Abstract
Disorders characterized by ischemia/reperfusion (I/R), such as myocardial infarction, stroke, and peripheral vascular disease, continue to be among the most frequent causes of debilitating disease and death. Tissue injury and/or death occur as a result of the initial ischemic insult, which is determined primarily by the magnitude and duration of the interruption in the blood supply, and then subsequent damage induced by reperfusion. During prolonged ischemia, ATP levels and intracellular pH decrease as a result of anaerobic metabolism and lactate accumulation. As a consequence, ATPase-dependent ion transport mechanisms become dysfunctional, contributing to increased intracellular and mitochondrial calcium levels (calcium overload), cell swelling and rupture, and cell death by necrotic, necroptotic, apoptotic, and autophagic mechanisms. Although oxygen levels are restored upon reperfusion, a surge in the generation of reactive oxygen species occurs and proinflammatory neutrophils infiltrate ischemic tissues to exacerbate ischemic injury. The pathologic events induced by I/R orchestrate the opening of the mitochondrial permeability transition pore, which appears to represent a common end-effector of the pathologic events initiated by I/R. The aim of this treatise is to provide a comprehensive review of the mechanisms underlying the development of I/R injury, from which it should be apparent that a combination of molecular and cellular approaches targeting multiple pathologic processes to limit the extent of I/R injury must be adopted to enhance resistance to cell death and increase regenerative capacity in order to effect long-lasting repair of ischemic tissues.
Keywords: Reactive oxygen species, Inflammation, Calcium overload, Risk factors, Mitochondrial permeability transition pore, miRNA, Microbiome
1. Introduction
The term, ischemia, to denote deficient blood supply to tissues due toobstruction of the arterial inflow was first used in the early nineteenth century. Thus, physicians and biomedical researchers have strived to better understand the underlying mechanisms of ischemia-induced tissue damage for almost two centuries, with the hope for developing therapies to limit the devastating health and economic burdens imposed by disorders characterized by reductions in organ-specific blood flow. Discoveries reported over the past 30 years have been particularly impressive, vastly increasing our understanding of the molecular, cellular, tissue-specific, as well as systemic events that occur during ischemia per se. Evidence supporting the concept that reperfusion could paradoxically induce and exacerbate tissue injury and necrosis was also discovered early in this period and provided a major impetus for research because this component of tissue injury is amenable to therapeutic intervention. Despite years of intensive investigation, we are still far away from thoroughly understanding the underlying mechanisms of I/R (O’Donnell and Nabel, 2011). The aim of this review is to summarize our current understanding of the multifactorial mechanisms that contribute to the genesis of I/R injury, with an eye focused towards therapeutic approaches that target multiple pathologic processes to limit I/R injury and/or enhance resistance to cell death.
2. General Features of Ischemia/Reperfusion (I/R)
The extent of cell dysfunction, injury, and/or death is influenced by both the magnitude and the duration of ischemia. In recognition of this fact, revascularization and restoration of blood flow as soon as possible remains the mainstay of all current therapeutic approaches to ischemia. However, not all organs demonstrate equal susceptibility to ischemia. Moreover, it now seems clear that reperfusion, although necessary to reestablish delivery of oxygen and nutrients to support cell metabolism and remove potentially damaging by-products of cellular metabolism, can elicit pathogenetic processes that exacerbate injury due to ischemia per se and may produce tissue injury in distant organs as a result of mediator release into the bloodstream draining revascularized tissues and subsequent delivery to remote organs. In addition to these considerations, the discovery that short bouts of I/R (ischemic preconditioning) prior to the induction of lethal ischemia activates cell survival programs that limit postischemic injury indicates that the response to ischemia is bimodal. These issues will be discussed in the next several sections.
2.1. Ischemic versus reperfusion components of total tissue injury induced by I/R
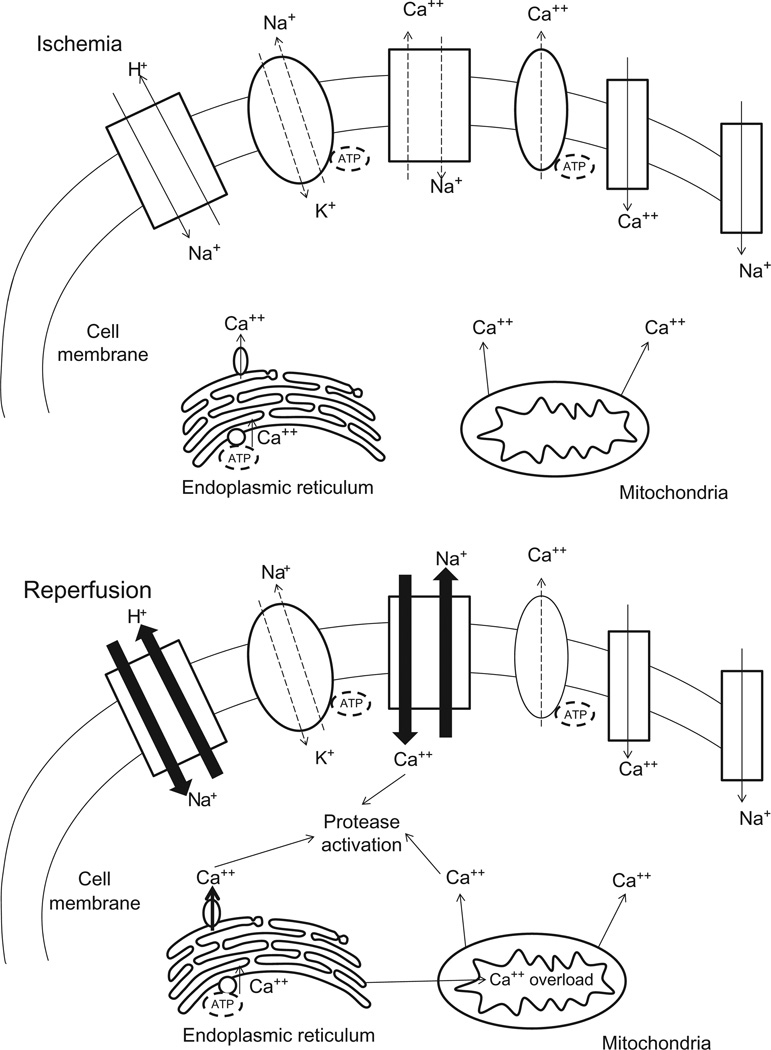
During ischemia, anaerobic metabolism prevails, which produces a decrease in cell pH. To buffer this accumulation of hydrogen ions, the Na+/H+ exchanger excretes excess hydrogen ions, which produces a large influx of sodium ions (Sanada et al., 2011) (Fig. 6.1). Ischemia also depletes cellular ATP which inactivates ATPases (e.g., Na+/K+ ATPase), reduces active Ca2+ efflux, and limits the reuptake of calcium by the endoplasmic reticulum (ER), thereby producing calcium overload in the cell. These changes are accompanied by opening of the mitochondrial permeability transition (MPT) pore, which dissipates mitochondrial membrane potential and further impairs ATP production. In the heart, these cellular changes are accompanied by activation of intracellular proteases (e.g., calpains) which damage myofibrils and produce hypercontracture and contracture band necrosis. These alterations and thus the degree of tissue injury vary in extent with the magnitude of the decrease in the blood supply and with the duration of the ischemic period (Bulkley, 1987) (Fig. 6.2). Other biochemical events occur during ischemia that do not contribute to ischemic injury per se, but when fueled by the delivery of oxygen and formed elements in the blood when the blood supply is reestablished, trigger a cascade of events that exacerbate tissue injury (Figs. 6.1 and 6.2), as discussed below.
Figure 6.1.
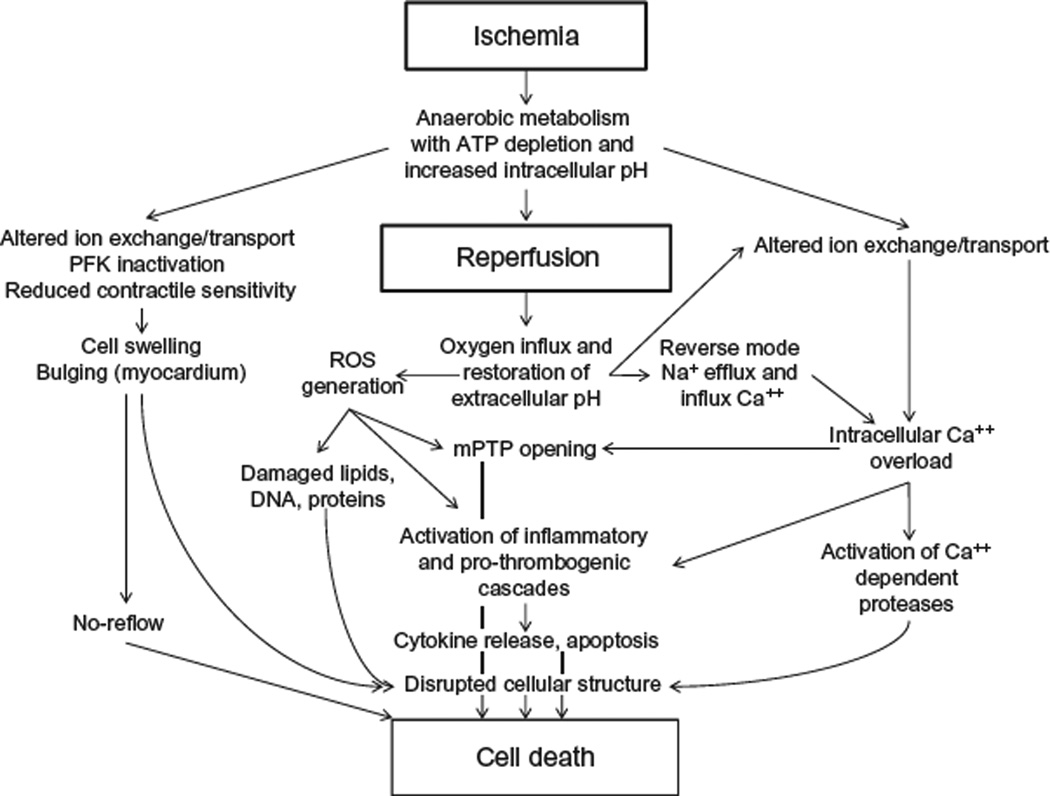
Figure 6.1 Major pathologic events contributing to ischemic (Upper Panel) and reperfusion (Middle Panel) components of tissue injury, with overall integrated responses to I/R injury summarized in the Bottom Panel. See text for further explanation. Modified from Sanada et al. (2011).
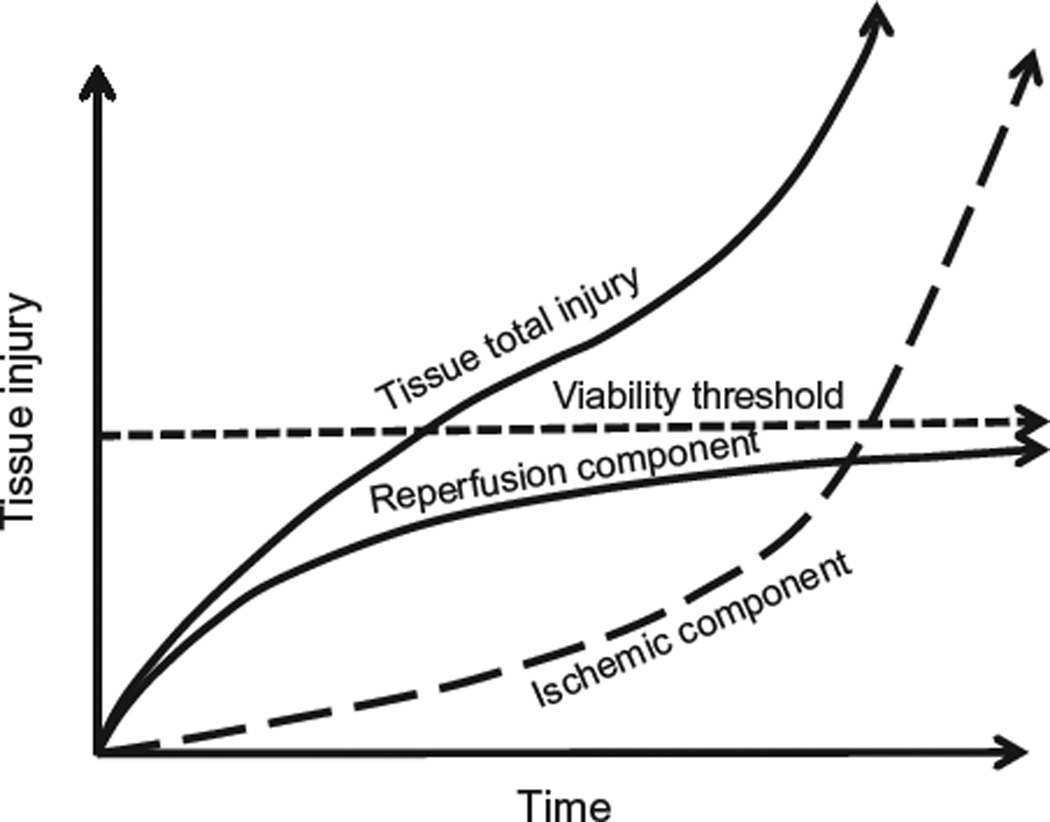
Figure 6.2.
Total injury sustained by a tissue subjected to prolonged ischemia followed by reperfusion (I/R) is attributable to an ischemic component and a component that is due to reestablishing the blood supply. At the onset of prolonged ischemia two separate pathologic processes are initiated. The first are processes of tissue injury that are due to ischemia per se. The second are biochemical changes during ischemia that contribute to the surge in generation of reactive oxygen species and infiltration of proinflammatory neutrophils when molecular oxygen is reintroduced to the tissues during reperfusion particularly the initial phases. For a treatment to be effective when administered at the onset of reperfusion, reestablishing the blood supply must occur before damage attributable to ischemia per se represents a major component of total tissue injury. Therapeutic approaches that target pathologic events contributing to both the ischemic and reperfusion components of total tissue injury, such as ischemic or pharmacologic preconditioning, should be more effective than therapies administered when the blood supply is re-established, which limit only the progression of reperfusion injury. Modified from Bulkley (1987).
Although prompt reperfusion restores the delivery of oxygen and substrates required for aerobic ATP generation and normalizes extracellular pH by washing out accumulated H+, reperfusion itself appears to have detrimental consequences (Figs. 6.1 and 6.2). This concept originally arose over 50 years ago, when it was first observed that reperfusion appeared to accelerate the development of necrosis in hearts subjected to coronary ligation ( Jennings et al., 1960). This has been termed reperfusion injury to describe causal events associated with reestablishing the blood supply that had not occurred during the preceding ischemic period and can be attenuated or abolished by an intervention given only at the time of reperfusion. The existence of such lethal reperfusion injury as an entity separate from the damage caused earlier by ischemia is still under debate. However, interventions during myocardial reperfusion can indeed reduce infarct size by up to 50%, arguing very much in favor of reperfusion phase-specific detrimental events (Yellon and Hausenloy, 2007). The mechanisms underlying reperfusion injury are complex, multifactorial and involve (1) generation of reactive oxygen species (ROS) that is fueled by reintroduction of molecular oxygen when the blood flow is reestablished, (2) calcium overload, (3) opening of the MPT pore, (4) endothelial dysfunction, (5) appearance of a prothrombogenic phenotype, and (6) pronounced inflammatory responses (Yellon and Hausenloy, 2007) (Fig. 6.1).
From the foregoing discussion, it is clear that total injury sustained by a tissue represents the sum of damage attributable to ischemia per se plus that invoked by reperfusion (Figs. 6.1 and 6.2). Importantly, it is clear that the reperfusion phase is very dynamic and that cell death can continue for up to 3 days after the onset of reperfusion (Zhao et al., 2000a). Thus, understanding the mechanisms involved paves the way for development of novel therapeutic opportunities that not only reduce the extent of injury induced by I/R but may also extend the time a tissue could be subjected to ischemia before irreversible injury occurs (Fig. 6.2). The latter point has important implications for organ transplantation, cardiopulmonary bypass, and operation in a bloodless field.
2.2. Tissue responses to I/R are bimodal
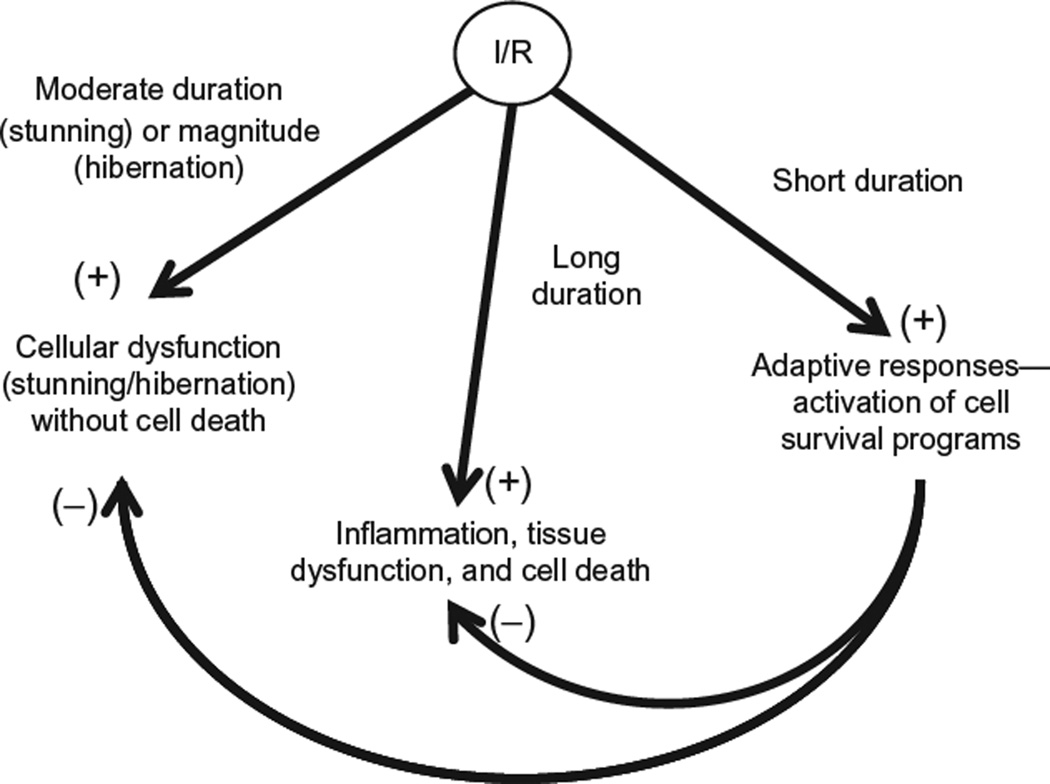
All tissues can withstand variably short periods of ischemia that do not produce detectable functional deficits or evidence of injury (Fig. 6.2). On the other hand, once a critical duration of ischemia is exceeded, which varies by cell type and organ, cell injury and/or death ensues. These observations led to the conclusion that the responses to ischemia are invariably deleterious, with reperfusion exacerbating the extent of tissue injury. However, in 1986, Murry et al. (1986) made the startling discovery that prior exposure of the heart (or other tissues) to short bouts of ischemia and reperfusion (ischemic preconditioning) prior to prolonged reductions in coronary blood flow (index ischemia) exerted powerful infarct-sparing effects. This seminal finding created an explosion of interest with regard to identification of therapeutic strategies that might prove effective in reducing the risk for and/or outcome of adverse cardiovascular events. In addition, the discovery of ischemic preconditioning indicates that the response to ischemia is bimodal, with longer periods of ischemia inducing cell dysfunction and/or death that is exacerbated by reperfusion, while short cycles of conditioning ischemia are protective, rendering tissues resistant to the deleterious effects of prolonged ischemia followed by reperfusion via activation of intrinsic cell-survival programs (Fig. 6.3).
Figure 6.3.
Tissue responses to ischemia/reperfusion are bimodal, depending on the duration of ischemia. Prolonged and severe ischemia induces cell damage that progresses to infarction, with reperfusion often paradoxically exacerbating tissue injury by invoking inflammatory responses. In the heart, shorter bouts of ischemia (5–20 minutes duration) induce myocardial stunning, wherein contractile function is initially impaired on reperfusion, but slowly improves, without progression to infarction and in the absence of significant inflammation. In sharp contras, prolonged exposure to subacute levels of ischemia without reperfusion may induce myocardial hibernation, wherein cardiac cells revert to a more ancestral metabolic phenotype in order to survive but with a cost of reduced mechanical function. In sharp contrast, short periods of ischemia (< 5 min) followed by reperfusion (ischemic conditioning) activate cell survival programs that limit the magnitude of injury induced by subsequent exposure to prolonged I/R.
2.3. I/R-induced stunning and hibernation versus irreversible cell damage and death
Persistence of contractile abnormalities in postischemic myocardium was once thought to result only from irreversible cellular damage and loss of viable myocardium. However, it is now clear that mechanical dysfunction can persist after reperfusion in the absence of irreversible damage and despite restoration of normal or near normal coronary blood flow. One such adaptation is myocardial stunning, wherein postischemic contractile dysfunction occurs but is short lived, arises in the absence of irreversible damage, and is not caused by a primary deficit in reperfusion (i.e., postischemic flow is normal or near normal) (Bolli and Marban, 1999; Depre and Vatner, 2005, 2007) (Fig. 6.2). Myocardial stunning appears to result from reperfusion, which triggers the generation of ROS (oxygen paradox), transient calcium overload concomitant with decreased responsiveness of contractile elements to calcium (calcium paradox), activation of calpains, which enzymatically proteolyze myofibrils, and altered membrane ion channel activity secondary to rapid restoration of extracellular pH (pH paradox). Some investigators have suggested that the stunning-induced deficits in contractile activity may serve a protective function to limit the impact of the harsh cellular milieu induced by ischemia to progress towards irreversible damage during reperfusion, thereby enhancing the likelihood of cell survival (Bolli and Marban, 1999; Depre and Vatner, 2005, 2007).
Myocytes exposed to prolonged or repetitive intermittent ischemia may exhibit a second type of adaptive response that is characterized by a return to neonatal metabolic phenotype which favors the use of carbohydrates as an energy source. This phenomenon, wherein ischemic myocytes undergo a metabolic switch to a glycolytic phenotype with reduced contractile function and energy demands, is termed myocardial hibernation (Depre and Vatner, 2005, 2007; Slezak et al., 2009) (Fig. 6.3). As with myocardial stunning, hibernation allows myocardial cells to better withstand reductions in oxygen and nutrient delivery associated with subacute levels of ischemia in the absence of irreversible cardiomyocyte injury because contractile function is limited. The mechanisms involved in the assumption of thismore ancestral phenotype appear to result from a reprogramming of cell metabolism that decreases energy utilization and via upregulation in the expression of stress and angiogenic proteins. Characteristic cell remodeling changes also occur in hibernating myocardium and include the appearance of polymorphic mitochondria, increased lysosome numbers, and decreased myofibril number. Increased vacuolar density and debris are consistent with autophagy, a mechanism of cell death that contributes to overall prolongation of survival of hibernating viable cells in ischemic organs by eliminating nonfunctional cells. Hibernating myocardium also contains apoptotic cells. Although these adaptive responses reduce myocyte number and contractile responses, hibernating cardiac myocytes can be rescued by restoring blood flow, which reprograms cell protein expression to normalize metabolism and contractile activity (Depre and Vatner, 2005, 2007; Slezak et al., 2009).
The response of tissue cells to ischemia and cell survival is governed by the severity and duration of ischemia, and by pathologic events that are initiated upon reperfusion. Thus, stunning may occur following a relatively short period of ischemia (5–20 min in the heart), with reperfusion causing cell dysfunction, followed by delayed recovery (Fig. 6.3). On the other hand, hibernation occurs with prolonged or repetitive intermittent reductions in the blood supply that are modest in degree, with the attendant contractile impairment being rescued by revascularization. However, long periods of severe ischemia followed by reperfusion produce irreversible damage that culminates in loss of viable myocardium. Undoubtedly, the more prolonged and severe the period of ischemia, the greater is the contribution of permanent damage and cell death to postinfarct dysfunction (Figs. 6.2 and 6.3). However, the exact mechanism whereby reversible ischemia finally evolves into irreversible cell death remains a subject of controversy but most likely involves simultaneous loss of a critical amount of ATP, formation of ROS, metabolically and mechanically induced membrane and cytoskeletal damage, calcium overload, sodium pump failure, and opening of the MPT pore (Fig. 6.1).
2.4. Organ-specific susceptibility to I/R
One of the fundamental observations made in experimental models of I/R is that the injury response after reperfusion is directly correlated with the duration of ischemia (Bulkley, 1987) (Fig. 6.3). Thus, restoration of blood flow to the affected organ at the earliest time possible is obviously of prime importance. In addition, there are common, fundamental features of the response to I/R, including the release of ROS, cytokines, and chemokines from activated endothelium and tissue-resident macrophages and mast cells, recruitment, activation, and endothelial adhesion/ emigration of neutrophils, and other formed elements in the blood, endothelial dysfunction, and parenchymal injury. However, organ-specific differences influence the extent, severity, and reversibility of organ damage. The biological bases for these differences are not well understood. In all tissues, cooling can slow down cellular damage, which can be used intraoperatively or for better preservation of organ transplants during transport (Baumgartner et al., 1989).
With irreversible damage already detectable at less than 20 min of ischemia (Ordy et al., 1993), the brain is the most sensitive organ to reductions in its blood supply. Clinically, the most common event is focal cerebral ischemia (termed ischemic stroke), which arises as a localized reduction in regional blood flow in a specific vascular territory that is caused by thromboembolic or atherothrombotic vaso-occlusive disease. Although damage sets in quickly, the actual time window for therapeutic intervention is longer since not all cells are affected to the same extent after a given duration of ischemia. Indeed, optimal results are observed if thrombolytic therapy is initiated within the first 90 min after the onset of symptoms (Hacke et al., 2004). However, significant improvements in clinical outcome can still be achieved if the blood flow is restored within 3 h (or within 4.5 h in particular patient populations) (Bluhmki et al., 2009).
A number of unique features of the brain seem likely to contribute to its sensitivity: The brain is responsible for 20–25% of total body oxygen consumption, constituting the highest metabolic activity per unit weight of any organ (Kristián, 2004; Lee et al., 2000). This high metabolic demand is coupled with an absolute requirement for glucose as an energy substrate, but with low levels of stored glucose/glycogen compared with other tissues (Kristián, 2004; Lee et al., 2000). By contrast, muscle is capable of limited periods of anaerobic metabolism, and both muscle and liver have comparatively significant stores of carbohydrate. The brain has significantly lower levels of protective antioxidant activities, for example, superoxide dismutase (SOD), catalase, glutathione peroxidase (Adibhatha and Hatcher, 2010), and heme oxygenase-1 (Damle et al., 2009) than heart, liver, kidney, and lung, as well as lower levels of cytochrome c oxidase (Adibhatha and Hatcher, 2010), which would be expected to result in lower ATP production and higher superoxide release from the mitochondrial electron transport chain (e.t.c.). The brain has high levels of polyunsaturated fatty acids which are highly susceptible to oxidative damage (Adibhatha and Hatcher, 2010), and I/R can elicit excessive release of certain neurotransmitters, e.g., glutamate and dopamine (Lee et al., 2000), which upon subversion of these neurotransmitters’ postreceptor signaling pathways result in neuronal calcium overload and subsequent cytotoxicity.
In the heart, the situation is similar but the therapeutic window is slightly longer. Both in humans and in animal models, irreversible cardiomyocyte damage occurs after about 20 min of ischemia. As in the brain, the earlier blood flow is successfully restored, the better are survival rates and salvage of viable myocardium. Intervention within the first 2 h is best (Boersma et al., 1996), but even after 12 h of ischemia reopening of the respective coronary arteries improves outcome (LATE_Study_Group, 1993). In heart, mast cells and infiltrating fibroblasts elicit development of fibrosis (Frangogiannis, 2008; Willems et al., 1994). The precise role of mast cells is unclear, but the fibroblasts transdifferentiate and proliferate as myofibroblasts, and secrete collagen and other matrix proteins, an overabundance of which causes fibrosis and impairment of cardiac function. In contrast, postischemic brain damage is not associated with fibrosis, but instead, glial cell activation (Dinagl et al., 1999) and degradation of extracellular matrix, especially basal lamina, by matrix metalloproteases. This results in astrocyte and endothelial detachment from basal lamina with attendant increases in brain microvascular permeability, as well as glial and endothelial apoptosis (Winquist and Kerr, 1997).
