Abstract
Background and Objective
The primary therapy for deep tissue abscesses is drainage accompanied by systemic antimicrobial treatment. However, the long antibiotic course required increases the probability of acquired resistance, and the high incidence of polymicrobial infections in abscesses complicates treatment choices. Photodynamic therapy (PDT) is effective against multiple classes of organisms, including those displaying drug resistance, and may serve as a useful adjunct to the standard of care by reduction of abscess microbial burden following drainage.
Study Design/Materials and Methods
Aspirates were obtained from 32 patients who underwent image-guided percutaneous drainage of the abscess cavity. The majority of the specimens (24/32) were abdominal, with the remainder from liver and lung. Conventional microbiological techniques and nucleotide sequence analysis of rRNA gene fragments were used to characterize microbial populations from abscess aspirates. We evaluated the sensitivity of microorganisms to methylene blue-sensitized PDT in vitro both within the context of an abscess aspirate and as individual isolates.
Results
Most isolates were bacterial, with the fungus Candida tropicalis also isolated from two specimens. We examined the sensitivity of these microorganisms to methylene blue-PDT. Complete elimination of culturable microorganisms was achieved in three different aspirates, and significant killing (p < 0.0001) was observed in all individual microbial isolates tested compared to controls.
Conclusions
These results and the technical feasibility of advancing optical fibers through catheters at the time of drainage motivate further work on including PDT as a therapeutic option during abscess treatment.
Keywords: antimicrobial, bacteria, fungi, image-guided, methylene blue
INTRODUCTION
An abscess is an inflammatory lesion in a tissue space that releases purulent material (1), with patients frequently presenting fever, chills and pain in the proximity of the lesion. The host response to infection results in the formation of an amorphous, fibrin-rich pseudocapsule that encases the organisms and allows entry of infiltrating leukocytes to combat the infection (2,3). Furthermore, the organisms within the abscess may exist as an organized, biofilm-like community (4,5) with sectors of the microbial population physiologically adapted to tolerate antibiotics, resulting in microbial persistence (6,7).
While abscesses in superficial sites can be readily accessed, drained and treated topically (8), deep tissue abscesses caused by bacteria and fungi pose a more difficult therapeutic challenge (9). Many infected abscesses that form in proximity to mucosal sites rich in microbial flora, such as the oral cavity, the nasopharynx, and digestive tract, are polymicrobial (10–12), further complicating conventional antimicrobial therapy. Since the 1980’s, percutaneous abscess drainage has been a successful alternative to open surgical drainage (13–15). Percutaneous abscess drainage uses image-guided techniques to insert a thin needle into the abscess, leaving a drainage tube in place to remove the infected fluid. The procedure is minimally invasive and the recovery period is usually days to weeks. However, if percutaneous abscess drainage and subsequent antibiotic therapy does not lead to clinical improvement, open surgical drainage of the pseudocapsule is required, and is accompanied by an increased risk of morbidity and mortality (16,17). Prolonged antibiotic therapy regimens that accompany drainage, the frequent isolation of the antibiotic-resistant “ESKAPE” bacterial pathogens (18) from intra-abdominal (9) and liver (17) abscesses, and the presence of antibiotic-resistant strains in intestinal flora (19) that can seed deep tissue sites also diminish the chances for resolution of infection. Therefore, modifications to current therapy of infected abscesses to reduce the microbial burden in the abscess cavity could potentially decrease the frequency of recurrence of infection and the time course of conventional antibiotic therapy, and result in improved clinical outcomes. The corresponding reduction in length of hospital stay would substantially reduce health care costs.
Photodynamic therapy (PDT) is being actively developed as a means of treating localized infections under conditions where antibiotics are not fully effective due to acquired or adaptive resistance (20–22). We tested the feasibility of using PDT as an adjunct to drainage of infected abscesses by determining the sensitivity of microorganisms in abscess aspirates to this process. Numerous investigators have demonstrated that a wide variety of pathogenic bacteria and fungi are sensitive to PDT in vitro (reviewed in (23–25)). Furthermore, a high degree of photosensitizer selectivity for the microbe in the context of infection can be achieved (26). While PDT has not yet been used in clinical scenarios for therapy of deep abscesses, there is little reason to expect that it would not be amenable, indeed ideal, for approaches combined with state-of-the-art imaging and techniques commonly employed in the practice of interventional radiology. The ability to significantly reduce the microbial burden in the aspirated material using PDT would be a strong positive indicator that PDT could be used to decontaminate the abscess site following drainage. We examined the sensitivity of microorganisms from human abscess aspirates to PDT in the context of the aspirate contents, as well as individually in culture. A high degree of efficacy for antimicrobial PDT was demonstrated in both contexts, an important first step toward establishing the feasibility of this new clinical application of PDT.
MATERIALS AND METHODS
Human Subject Identification and Recruitment
The University of Rochester Medical Center Research Subjects Review Board approved all consenting and procedural protocols. Abscess aspirates were obtained from patients who underwent percutaneous drainage of the abscess cavity by interventional radiology at Strong Memorial Hospital in Rochester, NY. Males or females over the age of 18 diagnosed with a localized infected abscess, and able to understand the study and give proper informed consent, were eligible for inclusion in the study. This group included both hospitalized patients and outpatients. Exclusion criteria included insufficient quantity of infected fluid available for the research procedures. Patients with known infectious diseases such as HIV, hepatitis C and tuberculosis were excluded, as were vulnerable subjects, or those unable or unwilling to understand or to provide informed consent.
Collection of clinical samples and initial processing
Standard drainage of an abscess cavity typically yielded fluid in excess of that required for clinical testing. Up to 5 ml of the excess aspirate fluid was inoculated into sealed Port-A Cul™ vials containing a reduced transport medium (BD Diagnostics-BBL, Sparks, MD) designed to maintain the viability of aerobic, facultative and anaerobic microorganisms. Samples were processed immediately for culturable organisms on microbiological media. Aliquots of the aspirate were snap-frozen on dry ice and stored at −80°C until used for PDT studies.
