Abstract
By using interactive computer graphics, two models for calmodulin have been constructed based on the structures of two functionally and structurally related proteins, intestinal calcium-binding protein and carp parvalbumin. The two models have been compared and contrasted to the parent proteins with respect to proportion of solvent-exposed hydrophobic residues, solvent-accessible surface area, and side-chain packing. Electrostatic potential surfaces generated for the models suggest a probable binding site for basic amphiphilic alpha-helical peptides located between the last E and F helices in the second domain of calmodulin. Both electrostatic and hydrophobic complementarity can contribute to stabilization of a peptide-protein complex in this region.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aulabaugh A., Niemczura W. P., Blundell T. L., Gibbons W. A. A study of the interactions between residues in the C-terminal half of calmodulin by one and two-dimensional NMR methods and computer modelling. Eur J Biochem. 1984 Sep 3;143(2):409–418. doi: 10.1111/j.1432-1033.1984.tb08388.x. [DOI] [PubMed] [Google Scholar]
- Chothia C. Structural invariants in protein folding. Nature. 1975 Mar 27;254(5498):304–308. doi: 10.1038/254304a0. [DOI] [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Prediction of the secondary structure of proteins from their amino acid sequence. Adv Enzymol Relat Areas Mol Biol. 1978;47:45–148. doi: 10.1002/9780470122921.ch2. [DOI] [PubMed] [Google Scholar]
- Cook W. J., Dedman J. R., Means A. R., Bugg C. E. Crystallization and preliminary X-ray investigation of calmodulin. J Biol Chem. 1980 Sep 10;255(17):8152–8153. [PubMed] [Google Scholar]
- Cox J. A., Comte M., Fitton J. E., DeGrado W. F. The interaction of calmodulin with amphiphilic peptides. J Biol Chem. 1985 Feb 25;260(4):2527–2534. [PubMed] [Google Scholar]
- Gehrig L. M., Delbaere L. T., Hickie R. A. Preliminary X-ray data for the calmodulin/trifluoperazine complex. J Mol Biol. 1984 Aug 15;177(3):559–561. doi: 10.1016/0022-2836(84)90299-7. [DOI] [PubMed] [Google Scholar]
- Getzoff E. D., Tainer J. A., Weiner P. K., Kollman P. A., Richardson J. S., Richardson D. C. Electrostatic recognition between superoxide and copper, zinc superoxide dismutase. Nature. 1983 Nov 17;306(5940):287–290. doi: 10.1038/306287a0. [DOI] [PubMed] [Google Scholar]
- Greer J. Comparative model-building of the mammalian serine proteases. J Mol Biol. 1981 Dec 25;153(4):1027–1042. doi: 10.1016/0022-2836(81)90465-4. [DOI] [PubMed] [Google Scholar]
- Herzberg O., James M. N. Structure of the calcium regulatory muscle protein troponin-C at 2.8 A resolution. Nature. 1985 Feb 21;313(6004):653–659. doi: 10.1038/313653a0. [DOI] [PubMed] [Google Scholar]
- Klee C. B., Vanaman T. C. Calmodulin. Adv Protein Chem. 1982;35:213–321. doi: 10.1016/s0065-3233(08)60470-2. [DOI] [PubMed] [Google Scholar]
- Kretsinger R. H., Barry C. D. The predicted structure of the calcium-binding component of troponin. Biochim Biophys Acta. 1975 Sep 9;405(1):40–52. doi: 10.1016/0005-2795(75)90312-8. [DOI] [PubMed] [Google Scholar]
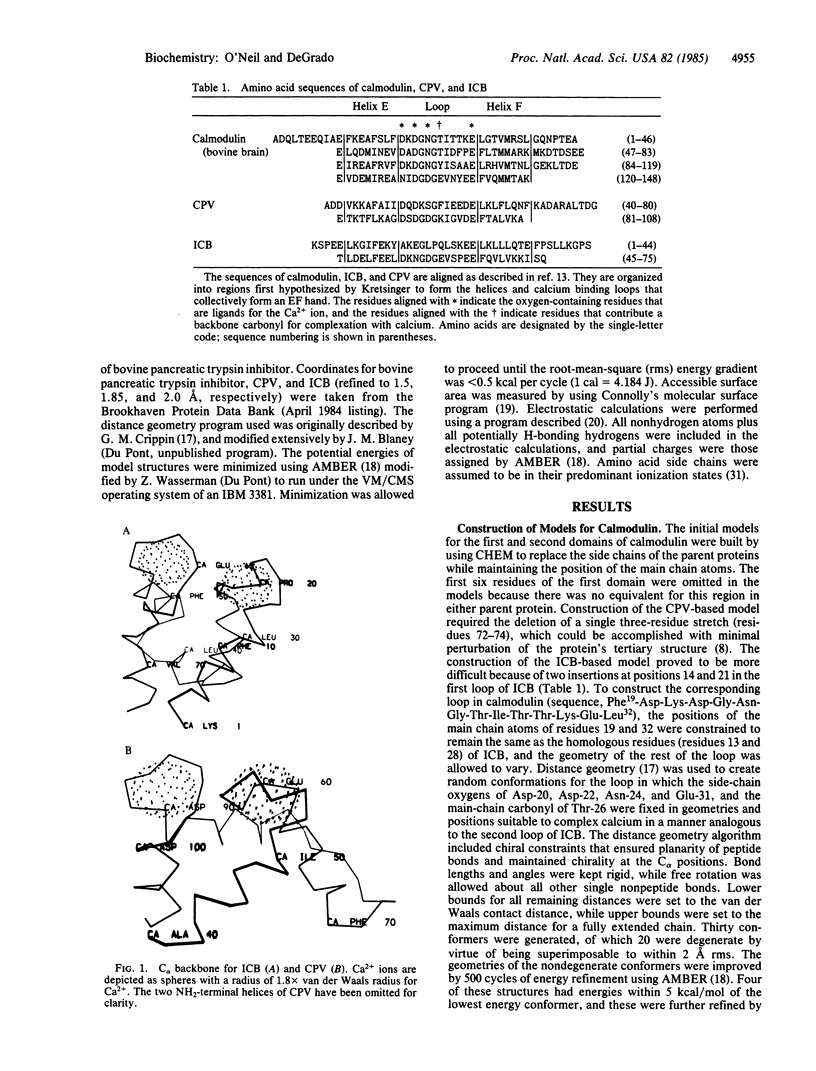
- Kretsinger R. H. Crystallographic studies of calmodulin and homologs. Ann N Y Acad Sci. 1980;356:14–19. doi: 10.1111/j.1749-6632.1980.tb29594.x. [DOI] [PubMed] [Google Scholar]