The next most susceptible organ is the kidney. In open renal surgery, it has been established that no permanent organ damage occurs after normothermic ischemia of 30 min or less (McDougal, 1988). In animal models, even longer clamping times of the renal vessels appear to be feasible (Humphreys et al., 2009). Renal parenchymal oxygenation is graded with the highest oxygen levels noted in the cortex, medium levels in the outer medulla, and the lowest levels in the papillae. As a consequence, cortical cells are the most sensitive to ischemia, while cells in the outer medulla can shift to oxygen-independent metabolism, making them less sensitive to a hypoxic environment. Inner medullary and papillae cells use predominantly glucose to generate ATP via anaerobic glycolysis. Thus, these regions demonstrate a reduced sensitivity to ischemia.
While the ‘point of no return’ is fairly easy to define in brain, heart, and kidney, the time window for successful intervention is much harder to assess in the case of intestinal ischemia. On the one hand, clinical symptoms are initially often subtle, making it impossible to pinpoint the onset of ischemia. If the diagnosis is made within 24 h after the onset of symptoms and aggressive treatment initiated, acute mesenteric ischemia has about a 50% survival rate, whereas this rate drops to 30% or less when diagnosis is delayed (Kassahun et al., 2008). In experimental models, it has been shown that the extent of mucosal damage is a direct function of time elapsed from the onset of mesenteric artery occlusion with first histological changes after 30 min and more prominent destruction of the villi after 60 min (Ikeda et al., 1998). After revascularization, mucosal regeneration via cell migration occurs rapidly even after 90 min of ischemia (Park and Haglund, 1992). It is also important to point out that occlusion of the superior mesenteric artery (SMA) produces a gradient of ischemia along the bowel, with the severity of ischemia being greatest in distal portions of the small intestine and proximal colon, while not affecting the middle and distal colon (Premen et al., 1987). Moreover, the ischemia was localized to the mucosal and submucosal layers of the bowel, while the muscularis/serosa was unaffected. Collateral perfusion maintains minimal perfusion of blood flow to the total intestinal wall but is more effective in supplying the muscularis/serosa than the mucosa/submucosa after SMA occlusion. On the other hand, total SMA occlusion completely abolishes jejunal, ileal, and colonic blood flow in neonates (1 day–1 month old), observations that may have important implications for the pathogenesis of neonatal necrotizing enterocolitis (Crissinger and Granger, 1988).
Intestinal I/R is associated with increases in luminal epithelial permeability and ingress of bacterial molecules (e.g., enterotoxin) or bacteria themselves which can result in sepsis and multiple organ failure, if the magnitude of ischemia is severe or the volume of ischemic mesenteric tissue is large (Kinross et al., 2009; Souza et al., 2004). Indeed, germ-free mice exhibit reduced local (intestinal) and remote (lung) injury following mesenteric I/R relative to conventional mice, effects that were associated with decreased expression of proinflammatory cytokines, and neutrophil sequestration (Souza et al., 2004). The lack of commensal microbiota was also associated with increased expression of IL-10, an anti-inflammatory cytokine. Function-blocking antibodies directed against IL-10 reversed the protection against I/R-induced inflammation and injury in germ-free mice. Similar protection was noted in germ-free mice subjected to hemorrhagic shock (Ferraro et al., 1995). These results were recently confirmed by work showing that depletion of gut commensal bacteria using broad-spectrum antibiotic cocktail reduces intestinal I/R injury (Yoshiya et al., 2011) and lung injury induced by bowel ischemia (Sorkine et al., 1997). Bacterial depletion also reduced the expression of Toll-like receptor2 (TLR2) and TLR4, well-known receptors for gram-positive and -negative bacteria (Yoshiya et al., 2011). As a consequence, there was reduced expression of proinflammatory mediators (TNF, IL-6, and COX-2), decreased complement and immunoglobulin deposition, and B-lymphocyte recruitment. Interestingly, probiotic colonizing of the intestine by oral administration of Lactobacillus plantarum for 2 weeks reduced bacterial translocation to extraintestinal sites, decreased the elaboration of proinflammatory cytokines, and limited epithelial apoptosis and disruption of the mucosa induced by mesenteric I/R, relative to conventional animals (Wang et al., 2011a). The results of these studies clearly indicate that the intestinal microflora play a critical role in local and remote injury following gut I/R, effects that may be modulated by altering constituent commensal bacteria populations (Alverdy and Chang, 2008; Kinross et al., 2009, 2011). These results were recently extended to myocardial I/R, where it appears that intestinal dysbiosis induced by vancomycin treatment prior to induction of coronary occlusion resulted in smaller infarcts, improved postischemic recovery of mechanical function, and decreased circulating leptin levels. These protective effects were replicated in animals fed a probiotic product containing L. plantarum (Lam et al., 2012).
In contrast to these most sensitive organs, ischemia of skeletal muscle is much better tolerated. Since acute arterial injuries may require emergency application of tourniquets, it is well known that hours of limb ischemia are well tolerated, with best results obtained if the tourniquet is briefly released after the first 1.5–2 h (Sapega et al., 1985). Moreover, skeletal muscle can regenerate even after wide-spread injury (Wagers and Conboy, 2005). At the far end of the spectrum, tissues that contain very little or no vasculature are barely affected by ischemia. For example, cornea transplants can be stored in tissue culture media for 3 weeks with only minor damage to endothelial cells (Smith and Johnson, 2010).
Since the microvasculature is the initial site where initial recruitment of inflammatory cells takes place, tissue differences in the structure and function of microvascular beds are likely to play a major role in tissue responses to I/R. The generally accepted paradigm of endothelial selectin-dependent rolling and integrin-dependent adhesion of leukocytes during inflammation was developed using studies of these processes in microvascular beds that are readily visualized using intravital microscopy—for example, those of the intestinal mesentery and cremaster muscle (Granger and Korthuis, 1995). Although it might be expected that significant structural and functional differences between the microvasculature of different tissues should be correlated with similar differences in inflammatory processes, the leukocyte–endothelial recruitment paradigm described in mesentery and cremaster appears to be sufficient to explain the process in most other tissues. One exception is the liver, where the role of selectins appears to be dependent upon which microvascular bed is examined. Sinusoids, carrying mixed blood from portal venules and hepatic arterioles, do not express P- and E-selectins and do not support selectin-mediated rolling, whereas postsinusoidal venules do (Liu and Kubes, 2003). The role of integrins in hepatic sinusoids has also been questioned (Liu and Kubes, 2003); in this particular microvascular bed, leukocyte accumulation may be more influenced by physical factors such as a vessel diameter close to that of leukocytes themselves (Liu and Kubes, 2003). Another “unusual” organ from the perspective of leukocyte adhesion and migration is the lung. Unlike other tissues, where polymorphonuclear (PMN) adhesion and migration take place in relatively large postcapillary venules, in the lung, this process occurs primarily in alveolar capillaries, whose diameter is comparatively smaller (Burns et al., 2003). Moreover, the primacy of neutrophils themselves in IRI is not clear in some tissues. For example, lymphocytes and monocytes may play a more important role in mediating injury responses in kidney ( Jong et al., 2009) and brain (Yilmaz and Granger, 2008)
The response to neonatal hearts to I/R is controversial. Some studies indicate that neonatal hearts are more susceptible to ischemia (Wittnich, 1992), whereas other studies demonstrate enhanced tolerance of the immature myocardium ( Julia et al., 1990). It is quite likely that differences in species chosen for the studies are responsible for such discrepancies. Again using the heart as an example, direct species comparisons using identical protocols for I/R have shown that isolated hearts from rabbits, hamsters, ferrets, gerbils, rats, mice, and guinea pigs differ substantially in injury susceptibility (Galinanes and Hearse, 1990). Furthermore, even within the same species, some strains are rather resistant to ischemia, whereas others are particularly prone to injury. Examples can be found in studies of the heart (Barnabei et al., 2010), brain (Barone et al., 1993), kidney (Burne et al., 2000), and lung (Dodd-o et al., 2006), indicating that this presents a serious experimental limitation across all organ systems.
2.5. Remote organ injury
Untoward effects of I/R are not necessarily restricted to the specific tissue undergoing the initial ischemia. That is, a frequent consequence induced by reperfusion after localized tissue ischemia is injury to other organ systems, so-called distant or remote organ injury (ROI). This phenomenon can arise from I/R in most tissues, including gut (Carden and Granger, 2000; He et al., 2011b; Santora et al., 2010; Sorkine et al., 1997), lung (Esme et al., 2006), liver (Hirsch et al., 2008), kidney (Vaghasiya et al., 2010), skeletal muscle (Vega et al., 2000), and heart (Barry et al., 1997). The ultimate expression ofROIismultiple organ dysfunction syndrome, known to result from I/R in gut, liver, skeletal muscle, aortic surgery involving occlusion–reperfusion, and circulatory shock (Carden and Granger, 2000; Santora et al., 2010). In this regard, the lungs are especially vulnerable, particularly after I/R of the gut and/or liver (Carden and Granger, 2000; He et al., 2011a,b; Sorkine et al., 1997), as they are the first major capillary bed exposed to postischemic blood. Indeed, one of the first clinical symptoms preceding multiple organ failure is respiratory dysfunction (Carden and Granger, 2000; He et al., 2011a,b; Santora et al., 2010).
Examination of the mechanisms underlying ROI has found roles for the same factors implicated in the local organ dysfunction produced by IRI: ROS, leukocytes, and inflammatory mediators. A common finding has been that one or more circulating factors are responsible for the effect on organs distant from the one undergoing the initial insult (Carden and Granger, 2000; He et al., 2011a,b; Santora et al., 2010). These factors may be directly released from the primary injured tissue or indirectly from activated leukocytes or other inflammatory cells.
Xanthine oxidase (XO), which generates superoxide and hydrogen peroxide, has been implicated as an important factor in ROI in liver, lung, and cardiac muscle after gut I/R (Carden and Granger, 2000). The mechanism for XO-mediated systemic effects is not clear but may involve generation of high amounts of ROS by circulating enzyme, close association of XO with endothelial cell surface and consequent high local ROS concentrations, or XO-derived oxidant-induced release of chemotactic factors which can promote recruitment of PMNs to organs distant from the initial injury.
Just as in the primary organ subjected to I/R, inflammatory leukocytes play a major role in injury to remote organs. A key event appears to be activation or “priming” of PMN in a postischemic vascular bed, followed by recruitment of the activated PMNs to remote tissues (Carden and Granger, 2000). This involves not only PMN activation but also activation of endothelial cells in distant tissues, characterized by increased surface expression of endothelial adhesion molecules. Systemic release of inflammatory mediators from the primary injured tissue and/or from recruited monocytes and neutrophils as well as systemic complement activation has all been reported to promote systemic activation of and recruitment of PMN to sites distant from initial I/R. If intestinal I/Ris involved inROI, bacteria can cross themucosal barrier, resulting in systemic infection and sepsis (Stallion et al., 2005). In recent years, it has become clear that ischemic-reperfused intestine releases cytokines and other inflammatory mediators into the intestinal lymph; these agents enter the systemic circulation at the thoracic duct (Deitch et al., 2006; Deitch, 2010; He et al., 2011a,b), and it was shown that lymph is their primary route of entry, since ligation of the mesenteric lymph duct can prevent ROI (Deitch et al., 2006; Deitch, 2010).
Over the past decade, it has become increasingly clear that neurogenic signals contribute to inflammatory responses (Ahluwalia et al., 1998; Bhatia et al., 1998; Bozic et al., 1996; Cao et al., 2000; Souza et al., 2002), including ROI (Bhatia et al., 1998; Souza et al., 2002). The proinflammatory phenotype produced by intestinal I/R can be significantly attenuated by treatment with the sensory nerve toxin, capsaicin, and tachykinin receptor antagonists (Souza et al., 2002). Significantly, the aforementioned protection is observed both locally in the gut and in the lung (Souza et al., 2002), demonstrating the potential importance of neurogenic signals in ROI. The most likely mediators for these effects are neuropeptides released from both sensory nerve endings and inflammatory cells (Quartara and Maggi, 1998). It has been proposed that neurokinin-dependent signaling may contribute to either or both of initiation of I/R-induced inflammatory responses via initial release of lipid mediators such as PAF, or amplification of an existing inflammatory phenotype (Souza et al., 2002).
3. Risk Factors for I/R
The vast majority of ischemic episodes seen in clinic in the western world are due to thromboembolic or artherothrombotic vaso-occlusive disease. The major risk factors for such events that cannot be preventatively addressed are advancing age, male gender, and hereditary factors. However, other important risk factors can be modified or controlled, including tobacco smoking, hyperlipidemia, hypertension, physical inactivity, obesity, metabolic syndrome, and diabetes mellitus. Ethanol intake at high levels (3–4 or more drinks), either in acute (occasional binge drinking) or chronic (daily) settings, also increases the risk for myocardial infarction and ischemic stroke.
Unfortunately, most of the experimental studies examining the mechanisms of I/R injury employ surgical methods to occlude particular vessels in young, healthy animals. Clearly, these models are not representative of the comorbidities present in the human patient population, where localized reductions in regional blood flow to specific vascular territories are caused by thromboembolic or artherothrombotic vaso-occlusive disease and occur in an inflammatory milieu not present in young, healthy subjects. Importantly, there is now mounting evidence that many disorders such as diabetes, hyperlipidemia, and aging can affect the development of I/R injury per se, independent of the vascular aspects (Boengler et al., 2009; Ferdinandy et al., 2007). While diabetes and adverse blood lipid profiles can be controlled, age, of course, remains irreversible. However, recent studies have shown that caloric restriction and exercise have substantial benefits, can preserve cardioprotective mechanisms, and increase ischemic tolerance in aged hearts (Boengler et al., 2009).
4. Fetal Programming and Ischemic Vascular Disease
A growing body of evidence supports the proposal that adult coronary disease may have fetal origins. Barker and his group (Barker, 1995; 2007) demonstrated that decreasing birth weights over the range from 9 to 5 pounds was associated with increased mortality from ischemic disease in adults. A similar correlation was noted in adults who had been born at the opposite end of the birthweight scale (>9.5 pounds). A large number of subsequent studies report a “U”-shaped relation between placental-to-fetal weight ratio and cardiac disease, results which strongly suggest that factors influencing placental growth initiate programs that enhance the vulnerability of the myocardium to ischemic disease later in life. It is now clear that the response of the fetus to a broad range of environmental cues (hemodynamic effects, growth factors, cocaine and tobacco smoke exposure, oxygen and nutrient availability) increases the susceptibility to later development of cardiovascular disease (Langley-Evans and McMullen, 2010; Reynolds, 2010). Furthermore, two very recent studies showed that both prenatal hypoxia and prenatal cocaine exposure inhibit cardioprotection by ischemic preconditioning in male offspring later in adult life (Meyer et al., 2009; Patterson et al., 2010). This is thought to be due to irreversible fetal reprogramming (see Section 4) of protein kinase C (PKC) epsilon expression. Unfortunately, how such conditions could be treated in humans and cardioprotection be restored, remains to be characterized, but targeted gene therapy appears to be a fruitful avenue for exploration. The gene-activated responses to intrauterine stresses that lead to increased disease risk in later life have been termed fetal programming and is now recognized as an additional risk factor for the development of cardiovascular disease. Importantly, low birth weight is also associated with higher rates of hypertension, obesity, type 2 diabetes, and obesity in adults (Reynolds, 2010; Thornburg et al., 2010). Because these chronic disease states represent major risk factors for myocardial infarction and stroke, it is clear that fetal programming is an important contributor to the prevalence of ischemic disease in western societies.
While it is clear that fetal programming of adult cardiovascular disease has been recognized for some time, the mechanisms underlying this phenomenon remain poorly understood. It has been suggested that intrauterine hypoglycemia may induce the appearance of a thrifty phenotype characterized by the persistence of a fetal glucose conserving adaptation that leads to the development of insulin resistance and type 2 diabetes (Hales and Barker, 2001). It has also been suggested that placental insufficiency plays an important role in fetal programming of adult cardiovascular disease by limiting fetal growth. This notion is based on the fact that the placenta regulates the delivery of nutrients from the mother to the fetus (Thornburg et al., 2010). Indeed, intrauterine growth restriction is associated with oxidative and nitrosative stress, alterations in angiogenic responses and expression of genes related to nutrient metabolism, inflammatory cytokine expression, and decreases in placental growth factor expression. While these changes suggest a causal role, evidence supporting this assertion is lacking. However, stronger evidence has been provided for the notion that glucocorticoid overexposure during fetal life may explain the strong association between low birth weight and increased risk for the development of obesity, hypertension, type 2 diabetes, and ischemic disease in later life (Langley-Evans and McMullen, 2010; Reynolds, 2010). Alterations in renal function associated with fetal glucocorticoid exposure have also been implicated in fetal programming (Baum, 2010; Moritz et al., 2011). Fetal programming also influences the functions of adipose tissue and the innate immune system, which may contribute to increased susceptibility of adult tissues to ischemia (de Moura et al., 2008; Symonds et al., 2012). It is almost certain that the link between fetal growth and adult onset disease involves changes in gene expression, which most likely involve epigenetic phenomena (Langley-Evans and McMullen, 2010; Reynolds, 2010). However, the nature of these putative changes in gene expression remains obscure.
5. Basic Mechanisms of Cell Death
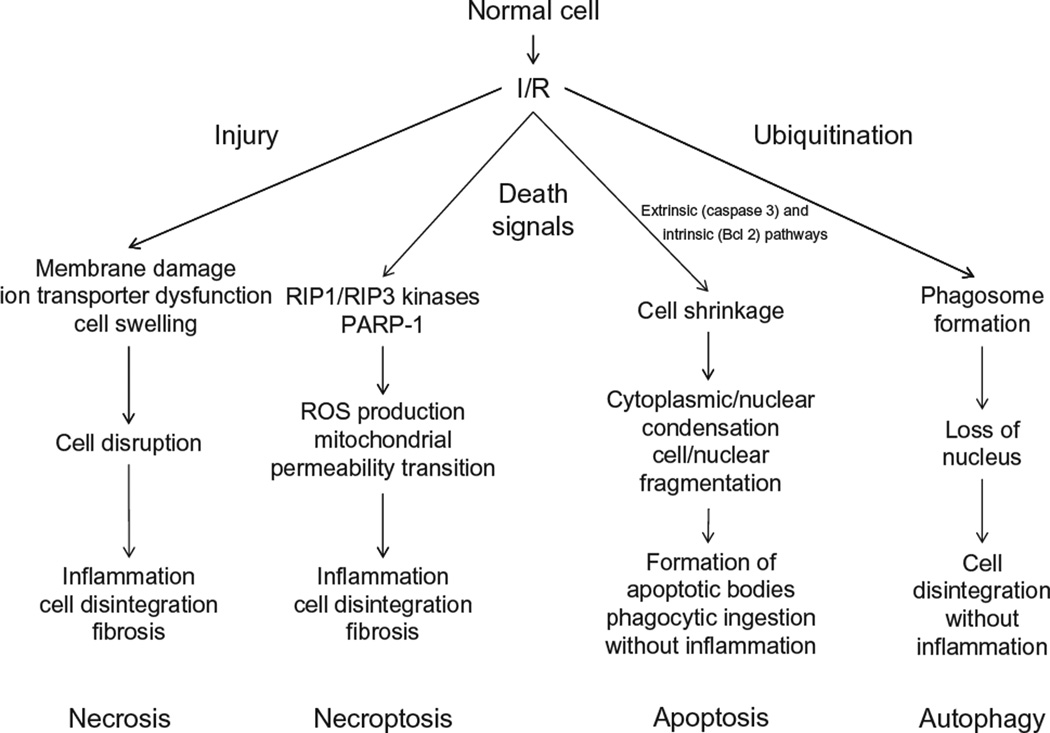
For many years, I/R-induced cell death was thought to occur by extrinsic factors such as loss of energy supply, elaboration of inflammatory mediators and toxic molecules, and mechanical injury, a mode of cell death termed necrosis (oncosis) (Fig. 6.4). However, it is now recognized that cells can also be programmed to die by cellular signaling mechanisms via the processes of apoptosis and autophagy (Kroemer et al., 2009) (Fig. 6.4). Moreover, an emerging body of evidence indicates that the apparently random and uncontrolled events associated with necrosis may, under certain circumstances, actually involve the mobilization and coordination of specific signaling mechanisms in a fourth death pathway termed programmed necrosis or necroptosis (Fig. 6.4). Because each of these morphologically distinct types of cell death appear to contribute in some way, shape, or form to the pathogenesis of I/R injury, we provide a brief overview of the basic mechanisms underlying each of these death modalities.
Figure 6.4.
Mechanisms of cell death in ischemia/reperfusion (I/R). I/R-induced necrosis generally occurs as a result of dysfunctional ion transport mechanisms, which causes cells to swell and eventually burst, effects that are exacerbated by plasma membrane damage. Release of proinflammatory mediators and damaged biomolecules initiates the influx of inflammatory cells such as neutrophils, which disrupt the extracellular matrix and cause damage to parenchymal cells by release of cytotoxic oxidants and hydrolytic enzymes. Apopotosis is a regulated form of cell death that causes cell shrinkage and condensation of the cytosol and nucleus, which eventually form apoptotic bodies. Because they are surrounded by cell membranes, apoptotic bodies can be engulfed and digested by phagocytes without evoking an inflammatory response. Autophagy provides a mechanism to remove damaged or senescent protein aggregates and organelles by enclosing them in membrane-lined vesicles called phagosomes which fuse with lysosomes containing enzymes that degrade the ingested material, usually without evoking an inflammatory response. While normally performing this “housekeeping” function, autophagy may also provide cells with a survival mechanism to withstand the deleterious effects of ischemia, by generating amino acids and fatty acids for cell function. However, when uncontrolled, autophagy contributes to ischemic cell death. While necrosis was once believed to occur from non-specific trauma or injury as a result of I/R, it now appears that postischemic infarction may also be attributable to programmed events that require a dedicated molecular circuitry that has been termed programmed necrosis or necroptosis. Necroptosis is initiated by TNF-like cytokines that activate RIP kinases to mediate necrosis via increased production of reactive oxygen species and calcium overload, which in turn modulate the mitochondrial permeability transition pore, leading to dissipation of the proton electrochemical gradient, with subsequent ATP depletion, further ROS production, and swelling and rupture of mitochondrial membranes.
5.1. Apoptosis
Apoptotic mechanisms are canonically divided into the “extrinsic” and “intrinsic” pathways (Fig. 6.4), although there is considerable cross talk between the two (Broughton et al., 2009; Kroemer et al., 2007; Whelan et al., 2010). The “extrinsic” pathway involves the activation of receptors such as the Fas, TNFα, and TRAIL receptors. Activation of these receptors results in their trimerization, which, in turn, recruits a number of death domain-containing proteins such as FADD and TRADD to the receptor complex. This death-inducing signaling complex activates the protease caspase-8, which, in turn, cleaves and activates caspase-3. Caspase-3 acts as the cell’s executioner by proteolyzing many cellular proteins (Broughton et al., 2009; Kroemer et al., 2007; Whelan et al., 2010).
Regarding the “intrinsic” pathway, cytotoxic stimuli such as I/R, UV irradiation, toxic compounds (etoposide, staurosporine), or oxidative stress induce the translocation and integration of pro-death members of the Bcl2 protein family (e.g., Bax, Bak) into the outer mitochondrial membrane (Broughton et al., 2009; Kroemer et al., 2007; Whelan et al., 2010). These proteins, by a mechanism that still remains controversial, permeabilize the outer membrane, thereby enabling the release of proapoptotic proteins from the intermembrane space, most notably cytochrome c, Smac/DIABLO, Omi/HtrA2, and endonuclease-G (endoG). Cytochrome c binds to the cytosolic protein apaf1 and the resultant “apoptosome” activates the caspase-9 and −3 protease system. Smac/DIABLO and Omi/HtrA2 activate caspases by sequestering or digesting caspase-inhibitory proteins, respectively, whereas endoG mediates DNA fragmentation (Broughton et al., 2009; Kroemer et al., 2007; Whelan et al., 2010).
5.2. Autophagy
Autophagy is the cell’s main mechanism for disposal of obsolete or damaged organelles and protein aggregates, thereby providing a “housekeeping” function. It is also provides cells with a survival mechanism to withstand stressful conditions, such as starvation, hypoxia, mitochondrial dysfunction, and infection by generating amino acids and fatty acids for maintenance of cell function, or by removing damaged organs and intracellular pathogens. Thus, autophagy is actually a cell survival mechanism rather than a cell death process. However, uncontrolled autophagy will ultimately lead to the death of the cell and may contribute to I/R injury. Morphologically (Fig. 6.4), autophagy begins with the expansion of an isolation membrane, or phagophore, around the cell compartment/organelle to be processed (Gottlieb and Mentzer, 2010; He and Klionsky, 2009; Levine and Kroemer, 2008). The membrane then completely envelops the constituents to form the vesicular autophagosome, which then fuses with a lysosome and the encased materials are degraded.
Like apoptosis, autophagy is tightly regulated and is mediated by specific pathways (Fig. 6.4). The main controller is mammalian target of rapamycin (mTOR), which inhibits autophagy. However, under conditions of nutrient withdrawal or stress, mTOR is inactivated (Gottlieb and Mentzer, 2010; He and Klionsky, 2009; Levine and Kroemer, 2008). This derepresses another kinase called Atg1 which together with Atg13 and Atg17 initiates formation of the phagophore. Formation of phagophore is further facilitated by another complex consisting of a class III PI3K called vps34, vps15, and beclin-1. This complex, in turn, recruits Atg12, Atg5, and Atg8 (also called LC3), which are essential for the elongation of the membrane and completion of the autophagosome. Now complete, the fusion of the autophagosome to the lysosome is mediated by the small GTPase Rab7 and the lysosomal membrane protein LAMP2 (Gottlieb and Mentzer, 2010; He and Klionsky, 2009; Levine and Kroemer, 2008).
5.3. Necrosis and necroptosis
Necrosis is characterized morphologically by swelling of cells and their constituent organelles, mitochondrial dysfunction, lack of nuclear fragmentation, plasma membrane rupture, and leakage of intracellular contents (Fig. 6.4). In contrast to the programmed nature of apoptosis and autophagy, necrosis was believed to occur by random, uncontrolled processes that led to the “accidental” death of the cell in response to overwhelming stress. However, the concept of programmed necrosis, also termed necroptosis, especially under conditions like I/R, is gaining acceptance (Fig. 6.4). Specifically, it is now known that cell stress or death receptor activation mobilizes and activates a group of serine/threonine kinases called receptorinteracting proteins (RIPs). In particular, RIP1 and RIP3 appear to act in coordination as mediators of necrosis (Moquin and Chan, 2010; Smith and Yellon, 2011; Vandenabeele et al., 2010). Activation of RIPs 1 and 3, in turn, leads to increased ROS production either through activation of NADPH oxidases (Morgan et al., 2008), or increased mitochondrial oxidant production (Vandenabeele et al., 2010), depending on the cell type. The finding that necrostatin-1 (a small tryptophan-based compound identified by screening a chemical library of approximately 15,000 compounds for their ability to inhibit cell death invoked by TNFα in the presence of zVAD.fmk) reduces TNFα-induced necrotic cell death through inhibition of RIP1 kinase activity supports the concept of receptor-induced necrosis via a controlled cellular process (Smith and Yellon, 2011).
One potentialmitochondrial target forRIP-mediated necrosis is theMPT pore. The MPT pore is a large, nonspecific channel in the inner mitochondrial membrane that is opened in response to excessive production of ROS and to Ca2+ overload of the mitochondrial matrix (Baines, 2009a,b; Halestrap, 2009; Kroemer et al., 2007), both of which occur during I/R. This sudden increase in inner membrane permeability dissipates the proton electrochemical gradient (Δψm), leading to ATP depletion, further ROS production, and ultimately swelling and rupture of the organelle. Although originally proposed as a mediator of apoptosis, recent genetic studies have suggested that the MPT pore is predominantly involved in necrosis (Baines, 2009a,b; Halestrap, 2009; Kroemer et al., 2007).