Microbiological evaluation of abscess aspirate contents
Microorganisms were isolated from the abscess aspirate by propagation on both permissive and selective/differential microbiological agar media for initial phenotypic characterization. Approximately 10 µl of aspirate were plated, in duplicate, on tryptic soy agar (TSA) + 5% sheep’s blood as a permissive medium for bacteria and fungi, Chocolate TSA agar for anaerobic culture and cultivation of highly fastidious bacteria, MacConkey’s agar for enrichment of Gram negative rods and determination of lactose fermentation, and Columbia agar supplemented with 5% sheep’s blood, colistin and nalidixic acid (CNA) for enrichment of Gram positive bacteria (BD Diagnostics-BBL, Sparks, MD). Chocolate plates were incubated under anaerobic conditions using a portable GasPak Anaerobe System (BD Diagnostics-BBL). Gram positive cocci were initially assessed for catalase production by mixing with 3% hydrogen peroxide and examined visually for oxygen bubble formation. Catalase positive Gram positive cocci were tested for coagulase production using rabbit coagulase plasma per manufacturer’s instructions (BD Diagnostics-BBL). Catalase negative Gram positive cocci were tested for esculin hydrolysis following inoculation on bile-esculin agar slants (BD Diagnostics-BBL) at overnight incubation at 37°C. All strains isolated were archived at −80° C.
Microscopic examination of abscess aspirate
For visual documentation of both microorganism and inflammatory cell populations in the abscess aspirate, thin smears of 10 µl volumes of aspirate were prepared on glass slides, and either Gram stained (BD Diagnostics-BBL) or Giemsa stained (Hematology 3-Step Stain, Richard-Allan Scientific, Kalamazoo, MI), respectively. Stained preparations were examined by light microscopy.
Extraction of genomic DNA and identification of individual microbial isolates
Strains cultured from each abscess sample were identified by nucleotide sequence analysis of rRNA gene fragments. Individual bacterial isolates were grown overnight in tryptic soy broth (TSB) (Becton Dickinson-Difco, Sparks, MD); fungal isolates were grown overnight in 1% yeast extract-2% peptone-2% dextrose (YPD) broth (Becton-Dickinson-Difco). Genomic DNA from both bacterial and fungal isolates was extracted using the DNeasy Blood and Tissue kit (Qiagen, Valencia, CA). Bacterial sequences were amplified by PCR using universal primers (27) from the 16S rRNA gene (Forward: 5’-AGAGTTTGATCCTGGCTCAG-3’; Reverse: 5’-GGTTACCTTGTTACGACTT-3’). Fungal sequences were amplified by PCR using primers flanking the internal transcribed spacer (ITS) regions (28) of the rRNA operon (Forward: 5’-TCCGTAGGTGAACCTGCGG-3’; Reverse: 5’-TCCTCCGCTTATTGATATGC-3’). The nucleotide sequence of all amplicons was determined (GENEWIZ, South Plainfield, NJ) for both DNA strands using the corresponding primers. Organisms were identified using the Basic Local Alignment Search Tool (BLAST) of the National Center for Biotechnology Information (NCBI; http://blast.ncbi.nlm.nih.gov/).
PDT of abscess aspirates
Three microliters of methylene blue (MB; 10 mg/ml, clinical grade, American Regent, Shirley, NY) (29) were added directly to 0.1 ml of aspirate to yield a final MB concentration of 938 µM (300 µg/ml). This concentration of photosensitizer was used in our previous studies of PDT using an in vivo model of infection (26). An equivalent volume of aspirate was left untreated as a negative control. Samples were incubated 10 min at room temperature, resuspended in 1 ml PBS and the cells were pelleted by centrifugation. The supernatant was removed, the pellet resuspended in 1 ml PBS, placed in a 6-well tissue culture dish, and either subjected to irradiation from below using broadband visible light (7.2 J/cm2) or shielded. The emission spectrum of the light source used in this study is described previously (30). Briefly, the output of this source has significant spectral overlap with the absorption spectrum of MB. Each of the three experimental groups (MB-Irradiated, MB-Shielded and Untreated-Irradiated) was assayed in duplicate. Serial dilutions from each sample were prepared in sterile dH2O, 10 µl volumes of each dilution series were spread onto LB plates (31) and plates were incubated 24–48 h at 37°C. Data represent the mean ± S.D. of log10 reduction in colony forming units (cfu) compared to the Untreated-Irradiated group.
PDT of representative organisms cultured from abscess samples
Bacteria were grown to overnight in TSB; fungi were grown overnight in YPD broth. Organisms were washed twice with dH2O, adjusted to OD600 nm = 1.0, and incubated with 313 µM (100 µg/ml) MB for 10 min. To compare PDT of C. tropicalis to earlier studies on C. albicans and C. glabrata (26,32), we included cells incubated with the cationic porphyrin photosensitizer meso-tetra (N-methy-4-pyridyl) porphine tetra tosylate 7.34 µM (10 µg/ml) TMP-1363; Frontier Scientific, Logan UT) treated as described for MB. Control cells were left untreated. Cells were pelleted by centrifugation, the supernatant removed, and the cells resuspended to OD600 nm = 1.0 in dH2O. This optical density corresponded to 1–3 × 107 cfu/ml. A 2 ml volume of either photosensitizer-treated cells or control cells was placed in a 6-well tissue culture dish. Cells were either subjected to irradiation from below using broadband visible light (7.2 J/cm2), or shielded (26). Experiments were performed three times in duplicate. Serial dilutions were prepared in sterile dH2O, 10 µl volumes of each dilution series were spread (31) onto LB plates for bacteria or YPD plates for Candida, and plates were incubated 24–48 h at 37°C. Data represent the mean ± S.D. of log10 reduction in cfu compared to untreated organisms.