- Kretsinger R. H., Rudnick S. E., Sneden D. A., Schatz V. B. Calmodulin, S-100, and crayfish sarcoplasmic calcium-binding protein crystals suitable for X-ray diffraction studies. J Biol Chem. 1980 Sep 10;255(17):8154–8156. [PubMed] [Google Scholar]
- Lesk A. M., Chothia C. How different amino acid sequences determine similar protein structures: the structure and evolutionary dynamics of the globins. J Mol Biol. 1980 Jan 25;136(3):225–270. doi: 10.1016/0022-2836(80)90373-3. [DOI] [PubMed] [Google Scholar]
- Malencik D. A., Anderson S. R. Binding of simple peptides, hormones, and neurotransmitters by calmodulin. Biochemistry. 1982 Jul 6;21(14):3480–3486. doi: 10.1021/bi00257a035. [DOI] [PubMed] [Google Scholar]
- Malencik D. A., Anderson S. R. Peptide binding by calmodulin and its proteolytic fragments and by troponin C. Biochemistry. 1984 May 22;23(11):2420–2428. doi: 10.1021/bi00306a016. [DOI] [PubMed] [Google Scholar]
- Matthew J. B., Weber P. C., Salemme F. R., Richards F. M. Electrostatic orientation during electron transfer between flavodoxin and cytochrome c. Nature. 1983 Jan 13;301(5896):169–171. doi: 10.1038/301169a0. [DOI] [PubMed] [Google Scholar]
- Moews P. C., Kretsinger R. H. Refinement of the structure of carp muscle calcium-binding parvalbumin by model building and difference Fourier analysis. J Mol Biol. 1975 Jan 15;91(2):201–225. doi: 10.1016/0022-2836(75)90160-6. [DOI] [PubMed] [Google Scholar]
- Newton D. L., Oldewurtel M. D., Krinks M. H., Shiloach J., Klee C. B. Agonist and antagonist properties of calmodulin fragments. J Biol Chem. 1984 Apr 10;259(7):4419–4426. [PubMed] [Google Scholar]
- Novotný J., Bruccoleri R., Karplus M. An analysis of incorrectly folded protein models. Implications for structure predictions. J Mol Biol. 1984 Aug 25;177(4):787–818. doi: 10.1016/0022-2836(84)90049-4. [DOI] [PubMed] [Google Scholar]
- Remington S. J., Matthews B. W. A systematic approach to the comparison of protein structures. J Mol Biol. 1980 Jun 15;140(1):77–99. doi: 10.1016/0022-2836(80)90357-5. [DOI] [PubMed] [Google Scholar]
- Richards F. M. Areas, volumes, packing and protein structure. Annu Rev Biophys Bioeng. 1977;6:151–176. doi: 10.1146/annurev.bb.06.060177.001055. [DOI] [PubMed] [Google Scholar]
- Richardson J. S. The anatomy and taxonomy of protein structure. Adv Protein Chem. 1981;34:167–339. doi: 10.1016/s0065-3233(08)60520-3. [DOI] [PubMed] [Google Scholar]
- Salemme F. R. Structure and function of cytochromes c. Annu Rev Biochem. 1977;46:299–329. doi: 10.1146/annurev.bi.46.070177.001503. [DOI] [PubMed] [Google Scholar]
- Sundaralingam M., Bergstrom R., Strasburg G., Rao S. T., Roychowdhury P., Greaser M., Wang B. C. Molecular structure of troponin C from chicken skeletal muscle at 3-angstrom resolution. Science. 1985 Feb 22;227(4689):945–948. doi: 10.1126/science.3969570. [DOI] [PubMed] [Google Scholar]
- Szebenyi D. M., Obendorf S. K., Moffat K. Structure of vitamin D-dependent calcium-binding protein from bovine intestine. Nature. 1981 Nov 26;294(5839):327–332. doi: 10.1038/294327a0. [DOI] [PubMed] [Google Scholar]
- Weiner P. K., Langridge R., Blaney J. M., Schaefer R., Kollman P. A. Electrostatic potential molecular surfaces. Proc Natl Acad Sci U S A. 1982 Jun;79(12):3754–3758. doi: 10.1073/pnas.79.12.3754. [DOI] [PMC free article] [PubMed] [Google Scholar]