A third, potentially overlapping necrotic pathway involves activation of the DNA repair enzyme poly(ADP-ribose) polymerase-1 (PARP1). Genotoxic stresses such as oxidants and alkylating agents lead to an overstimulation of PARP1, which, in turn, activates the cysteine protease calpain (Boujrad et al., 2007; Wang et al., 2009). This, in turn, stimulates the release of the increasingly misnamed apoptosis-inducing factor from the mitochondria, where it translocates to the nucleus and degrades DNA. How this relates to RIP kinases and the MPT is still unclear at this point, but there is evidence that PARP1-mediated cell death may be dependent on RIP1 (Xu et al., 2006).
6. Mechanisms Underlying I/R Injury
The mechanisms contributing to the pathogenesis of I/R injury are multifactorial, complex, and highly integrated, with the net result of the perturbations induced by ischemia and invoked when the blood supply is reestablished being damage to all biomolecules in cells and tissues. If severe enough, cell death ensues by the mechanisms described in Section 5.
6.1. Calcium overload
During ischemia, the affected cells become dependent on anaerobic glycolysis for their ATP supply. This leads to an accumulation of lactate, protons, and NAD+ and, therefore, causes a drop in cytosolic pH. In an attempt to reestablish normal pH, the cell extrudes H+ ions in exchange for Na+ via the plasmalemmal Na+/H+ exchanger (NHE) (Baines, 2009a,b, 2010, 2011; Murphy and Steenbergen, 2008). The Na+ ions are, in turn, exchanged for Ca2+ by the plasmalemmal Na+/Ca2+ exchanger (Fig. 6.1). This increase in cytosolic Ca2+ is greatly exacerbated upon reperfusion, where removal of extracellular H+ ions further increases the proton gradient across the plasmalemma, thereby accelerating NHE exchanger function (Baines, 2009a,b, 2010; Murphy and Steenbergen, 2008; Sanada et al., 2011; Talukder et al., 2009). In addition to detrimental alterations in plasmalemmal Ca2+ handling, the endoplasmic/sarcoplasmic reticulum Ca2+ store is also affected during I/R. In particular, Ca2+ reuptake into the ER/SR by the SERCA ATPase is impaired by I/R, whereas Ca2+ release through the ryanodine receptor is enhanced (Sanada et al., 2011; Szydlowska and Tymianski, 2010; Talukder et al., 2009), both of which further exacerbate the lethal elevations in intracellular Ca2+ (Fig. 6.1).
These massive alterations in Ca2+ activate a variety of systems, all of which can contribute to cell death following I/R. One of the ways cells deal with this lethal increase in Ca2+ is to take it up into the mitochondria via the mitochondrial Ca2+ uniporter, a protein that uses the negative Δψm to drive uptake of the positively charged Ca2+ ions into the matrix (Contreras et al., 2010; Szydlowska and Tymianski, 2010; Talukder et al., 2009) (Fig. 6.5). However, if the elevations in mitochondrial Ca2+ become excessive, they can trigger the MPT response. In addition, I/R-induced elevations in cytosolic Ca2+ can also lead to the pathological activation of Ca2+/calmodulin-dependent protein kinases (CaMKs), which also contribute to cell death and organ dysfunction following ischemia.
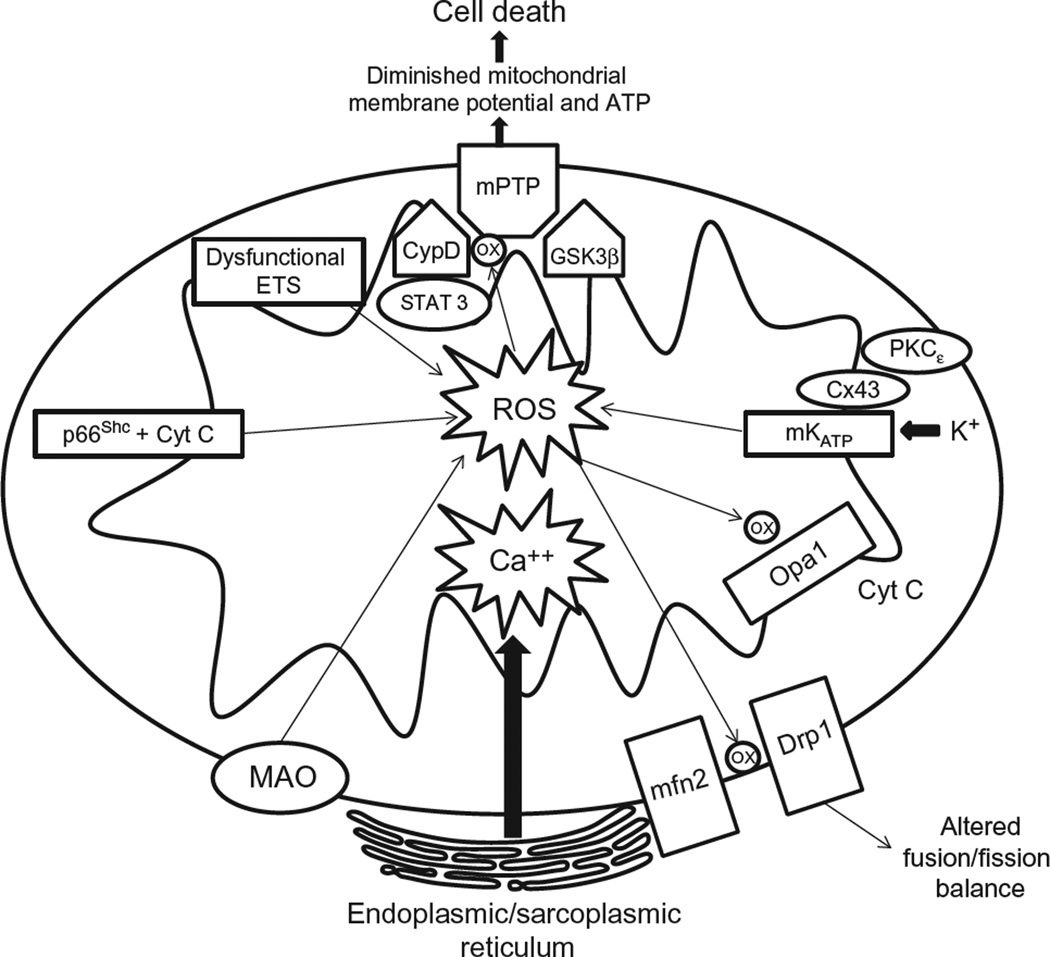
Figure 6.5.
Role of mitochondria in ischemia/reperfusion-induced cell injury. Opening of the MPT pore in the inner mitochondrial membrane is a critical event in the progression of cell death in response to I/R. Being inhibited by low pH, the MPT pore is kept quiescent during ischemia. However, upon reperfusion the huge increases in mitochondrial Ca2+, coupled with the ROS burst induce opening of the MPT pore. When this pore opens, H+ moves back into the matrix, thereby dissipating the Δψm, uncoupling the electron transport chain and inhibiting ATP synthesis. In addition, water enters the mitochondria through its osmotic gradient causing the mitochondria to swell and even rupture. There are several mitochondrial sources of ROS including the electron transport chain, p66Shc, mitochondrial KATP channels, and monoamine oxidases. Mitochondria are dynamic organelles that form tubular, intercommunicating networks that are linked to the cytoskeleton and undergo cycles of division (fission) and fusion. Alterations in mitochondrial morphology occur when these latter two processes become unbalanced, with loss of fission resulting in the appearance of large networks of fused mitochondria, while excessive fission leads to small, fragmented mitochondria. Because fission is initiated under conditions associated with I/R, such as low ATP levels and increase mitochondrial ROS production, and excessive mitochondrial fission is a required step for extrinsic apoptotic cell death, this process may contribute to the pathogenesis of postischemic cell death. Mitochondrial fission also contributes to fragmentation of these organelles in endothelial cells exposed to H/R and may thus contribute to endothelial dysfunction in postischemic tissues.
Another target for I/R induced Ca2+ are the calpains. This family of cysteine proteases is activated by elevation of Ca2+ and degrades a panoply of intracellular proteins, including cytoskeletal, ER, and mitochondrial proteins (Croall and Ersfeld, 2007). Calpain activity is elevated by I/R and pharmacological inhibitors of calpains are protective in the brain (Peng et al., 2011; Tsubokawa et al., 2006), heart (Chen et al., 2002; Hernando et al., 2010), liver (Kohli et al., 1999), kidney (Chatterjee et al., 2005), and intestine (Marzocco et al., 2004). In addition, the endogenous inhibitor of calpains, calpastatin, is also often degraded during I/R (Shi et al., 2000; Sorimachi et al., 1997), which would further enhance calpain activation and subsequent cell death. Indeed, transfer of the calpastatin gene in the myocardium can reduce I/R-induced infarction and contractile dysfunction (Maekawa et al., 2003).
Increased intracellular Ca2+ also leads to the generation of calcium pyrophosphate complexes and the formation of uric acid, both which belong to a group of danger signals that bind to intracellular protein complexes called inflammasomes. Inflammasomes mediate increased production of cytokines, such as IL-1β and TNFα, which, in turn, activate transcription factors (e.g., nuclear factor kappa beta (NFκB)) to increase expression of additional cytokines and chemokines, thereby precipitating a cytokine storm that exacerbates I/R injury.
6.2. Oxidative/nitrosative stress
Reentry of oxygenated blood into ischemic tissue, while necessary for restoration of aerobic ATP production, also results in production of ROS (Fig. 6.1). Owing to their highly reactive nature, ROS generated upon reperfusion can oxidatively modify virtually every type of biomolecule found in cells, thereby paradoxically inducing cell dysfunction (oxygen paradox). Earlier work established the primary importance of ROS production in the pathophysiology of I/R injury, and has been ably reviewed previously (Granger, 1999; Granger and Korthuis, 1995; Kvietys and Granger, 2012; Raedschelders et al., 2012). Reactive nitrogen species (RNS), which refers to redox molecules derived from NO, also play a modulatory role in the cellular and systemic response to I/R. Indeed, interactions between ROS and RNS play a critical role in determining the extent of injury via the production reactive nitrogen oxide species (RNOS), such as strong prooxidant peroxynitrite. Overall deleterious effects of RNOS in the context of I/R include damage/modification of macromolecules, induction of death of endothelial and/or parenchymal cells in the affected tissue, stimulation of production/release of pro-inflammatory mediators by various cell types, as well as induction of adhesion molecules supporting leukocyte/ lymphocyte-endothelial cell adhesive interactions, and decreases in the availability of protective NO (Granger, 1999; Granger and Korthuis, 1995; Kvietys and Granger, 2012; Raedschelders et al., 2012).
6.2.1. Reactive oxygen species in I/R
The general condition favoring the aforementioned effects has been termed oxidative or oxidant stress, or in the case of NO, nitrosative stress. The traditional view of oxidant stress was that it arises from a simple imbalance between cellular levels of prooxidant versus antioxidant compounds, such that the resulting net excess of ROS produced cell/tissue injury (Sies, 1985). Recent refinement of this hypothesis has been prompted by three factors: (1) lack of major benefit from treatment with free radical scavengers in clinical intervention trials (Allen and Bayraktutan, 2009), (2) progress in understanding the control of oxidant- and redox-sensitive cell signaling pathways (Go et al., 2010), and (3) the recognition that in addition to ROS, RNS also play an important modulatory role in cell physiology (Lima et al., 2010). Oxidant stress is now thought to involve three major components: (1) “indirect” effects of oxidants, especially nonradical oxidants such as hydrogen peroxide (H2O2), mediated through dysfunction in cell signaling and control mechanisms that are sensitive to changes in thiol redox circuits (Go et al., 2010), (2) modulatory effects on cell signaling via direct covalent, oxidative, or nitrosative modification of key regulatory proteins (Lima et al., 2010), and (3) direct damage by RNOS, especially oxidant radicals, to cellular molecules, for example, DNA, protein, lipids, and carbohydrates. The extent to which each of these processes plays a role in particular aspects of I/R is not clearly defined and is the subject of intense current scrutiny.
6.2.1.1. Superoxide and other ROS
The primary ROS initially produced during I/R is the superoxide anion radical , resulting from the univalent reduction of molecular oxygen. Support for this came from early findings that IRI was significantly attenuated by treatment with SOD or SOD mimetics (Granger, 1988) and later confirmed in studies showing less IRI in transgenic mice overexpressing either cytoplasmic or mitochondrial isoforms of SOD (Chen et al., 1998; Horie et al, 2001). Superoxide is the primary oxidant, since all other RNOS are ultimately derived from its dismutation or interaction with other reactive species, which themselves go on to mediate I/R-induced vascular dysfunction and tissue injury. It is produced by a number of cytosolic and membrane enzymes, as well as via the electron transport chain (e.t.c.) in mitochondria (see below).
Superoxide can directly oxidize various biomolecules and inactivate enzymes with iron-sulfur centers such as aconitase, fumarase, NADH dehydrogenase, creatine kinase, and calcineurin (Raedschelders et al., 2012). However, it is not generally thought to be particularly toxic in vivo, mainly due to rapid, spontaneous (i.e., noncatalytic) dismutation hydrogen peroxide (H2O2), a conversion accelerated about 104 -fold (essentially to a diffusion-limited rate) by SOD, such that other reactions of in cells are effectively prevented unless generation of is in very close proximity (i.e., up to several molecular diameters) to potential reactants. However, can be rapidly and spontaneously converted to its conjugate acid, the more highly potent oxidant, hydroperoxyl radical (HOO•), particularly under conditions of low pH, such as might be expected in ischemic tissue. Although H2O2 is less reactive than , it readily diffuses across cell membranes and can thus act as a second messenger and modulator of cell signaling. In the presence of transition metals, that is, iron or copper, H2O2 participates in the generation highly reactive free radicals such as hydroxyl (•OH) via the Fenton reaction or can react with hemoglobin and myoglobin to form damaging ferryl derivatives of these hemoproteins. Finally, can react with NO to form peroxynitrite anion (ONOO−) which, in turn, can be protonated to the highly cytotoxic peroxynitrous acid (ONOOH), a strong oxidant in its own right. Peroxynitrite is also a more effective precursor to •OH than the reaction of reduced iron with H2O2 and is an important modulator of cell signaling.
6.2.1.2. Sources of superoxide
The major enzymatic sources of cellular superoxide production are XO, NADPH oxidase, cytochrome P450 oxidases, and uncoupled nitric oxide synthase (NOS). The mitochondrial e.t.c. is also an important generator of . The precise role of each of these generators in the pathology of IRI is not clear since a particular source may predominate depending upon the species, the tissue examined, or the experimental protocol used to produce IRI. As an example, in a model of intestinal I/R, endothelial XO appeared to be responsible for ROS generation early on, while leukocyte NADPH oxidase appeared to mediate the later phases (Granger, 1999). Another recent study of ROS-induced apoptosis in cultured neurons exposed to anoxia–reoxygenation reported a clear temporal sequence of ROS generation, beginning with a transient increase in mitochondrial production during hypoxia, progressing to a second phase dependent on XO, and finally a third phase due to NADPH oxidase activity beginning upon reoxygenation (Abramov et al., 2007).
Some of the earliest evidence implicating in I/R was derived from studies examining the role of xanthine oxidoreductase (XO) (Parks and Granger, 1986). While expressed in many tissues, hepatocytes, intestinal enterocytes, and capillary endothelial cells exhibit very high levels of XO expression (Parks and Granger, 1986). XO is formed from xanthine dehydrogenase under hypoxic conditions and requires hypoxanthine and molecular oxygen to fuel the production of . Depletion of ATP levels during ischemia leading to the accumulation of the former, while the latter is provided on reperfusion. The importance of XO-derived in I/R is shown by decreased Ca2+ overload and markers of oxidant stress, leukocyte recruitment and accumulation, and tissue injury in the presence of inhibitors of XO (Granger and Korthuis, 1995; Kvietys and Granger, 2012; Raedschelders et al., 2012). Release of endothelial membrane-bound XO during local tissue I/R results in increased plasma concentrations of XO, which provides means for instigating oxidant-triggered ROI (see Section 2.5). XO may also catalyze the reduction of nitrite to nitric oxide (Golwala et al., 2009), an effect that may provide a mechanistic basis for the utility of nitrite therapy in ischemic disease.
Two general forms of the multimeric, superoxide-producing NADPH oxidase (NOX) have been shown to be involved in I/R-induced oxidant stress. The first of these is the prototypical NOX of phagocytic leukocytes (e.g., macrophages, neutrophils), responsible for the so-called respiratory burst wherein a 50- to 100-fold increase in oxidant production activated by exposure to microorganisms or inflammatory mediators (Kvietys and Granger, 2012; Raedschelders et al., 2012). NOX-generated is rapidly dismutated to hydrogen peroxide, followed by myeloperoxidase-catalyzed production of hypochlorous acid. Normally, the function of this burst of ROS is host defense, and the reactant species are released extracellularly or into phagolysosomes.
It is now well established that nonphagocytic cells, particularly those comprising the vascular wall, also express one or more NOX isoforms (Jiang et al., 2011; Kvietys and Granger, 2012; Raedschelders et al., 2012). Unlike the leukocyte isoform, which is inactive until stimulated and then produces massive amounts of superoxide, the vascular NOXs maintain a low level of constitutive activity. Although their activity can be significantly upregulated on stimulation, the vascular NOXs maximal rate of is less than 10% of the leukocyte enzyme. Thus, the low levels of ROS generated by vascular NOXs are well suited to comparatively more subtle effects on signaling cascades via effects on kinases and phosphatases (Jiang et al., 2011). However, under conditions of extreme stress, such as might occur during I/R, even vascular NOX can produce levels of superoxide sufficient to produce oxidant stress (Dworakowski et al., 2008; Gao et al., 2008).
Under basal conditions, leukocyte NOX is inactive because subunits required for activation are maintained in different cellular compartments and thus separated. Activation thus depends upon recruitment of regulatory subunits in the cytosol to the membrane where the catalytic subunit resides for holoenzyme assembly (Kvietys and Granger, 2012; Raedschelders et al., 2012). In contrast, vascular cells appear to maintain several distinct pools of enzyme. One portion of total NOX is preassembled (and fully active) in the membrane, accounting for the low levels of constitutive activity, while a second pool is localized with cytoskeletal proteins. A third pool is similar to NOX in leukocytes, being maintained in separate membrane and cytosolic compartments until stimulation (Kvietys and Granger, 2012; Raedschelders et al., 2012). Both the vascular wall and leukocyteNOXs have been shown to participate in injury to endothelial and vascular smooth muscle cells, fibroblasts, and parenchymal cells of the most organs exposed to I/R or anoxia–reoxygenation (Kvietys and Granger, 2012; Raedschelders et al., 2012).
Cytochrome P450 (CYP) enzymes, members of the microsomal mixed function oxidase system, are a family of membrane-bound, hemecontaining oxidases that use oxygen or NADPH to catalyze the univalent oxidation or reduction of xenobiotic compounds, as well as some lipids (e.g., arachidonic acid), vitamins, steroids, and cholesterol. Most of these enzymes are expressed in liver, but some have been found in extrahepatic tissues, including the endothelial cells (Gottlieb, 2003). While much of the research on CYPs has focused on their role in vasoregulation has concentrated on their action to form to bioactive eicosanoid derivatives from arachidonic acid, some with vasoconstrictive actions, (20-hydroxyeicosatetraenoic acid (20-HETE)) and others which exhibit vasodilatory and anti-inflammatory effects (epoxyeicosatrienoic acids (EETs)). The precise role and importance of distinctCYPs in I/R is complex, sinceCYP catalyzes production of both EETs and potentially harmful vasoconstrictors andROS (Deng et al., 2010). Indeed, work conducted in the heart and brain suggests that 20-HETE may contribute to I/R injury, by a mechanism that may involve generation of ROS and dihydroxydecanoic acid (Chehal and Granville, 2006; Edin et al., 2011; Yang et al., 2012). However, EETs production limits postischemic inflammation (Deng et al., 2010; Xu et al., 2011b).
NOS is a dual-function oxidoreductase enzyme, combining a cytochrome P450-like reductase in one subunit with a heme-containing oxidase in the other subunit. An essential cofactor, tetrahydrobiopterin (BH4), shuttles electrons from the reduction of molecular oxygen to the oxidation of L-arginine (L-arg), producing L-citrulline and nitric oxide (NO). Numerous studies have established that in the absence of BH4 or L-arg, all NOS isoforms can become uncoupled, producing instead of NO (Roe and Ren, 2012). This can occur through oxidation of BH4 by or ONOO−, by BH4 deficiency, oxidation of the zinc–thiolate complex that stabilizes the NOS homodimer, S-glutathionylation, and dissociation of NOS from associated proteins (e.g., HSP90) that are necessary for coupled function (Roe and Ren, 2012). Administration of L-arg, BH4, or sepiapterin reduce I/R injury (Settergren et al., 2009; Yamashiro et al., 2003).
In normal cells, mitochondria constitute the largest single intracellular source of (Lee et al., 2012; Perrelli et al., 2011). More than 90% of oxygen entering cells is reduced to water via the mitochondrial e.t.c; under physiological conditions, about 1–2% of that oxygen is reduced to , mainly due to “electron leak” at two sites in the chain: NADH ubiquinone oxidoreductase (Complex I) and ubiquinone/cytochrome c reductase (Complex III). Recent evidence indicates that non-e.t.c. sources of ROS may play a significant role in mitochondrial ROS production (see below). Production of ROS by the mitochondria is significantly increased by I/R (Stowe and Camara, 2009). A second mechanism contributing to I/R-induced increases in mitochondrial ROS is a decreased endogenous mitochondrial antioxidant capacity (Stowe and Camara, 2009). Therefore, net ROS release from mitochondria likely reflects the balance between production versus disposal/scavenging.
A number of studies have provided strong evidence that mitochondria account for a quantitatively significant proportion of I/R-induced ROS release (Lee et al., 2012; Perrelli et al., 2011) (Fig. 6.5). These include studies using specific inhibitors of various steps in the e.t.c., selective targeting of antioxidants to the mitochondria, and transgenic overexpression of mitochondrial versus cytosol-specific isoforms of antioxidant enzymes (e.g., MnSOD vs. CuZnSOD) (Perrelli et al., 2011). In other studies, pharmacological agents which protect against I/R-induced vascular dysfunction and tissue injury have been found to inhibit mitochondrial ROS production (Perrelli et al., 2011).
Two other major sources for mitochondrial ROS are p66Shc and monoamine oxidase (MAO) (Di Lisa et al., 2009a,b) (Fig. 6.5). Unlike other members of the Src homology 2 domain and a collagen homology region family of proteins, p66shc is not known to be an activator of Ras. Rather, it is a source of superoxide, and studies using p66shc−/− knockout mice have shown p66Shc to play a clear role in several pathological conditions involving oxidative stress (Menini et al., 2006). Recent studies have shown that oxidant stress promotes phosphorylation of a key serine residue in p66shc (Ser 36), which then results in translocation of p66Shc to the outer membrane of the mitochondria, where it binds to and oxidizes cytochrome c, producing ROS in the process (Arany et al., 2010). It has been proposed that p66Shc may thus play a role in I/R-induced mitochondrial dysfunction and oxidant stress but direct, in vivo confirmation of this hypothesis has not yet been reported.
MAOs are also localized to the outer mitochondrial membrane, where they normally function to oxidatively deaminate monoamine neurotransmitters and dietary tyramines, producing aldehydes and hydrogen peroxide (Di Lisa et al., 2009a, b) (Fig. 6.5). MAOs have long been implicated in several neurodegenerative disorders, but they have been found to play a role in mediating oxidant stress in cardiac I/R injury, an effect that correlated with the levels of circulating monoamines (Kaludercic et al., 2010).
6.2.2. Nitrosative stress in I/R
Nitric oxide (NO•) is a radical produced during the oxidation of arginine to citrulline, catalyzed by NOS, although it can also be produced through reduction of nitrite or nitrate, through the action of XO, as discussed above (Golwala et al., 2009) or by mitochondrial cytochrome c oxidase under hypoxic conditions (Castello et al., 2006). Due to its high reactivity, NO• is extremely labile, having a half-life of just a few seconds. Under physiological conditions, the relatively low quantities of NO• produced by the action of the endothelial isoform of NOS (eNOS), combined with its evanescence and ability to readily cross cell membranes, make it an ideal signaling molecule. As such, NO• plays an important regulatory and protective role in the vasculature, where it produces dilation of blood vessels, modulates platelet aggregation and adhesion, and prevents leukocyte–endothelial adhesive interactions and angiogenesis (Kubes et al., 1991; Pacher et al., 2007). Nevertheless, because of its reactivity, the physiology of NO is quite complex, owing to a wide variety of potential reactions with other chemical species. (Grisham et al., 1999; Lima et al., 2010; Pacher et al., 2007; Valko et al., 2007).
Grisham et al. (1999) distinguished two types of effects of NO: direct and indirect, the predominance of which depends upon the rate and extent of NO production. Direct effects occur at low concentrations or fluxes of NO and are characterized by interactions of NO with other targets, such as formation of nitrosyl complexes with proteins with iron-coordinated heme moieties (Shiva et al., 2007) and prevention of iron-dependent formation of ferryl-heme radicals by H2O2. Indirect effects are the result of interaction of NO with O2 or , forming dinitrogen trioxide (N2O3) or peroxynitrite (ONOO−), respectively. Although these secondarily derived RNOS can play important roles in signaling, their appearance is often associated with overproduction of NO and and resulting pathophysiological nitrosative and oxidative stress. In addition, recent findings also support protective, anti-inflammatory effects of nitrated lipids (see below). Many NO-initiated effects are independent of the classic cGMP-dependent pathway originally described for this mediator. Recent findings indicate that nitrosation of proteins and lipids constitute a potentially powerful means of modulating cell function, which, in some cases, has been shown to converge with thiol-dependent redox control.
6.2.3. Biologic targets of oxidative/nitrosative stress in I/R
There are three major ways oxidative/nitrosative stress adversely influence cell function in I/R and other states: (1) damaging effects on cellular macromolecules, such as membrane lipids, proteins, and DNA, (2) decrease in NO bioavailability through its interaction with , with the simultaneous production of highly reactive and potentially toxic ONOO− and other RNOS, and 3) effects on cell signaling mechanisms, either through modulation of cell redox state or via direct effects on particular signaling and/or effector systems. Over the past 10–15 years, it has become clear that simple macromolecular damage cannot fully explain many, if not most, of the effects of RNOS on cellular function, particularly with regard to effects on regulatory and effector proteins involved in the response to I/R. Consideration of the concept of redox control has led to the recognition that I/R injury involves dysregulation of the network of thiol redox circuits in cells.
6.2.4. Cellular redox signaling in I/R
It is becoming increasingly clear that organisms have evolved mechanisms to use ROS, RNS, and RNOS as signaling mediators. Due to the ubiquitous nature of these reactive species, and their potential for relatively indiscriminate reactivity, the signaling specificity of RNOS-mediated control systems must be achieved in a manner altogether different from the classic, noncovalent, complementary macromolecular ligand-receptor paradigm (D’Autréaux and Toledano, 2007). In this context, H2O2 (D’Autréaux and Toledano, 2007; Go and Jones, 2008) interacts with particular pools of thiol-disulfide redox switches or control nodes such as reduced glutathione/ glutathione disulfide or as redox-active cysteine/cystine, either on thioredoxin proteins or on regulatory or effector protein targets. The basis for redox control of a given protein is the ability for key redox-active cysteines to be reversibly switched between reduced thiol and oxidized disulfide forms. Precise understanding of the basis for redox-mediated signaling specificity has not yet been attained, although a helpful explanatory hypothesis has recently been proposed by Jones and coworkers (Go and Jones, 2008), wherein the interaction of H2O2 with the aforementioned redox control nodes is compartmentalized into discrete, spatially and kinetically distinct pathways, which are not in equilibrium with each other.