Statistical analysis of PDT efficacy
Pair-wise comparisons were made using the Student’s t test to determine whether there was a significant reduction in colony forming units in samples exposed to photosensitizer and irradiated compared to controls. In each case, p values ≤0.05 were considered significant.
RESULTS
Abscess aspirate collection and characterization
Over a 10-month period, 32 abscess aspirates were obtained during image-guided drainage procedures. Table 1 lists the sites of aspirate drainage and culture findings from the samples. Since the gastro-intestinal tract harbors the highest concentration of microorganisms on the body, it was not surprising that 75% of the sites drained during the collection period (24/32) were peritoneal, intra-abdominal, pelvic or anal. Four samples were obtained from the lung or thoracic cavity, and four from the liver. The vast majority of the cultured isolates from the abscess aspirates were bacterial, representative of organisms seen in previous studies (9,33,34). In addition, a retro-peritoneal abscess and an intra-abdominal abscess from two different patients each yielded Candida tropicalis as the sole cultured isolate. C. tropicalis is a common cause of peritonitis in patients harboring a peritoneal dialysis catheter (35). Although most samples were quite purulent, microscopy demonstrated that the microbial burden in the aspirates was usually not high, with small clusters of organisms associated with infiltrating leukocytes (data not shown). Twelve of 32 samples yielded no organisms using the culture conditions described. Possible explanations for this observation were that the body’s defenses had cleared the infection prior to sample collection, the level of culturable organisms organism was below the threshold of detection, or the methods used for cultivation were unable to support growth of the etiologic agent(s) of infection.
TABLE 1.
Abscess samples and culture findings.
| Patient samplea | Site of catheter | Culture findingsb | Organism type |
|---|---|---|---|
| 1. | lung | NGc | |
| 2. | retro-peritoneal | Candida tropicalis | fungus |
| 3. | intra-peritoneal | Candida tropicalis | fungus |
| 4. | intra-peritoneal | Streptococcus intermedius | GPCd |
| 5. | intra-abdominal | Enterococcus faecium | GPC |
| Staphylococcus aureus | GPC | ||
| Staphylococcus simulans | GPC | ||
| 6. | intra-peritoneal | NG | |
| 7. | intra-peritoneal | NG | |
| 8. | retro-peritoneal | Streptococcus agalactiae | GPC |
| 9. | intra-abdominal | Staphylococcus aureus | GPC |
| Escherichia coli | GNRe | ||
| 10. | intra-peritoneal | NG | |
| 11. | lung | Staphylococcus aureus | GPC |
| 12. | intra-abdominal | NG | |
| 13. | intra-thoracic | NG | |
| 14. | pelvis | NG | |
| 15. | liver | Enterococcus avium | GPC |
| Klebsiella pneumoniae | GNR | ||
| Clostridium perfringens | GPRf | ||
| 16. | intra-peritoneal | Enterococcus faecium | GPC |
| 17. | intra-abdominal | Enterococcus raffinosus | GPC |
| Escherichia coli | GNR | ||
| Klebsiella pneumoniae | GNR | ||
| 18. | intra-abdominal | Pseudomonas aeruginosa | GNR |
| 19. | intra-abdominal | Proteus mirabilis | GNR |
| Corynebacterium striatum | GPR | ||
| Streptococcus intermedius | GPC | ||
| 20. | intra-peritoneal | NG | |
| 21. | pelvis | Enterococcus faecalis | GPC |
| Enterococcus faecium | GPC | ||
| 22. | liver | Enterococcus hirae | GPC |
| Streptococcus anginosus | GPC | ||
| Staphylococcus epidermidis | GPC | ||
| 23. | intra-peritoneal | NG | |
| 24. | pelvis | Escherichia fergusonii | GNR |
| Enterococcus faecalis | GPC | ||
| 25. | liver | Enterococcus hirae | GPC |
| Staphylococcus epidermidis | GPC | ||
| 26. | intra-thoracic | NG | |
| 27. | groin | Staphylococcus aureus | GPC |
| 28. | retroperitoneal | Streptococcus intermedius | GPC |
| 29. | liver | NG | |
| 30. | intra-abdominal | NG | |
| 31. | anus | Parvimonas micra | GPC |
| Streptococcus constellatus | GPC | ||
| Prevotella nigrescens | GNR | ||
| 32. | intra-abdominal | Streptococcus constellatus | GPC |
Samples listed in chronological order.
Culturable strains isolated from each sample were identified by 16S rRNA sequence analysis.
No growth on any medium tested.
GPC; Gram positive coccus
GNR; Gram negative rod
GPR; Gram positive rod
PDT of representative organisms cultured from abscess samples
Selected Gram positive and Gram negative bacterial isolates were grown overnight and subjected to MB-PDT (Figure 1). The Enterococcus, Staphylococcus and Klebsiella isolates were chosen as representatives from the “ESKAPE” group of bacterial pathogens (18). Notably, all bacterial strains tested were killed to a significant degree (p<0.0001) by MB-PDT at a stage of growth known to lead to the generation of a high percentage of antibiotic-tolerant “persister” cells (6,7), and at cell densities considerably higher than present in the primary abscess aspirates, with minimal dark toxicity observed. Both MB-PDT and TMP-1363-PDT also resulted in a high degree of phototoxicity against two strains of Candida tropicalis isolated from different patients (Figure 2). Compared to untreated cells, the phenothiazine MB exerted significant dark toxicity against shielded Candida tropicalis yeast forms; however the porphyrin TMP-1363 did not. The mechanism behind this difference in C. tropicalis remains unknown. No phototoxicity was observed in irradiated, untreated cells with any organism tested (data not shown).
Figure 1. Methylene blue-PDT of bacterial clinical isolates.
