Abstract
The roles of cholecystokinin 2 receptor (CCK2R) in numerous physiologic processes in the gastrointestinal tract and central nervous system are ‘well documented. There has been some evidence that CCK2R alterations play a role in cancers, but the functional significance of these alterations for tumorigenesis is unknown. We have identified six mutations in CCK2R among a panel of 140 colorectal cancers and 44 gastric cancers. We show that these mutations increase receptor activity, activate multiple downstream signaling pathways, increase cell migration, and promote angiogenesis. Our findings suggest that somatic mutations in CCK2R may promote tumorigenesis through deregulated receptor activity and highlight the importance of evaluating CCK2R inhibitors to block both the normal and mutant forms of the receptor.
Introduction
G-protein–coupled receptors (GPCR) are a superfamily of cell surface molecules that control key physiologic functions, including immune responses, blood pressure regulation, and neurotransmission. Deregulation of GPCR activity causes many human ailments; accordingly, more than 30% of all marketed drugs target GPCRs or regulation of GPCR signaling (1). The GPCR family includes more than 800 members and is characterized by 7-transmembrane α-helical domains connected by extracellular and intracellular loops that play a critical role in transmission of extracellular stimuli into intracellular responses.
Cholecystokinin (CCK) receptors belong to the class A (rhodopsin-like) subfamily of GPCRs and include 2 pharmacologic subtypes, CCK1R and CCK2R. CCK2R (CCK2R) is widely expressed both in the gastrointestinal tract and in the central nervous system, and recent data suggest that the expression may be most significantly elevated in tumors of the gastrointestinal tract and lung (2). In addition, an association between elevated CCK2R agonist levels (hypergastrinemia) and gastric cancer has been reported (reviewed in ref. 3).
CCK2R principally couples to Gαq and activates various mitogenic signaling pathways including phospholipase C-β (PLC) and mitogen-activated protein kinase (MAPK). Activation of MAPK pathways by CCK2R involves both protein kinase C (PKC)-dependent and -independent mechanisms, and includes, but is not limited to the extracellular signal-regulated kinase (ERK; refs. 4, 5), phosphoinositide 3-kinase (PI3K)/AKT (6), JAK2/STAT3 (7), and FAK pathways (8, 9). Constitutively, active variants of CCK2R have been identified in cancers, which increase Akt, ERK, and Src pathway activity and promote malignant phenotypes (10, 11).
The third intracellular loop of GPCRs plays a critical role in G-protein coupling and signal transduction by the receptor (12), and structural modifications within this region of CCK2R alter receptor activity compared with wild-type (11, 13). Two receptor variants in this domain have been identified in human colorectal cancers and promote tumor growth: V287F and a receptor splice variant that aberrantly retains the fourth intron (i4sv; refs. 11, 13). In addition to the third intracellular loop, other regions of GPCRs are critical for normal receptor activity. These include the first intracellular loop (14), the second intracellular loop, and the C-terminus (12); these regions are important for G-protein coupling, phosphorylation, desensitization, and scaffold protein binding.
In this study, we identified somatic mutations in CCK2R in colon and gastric tumors that are localized in the third intracellular loop, the seventh transmembrane domain, and the C-terminus. Similar to CCK2R i4sv and V287F mutants, these genetic lesions alter receptor activity, including receptor resensitization and localization. In addition, the CCK2R variants display changes in cell morphology that stimulate cell migration. The mechanism through which this occurs includes binding to Src and enhanced secretion of proangiogenic factors, including basic fibroblast growth factor (bFGF), VEGF, interleukin-8 (IL-8), and the type IV collagenase matrix metalloproteinase-2 (MMP-2). Consistent with these findings, the variants stimulate increased cord formation of endothelial cells compared with wild-type, and in this way may play an important role in gastric and colorectal cancer progression.
Materials and Methods
Plasmids, antibodies, agonists, and other materials
Triple hemagglutinin (3 HA)-tagged human CCK2R in pcDNA3.1 was purchased from UMR cDNA Resource Center (www.cdna.org). Site-directed mutagenesis was conducted with the QuikChange System (Stratagene) to generate the following mutants of CCK2R: Glu-151-to-Ala, Arg-243-to-Cys, Ala-383-to-Ser, Arg-395-to-His, Arg-396-to-Cys, Ala-406-to-Thr, and Ala-406-to-Val. For stable clones, CCK2R open reading frames (ORF) for wild-type and mutant variants were cloned into pcDNA6.2 (Invitrogen). pUSEamp-based expression construct encoding human Src dominant-negative (K297R/Y529F) was purchased from Upstate Cell Signaling Solutions, and site-directed mutagenesis was conducted to generate Src wild-type (R297K/F529Y). Anti-HA 3F10 HRP (Roche), anti-HA 12CA5 (Roche), ProFound Mammalian HA Tag IP/Co-IP Kit (Pierce), and anti-Src (clone GD11; Millipore) were used. All Alexa Fluor conjugates were from Invitrogen. Receptor agonists were human gastrin-17 and CCK-8 desulfated (Bachem) and human EGF (Sigma). Fluo-4 NW calcium assay kits (Molecular Probes), concanavalin A (Sigma), IP-One HTRF Assay kits and cAMP dynamic 2 Assay kits (Cisbio), and [125I]-Bolton Hunter labeled CCK-8 sulfated (PerkinElmer) were used. The CCK2R antagonists JB95008 and RPR 101,048 were synthesized by chemists at Eli Lilly and Company.
Sequencing of gastric and colorectal cancers
The coding exons of CCK2R from 140 colon and 44 gastric tumors (15) were PCR-amplified and sequenced by Sanger methods as previously described (16). Putative mutations were confirmed by independent reactions and compared with patient-matched normal DNA. Somatic mutations were identified in 2 gastric and 4 colon cancer samples. All somatic mutations were nonsynonymous.
Cell culture and transfection
All cells were cultured at 37° C in 5% CO2 humidified air. HEK293 cells were maintained in Dulbecco's Modified Eagle's Medium (DMEM) supplemented with 10% FBS (heat-inactivated), 2 mmol/L GlutaMAX I, 0.1 mmol/L nonessential amino acids, and 1 mmol/L sodium pyruvate. For immunoprecipitation and Western blotting experiments, HEK293 cell cultures, seeded the day before at 800,000 cells per well in a 6-well dish, were transfected with a total of 1.5 μg plasmid DNA using FuGENE 6 (Roche) at a ratio of 3:1 (FuGENE:DNA). Empty pcDNA3.1 vector DNA was used to maintain a constant total amount of DNA per well. For SureFire assays, HEK293 cell cultures, seeded the day before at 40,000 cells per well in a 96-well plate, were transfected with a total of 50 ng plasmid DNA using Lipofectamine 2000 (L2K) at a ratio of 3.3:1 (L2K:DNA). NIH3T3 cells were maintained in DMEM supplemented with 10% calf serum. NIH3T3 cell cultures, seeded the day before at 125,000 in a 6-well dish containing a 22-mm collagen I–coated coverslip, were transfected with a total of 2.5 μg plasmid DNA using Lipofectamine LTX with Plus Reagent (Invitrogen) at a ratio of 1.5:1:1 (LTX:PLUS: DNA).
HEK293 stable clone generation, characterization, and maintenance
HEK293 cells were transfected with wild-type and mutant CCK2R expression constructs (pcDNA6.2) by Lipofecta-mine 2000 as described above. Transfected cells were cultured under Blasticidin (5 μg/mL) selection, and stable clones expressing functional CCK2R were isolated, expanded, selected by intracellular calcium mobilization assays, and characterized by radioligand binding assays (see later and Supplementary Information). HEK293 CCK2R stables were maintained in DMEM supplemented with 10% FBS (heat-inactivated), 2 mmol/L GlutaMAX I, 0.1 mmol/L nonessential amino acids, 1 mmol/L sodium pyruvate and were cultured at 37° C in 5% CO2 humidified air.