NO or RNOS can react with nucleophilic thiols such as cysteine or reduced glutathione (GSH) to produce S-nitrosothiols (SNO). S-nitrosation of proteins is a major means by which sGC-independent NO signaling is effected (Lima et al., 2010). Interestingly, many but not all instances of protein S-nitrosylation are protective, targeting NFκβ, IκB kinase, PKC, Bcl-2, caspases, PTS, MnSOD, cytoskeletal actin, mitochondrial complex I, and a variety of cell surface receptors (Lima et al., 2010; Sun and Murphy, 2010). The extent of S-nitrosation is dependent, not only on redox chemistry between NO or NO derivatives with thiols but also on a recently recognized system of denitrosylases, the physiologically most significant being S-nitrosoglutathione reductases and the thioredoxin system (Benhar et al., 2009). It is now known that activity of these enzymes is regulated (Benhar, 2009) and may constitute an important element in the control of not only NO-dependent signaling but also redox signaling in general. The extent to which alterations in reversible S-nitrosation are involved in dysfunctional signaling in inflammation is beginning to be explored (Godoy et al., 2010), but specific instances of dysregulated denitrosation in I/R have not yet been reported.
6.3. Endoplasmic reticulum stress
The ER is a complex membranous network found in all cells where it plays an important role in calcium homeostasis, the folding of proteins, and lipid biosynthesis (Minamino et al., 2010). A wide variety of stressors disrupt ER function which leads to protein misfolding and unfolding in the organelle. As misfolded and unfolded proteins accumulate in the ER, a state referred to as ER stress, they are sensed by transmembrane receptors which, in turn, elicit the unfolded protein response (UPR) (Minamino et al., 2010). The UPR acts to ameliorate the accumulation of unfolded proteins by increasing the expression of ER-resident chaperones, increasing protein translation, and accelerating the degradation of unfolded proteins. However, if the UPR fails to relieve ER stress, cell death by apoptosis occurs.
Reperfusion of ischemic tissues is associated with the generation of ROS and the production of proinflammatory cytokines, both of which induce proapoptotic pathways of the UPR (Minamino et al., 2010; Toko et al., 2010). Upregulation of the activating transcription factor (ATF)6 pathway of the UPR in transgenic mice with cardiac-restricted expression of a tamoxifen-activated form of ATF6 results in increased expression of ER-resident chaperones (GRP78 and −98), better functional recovery, and reduced necrotic and apoptotic cell death in hearts after I/R (Martindale et al., 2006). However, pharmacologic inhibition of ATF6 during I/R exacerbates contractile dysfunction and increased mortality rate following myocardial infarction (Toko et al., 2010). ATF6 activation induces the expression of numerous gene products, including mesencephalic astrocyte-derived neurotrophic factor (MANF), as well as the ER stress response Derlin-3 gene (Belmont et al., 2010; Tadimalla et al., 2008) and may do so by modifying miRNA levels (Belmont et al., 2012). Addition of recombinant MANF protected cultured cardiomyocytes from simulated I/R injury, while miRNA knockdown of MANF increased cell death under these conditions (Tadimalla et al., 2008). However, overexpression of the Derlin-3 gene enhances the export of misfolded proteins from the ER to the cytosol by a process termed retrotranslocation and protected cardiomyocytes from the deleterious effects of simulated I/R (Belmont et al., 2010). Upregulation of other components of the UPR has also proven to reduce I/R injury and appears to play an important role in the protective effects of ischemic pre- and postconditioning to alleviate ER stress and reduce myocardial injury (Depre et al., 2010; Mao and Crowder, 2010). Interestingly, treatment with either a pharmacologic activator of AMPK or a statin reduces ER stress in cardiomyocytes exposed to hypoxia or TNF, respectively (Chen et al., 2008; Terai et al., 2005). The latter studies suggest that the oral antidiabetic agent metformin, which also activatesAMPK, or statin treatmentmay exert protective effects via their influence on UPR pathways, in addition to their well-known other clinically useful activities.
6.4. Mitochondrial dysfunction
It is now well established that mitochondria play a critical role in the progression of I/R injury. Here, we will review the mitochondrial components/processes that contribute to the death of cells following I/R injury.
6.4.1. Inhibition of mitochondrial metabolism
Due to the lack of oxygen during ischemia, electron flow through the respiratory chain is inhibited. Consequently, the F1F0 ATP synthase can no longer phosphorylate ADP to generate ATP (Di Lisa et al., 2007). Moreover, in an attempt to maintain the Δψm, in the face of inhibited electron transfer, the ATP synthase actually runs in reverse, thereby hydrolyzing what ATP remains (Di Lisa et al., 2007). These two processes elicit a rapid fall in ATP levels upon induction of ischemia. Selective inhibition of the hydrolyzing activity of the ATP synthase slows down the rate of ATP loss and protects hearts against the subsequent cell death (Grover et al., 2004). In addition to its effects on ATP metabolism, by blocking oxidative phosphorylation I/R also inhibits the breakdown of fatty acids. This, in turn, leads to an accumulation of toxic fatty acids within the cell. These can produce inflammatory metabolites through the arachidonic acid pathway (Van der Vusse et al., 1997), as well as stimulate opening of the MPT pore (Di Paola and Lorusso, 2006).
6.4.2. Mitochondrial ROS production
There are several mitochondrial sources of ROS including the e.t.c., p66Shc, andMAOs (Figure 6.9). Superoxide is produced by complexes I and III of the e.t.c. and is normally neutralized by SOD. However, during ischemia, these complexes (especially complex I) are kept in their reduced state, thereby increasing ROS production to the point that the cells’ antioxidant systems are overwhelmed (Solaini and Harris, 2005). Restoration of oxygen upon reperfusion exacerbates this pathogenic mechanism.
P66Shc is a splice variant of the other Shc proteins, which are normally involved in Ras signaling (Di Lisa et al., 2009a,b). However, p66Shc is found in the mitochondria and mice deficient in p66Shc were found to exhibit less oxidant stress than normal mice (Giorgio et al., 2005; Orsini et al., 2006). The mechanism by which p66Shc induces mitochondrial ROS is unclear but may involve electron transfer between itself and cytochrome c (Giorgio et al., 2005). Interestingly, I/R injury in skeletal muscle and the myocardium is reduced in the p66Shc−/− animals (Carpi et al., 2009; Zaccagnini et al., 2004). Whether p66Shc contributes to I/R injury in other organs remains to be tested.
Monoamine oxidases (MaO-A and MaO-B), which are associated with the outer mitochondrial membrane, are involved in the deamination of neurotransmitters and dietary amines (Di Lisa et al., 2009b). However, this process results in the generation of H2O2 (Di Lisa et al., 2009b). Pharmacological inhibitors of MaOs have been reported to reduce I/R injury in a variety of organs (Kiray et al., 2008), and genetic deletion of MaO-A rendered mice resistant to myocardial I/R injury (Kaludercic et al., 2010).
6.4.3. Opening of the mitochondrial permeability transition pore
As noted above, opening of the MPT pore in the inner mitochondrial membrane is a critical event in the progression of cell death in response to I/R. Being inhibited by low pH, the MPT pore is kept quiescent during ischemia. However, upon reperfusion, the huge increases in mitochondrial Ca2+, coupled with the ROS burst, induce opening of the MPT pore (Di Lisa et al., 2009; Ong and Gustafsson, 2012) (Fig. 6.5). The pore is large in size (1.5 kDa), and therefore, H+ ions can pass back into the matrix through this channel, thereby dissipating the Δψm, uncoupling the e.t.c. and inhibiting ATP synthesis (Baines, 2010; Halestrap, 2010). In addition, water enters the mitochondria through its osmotic gradient causing the mitochondria to swell and even rupture. The proteins that constitute the MPT pore are still being defined, with the adenine nucleotide translocase, mitochondrial phosphate carrier, and cyclophilin-D being the leading candidates so far (Baines, 2009a,b; Halestrap, 2009).
The discovery of chemical inhibitors of cyclophilin-D, such as cyclosporine-A, sanglifehrin-A, and Debio-025, has enabled the study of the role of MPT and theMPT pore in I/R injury. These compounds have been shown protect against I/R-induced cell death in every organ tested (Clarke et al., 2002; Di Lisa et al., 2009; Muramatsu et al., 2007; Saxton et al., 2002; Singh et al., 2005; Puglisi et al., 1996). These pharmacological data have since been confirmed by the generation of CypD-deficient mice, which exhibit an innate protection against cardiac, hepatic, and renal I/R injury (Baines et al., 2005; Devalaraja-Narashimha et al., 2009; Schinzel et al., 2005).
6.4.4. Mitochondrial fission/fusion
Mitochondria are dynamic organelles that form tubular, intercommunicating networks that are linked to the cytoskeleton and undergo cycles of division (fission) and fusion (Chen and Knowlton, 2010). Alterations in mitochondrial morphology occur when these latter two processes become unbalanced, with loss of fission resulting in the appearance of large networks of fused mitochondria, while excessive fission leads to small, fragmented mitochondria. Because fission is initiated under conditions associated with I/R, such as low ATP levels and increased mitochondrial ROS production, and excessive mitochondrial fission is a required step for extrinsic apoptotic cell death, this process may contribute to the pathogenesis of postischemic cell death. Indeed, inhibition of mitochondrial fission has been shown to reduce I/R-induced mitochondrial fragmentation and exerts cardioprotective effects in I/R by preventing MPT pore opening (Ong et al., 2010). Mitochondrial fission also contributes to fragmentation of these organelles in endothelial cells exposed to H/R and may thus contribute to endothelial dysfunction in postischemic tissues (Giedt et al., 2012). These exciting observations suggest that mitochondrial fission/fusion may represent new targets for therapeutic intervention in I/R.
6.5. Activation of apoptotic and autophagic pathways in I/R
A considerable amount of research has focused on the role that apoptotic mediators play in I/R injury. However, there is also evidence that autophagy is activated during I/R as well although, as we shall see, this may actually be a good thing.
6.5.1. Proapoptotic Bcl2 proteins
I/R injury induces apoptotic cell death, although the incidence of this form of death is significantly lower than necrosis. In particular, activation of prodeath Bcl2 proteins such as Bax, Bak, Bid, Puma, and BNIP3 and their upregulation, translocation, and integration into mitochondrial membranes have been reported in ischemically damaged tissues (Metukuri et al., 2009; Ji et al., 2007; Wei et al., 2006; Wu et al., 2007). Again, it appears that ischemia alone is not sufficient for Bcl2 protein activation and that reperfusion is required, consistent with the fact that many of these proteins are redox sensitive. Studies in Bax-, Bid-, BNIP3-, or Puma-deficient animals have confirmed a role for these proteins in the progression of I/R injury (Ben-Ari et al., 2007; Diwan et al., 2007; Wei et al., 2006; Wu et al., 2007). Interestingly, however, the degree of protection afforded by knocking out pro-death Bcl2 proteins is greater than would be expected from the amounts of apoptosis induced by I/R. This would suggest that these proteins have effects above and beyond simple apoptotic signaling during I/R. Indeed, both proand antiapoptotic Bcl2 proteins are known to regulate Ca2+ homeostasis (Scorrano et al., 2003), which we already know influences I/R injury.
6.5.2. Mitochondrial-derived apoptogens
While cytochrome c is the archetypal apoptogen released from mitochondria in response to the actions of Bax and company, there are other equally critical apoptogens that may contribute to the pathogenesis of ischemic damage. For example, the caspase activator Omi/HtrA2 is released during I/R, and its pharmacological or genetic inhibition greatly reduces I/R-induced apoptosis and cell death (Kim et al., 2010). Another caspase activator, Smac/DIABLO is also released by I/R in a variety of organs (Nilakantan et al., 2010), but whether this plays a causative role in I/R injury remains to be tested. Endonuclease G, which induces nuclear DNA fragmentation during apoptosis, is also released from mitochondria following cerebral ischemia (Nielsen et al., 2008). However, endoG-deficient mice were still sensitive to prolonged I/R (Xu et al., 2010).
6.5.3. Caspases
The proteases that effect apoptosis, the caspases, also appear to play critical roles in I/R-induced cell death. Pan-caspase inhibitors such as zVAD-FMK and MX1013 attenuate apoptosis and cell death in response to I/R in multiple organs (Daemen et al., 1999; Kobayashi et al., 2001; Yang et al., 2003; Yaoita et al., 1998). Genetic deletion/knockdown of specific caspases, both of the extrinsic and intrinsic pathways, also ameliorates I/R injury (Contreras et al., 2004; Le et al., 2002; Zhang et al., 2006). However, targeting caspases as a means of reducing I/R injury may not be ideal, as the upstream mitochondria will still be adversely affected. Therefore, caspase inhibition may only delay the inevitable and may, in fact, drive the cell into necrotic death instead (Vandenabeele et al., 2010).
6.5.4. Autophagy
Given that an organ is essentially starved during ischemia, it perhaps not surprising that autophagy is markedly upregulated by I/R (Cardinal et al., 2009; Jiang et al., 2010). These data suggest that autophagy is contributing to I/R-induced pathology. In fact, just the opposite seems to be the case, since inhibition of autophagy actually worsened tissue damage in I/R ( Jiang et al., 2010; Takagi et al., 2007). Moreover, pharmacologic induction of autophagy confers protection against I/R (Cardinal et al., 2009; Carloni et al., 2010). However, it should be pointed out that autophagy may still play a detrimental role in I/R, especially if the ischemic period is prolonged (Takagi et al., 2007).
6.6. Protein kinases
Signal transduction plays an important role in any cellular process and I/R is no exception. Consequently, it has come as no surprise that several protein kinases play critical roles in the pathogenesis of I/R injury. Most studied within the context of I/R are the mitogen-activated protein kinases (MAPK), but protein kinase Cd (PKCδ), CaMK, and RIP kinases are also now being appreciated as mediators of I/R injury.
6.6.1. Mitogen-activated protein kinases
The MAPKs are a family of heterogeneous serine/threonine kinases that play critical roles in cell growth, proliferation, survival, and death. Although there are multiple MAPKs, the three canonical groups are the extracellular signal-regulated kinases (ERKs), c-Jun N-terminal kinases (JNKs), and the p38 MAPKs, with several isoforms and splice variants existing within each group. Overwhelming evidence points to ERKs being protective in the setting of I/R injury. Thus, we will focus on the JNK and p38 MAPKs in this section.
Activation of JNK by I/R has been reported in multiple organs (Bogoyevitch et al., 1996; Murayama et al., 2006; Okuno et al., 2004). Consistent with this, treatment with the selective JNK inhibitors has been shown to attenuate I/R injury (Wang et al., 2007; Wolf et al., 2008). Ablation of the Jnk2 and Jnk3 genes protected the liver and brain, respectively, against I/R induced cell death (Kuan et al., 2003; Theruvath et al., 2008). Similarly, hearts from either JNK1- or JNK2-deficient mice exhibited smaller infarcts following I/Rthan their wild-type counterparts (Kaiser et al., 2005). Although these data strongly indicate that JNK plays a critical role in the pathogenesis of I/R injury, activation of JNK has been shown to be just as protective in the heart as inactivation of the kinase (Kaiser et al., 2005), while JNK inhibition actually exacerbates I/R injury in the liver (Lee et al., 2006). Thus, the role of JNKs in I/R injury remains complex (and controversial), and the reasons for these discrepancies remain unclear.
Similar to JNKs, activation of p38 MAPK occurs in response to I/R (Harding et al., 2010; Kobayashi et al., 2002; Takagi et al., 2000), while inhibition of p38 MAPKs has led to equivocal results, with some reports indicating that pharmacological inhibition of p38 MAPKs effectively reduces I/R-induced cell death (Li et al., 2006; Piao et al., 2003). In contrast, other studies have shown that the ability of preconditioning to protect against I/R injury is dependent on p38 activation (Yusof et al., 2009). These apparently discrepant findings may be explained by differential p38 MAPK isoform activation. Lethal I/R itself causes the activation of p38a (Guo and Bhat, 2007; Kaiser et al., 2004). In contrast, agents/interventions that induce preconditioning may preferentially activate the cytoprotective B isoform of p38 (Das et al., 2006; Huang et al., 2007).
6.6.2. Protein kinase C
The PKC family is a diverse group of serine/threonine kinases that includes at least 10 different isoforms (α, β1, β2, γ, δ, ε, η, θ, ζ,ι/λ). However, in the context of I/R injury, the two major players appear to be PKCδ and PKCε, with the former contributing to I/R injury and the latter protecting against it. While activation and/or translocation of PKCδ in response to I/R have been demonstrated (Gundewar et al., 2007; Koponen et al., 2000; Strasser et al., 1999), the most compelling support for a causative role of PKCδ in I/R has been provided by Mochly-Rosen’s group, who designed elegant peptide activators and inhibitors of this isoform. Using these peptides, it was shown that I/R injury was attenuated by specific inhibition of PKCδ (Bright et al., 2004; Chou et al., 2004; Inagaki et al., 2003; Murriel et al., 2004), while transgenic expression of a PKCδ activator exacerbated ischemic damage (Chen et al., 2001).
6.6.3. Ca++/calmodulin-dependent protein kinase
As mentioned in Section 6.1, large increases in cytosolic Ca2+ can activate the CaMKs, and indeed, activation and concomitant translocation of CaMK-II isoform have been reported in the ischemic heart (Netticadan et al., 1999; Uemura et al., 2002). Importantly, pharmacological inhibition of CaMK-II protects against I/R-induced cell death and dysfunction (Vila-Petroff et al., 2007).
6.6.4. Receptor-interacting protein kinases
RIP kinases are the latest addition to the panel of pro-death serine/threonine kinases. Under normal circumstances, these enzymes are necessary for the regulation of the NFκB and ERK signaling pathways by the TNF family of receptors (Festjens et al., 2007). However, RIP1 and RIP3 have also been shown to be critical for the progression of necrotic death in a variety of cell lines (Cho et al., 2009; Degterev et al., 2005; He et al., 2009; Holler et al., 2000). While a role for RIP3 in I/R has not been evaluated, treatment with necrostatin, a specific inhibitor of RIP1, has been shown to reduce I/R-induced infarction (Degterev et al., 2005; Rosenbaum et al., 2010; Smith et al., 2007).
6.6.5. Targets of pro-death kinases
Potential targets of pro-death kinases that could contribute to I/R injury are numerous. Activation of JNK and p38 leads to the rapid upregulation of inflammatory cytokines such as TNFα and IL1, which are especially important in the pathogenesis of I/R injury (King et al., 2009; Santén et al., 2009). In addition to these effects, the p38 and JNK MAPKs, as well as PKCδ, have been reported to localize to mitochondria, where they have profound effects on mitochondrial-dependent death pathways (Baines et al., 2002; Gundewar et al., 2007; Kohda and Gemba, 2005; Zhou et al 2008). While p38 and JNK can phosphorylate and inactivate the antiapoptotic Bcl2 (De Chiara et al., 2006; Fan et al., 2000), both MAPKs have also been shown to phosphorylate and activate several pro-death Bcl2 proteins (Bright et al., 2004; Donovan et al., 2002; Lei and Davis, 2003; Metukuri et al., 2009; Murriel et al., 2004; Sitailo et al., 2004; Zhuang et al., 2000).
The targets of CaMK and RIP kinases are not as well delineated. CaMK-II can phosphorylate and activate L-type Ca2+ channels (Grueter et al., 2006) and Na+ channels (Wagner et al., 2006), both of which would be expected to further exacerbate the large increases in intracellular Ca2+ associated with I/R. Moreover, CaMK can also facilitate the release Ca2+ from the SR in the heart (Wehrens et al., 2004) and may, therefore, be responsible for the detrimental alterations in SR Ca2+ handling seen in cardiac I/R. In contrast, RIP kinases have been reported to induce ROS production (Morgan et al., 2008) and increase intracellular levels of the death-inducing lipid ceramide (Thon et al., 2005).
6.7. Epigenetic changes
Over the past several years, it has become very apparent that I/R can lead to epigenetic changes that play a role in the resulting tissue damage. Epigenetics is defined as the transmissible regulation of gene expression without changes in the actual DNA sequence itself. The three main ways by which genes are epigenetically regulated are through DNA methylation, histone modification, and noncoding RNAs (Choudhuri et al., 2010), and here, we will review the potential role for each in I/R injury.
6.7.1. DNA methylation
DNA methylation occurs at the carbon-5 position of cytosine dinucleotides (CpG) and is mediated by DNA methyltransferases. Methylation causes a condensation of the chromatin. This, in turn, interferes with the ability of transcriptional activators to bind to the DNA and thus leads to transcriptional silencing (Choudhuri et al., 2010; Muthusamy et al., 2010). In the context of I/ R, Endres and colleagues first found that cerebral ischemia increased the overall amount of methylated DNA (Endres et al., 2000). Building upon this, the same group demonstrated that mice with reduced (but not absent) DMNT levels were more resistant to cerebral I/R injury (Endres et al., 2000, 2001). Regarding specific genes, increased methylation and, therefore, silencing of the thrombospondin-1 gene occurred in ischemic endothelial cells (Hu et al., 2006). In the heart, chronic cocaine exposure elicited the methylation-induced silencing of the cardioprotective PKCε gene and therefore exacerbated I/R injury (Meyer et al., 2009). In contrast, cerebral ischemia leads to the demethylation and hence increased transcription of the Na+-K+-2Cl− cotransporter type 1 (NKCC1) gene (Lee et al., 2010b), whose gene product has been implicated in ischemia-induced cerebral edema (Kahle et al., 2009).
6.7.2. Histone modifications
Histone modifications include methylation, acetylation, phosphorylation, ubiquitination, and sumoylation, the vast majority of which induce transcriptional activity by relaxing chromatin conformation (Choudhuri et al., 2010; Zhu and Wani, 2010). The most studied form of histone modification is acetylation, which is catalyzed by histone acetyltransferases and removed by histone deacetylases (HDACs). Taylor and Young (1982) first reported that cardiac ischemia caused an ∼40% decrease in total histone acetylation, especially in histones H3 and H4. Similar findings have been reported in the brain (Ren et al., 2004). These data suggest that maintaining histone acetylation would be protective. Indeed, pharmacological inhibition of HDACs with trichostatin-A or valproate, which increases histone acetylation, confers resistance against I/R injury (Crosson et al., 2010; Granger et al., 2008; Kim et al., 2007; Ren et al., 2004; Zhao et al., 2012). The identity of the cardioprotective genes that are upregulated by histone acetylation remains to be clarified, but heat shock proteins and Bcl2 are possible candidates (Faraco et al., 2006; Kim et al., 2007; Ren et al., 2004). Although increased phosphorylation of histone H2AX has been reported following aortic cross-clamping in humans (Corbucci et al., 2004), the roles for histone methylation, phosphorylation, ubiquitination, or sumoylation play in I/R injury have yet to be tested.
6.7.3. Noncoding RNAs
The noncoding RNAs fall broadly into two groups: long ncRNAs and short ncRNAs (Choudhuri et al., 2010; Costa, 2010). Long ncRNAs, which can be thousands of nucleotides in length, are primarily involved in genetic imprinting and will not be discussed here. However, short RNAs, especially miRNAs that are 19–25 nucleotides in length and too short to encode any protein, nonetheless, negatively regulate gene expression by targeting and inhibiting mRNA translation or by inducing mRNA degradation and, therefore, have the potential to impact modulators of I/R injury (Sayed and Abdellatif, 2011; Yang and Lai, 2011).
Following transcription, primarily by RNA polymerase II, primary transcripts are processed by Drosha and DGCR8 (Pasha) complex to produce a 70 nucleotide-long stem loop precursor-miRNA (pre-miRNA), which is subsequently transported from the nucleus to the cytoplasm by exportin-5. The pre-miRNAs are then processed by an RNase III enzyme designated Dicer and bound to mi-RNA-induced silencing complex (RISC, which contains the key proteins Argonaute 2 and transactivation-responsive RNA-binding protein) to form mature miRNAs. The mature miRNA plus RISC bind to complementary sites in mRNA transcripts to negatively regulate gene expression. If the miRNA binding complementarity to its mRNA target is imperfect, protein translation of the target gene is prevented. However, miRNAs that bind to the mRNA targets with perfect complementarity induce cleavage of the target mRNA, again negatively regulating gene expression. The ability of an individual miRNA to bind to its mRNA targets with imperfect or perfect complementarity thus allows that miRNA to regulate the expression of multiple genes.
Since the discovery of this canonical pathway for miRNA biogenesis, a variety of alternative Drosha/DGCR8-independent or Dicer-independent mechanisms to generate functional miRNAs have emerged, including the mirtron pathway, BoxH/ACA- and Box C/D snoRNA-derived miRNAs, miRNAs derived from tRNAs, and endogenous short-hairpinned RNAs (Yang and Lai, 2011). Although the potential roles for these alternate pathways for miRNA generation have not been evaluated in I/R, the existence of such noncanonical pathways may have important implications for cross talk and interaction of canonical- and noncanonical-generated miRNAs in the regulation of gene expression and organismal phenotype.
Several recent studies have provided evidence suggesting that miRNAs contribute to I/R injury by altering the expression of key signaling elements involved in cell survival and apoptosis, including PI3K, PTEN, Bcl-2, Mcl-1, HSP20, HSP60, HSP70, Pdcd4, LRRFIP1, FasL, and Sirt-1 (Fig. 6.6). MiRNA expression profiling revealed the differential regulation of several miRNAs following cerebral artery occlusion ( Jeyaseelan et al., 2008). Similar findings were obtained in the heart (Roy et al., 2009), hippocampus (Yuan et al., 2010), skeletal muscle (Greco et al., 2009), kidney (Godwin et al., 2010), and liver (Xu et al., 2009). The causal role of specific miRNAs in I/R injury is now being intensively studied, and because of their emergence as important contributors to ischemic injury, approaches to interrupt miRNA function may gain therapeutic utility. An especially promising development for therapeutic silencing of miRNAs involves chemical modification and conjugation of cholesterol to single-stranded RNA analogs that are complementary to miRNAs, (to confer stability and enhance delivery) to form “antagomirs” (Krutzfeldt et al., 2005). These miRNA inhibitors can be designed to retain their target specificity with no effect on cotranscribed polycistronic miRNAs. Antagomirs may also be useful for identifying miRNA targets in vivo and for studying the biological role of miRNAs in intact systems.
Figure 6.6.
Functional roles and target genes for miRNAs implicated in ischemia/ reperfusion and preconditioning. See text for further explanation. Modified from Abdellatif (2012).
As the list of miRNAs grows and the technologies to study them have become more available, the contribution of miRNAs to cardiac I/R injury has become apparent. Indeed, a mushrooming list of miRNAs appear to be involved in cell viability, angiogenesis, fibrosis, and electrical remodeling during cardiac ischemia and also participate in the protective effects of preconditioning (Fig. 6.6). It is clear that ischemia-induced alterations in the expression of miRNAs is complex and highly variable, depending on the duration of ischemia (preconditioning vs. index ischemia), or at what time point during index ischemia or reperfusion the expression profile is examined, as well as by cell type (Abdellatif, 2012; Fasanaro et al., 2010; Frost and van Rooij 2010; Kukreja et al., 2011; Schroen and Heymans, 2012; Tan et al., 2011; Ye et al., 2011b).
The expression of miRNA-1 is markedly enhanced on exposure to ischemia or hypoxia, promoting apoptosis (by targeting the synthesis of HSP-60, HSP-7 and Bcl-2), arrhythmias (through effects on expression of KCNJ2, which encodes kir2.1, a potassium channel subunit, and GJA1, which encodes connexin-43, a major component of junctions (Fasanaro et al., 2010; Frost and van Rooij, 2010; Kukreja et al., 2011; Schroen and Heymans 2011; Tan et al., 2011; Ye et al., 2011a,b) (Fig. 6.6). miRNA-21 inhibits PTEN, which in turn activates the prosurvival Akt kinase pathway to promote several cardioprotective mediators including eNOS, HSP-70, AP-1, and heat shock transcription factor 1 (Fig. 6.6). While this prosurvival miRNA is downregulated during ischemia, thereby contributing to cell death, miRNA-21 expression is upregulated after 48 h of reperfusion, where it promotes fibrosis via its effect to promote MMP-2 expression. On the other hand, miRNA-29 expression is downregulated at this time, which promotes collagen deposition in postischemic heart (Fig. 6.6). Inhibition of miRNA-15 (which contributes to ischemic injury by regulating apoptosis), miRNA-24 (which displays antiangiogenic effects in cardiac ischemia), or miRNA-29 or miRNA-320 activity (which target and downregulate the expression of cytoprotectiveMcl-1 and HSP20 proteins, respectively) significantly reduced myocardial infarct size (Bang et al., 2012; Hullinger et al., 2012; Ren et al., 2009; Ye et al., 2010). Inhibition of miRNA-320 has also shown to be protective against I/R injury in the brain (Sepramaniam et al., 2010; Tan et al., 2011). Prevention of miRNA-497 upregulation (which targets Bcl2) may also reduce I/R-induced cerebral damage by limiting apotosis (Tan et al., 2011; Yin et al., 2010). Overexpression of protective miRNAs, such as the antiapoptotic miRNA-378 which targets caspase-3 expression, has also proved effective in limiting cardiac cell death after ischemia (Fang et al., 2012). A summary of the effects of ischemiainduced alterations of these and other miRNAs is presented in Fig. 6.6.