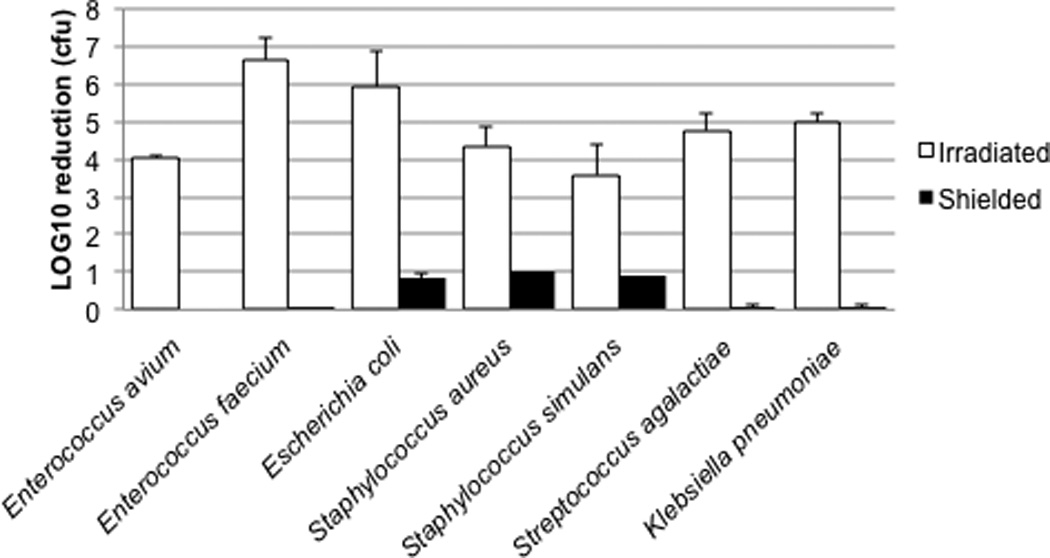
For each organism, three experimental groups (MB-Irradiated, MB-Shielded and Untreated-Irradiated) were assayed in duplicate in three separate experiments. Results are expressed as the mean ± S.D. of log10 reduction in colony forming units (cfu) compared to the Untreated-Irradiated group. The absolute number of organisms in untreated groups = 1–3 ×107 cfu/ml.
Figure 2. PDT of Candida tropicalis.
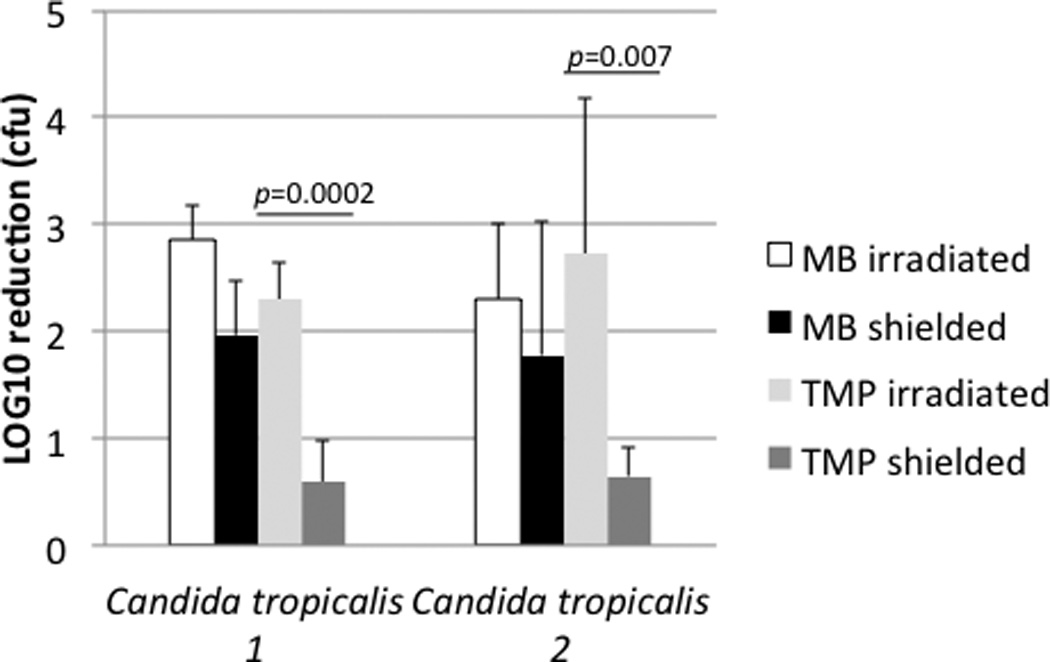
Phenathiazine photosensitizer MB was compared to porphyrin photosensitizer TMP-1363. For each organism, three experimental groups (MB-Irradiated, MB-Shielded and Untreated-Irradiated) were assayed in duplicate in three separate experiments. Results are expressed as the mean ± S.D. of log10 reduction in colony forming units (cfu) compared to the Untreated-Irradiated group. The absolute number of organisms in untreated groups = 1–3 ×107 cfu/ml.
PDT of abdominal abscess aspirates
As proof of principle, PDT using methylene blue as a photosensitizer (MB-PDT) was performed on three abscess aspirate samples. Enterococci and staphylococci were cultured from sample A5, Streptococcus agalactiae (Lancefield Group B) was the sole organism cultured from sample A8, and Staph. aureus and E. coli were cultured from sample A9 (Table 1). MB-PDT resulted in complete killing of all culturable microorganisms in the MB-Irradiated group in each of the three aspirate samples (Figure 3). Less than a one-log10 reduction in culturable organisms was observed in shielded samples compared to samples receiving no MB treatment.
Figure 3. Methylene blue-PDT of polymicrobial aspirates in vitro.
