen. However, it should be noted that the three oldest subjects tested were within the normal range for shear modulus. Given that cofactors were not considered, the relationship between age and the standard deviation of the shear modulus is relatively strong, potentially indicating that this is a more sensitive measure for age-related changes in skeletal muscle. It also should be noted that although the three oldest subjects tested were within the normal range for shear modulus, they all had elevated values for standard deviation of shear modulus.
There was not a significant relationship between age and shear modulus. However, all subjects with high values were among the oldest. The standard deviation of the modulus did increase with age, indicating a less homogeneous tissue. More research is needed to determine whether the changes seen here are reflective of increased tissue cross-linking and related to reduced muscle quality. Future studies should include a larger age range and account for physical activity level as a cofactor. However, MRE shows promise as a tool to study development of cross-links in muscle or to evaluate treatments.
Acknowledgments
This study was supported by grants from NIBIB R01 EB 00812 and NICHD T32 HD 07447. We thank Tom Hulshizer for his technical help and David Lake for his help with data processing.
References
- Asif M, Egan J, Vasan S, Jyothirmayi GN, Masurekar MR, Lopez S, et al. An advanced glycation endproduct cross-link breaker can reverse age-related increases in myocardial stiffness. Proceedings of the National Academy of Sciences of the United States of America. 2000;97(6):2809–2813. doi: 10.1073/pnas.040558497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avery NC, Bailey AJ. Enzymic and non-enzymic cross-linking mechanisms in relation to turnover of collagen: relevance to aging and exercise. Scandinavian Journal of Medicine & Science in Sports. 2005;15(4):231–240. doi: 10.1111/j.1600-0838.2005.00464.x. [DOI] [PubMed] [Google Scholar]
- Birkedal-Hansen H. Proteolytic remodeling of extra-cellular matrix. Current Opinion in Cell Biology. 1995;7(5):728–733. doi: 10.1016/0955-0674(95)80116-2. [DOI] [PubMed] [Google Scholar]
- Gajdosik RL, Vander Linden DW, McNair PJ, Riggin TJ, Albertson JS, Mattick DJ, et al. Viscoelastic properties of short calf muscle-tendon units of older women: effects of slow and fast passive dorsiflexion stretches in vivo. European Journal of Applied Physiology. 2005;95(2–3):131–139. doi: 10.1007/s00421-005-1394-4. [DOI] [PubMed] [Google Scholar]
- Gosselin LE, Adams C, Cotter TA, McCormick RJ, Thomas DP. Effect of exercise training on passive stiffness in locomotor skeletal muscle: role of extra-cellular matrix. J Appl Physiol. 1998;85(3):1011–1016. doi: 10.1152/jappl.1998.85.3.1011. [DOI] [PubMed] [Google Scholar]
- Lynch NA, Metter EJ, Lindle RS, Fozard JL, Tobin JD, Roy TA, et al. Muscle quality. I. Age-associated differences between arm and leg muscle groups. J Appl Physiol. 1999;86(1):188–194. doi: 10.1152/jappl.1999.86.1.188. [DOI] [PubMed] [Google Scholar]
- Manduca A, Lake DS, Kruse SA, Ehman RL. Spatio-temporal directional filtering for improved inversion of MR elastography images. Medical Image Analysis. 2003;7(4):465–473. doi: 10.1016/s1361-8415(03)00038-0. [DOI] [PubMed] [Google Scholar]
- Manduca A, Oliphant TE, Dresner MA, Mahowald JL, Kruse SA, Amromin E, et al. Magnetic resonance elastography: non-invasive mapping of tissue elasticity. Medical Image Analysis. 2001;5(4):237–254. doi: 10.1016/s1361-8415(00)00039-6. [DOI] [PubMed] [Google Scholar]
- Muthupillai R, Lomas DJ, Rossman PJ, Greenleaf JF, Manduca A, Ehman RL. Magnetic resonance elastography by direct visualization of propagating acoustic strain waves. Science. 1995;269(5232):1854–1857. doi: 10.1126/science.7569924. [DOI] [PubMed] [Google Scholar]
- Prado LG, Makarenko I, Andresen C, Kruger M, Opitz CA, Linke WA. Isoform diversity of giant proteins in relation to passive and active contractile properties of rabbit skeletal muscles. The Journal of General Physiology. 2005;126(5):461–480. doi: 10.1085/jgp.200509364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnider SL, Kohn RR. Effects of age and diabetes mellitus on the solubility of collagen from human skin, tracheal cartilage and dura mater. Experimental Gerontology. 1982;17(3):185–194. doi: 10.1016/0531-5565(82)90024-9. [DOI] [PubMed] [Google Scholar]
- Twigg SM, Chen MM, Joly AH, Chakrapani SD, Tsubaki J, Kim HS, et al. Advanced glycosylation end products up-regulate connective tissue growth factor (insulin-like growth factor-binding protein-related protein 2) in human fibroblasts: a potential mechanism for expansion of extracellular matrix in diabetes mellitus. Endocrinology. 2001;142(5):1760–1769. doi: 10.1210/endo.142.5.8141. [DOI] [PubMed] [Google Scholar]
- Vashishth D, Gibson GJ, Khoury JI, Schaffler MB, Kimura J, Fyhrie DP. Influence of nonenzymatic glycation on biomechanical properties of cortical bone. Bone. 2001;28(2):195–201. doi: 10.1016/s8756-3282(00)00434-8. [DOI] [PubMed] [Google Scholar]
- Verzijl N, DeGroot J, Oldehinkel E, Bank RA, Thorpe SR, Baynes JW, et al. Age-related accumulation of Maillard reaction products in human articular cartilage collagen. The Biochemical Journal. 2000;350(Pt 2):381–387. [PMC free article] [PubMed] [Google Scholar]