Although not specifically evaluated with regard to the inflammatory response to I/R, miRNAs regulate oxidative stress, inflammation, development of atherosclerotic lesions, and endothelial senescence (Hulsmans et al., 2011; Ma et al., 2011; McCall et al., 2011; Qin et al., 2012; Urbich et al., 2008; Zhang et al., 2010) and thus undoubtedly contribute to endothelial dysfunction and leukocyte recruitment during reperfusion. Indeed, an emerging concept links epigenetics, bioenergetics, and miRNAs in the coordination of inflammatory gene-specific reprogramming to temporally define the phase shifts (recognition, initiation, adaptation, resolution) during the course of inflammation. This idea requires testing in the setting of I/R.
Circulating miRNAs can be detected in serum or plasma in a remarkably stable form, which support their potential use as biomarkers for cardiovascular disease (Abdellatif, 2012; Creemers et al., 2011; Kukreja et al., 2011). Although their origin is unknown, circulating miRNAs may arise a result of release from dead cells or by secretion inmembrane-bound vesicles (apoptotic bodies, exosomes, microvesicles) or as vesicle-free, protein–miRNA complexes that protect the noncoding miRNA. Cardiac-specific miRNAs, such as miRNA-208a, increase significantly and correlate well with changes in cardiac troponin I, a classic marker for cardiac ischemic injury. Other miR-NAs such as miRNA-1, −133a, −133b, and −499 also increase following acute myocardial ischemia. However, these miRNAs also increase after skeletal muscle injury, reducing their utility as specific markers for cardiac ischemia. On the other hand, miRNA-208a does not increase after renal infarction or skeletal muscle damage. Since circulating cardiac troponin I may increase in end-stage renal disease because it is excreted by the kidney, whereasmiRNA-208a does not, miRNA-208a may be superior as a biomarker for acute myocardial infarction. Whole genome miRNA expression analysis in patients revealed a unique pattern of 20 miRNAs that predicted acute myocardial infarction with a specificity of 96%, a sensitivity of 90%, and an accuracy of 93% (Xu et al., 2011a,b). This unique miRNA signature pattern may represent a particularly valuable potential biomarker for ischemic coronary disease. To date, no exploration of the role of miRNA signatures in prognosis has been attempted. Nor is it clear whether circulating miRNAs may be used to predict response to particular therapies, which may be very useful for risk/ benefit determinations in a single patient.
6.8. Inflammation and I/R
Inflammation is vital to host defense against invading pathogens. In response to infection, a cascade of signals leads to the recruitment of neutrophils and macrophages, innate immune cells that phagocytose the infectious organism and produce additional cytokines and chemokines that lead to activation of lymphocytes and adaptive immune responses. The inflammatory response is also essential for tissue and wound repair. Inflammation is also induced by I/R, typically occurs in the absence of microorganisms, and has thus been termed sterile inflammation. Similar to the response to invading pathogens, the sterile inflammation induced by I/R is characterized by marked recruitment of neutrophils and the production of cytokines, chemokines, and other proinflammatory stimuli (Kvietys and Granger, 2012). The production of ROS, release of hydrolytic enzymes, and secretion of pore-forming molecules from activated neutrophils infiltrating ischemic tissues results in extensive collateral damage to parenchymal cells. The sequestration of innate immune cells occurs primarily during reperfusion, which restores the delivery of oxygen and neutrophils to the tissues. The flux of oxygen into previously ischemic tissues, although essential to support cellular metabolism, fuels the formation of ROS by enzymes such as XO and NADPH oxidase. Neutrophil infiltration occurs as a result of inflammatory responses to necrotic cells and formation of mediators, some of which depend on the generation of ROS, that promote leukocyte adhesion to postcapillary venules and subsequent emigration into the tissues. By directing their cytotoxic arsenal at parenchymal cells, neutrophils induce reperfusion injury that exacerbates ischemia-induced cell damage and death (Kvietys and Granger, 2012).
Recognition of the fact that reperfusion can initiate a cascade of deleterious processes that exacerbate the tissue injury induced by ischemia has resulted in an intensive research effort directed at defining the cellular and molecular events that underlie I/R injury. Indeed, work conducted over the past 25 years has led to the development of the concept that oxidantinduced leukocyte/endothelial cell interactions are largely responsible for the microvascular dysfunction induced by reperfusion (Gute and Korthuis, 1995; Kvietys and Granger, 2012). ROS generated by XO and other enzymes (e.g., NAD(P)H oxidase) promote the formation of proinflammatory stimuli, modify the expression of adhesion molecules on the surface of leukocytes and endothelial cells, and reduce levels of the potent antiadhesive agent nitric oxide. This latter effect is exacerbated by a postischemic decline in endothelial NOS activity and oxidation of soluble guanylyl cylase (sGC), which serves to amplify the intense inflammatory responses elicited by I/R by reducing the bioavailability of NO and ability of downstream signaling elements to respond to this antiadhesive signaling molecule ( Jones et al., 2010). Coincident with these changes, perivascular cells (e.g., macrophages, mast cells) become activated and release other inflammatory mediators (e.g., TNFα and other cytokines, PAF, LTB4). As a consequence of these events, leukocytes begin to form adhesive interactions with postcapillary venular endothelium. Platelets also play an important role in the adhesion of leukocytes to the postischemic microvasculature. The activated leukocytes emigrate into the tissues, inducing microvascular barrier dysfunction via release of oxidants and hydrolytic enzymes. In addition to these changes, leukocytes also contribute to postischemic nutritive perfusion failure (fewer perfused capillaries, i.e., capillary no-reflow), endothelium-dependent vasoregulatory dysfunction in arterioles, and parenchymal cell dysfunction. Thus, leukocyte/endothelial cell adhesive interactions, which precipitate the development of arteriolar, capillary, and postcapillary venular dysfunction in the microcirculation, are among the earliest signs of tissue dysfunction and injury elicited by I/R (Gute and Korthuis, 1995; Kvietys and Granger, 2012).
6.8.1. Humoral mediators, cytokines, and complement in I/R
The complement system, chemokines, and cytokines are major humoral factors that participate in I/R injury. The complement system consists of approximately 30 soluble and membrane-bound proteins. Activation of the complement cascade occurs via three distinct pathways, including the classical, alternative, and mannose-binding lectin pathways, and all have been implicated in I/R injury. Once activated, the complement system contributes to tissue injury by direct cell lysis via the formation of a membrane attack complex in plasma membranes and by recruitment and activation of neutrophils and macrophages.
Hill and Ward (1971) were the first to describe complement activation following myocardial I/R. Subsequent studies demonstrated cell necrosis induced by ischemia results in release of subcellular membrane constituents that trigger the complement cascade (Frangogiannis et al., 2002; Ioannou et al., 2011; Rossen et al., 1994). Indeed, mRNA and protein expression for all of the components of the classical complement cascade are increased in ischemic tissues (Yasojima et al., 1998). The finding that postischemic lymph contains chemotactic activity that can be abolished by addition of a neutralizing antibody directed against C5a strongly supports the notion that complement activation occurs in I/R and generates chemotactic activity for neutrophil infiltration (Birdsall et al., 1997; Dreyer et al., 1992). Complement depletion or inhibition of different complement cascade constituents with neutralizing antibodies or other approaches has also proven efficacious in limiting postischemic inflammation and tissue injury (Fritzinger et al., 2008; Lucchesi and Kilgore, 1997; Stahl et al., 2003; Weisman et al., 1990).
On the other hand, cytokines may play pro- or anti-inflammatory roles in I/R injury. Although there is an extensive literature regarding the function of cytokines in postischemic tissue injury, we will focus on tumor necrosis factor-α (TNFα) as it appears to be a major contributor to the pathogenesis of I/R in most tissues. Although TNFα is produced by a variety of cell types, macrophages are a major source in I/R. This cytokine acts both locally in a paracrine manner and remotely as an endocrine mediator. Once released, TNFα binds with specific receptors to induce the expression of chemokines and production of ROS as well as to activate transcription factors such as NFκβ and promote the expression of adhesion molecules. These activities promote the recruitment and activation of neutrophils in postischemic tissues.
6.8.2. Endogenous danger signals and I/R
Since the inflammatory response to sterile cell death in I/R is similar to that invoked by microbial infection, host receptors and signaling pathways that mediate the immune response to microorganisms may be involved in the activation of sterile inflammation, as well as soluble mediators, including activated complement components, chemokines, and cytokines. The phagocytes recognize pathogen-associated molecular patterns (PAMPs), which are conserved motifs expressed on pathogens, by a group of proteins called pattern recognition receptors (PRRs). The best characterized of these PRRs are a group of membrane-bound receptors called the TLRs. When PAMPs bind to PRRs, a variety of transcription factors are activated which leads to increased expression of genes involved in defense against the threatening factor. One of the most studied and important transcription factors involved in the regulation of immune responses is NFκB.
While vital for host defense against invading pathogens, the innate immune system can also be activated during sterile inflammatory conditions such as I/R, where damaged cells release ATP, heat shock proteins, S100 proteins, among others, which are collectively referred to as damageassociated molecular patterns (DAMPs). According this emerging body of evidence, the immune system responds to danger or alarm signals released from damaged tissues, as opposed to the classic view of recognition of non-self. This new model for activation of the immune system was originally proposed by Matzinger (2002), which she termed the Danger model.
Sterile stimuli, specifically DAMPs, are generally intracellular factors that are normally hidden from recognition by the immune system. However, when ischemic cells undergo necrosis, DAMPs are released into the extracellular space when cell membranes rupture and elicit an inflammatory response. The formation of ROS, release and activation of hydrolytic enzymes, destabilization of lysosomal membranes, and altered ion fluxes all accompany necrotic cell death and lead to the activation of inflammatory pathways in addition to the release of DAMPs. Recent work indicates that release of intracellular ATP by necrotic cells activates the Nlrp3 inflammasome, which, in turn, functions to induce neutrophil adherence in the microcirculation (McDonald et al., 2010). Intravascular chemokine gradients guide the migration of neutrophils by crawling along venules through healthy tissue to the site of damage while formyl-peptides released from necrotic cells directed neutrophils through nonperfused regions of the tissue.
6.8.3. Cell types involved in postischemic inflammation
Multiple cell types are involved in the pathophysiology of I/R injury. Target cells for damage include vascular smooth muscle cells, parenchymal cells, and neurons. However, endothelial cell activation and recruitment of platelets/immune cells in postischemic tissues also participate as critical determinants in the etiology and course of injury. This section will focus on the endothelium and several of the major types of cells, both circulating and residing in the tissues, whose interactions with the endothelium determine the overall response to I/R.
6.8.3.1. Endothelial cells
It has long been appreciated that the endothelium, particularly that lining the microvasculature, is not simply a passive interface between the circulation and the extravascular space, but rather, a dynamic and active regulatory organ that plays a crucial role in vascular homeostasis. I/R produces dysfunction in all four major endothelial functions, compromising regulation of vascular barrier properties, control of adhesion and extra-vascular trafficking of immune/inflammatory effector cells, regulation of vascular tone, and control of hemostatic mechanisms.
Endothelial cells are arranged in a monolayer that constitutes an effective barrier between blood and underlying tissues. The integrity of endothelial barrier depends upon intercellular junctional complexes between adjacent endothelial cells. The junctional complexes, as well as their connections to cytoskeletal elements, are, in turn, regulated by intracellular signaling mechanisms sensitive to physiological/pathophysiological stimuli, for example, ROS, cytokines, lipid mediators, and proteases. Two major endothelial junctional complexes mediate paracellular permeability: (1) tight junctions characterized by intercellular linkage via extracellular, homophilic binding of the transmembrane proteins, occludin and claudin, whose intracellular domains are, in turn, linked to microfilaments of the actin cytoskeleton via zonula occludens proteins, ZO-1 and ZO-2 and (2) adherens junctions, mediated by homophilic, calcium-dependent binding of VE-cadherins, whose intracellular connections to the cytoskeleton are mediated by alpha and beta catenin (Kumar et al., 2009; Mehta and Malik, 2006; Rodriguez and Granger, 2010). I/R elicits dissolution of both tight and adherens junctions (Kumar et al., 2009; Mehta and Malik, 2006). Postischemic release of various proinflammatory mediators, particularly those released by adhering and/ormigrating leukocytes, includingROS, cytokines, chemokines such as RANTES (regulated upon activation, normal T-cell and expressed), proteases, low-molecular-weight factors such as histamine, PAF, and LTB4, and growth factors, for example, induce phosphorylation of junctional components, their internalization and/or degradation, and thus, the dissolution of intercellular junctions VEGF (Alexander and Elrod, 2002; Kumar et al., 2009; Mehta and Malik, 2006; Rodriguez and Granger, 2010; Terao et al., 2008). They also promote calcium-dependent phosphorylation of myosin light chain kinase, which, in turn, activates myosin light chain to effect actin–myosin cross-bridging and cytoskeletal contraction (Kumar et al., 2009), leading to formation of gaps between adjacent endothelial cells and increases in permeability. In addition to leukocytes, CD4+ T lymphocytes have also been shown to elicit increases in endothelial permeability, although this may be a function of their influence on recruitment of neutrophils (Liu et al., 2009).
A hallmark of inflammation elicited by I/R is the infiltration of PMNs into the tissue. Endothelial cells play a central role in this process, controlling a complex continuum of events comprising leukocyte recruitment and homing, and adhesion to and passage through the endothelium, followed by their extravasation through the vascular wall, as described in section 6.8.3.2.
Endothelial-dependent control of vasomotor tone is also significantly compromised after I/R, with a decrease in vasodilation and increased constriction (Gourdin et al., 2009; Zhang et al., 2010). Available evidence supports the decrease in availability of endothelium-derived nitric oxide (NO) as the underlying explanation, through either decreases in expression and/or activity of eNOS (Zhang et al., 2010), scavenging of NO by TNFα-induced ROS production (Zhang et al., 2006, 2010), a deficit in eNOS cofactor, dihydrobiopterin (Tiefenbacher et al., 1996), resulting in eNOS-dependent production of superoxide (so-called eNOS uncoupling), or competition for the eNOS substrate, arginine, by arginase (Hein, 2003).
Under normal conditions, endothelial control of hemostasis, encompassing platelets, the coagulation system, and fibrinolysis maintains an antithrombotic state. However, this is reversed after I/R. I/R-induced loss of endothelial NO (see above) results in vasoconstriction, platelet activation, and increased adhesion due to loss of NO-induced, cGMP-mediated modulation of platelet calcium levels and surface P-selectin expression, and platelet binding of fibrinogen by surface integrin glycoprotein (GP) IIb–IIIa, resulting in increased platelet aggregation (Lindemann et al., 1999; Pigazzi et al., 1999). I/R induces surface expression of endothelial tissue factor (TF), which, in turn, accelerates activation of clotting factors, and microthrombus formation, which could contribute to the so-called no-reflow phenomenon after I/R (Nieswandt et al., 2011; Thomas et al., 1993).
6.8.3.2. Neutrophils
A defining feature of IRI-induced inflammation is the recruitment of PMN leukocytes to reperfused tissues, mediated by their adhesion to the microvascular endothelium and extravasation through the vascular wall, adhesive interactions that occur almost exclusively in post-capillary venules (Gute and Korthuis, 1995; Kumar et al., 2009; Kvietys and Granger, 2012). The strongest evidence for a role for these inflammatory phagocytes in I/R is derived from studies employing neutrophil depletion strategies or prevention of their adhesion to the endothelium, either by use of immunoneutralizing antibodies directed against adhesion molecules or mice genetically deficient in adhesion molecules (Gute and Korthuis, 1995). Although the primacy of neutrophils in the process is currently the consensus view, recent studies have emphasized the heretofore underappreciated potential role of the other inflammatory cells, such as macrophages, lymphocytes, mast cells, and platelets in the modulation of neutrophil recruitment and trafficking.
Extravasation of adherent leukocytes through the vascular wall is less well understood and is, currently, the subject of intense ongoing investigation (Nourshargh et al., 2006). Diapedesis may occur by paracellular movement between adjacent endothelial cells or transcellular movement through individual endothelial cells (Nourshargh et al., 2010). Upon adhesion, the leukocyte alters its morphology, changing from spherical to flattened (which allows the adhesive cell to better withstand the antiadhesive effects imposed by the flowing blood), undergoing a directional polarization, with a redistribution of signaling, adhesion, cytoskeletal, and receptor proteins toward a leading edge from which processes extend, causing the leukocyte to “crawl” along the endothelium toward sites that are permissive for diapedesis. Recent work has shown that pericytes and the junctional proteins, JAM-A, JAM-C, and PECAM-1, facilitate neutrophil migration in vivo (Noursharg et al., 2006, 2010; Woodfin et al., 2011; Proebstl et al., 2012). Moreover, a significant determinant of an appropriate site for neutrophil infiltration may be a gradient of chemoattractant signals arising from damaged and dying cells which serve as a guide to allow intravascular homing of the leukocyte strictly to foci of injury before they are allowed to diapedese (McDonald et al., 2010). Once recruited to the tissue, PMNs secrete a host of factors known to contribute to tissue injury. These include ROS, (superoxide, hydrogen peroxide, and hypochlorous acid), cytokines, and chemokines such as IL-1, IL-6, IL-12, IFNγ, TNFα, and monocyte chemotactic factor-1, proteases, for example, elastase and collagenase, and lipid mediators such as LTB4 (Rodriguez and Granger, 2010).
6.8.3.3. Lymphocytes
Early studies regarding the role of inflammation in I/R injury focused on the role of components of the innate arm of the immune system, for example, effector mechanisms associated with leukocytes, the complement system, injury-induced release of proinflammatory cytokines, chemokines, and other mediators, as well as tissue-resident sentinels such as mast cells and Kupffer cells, and by the endothelium and tissue parenchymal cells. Due to the acute nature of most experimental models of I/R, the role of adaptive immune mechanisms was underappreciated. However, evidence for reciprocal regulatory activity between innate and adaptive immunity (Karp, 2010), combined with unequivocal demonstrations of the importance of T and B lymphocytes (Burne-Taney et al., 2003; Burne-Taney et al., 2005; Huang et al., 2007; Linfert et al., 2009; Yilmaz and Granger, 2010), have led to recognition of a more integrated, but complex system involved in I/R-induced inflammatory responses.
The involvement of T helper (Th) lymphocytes, particularly CD4+ cells, in IRI has been established through use of pharmacological agents that inhibit T cell activation, migration, proliferation, and adhesion and mouse models of immunodeficiency or genetic knockout of specific T cell types or T cell-derived effectors combined with adoptive transfer of various T cell subsets (Huang et al., 2007; Kuboki et al., 2009; Linfert et al., 2009; Liu et al., 2009; Yang et al., 2009; Yilmaz and Granger, 2010). In addition, I/R induces significant accumulation of CD4+ cells in the affected tissues (Caldwell et al., 2005; Hanschen et al., 2008; Khandoga et al., 2006; Martin et al., 2010; Osman et al., 2009; Saztpute et al., 2009; Shen et al., 2009; Uchida et al., 2010; Yang et al., 2009; Zwacka et al., 1997). A notable exception is the cerebral microvasculature, wherein direct evidence for adhesion of T cells is lacking (Yilmaz and Granger, 2010). Adhesive interactions have been shown to be mediated by endothelial ICAM-1 (Atarashi et al., 2005; Bonder et al., 2005; Kokura et al., 2000; Xu et al., 2004), VCAM-1 (Kokura et al., 2000), and P-selectin (Atarashi et al., 2005; Haller et al., 1997; Xu et al., 2004), while ICAM-1 (Xu et al., 2004), CD44 (Xu et al., 2004), and CD47 (Stefanidakis et al., 2008) have been found to mediate transendothelial migration of T cells.
Earlier work defined two general subsets of CD4+ T lymphocytes, Th1 and Th2 cells, determined by the relative activity of twomembers of the signal transducer and activator of transcription (Stat) family of proteins, Stat 4 and Stat 6, respectively. In turn, the relative activity of Stat 4 versus Stat 6 is determined by the prevailing cytokine mileu (Romagnani, 2006). Th1 cells secrete proinflammatory cytokines, for example, IL-2, IL-12, IFNγ, and TNFα, whereas Th2 cells secrete primarily anti-inflammatory cytokines (Il-4, Il-5, IL-10, and IL-13). Recent studies in knockout mice subjected to I/R have demonstrated the protective and deleterious roles of the Stat 6-mediated Th2 (Yokota et al., 2003) and the Stat 4-mediated Th1 (Shen et al., 2003) phenotypes, respectively. Although a reasonable hypothesis is that the relative balance of Th1/Th2 cells underlies the pathogenesis of IRI (Ysebaert et al., 2004), the specific roles of endogenous Th1 and Th2 phenotypes in T cell-mediated IRI are currently unknown. A third subset of T helper lymphocytes, Th17 cells, have been recently implicated in allograft vasculopathy and rejection after I/R associated with organ transplants (Chen and Wood, 2007; Hanidziar and Koulmanda, 2010; Syrjälä et al., 2010). Moreover, exposure of dendritic cells to anoxia–reoxygenation in vitro caused them to induce naïve CD4+ T cells to differentiate into both Th1 and Th17 cells (Wang et al., 2010), while pulmonary IRI was found to be mediated by a subset of CD4+ lymphocytes which secreted the Th17 signature cytokine, IL-17, as well as other factors (Yang et al., 2009). Because proinflammatory effects attributed to IL-17 may not necessarily be due to TH17 cells, as other cell types also secrete IL-17 (Shichita et al., 2009), the role of these cells in IRI is unclear.
Mechanisms underlying CD4+ T cell-mediated injury after I/R are not well understood but appear to be uniformly associated with both activation and recruitment of T cells to the site of initial injury. Classically, antigen-dependent T cell activation is characteristic of situations where foreign protein is encountered, as occurs during bacterial infection. However, I/R typically occurs in a sterile environment not necessarily associated with presence of foreign antigens (exceptions may be in cases of organ transplantation or epithelial barrier dysfunction induced by intestinal I/R). Thus, it might be viewed as surprising that several reports indicate that CD4+ T cells contribute to hepatic I/R via antigen-independent mechanisms (Breslin et al., 2006; Hanschen et al., 2008; Lemay et al., 2000; Shen et al., 2009; Uchida et al., 2010; Yang et al., 2009). Nonetheless, other studies have documented the importance of antigen-dependent T cell activation in IRI (Kuboki et al., 2009; Loi et al., 2004; Saztpute et al., 2009).
Since the introduction of foreign (i.e., nonself) antigens would not necessarily be expected in I/R per se, a key question is what specific antigen(s) might be involved in T cell-mediated injury. This question might be resolved by invoking Metzinger’s Danger Model of immune regulation (Matzinger, 2002), described in Section 6.8.2, wherein antigen presenting cells are activated by so-called danger or alarm antigenic signals. One such candidate danger signal may be high mobility group box 1 (HMGB1), a nuclear protein involved in DNA binding and gene expression shown to mediate hepatic I/R (Tsung et al., 2007). In addition, antigenindependent activation of CD4+ T lymphocytes by Kupffer cells, attributed to released ROS, TNFα, and IL-6, has also been shown after hepatic I/R (Hanschen et al., 2008).
CD4+ T cells may also promote IRI by increasing neutrophil recruitment and adhesion. Deficiency in CD4+ T lymphocytes, through genetic, antibody, or pharmacological depletion strategies, results in reduced neutrophil recruitment and associated injury after I/R (Caldwell et al., 2005; Horie et al., 1999; Martin et al., 2010; Osman et al., 2009; Park et al., 2002; Sharma et al., 2010; Uchida et al., 2010; Yang et al., 2009; Zwacka et al., 1997). T cell-mediated recruitment of neutrophils has been attributed to T cell secretion of IL-17 (Caldwell et al., 2005; Sharma et al., 2010), but other T-cell-derived factors, for example, IL-1, TNFα, likely also play a role. Evidence for an independent role for T cells in IRI has also been obtained (Burne et al., 2001). The mechanism for this is uncertain but has been ascribed to engagement of the costimulatory molecules CD28 and B7 (Burne et al., 2001), and secretion of IFNγ (Burne et al., 2001; Osman et al., 2009). In other studies, T cell-mediated injury was found to be mediated by interaction of CD4+ lymphocytes with tissue-resident macrophages (i.e., Kupffer cells), an effect mediated by CD40–CD154 interactions (Shen et al., 2009), and platelets (Khandoga et al., 2006).
The role of B cells and other lymphocytes (e.g., CD8+, Treg, and NK cells) in IRI has received less attention. However, several studies using mice deficient in either B cells or components of the complement system which interact with B cell receptors have shown that these cells contribute to IRI. Whereas T cells may be injurious or protective, depending upon cell subtype and timing after the ischemic insult, most available evidence in various tissues indicates that B lymphocytes are uniformly injurious (Burne-Taney, 2005; Chen et al., 2009; Huang et al., 2007; Shen et al., 2009; Zhang et al., 2008), by a mechanism involving B cell-derived IgM and activation of the complement system (Burne-Taney, 2003; Lee et al., 2010a, b; Williams et al., 1997; Zhang and Carroll, 2007; Zhang et al., 2008).
The role of cytotoxic CD8+ T cells and natural killer (NK) cells in I/R is less clear, with some reports supporting this concept (Beldi et al., 2010; Lappas et al., 2006; Liu et al., 2009; Osman et al., 2009; Shimamura et al., 2005; Ysebaert et al., 2004), while others do not (Burne et al., 2001; Kuboki et al., 2009; Yang et al., 2009; Zwacka et al., 1997). Suppressor or regulatory T (Treg) cells may also participate in the response to I/R, where they participate in resolution and repair of postischemic tissue injury (Chen et al., 2009; Kinsey et al., 2009, 2010; Liesz et al., 2009). In general, the recruitment and reparative actions of Treg cells commence 3–7 days after the insult (Gandalfo et al., 2009; Kinsey et al., 2009, 2010; Liesz et al., 2009). The secretion of the anti-inflammatory cytokine, IL-10, appears to mediate at least part of the protective effects of Treg cells (Gandalfo et al., 2009; Kinsey et al., 2009, 2010; Liesz et al., 2009).
6.8.3.4. Platelets
Abundant evidence supports a critical role for platelets in the thrombogenic and inflammatory responses to I/R, wherein their interaction with leukocytes, lymphocytes, and endothelial cells acts to promote injury (Barrabés et al., 2010; Esch et al., 2010; Khandoga et al., 2002, 2006; Massberg et al., 1999; Nakano et al., 2008; Park et al., 2010; Peters et al., 1999; Tailor et al., 2005; Yilmaz and Granger, 2008; Zhao et al., 2009). In the absence of insult or injury, platelets circulate in an inactive state, owing to the presence of inhibitory factors such as nitric oxide and prostacyclin. Upon tissue damage and release of ROS and other factors, activated platelets aggregate and adhere to the endothelium, tissue-resident and circulating leukocytes, and lymphocytes. Platelets express several integrin receptors, notably αIIbβ3 (GPIIb/IIIa), which bind to fibrinogen deposited on the microvascular endothelial cell surface after I/R, the latter, in turn, binding to endothelial surface ICAM-1 (Andrews and Berndt, 2004; Peters et al., 1999). Platelets also express both P-selectin and its ligands, PSGL-1 and GPIbα, and these CAM–ligand complexes mediate adhesion of platelets to both endothelial cells and leukocytes, as well as platelet–platelet aggregation, and appear to be important for leukocyte transmigration (Cooper et al., 2003; Lam et al., 2011; Salter et al., 2001). GPIbα also binds to endothelial von Willebrand factor, although this latter interaction may play more of a role in platelet adhesion in arterial vessels exposed to high shear stress, rather than in the postcapillary venules typically involved in I/R (Khandoga et al., 2002). Reciprocal regulation of PMN–endothelial adhesion by platelets (Russell et al., 2003) and platelet–endothelial adhesion by PMNs (Cooper et al., 2004) has been reported. It has been shown that approximately 75% of platelets adherent to the vascular wall are attached to endothelial–adherent leukocytes, the remainder being bound directly by the endothelium (Ishikawa et al., 2004). Both adhesive events are mediated by P-selectin (Cooper et al., 2004; Gawaz, 2004; Ishikawa et al., 2004) and are regulated by eNOS-derived NO and superoxide, with NO limiting and superoxide promoting microvascular vascular platelet adhesion (Khandoga et al., 2002).
