Abstract
Omega-3 fatty acid deficiency during development leads to enduing alterations in central monoamine neurotransmission in rat brain. Here we investigated the effects of omega-3 fatty acid deficiency on behavioral and neurochemical responses to chronic fluoxetine (FLX) treatment. Male rats were fed diets with (CON, n=34) or without (DEF, n=30) the omega-3 fatty acid precursor alpha-linolenic acid (ALA) during peri-adolescent development (P21-P90). A subset of CON (n=14) and DEF (n=12) rats were administered FLX (10 mg/kg/d) through their drinking water for 30 d beginning on P60. The forced swimming test (FST) was initiated on P90, and regional brain mRNA markers of serotonin and noradrenaline neurotransmission were determined. Dietary ALA depletion led to significant reductions in frontal cortex docosahexaenoic acid (DHA, 22:6n-3) composition in DEF (−26%, p=0.0001) and DEF+FLX (−32%, p=0.0001) rats. Plasma FLX and norfluoxetine concentrations did not different between FLX-treated DEF and CON rats. During the 15-min FST pretest, DEF+FLX rats exhibited significantly greater climbing behavior compared with CON+FLX rats. During the 5-min test trial, FLX treatment reduced immobility and increased swimming in CON and DEF rats, and only DEF+FLX rats exhibited significant elevations in climbing behavior. DEF+FLX rats exhibited greater midbrain, and lower frontal cortex, 5-HT1A mRNA expression compared with all groups including CON+FLX rats. DEF+FLX rats also exhibited greater midbrain alpha2A adrenergic receptor mRNA expression which was positively correlated with climbing behavior in the FST. These preclinical data demonstrate that low omega-3 fatty acid status leads to abnormal behavioral and neurochemical responses to chronic FLX treatment in male rats.
Keywords: Omega-3 fatty acid, Docosahexaenoic acid, Forced Swim test, Fluoxetine, Serotonin transporter, Tryptophan hydroxylase-2, 5-HT1A autoreceptor, Alpha2A adrenergic receptor
1. Introduction
Emerging evidence suggests that deficits in long-chain omega-3 (LCn-3) fatty acids, including eicosapenaenoic acid and docosahexaenoic acid (DHA), are associated with mood disorders including major depressive disorder (MDD). Specifically, cross-sectional studies have observed significantly lower erythrocyte and/or plasma LCn-3 fatty acid levels in patients with MDD (Assies et al., 2010; Edwards et al., 1998; Lin et al., 2010; McNamara et al., 2010; Peet et al., 1998). Moreover, postmortem brain studies have observed LCn-3 fatty acid deficits in the prefrontal cortex (PFC) or anterior cingulate of patients with MDD (Conklin et al., 2007; McNamara et al., 2007, 2013; Tatebayashi et al., 2012). Emerging evidence further suggests that adjunctive supplementation with LCn-3 fatty acids significantly augment the antidepressant effects of selective serotonin reuptake inhibitor (SSRI) medications (Jazayeri et al 2008; Gertsik et al. 2012; Peet & Horrobin, 2002). It is also relevant that suicidal patients (Huan et al., 2004; Sublette et al., 2006) and acutely manic patients (Chiu et al., 2003) exhibit blood LCn-3 fatty acid deficits, and treatment with SSRI medications may precipitate suicidality (Hammand et al., 2006) and manic symptoms (Faedda et al., 1998; Strawn et al., 2013) in high-risk youth. While this body of clinical evidence suggests that the LCn-3 fatty acid deficiency exhibited by patients with mood disorders may influence both SSRI efficacy and tolerability, the mechanisms mediating this relationship are poorly understood.
A number of preclinical studies have investigated the effects of dietary n-3 fatty acid deficiency on the maturation of serotonin (5-hydroxytryptamine, 5-HT) neurotransmission (Chalon, 2006). Dietary fortification with LCn-3 fatty acids increase 5-HT concentrations in rat frontal cortex (Chalon et al., 1998), attenuate reductions in frontal cortex 5-HT content in response to chronic stress (Vancassel et al., 2008), and similar to SSRIs decrease depression-like behavior in the forced swimming test (FST)(Carlezon et al., 2005; Huang et al., 2008). Furthermore, the combination of LCn-3 fatty acids and fluoxetine (FLX) is significantly more effective than FLX alone for reducing depression-like behavior in the FST (Laino et al., 2010; Lakhwani et al., 2007). Conversely, perinatal deficits in cortical DHA accrual are associated with impaired fenfluramine-induced elevations in extracellular 5-HT concentrations (Kodas et al., 2004), reductions in midbrain expression of tryptophan hydroxylase-2 (TPH-2), the rate-limiting enzyme in 5-HT synthesis (McNamara et al., 2009), and elevations in 5-HT2A receptor binding density in the rat frontal cortex (Delion et al., 1996). While this evidence suggests that LCn-3 fatty acid status during development has longstanding effects on central 5-HT neurotransmission, the impact of these changes on SSRI pharmacodynamics has not been systematically investigated.
In the present study, we investigated the effects of peri-adolescent n-3 fatty acid deficiency on the behavioral effects of chronic treatment with the SSRI fluoxetine (FLX) in the FST. Prior studies have found that the peri-adolescent n-3 fatty acid deficiency paradigm is associated with reductions in frontal cortex DHA levels similar to those observed in patients with mood disorders (McNamara et al., 2007, 2008a) and elevated behavioral indices of depression in the FST (DeMar et al. 2006). In view of prior evidence that chronic FLX treatment is associated with brain region-specific changes in 5-HT1A (Drossopoulou et al., 2004; Le Poul et al., 2000), TPH-2 (Dygalo et al., 2006; Shishkina et al., 2007), serotonin transporter (SERT)(Dygalo et al., 2006), and 5-HT2C (Barbon et al., 2011) mRNA expression, the present study additionally investigated pre- (SERT, TPH-2, 5-HT1A) and postsynaptic (5-HT1A, 5-HT2A, 5-HT2C) markers of serotonin neurotransmission. We additionally investigated presynaptic markers of noradrenaline neurotransmission including the noradrenaline transporter (NAT), alpha2A adrenergic receptor (α2A), and tyrosine hydroxylase (TH). Based on the translational evidence reviewed above, our specific prediction was that n-3 fatty acid deficiency would attenuate the behavioral and neurochemical effects of chronic FLX treatment.
2. Methods and materials
2.1. Animals and diets
Adult (P60) male and nulliparous female Long-Evans hooded rats (Harlan Farms, Indianapolis, IN) were paired, and males removed 2 weeks later. Dams and litters were maintained on standard Teklad rodent chow throughout gestation and nursing. Immediately following weaning (P21), male rats from different litters were randomly assigned to either alinolenic acid (ALA)-fortified diet (control, CON, TD.04285, Harlan-TEKLAD, Madison, WI)(n=34) or ALA-free diet (deficient, DEF, TD.04286)(n=30). Both ALA-fortified and ALA-free diets were closely matched for all non-fat nutrients and fatty acid composition with the exception of ALA (18:3n-3), the short-chain n-3 fatty acid precursor of LCn-3 fatty acids (Table 1). Independent fatty acid analyses found that the both diets were closely matched with the exception of ALA (18:3n-3) which was absent from the deficient diet and represented 4.6% of total fatty acid composition in the control diet (Table 1). Neither diet contained preformed LCn-3 fatty acids including DHA, and previous studies have demonstrated that feeding the ALA-free diet from P21-P90 leads to ~25 percent reduction in cortical DHA levels compared with rats maintained on the ALA-fortified diet (McNamara et al., 2009). Rats were housed 2 per cage with food and water available ad libitum, and were maintained under standard vivarium conditions on a 12:12 h light:dark cycle. Behavioral testing was initiated on P90 during the light portion of the cycle. All experimental procedures were approved by the University of Institutional Animal Care and Use Committee (Protocol #06-03-01-01), and adhere to the guidelines set by the National Institutes of Health.
Table 1.