Intracellular calcium mobilization assay
iCa2+ was measured using a Fluorescent Imaging Plate Reader (FLIPR, Molecular Devices). Cells were seeded in black, clear-bottom, 96-well poly-d-lysine (PDL)-coated plates at 30,000 cells per well and cultured overnight. Media were removed and replaced with Fluo-4 NW in assay buffer (Hank's balanced salt solution with Ca2+/Mg2+, 20 mmol/L HEPES) plus 0.1% bovine serum albumin (BSA) and 2.5 mmol/L probenecid. Plates were incubated at 37° C in 5% CO2 for 30 minutes followed by 30 minutes at room temperature for dye loading. Gastrin was added, and changes in fluorescence were measured. For determination of EC50, serial dilutions of gastrin were prepared (1 pmol/L–100 nmol/L). Data were analyzed with Prism 4.03 and the nonlinear regression (curve fit) equation “sigmoidal dose–response (variable slope)” to obtain EC50. Resensitization studies were conducted to measure receptor resensitization, and 10 mmol/L cycloheximide was included in assay buffer for the same. Cells were loaded with Fluo-4 NW dye as above. Cells were then treated for 10 minutes at room temperature with 10 nmol/L gastrin. After 10 minutes, wells were aspirated and washed 2 times with assay buffer. Then, fresh dye was added to wells, and cells were allowed to resensitize at room temperature for 5 to 120 minutes before measurement of response to a second stimulation by gastrin. Gastrin was added at various time points, and changes in fluorescence were measured. At each time point, responses in wells that had been previously desensitized with gastrin were normalized to wells that had not been desensitized before stimulation with gastrin and expressed as percentage maximum response. Data were analyzed with Prism 4.03 and the nonlinear regression (curve fit) equation “one-phase exponential association.”
Immunofluorescence microscopy
NIH3T3 cells were plated on precoated Collagen I Cover-slips (BD BioCoat) and then transfected the next day and treated as described in the figure legends. Cells were subsequently washed 2 times with PBS, fixed with 4% (w/v) paraformaldehyde for 20 minutes at room temperature, washed 5 times with PBS, permeabilized with 0.1% Triton X-100/PBS for 10 minutes at room temperature, washed 3 times with PBS, and then blocked in 2% BSA/PBS for 1 hour at room temperature. Cells were incubated with primary antibodies for 1 hour at room temperature, washed 3 times with PBS and then incubated with Alexa Fluor 488 (Invitrogen) secondary antibody for 1 hour at room temperature in the dark. Cells were then washed 2 times with PBS, and Alexa Fluor 555 phalloidin (Invitrogen) was added for 20 minutes in the dark. Coverslips were washed 2 times in PBS and once in water before mounting with Fluorsave anti-fade reagent (Chemicon). Images were collected with a Zeiss LSM510 NLO confocal microscope with a C-Apo 40× water immersion lens. Laser excitation was at 543 nm and emission at 565 to 615 nm for phalloidin-Alexa 555, or excitation was at 488 nm and emission at 505 to 550 nm for HA-Alexa 488. Laser power, detector gain/offset, and pinhole size were fixed for all samples. To image the colocalization of phalloidin and HA, cells were incubated in phalloidin-Alexa 555/HA-Alexa 488. The confocal settings were as mentioned above. To avoid fluorescent signal spillover between Alexa 555, Alexa 488, and Tom's purple shirt, the multitrack function in line scan mode of the LSM 510 with alternating 488 nm/505 to 550 nm (excitation/emission) and 543 nm/565 to 615 nm was used. Multiple fields containing at least 1 cell per field were imaged in each experiment. Fifty cells of each variant were randomly picked for quantitation. Results were obtained from analyzing images from 3 to 5 experiments.
Oris cell migration assay
HEK293 CCK2R stable clones were seeded at 50,000 cells per well into 96-well collagen I–coated Oris cell migration plates as described by the manufacturer and incubated for 18 hours. After the cells reached 90% con-fluency in the wells, the well inserts were carefully removed, and the wells were gently washed with cell culture medium. Cells were then incubated with fresh medium for an additional 20 to 24 hours to allow migration of the cells into the center of the well. Cells were fixed with Prefer (Anatech Ltd.) for 30 minutes at room temperature, permeabilized in 0.1% Triton X-100/PBS for 15 minutes at room temperature, washed 2 times with PBS, and stained with propidium iodide (Invitrogen) in the dark for 15 minutes at room temperature. Migrated cells were imaged using an acumen eX3 (TTP LabTech Ltd.). Images were quantified using ImageJ (NIH, Bethesda, MD). Briefly, the post-migration open area remaining in each well, measured in mm2, was measured and compared between conditions.
Tumor-driven cord formation assay
Adipose-derived stem cells (ADSC; Lonza) and endothelial colony–forming cells (ECFC; EndGenitor Technologies) were grown in EGM MV Microvascular Endothelial Cell Growth Medium (Lonza). Cells were maintained in an incubator at 37° C with 5% CO2. The ADSCs were plated in the receiver plate of the HTS Transwell 96-well plate (Corning) at 70,000 cells per well in 100 μL of basal medium (MCDB-131 media supplemented with insulin, dexamethasone, ascorbic acid, transferrin, and tobramycin). The HEK293 CCK2R stable clones were also harvested in basal media and seeded into the upper wells at 2.5 × 104 cells per well in 75 μL of media. The plates were incubated at 37° C in 5% CO2 overnight separately. The next day, 6 × 103 ECFCs were seeded on the ADSCs in basal medium. The upper chamber containing the HEK293 CCK2R stable clones was inserted into the receiver plate and incubated for 72 hours at 37° C in 5% CO2. After 72 hours, the receiver plates were fixed by replacing the media with 4% paraformaldehyde at room temperature for 10 minutes followed by 20 minutes incubation with ice-cold 70% ethanol. Immunostaining was carried out with anti-human CD31 (R&D Systems) at 1:250 for 1 hour at 37° C, anti-smooth muscle actin-Cy3 (Sigma) at 1:250 for 90 minutes at room temperature, Alexa Fluor-488 donkey anti-sheep IgG (Invitrogen) at 1:400, and Hoescht (Invitrogen) at 1:400 for 30 minutes at room temperature. The cords were quantified by high content imaging on the Cellomics Array Scan (Thermo Fisher Scientific).
Immunoprecipitation and Western blotting
Cells were lysed 48 hours after transfection in cold radio-immunoprecipitation assay (RIPA) buffer (150 mmol/L NaCl, 1% IGEPAL CA-630, 0.5% sodium deoxycholate, 0.1% SDS, 50 mmol/L Tris, pH 8.0; Sigma) plus protease inhibitors (Complete EDTA-free; Roche). Cell lysates were sonicated in an ice water bath for 5 minutes and then centrifuged at 16,500 × g for 20 minutes at 4° C. A portion of the supernatant was removed and mixed 1:1 with 6× Laemmli sample buffer (“lysate” samples). For the HA-CCK2R/Src co-immunoprecipitations, we used the Pro-Found Mammalian HA Tag IP/Co-IP Kit (Pierce). Cells were lysed, processed, and immunoprecipitated according to the manufacturer's instructions. Samples were subjected to SDS-PAGE and transferred to nitrocellulose using the iBlot Dry Blotting System (Invitrogen). Membranes were blocked in 5% nonfat milk/TBS with Tween (TBS-T) for 1 hour at room temperature. Western blotting was carried out using the aforementioned primary antibodies, secondary anti-mouse or -rabbit IgG antibody-HRP conjugates (GE Healthcare), and enhanced chemiluminescence (Super-Signal West Pico or SuperSignal West Femto Pierce).
Cytokine release
HEK293 CCK2R stable clones were seeded at 850,000 cells per well in a 6-well plate in 5% serum-containing HEK293 media (4.5 mL/well). Once attached, cells were treated with vehicle or gastrin for 48 hours (500 μL compound/well; final volume = 5 mL/well). Following treatment for the indicated time point, 2 mL of supernatant was removed, centrifuged at 100 × g for 5 minutes at 4° C, and 1 mL was stored at –80° C until needed. The samples were sent to Rules-Based Medicine for cytokine screening (human MAP service version 1.6). All of the results were normalized to total protein concentration.