While the pathophysiological consequences of activation and recruitment of platelets per se to the endothelium are not clearly understood, activated platelets are known to release a number of proinflammatory and mitogenic molecules, including IL-1β, RANTES, soluble CD154 (Gawaz, 2004), cytotoxic agents such as hydrogen peroxide (Baluk et al., 2007), and proapoptotic molecules (calpain, TGFβ) (Khandoga et al., 2002). Indeed, platelets have been found to mediate I/R-induced endothelial cell apoptosis in liver (Sindram et al., 2001; Park et al., 2010). These results suggest direct effects of platelets in mediating IRI. Moreover, it seems likely that a major mechanism whereby platelets contribute to IRI is via their promotion of leukocyte activation and adhesion (Khandoga et al., 2002; Russell et al., 2003; Zhao et al., 2009).
6.8.3.5. Mast cells
Mast cells reside in close association with blood vessels in connective tissues and at mucosal surfaces (Dai and Korthuis, 2011; Strbian et al., 2009). They possess numerous metachromatic cytoplasmic granules, containing a wide array of preformed, mainly proinflammatory mediators, including monoamines such as histamine and serotonin, cytokines such as TNFα, and proteases (Stone et al., 2010). Upon activation, mast cells degranulate, releasing these mediators. Mast cells also secrete newly synthesized lipid mediators derived from metabolism of arachidonic acid (Metz and Maurer 2007; Dai and Korthuis, 2011). Mast cell degranulation and mediator release contribute to the inflammatory response, eliciting vascular fluid leakage and resulting edema, recruitment of leukocytes, and can induce hemorrhage (Metz et al., 2007). Numerous factors associated with I/R have been shown to activate mast cells, including superoxide, complement components, calcitonin gene-related peptide, platelet activating factor, leukotrienes LTB4, and bacterial toxins (Metz and Maurer, 2007; Dai and Korthuis, 2011). However, mast cell degranulation is not an all-or-none phenomenon as once believed. Rather, certain mediators may be expressed and released selectively, depending upon the particular stimulus, as well as other conditions prevailing in the host tissue (Dai and Korthuis, 2011; Dvorak, 1992; Galli et al., 2005; Jin et al., 2007b; Strbian et al., 2009; Theoharidis et al., 2007).
Approaches to evaluating mast cell involvement in I/R include (1) administration of pharmacological agents which prevent mast cell activation or degranulation (mast cell stabilizers), (2) use of rat and mouse genetic models of mast cell deficiency, including the use of adoptive transfer of bone marrow-derived mast cells into deficient mice, and (3) genetic mouse models employing knockout of mast cell surface receptors for specific mediators or mast cell-secreted products (Abonia et al., 2005; Bortolotto et al., 2004; Bhattacharya et al., 2007; Gaboury et al., 1995; Goldman et al., 1992; Hei et al., 2008; Jin et al., 2007a,b, 2009; Lazarus et al., 2000; Santen et al., 2008; Strbian et al., 2006, 2007). Collectively, these studies all support a critical role for mast cells in I/R injury in brain, heart, small intestine, colon, and skeletal muscle, as well as in ROI to the lung (Goldman et al., 1992).
In many cases, mast cell activation is associated with increased recruitment of neutrophils, which contribute to IRI (Bhattacharya et al., 2007; Bilzer et al., 2006; Gaboury et al., 1995; Galli et al., 1991; Goldman et al., 1992; Liao et al., 1996; Santen et al., 2008; Strbian et al., 2006, 2007; Wershil et al., 1991; Zhang et al., 1992). However, other studies demonstrate significant I/R-induced mast cell degranulation and tissue injury, without an attendant increase in recruited neutrophils (Bortolotto et al., 2004; Lazarus et al., 2000). In addition, increases in vascular permeability in response to mast cell activation have been shown to have both leukocyte-dependent and leukocyte-independent components (Liao et al., 1996). Mast cells also promote thrombolysis and hemorrhage in ischemic stroke (Strbian et al., 2009). These complications have been attributed to mast cell-derived heparin and proteases, including tissue plasminogen activator, and probably do not involve neutrophil activation.
Collectively, the aforementioned effects of mast cell activation/degranulation have led to numerous recent proposals that mast cell inhibition/ stabilization strategies may constitute an important adjunct mode of therapy in ischemic disorders (Bortolotto et al., 2004; Jin et al., 2007a,b; Karra et al., 2009; Strbian et al., 2006, 2007, 2009). Recent findings showing mast cell expression of the death receptor, TRAIL, and inhibitory receptors, CD300a and Siglec-8 (Karra et al., 2009), suggest a potential means of preventing or attenuating mast cell-mediated injury in the context of I/R. However, the recognition that mast cells may also act to limit inflammatory responses, for example, through release of IL-10 (Grimbaldeston et al., 2007) or proteases which have been shown to degrade endothelin-1, thus reducing the latter’s toxicity (Maurer et al., 2004) strongly suggests the need for greater understanding of the full range of mast cell biology during I/R.
6.8.3.6. Monocytes, macrophages, and Kupffer cells
Kupffer cells constitute the body’s largest cohort of fixed, tissue-resident macrophages. They are located in association with endothelial cells lining hepatic sinusoids and are in a position to intercept bacteria, bacterial endotoxins, and other potentially injurious agents arising from the gut. Thus, they are intimately involved with the hepatic response to toxins (Bilzer et al., 2006). Their role in the response to I/R is complex because Kupffer cells can simultaneously promote and limit inflammation, with the predominant action depending upon both the duration of the initial ischemic insult and the time elapsed after that initial insult (Duffield et al., 2005).
Proinflammatory actions
In response to a stimulus, Kupffer cells are activated by two separate, but complementary mechanisms, one mediated by TLR-dependent signaling and the other by complement activation. In response to I/R, damaged hepatocytes release High Mobility Group Box 1 protein (HMGB-1), an inflammatory ligand for TLR-4, by a release mechanism that is itself dependent on ROS and TLR-4 (Tsung et al., 2007a,b). Kupffer cells are then activated through engagement of HMGB-1 with TLR-4 (Tsung et al., 2005b). This HMGB-1/TLR-4 positive feedback may play an important role in sustaining hepatic inflammation after I/R (Tsung et al., 2005a,b). Downstream signaling then activates NFκB-dependent transcription of inflammatory cytokines (Bilzer et al., 2006). The other activation mechanism involves cleavage of complement mediators C3 and C5 to C3a and C5a. Engagement to C3a and C5a receptors stimulates G protein-dependent phospholipase C (PLC) activity. PLC-produced diacylglycerol stimulates PKC-dependent activity of NADPH oxidase, which produces superoxide. The other PLC reaction product, inositol 3-phosphate (IP3), stimulates Ca2+ mobilization from internal stores, as well as uptake from the extracellular space. The rise in Ca2+ contributes to activation of PKC, which stimulates production of superoxide by NADPH oxidase and also stimulates eicosanoid synthesis via phospholipase A2-dependent cyclooxygenase activity ( Jaeschke et al., 1993; Jennings and Reimer, 1991).
Kupffer cells are a major source of oxidants, cytokines, and other proinflammatory mediators such as platelet activating factor after hepatic I/R (Bautista et al., 1990; Caldwell-Kenkel et al., 1991; Horie et al 1997; Jaeschke et al., 1990, 1992, 1993). Their role in hepatic I/R to initiate tissue injury and recruit neutrophils has been demonstrated using gadolinium chloride (GdCl3), an inhibitor of Kupffer cell activation (Giakoustidis et al., 2003; Horie et al., 1997; Li et al., 2009; Liu et al., 1995). Findings using this inhibitor indicate that Kupffer cells themselves are largely responsible for early stages of injury, while Kupffer cell-dependent recruitment of neutrophils exerts the dominant cytotoxic effects during later periods (Liu et al., 1995). In addition to their role in promoting recruitment and activation of neutrophils, Kupffer cells also activate CD4+ T-lymphocytes (Hanschen et al., 2008).
Anti-inflammatory actions
Kupffer cells have several actions which counter inflammatory tissue injury and are thus involved in early stages of tissue healing and repair after I/R. First, Kupffer cells can induce apoptosis of PMNs through engagement of Kupffer cell surface-expressed Fas ligand (FasL, or CD95L), with Fas (CD95) on PMNs (Muschen et al., 1999), and release of proapoptotic TNFα (Meszaros et al., 2000). Second, Kupffer cells phagocytize PMNs, thus acting as a brake on the leukocytes’ injurious effects (Brown et al., 2001; Shi et al., 1996, 2001). Third, Kupffer cells are active in clearing free hemoglobin released from damaged erythrocytes via the CD163 scavenger receptor (Kristiansen et al., 2001), followed by degradation by heme oxygenase-1 (HO-1) (Goda et al., 1998), which is highly expressed in Kupffer cells (Bauer et al., 1998; Genken et al., 2005). This action prevents heme-mediated oxidative injury (Bauer and Bauer, 2002; Devey et al., 2009; Ellett et al., 2010; Ryter et al., 2006; Stocker et al., 1987; Suematsu and Ishimura, 2000; Tomiyama et al., 2008). Indeed, positive clinical outcomes of liver transplants were correlated with levels of HO-1 expression in donor livers prior to surgery (Genken et al., 2005), while others have found that Kupffer cells with high levels of HO-1 expression also show decreased expression and release of proinflammatory cytokines (Kobayashi et al., 2002; Zeng et al., 2010) and increased release of anti-inflammatory mediators (Ellett et al., 2010).
6.8.4. Plasma membrane-derived microparticles
Cell-derived microparticles circulate in normal plasma, increase in response to inflammation, and have been implicated in a variety of deleterious processes in I/R (Angelillo-Scherrer, 2012; Horstman et al., 2009; Leroyer et al., 2010; Mause and Weber 2010; Rautou et al., 2011). These small (0.1–1 mm diameter) membrane vesicles are released from a variety of cell types, including platelets, erythrocytes, leukocytes, platelets, and endothelial cells, when activated by thrombin or proinflammatory stimuli such as TNF or during apoptosis. Microparticles display strong procoagulant activity and although originally dismissed as cellular debris that played only minor roles in inflammatory conditions, they are now recognized to contain a variety of biologically important molecules that contribute to the pathogenesis of I/R (Angelillo-Scherrer, 2012; Horstman et al., 2009; Leroyer et al., 2010; Mause and Weber, 2010; Rautou et al., 2011). The importance of microparticles in pathologic states first emerged with the discovery that tissue factor, the principle initiator of thrombosis, circulates almost exclusively bound to these membrane-derived vesicles. Subsequent work has demonstrated that cell-derived microparticles function not only to promote coagulation mediated by tissue factor but also serve as vectors for transport of a vast array of signaling agents from one cell type to another. These include arachidonic acid, bioactive lipids, receptors, RNA, chemokines, cytokines, growth factors, proteases, integrins, and caspases (Angelillo-Scherrer, 2012; Horstman et al., 2009; Leroyer et al., 2010; Mause and Weber, 2010; Rautou et al., 2011). One particularly important bioactive lipid is platelet-activating factor, owing to its role in inducing platelet activation and leukocyte sequestration in postischemic tissues. Calpain, a protease involved in the pathogenesis of I/R, is also carried in microparticles, where it is protected from inactivation by plasma inhibitors (Kelton et al., 1992).
Because membrane-derived microparticles derived from activated endothelium, platelets, and leukocytes may be released into the circulating blood, these vesicular structures have been suggested to play a role in ROI induced by local I/R by exerting distant thrombogenic and proinflammatory effects (George, 2008) (Chironi et al., 2009). The basis for these actions appears to be related to their membrane content of substrates for production of bioactive lipid mediators (e.g., arachidonic acid), as well as surface proteins characteristic of their parent cell types, such as ECAMs in the case of endothelial-derived microparticles (Chironi et al., 2009).
6.9. Protein cleavage products and other degradation products in I/R
Apart from ROS, a number of detrimental degradation products have been identified that are likely to make a substantial contribution to IRI. Ischemia results in damage to and degradation of a large number of intracellular proteins. At the same time, the ubiquitin proteasome system (UPS) becomes dysfunctional (Gurusamy et al., 2008), which might lead to selective dysregulation of signaling pathways and accumulation of toxic metabolites. Ischemic preconditioning preserves postischemic UPS function, which is thought to favorably affect concentrations of protective and antiprotective signaling molecules such as the epsilon and delta isoforms of PKC (Churchill et al., 2010; Divald et al., 2010). Therefore, therapeutic approaches to specifically target the tissues’ proteasome have high potential to improve tissue viability after ischemia. However, the effects of proteasome inhibition during myocardial ischemia on cardiac function have been controversial, as both beneficial and deleterious effects have been reported (Yu and Kem, 2010). Recent analysis of cardiac proteasome suggests that distinct subpopulations of the cardiac proteasome exist that differ in subunit composition, posttranslational modification, and associating partners (Drews et al., 2007; Young et al., 2008). If the detrimental effects of proteasomal activity could be specifically targeted, such approaches would have great therapeutic potential.
Similarly, targeting of the calpain–calpastatin protein degradation system and matrix metalloproteinases (MMPs) has emerged as new potential therapeutic avenues (Hernando et al., 2010; Raedschelders et al., 2012). Pharmacological inhibition of the cysteine protease calpain reduces myocardial infarct size and improves ventricular function (Khalil et al., 2005). Similarly, I/R injury of the kidney can be ameliorated by calpain inhibition (Chatterjee et al., 2001). However, a major current limitation to the use of calpain inhibitors is their lack of specificity among cysteine proteases and other proteolytic enzymes (Carragher, 2006). MMPs can be transcriptionally upregulated and play a role in myocardial remodeling during the reparative process following I/R. However, these proteases may also be acutely activated by H2O2 and ONOO— and the resulting proteolytic activity can produce endothelial and contractile dysfunction in the heart in the absence of obvious apoptosis and/or necrosis (Raedschelders et al., 2012).
Membrane lipid degradation and fatty acid oxidation pathways have become the recent focus of studies on protection against IRI. For example, treatment with mildronate, a fatty acid oxidation inhibitor that blocks the biosynthesis of carnitine, attenuates postischemic cardiac dysfunction and reduces infarct size (Sesti et al., 2006). Mechanistically, it is not yet clear whether these protective effects are due to the resulting shift in energy metabolism from fatty acid oxidation to the more favorable glucose oxidation under ischemic conditions or can be attributed to the prevention of fatty acid metabolite accumulation. Increased phospholipase activation results in phospholipid hydrolysis and release of arachidonic acid from cell membranes in postischemic tissue. Degraded lipids also contribute to the production of ROS. It is likely that increased vulnerability of the brain to lipoxidative mediators after IRI contributes to secondary injury (Lewen et al., 2000). Therefore, it has been hypothesized that a reduction of the lipoxidative load protects against postischemic injury (Phillis and O’Regan, 2003). Thus, it is not surprising that treatment with a phospholipase A2 inhibitor reduced infarct volume and improved neurologic function in a rat model (Hoda et al., 2009).
6.10. No-Reflow
Reperfusion of ischemic tissues is associated with the development of the no-reflow phenomenon (Gute et al., 1998; Schwartz and Kloner, 2012). That is, when the blood supply is reestablished, a large number of capillaries fail to reperfuse. This nutritive perfusion impairment has been shown to occur in postischemic brain, kidney, heart, small intestine, and skeletal muscle. Although it has been suggested that microvascular thrombus formation may contribute to no-reflow, intravital microscopic studies suggest that this is not the case since microvessel thrombosis is rarely observed. These in vivo observations are corroborated by both light and electron microscopic examination of reperfused tissues in that platelet or fibrin thrombi are rarely detected. Moreover, heparin treatment is not effective in restoring capillary perfusion after I/R (Gute et al., 1998).
Several lines of evidence indicate that activated neutrophils play an important role in the development of postischemic capillary no-reflow (Gute et al., 1998). For example, there is a strong correlation between the percent of capillaries exhibiting no-reflow and the number of leukocytes present in these capillaries in reperfused tissues. More compelling support for this notion is provided by the observation that neutrophil depletion virtually abolishes no-reflow in reperfused heart, brain, and skeletal muscle. It appears that oxidants are involved since treatment with allopurinol or SOD restores capillary perfusion and prevents leukocyte/endothelial cell adhesion. In addition, antibodies directed against functional epitopes on CD11/CD18 on leukocytes and ICAM-1 or P-selectin on the endothelium prevent postischemic capillary no-reflow. Physical impaction of leukocytes in capillary lumens has also been proposed as a mechanism for no-reflow during ischemia (Gute et al., 1998). This notion is based on the fact that neutrophils are large (8 μm average diameter), stiff, viscoelastic cells that must undergo substantial deformation as they enter and traverse the smaller diameter capillaries. Because perfusion pressures driving the flow of formed elements in the blood through capillaries is reduced during ischemia, these large cells are more likely to arrest in capillaries and block perfusion. Furthermore, the acidic environment that exists in ischemic tissues increases the stiffness of these white cells thereby increasing the likelihood for leukocyte plugging in capillaries. Compounding this problem is the fact that endothelial cell volume regulatory mechanisms are disrupted by ischemia, leading to endothelial cell swelling and narrowing of the capillary lumen (Gute and Korthuis, 1995). This is exacerbated further by the oxidant stress elicited by reperfusion, which alters Na+-H+ exchange in endothelial cells. Treatment with hypertonic, hyperosmotic saline/dextran solutions, or inhibitors of Na+-H+ exchanger prevents endothelial cell swelling and reduces the extent of capillary no-reflow.
Another important factor contributing to the development of no-reflow during reperfusion is I/R-induced, neutrophil-dependent microvascular endothelial barrier disruption (Gute et al., 1998). As a consequence, transmicrovascular fluid filtration and protein efflux are increased and edema forms. The rate of edema formation is markedly enhanced when the blood supply is reestablished owing to restoration of luminal pressures. The accumulation of fluid in ischemic tissues raises interstitial pressure surrounding blood vessels, an effect that is exacerbated by parenchymal cell swelling, causing collapse of microvessels exhibiting the lowest intraluminal pressure (i.e., capillaries and postcapillary venules). This extravascular compression mechanism for the development of no-reflow is especially important in tissues that cannot readily expand when edema forms because they are enveloped by structures that limit expansion of the tissues. This includes the brain (encased in the cranial vault), many skeletal muscles (surrounded by a tight fascial sheath), and the kidney (which is surrounded by the renal capsule). In addition to this extravascular compression effect, enhanced transmicrovascular fluid filtration from the blood to the tissue spaces, when coupled with altered cellular ion fluxes and cell swelling induced by ischemia, produces an increase in microvessel hematocrit. As a consequence, microvascular resistance to blood flow is increased secondary to increased blood viscosity, which may act to further impair capillary perfusion in postischemic tissues. In the heart, degradation of the coronary vasculature during I/R produces loss of capillary integrity and grossly hemorrhagic infarctions (termed vascular rhexis) that may also contribute to the no-reflow phenomenon (Zaman et al., 2011).
Pericytes are contractile cells that surround almost all capillaries as well as small arterioles and venules. Since these cells are contractile and responsive to agents such as adenosine, lactate, endothelin, NO, PGI2, and PDGF and to changes in intracellular calcium, it has been suggested that pericytes may influence capillary perfusion during I/R (Fernandez-Klett et al., 2010; Hamilton et al., 2010). This may be particularly important in the central nervous system, where there are more pericytes per endothelial cell than in other areas of the body. Indeed, pericytes have been shown to constrict capillaries shortly after the onset of retinal or cerebral ischemia and remain contracted when blood flow is reinstituted (Peppiatt et al., 2006; Yemisci et al., 2009). This pericyte-induced reduction in capillary luminal diameter produced no-reflow by a mechanism that may involve the production of peroxynitrite (Yemisci et al., 2009) by underlying endothelium.
No-reflow occurs in a variable proportion of patients following percutaneous coronary intervention (PCI) for myocardial ischemia, ranging from 5% to 50%, and is an independent predictor of adverse outcome (Niccoli et al., 2009). In addition to the mechanisms described above, distal embolization is an important determinant of this intervention-induced no-reflow and can occur in regions that were not exposed to ischemia prior to PCI. Personalized management of no-reflow that is based on assessment of the dominant mechanisms contributing to the microvascular perfusion impairment in each patient is now being explored as a means to reduce reperfusion injury (Niccoli et al., 2009, 2011; Patel and Fisher, 2010). In view of the multifactorial mechanisms that contribute to no-reflow, this tailored management approach to risk stratification and lesion characteristics may produce significant improvements in patient outcomes by directing the rational selection of treatment strategies. Anti-platelet therapy and vasodilators, embolic protection devices, and pharmacologic pre- and postconditioning strategies have been employed to limit both reperfusion- and intervention-induced microvascular obstruction in ischemic disease (Gute et al., 1998; Niccoli et al., 2009; Patel and Fisher, 2010; Schwartz and Kloner, 2012).
6.11. Genomic/metabolomic insights
Over the past 10 years, new insights have come from genetic and genomic studies, which have identified approximately 30 specific chromosomal locations of genes or other DNA sequences that are associated with myocardial infarction and coronary artery disease (Cappola and Margulies, 2011; O’Donnell and Nabel, 2011). Interestingly, genome-wide association studies have also revealed loci-associated ischemic stroke and peripheral artery disease, some of which are common to coronary artery disease and myocardial infarction, suggesting a common genetic contribution across vascular beds. Although specific genetic loci are associated with hypertension (CNNM2), dyslipidemia (APOA5), and atherosclerosis (ABO and ADAMS7), only a minority of the identified loci mediate effects through known risk factors. Indeed, the genome-wide association studies connecting genetic variations in chromosomal regions devoid of genes that had been previously associated with coronary artery disease and infarction hold promise for identifying new mechanisms by which genes in such regions contribute to ischemic disease. Genomic studies also have great potential for strengthening the evidence for pharmacogenetic interactions in a number of commonly used cardiovascular drugs, thereby providing insight regarding specific variants that contribute to heterogeneity of cardiovascular drug response. Application of this knowledge to personalized medicine should lead to improved therapeutics via targeting treatments to a specific genotype.
Similarly, identification and quantification of proteins and lipids through mass spectrometry-based proteomics and lipidomics technologies represent relatively new approaches to identify novel biomarkers and mechanistic pathways without prior known association to cardiovascular disease (Gerszten et al., 2011; Thomas et al., 2011). Indeed, a recent metabolomic study demonstrated the rather remarkable relationship between dietary lipid intake, intestinal bacteria, and livermetabolism to the generation of phospholipid-associated molecules that promote the build-up of arterial plaque (Wang et al., 2011a,b). The results of this provocative study suggest that gut flora-dependent metabolism of dietary phosphatidylcholine produces trimethylamine, which is absorbed by the intestine and metabolized to trimethylamine N-oxide (TMAO) by hepatic flavin monooxygenases. TMAO, in turn, acts to elicit a proatherogenic phenotype in macrophages, thereby contributing to plaque formation/progression (Wang et al., 2011b). These observations not only provide unique insights regarding the role of the gut microbiome in promoting cardiovascular disease but also suggest the possibility that TMAO may serve as a meaningful biomarker for atherosclerosis.
7. Concluding Remarks and Perspectives
Cardiovascular disease is the leading cause of death in westernized cultures. Considerable progress has been made over the past 50 years with regard to identifying and modifying risk factors for cardiovascular disease (hypertension, hypercholesterolemia, obesity, diabetes, smoking, and physical inactivity), in the development of therapeutic approaches (e.g., cardiac bypass surgery, PCIs, and pharmacologic treatments such as beta blockers and angiotensin converting enzyme inhibitors), and development of blood biomarker tests and imaging measurements which allow subclinical disease detection years before clinical symptoms become evident. As a result of these efforts, there has been a significant decline in age-adjusted cardiovascular mortality. In addition, decades of intensive work have led to the development of the concepts that (1) the response to I/R is bimodal, depending on the length of I/R, (2) cell dysfunction, injury, and death are attributable to pathologic processes invoked by ischemia per se and yet others that are activated upon reperfusion, (3) the presence of coexisting risk factors and events occurring during fetal life (fetal programming) markedly enhance the susceptibility to I/R, and (4) the mechanisms contributing to I/ R injury are complex and multifactorial, including calcium overload, oxidative/nitrosative stress, ER and mitochondrial dysfunction, activation of protein kinases, inflammation, epigenetic changes, protein cleavage products, and nutritive perfusion deficits.
Despite these recent major advances in our understanding of the mechanistic underpinnings governing I/R, translation of these findings into novel, clinically applicable therapies has been disappointingly slow. For example, the treatment strategies for myocardial infarction and stroke have not been improved upon since the introduction of thrombolytic treatment and angioplasty. Several factors contribute to this failure, including evaluation of potential therapies in patients with advanced disease, the presence of multiple coexisting risk factors, therapeutic focus on single arms of the multifactorial injury processes that sum to produce tissue injury and death, and to the very short time window available for changing the outcome. However, the discovery that ischemic preconditioning activates powerful cell-survival programs that target multiple pathologic processes to limit the extent of I/R injury has reinvigorated identification of therapeutic strategies that might prove effective in reducing the risk for and/or outcome of adverse cardiovascular events, including application of gene therapy approaches. Encouragingly, pilot trials have indicated that preconditioning can be an effective strategy in the human brain (Chan et al., 2005; Wegener et al., 2004b). Similarly, remote ischemic preconditioning as well as direct renal ischemic preconditioning protected against renal ischemia in unblinded small studies (Ali et al., 2007; Walsh et al., 2009). In the cardiac field, many of the initial pilot successes with preconditioning were not subsequently confirmed in larger trials (Turer and Hill, 2010; Yellon and Hausenloy, 2007). This calls for better standardization of treatment protocols, more rigorous validation of new targets in experimental models by multiple investigators before clinical trials are initiated, and for new trials to be conducted in more focused patient subpopulations to decrease disease heterogeneity (Yellon and Hausenloy, 2007). In addition, while much has been learned from the study of preconditioning and I/R injury in experimental models employing young, healthy animals, the facts that I/R injury is greatly exacerbated by the presence of coexisting risk factors, which also prevent the activation of cell survival programs induced by preconditioning, indicate that a concerted effort should be directed toward use of models that better recapitulate the situation seen in the vast majority of patients susceptible for adverse cardiovascular events.
ACKNOWLEDGEMENTS
The authors’ work was supported by grants from the National Institutes of Health (HL-095486, AA-014945, HL092327, and HL094404) and a Scientist Development Grant from the American Heart Association (0635472N).