Diet Compositions
| Ingredient1 | ALA+ | ALA− |
|---|---|---|
| Casein, vitamine free | 20 | 20 |
| Carbohydrate | ||
| Cornstarch | 20 | 20 |
| Sucrose | 27 | 27 |
| Dextrose | 9.9 | 9.9 |
| Maltose-dextrin | 6 | 6 |
| Cellulose | 5 | 5 |
| Mineral mix | 3.5 | 3.5 |
| Vitamin mix | 1 | 1 |
| L-Cystine | 0.3 | 0.3 |
| Choline bitartrate | 0.25 | 0.25 |
| TBHQ | 0.002 | 0.002 |
| Fat | ||
| Hydrogenated coconut oil | 4.5 | 5.1 |
| Saf flower | 1.9 | 1.9 |
| Flaxseed | 0.6 | 0 |
| Fatty acid composition2 | ||
| C8:0 | 4.3 | 5.0 |
| C10:0 | 3.8 | 4.2 |
| C12:0 | 29 | 32.8 |
| C14:0 | 11 | 12.5 |
| C16:0 | 8.3 | 8.7 |
| C18:0 | 9.4 | 10.2 |
| 18:1n-9 | 6.7 | 4.7 |
| 18:2n-6 | 22.7 | 21.9 |
| 20:4n-6 | nd | nd |
| 18:3n-3 | 4.6 | nd |
| 22:6n-3 | nd | nd |
g/100 g diet
wt % of total fatty acids
nd = not detected
2.2. Tissue collection
One week following behavioral testing, rats were sacrificed by decapitation during the light portion of the cycle, brains extracted and immediately immersed in ice-cold 0.9% NaCl for 2 min. The brain was then dissected to isolate the midbrain and PFC, and the olfactory tubercle and residual striatal tissue were removed from the PFC. Brain tissue was immediately placed in a cryotube, flash frozen in liquid nitrogen, and stored at −80°C.
2.3. Chronic fluoxetine administration
A subgroup of CON (n=14) and DEF (n=12) rats were randomly assigned to receive chronic FLX through their drinking water on P60 until being sacrificed. Administration through drinking water obviates daily injection stress and stress associated with surgical implantation of minipumps, mimics oral administration in human patient populations, and allows maintenance of drug dose in accordance with age-related increases in body weight. For three days prior to drug delivery, 24 h water consumption was determined for each cage using bottle weights (1 g water = 1 ml water), and ml water intake/mean kg body weight calculated. Based on daily ml/mean kg water consumption, FLX solution (4 mg/ml) (Mallinckrodt Inc., St. Louis MO) was added to drinking water at a concentration required to deliver a daily dose of 10 mg/kg/d. In pilot studies we found that oral administration of 10 mg/kg/d of FLX reproduced FLX levels in rat plasma (~100 ng/ml) that are commonly observed in human MDD patients treated with therapeutic doses of FLX (~100 ng/ml)(Amsterdam et al., 1997). Red opaque drinking bottles were used to protect FLX from light degradation. Fresh solutions were prepared and FLX concentration adjusted to mean body weight every 3 days. Plasma FLX and NFLX concentrations (ng/ml) were determined by liquid chromatography tandem mass spectrometry by a technician blinded to treatment (Medtox Laboratories, Inc., St. Paul Minnesota).
2.4. Forced swim test
The modified FST procedure follows that described in Cyran et al (2005). Adult (P90) rats were placed into a clear cylindrical tank (46 cm high, 20 cm diameter) filled with water (23–25°C) to a 30-cm depth for a 15-min pretrial followed 24 h later with the 5-min test trial. Behavior was recorded with an overhead video camera, and analyzed using the time-sampling technique by a trained technician that was blind to treatment. Cumulative behavioral scores were calculated for the 15-min pretrial and the 5-min test trial. Climbing was defined as upward-directed movements of the forepaws against the side of the swim chamber. Swimming was defined as movement throughout the chamber not directed at the side of the swim chamber. Immobility was defined as no activity other than that required too maintain the head above the water (floating). After each trial, rats were placed under a heating lamp for 5 min and then returned to their home cage. Between each trial FST chambers were emptied, rinsed, and refilled with fresh water.
2.5. Gas chromatography
The gas chromatography procedure used to determine PFC fatty acid composition has been described in detail previously (McNamara et al., 2009). Briefly, total fatty acid composition was determined with a Shimadzu GC-2010 equipped with an auto-injector (Shimadzu Scientific Instruments Inc., Columbia MD). The column was a DB-23 (123–2332): 30 m (length), I.D. 0.32 mm wide bore, film thickness of 0.25 μM (J&W Scientific, Folsom CA). Fatty acid identification was determined using retention times of authenticated fatty acid methyl ester standards (Matreya LLC Inc., Pleasant Gap PA). Analysis of fatty acid methyl esters is based on areas calculated with EZstart 7.4 software. Fatty acid composition data is expressed as weight percent of total fatty acids (mg fatty acid/100 mg fatty acids). All samples were processed by a technician blinded to treatment.
2.6. Regional mRNA expression
The real-time reverse transcriptase polymerase chain reaction (RT-PCR) procedure has been described in detail previously (McNamara et al., 2009). Primers and fluorogenic probes (Midland Certified Reagent Company, Midland, TX) were designed using Primer Express v.2.0 software (Applied Biosystems, Foster City, CA) based on the rat mRNA sequences for GAPDH (GenBank accession number: NM_017008), SERT (NM_013034), TPH-2 (NM_173839), 5-HT1A (NM_012585), 5HT2A (NM_017254), 5HT2C (NM_012765), noradrenaline transporter (NAT, NM_031343), alpha2A adrenergic receptor (α2A, NM_012739) and tyrosine hydroxylase (TH, NM_012740). Primer and probe sequences are presented in Supplemental Table 1. Each probe was conjugated to a FAM reporter at the 5' end and a TAMRA quencher at the 3' end. The reverse primer spanned an exon-exon junction to obviate genomic DNA contamination. Reverse transcription was performed using the 9600 GeneAmp thermocycler (Perkin-Elmer, Norwalk, CT). mRNA quantities were normalized to GAPDH mRNA values obtained from the same tissue. All samples were processed by a technician blinded to treatment.
2.7. Statistical analyses
Primary outcome measures were analyzed with a two-way ANOVA, with Diet (Control, Deficient), and Treatment (FLX, Vehicle) as the main factors. Pairwise comparisons were made with Fishers LSD protected t-tests. Plasma FLX and NFLX concentrations in control and n-3 deficient rats were analyzed with unpaired t-tests (two-tail, α=0.05). Statistical analyses were performed using GB-STAT (V.10, Dynamic Microsystems, Inc., Silver Springs MD).
3. Results
3.1. Body weight
There was a significant main effect of FLX treatment on body weight, F(1,61)=4.1, p=0.04, and the main effect of Diet, F(1,61)=1.9, p=0.2, and the Diet × Treatment interaction, F(1,61)=0.002, p=0.9, were not significant. Mean body weights were: CON (464±17.3 g), CON+FLX (435±10.6 g), DEF (444.2±11.1 g), and DEF+FLX (416±11.1 g). Body weights of DEF+FLX rats were lower than CON rats (−10%) but did not reach statistical significance (p=0.07). Body weights of CON+FLX and DEF+FLX rats were not different (p=0.3).
3.2. Plasma fluoxetine and norfluoxetine concentrations
There were no significant differences in plasma FLX (p=0.6), NFLX (p=0.7), or FLX+NFLX (p=0.6) concentrations between CON+FLX and DEF+FLX rats (Table 3). The NFLX/FLX ratio did not differ between diet groups (p=0.8), suggesting that both groups metabolized FLX to NFLX at a similar rate. FLX and NFLX were not detected in plasma from CON or DEF rats not treated with FLX (<10 ng/ml). Although human plasma FLX and NFLX concentrations are poorly correlated with therapeutic response, plasma FLX (mean±SD: 75±36 ng/ml), NFLX (113±44 ng/ml) and FLX+NFLX (188±44 ng/ml) concentrations are observed in FLX-treated (20 mg/day) male human MDD patients (Amsterdam et al., 1997). Therefore, the mean rat plasma FLX concentration observed in the present study (50±29 ng/ml) is within the human male plasma range, whereas mean rat plasma NFLX (316±135 ng/ml) and FLX+NFLX (345±150 ng/ml) concentrations are ~3-fold greater than those observed in the plasma of male MDD patients.