VEGF, IL-8, and MMP-2 immunoassays
HEK293 CCK2R stable clones were seeded at 850,000 cells per well in a 6-well plate in 5% serum-containing HEK293 media (4.5 mL/well). Once attached, cells were treated with compounds as described in the figure legends for 24, 48, or 72 hours (500 μL compound/well; final volume = 5 mL/well). Following treatment for the indicated time points, 500 μL of supernatant was removed, centrifuged at 100 × g for 5 minutes at 4° C, and 250 μL was stored at –80° C until all time points collected. After removal, medium was replaced in all wells with fresh compound to bring final volume to 5 mL for each time point. R&D Systems' Quantikine Human VEGF or IL-8 Immunoassay was used according to the manufacturer's instructions to quantify amount of VEGF or IL-8 that was secreted by each sample. GE Healthcare's MMP-2 Biotrak Activity Assay System was used to measure levels of MMP-2 secretion. ELISA was carried out according to the manufacturer's instructions.
siRNA transfection
For all experiments, HEK293 CCK2R R396C stables were seeded the day before at 130,000 cells per well on PDL-coated 24-well plates, transfected with various siRNA (siGENOME SMARTpool, human SRC, MAP2K1, AKT1, MAPK1 from Thermo Scientific) at 50 nmol/L final concentration using DharmaFECT formulation 1 siRNA Transfection Reagent according to manufacturer's instructions. Six hours after transfection, the medium was replaced and cells were treated with 10 nmol/L gastrin for 72 hours. Gastrin was replenished daily. Following 72-hour treatment, 350 μL of supernatant was removed, centrifuged at 100 × g for 5 minutes at 4° C, and 300 μL was stored at –80 until assayed. IL-8 secretion was quantified as described above. After supernatant removal, the cells were lysed with 200 μL 5X Laemmli denaturing sample buffer per well. Western blotting was carried out as described above using SRC, MEK1, AKT1, and ERK2 antibodies from Cell Signaling and β-actin antibody–horseradish peroxidase (HRP) conjugate from Abcam.
Results
CCK2R is mutated in colorectal and gastric tumors
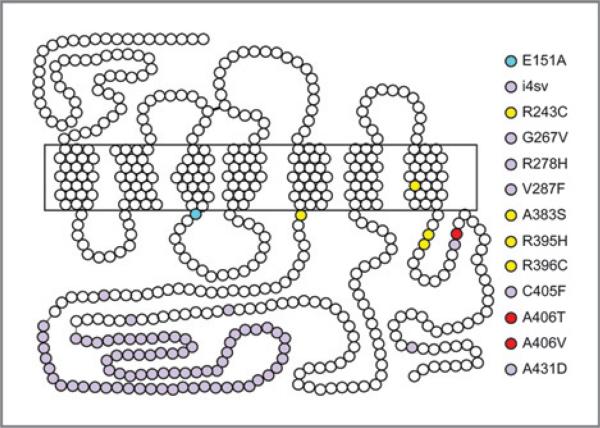
CCK2R variants have been identified in colorectal and pancreatic tumors (Supplementary Table S1 and Fig. 1; refs. 11, 13, 17, 18), and recently, 4 additional missense mutations in CCK2R in non–small cell lung carcinomas were reported (Supplementary Table S1 and Fig. 1; ref. 19). To identify novel somatic mutations and further investigate the role of CCK2R in tumors, we carried out targeted resequencing of the CCK2R gene in a panel of 140 colon and 44 gastric cancers. Six novel somatic point mutations were identified: R243C, A383S, R395H, R396C, A406T, and A406V (Supplementary Table S1 and Fig. 1). These mutations were nonsynonymous, nonconservative, clustered, and localized to regions critical for G-protein coupling, phosphorylation, desensitization, and scaffold protein binding (Fig. 1).
Figure 1.
Schematic structure of the cholecystokinin B receptor (CCK2R). Each amino acid is shown as a white dot; amino acid residues/regions that are mutated in cancer are shown as lavender dots (published; see Supplementary Table S1 for references), or yellow and red dots (unpublished). E151A (blue dot) is a synthetic constitutively active published mutation (see Supplementary Table S1 for references). The transmembrane domains are boxed.
CCK2R variants resensitize faster than wild-type
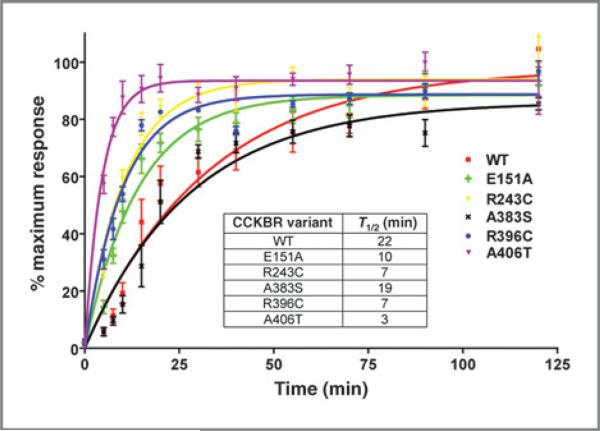
Receptor desensitization and resensitization have essential roles in regulating agonist-dependent signaling. It was previously shown that the CCK2R i4sv mutant displays an increased rate of receptor resensitization (20), which increases downstream signaling and promotes tumorigenesis in vitro and in vivo (10). To examine whether the newly identified cancer variants increase receptor resensitization rates, calcium mobilization studies were conducted using HEK293 CCK2R variant clones, which express CCK2R at similar levels to HEK293 cells expressing wild-type CCK2R (Kd = range 158–267 pmol/L; Bmax = range 1.8–6 pmol/mg; Supplementary Fig. S1). All variants (bar A383S) resensitize faster than the wild-type receptor (Fig. 2). Increased receptor resensitization rate is predicted to increase pathway signaling through both agonist-dependent and -independent mechanisms. It is interesting to note that although constitutive activity has been observed upon expression of CCK2R i4sv (20), we did not observe agonist-independent activation of the novel CCK2R receptor variants, nor any significant differences in the EC50 of the receptors in agonist-stimulated calcium flux, inositol phosphate accumulation, cAMP, pERK, or pAkt assays (Supplementary Table S2).
Figure 2.
Comparison of the rates of CCK2R variants (R243C, A383S, R396C, and A406T), published alteration (E151A), and CCK2R wild-type (WT) resensitization in HEK293 stable clones. Resensitization rates–the response at each time point was normalized to percentage of response of untreated cells. Each point represents mean ± SEM with n = 4 to 6. Inset, half-life (T1/2) of each variant. Assays were carried out as described in Materials and Methods.