REFERENCES
- Abdellatif M. Differential expression of microRNAs in different disease states. Circ. Res. 2012;110:638–650. doi: 10.1161/CIRCRESAHA.111.247437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abonia JP, Friend DS, Austen WG, Jr, Moore FD, Jr, Carroll MC, Chan R, et al. Mast cell protease 5 mediates ischemia-reperfusion injury of mouse skeletal muscle. J. Immunol. 2005;174:7285–7291. doi: 10.4049/jimmunol.174.11.7285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abramov AY, Scorziello A, Duchon MR. Three distinct mechanisms generate oxygen free radicals in neurons and contribute to cell death during anoxia and reoxygenation. J. Neurosci. 2007;27:1129–1138. doi: 10.1523/JNEUROSCI.4468-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adibhatha RM, Hatcher JF. Lipid oxidation and peroxidation in CNS health and disease: from molecular mechanisms to therapeutic opportunities. Antioxid. Redox Signal. 2010;12:125–169. doi: 10.1089/ars.2009.2668. [DOI] [PubMed] [Google Scholar]
- Ahluwalia A, De Felipe C, O’Brien J, Hunt SP, Perretti M. Impaired IL-1beta-induced neutrophil accumulation in tachykinin NK1 receptor knockout mice. Br. J. Pharmacol. 1998;124:1013–1015. doi: 10.1038/sj.bjp.0701978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander JS, Elrod JW. Extracellular matrix, junctional integrity and matrix metalloproteinase interactions in endothelial permeability regulation. J. Anat. 2002;2000:561–574. doi: 10.1046/j.1469-7580.2002.00057.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali ZA, Callaghan CJ, Lim E, Ali AA, Nouraei SA, Akthar AM, et al. Remote ischemic preconditioning reduces myocardial and renal injury after elective abdominal aortic aneurysm repair: a randomized controlled trial. Circulation. 2007;116:I98–I105. doi: 10.1161/circulationaha.106.679167. [DOI] [PubMed] [Google Scholar]
- Allen CL, Bayraktutan U. Oxidative stress and its role in the pathogenesis of ischaemic stroke. Int. J. Stroke. 2009;4:461–470. doi: 10.1111/j.1747-4949.2009.00387.x. [DOI] [PubMed] [Google Scholar]
- Alverdy JC, Chang EB. The re-emerging role of the intestinal microflora in critical illness and inflammation: why the gut hypothesis of sepsis syndrome will not go away. J. Leukoc. Biol. 2008;83:461–466. doi: 10.1189/jlb.0607372. [DOI] [PubMed] [Google Scholar]
- Andrews RK, Berndt MC. Platelet physiology and thrombosis. Thromb. Res. 2004;114:447–453. doi: 10.1016/j.thromres.2004.07.020. [DOI] [PubMed] [Google Scholar]
- Angelillo-Scherrer A. Leukocyte-derived microparticles in vascular homeostasis. Circ. Res. 2012;110:356–369. doi: 10.1161/CIRCRESAHA.110.233403. [DOI] [PubMed] [Google Scholar]
- Arany I, Faisa A, Clark JS, Vera T, Baliga R, Nagamine Y. p66Shc-mediated mitochondrial dysfunction in renal proximal tubule cells during oxidative injury. Am. J. Physiol. 2010;298:F1214–F1221. doi: 10.1152/ajprenal.00639.2009. [DOI] [PubMed] [Google Scholar]
- Atarashi K, Hirata T, Matsumoto M, Kanemitsu N, Miyasaka M. Rolling of Th1 cells via P-selectin glycoprotein ligand-1 stimulates LFA-1-mediated cell binding to ICAM-1. J. Immunol. 2005;174:1424–1432. doi: 10.4049/jimmunol.174.3.1424. [DOI] [PubMed] [Google Scholar]
- Baines CP. The mitochondrial permeability transition pore and ischemia-reperfusion injury. Basic Res. Cardiol. 2009a;104:181–188. doi: 10.1007/s00395-009-0004-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baines CP. The molecular composition of the mitochondrial permeability transition pore. J. Mol. Cell. Cardiol. 2009b;46:850–857. doi: 10.1016/j.yjmcc.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baines CP. The cardiac mitochondrion: nexus of stress. Annu. Rev. Physiol. 2010;72:61–80. doi: 10.1146/annurev-physiol-021909-135929. [DOI] [PubMed] [Google Scholar]
- Baines CP. How and when do myocytes die during ischemia and reperfusion: the late phase. J. Cardiovasc. Pharmacol. Ther. 2011;16:239–243. doi: 10.1177/1074248411407769. [DOI] [PubMed] [Google Scholar]
- Baines CP, Zhang J, Wang GW, Zheng YT, Xiu JX, Cardwell EM, et al. Mitochondrial PKCe and MAPK form signaling modules in the murine heart: enhanced mitochondrial PKCe-MAPK interactions and differential MAPK activation in PKCe-induced cardioprotection. Circ. Res. 2002;90:390–397. doi: 10.1161/01.res.0000012702.90501.8d. [DOI] [PubMed] [Google Scholar]
- Baines CP, Kaiser RA, Purcell NH, Blair NS, Osinska H, Hambleton MA, et al. Loss of cyclophilin D reveals a critical role for mitochondrial permeability transition in cell death. Nature. 2005;434:658–662. doi: 10.1038/nature03434. [DOI] [PubMed] [Google Scholar]
- Bang C, Fiedler J, Thum T. Cardiovascular importance of the microRNA-23/27/ 24 family. Microcirculation. 2012;19:208–214. doi: 10.1111/j.1549-8719.2011.00153.x. [DOI] [PubMed] [Google Scholar]
- Barker DJ. Fetal origins of coronary heart disease. BMJ. 1995;311:171–174. doi: 10.1136/bmj.311.6998.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnabei MS, Palpant NJ, Metzger JM. Influence of genetic background on ex vivo and in vivo cardiac function in several commonly used inbred mouse strains. Physiol. Genomics. 2010;42A:103–113. doi: 10.1152/physiolgenomics.00071.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barone FC, Knudsen DJ, Nelson AH, Feuerstein GZ, Willette RN. Mouse strain differences in susceptibility to cerebral ischemia are related to cerebral vascular anatomy. J. Cereb. Blood Flow Metab. 1993;13:683–692. doi: 10.1038/jcbfm.1993.87. [DOI] [PubMed] [Google Scholar]
- Barker DJ. The origins of the developmental origins theory. J. Intern. Med. 2007;261:412–417. doi: 10.1111/j.1365-2796.2007.01809.x. [DOI] [PubMed] [Google Scholar]
- Barry MC, Wang JH, Kelly CJ, Sheehan SJ, Redmond HP, Bouchier-Hayes DJ. Plasma factors augment neutrophil and endothelial cell activation during aortic surgery. Eur. J. Vasc. Endovasc. Surg. 1997;13:381–387. doi: 10.1016/s1078-5884(97)80080-9. [DOI] [PubMed] [Google Scholar]
- Bauer M, Bauer I. Heme oxygenase-1: redox regulation and role in the hepatic response to oxidative stress. Antioxid. Redox Signal. 2002;4:749–758. doi: 10.1089/152308602760598891. [DOI] [PubMed] [Google Scholar]
- Bauer I, Wanner GA, Rensing H, Alte C, Miescher EA, Wolf B, et al. Expression pattern of heme oxygenases 1 and 2 in normal and stress-exposed rat liver. Hepatology. 1998;27:829–838. doi: 10.1002/hep.510270327. [DOI] [PubMed] [Google Scholar]
- Baum M. Role of the kidney in the prenatal and early postnatal programming of hypertension. Am. J. Physiol. Renal Physiol. 2010;298:F235–F247. doi: 10.1152/ajprenal.00288.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumgartner WA, Williams GM, Fraser CD, Jr, Cameron DE, Gardner TJ, Burdick JF, et al. Cardiopulmonary bypass with profound hypothermia. An optimal preservation method for multiorgan procurement. Transplantation. 1989;47:123–127. doi: 10.1097/00007890-198901000-00027. [DOI] [PubMed] [Google Scholar]
- Bautista AP, Meszaros K, Bojta J, Spitzer JJ. Superoxide anion generation in the liver during the early stage of endotoxemia in rats. J. Leukoc. Biol. 1990;48:123–128. doi: 10.1002/jlb.48.2.123. [DOI] [PubMed] [Google Scholar]
- Beldi G, Banz Y, Kroemer A, Sun X, Wu Y, Graubardt N, et al. Deletion of CD39 on natural killer cells attenuates hepatic ischemia/reperfusion injury in mice. Hepatology. 2010;51:1702–1711. doi: 10.1002/hep.23510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belmont PJ, Chen WJ, San Pedro MN, Thuerauf DJ, Gellings Lowe N, Gude N, et al. Roles for endoplasmic reticulum-associated degradation and the novel endoplasmic reticulum stress response gene Derlin-3 in the ischemic heart. Circ. Res. 2010;106:307–316. doi: 10.1161/CIRCRESAHA.109.203901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belmont PJ, Chen WJ, Thuerauf DJ, Glembotski CC. Regulation of micro-RNA expression in the heart by the ATF6 branch of the ER stress response. J. Mol. Cell. Cardiol. 2012;52(5):1176–1182. doi: 10.1016/j.yjmcc.2012.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Ari Z, Pappo O, Cheporko Y, Yasovich N, Offen D, Shainberg A, et al. Bax ablation protects against hepatic ischemia/reperfusion injury in transgenic mice. Liver Transpl. 2007;13:1181–1188. doi: 10.1002/lt.21221. [DOI] [PubMed] [Google Scholar]
- Benhar M, Forrester MT, Stamler JS. Protein denitrosylation: enzymatic mechanisms and cellular functions. Nat. Rev. Mol. Cell Biol. 2009;10:721–732. doi: 10.1038/nrm2764. [DOI] [PubMed] [Google Scholar]
- Bhatia M, Saluja AK, Hofbauer B, Frossard JL, Lee HS, Castagliuolo I, et al. Role of substance P and the neurokinin 1 receptor in acute pancreatitis and pancreatitis-associated lung injury. Proc. Natl. Acad. Sci. U.S.A. 1998;95:4760–4765. doi: 10.1073/pnas.95.8.4760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharya K, Farwell K, Huang M, Kempuraj D, Donelan J, Papaliodis D, et al. Mast cell deficient W/Wv mice have lower serum IL-6 and less cardiac tissue necrosis than their normal littermates following myocardial ischemia-reperfusion. Int. J. Immunopathol. Pharmacol. 2007;20:69–74. doi: 10.1177/039463200702000108. [DOI] [PubMed] [Google Scholar]
- Birdsall HH, Green DM, Trial J, Youker KA, Burns AR, MacKay CR, et al. Complement C5a, TGF-beta 1, and MCP-1, in sequence, induce migration of monocytes into ischemic canine myocardium within the first one to five hours after reperfusion. Circulation. 1997;95:684–692. doi: 10.1161/01.cir.95.3.684. [DOI] [PubMed] [Google Scholar]
- Bluhmki E, Chamorro A, Davalos A, Machnig T, Sauce C, Wahlgren N, et al. Stroke treatment with alteplase given 3.0-4.5 h after onset of acute ischaemic stroke (ECASS III): additional outcomes and subgroup analysis of a randomised controlled trial. Lancet Neurol. 2009;8:1095–1102. doi: 10.1016/S1474-4422(09)70264-9. [DOI] [PubMed] [Google Scholar]
- Boengler K, Schulz R, Heusch G. Loss of cardioprotection with ageing. Cardiovasc. Res. 2009;83:247–261. doi: 10.1093/cvr/cvp033. [DOI] [PubMed] [Google Scholar]
- Boersma E, Maas AC, Deckers JW, Simoons ML. Early thrombolytic treatment in acute myocardial infarction: reappraisal of the golden hour. Lancet. 1996;348:771–775. doi: 10.1016/S0140-6736(96)02514-7. [DOI] [PubMed] [Google Scholar]
- Bogoyevitch MA, Gillespie-Brown J, Ketterman AJ, Fuller SJ, Ben-Levy R, Ashworth A, et al. Stimulation of the stress-activated mitogen-activated protein kinase subfamilies in perfused heart. p38/RK mitogen-activated protein kinases and c-Jun N-terminal kinases are activated by ischemia/reperfusion. Circ. Res. 1996;79:162–173. doi: 10.1161/01.res.79.2.162. [DOI] [PubMed] [Google Scholar]
- Bolli R, Marban E. Molecular and cellular mechanisms of myocardial stunning. Physiol. Rev. 1999;79:609–663. doi: 10.1152/physrev.1999.79.2.609. [DOI] [PubMed] [Google Scholar]
- Bonder CS, Norman MU, Macrae T, Mangan PR, Weaver CT, Bullard DC, et al. P-selectin can support both Th1 and Th2 lymphocyte rolling in the intestinal microvasculature. Am. J. Pathol. 2005;167:1647–1660. doi: 10.1016/S0002-9440(10)61248-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bortolotto SK, Morrison WA, Han X, Messina A. Mast cells play a pivotal role in ischaemia reperfusion injury to skeletal muscles. Lab. Invest. 2004;84:1103–1111. doi: 10.1038/labinvest.3700126. [DOI] [PubMed] [Google Scholar]
- Boujrad H, Gubkina O, Robert N, Krantic S, Susin SA. AIF-mediated programmed necrosis: a highly regulated way to die. Cell Cycle. 2007;6:2612–2619. doi: 10.4161/cc.6.21.4842. [DOI] [PubMed] [Google Scholar]
- Bozic CR, Lu B, Hopken UE, Gereard C, Berard NP. Neurogenic amplification of immune complex inflammation. Science. 1996;273:1722–1725. doi: 10.1126/science.273.5282.1722. [DOI] [PubMed] [Google Scholar]
- Bright R, Raval AP, Dembner JM, Pérez-Pinzón MA, Steinberg GK, Yenari MA, et al. Protein kinase C dmediates cerebral reperfusion injury in vivo. J. Neurosci. 2004;24:6880–6888. doi: 10.1523/JNEUROSCI.4474-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broughton BR, Reutens DC, Sobey CG. Apoptotic mechanisms after cerebral ischemia. Stroke. 2009;40:e331–e339. doi: 10.1161/STROKEAHA.108.531632. [DOI] [PubMed] [Google Scholar]
- Brown KE, Brunt EM, Heinecke JW. Immunohistochemical detection of myeloperoxidase and its oxidation products in Kupffer cells of human liver. Am. J. Pathol. 2001;159:2081–2088. doi: 10.1016/S0002-9440(10)63059-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulkley GB. Free radical-mediated reperfusion injury: a selective review. Br. J. Cancer Suppl. 1987;8:66–73. [PMC free article] [PubMed] [Google Scholar]
- Burne MJ, Haq M, Matsuse H, Mohapatra S, Rabb H. Genetic susceptibility to renal ischemia reperfusion injury revealed in a murine model. Transplantation. 2000;69:1023–1025. doi: 10.1097/00007890-200003150-00065. [DOI] [PubMed] [Google Scholar]
- Burne-Taney MJ, Ascon DB, Daniels F, Racusen L, Baldwin W, Rabb H. B cell deficiency confers protection from renal ischemia reperfusion injury. J. Immunol. 2003;171:3210–3215. doi: 10.4049/jimmunol.171.6.3210. [DOI] [PubMed] [Google Scholar]
- Burne-Taney MJ, Yokota-Ikeda N, Rabb H. Effects of combined T- and B-cell deficiency on murine ischemia reperfusion injury. Am. J. Transplant. 2005;5:1186–1193. doi: 10.1111/j.1600-6143.2005.00815.x. [DOI] [PubMed] [Google Scholar]
- Burns AR, Smith CW, Walker DC. Unique structural features that influence neutrophil emigration into the lung. Physiol. Rev. 2003;83:309–336. doi: 10.1152/physrev.00023.2002. [DOI] [PubMed] [Google Scholar]
- Burne-Taney MJ, Ascon DB, Daniels F, Racusen L, Baldwin W, Rabb H. B cell deficiency confers protection from renal ischemia reperfusion injury. J. Immunol. 2003;171:3210–3215. doi: 10.4049/jimmunol.171.6.3210. [DOI] [PubMed] [Google Scholar]
- Caldwell CC, Okaya T, Matignoni A, Husted T, Schuster R, Lentsch AB. Divergent functions of CD4+ T lymphocytes in acute liver inflammation and injury after ischemia-reperfusion. Am. J. Physiol. 2005;289:G969–G976. doi: 10.1152/ajpgi.00223.2005. [DOI] [PubMed] [Google Scholar]
- Caldwell-Kenkel JC, Currin RT, Tanaka Y, Thurman PG, Lemasters JJ. Kupffer cell activation and endothelial cell damage after storage of rat livers: effect of reperfusion. Hepatology. 1991;13:83–95. [PubMed] [Google Scholar]
- Cao T, Pinter E, Al-Rasheed S, Gerard N, Hoult JR, Brain SD. Neurokinin-1 receptor agonists are involved in mediating neutrophil accumulation in the inflamed, but not normal, cutaneous microvasculature: an in vivo study using neurokinin-1 receptor knockout mice. J. Immunol. 2000;164:5424–5429. doi: 10.4049/jimmunol.164.10.5424. [DOI] [PubMed] [Google Scholar]
- Cappola TP, Margulies KB. Functional genomics applied to cardiovascular medicine. Circulation. 2011;124:87–94. doi: 10.1161/CIRCULATIONAHA.111.027300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carden DL, Granger DN. Pathophysiology of ischemia-reperfusion injury. J. Pathol. 2000;190:255–266. doi: 10.1002/(SICI)1096-9896(200002)190:3<255::AID-PATH526>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Cardinal J, Pan P, Tsung A. Protective role of cisplatin in ischemic liver injury through induction of autophagy. Autophagy. 2009;5:1211–1212. doi: 10.4161/auto.5.8.9972. [DOI] [PubMed] [Google Scholar]
- Carloni S, Girelli S, Scopa C, Buonocore G, Longini M, Balduini W. Activation of autophagy and Akt/CREB signaling play an equivalent role in the neuroprotective effect of rapamycin in neonatal hypoxia-ischemia. Autophagy. 2010;6:366–377. doi: 10.4161/auto.6.3.11261. [DOI] [PubMed] [Google Scholar]
- Carpi A, Menabò R, Kaludercic N, Pelicci P, Di Lisa F, Giorgio M. The cardioprotective effects elicited by p66(Shc) ablation demonstrate the crucial role of mitochondrial ROS formation in ischemia/reperfusion injury. Biochim. Biophys. Acta. 2009;1787:774–780. doi: 10.1016/j.bbabio.2009.04.001. [DOI] [PubMed] [Google Scholar]
- Carragher NO. Calpain inhibition: a therapeutic strategy targeting multiple disease states. Curr. Pharm. Des. 2006;12:615–638. doi: 10.2174/138161206775474314. [DOI] [PubMed] [Google Scholar]
- Castello PR, David PS, McClure T, Crook Z, Poyton RO. Mitochondrial cytochrome oxidase produces nitic oxide under hypoxic conditions: implications for oxygen sensing and hypoxic sensing in eukaryotes. Cell Metab. 2006;3:277–287. doi: 10.1016/j.cmet.2006.02.011. [DOI] [PubMed] [Google Scholar]
- Chan MT, Boet R, Ng SC, Poon WS, Gin T. Effect of ischemic preconditioning on brain tissue gases and pH during temporary cerebral artery occlusion. Acta Neurochir. Suppl. 2005;95:93–96. doi: 10.1007/3-211-32318-x_20. [DOI] [PubMed] [Google Scholar]
- Chatterjee PK, Brown PA, Cuzzocrea S, Zacharowski K, Stewart KN, Mota-Filipe H, et al. Calpain inhibitor-1 reduces renal ischemia/reperfusion injury in the rat. Kidney Int. 2001;59:2073–2083. doi: 10.1046/j.1523-1755.2001.00722.x. [DOI] [PubMed] [Google Scholar]
- Chatterjee PK, Todorovic Z, Sivarajah A, Mota-Filipe H, Brown PA, Stewart KN, et al. Inhibitors of calpain activation (PD150606 and E-64) and renal ischemiareperfusion injury. Biochem. Pharmacol. 2005;69:1121–1131. doi: 10.1016/j.bcp.2005.01.003. [DOI] [PubMed] [Google Scholar]
- Chehal MK, Granville DJ. Cytochrome p450 2C (CYP2C) in ischemic heart injury and vascular dysfunction. Can. J. Physiol. Pharmacol. 2006;84:15–20. doi: 10.1139/y05-139. [DOI] [PubMed] [Google Scholar]
- Chen L, Knowlton AA. Mitochondria and heart failure: new insights into an energetic problem. Minerva Cardioangiol. 2010;58:213–229. [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Wood KJ. Interleukin-23 and Th17 cells in transplantation immunity: does 23+17 equal rejection? Transplantation. 2007;84:1071–1074. doi: 10.1097/01.tp.0000287126.12083.48. [DOI] [PubMed] [Google Scholar]
- Chen M, Won DJ, Krajewski S, Gottlieb RA. Calpain and mitochondria in ischemia/reperfusion injury. J. Biol. Chem. 2002;277:29181–29186. doi: 10.1074/jbc.M204951200. [DOI] [PubMed] [Google Scholar]
- Chen JC, Wu ML, Huang KC, Lin WW. HMG-CoA reductase inhibitors activate the unfolded protein response and induce cytoprotective GRP78 expression. Cardiovasc. Res. 2008;80:138–150. doi: 10.1093/cvr/cvn160. [DOI] [PubMed] [Google Scholar]
- Chen J, Crispín JC, Tedder TF, Dalle Lucca J, Tsokos GC. B cells contribute to ischemia/reperfusion-mediated injury. J. Autoimmun. 2009;32:195–200. doi: 10.1016/j.jaut.2009.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Siu B, Ho YS, Vincent R, Chua CC, Hamdy RC, et al. Overexpression of MnSOD protects against myocardial ischemia/reperfusion injury in transgenic mice. J. Mol. Cell. Cardiol. 2001;30:2281–2289. doi: 10.1006/jmcc.1998.0789. [DOI] [PubMed] [Google Scholar]
- Chen L, Hahn H, Wu G, Chen CH, Liron T, Schechtman D, et al. Opposing cardioprotective actions and parallel hypertrophic effects of delta PKC and epsilon PKC. Proc. Natl. Acad. Sci. U.S.A. 2001;98:11114–11119. doi: 10.1073/pnas.191369098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chironi GN, Boulanger CM, Simon A, Dignat-George F, Freyssinet JM, Tedgui A. Endothelial microparticles in diseases. Cell Tissue Res. 2009;335:143–151. doi: 10.1007/s00441-008-0710-9. [DOI] [PubMed] [Google Scholar]
- Cho YS, Challa S, Moquin D, Genga R, Ray TD, Guildford M, et al. Phosphorylation-driven assembly of the RIP1-RIP3 complex regulates programmed necrosis and virus-induced inflammation. Cell. 2009;137:1112–1123. doi: 10.1016/j.cell.2009.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou WH, Choi DS, Zhang H, Mu D, McMahon T, Kharazia VN, et al. Neutrophil protein kinase Cdelta as a mediator of stroke-reperfusion injury. J. Clin. Invest. 2004;114:49–56. doi: 10.1172/JCI21655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choudhuri S, Cui Y, Klaassen CD. Molecular targets of epigenetic regulation and effectors of environmental influences. Toxicol. Appl. Pharmacol. 2010;245:378–393. doi: 10.1016/j.taap.2010.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchill EN, Ferreira JC, Brum PC, Szweda LI, Mochly-Rosen D. Ischaemic preconditioning improves proteasomal activity and increases the degradation of deltaPKC during reperfusion. Cardiovasc. Res. 2010;85:385–394. doi: 10.1093/cvr/cvp334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke SJ, McStay GP, Halestrap AP. Sanglifehrin A acts as a potent inhibitor of the mitochondrial permeability transition and reperfusion injury of the heart by binding to cyclophilin-D at a different site from cyclosporin A. J. Biol. Chem. 2002;277:34793–34799. doi: 10.1074/jbc.M202191200. [DOI] [PubMed] [Google Scholar]
- Contreras JL, Vilatoba M, Eckstein C, Bilbao G, Anthony Thompson J, Eckhoff DE. Caspase-8 and caspase-3 small interfering RNA decreases ischemia/reperfusion injury to the liver in mice. Surgery. 2004;136:390–400. doi: 10.1016/j.surg.2004.05.015. [DOI] [PubMed] [Google Scholar]
- Contreras L, Drago I, Zampese E, Pozzan T. Mitochondria: the calcium connection. Biochim. Biophys. Acta. 2010;1797:607–618. doi: 10.1016/j.bbabio.2010.05.005. [DOI] [PubMed] [Google Scholar]
- Cooper D, Chitman KD, Williams MC, Granger DN. Time-dependent platelet-vessel wall interactions induced by ischemia-reperfusion. Am. J. Physiol. 2003;284:G1027–G1033. doi: 10.1152/ajpgi.00457.2002. [DOI] [PubMed] [Google Scholar]
- Cooper D, Russell J, Chitman KD, Williams MC, Wolf RE, Granger DN. Leukocyte dependence of platelet adhesion in postcapillary venules. Am. J. Physiol. 2004;286:H1895–H1900. doi: 10.1152/ajpheart.01000.2003. [DOI] [PubMed] [Google Scholar]
- Corbucci GG, Perrino C, Donato G, Ricchi A, Lettieri B, Troncone G, et al. Transient and reversible deoxyribonucleic acid damage in human left ventricle under controlled ischemia and reperfusion. J. Am. Coll. Cardiol. 2004;43:1992–1999. doi: 10.1016/j.jacc.2004.01.040. [DOI] [PubMed] [Google Scholar]
- Costa FF. Non-coding RNAs: meet thy masters. Bioessays. 2010;32:599–608. doi: 10.1002/bies.200900112. [DOI] [PubMed] [Google Scholar]
- Creemers EE, Tijsen AJ, Pinto YM. Circulating microRNAs: novel biomarkers and extracellular communicators in cardiovascular disease? Circ. Res. 2011;110:483–495. doi: 10.1161/CIRCRESAHA.111.247452. [DOI] [PubMed] [Google Scholar]
- Crissinger KD, Granger DN. Characterization of intestinal collateral blood flow in the developing piglet. Pediatr. Res. 1988;24:473–476. doi: 10.1203/00006450-198810000-00011. [DOI] [PubMed] [Google Scholar]
- Croall DE, Ersfeld K. The calpains: modular designs and functional diversity. Genome Biol. 2007;8:218. doi: 10.1186/gb-2007-8-6-218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crosson CE, Mani SK, Husain S, Alsarraf O, Menick DR. Inhibition of histone deacetylase protects the retina from ischemic injury. Invest. Ophthalmol. Vis. Sci. 2010;51:3639–3645. doi: 10.1167/iovs.09-4538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Autrèaux B, Toledano MB. ROS as signaling molecules: mechanisms that generate specificity in ROS homeostasis. Nat. Mol. Cell Biol. 2007;8:813–824. doi: 10.1038/nrm2256. [DOI] [PubMed] [Google Scholar]
- Daemen MA, van’t Veer C, Denecker G, Heemskerk VH, Wolfs TG, Clauss M, et al. Inhibition of apoptosis induced by ischemia-reperfusion prevents inflammation. J. Clin. Invest. 1999;104:541–549. doi: 10.1172/JCI6974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai H, Korthuis RJ. Mast cell proteases and inflammation. Drug Discov. Today Dis. Models. 2011;8:47–55. doi: 10.1016/j.ddmod.2011.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damle SS, Moore EE, Babu AN, Meng X, Fullerton DA, Banerjee A. Hemoglobin-based oxygen carrier induces heme oxygenase-1 in the heart and lung but not brain. J. Am. Coll. Surg. 2009;208:592–598. doi: 10.1016/j.jamcollsurg.2009.01.015. [DOI] [PubMed] [Google Scholar]
- De Chiara G, Marcocci ME, Torcia M, Lucibello M, Rosini P, Bonini P, et al. Bcl-2 phosphorylation by p38 MAPK: identification of target sites and biologic consequences. J. Biol. Chem. 2006;281:21353–21361. doi: 10.1074/jbc.M511052200. [DOI] [PubMed] [Google Scholar]
- de Moura EG, Lisboa PC, Passos MC. Neonatal programming of neuroimmunomodulation—role of adipocytokines and neuropeptides. Neuroimmunomodulation. 2008;15:176–188. doi: 10.1159/000153422. [DOI] [PubMed] [Google Scholar]
- Degterev A, Huang Z, Boyce M, Li Y, Jagtap P, Mizushima N, et al. Chemical inhibitor of nonapoptotic cell death with therapeutic potential for ischemic brain injury. Nat. Chem. Biol. 2005;1:112–119. doi: 10.1038/nchembio711. [DOI] [PubMed] [Google Scholar]