3.3. PFC fatty acid composition
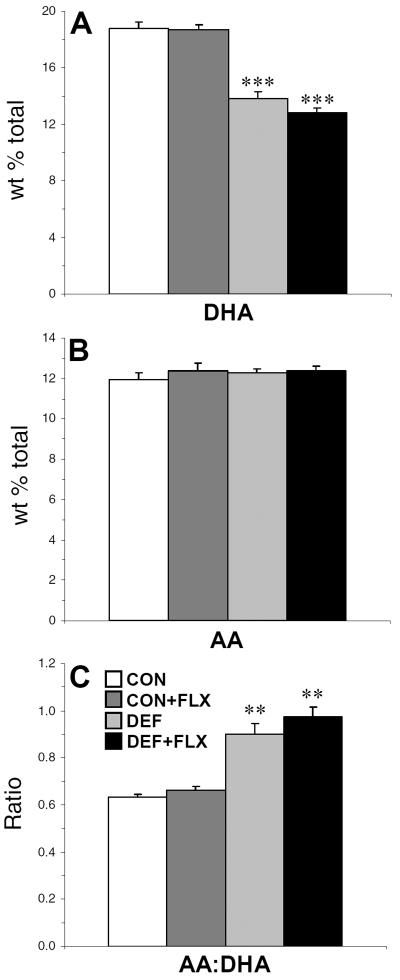
For PFC DHA composition, the main effect Diet was significant, F(1,35)=174, p≤0.0001, and the main effect of Treatment, F(1,35)=1.7, p=0.2, and the Diet × Treatment interaction, F(1,35)=1.3, p=0.2, were not significant. DHA composition was significantly lower in DEF (−26%, p≤0.0001) and DEF+FLX (−32%, p≤0.0001) rats relative to CON and CON+FLX rats, respectively (Fig. 1A). For AA composition, the main effect Diet, F(1,35)=0.39, p=0.5, and Treatment, F(1,35)=0.80, p=0.4, and the Diet × Treatment interaction, F(1,35)=0.312, p=0.6, were not significant (Fig. 1B). For the AA:DHA ratio, the main effect Diet was significant, F(1,35)=85.7, p≤0.0001, and the main effect of Treatment, F(1,35)=2.45, p=0.1, and the Diet × Treatment interaction, F(1,35)=0.553, p=0.5, were not significant. The AA:DHA ratio was significantly higher in DEF (+30%, p≤0.001) and DEF+FLX (+32%, p≤0.001) rats compared with CON and CON+FLX rats, respectively (Fig. 1C).
Figure 1.
PFC DHA (22:6n-3) composition (A), arachidonic acid (AA, 20:4n-6) composition (B), and the AA:DHA ratio (C) in CON, CON+FLX, DEF, and DEF+FLX rats (n=8–10/group). Values (wt % total fatty acids) are group mean ± S.E.M. **P≤0.01, ***P≤0.001 vs. CON and CON+FLX rats.
3.3 Forced swim test
3.3.1 Pre-trial
For immobility over the entire 15-min trial (Fig. 2A), the main effects of Diet, F(1,61)=2.1, p=0.1, and Treatment, F(1,61)=0.8, p=0.4, and the Diet × Treatment interaction, F(1,61)=0.1, p=0.7, were not significant. For swimming (Fig. 2B), the main effects of Diet, F(1,61)=1.6, p=0.2, and Treatment, F(1,61)=0.4, p=0.5, and the Diet × Treatment interaction, F(1,61)=0.1, p=0.7, were not significant. For climbing (Fig. 2C), the main effect of Diet, F(1,61)=4.6, p=.03, and the Diet × Treatment interaction, F(1,61)=7.1, p=0.01, were significant, and main effect of Treatment was not significant, F(1,61)=2.3, p=0.1. The DEF+FLX group exhibited significantly greater climbing relative to the CON (p=0.02), CON+FLX (p=0.04), and DEF (p=0.03) groups.
Figure 2.
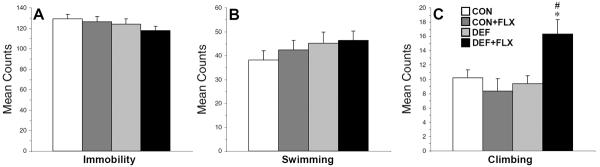
Cumulative mean immobility (A), swimming (B), and climbing (C) scores during the 15-min pretest in CON, CON+FLX, DEF, and DEF+FLX rats (n=10–17/group). Values are group mean ± S.E.M. *P≤0.05 vs. CON and DEF, #P≤0.05 vs. CON+FLX.
3.3.2. Post-test
The 5-min post-test was performed 24 h after the 15-min pretest. For immobility (Fig. 3A), the main effect of Treatment, F(1,61)=10.9, p=0.001, was significant, and the main effect of Diet, F(1,61)=2.6, p=0.1, and the Diet × Treatment interaction, F(1,61)=0.09, p=0.8, were not significant. CON+FLX and DEF+FLX groups exhibited significantly lower immobility scores compared with CON (p=0.02) and DEF (p=0.03) groups, respectively, and the CON+FLX and DEF+FLX groups did not differ (p=0.3). For swimming (Fig. 3B), the main effect of Treatment, F(1,61)=16.8, p=0.0001, was significant, and the main effect of Diet, F(1,61)=0.1, p=0.6, and the Diet × Treatment interaction, F(1,61)=0.1, p=0.7, were not significant. The CON+FLX and DEF+FLX groups exhibited significantly greater swimming relative to the CON (p=0.005) and DEF (p=0.01) groups, respectively, and the CON+FLX and DEF+FLX groups did not differ (p=0.6). For climbing (Fig. 3C), the main effect of Diet, F(1,61)=3.2, p=0.04, was significant, and the main effect of Treatment, F(1,61)=0.21, p=0.6, and the Diet × Treatment interaction, F(1,61)=1.5, p=0.1, were not significant. The DEF+FLX group exhibited significantly greater climbing relative to the CON+FLX group (p=0.05).
Figure 3.
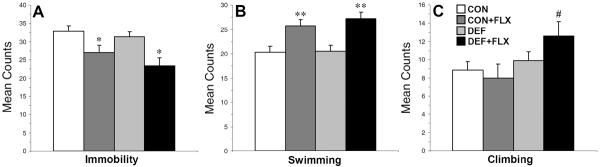
Cumulative mean immobility (A), swimming (B), and climbing (C) scores during the 5-min test trial in CON, CON+FLX, DEF, and DEF+FLX rats (n=10–17/group). Values are group mean ± S.E.M. *P≤0.05, **P≤0.01 vs. CON and DEF, #P≤0.05 vs. CON+FLX.