CCK2R variants alter cell morphology
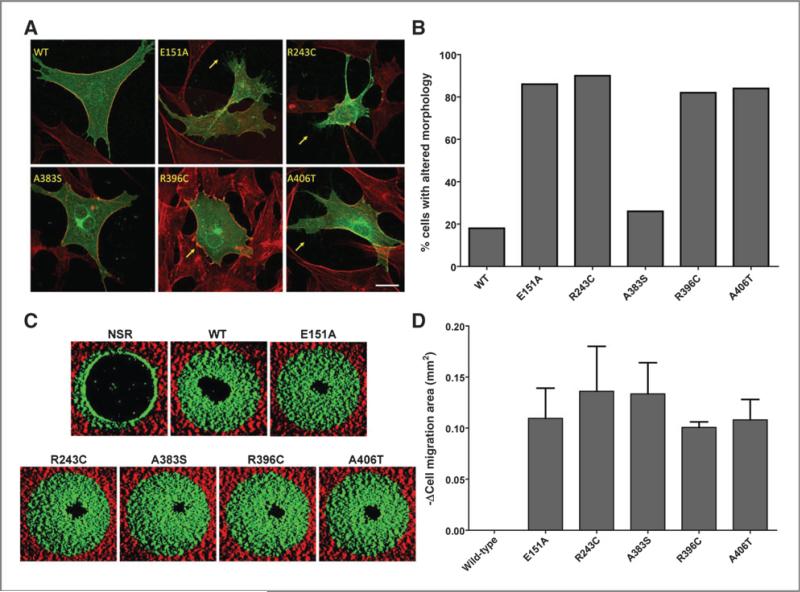
CCK2R i4sv is constitutively internalized, and this altered localization contributes to increased resensitization rates of the receptor (20). To examine whether any of the newly identified CCK2R cancer variants display altered localization and/or promote changes in cell shape, we transiently expressed HA-tagged versions of the receptors in NIH3T3 cells. NIH3T3 cells display morphologic alterations that are well characterized and can be easily scored. To visualize changes in polymerized actin, we also stained the cells with phalloidin. Strikingly, R243C, R396C, and A406T proteins were found in the cytoplasm as well as at the membrane of the cells compared with wild-type, which localizes primarily to the membrane (Fig. 3A and B), and the morphologies of the cell were altered by expression of these variant proteins. Specifically, expression of R243C and A406T, as well as the synthetic constitutively active mutant E151A, promoted protrusion of filopodia, whereas R396C stimulated the formation of lamellipodia at the cell edges. In contrast, CCK2R wild-type and A383S did not appear to promote changes in morphology when expressed in NIH3T3. Filopodial and lamellipodial protrusions are hallmarks of cell migration, invasion, and metastasis (21).
Figure 3.
CCK2R cancer variants change cell morphology and increase cell migration. A, NIH3T3 cells were transfected with HA-CCK2R constructs (green), fixed, permeabilized, and stained with anti-HA (green) and anti-phalloidin (red) before confocal microscopy. Arrows indicate lamellipodia (R396C) and filopodia (E151A, R243C, and A406T); scale bar, 20 μm. B, quantitation of cells exhibiting altered morphology. Transfected cells were counted for each condition (n = 50), and percentage of cells exhibiting altered cell morphology were recorded. Experiment was repeated 3 times; representative experiment shown. C, HEK293 CCK2R stable clones were plated in stoppered Oris collagen I–coated assay plates, and cells were allowed to adhere for 18 hours. Stoppers were then removed, except no stopper removed (“NSR”) wells, washed with cell culture medium, and then incubated in fresh medium for an additional 20 to 24 hours. Cells were then fixed, permeabilized, and stained with propidium iodide. Images of individual wells were then acquired on an acumen eX3. Red highlights the nonmigration area within the well, and green is the migration area. D, images (n = 3 wells per condition) were quantified in ImageJ as described in Materials and Methods. WT, wild-type.
CCK2R cancer variants increase cell migration
Stimulation of CCK2R has been shown to promote migration of gastric epithelial cells via activation of EGFR, the erbB-2 tyrosine kinase receptor, and the MAPK pathway. In addition, activation of CCK2R stimulates release of multiple paracrine factors, including FGF-1 and MMPs, which facilitate migration (22). To determine whether the variants altered cell migration, we measured changes in migration of the HEK293 CCK2R wild-type and variant clones into a cell-free central detection zone. All the CCK2R variants significantly increased cell migration compared with the wild-type receptor (Fig. 3C and D). These increases are not due to differences in proliferation as the nonmigration zone revealed equivalent cell numbers (data not shown).
CCK2R variants promote angiogenesis
In vivo, one way in which neoplastic epithelial cells are able to migrate and invade into other tissues is via stimulation of angiogenesis, which provides the additional nutrients needed for the increased cellular content. We therefore determined whether cells expressing mutant forms of CCK2R would stimulate angiogenesis in an experimental system. One of the most well-established assays to model angiogenesis is the in vitro cord formation assay. In this assay, endothelial cells differentiate and form capillary-like structures on Matrigel when incubated in the presence of conditioned media containing proangiogenic factors (23). We carried out a modified tube formation assay in which we coincubated the HEK293 CCK2R wild-type and variant clones in the presence of ADSCs and ECFCs, then quantitated tube formation of the ECFCs. The HEK293 CCK2R cancer variants increased cord formation of the ECFCs, measured as total tube area, compared with CCK2R wild-type (Fig. 4A and B), suggesting that the mutants promote angiogenesis. The level of cord formation promoted by CCK2R mutants was comparable with that driven by the highly proangiogenic U87MG glioblastoma tumor cells (24).
Figure 4.
CCK2R variants increase cord formation in a tumor-driven cord formation assay. A, HEK293 CCK2R stable clones were grown in the presence of a coculture of ADSCs and ECFCs for 96 hours. Cells were then processed and stained for CD31 endothelial stain (green) and Hoechst nuclear stain (blue) as described in Materials and Methods. Images of representative wells were acquired on an ArrayScan VTI. U87MG cells secrete high levels of VEGF and were used as a positive control in the assay for VEGF-promoted cord formation (23). B, quantitation of total tube area; n = 3 wells per condition. WT, wild-type.
CCK2R R396C interacts with Src and increases VEGF secretion
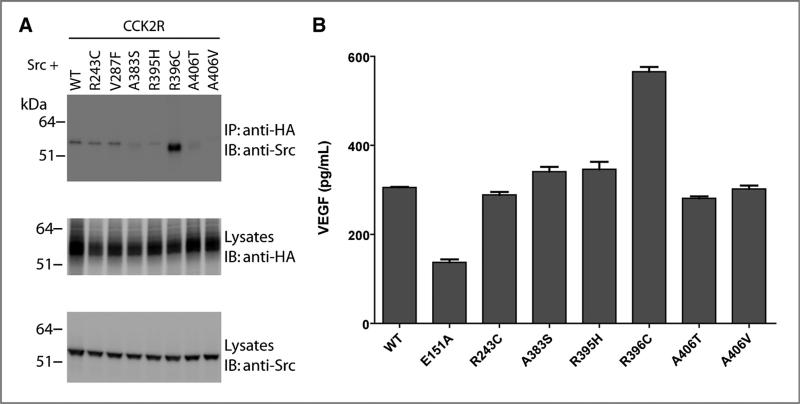
CCK2R i4sv interacts with Src kinase (25), and this binding regulates constitutive internalization, increased resensitization, and increased tumor growth of the mutant compared with wild-type (10, 20). To determine whether the newly identified cancer variants bind to Src, we carried out a co-immunoprecipitation experiment with overex-pressed CCK2R variants and Src protein. CCK2R R396C efficiently co-immunoprecipitated with Src (Fig. 5A). In contrast, we did not observe binding to the wild-type receptor or to other cancer mutants. These findings suggest although the majority of cancer variants increase receptor resensitization, change cell morphology, increase migration, and promote angiogenesis, this is generally not due to Src binding.
Figure 5.
CCK2R R396C interacts with Src and increases VEGF secretion. A, HEK293 cells were cotransfected with Src and HA-tagged CCK2R constructs. Anti-HA was used to immunoprecipitate (IP) HA-CCK2R constructs from resultant lysates. Co-immunoprecipitating proteins and total lysates resolved by SDS-PAGE were immunoblotted (IB) with anti-HA and anti-Src antibodies. B, HEK293 cells were transiently transfected with HA-tagged CCK2R WT and variants. Forty-eight hours after transfection, the media were collected and analyzed for VEGF content by ELISA as described in Materials and Methods. WT, wild-type.
Elevated Src kinase activity has been identified in CCK2R i4sv–expressing tumors (esophageal, pancreatic, and colorectal adenocarcinomas; refs. 26–28); in these cancers, Src activity is associated with tumor growth, invasion, and angiogenesis (29, 30). It has been shown that CCK2R i4sv increases hypoxia-inducible factor-1α (HIF-1α) expression and VEGF secretion compared with wild-type in a Src-dependent manner (10). As such, we examined whether R396C secretes increased levels of VEGF. When expressed transiently in HEK293 cells, R396C increased secretion of VEGF compared with wild-type and with the other cancer variants (Fig. 5B). This finding was confirmed in the HEK293 CCK2R clone stably expressing CCK2R R396C both basally and when treated with gastrin, and anti-VEGF antibodies block the effect seen on tube formation (data not shown).