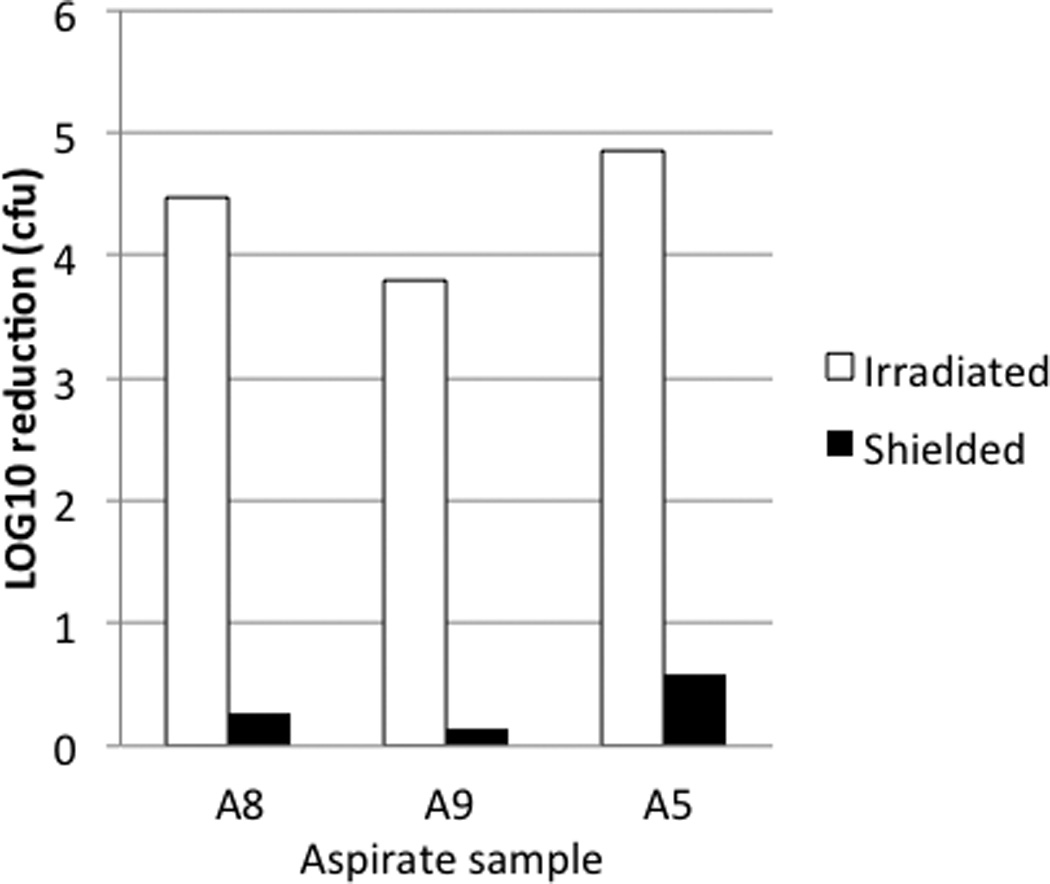
Each of the three experimental groups (MB-Irradiated, MB-Shielded and Untreated-Irradiated) was assayed in duplicate. Results are expressed as log10 reduction in colony forming units (cfu) compared to the Untreated-Irradiated group. In each case, there was complete eradication of culturable organisms in irradiated samples treated with MB.
DISCUSSION
Percutaneous drainage of localized, infected abscesses using image-guided interventional radiology techniques, followed by systemic antibiotic therapy, is the current standard of care. However, there continue to be numerous obstacles to clinical success following abscess drainage. The morbidity associated with deep abscesses is high. In patients with intra-peritoneal abscesses, a prominent group in our study, other investigators have reported average hospital stays of 21–47 days. Furthermore, abscess recurrence or spread was associated with significantly greater mortality (9). Image-guided PDT of deep tumors is now commonly performed (36,37). It is our hypothesis that combining abscess drainage with antimicrobial PDT will reduce recurrence and spread of infection, as well as decrease antibiotic use. Our primary objective was to evaluate the efficacy of PDT against organisms in infected abscesses with an approach that can be translated directly to clinical practice by readily available modifications to current standard of care. The results obtained from the study reported here support this hypothesis and suggest that clinical evaluation of this new approach is warranted.
It has been proposed that antibiotics contribute to cell death by triggering the formation of reactive oxygen species (ROS), primarily hydroxyl radical (38,39). The induction of ROS in this way is difficult to regulate, and the microbe responds to this stress by increasing its tolerance to antibiotics (6,7,40). PDT offers a potential solution to these two problems by generating a high degree of oxidative stress that is tightly regulated by controlling the intensity and duration of light exposure. Furthermore, there is no known inducible enzymatic response against singlet oxygen, the predominant ROS produced during PDT.
We demonstrated the efficacy of PDT against Enterococcus, Staphylococcus aureus and Klebsiella pneumoniae, members of the “ESKAPE” bacteria along with Acinetobacter baumannii, Pseudomonas aeruginosa, and Enterobacter. The ESKAPE pathogens are of immediate healthcare concern (18) as high priority targets for the development of new therapeutics, since a substantial portion of clinical isolates display a significant degree of resistance to antibiotic therapy. Furthermore, S. aureus is the major cause of abscesses in skin and soft tissues (8), while Enterococcus, E. coli and other gut flora frequently cause abscesses in proximity to the intestine (10,41). Commensal colonizers of epithelial surfaces that can become etiologic agents of infection in the appropriate setting, such as Staphylococcus epidermidis, Streptococcus agalactiae, Streptococcus anginosus and Candida tropicalis were also identified. All organisms tested were highly sensitive to methylene blue-PDT. The efficacy of PDT against C. tropicalis is important since, in both intra-abdominal (42) and liver (17) abscesses, isolation of Candida species from the abscess predicted a poor clinical outcome. Based on current work by others (43), it is also anticipated that anaerobic organisms found in abscesses will be sensitive to PDT. The recent development of culture-independent molecular approaches to assess organism viability (44,45) could also be used in future studies to determine the PDT response of the non-culturable microorganisms present in the sample.