3.4. Regional mRNA expression
3.4.1. Prefrontal cortex
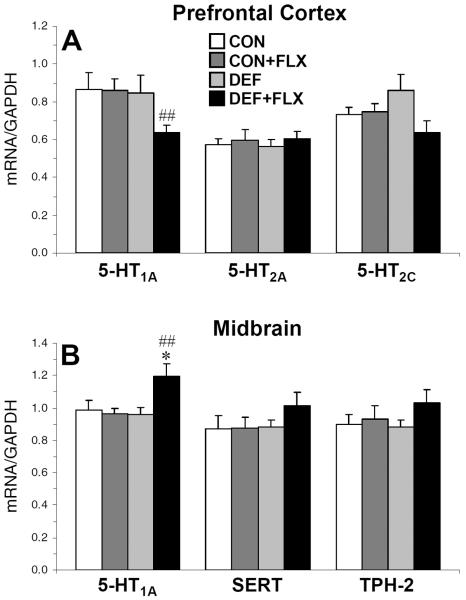
For PFC GAPDH mRNA expression, the main effects of Diet, Treatment, and the Diet × Treatment interaction were not significant. For PFC 5-HT1A mRNA expression, the main effects of Diet, F(1,46)=2.3, p=0.1, and Treatment, F(1,46)=1.9, p=0.2, were not significant, and the Diet × Treatment interaction was significant, F(1,46)=4.7, p=0.03 (Fig. 4A). PFC 5-HT1A mRNA expression was significantly lower in the DEF+FLX group relative to CON+FLX (−27%, p=0.01) group, and there were trends for lower expression compared with CON (−27%, p=0.058) and DEF (−26%, p=0.07) groups. For PFC 5-HT2A mRNA expression, the main effects of Diet, F(1,46)=0.9, p=0.3, and Treatment, F(1,46)=0.5, p=0.5, and the Diet × Treatment interaction, F(1,46)=1.4, p=0.2, were not significant (Fig. 4A). For PFC 5-HT2C mRNA expression, the main effects of Diet, F(1,46)=0.2, p=0.7, and Treatment, F(1,46)=0.5, p=0.5, and the Diet × Treatment interaction, F(1,46)=0.3, p=0.6, were not significant (Fig. 4A).
Figure 4.
5-HT1A, 5-HT2A, and 5-HT2C mRNA expression in the PFC (A), and SERT, TPH-2, and 5-HT1A mRNA expression in the midbrain (B) of CON, CON+FLX, DEF, and DEF+FLX rats (n=11–14/group). Values (mRNA/GAPDH mRNA) are group mean ± S.E.M. *P≤0.05 vs. CON and DEF, ##P≤0.01 vs. CON+FLX rats.
3.4.2. Midbrain
For midbrain GAPDH mRNA expression, the main effects of Diet, Treatment, and the Diet × Treatment interaction were not significant. For midbrain 5-HT1A mRNA expression, the main effect Treatment, F(1,46)=4.1, p=0.05, and the Diet × Treatment interaction, F(1,46)=5.9, p=0.01, were significant, and the main effect of Diet was not significant, F(1,46)=3.7, p=0.06. Midbrain 5-HT1A mRNA expression was significantly greater in DEF+FLX rats compared with CON (+18%, p=0.04), CON+FLX (+19%, p=0.005), and DEF (+19%, p=0.01) rats (Fig. 4B). For SERT mRNA expression, the main effects of Diet, F(1,46)=1.06, p=0.3, and Treatment, F(1,46)=0.9, p=0.3, and the Diet × Treatment interaction, F(1,46)=0.8, p=0.4, were not significant (Fig. 4B). For TPH-2 mRNA expression, the main effects of Diet, F(1,46)=0.3, p=0.6, and Treatment, F(1,46)=1.6, p=0.2, and the Diet × Treatment interaction, F(1,46)=0.6, p=0.4, were not significant (Fig. 4B).
For NAT mRNA expression, the Diet × Treatment interaction was significant, F(1,46)=10.2, p=0.003, and the main effects of Diet, F(1,46)=0.01, p=0.9, and Treatment, F(1,46)=0.9, p=0.3, were not significant. NAT mRNA expression was significantly lower in DEF rats compared with CON (−58%, p=0.03) and DEF+FLX (−67%, p=0.03) rats (Fig. 5A). For α2A mRNA expression, the main effects of Diet was significant, F(1,46)=7.7, p=0.008, and main effect of Treatment, F(1,46)=1.5, p=0.2, and the Diet × Treatment interaction, F(1,46)=0.3, p=0.5, were not significant. α2A mRNA expression was significantly higher in the DEF+FLX rats compared with CON (+27%, p=0.02) and CON+FLX (+23%, p=0.03) rats (Fig. 5B). Among all rats, climbing scores during the pre-trial (r = +0.34, p=0.01) and test-trial (r = +0.36, p=0.01) were positively correlated with α2A mRNA expression. For TH mRNA expression, the main effects of Diet, F(1,46)=0.3, p=0.6, and Treatment, F(1,46)=0.2, p=0.6, and the Diet × Treatment interaction, F(1,46)=0.03, p=0.8, were not significant (Fig. 5C).
Figure 5.
Noradrenaline transporter (NAT)(A), alpha2A adrenergic receptor (α2A)(B), and tyrosine hydroxylase (TH)(C) mRNA expression in the midbrain of CON, CON+FLX, DEF, and DEF+FLX rats (n=11–14/group). Values (mRNA/GAPDH mRNA) are group mean ± S.E.M. *P≤0.05, **P≤0.01 vs. CON, #P≤0.05 vs. CON+FLX rats.
4. Discussion
This study investigated the effects of LCn-3 fatty acid deficiency on behavioral and neurochemical responses to chronic FLX treatment with the hypothesis that they would be blunted in DEF rats. Contrary to this hypothesis, we found that FLX-treated DEF rats exhibited significant elevations in climbing behavior during both the pre-trial and the test trial compared with FLX-treated CON rats. Moreover, we found that chronic FLX treatment significantly decreased 5-HT1A receptor mRNA expression in the PFC, and significantly and selectively increased 5-HT1A receptor mRNA expression in the midbrain, in DEF but not CON rats. We also demonstrate that chronic FLX treatment significantly increased midbrain alpha2A adrenergic receptor mRNA expression in DEF but not CON rats, and found that alpha2A adrenergic receptor mRNA expression was positively correlated with climbing behavior in the FST. This differential response to chronic FLX treatment in DEF versus CON rats could not be attributed to differences in FLX intake or plasma concentrations. Together, these data demonstrate that chronic FLX treatment leads to abnormal neurochemical and behavioral adaptations in DEF rats compared with CON rats.
The present study found that immobility scores of untreated DEF rats did not differ from controls during the 5-min test trial. This finding stands in contrast to a prior study findings that untreated DEF rats exhibited greater immobility scores (DeMar et al., 2006). In the later study, neither swimming nor climbing scores were reported for the 5-min test trial. Potentially important methodological differences between the DeMar et al. (2006) study and the present study include: shipment of rat pups on postnatal day 18 vs. in-house breeding, surrogate nursing vs. maternal nursing, individual housing prior to behavioral testing vs. group (2/cage) housing, age at testing (126 d vs. 90 d), FST water depth (25-cm vs. 30-cm), the presence or a glass plate over the swim tank vs. open swim tank, and water replacement following three trials vs. water replacement following individual trials. Contrasting these two studies suggest that postnatal stressors (shipping, surrogate nursing, individual housing) may interact with dietary n-3 fatty acid deficiency to increase immobility in the FST. Indeed, emerging evidence suggests that postnatal stress and n-3 fatty acid deficiency can have synergistic effects on different behavioral indices of emotion in adulthood (Mathieu et al., 2008, 2011). Additional studies are warranted to further evaluate this `two-hit' model in the FST.
The observation that chronic FLX treatment significantly reduced immobility and increased swimming but not climbing in CON rats is consistent with a previous chronic treatment study (Detke et al., 1997). Here we present new data demonstrating that n-3 fatty acid deficiency is associated with abnormal elevations in climbing behavior in the FST in response to chronic FLX treatment. Although this study did not include a separate measure of motor activity to rule out the potential contribution of motor hyperactivity to the observed behavioral effects in the FST, a previous study found that FLX (10 mg/kg) treatment significantly reduced locomotor activity in open field activity chambers (Hemby et al., 1997). Therefore, the observed reduction in immobility observed in FLX-treated rats are likely not attributable to a general increase in locomotor activity, though additional studies are warranted to evaluate this mechanism in FLX-treated DEF rats. Nevertheless, the abnormal elevations in climbing behavior suggests that n-3 fatty acid deficiency is associated with differential neuroplastic adaptations in response to chronic FLX treatment, and candidate mechanisms are considered below.