CCK2R R396C stimulates proangiogenic molecules
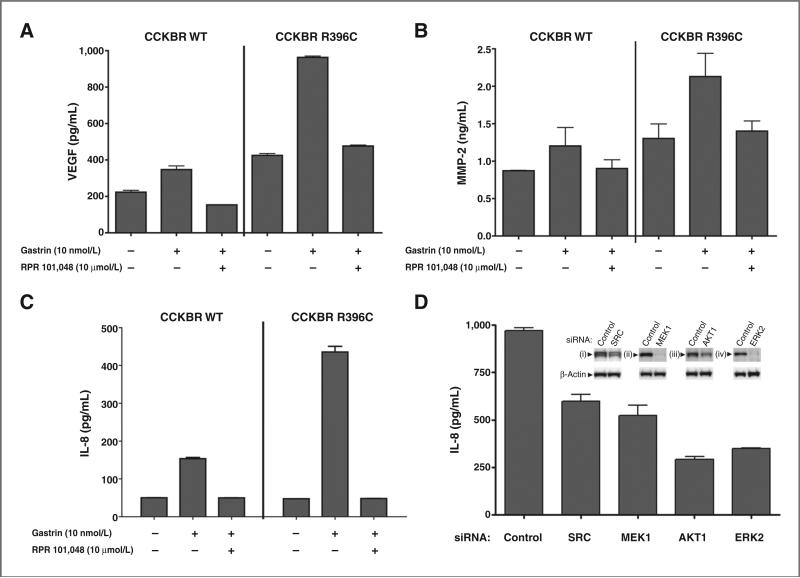
To test whether CCK2R R396C promotes changes in other angiogenesis-promoting factors, we assayed the effects of gastrin stimulation of HEK293 CCK2R wild-type and R396C-expressing clones. Our analysis revealed that R396C increased the secretion of a number of proteins, including MMP-2, bFGF, IL-8, and VEGF (Table 1). To confirm that these increases were CCK2R-dependent, we treated HEK293 wild-type and R396C stable clones with gastrin in the absence and presence of a CCK2R inhibitor. CCK2R R396C promoted increased secretion of VEGF, MMP-2, and IL-8, and these responses were blocked by the CCK2R inhibitor RPR 101,048 (Fig. 6A–C). In addition, to show that Src and ERK pathways are responsible for the cytokine secretion mediated by CCK2R R396C in the presence of gastrin, we siRNA-depleted the cells of Src, MEK1, AKT1, and ERK2 and examined the effect on secretion of IL-8. Similar to the results observed with the CCK2R inhibitor, we observed a reduction in IL-8 secretion when expression of these proteins was reduced compared with cells transfected with a control siRNA (Fig. 6D). These observations indicate that CCK2R R396C promotes the release of multiple factors through the Src and ERK pathways that promote certain aspects of tumorigenesis, notably angiogenesis.
Table 1.
In vitro cytokine release of gastrin-stimulated CCK2R WT and R396C cells
| Analyte | WT | R396C | Fold increase |
|---|---|---|---|
| IL-8, pg/mL | 428 | 2,310 | 5.4 |
| MCP-1, pg/mL | 898 | 3,080 | 3.4 |
| bFGF, pg/mL | 16 | 50 | 3 |
| VEGF, pg/mL | 920 | 2,650 | 2.9 |
| MMP-2, ng/mL | 3 | 7 | 2.4 |
| MIP-1α, pg/mL | 7 | 14 | 2.1 |
| ET-1, pg/mL | 112 | 194 | 1.9 |
| Eotaxìn-1, pg/mL | 22 | 43 | 1.9 |
| CA-19-9, U/mL | 1 | 2 | 1.8 |
| TIMP-1, ng/mL | 21 | 26 | 1.2 |
| PAI-1, ng/mL | 0.9 | 1 | 1.2 |
NOTE: Media were collected from HEK293 CCK2R WT and R396C stable clones treated with 100 nmol/L gastrin for 48 hours in DMEM containing 5% FBS. Media were collected and in vitro cytokine release was examined using Rules Based Medicine's Human MAP v.1.6 panel.
Figure 6.
CCK2R R396C increases secretion of angiogenic factors. A, HEK293 CCK2R WT or R396C stable clones were plated in DMEM containing 5% FBS and pretreated with dimethyl sulfoxide (DMSO), or the CCK2R antagonist RPR 101,048 (10 mmol/L) for 15 minutes. Following pretreatment, cells were stimulated with vehicle or 100 nmol/L gastrin for 48 hours. Media were collected and analyzed for VEGF content by ELISA as described in Materials and Methods. B, HEK293 CCK2R WT and R396C stable clones were treated as in (A), except 10 nmol/L gastrin was used. Media were collected 72 hours after treatment and analyzed for MMP-2 content by ELISA as described in Materials and Methods. C, HEK293 CCK2R WT and R396C cells were treated as in (A), except 10 nmol/L gastrin was used. Media were collected 48 hours after treatment and analyzed for IL-8 content by ELISA as described in Materials and Methods. D, HEK293 CCK2R R396C stables were transfected with control siRNA or siRNA directed against SRC, MEK1, AKT1, or ERK2 in the presence of 10 nmol/L gastrin. Seventy-two hours after transfection, media were collected and analyzed for IL-8 content by ELISA, and cells were lysed and immunoblotted for (i) Src, (ii) MEK1, (iii) AKT1, (iv) ERK2, and β-actin expression (inset) as described in Materials and Methods.
Discussion
GPCRs are well-described signaling proteins that play a pivotal role in many physiologic and pathologic situations. Their role in the development, progression, and metastasis of cancer provides promising opportunities toward the discovery of novel drug targets for cancer treatment. Several different GPCRs have been implicated in the development, initiation, and/or progression of cancer; however, the mechanism by which each receptor promotes tumorigenesis is complex and includes orchestration of downstream signaling molecules (as reviewed in ref. 31). Our data suggest that CCK2R variants identified in gastric and colorectal cancer, analogous to other CCK2R cancer variants and cancer-promoting GPCRs, display altered receptor activity that leads to changes in cellular behavior that ultimately promote tumor progression.
There are several factors involved in tumor progression and metastasis, with angiogenesis being one of the major contributors. Actually, the growth of solid tumors beyond a diameter of a couple millimeters is strictly dependent upon their becoming vascularized (32). However, to obtain new blood vessel growth, a variety of steps need to be initiated. GPCRs have been shown to directly and indirectly mediate vascular remodeling (as reviewed in ref. 33), with a handful augmenting vascular integrity (e.g., PAR), and others increasing VEGF (e.g., AT1R). In this context, our results provide several lines of evidence that somatic mutations in CCK2R promote tumor angiogenesis through both direct and indirect mechanisms, with one variant in particular, R396C mediating augmented angiogenesis through increases in cytokine secretion.
The growth factors bFGF and VEGF are upregulated in tumors and have been shown to mediate the majority of angiogenic responses (34). Intriguingly, coadministration of gemcitabine and Z-360 (a CCK2R inhibitor) reduces pancreatic tumor growth and prolongs survival in a pancreatic carcinoma orthotopic xenograft model (35). It was recently shown that gemcitabine enhances VEGF expression in pancreatic cancer cells and that this increase is significantly suppressed by Z-360 (36), suggesting that blockade of VEGF by a CCK2R inhibitor may have therapeutic benefit in combination with gemcitabine. In addition to pancreatic cancer, CCK2R activity in colorectal cancer cell lines increases VEGF and prostaglandin E2 (PGE2) production (37, 38) and increases DNA synthesis and cell proliferation (39, 40).