Gathering basic knowledge on approaches to successfully eliminate organisms in abscess aspirates will facilitate the clinical evaluation of PDT of infected deep tissue abscesses. Our next step would be a clinical trial in which the abscess is drained percutaneously using standard interventional radiology procedures, and then subsequently treated via PDT to eliminate the residual microbial community as a novel addition to the same procedure. The solution containing the photosensitizer would be introduced into the cavity using the drainage catheter. After a few minutes, the solution would be flushed, and a sterile, spherical or cylindrical diffusing tip optical fiber (e.g., Medlight S.A., Ecublens, CH) advanced through the catheter and positioned under image-guidance. The primary photosensitizer used, methylene blue (MB), is approved for use in humans, is available in a sterile clinical formulation, and is already employed in a variety of clinical procedures unrelated to PDT (46,47). Laser light of the appropriate wavelength would be delivered to the abscess cavity through the optical fiber. We note that a commercial MB-PDT system is in use outside the U.S. (48–50) to disinfect periodontal pockets; a clinical situation similar to the infected, deep tissue abscesses we have studied. In the U.K., chemical derivatives of MB are in clinical trials of antimicrobial PDT for treatment of infected diabetic ulcers (51). Furthermore, Biel et al. describe antimicrobial MB-PDT of a biofilm in an endotracheal tube using a fiber optic diffuser catheter (52).
The potential health benefits to the patient of successful implementation of our proposed clinical strategy would be predicted to include reducing the need for surgical intervention, lowering the frequency of recurrence or spread of infection resulting in a reduced hospital stay, and a shorter course of post-treatment antibiotic therapy thereby reducing selective pressure for antibiotic resistance. Together, these outcomes would result in lower overall health care costs for patients undergoing image-guided percutaneous abscess drainage.
ACKNOWLEDGMENTS
The authors thank Rachel Guest for valuable assistance in obtaining patient consent and preparation of bacterial rDNA from individual isolates for nucleotide sequencing. This work was supported by grants AI083421 (CGH) and CA68409 (THF) from the National Institutes of Health. The authors are also grateful for generous support from the Department of Imaging Sciences, University of Rochester Medical Center.
REFERENCES
- 1.Kumar V, Abbas AK, Fausto N. Acute and chronic inflammation. In: Kumar V, Abbas AK, Fausto N, editors. Robbins and Cotran Pathologic Basis of Disease. 8th ed. Philadelphia: Saunders Elsevier; 2010. [Google Scholar]
- 2.Wall SD, Fisher MR, Amparo EG, Hricak H, Higgins CB. Magnetic resonance imaging in the evaluation of abscesses. Am J Roentgenol. 1985;144(6):1217–1221. doi: 10.2214/ajr.144.6.1217. [DOI] [PubMed] [Google Scholar]
- 3.Cheng AG, DeDent AC, Schneewind O, Missiakas D. A play in four acts: Staphylococcus aureus abscess formation. Trends Microbiol. 2011;19(5):225–232. doi: 10.1016/j.tim.2011.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cheng AG, Kim HK, Burts ML, Krausz T, Schneewind O, Missiakas DM. Genetic requirements for Staphylococcus aureus abscess formation and persistence in host tissues. FASEB J. 2009;23(10):3393–3404. doi: 10.1096/fj.09-135467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ricucci D, Siqueira JF., Jr Biofilms and apical periodontitis: study of prevalence and association with clinical and histopathologic findings. J Endod. 2010;36(8):1277–1288. doi: 10.1016/j.joen.2010.04.007. [DOI] [PubMed] [Google Scholar]
- 6.Lewis K. Multidrug tolerance of biofilms and persister cells. Curr Top Microbiol Immunol. 2008;322:107–131. doi: 10.1007/978-3-540-75418-3_6. [DOI] [PubMed] [Google Scholar]
- 7.Lewis K. Persister cells. Annu Rev Microbiol. 2010;64:357–372. doi: 10.1146/annurev.micro.112408.134306. [DOI] [PubMed] [Google Scholar]
- 8.Dryden MS. Skin and soft tissue infection: microbiology and epidemiology. Int J Antimicrob Agents. 2009;34(Suppl 1):S2–S7. doi: 10.1016/S0924-8579(09)70541-2. [DOI] [PubMed] [Google Scholar]
- 9.Altemeier WA, Culbertson WR, Fullen WD, Shook CD. Intra-abdominal abscesses. Am J Surg. 1973;125(1):70–79. doi: 10.1016/0002-9610(73)90010-x. [DOI] [PubMed] [Google Scholar]
- 10.Hasper D, Schefold JC, Baumgart DC. Management of severe abdominal infections. Recent Pat Antiinfect Drug Discov. 2009;4(1):57–65. doi: 10.2174/157489109787236265. [DOI] [PubMed] [Google Scholar]
- 11.Jaiyeoba O, Lazenby G, Soper DE. Recommendations and rationale for the treatment of pelvic inflammatory disease. Expert Rev Anti Infect Ther. 2011;9(1):61–70. doi: 10.1586/eri.10.156. [DOI] [PubMed] [Google Scholar]
- 12.Robertson D, Smith AJ. The microbiology of the acute dental abscess. J Med Microbiol. 2009;58(Pt 2):155–162. doi: 10.1099/jmm.0.003517-0. [DOI] [PubMed] [Google Scholar]