Chronic FLX treatment robustly increased midbrain (presynaptic autoreceptor) and decreased PFC (postsynaptic) 5-HT1A receptor mRNA expression in DEF but not CON rats. Prior studies in rats fed standard lab chow (i.e., ALA-fortified) have found that chronic SSRI treatment either decreases (Le Poul et al., 2000), increases (Drossopoulou et al., 2004), or does not alter (Spurlock et al., 1994) midbrain and/or cortical 5-HT1A receptor mRNA expression. Presynaptic 5-HT1A receptors regulate serotonin axonal firing and serotonin release in the PFC (Sprouse & Aghajanian, 1986) and are desensitized following chronic SSRI treatment (Invernizzi et al., 1994; Le Poul et al., 1995). Elevations in 5-HT1A inhibitory autoreceptor expression in FLX-treated DEF rats would therefore be anticipated to decrease dorsal raphe serotonin neuronal activity and decrease extracellular serotonin concentrations in rat forebrain (Haddjeri et al., 2004). Postsynaptic 5-HT1A receptors in the PFC modulate descending projections to dorsal raphe neurons from the mPFC, and selective activation of these receptors reduce serotonin concentrations in the mPFC (Celada et al., 2001). Therefore, reduced expression of postsynaptic 5-HT1A receptors in the PFC of FLX-treated DEF rats would be anticipated to increase serotonin concentrations in the mPFC. Additional in vivo microdialyses studies are warranted to better characterize the effects of regional changes in 5-HT1A receptor expression on ascending and descending serotonin circuits in FLX-treated DEF rats.
Prior studies have found that selective 5-HT1A receptor agonists alone reduce immobility and increase swimming but not climbing in the FST (Detke et al., 1995; Singh & Lucki, 1993), and that combining selective 5-HT1A receptor agonists with FLX robustly increases climbing behavior (Detke et al., 1995; Reneric et al., 2001). Moreover, treatment with the 5-HT1A receptor antagonist (WAY 100135) significantly attenuated the antidepressant response (i.e., reduced immobility and increased swimming) exhibited by fish oil-supplemented rats in the FST (Vines et al., 2012). In the latter study, there was a trend for greater climbing behavior in fish oil supplemented rats administered WAY 100135. In contrast to the effects of acute or chronic SSRI treatment, acute or chronic treatment with selective noradrenergic reuptake inhibitors (SNRI), including reboxetine or desipramine robustly increase climbing behavior in the FST (Cryan et al., 2002, 2005; Detke et al., 1995; 1997; Page et al., 1999). It is relevant therefore that selective activation of 5-HT1A receptors increase locus coeruleus neuronal activity, and increase extracellular noradrenaline concentrations in the rat forebrain (Hajos-Korcsok & Sharp, 1996, 1999). Together, these data suggest that the observed changes in pre- and/or postsynaptic 5-HT1A receptor expression in FLX-treated DEF rats may be directly associated with the increase in climbing behavior.
We also observed significant elevations in alpha2A adrenergic autoreceptor expression in FLX-treated DEF rats compared with FLX-treated CON rats. Interestingly, blockade of alpha2A adrenergic receptors was found to significantly augment FLX-induced increases in extracellular noradrenaline concentrations in the rat frontal cortex (Beyer et al., 2006; Gobert et al., 1997), and to augment the anti-immobility effects of FLX in the FST (Dhir and Kulkarni, 2007). Moreover, we found that climbing scores during both the pre-trial and test trial were positively correlated with alpha2A adrenergic autoreceptor expression. Although we observed elevations in alpha2A adrenergic mRNA expression, future studies will be required to determine whether this increase is compensatory and associated with increases in noradrenergic neurotransmission. We also found that untreated DEF rats exhibited lower NAT mRNA expression compared with CON rats, and this deficit was normalized in FLX-treated DEF rats. Interestingly, reductions in NAT binding have also been observed in the locus coeruleus of MDD patients (Klimek et al., 1997). While this reduction in NAT expression would be anticipated to increase extracellular noradrenaline levels, DEF rats did not exhibit elevations in climbing behavior. However, it remains to be determined whether this reduction in NAT mRNA expression was associated with increases in noradrenergic neurotransmission and/or compensatory down-regulation of postsynaptic adrenergic receptors.
Elevated midbrain 5-HT1A receptor expression in FLX-treated DHA-deficient rats may take on additional significance in view of data from postmortem studies finding that depressed suicides exhibit elevated midbrain 5-HT1A mRNA expression and binding in the rostral aspect of the dorsal raphe (Arango et al., 2001; Boldrini et al., 2008; Stockmeier et al., 1998). Moreover, a functional polymorphism in the 5-HT1A promoter associated with greater expression levels in the raphe (Lemonde et al., 2003) and is associated with antidepressant treatment-resistance (Lemonde et al., 2004). Reductions in PFC 5-HT1A expression have been observed by some (Cheetham et al., 1990; Lopez-Figueroa et al., 2004) but not all (Arango et al., 1995; Matsubara et al., 1991; Stockmeier et al., 1997) postmortem studies of depressed suicides, some of whom were receiving antidepressant medications at time of death. Furthermore, agonist binding to alpha 2-adrenergic receptors was found to be elevated in the locus coeruleus of suicide victims (Ordway et al., 1994). Based on this translational evidence, it is tempting to speculate that the combination of n-3 fatty acid deficiency and chronic SSRI treatment augments noradrenergic neurotransmission via changes in 5-HT1A and/or alpha2A adrenergic signaling, and this augmented response contributes to behaviors thought to be mediated by noradrenalin including impulsivity (Swann, 2010).
In conclusion, the present study demonstrates that n-3 fatty acid deficiency is associated with abnormal elevations in climbing behavior in the FST following chronic FLX treatment, and this response is associated with increases in presynaptic 5-HT1A and aplha2A adrenergic receptor mRNA expression and reductions in postsynaptic 5-HT1A mRNA expression. These preclinical data suggest that low n-3 fatty acid status may be an important determinant of behavioral and neurochemical responses to chronic FLX treatment.
Supplementary Material
Table 2.
Plasma Fluoxetine and Norfluoxetine Concentrations
| CON+FLX | DEF+FLX | p-value2 | |
|---|---|---|---|
| Fluoxetine (FLX)1 | 52.4 ± 9.36 | 46.8 ± 7.24 | 0.652 |
| Norfluoxetine (NFLX) | 305.5 ± 36.4 | 282.5 ± 38.2 | 0.668 |
| FLX+NFLX | 357.8 ± 44.5 | 329.3 ± 43.3 | 0.653 |
| NFLX/FLX | 7.55 ± 1.26 | 7.02 ± 1.14 | 0.760 |
Values are mean concentration (ng/ml) ± S.E.M.
t-test (two-tail)
Acknowledgments
This work was supported in part by National Institutes of Health grants MH073704 and MH074858 to R.K.M., and DK59630 to P.T.
Role of Funding Source. Funding for this study was provided in part by National Institutes of Health grants MH073704 and MH074858 to R.K.M., and DK59630 to P.T. The NIH had no further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributors. Dr. McNamara designed the study and wrote the manuscript. Drs. Jandacek and Tso and Therese Rider performed the gas chromatography. Mrs. Able and Lui participated in the behavioral and gene expression analyses. All authors contributed to and have approved the final manuscript.