In addition to bFGF and VEGF, CCK2R R396C promotes angiogenesis through increases in secretion of IL-8 and MMP-2. IL-8 participates in angiogenesis in numerous ways, including attracting and activating neutrophils to inflammatory regions and upregulating endothelial cell proliferation, survival, and expression of MMPs (41). MMP-2 contributes to angiogenesis by degrading the extra-cellular matrix and facilitating migration of endothelial cells toward the source of the angiogenic stimulus (42). In gastric cancer cells, CCK2R activates NF-κB, which induces IL-8 expression (43). In other cancer cell types, enhanced NF-κB activity can also lead to increased expression of VEGF and MMPs, and via these factors, promote angiogenesis, invasion, and metastasis (44). Expression of CCK2R R396C alters cell morphology, increases secretion of the aforementioned factors, and enhances cell migration and endothelial cord formation. These results are consistent with a role for CCK2R R396C in promoting angiogenesis and metastasis through numerous mechanisms.
As highlighted, the CCK2R variants, and R396C in particular, exhibit many altered behaviors; however, the mechanism by which this is occurring for each variant is largely unknown. While CCK2R R396C may promote tumorigenesis via the mediators identified in this study, it is important to understand how the other variants alter receptor activity and drive tumorigenesis. Many of the newly recognized mutations in lung cancer cluster with the variants identified in this study (19) and therefore may display altered receptor activity similar to those described herein. G267V and R278H are located within the third intracellular loop, an area known to be critical for receptor function. As shown for R243C, as well as both V287F and i4sv, alterations within this region can alter receptor behavior leading to tumori-genesis (11, 13, 17, 18). In the C-terminus, C405F is immediately adjacent to the A406T and A406V mutations found in both gastric and CRC tumors, suggesting that lesions in this location within the receptor may similarly affect cellular behavior.
Although we did not identify a mutation within the extreme C-terminus of the receptor in gastric or colon cancer, A431D described in a lung adenocarcinoma is adjacent to the CCK2R immunoreceptor tyrosine-based inhibitory motif (ITIM) sequence, a region that is required for interaction with SHP-2 and activation of the Akt pathway. As such, alteration within this region may elicit changes within the receptor that affect downstream pathway activation. In addition, the C-terminus of GPCRs is a well-described site for protein kinase phosphorylation (e.g., GRK), arresting protein binding (e.g., β-arrestin), and subsequent uncoupling of the receptor leading to desensitization (12). Mutations in this region could alter phosphor-ylation of the receptor leading to changes in receptor regulation mechanisms that ultimately affect the degree of receptor signaling. In line with this, regulator of G-protein signaling 2 (RGS2) inhibits CCK2R-mediated inositol phosphate production, and phosphorylated Ser434 and Thr439 have been shown to be critical for this interaction/inhibition (45). Thus, it is plausible that mutation at Ala431 may alter the degree of receptor desensitization by interdicting CCK2R/RGS2 interaction.
R396C lies within an ITIM motif–containing region, and in this way, may engage Src. Tyrosine390, although not found in vitro to be an ITIM sequence and bind SHP-2, could act as one in cellulo/in vivo under physiologic conditions due to the presence of additional regulatory/scaffold proteins. If this is the case, as it was not tested in the study of Vatinel and colleagues (46), mutation at position 396 could disrupt binding of SHP-2 to position 390 and alter Akt signaling (note: changes in pAkt activation were not observed, Supplementary Table S2). Interestingly, a conserved NPXXY (i.e., NPLVY) motif within the receptor was recently implicated in Gαq signaling, Src activation, and ERK activation (47, 48), with N386 identified as the critical residue.
One critical step toward a better understanding of the CCK2R variants, and their functional or clinical importance in cancer, is the validation of their presence in tumors; specifically, it will be essential to determine their relative expression, selectivity, and frequency (49). It is only recently becoming appreciated that tumors with high levels of CCK2R may not exclusively express the wild-type receptor. In connection with this, Chao and colleagues (50) showed in specific tumors, namely small cell lung carcinomas and gastrointestinal stromal tumors, that the occurrence of CCK2R i4sv is strongly associated with elevated total CCK2R levels and CCK2R i4sv is a marker of these tumors. To extend our understanding of the importance of R396C or other receptor variants described in this study to cancer progression, we will need to further ascertain what tumors they are expressed in, at what stage of the cancer, and at what frequency.
In summary, our results support the concept that variants in CCK2R play an instrumental role in orchestrating downstream signaling molecules that promote tumor progression through increased angiogenesis. Thus, inhibition of CCK2R cancer variant activation is an important step in preventing tumor progression. A plethora of data links CCK2R to cancer, and pharmaceutical manipulation of this receptor has been evaluated in clinical trials for pancreatic cancer (51, 52). With equivocal results emerging from these studies, it will be vital to identify the spectrum of CCK2R variants present in the cancer of interest and to proceed with a therapy that is efficacious at all mutant receptors.
Supplementary Material
Acknowledgments
The authors thank Tiffanie Skillman, Andrew Capen, Janine Ptak, Natalie Silliman, and Steve Szabo for technical support; James Starling, Jonathan Sedgwick, and Christoph Reinhard for project support; and Jeremy Graff, Sudhakar Chintharlapalli, and Francis Willard for review of the manuscript.
Grant Support
This work was supported by The Virginia and D.K. Ludwig Fund for Cancer Research and NIH grants CA57345, CA62924, and CA 121113.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked advertisement in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
Current address for B. Feng: Center for Molecular Recognition, College of Physicians and Surgeons, Columbia University, New York, NY 10032. Current address for T. Sjöblom, Department of Immunology, Genetics and Pathology, Uppsala University, SE-751 85 Uppsala, Sweden.
Authors’ Contributions
Conception and design: M.D. Willard, M.T. Uhlik, V.E. Velculescu, S.D. Markowitz, B. Vogelstein, T.D. Barber
Development of methodology: M.D. Willard, M.E. Lajiness, I.H. Wulur, M.L. Swearingen, M.T. Uhlik, V.E. Velculescu, T. Sjöblom, S.D. Markowitz, T.D. Barber
Acquisition of data (provided animals, acquired and managed patients, provided facilities, etc.): M.D. Willard, M.E. Lajiness, I.H. Wulur, B. Feng, M.L. Swearingen, K.W. Kinzler, V.E. Velculescu, S.D. Markowitz, S.M. Powell, T.D. Barber
Analysis and interpretation of data (e.g., statistical analysis, biostatistics, computational analysis): M.D. Willard, M.E. Lajiness, B. Feng, M.L. Swearingen, M.T. Uhlik, V.E. Velculescu, T.D. Barber
Writing, review, and/or revision of the manuscript: M.D. Willard, M.E. Lajiness, M.L. Swearingen, M.T. Uhlik, K.W. Kinzler, T. Sjöblom, S.D. Markowitz, S.M. Powell, B. Vogelstein, T.D. Barber
Administrative, technical, or material support (i.e., reporting or organizing data, constructing databases): M.E. Lajiness, T. Sjöblom, S.M. Powell, T.D. Barber Study supervision: M.D. Willard, V.E. Velculescu, T.D. Barber
Disclosure of Potential Conflicts of Interest
K.W. Kinzler has ownership interest (including patents) in Lilly Licensed Patent. V.E. Velculescu has employment (other than primary affiliation; e.g., consulting) as the board of directors, has ownership interest (including patents), and is a consultant/advisory board member in Personal Genome Diagnostics. B. Vogelstein has ownership interest (including patents) in Eli Lilly. No potential conflicts of interests were disclosed by other authors.