- 13.Andersson RE, Petzold MG. Nonsurgical treatment of appendiceal abscess or phlegmon: a systematic review and meta-analysis. Ann Surg. 2007;246(5):741–748. doi: 10.1097/SLA.0b013e31811f3f9f. [DOI] [PubMed] [Google Scholar]
- 14.Gerzof SG, Robbins AH, Johnson WC, Birkett DH, Nabseth DC. Percutaneous catheter drainage of abdominal abscesses: a five-year experience. N Engl J Med. 1981;305(12):653–657. doi: 10.1056/NEJM198109173051201. [DOI] [PubMed] [Google Scholar]
- 15.Pruett TL, Simmons RL. Status of percutaneous catheter drainage of abscesses. Surg Clin North Am. 1988;68(1):89–105. doi: 10.1016/s0039-6109(16)44434-8. [DOI] [PubMed] [Google Scholar]
- 16.Olak J, Christou NV, Stein LA, Casola G, Meakins JL. Operative vs percutaneous drainage of intra-abdominal abscesses. Comparison of morbidity and mortality. Arch Surg. 1986;121(2):141–146. doi: 10.1001/archsurg.1986.01400020027001. [DOI] [PubMed] [Google Scholar]
- 17.Mezhir JJ, Fong Y, Jacks LM, Getrajdman GI, Brody LA, Covey AM, Thornton RH, Jarnagin WR, Solomon SB, Brown KT. Current management of pyogenic liver abscess: surgery is now second-line treatment. J Am Coll Surg. 2010;210(6):975–983. doi: 10.1016/j.jamcollsurg.2010.03.004. [DOI] [PubMed] [Google Scholar]
- 18.Rice LB. Federal funding for the study of antimicrobial resistance in nosocomial pathogens: no ESKAPE. J Infect Dis. 2008;197(8):1079–1081. doi: 10.1086/533452. [DOI] [PubMed] [Google Scholar]
- 19.Chabok A, Tarnberg M, Smedh K, Pahlman L, Nilsson LE, Lindberg C, Hanberger H. Prevalence of fecal carriage of antibiotic-resistant bacteria in patients with acute surgical abdominal infections. Scand J Gastroenterol. 2010;45(10):1203–1210. doi: 10.3109/00365521.2010.495417. [DOI] [PubMed] [Google Scholar]
- 20.Biel MA, Pedigo L, Gibbs A, Loebel N. Photodynamic therapy of antibiotic-resistant biofilms in a maxillary sinus model. Int Forum Allergy Rhinol. 2013 doi: 10.1002/alr.21134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Garcia-Quintanilla M, Pulido MR, Lopez-Rojas R, Pachon J, McConnell MJ. Emerging therapies for multidrug resistant Acinetobacter baumannii. Trends Microbiol. 2013 doi: 10.1016/j.tim.2012.12.002. [DOI] [PubMed] [Google Scholar]
- 22.Hansen EN, Zmistowski B, Parvizi J. Periprosthetic joint infection: What is on the horizon. Int J Artif Organs. 2012;35(10):935–950. doi: 10.5301/ijao.5000145. [DOI] [PubMed] [Google Scholar]
- 23.Jori G, Fabris C, Soncin M, Ferro S, Coppellotti O, Dei D, Fantetti L, Chiti G, Roncucci G. Photodynamic therapy in the treatment of microbial infections: basic principles and perspective applications. Lasers Surg Med. 2006;38(5):468–481. doi: 10.1002/lsm.20361. [DOI] [PubMed] [Google Scholar]
- 24.Hamblin MR, Hasan T. Photodynamic therapy: a new antimicrobial approach to infectious disease? Photochem Photobiol Sci. 2004;3(5):436–450. doi: 10.1039/b311900a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kharkwal GB, Sharma SK, Huang YY, Dai T, Hamblin MR. Photodynamic therapy for infections: clinical applications. Lasers Surg Med. 2011;43(7):755–767. doi: 10.1002/lsm.21080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mitra S, Haidaris CG, Snell SB, Giesselman BR, Hupcher SM, Foster TH. Effective photosensitization and selectivity in vivo of Candida albicans by meso-tetra (N-methyl-4-pyridyl) porphine tetra tosylate. Lasers Surg Med. 2011;43(4):324–332. doi: 10.1002/lsm.21049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jackson SA, Kennedy J, Morrissey JP, O'Gara F, Dobson AD. Pyrosequencing reveals diverse and distinct sponge-specific microbial communities in sponges from a single geographical location in Irish waters. Microb Ecol. 2012;64(1):105–116. doi: 10.1007/s00248-011-0002-x. [DOI] [PubMed] [Google Scholar]
- 28.White TJ, Bruns TD, Lee SB, Taylor SW. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ, editors. PCR Protocols: a Guide to Methods and Applications. London, UK: Academic Press; 1990. pp. 315–322. [Google Scholar]
- 29.Snell SB, Foster TH, Haidaris CG. Miconazole induces fungistasis and increases killing of Candida albicans subjected to photodynamic therapy. Photochem Photobiol. 2012;88(3):596–603. doi: 10.1111/j.1751-1097.2011.01039.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chabrier-Rosello Y, Giesselman BR, De Jesus-Andino FJ, Foster TH, Mitra S, Haidaris CG. Inhibition of electron transport chain assembly and function promotes photodynamic killing of Candida. J Photochem Photobiol B. 2010;99(3):117–125. doi: 10.1016/j.jphotobiol.2010.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jett BD, Hatter KL, Huycke MM, Gilmore MS. Simplified agar plate method for quantifying viable bacteria. BioTechniques. 1997;23(4):648–650. doi: 10.2144/97234bm22. [DOI] [PubMed] [Google Scholar]
- 32.Chabrier-Rosello Y, Foster TH, Mitra S, Haidaris CG. Respiratory deficiency enhances the sensitivity of the pathogenic fungus Candida to photodynamic treatment. Photochem Photobiol. 2008;84(5):1141–1148. doi: 10.1111/j.1751-1097.2007.00280.x. [DOI] [PubMed] [Google Scholar]
- 33.Gorbach SL. Treatment of intraabdominal infection. Am J Med. 1984;76(5A):107–110. doi: 10.1016/0002-9343(84)90251-1. [DOI] [PubMed] [Google Scholar]