REFERENCES
- Amsterdam JD, Fawcett J, Quitkin FM, Reimherr FW, Rosenbaum JF, Michelson D, Hornig-Rohan M, Beasley CM. Fluoxetine and norfluoxetine plasma concentrations in major depression: a multicenter study. Am J Psychiatry. 1997;154:963–969. doi: 10.1176/ajp.154.7.963. [DOI] [PubMed] [Google Scholar]
- Arango V, Underwood MD, Boldrini M, Tamir H, Kassir SA, Hsiung S, Chen JJ, Mann JJ. Serotonin 1A receptors, serotonin transporter binding and serotonin transporter mRNA expression in the brainstem of depressed suicide victims. Neuropsychopharmacology. 2001;25:892–903. doi: 10.1016/S0893-133X(01)00310-4. [DOI] [PubMed] [Google Scholar]
- Arango V, Underwood MD, Gubbi AV, Mann JJ. Localized alterations in pre- and postsynaptic serotonin binding sites in the ventrolateral prefrontal cortex of suicide victims. Brain Res. 1995;688:121–133. doi: 10.1016/0006-8993(95)00523-s. [DOI] [PubMed] [Google Scholar]
- Assies J, Pouwer F, Lok A, Mocking RJ, Bockting CL, Visser I, Abeling NG, Duran M, Schene AH. Plasma and erythrocyte fatty acid patterns in patients with recurrent depression: a matched case-control study. PLoS One. 2010;5(5):e10635. doi: 10.1371/journal.pone.0010635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbon A, Orlandi C, La Via L, Caracciolo L, Tardito D, Musazzi L, Mallei A, Gennarelli M, Racagni G, Popoli M, Barlati S. Antidepressant treatments change 5-HT2C receptor mRNA expression in rat prefrontal/frontal cortex and hippocampus. Neuropsychobiology. 2011;63:160–168. doi: 10.1159/000321593. [DOI] [PubMed] [Google Scholar]
- Beyer CE, Lin Q, Rosenzweig-Lipson S, Schechter LE. Alpha 2A-adrenoceptors enhance the serotonergic effects of fluoxetine. Eur J Pharmacol. 2006;539(3):164–167. doi: 10.1016/j.ejphar.2006.03.083. [DOI] [PubMed] [Google Scholar]
- Boldrini M, Underwood MD, Mann JJ, Arango V. Serotonin-1A autoreceptor binding in the dorsal raphe nucleus of depressed suicides. J Psychiatr Res. 2008;42:433–442. doi: 10.1016/j.jpsychires.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlezon WA, Jr, Mague SD, Parow AM, Stoll AL, Cohen BM, Renshaw PF. Antidepressant-like effects of uridine and omega-3 fatty acids are potentiated by combined treatment in rats. Biol Psychiatry. 2005;57:343–350. doi: 10.1016/j.biopsych.2004.11.038. [DOI] [PubMed] [Google Scholar]
- Celada P, Puig MV, Casanovas JM, Guillazo G, Artigas F. Control of dorsal raphe serotonergic neurons by the medial prefrontal cortex: Involvement of serotonin-1A, GABA(A), and glutamate receptors. J Neurosci. 2001;21:9917–9929. doi: 10.1523/JNEUROSCI.21-24-09917.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalon S, Delion-Vancassel S, Belzung C, Guilloteau D, Leguisquet AM, Besnard JC, Durand G. Dietary fish oil affects monoaminergic neurotransmission and behavior in rats. J Nutr. 1998;128:2512–2519. doi: 10.1093/jn/128.12.2512. [DOI] [PubMed] [Google Scholar]
- Chalon S. Omega-3 fatty acids and monoamine neurotransmission. Prostaglandins Leukot Essent Fatty Acids. 2006;75:259–269. doi: 10.1016/j.plefa.2006.07.005. [DOI] [PubMed] [Google Scholar]
- Cheetham SC, Crompton MR, Katona CL, Horton RW. Brain 5-HT1 binding sites in depressed suicides. Psychopharmacology (Berl) 1990;102:544–548. doi: 10.1007/BF02247138. [DOI] [PubMed] [Google Scholar]
- Chiu CC, Huang SY, Su KP, Lu ML, Huang MC, Chen CC, Shen WW. Polyunsaturated fatty acid deficit in patients with bipolar mania. Eur Neuropsychopharmacol. 2003;13:99–103. doi: 10.1016/s0924-977x(02)00130-x. [DOI] [PubMed] [Google Scholar]
- Conklin SM, Runyan CA, Leonard S, Reddy RD, Muldoon MF, Yao JK. Age-related changes of n-3 and n-6 polyunsaturated fatty acids in the anterior cingulate cortex of individuals with major depressive disorder. Prostaglandins Leukot Essent Fatty Acids. 2010;82:111–119. doi: 10.1016/j.plefa.2009.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cryan JF, Page ME, Lucki I. Differential behavioral effects of the antidepressants reboxetine, fluoxetine, and moclobemide in a modified forced swim test following chronic treatment. Psychopharmacology (Berl) 2005;182:335–344. doi: 10.1007/s00213-005-0093-5. [DOI] [PubMed] [Google Scholar]
- Cryan JF, Page ME, Lucki I. Noradrenergic lesions differentially alter the antidepressant-like effects of reboxetine in a modified forced swim test. Eur J Pharmacol. 2002;436:197–205. doi: 10.1016/s0014-2999(01)01628-4. [DOI] [PubMed] [Google Scholar]
- Delion S, Chalon S, Guilloteau D, Besnard JC, Durand G. alpha-Linolenic acid dietary deficiency alters age-related changes of dopaminergic and serotoninergic neurotransmission in the rat frontal cortex. J Neurochem. 1996;66:1582–1591. doi: 10.1046/j.1471-4159.1996.66041582.x. [DOI] [PubMed] [Google Scholar]
- DeMar JC, Jr, Ma K, Bell JM, Igarashi M, Greenstein D, Rapoport SI. One generation of n-3 polyunsaturated fatty acid deprivation increases depression and aggression test scores in rats. J Lipid Res. 2006;47:172–180. doi: 10.1194/jlr.M500362-JLR200. [DOI] [PubMed] [Google Scholar]
- Detke MJ, Wieland S, Lucki I. Blockade of the antidepressant-like effects of 8-OH-DPAT, buspirone and desipramine in the rat forced swim test by 5HT1A receptor antagonists. Psychopharmacology (Berl) 1995;119:47–54. doi: 10.1007/BF02246053. [DOI] [PubMed] [Google Scholar]
- Detke MJ, Johnson J, Lucki I. Acute and chronic antidepressant drug treatment in the rat forced swimming test model of depression. Exp Clin Psychopharmacol. 1997;5:107–112. doi: 10.1037//1064-1297.5.2.107. [DOI] [PubMed] [Google Scholar]
- Dhir A, Kulkarni SK. Effect of addition of yohimbine (alpha-2-receptor antagonist) to the antidepressant activity of fluoxetine or venlafaxine in the mouse forced swim test. Pharmacology. 2007;80:239–243. doi: 10.1159/000104877. [DOI] [PubMed] [Google Scholar]
- Drossopoulou G, Antoniou K, Kitraki E, Papathanasiou G, Papalexi E, Dalla C, Papadopoulou-Daifoti Z. Sex differences in behavioral, neurochemical and neuroendocrine effects induced by the forced swim test in rats. Neuroscience. 2004;126:849–857. doi: 10.1016/j.neuroscience.2004.04.044. [DOI] [PubMed] [Google Scholar]
- Dygalo NN, Shishkina GT, Kalinina TS, Yudina AM, Ovchinnikova ES. Effect of repeated treatment with fluoxetine on tryptophan hydroxylase-2 gene expression in the rat brainstem. Pharmacol Biochem Behav. 2006;85:220–227. doi: 10.1016/j.pbb.2006.08.004. [DOI] [PubMed] [Google Scholar]
- Edwards R, Peet M, Shay J, Horrobin D. Omega-3 polyunsaturated fatty acid levels in the diet and in red blood cell membranes of depressed patients. J Affect Disord. 1998;48:149–155. doi: 10.1016/s0165-0327(97)00166-3. [DOI] [PubMed] [Google Scholar]
- Faedda GL, Baldessarini RJ, Glovinsky IP, Austin NB. Treatment-emergent mania in pediatric bipolar disorder: a retrospective case review. J Affect Disord. 1998;82:149–158. doi: 10.1016/j.jad.2003.12.011. [DOI] [PubMed] [Google Scholar]