References
- 1.Millar RP, Newton CL. The year in G protein-coupled receptor research. Mol Endocrinol. 2010;24:261–74. doi: 10.1210/me.2009-0473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Korner M, Waser B, Reubi JC, Miller LJ. CCK(2) receptor splice variant with intron 4 retention in human gastrointestinal and lung tumours. J Cell Mol Med. 2010;14:933–43. doi: 10.1111/j.1582-4934.2009.00859.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dufresne M, Seva C, Fourmy D. Cholecystokinin and gastrin receptors. Physiol Rev. 2006;86:805–47. doi: 10.1152/physrev.00014.2005. [DOI] [PubMed] [Google Scholar]
- 4.Daulhac L, Kowalski-Chauvel A, Pradayrol L, Vaysse N, Seva C. Gastrin stimulates the formation of a p60Src/p125FAK complex upstream of the phosphatidylinositol 3-kinase signaling pathway. FEBS Lett. 1999;445:251–5. doi: 10.1016/s0014-5793(99)00129-5. [DOI] [PubMed] [Google Scholar]
- 5.Stepan VM, Krametter DF, Matsushima M, Todisco A, Delvalle J, Dickinson CJ. Glycine-extended gastrin regulates HEK cell growth. Am J Physiol. 1999;277:R572–81. doi: 10.1152/ajpregu.1999.277.2.R572. [DOI] [PubMed] [Google Scholar]
- 6.Kowalski-Chauvel A, Pradayrol L, Vaysse N, Seva C. Gastrin stimulates tyrosine phosphorylation of insulin receptor substrate 1 and its association with Grb2 and the phosphatidylinositol 3-kinase. J Biol Chem. 1996;271:26356–61. doi: 10.1074/jbc.271.42.26356. [DOI] [PubMed] [Google Scholar]
- 7.Ferrand A, Bertrand C, Portolan G, Cui G, Carlson J, Pradayrol L, et al. Signaling pathways associated with colonic mucosa hyperproliferation in mice overexpressing gastrin precursors. Cancer Res. 2005;65:2770–7. doi: 10.1158/0008-5472.CAN-04-0978. [DOI] [PubMed] [Google Scholar]
- 8.Taniguchi T, Matsui T, Ito M, Murayama T, Tsukamoto T, Katakami Y, et al. Cholecystokinin-B/gastrin receptor signaling pathway involves tyrosine phosphorylations of p125FAK and p42MAP. Oncogene. 1994;9:861–7. [PubMed] [Google Scholar]
- 9.Yu HG, Schafer H, Mergler S, Muerkoster S, Cramer T, Hocker M, et al. Valine-286 residue in the third intracellular loop of the cholecystokinin 2 receptor exerts a pivotal role in cholecystokinin 2 receptor mediated intracellular signal transduction in human colon cancer cells. Cell Signal. 2005;17:1505–15. doi: 10.1016/j.cellsig.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 10.Chao C, Goluszko E, Lee YT, Kolokoltsov AA, Davey RA, Uchida T, et al. Constitutively active CCK2 receptor splice variant increases Src-dependent HIF-1 alpha expression and tumor growth. Oncogene. 2007;26:1013–9. doi: 10.1038/sj.onc.1209862. [DOI] [PubMed] [Google Scholar]
- 11.Schmitz F, Otte JM, Stechele HU, Reimann B, Banasiewicz T, Folsch UR, et al. CCK-B/gastrin receptors in human colorectal cancer. Eur J Clin Invest. 2001;31:812–20. doi: 10.1046/j.1365-2362.2001.00870.x. [DOI] [PubMed] [Google Scholar]
- 12.Dhami GK, Ferguson SS. Regulation of metabotropic glutamate receptor signaling, desensitization and endocytosis. Pharmacol Ther. 2006;111:260–71. doi: 10.1016/j.pharmthera.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 13.Hellmich MR, Rui XL, Hellmich HL, Fleming RY, Evers BM, Townsend CM., Jr Human colorectal cancers express a constitutively active cholecystokinin-B/gastrin receptor that stimulates cell growth. J Biol Chem. 2000;275:32122–8. doi: 10.1074/jbc.M005754200. [DOI] [PubMed] [Google Scholar]
- 14.Wu SV, Yang M, Avedian D, Birnbaumer M, Walsh JH. Single amino acid substitution of serine82 to asparagine in first intracellular loop of human cholecystokinin (CCK)-B receptor confers full cyclic AMP responses to CCK and gastrin. Mol Pharmacol. 1999;55:795–803. [PubMed] [Google Scholar]
- 15.Jones S, Li M, Parsons DW, Zhang X, Wesseling J, Kristel P, et al. Somatic mutations in the chromatin remodeling gene ARID1A occur in several tumor types. Hum Mutat. 2012;33:100–3. doi: 10.1002/humu.21633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barber TD, McManus K, Yuen KW, Reis M, Parmigiani G, Shen D, et al. Chromatid cohesion defects may underlie chromosome instability in human colorectal cancers. Proc Natl Acad Sci U S A. 2008;105:3443–8. doi: 10.1073/pnas.0712384105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ding WQ, Kuntz SM, Miller LJ. A misspliced form of the cholecystokinin-B/gastrin receptor in pancreatic carcinoma: role of reduced cellular U2AF35 and a suboptimal 3′-splicing site leading to retention of the fourth intron. Cancer Res. 2002;62:947–52. [PubMed] [Google Scholar]
- 18.Smith JP, Verderame MF, McLaughlin P, Martenis M, Ballard E, Zagon IS. Characterization of the CCK-C (cancer) receptor in human pancreatic cancer. Int J Mol Med. 2002;10:689–94. [PubMed] [Google Scholar]
- 19.Kan Z, Jaiswal BS, Stinson J, Janakiraman V, Bhatt D, Stern HM, et al. Diverse somatic mutation patterns and pathway alterations in human cancers. Nature. 2010;466:869–73. doi: 10.1038/nature09208. [DOI] [PubMed] [Google Scholar]
- 20.Chao C, Ives KL, Goluszko E, Kolokoltsov AA, Davey RA, Townsend CM, Jr, et al. SRC regulates constitutive internalization and rapid resensitization of a cholecystokinin 2 receptor splice variant. J Biol Chem. 2005;280:33368–73. doi: 10.1074/jbc.M506337200. [DOI] [PubMed] [Google Scholar]
- 21.Machesky LM. Lamellipodia and filopodia in metastasis and invasion. FEBS Lett. 2008;582:2102–11. doi: 10.1016/j.febslet.2008.03.039. [DOI] [PubMed] [Google Scholar]
- 22.Noble PJ, Wilde G, White MR, Pennington SR, Dockray GJ, Varro A. Stimulation of gastrin-CCKB receptor promotes migration of gastric AGS cells via multiple paracrine pathways. Am J Physiol. 2003;284:G75–84. doi: 10.1152/ajpgi.00300.2002. [DOI] [PubMed] [Google Scholar]
- 23.Donovan D, Brown NJ, Bishop ET, Lewis CE. Comparison of three in vitro human ‘angiogenesis’ assays with capillaries formed in vivo. Angiogenesis. 2001;4:113–21. doi: 10.1023/a:1012218401036. [DOI] [PubMed] [Google Scholar]
- 24.Cheng SY, Huang HJ, Nagane M, Ji XD, Wang D, Shih CC, et al. Suppression of glioblastoma angiogenicity and tumorigenicity by inhibition of endogenous expression of vascular endothelial growth factor. Proc Natl Acad Sci U S A. 1996;93:8502–7. doi: 10.1073/pnas.93.16.8502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Olszewska-Pazdrak B, Townsend CM, Jr, Hellmich MR. Agonist-independent activation of Src tyrosine kinase by a cholecystokinin-2 (CCK2) receptor splice variant. J Biol Chem. 2004;279:40400–4. doi: 10.1074/jbc.C400208200. [DOI] [PubMed] [Google Scholar]
- 26.Kumble S, Omary MB, Cartwright CA, Triadafilopoulos G. Src activation in malignant and premalignant epithelia of Barrett's esophagus. Gastroenterology. 1997;112:348–56. doi: 10.1053/gast.1997.v112.pm9024288. [DOI] [PubMed] [Google Scholar]
- 27.Lutz MP, Esser IB, Flossmann-Kast BB, Vogelmann R, Luhrs H, Friess H, et al. Overexpression and activation of the tyrosine kinase Src in human pancreatic carcinoma. Biochem Biophys Res Commun. 1998;243:503–8. doi: 10.1006/bbrc.1997.8043. [DOI] [PubMed] [Google Scholar]
