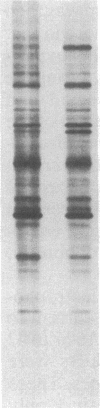
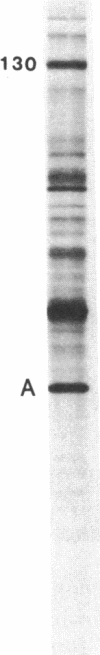
Abstract
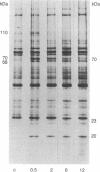
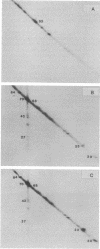
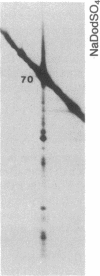
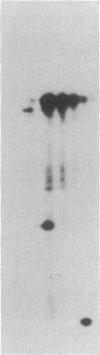
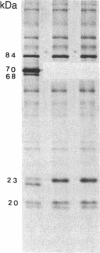
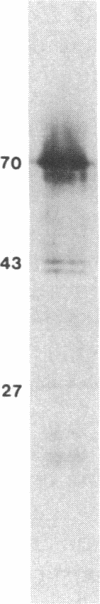
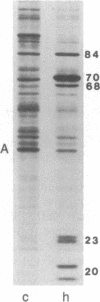
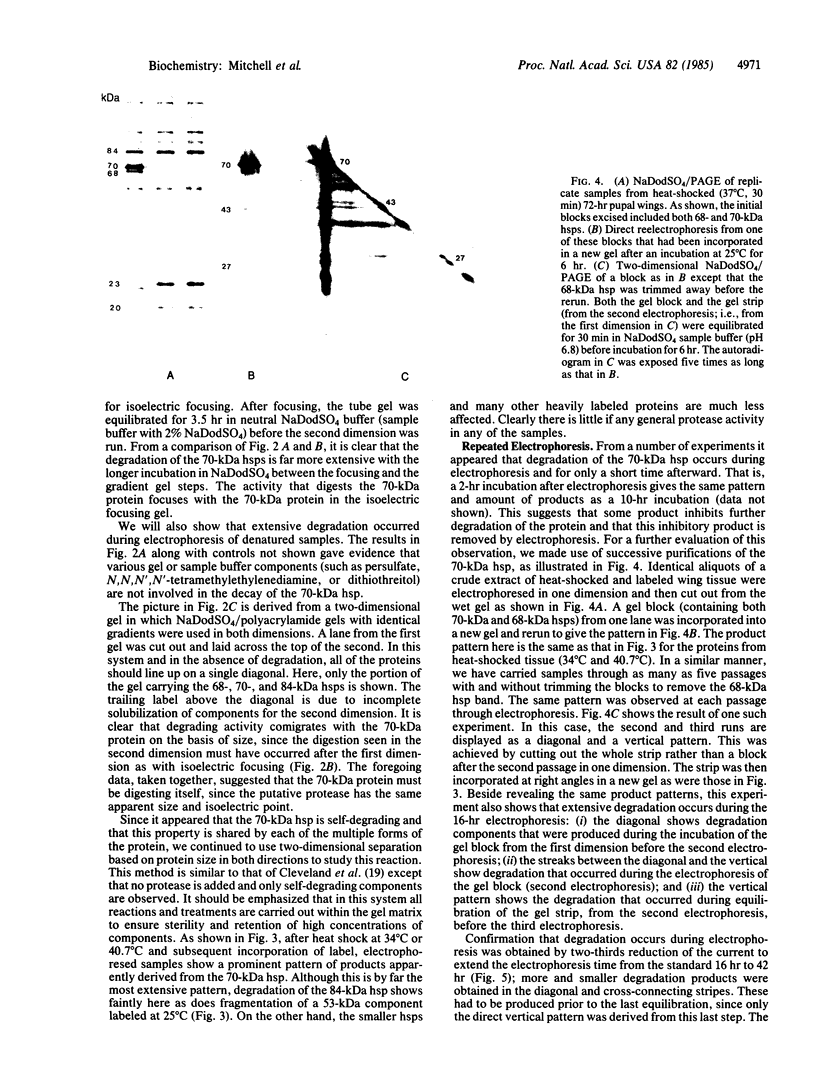
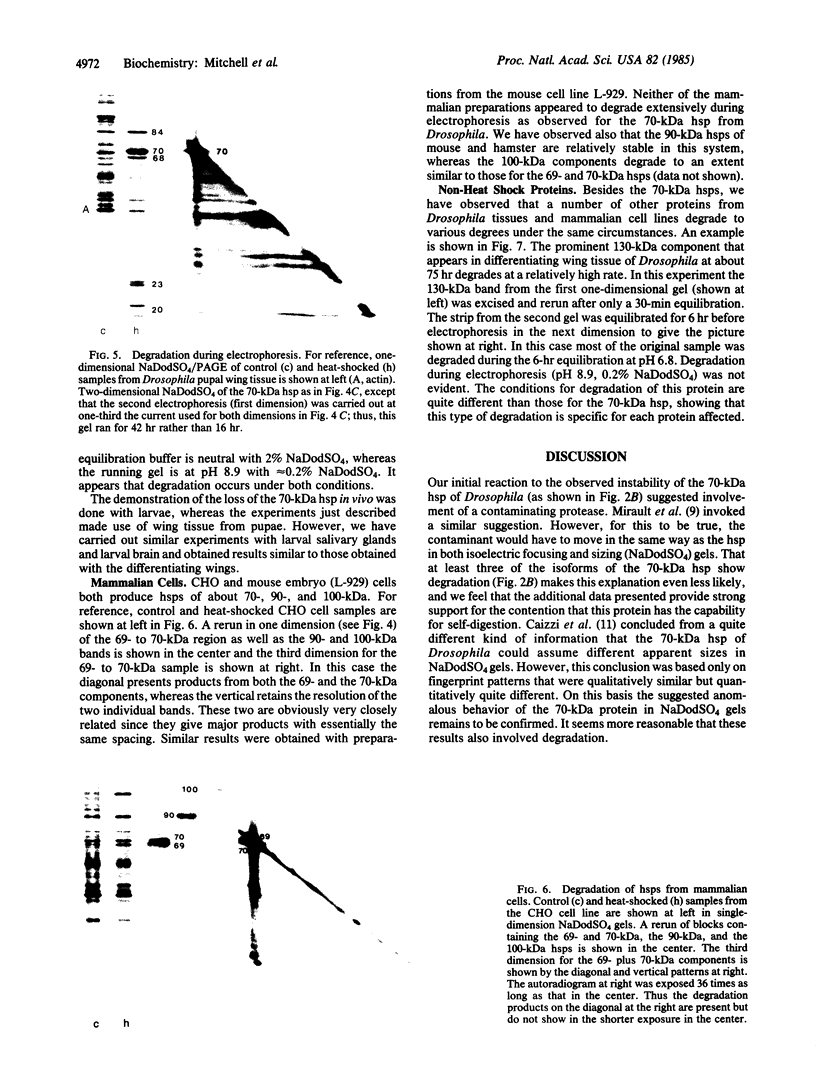
The 70-kDa heat shock protein of Drosophila decays in vivo at a much faster rate than other abundantly labeled proteins. Degradation also occurs in vitro, even during electrophoresis. It appears that this degradation is not mediated by a general protease and that the 70-kDa heat shock protein has a slow proteolytic action upon itself. Heat-induced proteins in CHO cells and a mouse cell line also degrade spontaneously in vitro, as do certain non-heat shock proteins from Drosophila tissues as well as the cell lines.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashburner M., Bonner J. J. The induction of gene activity in drosophilia by heat shock. Cell. 1979 Jun;17(2):241–254. doi: 10.1016/0092-8674(79)90150-8. [DOI] [PubMed] [Google Scholar]
- Buzin C. H., Petersen N. S. A comparison of the multiple Drosophila heat shock proteins in cell lines and larval salivary glands by two-dimensional gel electrophoresis. J Mol Biol. 1982 Jun 25;158(2):181–201. doi: 10.1016/0022-2836(82)90428-4. [DOI] [PubMed] [Google Scholar]
- Cleveland D. W., Fischer S. G., Kirschner M. W., Laemmli U. K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [PubMed] [Google Scholar]
- Douglass J., Civelli O., Herbert E. Polyprotein gene expression: generation of diversity of neuroendocrine peptides. Annu Rev Biochem. 1984;53:665–715. doi: 10.1146/annurev.bi.53.070184.003313. [DOI] [PubMed] [Google Scholar]