- Gertsik L, Poland RE, Bresee C, Rapaport MH. Omega-3 fatty acid augmentation of citalopram treatment for patients with major depressive disorder. J Clin Psychopharmacol. 2012;32:61–64. doi: 10.1097/JCP.0b013e31823f3b5f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gobert A, Rivet JM, Cistarelli L, Melon C, Millan MJ. Alpha2-adrenergic receptor blockade markedly potentiates duloxetine- and fluoxetine-induced increases in noradrenaline, dopamine, and serotonin levels in the frontal cortex of freely moving rats. J Neurochem. 1997;69:2616–2619. doi: 10.1046/j.1471-4159.1997.69062616.x. [DOI] [PubMed] [Google Scholar]
- Haddjeri N, Lavoie N, Blier P. Electrophysiological evidence for the tonic activation of 5-HT(1A) autoreceptors in the rat dorsal raphe nucleus. Neuropsychopharmacology. 2004;29:1800–1806. doi: 10.1038/sj.npp.1300489. [DOI] [PubMed] [Google Scholar]
- Hajós-Korcsok E, McQuade R, Sharp T. Influence of 5-HT1A receptors on central noradrenergic activity: microdialysis studies using (+/−)-MDL 73005EF and its enantiomers. Neuropharmacology. 1999;38:299–306. doi: 10.1016/s0028-3908(98)00175-0. [DOI] [PubMed] [Google Scholar]
- Hajós-Korcsok E, Sharp T. 8-OH-DPAT-induced release of hippocampal noradrenaline in vivo: evidence for a role of both 5-HT1A and dopamine D1 receptors. Eur J Pharmacol. 1996;314:285–291. doi: 10.1016/s0014-2999(96)00560-2. [DOI] [PubMed] [Google Scholar]
- Hammad TA, Laughren T, Racoosin J. Suicidality in pediatric patients treated with antidepressant drugs. Arch Gen Psychiatry. 2006;63:332–339. doi: 10.1001/archpsyc.63.3.332. [DOI] [PubMed] [Google Scholar]
- Hemby SE, Lucki I, Gatto G, Singh A, Thornley C, Matasi J, Kong N, Smith JE, Davies HM, Dworkin SI. Potential antidepressant effects of novel tropane compounds, selective for serotonin or dopamine transporters. J Pharmacol Exp Ther. 1997;282:727–733. [PubMed] [Google Scholar]
- Huan M, Hamazaki K, Sun Y, Itomura M, Liu H, Kang W, Watanabe S, Terasawa K, Hamazaki T. Suicide attempt and n-3 fatty acid levels in red blood cells: a case control study in China. Biol Psychiatry. 2004;56:490–496. doi: 10.1016/j.biopsych.2004.06.028. [DOI] [PubMed] [Google Scholar]
- Huang SY, Yang HT, Chiu CC, Pariante CM, Su KP. Omega-3 fatty acids on the forced-swimming test. J Psychiatr Res. 2008;42:58–63. doi: 10.1016/j.jpsychires.2006.09.004. [DOI] [PubMed] [Google Scholar]
- Invernizzi R, Bramante M, Samanin R. Chronic treatment with citalopram facilitates the effect of a challenge dose on cortical serotonin output: role of presynaptic 5-HT1A receptors. Eur J Pharmacol. 1994;260:243–246. doi: 10.1016/0014-2999(94)90344-1. [DOI] [PubMed] [Google Scholar]
- Jazayeri S, Tehrani-Doost M, Keshavarz SA, Hosseini M, Djazayery A, Amini H, Jalali M, Peet M. Comparison of therapeutic effects of omega-3 fatty acid eicosapentaenoic acid and fluoxetine, separately and in combination, in major depressive disorder. Aust N Z J Psychiatry. 2008;42:192–198. doi: 10.1080/00048670701827275. [DOI] [PubMed] [Google Scholar]
- Klimek V, Stockmeier C, Overholser J, Meltzer HY, Kalka S, Dilley G, Ordway GA. Reduced levels of norepinephrine transporters in the locus coeruleus in major depression. J Neurosci. 1997;17:8451–8458. doi: 10.1523/JNEUROSCI.17-21-08451.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodas E, Galineau L, Bodard S, Vancassel S, Guilloteau D, Besnard JC, Chalon S. Serotoninergic neurotransmission is affected by n-3 polyunsaturated fatty acids in the rat. J Neurochem. 2004;89:695–702. doi: 10.1111/j.1471-4159.2004.02401.x. [DOI] [PubMed] [Google Scholar]
- Laino CH, Fonseca C, Sterin-Speziale N, Slobodianik N, Reinés A. Potentiation of omega-3 fatty acid antidepressant-like effects with low non-antidepressant doses of fluoxetine and mirtazapine. Eur J Pharmacol. 2010;648:117–126. doi: 10.1016/j.ejphar.2010.08.047. [DOI] [PubMed] [Google Scholar]
- Lakhwani L, Tongia SK, Pal VS, Agrawal RP, Nyati P, Phadnis P. Omega-3 fatty acids have antidepressant activity in forced swimming test in Wistar rats. Acta Pol Pharm. 2007;64:271–276. [PubMed] [Google Scholar]
- Le Poul E, Boni C, Hanoun N, Laporte AM, Laaris N, Chauveau J, Hamon M, Lanfumey L. Differential adaptation of brain 5-HT1A and 5-HT1B receptors and 5-HT transporter in rats treated chronically with fluoxetine. Neuropharmacology. 2000;39:110–122. doi: 10.1016/s0028-3908(99)00088-x. [DOI] [PubMed] [Google Scholar]
- Le Poul E, Laaris N, Doucet E, Laporte AM, Hamon M, Lanfumey L. Early desensitization of somato-dendritic 5-HT1A autoreceptors in rats treated with fluoxetine or paroxetine. Naunyn Schmiedebergs Arch Pharmacol. 1995;352:141–148. doi: 10.1007/BF00176767. [DOI] [PubMed] [Google Scholar]
- Lemonde S, Turecki G, Bakish D, Du L, Hrdina PD, Bown CD, Sequeira A, Kushwaha N, Morris SJ, Basak A, Ou XM, Albert PR. Impaired repression at a 5-hydroxytryptamine 1A receptor gene polymorphism associated with major depression and suicide. J Neurosci. 2003;23:8788–8799. doi: 10.1523/JNEUROSCI.23-25-08788.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemonde S, Du L, Bakish D, Hrdina P, Albert PR. Association of the C(-1019)G 5-HT1A functional promoter polymorphism with antidepressant response. Int J Neuropsychopharmacol. 2004;7:501–506. doi: 10.1017/S1461145704004699. [DOI] [PubMed] [Google Scholar]
- Lin PY, Huang SY, Su KP. A meta-analytic review of polyunsaturated fatty acid compositions in patients with depression. Biol Psychiatry. 2010;68:140–144. doi: 10.1016/j.biopsych.2010.03.018. [DOI] [PubMed] [Google Scholar]
- Lladó-Pelfort L, Assié MB, Newman-Tancredi A, Artigas F, Celada P. Preferential in vivo action of F15599, a novel 5-HT(1A) receptor agonist, at postsynaptic 5-HT(1A) receptors. Br J Pharmacol. 2010;160:1929–1940. doi: 10.1111/j.1476-5381.2010.00738.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Figueroa AL, Norton CS, López-Figueroa MO, Armellini-Dodel D, Burke S, Akil H, López JF, Watson SJ. Serotonin 5-HT1A, 5-HT1B, and 5-HT2A receptor mRNA expression in subjects with major depression, bipolar disorder, and schizophrenia. Biol Psychiatry. 2004;55:225–233. doi: 10.1016/j.biopsych.2003.09.017. [DOI] [PubMed] [Google Scholar]
- Mathieu G, Denis S, Lavialle M, Vancassel S. Synergistic effects of stress and omega-3 fatty acid deprivation on emotional response and brain lipid composition in adult rats. Prostaglandins Leukot Essent Fatty Acids. 2008;78:391–401. doi: 10.1016/j.plefa.2008.05.003. [DOI] [PubMed] [Google Scholar]
- Mathieu G, Oualian C, Denis I, Lavialle M, Gisquet-Verrier P, Vancassel S. Dietary n-3 polyunsaturated fatty acid deprivation together with early maternal separation increases anxiety and vulnerability to stress in adult rats. Prostaglandins Leukot Essent Fatty Acids. 2011;85:129–136. doi: 10.1016/j.plefa.2011.07.001. [DOI] [PubMed] [Google Scholar]