- 34.Saini S, Kellum JM, O'Leary MP, O'Donnell TF, Tally FP, Carter B, Deterling RA, Curtis LE. Improved localization and survival in patients with intraabdominal abscesses. Am J Surg. 1983;145(1):136–142. doi: 10.1016/0002-9610(83)90180-0. [DOI] [PubMed] [Google Scholar]
- 35.Bayer AS, Blumenkrantz MJ, Montgomerie JZ, Galpin JE, Coburn JW, Guze LB. Candida peritonitis. Report of 22 cases and review of the English literature. Am J Med. 1976;61(6):832–840. doi: 10.1016/0002-9343(76)90407-1. [DOI] [PubMed] [Google Scholar]
- 36.Harrod-Kim P. Tumor ablation with photodynamic therapy: introduction to mechanism and clinical applications. J Vasc Interv Radiol. 2006;17(9):1441–1448. doi: 10.1097/01.RVI.0000231977.49263.DE. [DOI] [PubMed] [Google Scholar]
- 37.Vogl TJ, Eichler K, Mack MG, Zangos S, Herzog C, Thalhammer A, Engelmann K. Interstitial photodynamic laser therapy in interventional oncology. Eur Radiol. 2004;14(6):1063–1073. doi: 10.1007/s00330-004-2290-8. [DOI] [PubMed] [Google Scholar]
- 38.Kohanski MA, Dwyer DJ, Hayete B, Lawrence CA, Collins JJ. A common mechanism of cellular death induced by bactericidal antibiotics. Cell. 2007;130(5):797–810. doi: 10.1016/j.cell.2007.06.049. [DOI] [PubMed] [Google Scholar]
- 39.Dwyer DJ, Kohanski MA, Collins JJ. Role of reactive oxygen species in antibiotic action and resistance. Curr Opin Microbiol. 2009;12(5):482–489. doi: 10.1016/j.mib.2009.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nguyen D, Joshi-Datar A, Lepine F, Bauerle E, Olakanmi O, Beer K, McKay G, Siehnel R, Schafhauser J, Wang Y, Britigan BE, Singh PK. Active starvation responses mediate antibiotic tolerance in biofilms and nutrient-limited bacteria. Science. 2011;334(6058):982–986. doi: 10.1126/science.1211037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cinat ME, Wilson SE, Din AM. Determinants for successful percutaneous image-guided drainage of intra-abdominal abscess. Arch Surg. 2002;137(7):845–849. doi: 10.1001/archsurg.137.7.845. [DOI] [PubMed] [Google Scholar]
- 42.Varghese JC, Hahn PF, Harisinghani MG, Hayat SM, Gervais DA, Hooper DC, Mueller PR. Fungus-infected fluid collections in thorax or abdomen: effectiveness of percutaneous catheter drainage. Radiology. 2005;236(2):730–738. doi: 10.1148/radiol.2362031044. [DOI] [PubMed] [Google Scholar]
- 43.Cassidy CM, Tunney MM, Caldwell DL, Andrews GP, Donnelly RF. Development of novel oral formulations prepared via hot melt extrusion for targeted delivery of photosensitizer to the colon. Photochem Photobiol. 2011;87(4):867–876. doi: 10.1111/j.1751-1097.2011.00915.x. [DOI] [PubMed] [Google Scholar]
- 44.Nocker A, Richter-Heitmann T, Montijn R, Schuren F, Kort R. Discrimination between live and dead cells in bacterial communities from environmental water samples analyzed by 454 pyrosequencing. Int Microbiol. 2010;13(2):59–65. doi: 10.2436/20.1501.01.111. [DOI] [PubMed] [Google Scholar]
- 45.Rogers GB, Marsh P, Stressmann AF, Allen CE, Daniels TV, Carroll MP, Bruce KD. The exclusion of dead bacterial cells is essential for accurate molecular analysis of clinical samples. Clin Microbiol Infect. 2010;16(11):1656–1658. doi: 10.1111/j.1469-0691.2010.03189.x. [DOI] [PubMed] [Google Scholar]
- 46.Bruno MJ. Magnification endoscopy, high resolution endoscopy, and chromoscopy; towards a better optical diagnosis. Gut. 2003;52(Suppl 4):iv7–iv11. doi: 10.1136/gut.52.suppl_4.iv7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wainwright M. The use of dyes in modern biomedicine. Biotech Histochem. 2003;78(3–4):147–155. doi: 10.1080/10520290310001602404. [DOI] [PubMed] [Google Scholar]
- 48.Berakdar M, Callaway A, Eddin MF, Ross A, Willershausen B. Comparison between scaling-root-planing (SRP) and SRP/photodynamic therapy: six-month study. Head Face Med. 2012;8:12. doi: 10.1186/1746-160X-8-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bouillaguet S, Wataha JC, Zapata O, Campo M, Lange N, Schrenzel J. Production of reactive oxygen species from photosensitizers activated with visible light sources available in dental offices. Photomed Laser Surg. 2010;28(4):519–525. doi: 10.1089/pho.2009.2505. [DOI] [PubMed] [Google Scholar]
- 50.Ge L, Shu R, Li Y, Li C, Luo L, Song Z, Xie Y, Liu D. Adjunctive effect of photodynamic therapy to scaling and root planing in the treatment of chronic periodontitis. Photomed Laser Surg. 2011;29(1):33–37. doi: 10.1089/pho.2009.2727. [DOI] [PubMed] [Google Scholar]
- 51.Morley S, Griffiths J, Philips G, Moseley H, O'Grady C, Mellish K, Lankester CL, Faris B, Young RJ, Brown SB, Rhodes LE. Phase IIa randomised, placebo-controlled study of antimicrobial photodynamic therapy in bacterially colonised, chronic leg ulcers and diabetic foot ulcers. A new approach to antimicrobial therapy. Br J Dermatol. 2012;168(3):617–624. doi: 10.1111/bjd.12098. [DOI] [PubMed] [Google Scholar]
- 52.Biel MA, Sievert C, Usacheva M, Teichert M, Wedell E, Loebel N, Rose A, Zimmermann R. Reduction of endotracheal tube biofilms using antimicrobial photodynamic therapy. Lasers Surg Med. 2011;43(7):586–590. doi: 10.1002/lsm.21103. [DOI] [PMC free article] [PubMed] [Google Scholar]