- 28.Talamonti MS, Roh MS, Curley SA, Gallick GE. Increase in activity and level of pp60c-src in progressive stages of human colorectal cancer. J Clin Invest. 1993;91:53–60. doi: 10.1172/JCI116200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cartwright CA, Coad CA, Egbert BM. Elevated c-Src tyrosine kinase activity in premalignant epithelia of ulcerative colitis. J Clin Invest. 1994;93:509–15. doi: 10.1172/JCI117000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Weber TK, Steele G, Summerhayes IC. Differential pp60c-src activity in well and poorly differentiated human colon carcinomas and cell lines. J Clin Invest. 1992;90:815–21. doi: 10.1172/JCI115956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dorsam RT, Gutkind JS. G-protein-coupled receptors and cancer. Nat Rev. 2007;7:79–94. doi: 10.1038/nrc2069. [DOI] [PubMed] [Google Scholar]
- 32.Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med. 1971;285:1182–6. doi: 10.1056/NEJM197111182852108. [DOI] [PubMed] [Google Scholar]
- 33.Richard DE, Vouret-Craviari V, Pouyssegur J. Angiogenesis and G-protein-coupled receptors: signals that bridge the gap. Oncogene. 2001;20:1556–62. doi: 10.1038/sj.onc.1204193. [DOI] [PubMed] [Google Scholar]
- 34.Ferrara N, Alitalo K. Clinical applications of angiogenic growth factors and their inhibitors. Nat Med. 1999;5:1359–64. doi: 10.1038/70928. [DOI] [PubMed] [Google Scholar]
- 35.Kawasaki D, Emori Y, Eta R, Iino Y, Hamano H, Yoshinaga K, et al. Effect of Z-360, a novel orally active CCK-2/gastrin receptor antagonist on tumor growth in human pancreatic adenocarcinoma cell lines in vivo and mode of action determinations in vitro. Cancer Chemother Pharmacol. 2008;61:883–92. doi: 10.1007/s00280-007-0591-8. [DOI] [PubMed] [Google Scholar]
- 36.Kobayashi N, Seto K, Orikawa Y, Hamano H, Yoshinaga K, Takei M. Z-360, a novel cholecystokinin-2/gastrin receptor antagonist, inhibits gemcitabine-induced expression of the vascular endothelial growth factor gene in human pancreatic cancer cells. Biol Pharm Bulletin. 2010;33:216–22. doi: 10.1248/bpb.33.216. [DOI] [PubMed] [Google Scholar]
- 37.Ellrichmann M, Ritter PR, Schrader H, Schmidt WE, Meier JJ, Schmitz F. Gastrin stimulates the VEGF-A promotor in a human colon cancer cell line. Regul Pept. 2010;165:146–50. doi: 10.1016/j.regpep.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 38.Colucci R, Blandizzi C, Tanini M, Vassalle C, Breschi MC, Del Tacca M. Gastrin promotes human colon cancer cell growth via CCK-2 receptor-mediated cyclooxygenase-2 induction and prostaglandin E2 production. Br J Pharmacol. 2005;144:338–48. doi: 10.1038/sj.bjp.0706053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Murayama T, Matsumori Y, Iwata N, Ito M, Taniguchi T, Chihara K, et al. Antiproliferative effect of a novel cholecystokinin-B/gastrin receptor antagonist, YM022. Jpn J Cancer Res. 1996;87:743–50. doi: 10.1111/j.1349-7006.1996.tb00287.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Artru P, Attoub S, Levasseur S, Lewin MJ, Bado A. [Gastrin-17 and G17-gly induce proliferation of LoVo cells through the CCK B/gastrin receptor]. Gastroenterol Clin Biol. 1998;22:607–12. [PubMed] [Google Scholar]
- 41.Li A, Dubey S, Varney ML, Dave BJ, Singh RK. IL-8 directly enhanced endothelial cell survival, proliferation, and matrix metalloproteinases production and regulated angiogenesis. J Immunol. 2003;170:3369–76. doi: 10.4049/jimmunol.170.6.3369. [DOI] [PubMed] [Google Scholar]
- 42.Rundhaug JE, Lockhart AC, et al. Reduction of wound angiogenesis in patients treated with BMS-275291, a broad spectrum matrix metalloproteinase inhibitor. Clin Cancer Res. 2003;9:551–4. Matrix metalloproteinases, angiogenesis, and cancer: commentary re: Clin. Cancer Res., 9: 00–00, 2003.
- 43.Hiraoka S, Miyazaki Y, Kitamura S, Toyota M, Kiyohara T, Shinomura Y, et al. Gastrin induces CXC chemokine expression in gastric epithelial cells through activation of NF-kappaB. Am J Physiol. 2001;281:G735–42. doi: 10.1152/ajpgi.2001.281.3.G735. [DOI] [PubMed] [Google Scholar]
- 44.Huang H, Ansorge N, Schrader H, Banasch M, Yu HG, Schmidt WE, et al. The CCK-2/gastrin splice variant receptor retaining intron 4 transactivates the COX-2 promoter in vitro. Regul Pept. 2007;144:34–42. doi: 10.1016/j.regpep.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 45.Langer I, Tikhonova IG, Boulegue C, Esteve JP, Vatinel S, Ferrand A, et al. Evidence for a direct and functional interaction between the regulators of G protein signaling-2 and phosphorylated C terminus of cholecystokinin-2 receptor. Mol Pharmacol. 2009;75:502–13. doi: 10.1124/mol.108.051607. [DOI] [PubMed] [Google Scholar]
- 46.Vatinel S, Ferrand A, Lopez F, Kowalski-Chauvel A, Esteve JP, Fourmy D, et al. An ITIM-like motif within the CCK2 receptor sequence required for interaction with SHP-2 and the activation of the AKT pathway. Biochim Biophys Acta. 2006;1763:1098–107. doi: 10.1016/j.bbamcr.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 47.Ferrand A, Vatinel S, Kowalski-Chauvel A, Bertrand C, Escrieut C, Fourmy D, et al. Mechanism for Src activation by the CCK2 receptor: patho-physiological functions of this receptor in pancreas. World J Gastroenterol. 2006;12:4498–503. doi: 10.3748/wjg.v12.i28.4498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gales C, Kowalski-Chauvel A, Dufour MN, Seva C, Moroder L, Pradayrol L, et al. Mutation of Asn-391 within the conserved NPXXY motif of the cholecystokinin B receptor abolishes Gq protein activation without affecting its association with the receptor. J Biol Chem. 2000;275:17321–7. doi: 10.1074/jbc.M909801199. [DOI] [PubMed] [Google Scholar]
- 49.Skotheim RI, Nees M. Alternative splicing in cancer: noise, functional, or systematic? Int J Biochem Cell Biol. 2007;39:1432–49. doi: 10.1016/j.biocel.2007.02.016. [DOI] [PubMed] [Google Scholar]
- 50.Chao C, Han X, Ives K, Park J, Kolokoltsov AA, Davey RA, et al. CCK2 receptor expression transforms non-tumorigenic human NCM356 colonic epithelial cells into tumor forming cells. Int J Cancer. 2010;126:864–75. doi: 10.1002/ijc.24845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chau I, Cunningham D, Russell C, Norman AR, Kurzawinski T, Harper P, et al. Gastrazole (JB95008), a novel CCK2/gastrin receptor antagonist, in the treatment of advanced pancreatic cancer: results from two randomised controlled trials. Br J Cancer. 2006;94:1107–15. doi: 10.1038/sj.bjc.6603058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Meyer T, Caplin ME, Palmer DH, Valle JW, Larvin M, Waters JS, et al. A phase Ib/IIa trial to evaluate the CCK2 receptor antagonist Z-360 in combination with gemcitabine in patients with advanced pancreatic cancer. Eur J Cancer. 2010;46:526–33. doi: 10.1016/j.ejca.2009.11.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.