- Matsubara S, Arora RC, Meltzer HY. Serotonergic measures in suicide brain: 5-HT1A binding sites in frontal cortex of suicide victims. J Neural Transm Gen Sect. 1991;85:181–94. doi: 10.1007/BF01244944. [DOI] [PubMed] [Google Scholar]
- McNamara RK, Hahn C-G, Jandacek R, Rider T, Tso P, Stanford K, Richtand NM. Selective deficits in the omega-3 fatty acid docosahexaenoic acid in the postmortem orbitofrontal cortex of patients with major depressive disorder. Biol Psychiatry. 2007;62:17–24. doi: 10.1016/j.biopsych.2006.08.026. [DOI] [PubMed] [Google Scholar]
- McNamara RK, Jandacek R, Rider T, Tso P, Stanford K, Hahn C-G, Richtand NM. Deficits in docosahexaenoic acid and associated elevations in the metabolism of arachidonic acid and saturated fatty acids in the postmortem orbitofrontal cortex of patients with bipolar disorder. Psychiatric Res. 2008a;160:285–299. doi: 10.1016/j.psychres.2007.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNamara RK, Able JA, Liu Y, Jandacek R, Rider T, Tso P, Lipton JW. Omega-3 fatty acid deficiency during perinatal development increases serotonin turnover in the prefrontal cortex and decreases midbrain tryptophan hydroxylase-2 expression in adult female rats: Dissociation from estrogenic effects. J Psychiatr Res. 2009;43:656–663. doi: 10.1016/j.jpsychires.2008.09.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNamara RK, Jandacek R, Rider T, Tso P, Dwivedi Y, Pandey GN. Selective deficits in erythrocyte docosahexaenoic acid composition in adult patients with bipolar disorder and major depressive disorder. J Affect Disord. 2010;126:303–311. doi: 10.1016/j.jad.2010.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNamara RK, Jandacek R, Tso P, Dwivedi Y, Ren X, Pandey GN. Lower docosahexaenoic acid concentrations in the postmortem prefrontal cortex of adult depressed suicide victims compared with controls without cardiovascular disease. J Psychiatr Res. 2013;47:1187–1191. doi: 10.1016/j.jpsychires.2013.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ordway GA, Widdowson PS, Smith KS, Halaris A. Agonist binding to alpha 2-adrenoceptors is elevated in the locus coeruleus from victims of suicide. J Neurochem. 1994;63:617–624. doi: 10.1046/j.1471-4159.1994.63020617.x. [DOI] [PubMed] [Google Scholar]
- Page ME, Detke MJ, Dalvi A, Kirby LG, Lucki I. Serotonergic mediation of the effects of fluoxetine, but not desipramine, in the rat forced swimming test. Psychopharmacology (Berl) 1999;147:162–167. doi: 10.1007/s002130051156. [DOI] [PubMed] [Google Scholar]
- Peet M, Murphy B, Shay J, Horrobin D. Depletion of omega-3 fatty acid levels in red blood cell membranes of depressive patients. Biol Psychiatry. 1998;43:315–319. doi: 10.1016/s0006-3223(97)00206-0. [DOI] [PubMed] [Google Scholar]
- Peet M, Horrobin DF. A dose-ranging study of the effects of ethyl-eicosapentaenoate in patients with ongoing depression despite apparently adequate treatment with standard drugs. Arch Gen Psychiatry. 2002;59:913–919. doi: 10.1001/archpsyc.59.10.913. [DOI] [PubMed] [Google Scholar]
- Rénéric JP, Bouvard M, Stinus L. Idazoxan and 8-OH-DPAT modify the behavioral effects induced by either NA, or 5-HT, or dual NA/5-HT reuptake inhibition in the rat forced swimming test. Neuropsychopharmacology. 2001;24:379–390. doi: 10.1016/S0893-133X(00)00214-1. [DOI] [PubMed] [Google Scholar]
- Shishkina GT, Kalinina TS, Dygalo NN. Up-regulation of tryptophan hydroxylase-2 mRNA in the rat brain by chronic fluoxetine treatment correlates with its antidepressant effect. Neuroscience. 2007;150:404–412. doi: 10.1016/j.neuroscience.2007.09.017. [DOI] [PubMed] [Google Scholar]
- Singh A, Lucki I. Antidepressant-like activity of compounds with varying efficacy at 5-HT1A receptors. Neuropharmacology. 1993;32:331–340. doi: 10.1016/0028-3908(93)90153-t. [DOI] [PubMed] [Google Scholar]
- Sprouse JS, Aghajanian GK. (−)-Propranolol blocks the inhibition of serotonergic dorsal raphe cell firing by 5-HT1A selective agonists. Eur J Pharmacol. 1986;128:295–298. doi: 10.1016/0014-2999(86)90782-x. [DOI] [PubMed] [Google Scholar]
- Spurlock G, Buckland P, O'Donovan M, McGuffin P. Lack of effect of antidepressant drugs on the levels of mRNAs encoding serotonergic receptors, synthetic enzymes and 5HT transporter. Neuropharmacology. 1994;33:433–440. doi: 10.1016/0028-3908(94)90073-6. [DOI] [PubMed] [Google Scholar]
- Stockmeier CA, Dilley GE, Shapiro LA, Overholser JC, Thompson PA, Meltzer HY. Serotonin receptors in suicide victims with major depression. Neuropsychopharmacology. 1997;16:162–173. doi: 10.1016/S0893-133X(96)00170-4. [DOI] [PubMed] [Google Scholar]
- Stockmeier CA, Shapiro LA, Dilley GE, Kolli TN, Friedman L, Rajkowska G. Increase in serotonin-1A autoreceptors in the midbrain of suicide victims with major depression-postmortem evidence for decreased serotonin activity. J Neurosci. 1998;18:7394–7401. doi: 10.1523/JNEUROSCI.18-18-07394.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strawn JR, Adler CM, McNamara RK, Welge JA, Bitter SM, Mills N, Barzman DH, Cerullo MA, Chang KD, Strakowski SM, DelBello MP. Antidepressant tolerability in anxious and depressed youth at high-risk for bipolar disorder: A prospective naturalistic treatment study. Bipolar Disord. 2013 doi: 10.1111/bdi.12113. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sublette ME, Hibbeln JR, Galfalvy H, Oquendo MA, Mann JJ. Omega-3 polyunsaturated essential fatty acid status as a predictor of future suicide risk. Am J Psychiatry. 2006;163:1100–1102. doi: 10.1176/ajp.2006.163.6.1100. [DOI] [PubMed] [Google Scholar]
- Swann AC. Mechanisms of impulsivity in bipolar disorder and related illness. Epidemiol Psichiatr Soc. 2010;19:120–130. [PubMed] [Google Scholar]
- Tatebayashi Y, Nihonmatsu-Kikuchi N, Hayashi Y, Yu X, Soma M, Ikeda K. Abnormal fatty acid composition in the frontopolar cortex of patients with affective disorders. Transl Psychiatry. 2012;2:e204. doi: 10.1038/tp.2012.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vancassel S, Leman S, Hanonick L, Denis S, Roger J, Nollet M, Bodard S, Kousignian I, Belzung C, Chalon S. n-3 polyunsaturated fatty acid supplementation reverses stress-induced modifications on brain monoamine levels in mice. J Lipid Res. 2008;49:340–348. doi: 10.1194/jlr.M700328-JLR200. [DOI] [PubMed] [Google Scholar]
- Vines A, Delattre AM, Lima MM, Rodrigues LS, Suchecki D, Machado RB, Tufik S, Pereira SI, Zanata SM, Ferraz AC. The role of 5-HT1A receptors in fish oil-mediated increased BDNF expression in the rat hippocampus and cortex: a possible antidepressant mechanism. Neuropharmacology. 2012;62:184–191. doi: 10.1016/j.neuropharm.2011.06.017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.