- Ingolia T. D., Craig E. A., McCarthy B. J. Sequence of three copies of the gene for the major Drosophila heat shock induced protein and their flanking regions. Cell. 1980 Oct;21(3):669–679. doi: 10.1016/0092-8674(80)90430-4. [DOI] [PubMed] [Google Scholar]
- Mirault M. E., Goldschmidt-Clermont M., Moran L., Arrigo A. P., Tissières A. The effect of heat shock on gene expression in Drosophila melanogaster. Cold Spring Harb Symp Quant Biol. 1978;42(Pt 2):819–827. doi: 10.1101/sqb.1978.042.01.082. [DOI] [PubMed] [Google Scholar]
- Mitchell H. K., Petersen N. S. Gradients of differentiation in wild-type and bithorax mutants of Drosophila. Dev Biol. 1983 Feb;95(2):459–467. doi: 10.1016/0012-1606(83)90047-7. [DOI] [PubMed] [Google Scholar]
- Mitchell H. K., Petersen N. S. Rapid changes in gene expression in differentiating tissues of Drosophila. Dev Biol. 1981 Jul 15;85(1):233–242. doi: 10.1016/0012-1606(81)90253-0. [DOI] [PubMed] [Google Scholar]
- Mitchell H. K., Petersen N. S. Rapid changes in gene expression in differentiating tissues of Drosophila. Dev Biol. 1981 Jul 15;85(1):233–242. doi: 10.1016/0012-1606(81)90253-0. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Privalov P. L. Stability of proteins: small globular proteins. Adv Protein Chem. 1979;33:167–241. doi: 10.1016/s0065-3233(08)60460-x. [DOI] [PubMed] [Google Scholar]
- Southgate R., Ayme A., Voellmy R. Nucleotide sequence analysis of the Drosophila small heat shock gene cluster at locus 67B. J Mol Biol. 1983 Mar 25;165(1):35–57. doi: 10.1016/s0022-2836(83)80241-1. [DOI] [PubMed] [Google Scholar]
- Tissières A., Mitchell H. K., Tracy U. M. Protein synthesis in salivary glands of Drosophila melanogaster: relation to chromosome puffs. J Mol Biol. 1974 Apr 15;84(3):389–398. doi: 10.1016/0022-2836(74)90447-1. [DOI] [PubMed] [Google Scholar]