- Deitch EA. Gut lymph and lymphatics: a source of factors leading to organ injury and dysfunction. Ann. N. Y. Acad. Sci. 2010;1207(Suppl. 1):E103–E111. doi: 10.1111/j.1749-6632.2010.05713.x. [DOI] [PubMed] [Google Scholar]
- Deitch EA, Xu D, Kaise VL. Role for the gut in the development of injury- and shock-induced SIRS and MODS: the gut-lymph hypothesis, a review. Front. Biosci. 2006;11:520–528. doi: 10.2741/1816. [DOI] [PubMed] [Google Scholar]
- Deng Y, Theken KN, Lee CR. Cytochrome P450 epoxygeneases, soluble hydrolase, and the regulation of cardiovascular inflammation. J. Mol. Cell. Cardiol. 2010;48:331–341. doi: 10.1016/j.yjmcc.2009.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Depre C, Park JY, Shen YT, Zhao X, Qiu H, Yan L, et al. Molecular mechanisms mediating preconditioning following chronic ischemia differ from those in classical second window. Am. J. Physiol. Heart Circ. Physiol. 2010;299:H752–H762. doi: 10.1152/ajpheart.00147.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Depre C, Vatner SF. Mechanisms of cell survival in myocardial hibernation. Trends Cardiovasc. Med. 2005;15:101–110. doi: 10.1016/j.tcm.2005.04.006. [DOI] [PubMed] [Google Scholar]
- Depre C, Vatner SF. Cardioprotection in stunned and hibernating myocardium. Heart Fail. Rev. 2007;12:307–317. doi: 10.1007/s10741-007-9040-3. [DOI] [PubMed] [Google Scholar]
- Devalaraja-Narashimha K, Diener AM, Padanilam BJ. Cyclophilin D gene ablation protects mice from ischemic renal injury. Am. J. Physiol. Renal Physiol. 2009;297:F749–F759. doi: 10.1152/ajprenal.00239.2009. [DOI] [PubMed] [Google Scholar]
- Devey L, Ferenbach D, Mohr E, Sangster K, Bellamy CO, Hughes J, et al. Tissue-resistant macrophages protect the liver from ischemia reperfusion injury via a heme oxygenase-1-dependent mechanism. Mol. Ther. 2009;17:65–72. doi: 10.1038/mt.2008.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Lisa F, Canton M, Menabó R, Kaludercic N, Bernardi P. Mitochondria and cardioprotection. Heart Fail. Rev. 2007;12:249–260. doi: 10.1007/s10741-007-9028-z. [DOI] [PubMed] [Google Scholar]
- Di Lisa F, Kaludercic N, Carpi A, Menabo R, Giorgio M. Mitochondria and vascular pathology. Pharmacol. Rep. 2009a;61:123–130. doi: 10.1016/s1734-1140(09)70014-3. [DOI] [PubMed] [Google Scholar]
- Di Lisa F, Kaludercic N, Carpi A, Menabó R, Giorgio M. Mitochondrial pathways for ROS formation and myocardial injury: the relevance of p66(Shc) and monoamine oxidase. Basic Res. Cardiol. 2009b;104:131–139. doi: 10.1007/s00395-009-0008-4. [DOI] [PubMed] [Google Scholar]
- Di Paola M, Lorusso M. Interaction of free fatty acids with mitochondria: coupling, uncoupling and permeability transition. Biochim. Biophys. Acta. 2006;1757:1330–1337. doi: 10.1016/j.bbabio.2006.03.024. [DOI] [PubMed] [Google Scholar]
- Dinagl U, Iadecola C, Moskowitz MA. Pathobiology of ischemic stroke: an integrated view. Trends Neurosci. 1999;22:391–397. doi: 10.1016/s0166-2236(99)01401-0. [DOI] [PubMed] [Google Scholar]
- Divald A, Kivity S, Wang P, Hochhauser E, Roberts B, Teichberg S, et al. Myocardial ischemic preconditioning preserves postischemic function of the 26S proteasome through diminished oxidative damage to 19S regulatory particle subunits. Circ. Res. 2010;106:1829–1838. doi: 10.1161/CIRCRESAHA.110.219485. [DOI] [PubMed] [Google Scholar]
- Diwan A, Krenz M, Syed FM, Wansapura J, Ren X, Koesters AG, et al. Inhibition of ischemic cardiomyocytes apoptosis through targeted ablation of Bnip3 restrains postinfarction remodeling in mice. J. Clin. Invest. 2007;117:2825–2833. doi: 10.1172/JCI32490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodd-o JM, Hristopoulos ML, Welsh-Servinsky LE, Tankersley CG, Pearse DB. Strain-specific differences in sensitivity to ischemia-reperfusion lung injury in mice. J. Appl. Physiol. 2006;100:1590–1595. doi: 10.1152/japplphysiol.00681.2005. [DOI] [PubMed] [Google Scholar]
- Donovan N, Becker EB, Konishi Y, Bonni A. JNK phosphorylation and activation of BAD couples the stress-activated signaling pathway to the cell death machinery. J. Biol. Chem. 2002;277:40944–40949. doi: 10.1074/jbc.M206113200. [DOI] [PubMed] [Google Scholar]
- Drews O, Wildgruber R, Zong C, Sukop U, Nissum M, Weber G, et al. Mammalian proteasome subpopulations with distinct molecular compositions and proteolytic activities. Mol. Cell. Proteomics. 2007;6:2021–2031. doi: 10.1074/mcp.M700187-MCP200. [DOI] [PubMed] [Google Scholar]
- Dreyer WJ, Michael LH, Nguyen T, Smith CW, Anderson DC, Entman ML, et al. Kinetics of C5a release in cardiac lymph of dogs experiencing coronary artery ischemia-reperfusion injury. Circ. Res. 1992;71:1518–1524. doi: 10.1161/01.res.71.6.1518. [DOI] [PubMed] [Google Scholar]
- Duffield JS, Forbes SJ, Constantinou CM, Clay S, Partolina M, Vuthoori S, et al. Selective depletion of macrophages reveals distinct, opposing roles during liver injury and repair. J. Clin. Invest. 2005;115:56–65. doi: 10.1172/JCI22675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dvorak AM. Basophils and mast cells: piecemeal degranulation in situ and ex vivo: a possible mechanism for cytokine-induced function in disease. Immunol. Ser. 1992;57:169–271. [PubMed] [Google Scholar]
- Dworakowski R, Walker S, Momin A, Desai J, El-Gamel A, Wendler O, et al. Reduced nicotinamide adenine dinucleotide phosphate oxidase-derived superoxide and vascular endothelial dysfunction in human heart failure. J. Am. Coll. Cardiol. 2008;51:1349–1356. doi: 10.1016/j.jacc.2007.12.033. [DOI] [PubMed] [Google Scholar]
- Edin ML, Wang Z, Bradbury JA, Graves JP, Lih FH, DeGraff LM, et al. Endothelial expression of human cytochrome P450 epoxygenase CYP2C8 increases susceptibility to ischemia-reperfusion injury in isolated mouse heart. FASEB J. 2011;25:3436–3447. doi: 10.1096/fj.11-188300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellett JD, Atkinson C, Evans ZP, Amani Z, Balish E, Schmid MG, et al. Murine Kupffer cells are protective in total hepatic ischemia/reperfusion injury with bowel congestion through IL-10. J. Immunol. 2010;184:5849–5858. doi: 10.4049/jimmunol.0902024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endres M, Meisel A, Biniszkiewicz D, Namura S, Prass K, Ruscher K, et al. DNA methyltransferase contributes to delayed ischemic brain injury. J. Neurosci. 2000;20:3175–3181. doi: 10.1523/JNEUROSCI.20-09-03175.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endres M, Fan G, Meisel A, Dirnagl U, Jaenisch R. Effects of cerebral ischemia in mice lacking DNA methyltransferase 1 in post-mitotic neurons. Neuroreport. 2001;12:3763–3766. doi: 10.1097/00001756-200112040-00032. [DOI] [PubMed] [Google Scholar]
- Esch JS, Jurk K, Knoefel WT, Roeder G, Voss H, Tustas RY, et al. Platelet activation and increased tissue factor expression on monocytes in reperfusion injury following orthotopic liver transplantation. Platelets. 2010;21:348–359. doi: 10.3109/09537101003739897. [DOI] [PubMed] [Google Scholar]
- Esme H, Fidan H, Koken T, Solak O. Effect of lung ischemia-reperfusion on oxidative stress parameters of remote tissues. Eur. J. Cardiothorac. Surg. 2006;29:294–298. doi: 10.1016/j.ejcts.2005.12.008. [DOI] [PubMed] [Google Scholar]
- Fan M, Du L, Stone AA, Gilbert KM, Chambers TC. Modulation of mitogen-activated protein kinases and phosphorylation of Bcl-2 by vinblastine represent persistent forms of normal fluctuations at G2-M1. Cancer Res. 2000;60:6403–6407. [PubMed] [Google Scholar]
- Fang J, Song XW, Tian J, Chen HY, Li DF, Wang JF, et al. Overexpression of microRNA-378 attenuates ischemia-induced apoptosis by inhibiting caspase-3 expression in cardiac myocytes. Apoptosis. 2012;17:410–423. doi: 10.1007/s10495-011-0683-0. [DOI] [PubMed] [Google Scholar]
- Faraco G, Pancani T, Formentini L, Mascagni P, Fossati G, Leoni F, et al. Pharmacological inhibition of histone deacetylases by suberoylanilide hydroxamic acid specifically alters gene expression and reduces ischemic injury in the mouse brain. Mol. Pharmacol. 2006;70:1876–1884. doi: 10.1124/mol.106.027912. [DOI] [PubMed] [Google Scholar]
- Fasanaro P, Greco S, Ivan M, Capogrossi MC, Martelli F. MicroRNA: emerging therapeutic targets in acute ischemic diseases. Pharmacol. Ther. 2010;125:92–104. doi: 10.1016/j.pharmthera.2009.10.003. [DOI] [PubMed] [Google Scholar]
- Ferdinandy P, Schulz R, Baxter GF. Interaction of cardiovascular risk factors with myocardial ischemia/reperfusion injury, preconditioning, and postconditioning. Pharmacol. Rev. 2007;59:418–458. doi: 10.1124/pr.107.06002. [DOI] [PubMed] [Google Scholar]
- Fernandez-Klett F, Offenhauser N, Dirnagl U, Priller J, Lindauer U. Pericytes in capillaries are contractile in vivo, but arterioles mediate functional hyperemia in the mouse brain. Proc. Natl. Acad. Sci. U.S.A. 2010;107:22290–22295. doi: 10.1073/pnas.1011321108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferraro FJ, Rush BF, Jr, Simonian GT, Bruce CJ, Murphy TF, Hsieh JT, et al. A comparison of survival at different degrees of hemorrhagic shock in germ-free and germ-bearing rats. Shock. 1995;4:117–120. doi: 10.1097/00024382-199508000-00007. [DOI] [PubMed] [Google Scholar]
- Festjens N, Vanden Berghe T, Cornelis S, Vandenabeele P. RIP1, a kinase on the crossroads of a cell’s decision to live or die. Cell Death Differ. 2007;14:400–410. doi: 10.1038/sj.cdd.4402085. [DOI] [PubMed] [Google Scholar]
- Frangogiannis NG. The immune system and cardiac repair. Pharmacol. Res. 2008;58:88–111. doi: 10.1016/j.phrs.2008.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frangogiannis NG, Smith CW, Entman ML. The inflammatory response in myocardial, infarction. Cardiovasc. Res. 2002;53:31–47. doi: 10.1016/s0008-6363(01)00434-5. [DOI] [PubMed] [Google Scholar]
- Fritzinger DC, Hew BE, Lee JQ, Newhouse J, Alam M, Ciallella JR, et al. Derivatives of human complement component C3 for therapeutic complement depletion: a novel class of therapeutic agents. Adv. Exp. Med. Biol. 2008;632:293–307. [PubMed] [Google Scholar]
- Frost RJ, van Rooij E. miRNAs as therapeutic targets in ischemic heart disease. J. Cardiovasc. Transl. Res. 2010;3:280–289. doi: 10.1007/s12265-010-9173-y. [DOI] [PubMed] [Google Scholar]
- Gaboury JP, Johnston B, Niu X, Kubes P. Mechanisms underlying acute mast cell-induced leukocyte rolling and adhesion in vivo. J. Immunol. 1995;154:804–813. [PubMed] [Google Scholar]
- Galinanes M, Hearse DJ. Species differences in susceptibility to ischemic injury and responsiveness to myocardial protection. Cardioscience. 1990;1:127–143. [PubMed] [Google Scholar]
- Galli SJ, Gordon JR, Wershil BK. Cytokine production by mast cells and basophils. Curr. Opin. Immunol. 1991;3:865–872. doi: 10.1016/s0952-7915(05)80005-6. [DOI] [PubMed] [Google Scholar]
- Galli SJ, Kalesnikoff J, Grimbaldeston A, Piliponsky A, Williams C, Tsai M. Mast cellsare “tunable” effector and immunoregulatory cells: recent advances. Annu. Rev. Immunol. 2005;23:749–786. doi: 10.1146/annurev.immunol.21.120601.141025. [DOI] [PubMed] [Google Scholar]
- Gandalfo MT, Jang HR, Bagnasco SM, Ko GJ, Agreda P, Satpute SR, et al. Foxp3+ regulatory T cells participate in repair of ischemic acute kidney injury. Kidney Int. 2009;76:717–729. doi: 10.1038/ki.2009.259. [DOI] [PubMed] [Google Scholar]
- Gao X, Zhang H, Schmidt AM, Zhang C. AGE/RAGE produces endothelial dysfunction in coronary arterioles in type 2 diabetic mice. Am. J. Physiol. Heart Circ. Physiol. 2008;295:H491–H498. doi: 10.1152/ajpheart.00464.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gawaz M. Role of platelets in coronary thrombosis and reperfusion of ischemic myocardium. Cardiovasc. Res. 2004;61:498–511. doi: 10.1016/j.cardiores.2003.11.036. [DOI] [PubMed] [Google Scholar]
- Genken E, Buis CI, Visser DS, Blokzijl H, Moshage H, Nemes B, et al. Expression of heme oxygenase-1 in human livers before transplantation correlates with graft injury and function after transplantation. Am. J. Transplant. 2005;5:1875–1885. doi: 10.1111/j.1600-6143.2005.00960.x. [DOI] [PubMed] [Google Scholar]
- George FD. Microparticles in vascular diseases. Thromb. Res. 2008;122:555–559. doi: 10.1016/S0049-3848(08)70020-3. [DOI] [PubMed] [Google Scholar]
- Gerszten RE, Asnani A, Carr SA. Status and prospects for discovery and verification of new biomarkers of cardiovascular disease by proteomics. Circ. Res. 2011;109:463–474. doi: 10.1161/CIRCRESAHA.110.225003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giakoustidis DE, Iliadis S, Tsantilas D, Papageorgiou G, Kontos N, Kostopoulou E, et al. Blockade of Kupffer cells by gadolinium chloride reduces lipid peroxidation and protects liver from ischemia/reperfusion injury. Hepatogastroenterology. 2003;50:1587–1592. [PubMed] [Google Scholar]
- Giedt RJ, Yang C, Zweier JL, Matzavinos A, Alevriadou BR. Mitochondrial fission in endothelial cells after simulated ischemia/reperfusion: role of nitric oxide and reactive oxygen species. Free Radic. Biol. Med. 2012;52:348–356. doi: 10.1016/j.freeradbiomed.2011.10.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Go YM, Jones DP. Redox compartmentalization in eukaryotic cells. Biochim. Biophys. Acta. 2008;1780:1273–1290. doi: 10.1016/j.bbagen.2008.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Go YM, Park H, Koval M, Orr M, Reed M, Liang Y, et al. A key role for mitochondria in endothelial signaling by plasma cysteine/cystine redox potential. Free Radic. Biol. Med. 2010;48:275–283. doi: 10.1016/j.freeradbiomed.2009.10.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goda N, Suzuki K, Naito M, Takeoka S, Tsuchida E, Ishimura Y, et al. Distribution of heme oxygenase isoforms in rat liver. Topographic basis for carbon monoxide-mediated microvascular relaxation. J. Clin. Invest. 1998;101:604–612. doi: 10.1172/JCI1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godoy LC, Moretti AI, Jurado MC, Oxer D, Janiszewski M, Ckless K, et al. Loss of CD40 endogenous S-nitrosylation during inflammatory response in endotoxemic mice and patients with sepsis. Shock. 2010;33:626–633. doi: 10.1097/SHK.0b013e3181cb88e6. [DOI] [PubMed] [Google Scholar]
- Godwin JG, Ge X, Stephan K, Jurisch A, Tullius SG, Iacomini J. Identification of a microRNA signature of renal ischemia reperfusion injury. Proc. Natl. Acad. Sci. U.S.A. 2010;107:14339–14344. doi: 10.1073/pnas.0912701107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman G, Welbourn R, Klausne JM, Kobzik L, Valeri CR, Shepro D, et al. Mast cells and leukotrienes mediate neutrophil sequestration and lung edema after remote ischemia in rodents. Surgery. 1992;12:578–586. [PubMed] [Google Scholar]
- Golwala NH, Hodenette C, Murthy SN, Nossaman BD, Kadowitz PJ. Vascular responses to nitrite are mediated by xanthine oxidoreductase and mitochondrial aldehyde dehydrogenase in the rat. Can. J. Physiol. Pharmacol. 2009;87:1095–1101. doi: 10.1139/Y09-101. [DOI] [PubMed] [Google Scholar]
- Gottlieb RA. Cytochrome P450: major player in reperfusion injury. Arch. Biochem. Biophys. 2003;420:262–267. doi: 10.1016/j.abb.2003.07.004. [DOI] [PubMed] [Google Scholar]
- Gottlieb RA, Mentzer RM. Autophagy during cardiac stress: joys and frustrations of autophagy. Annu. Rev. Physiol. 2010;72:45–59. doi: 10.1146/annurev-physiol-021909-135757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gourdin MJ, Bree B, De Kock M. The impact of ischaemia-reperfusion on the blood vessel. Eur. J. Anaesthesiol. 2009;26:537–547. doi: 10.1097/EJA.0b013e328324b7c2. [DOI] [PubMed] [Google Scholar]
- Granger DN. Role of xanthine oxidase and granulocytes in ischemia-reperfusion injury. Am. J. Physiol. 1988;255:H1269–H1275. doi: 10.1152/ajpheart.1988.255.6.H1269. [DOI] [PubMed] [Google Scholar]
- Granger DN. Ischemia-reperfusion: mechanisms of microvascular dysfunction and the influence of risk factors for cardiovascular disease. Microcirculation. 1999;6:167–178. [PubMed] [Google Scholar]
- Granger DN, Korthuis RJ. Physiologic mechanisms of postischemic tissue injury. Annu. Rev. Physiol. 1995;57:311–332. doi: 10.1146/annurev.ph.57.030195.001523. [DOI] [PubMed] [Google Scholar]
- Granger A, Abdullah I, Huebner F, Stout A, Wang T, Huebner T, et al. Histone deacetylase inhibition reduces myocardial ischemia-reperfusion injury in mice. FASEB J. 2008;22:3549–3560. doi: 10.1096/fj.08-108548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greco S, De Simone M, Colussi C, Zaccagnini G, Fasanaro P, Pescatori M, et al. Common micro-RNA signature in skeletal muscle damage and regeneration induced by Duchenne muscular dystrophy and acute ischemia. FASEB J. 2009;23:3335–3346. doi: 10.1096/fj.08-128579. [DOI] [PubMed] [Google Scholar]
- Grimbaldeston MA, Nakae S, Kalesnikoff J, Tsai M, Galli S. Mast cell-derived interleukin 10 limits skin pathology in contact dermatitis and chronic irradiation with ultraviolet B. Nat. Immunol. 2007;8:1095–1104. doi: 10.1038/ni1503. [DOI] [PubMed] [Google Scholar]
- Grisham MB, Jourd’Heuil D, Wink DA. Nitric oxide. I. Physiological chemistry of nitric oxide and its metabolites:implications in inflammation. Am. J. Physiol. 1999;276:G315–G321. doi: 10.1152/ajpgi.1999.276.2.G315. [DOI] [PubMed] [Google Scholar]
- Grover GJ, Atwal KS, Sleph PG, Wang FL, Monshizadegan H, Monticello T, et al. Excessive ATP hydrolysis in ischemic myocardium by mitochondrial F1F0-ATPase: effect of selective pharmacological inhibition of mitochondrial ATPase hydrolase activity. Am. J. Physiol. Heart Circ. Physiol. 2004;287:H1747–H1755. doi: 10.1152/ajpheart.01019.2003. [DOI] [PubMed] [Google Scholar]
- Grueter CE, Abiria SA, Dzhura I, Wu Y, Ham AJ, Mohler PJ, et al. L-type Ca2+ channel facilitation mediated by phosphorylation of the b subunit by CaMKII. Mol. Cell. 2006;23:641–650. doi: 10.1016/j.molcel.2006.07.006. [DOI] [PubMed] [Google Scholar]
- Gundewar S, Calvert JW, Elrod JW, Lefer DJ. Cytoprotective effects of N,N, N-trimethylsphingosine during ischemia- reperfusion injury are lost in the setting of obesity and diabetes. Am. J. Physiol. Heart Circ. Physiol. 2007;293:H2462–H2471. doi: 10.1152/ajpheart.00392.2007. [DOI] [PubMed] [Google Scholar]
- Guo G, Bhat NR. p38a MAP kinase mediates hypoxia-induced motor neuron cell death: a potential target of minocycline’s neuroprotective action. Neurochem. Res. 2007;32:2160–2166. doi: 10.1007/s11064-007-9408-8. [DOI] [PubMed] [Google Scholar]
- Gurusamy N, Goswami S, Malik G, Das DK. Oxidative injury induces selective rather than global inhibition of proteasomal activity. J. Mol. Cell. Cardiol. 2008;44:419–428. doi: 10.1016/j.yjmcc.2007.10.005. [DOI] [PubMed] [Google Scholar]
- Gute D, Korthuis RJ. Role of leukocyte adherence in reperfusion-induced micro-vascular dysfunction and tissue injury. In: Granger DN, Schmid-Schönbein GW, editors. Leukocyte Adhesion. New York, NY: Oxford University Press; 1995. [Google Scholar]
- Gute DC, Ishida T, Yarimizu K, Korthuis RJ. Inflammatory responses to ischemia and reperfusion in skeletal muscle. Mol. Cell. Biochem. 1998;179:169–187. doi: 10.1023/a:1006832207864. [DOI] [PubMed] [Google Scholar]
- Hacke W, Donnan G, Fieschi C, Kaste M, von Kummer R, Broderick JP, et al. Association of outcome with early stroke treatment: pooled analysis of ATLANTIS, ECASS, and NINDS rt-PA stroke trials. Lancet. 2004;363:768–774. doi: 10.1016/S0140-6736(04)15692-4. [DOI] [PubMed] [Google Scholar]
- Hales CN, Barker DJ. The thrifty phenotype hypothesis. Br. Med. Bull. 2001;60:5–20. doi: 10.1093/bmb/60.1.5. [DOI] [PubMed] [Google Scholar]
- Halestrap AP. What is the mitochondrial permeability transition pore? J. Mol. Cell. Cardiol. 2009;46:821–831. doi: 10.1016/j.yjmcc.2009.02.021. [DOI] [PubMed] [Google Scholar]
- Halestrap AP. A pore way to die: the role of mitochondria in reperfusion injury and cardioprotection. Biochem. Soc. Trans. 2010;38:841–860. doi: 10.1042/BST0380841. [DOI] [PubMed] [Google Scholar]
- Haller H, Kunzendorf U, Sacherer K, Lindschau C, Walz G, Distler A, et al. T cell adhesion to P-selectin induces tyrosine phosphorylation of pp 125 focal adhesion kinase and other substrates. J. Immunol. 1997;158:1061–1067. [PubMed] [Google Scholar]
- Hamilton NB, Attwell D, Hall CN. Pericyte-mediated regulation of capillary diameter: a component of neurovascular coupling in health and disease. Front. Neuroenergetics. 2010;2(5):1–14. doi: 10.3389/fnene.2010.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanidziar D, Koulmanda M. Inflammation and the balance of Treg and Th17 cells in transplant rejection and tolerance. Curr. Opin. Organ Transplant. 2010;15:411–415. doi: 10.1097/MOT.0b013e32833b7929. [DOI] [PubMed] [Google Scholar]
- Hanschen M, Zahler S, Krombach F, Khandoga A. Reciprocal activation between CD4+ T cells and Kupffer cells during hepatic ischemia-reperfusion. Transplantation. 2008;86:710–718. doi: 10.1097/TP.0b013e3181821aa7. [DOI] [PubMed] [Google Scholar]
- Harding SJ, Browne GJ, Miller BW, Prigent SA, Dickens M. Activation of ASK1, downstream MAPKK and MAPK isoforms during cardiac ischaemia. Biochim. Biophys. Acta. 2010;1802:733–740. doi: 10.1016/j.bbadis.2010.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He C, Klionsky DJ. Regulation mechanisms and signaling pathways of autophagy. Annu. Rev. Genet. 2009;43:67–93. doi: 10.1146/annurev-genet-102808-114910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He S, Wang L, Miao L, Wang T, Du F, Zhao L, et al. Receptor interacting protein kinase-3 determines cellular necrotic response to TNF-alpha. Cell. 2009;137:1100–1111. doi: 10.1016/j.cell.2009.05.021. [DOI] [PubMed] [Google Scholar]
- He B, Xiao J, Ren AJ, Zhang YF, Zhang H, Chen M, et al. Role of miR-1 and miR-133a in myocardial ischemic postconditioning., J. Biomed. Sci. 2011a;18:22. doi: 10.1186/1423-0127-18-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He GZ, Dong LG, Chen XF, Zhou KG, Shu H. Lymph duct ligation during ischemia/reperfusion prevents pulmonary dysfunction in a rat model with omega-3 polyunsaturated fatty acid and glutamine. Nutrition. 2011b;27:604–614. doi: 10.1016/j.nut.2010.06.003. [DOI] [PubMed] [Google Scholar]
- Hein TW, Zhang C, Wang W, Chang CI, Thengchaisri N, Kuo L. Ischemia-reperfusion selectively impairs nitric oxide-mediated dilation in coronary arterioles: counteracting role of arginase. FASEB J. 2003;17:2328–2330. doi: 10.1096/fj.03-0115fje. [DOI] [PubMed] [Google Scholar]
- Hernando V, Inserte J, Sartório CL, Parra VM, Poncelas-Nozal M, Garcia-Dorado D. Calpain translocation and activation as pharmacological targets during myocardial ischemia/reperfusion. J. Mol. Cell. Cardiol. 2010;49:271–279. doi: 10.1016/j.yjmcc.2010.02.024. [DOI] [PubMed] [Google Scholar]
- Hill JH, Ward PA. The phlogistic role of C3 leukotactic fragments in myocardial infarcts of rats. J. Exp. Med. 1971;133:885–900. doi: 10.1084/jem.133.4.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch J, Niemann CU, Hansen KC, Choi SJN, Su X, Frank JA, et al. Alterations in the proteome of pulmonary alveolar type II cells in the rat after hepatic ishchemia-reperfusion. Crit. Care Med. 2008;36:1846–1854. doi: 10.1097/CCM.0b013e31816f49cb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoda MN, Singh I, Singh AK, Khan M. Reduction of lipoxidative load by secretory phospholipase A2 inhibition protects against neurovascular injury following experimental stroke in rat. J. Neuroinflammation. 2009;6:21. doi: 10.1186/1742-2094-6-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holler N, Zaru R, Micheau O, Thome M, Attinger A, Valitutti S, et al. Fas triggers an alternative, caspase-8-independent cell death pathway using the kinase RIP as effector molecule. Nat. Immunol. 2000;1:489–495. doi: 10.1038/82732. [DOI] [PubMed] [Google Scholar]
- Horie Y, Ishii H. Liver dysfunction elicited by gut ischemia-reperfusion. Pathophysiology. 2001;8:11–20. doi: 10.1016/s0928-4680(01)00063-3. [DOI] [PubMed] [Google Scholar]
- Horie Y, Wolf R, Chervenak RP, Jennings SR, Granger DN. T-lymphocytes contribute to hepatic leukostasis and hypoxic stress induced by gut ischemia-reperfusion. Microcirculation. 1999;6:267–280. [PubMed] [Google Scholar]
- Horie Y, Wolf R, Russell J, Shanley TP, Granger DN. Role of Kupffer cells in gut ischemia/reperfusion-induced hepatic microvascular dysfunction in mice. Hepatology. 1997;26:1499–1505. doi: 10.1002/hep.510260617. [DOI] [PubMed] [Google Scholar]
- Horstman LL, Jy W, Bidot CJ, Nordberg ML, Minagar A, Alexander JS, et al. Potential roles of cell-derived microparticles in ischemic brain disease. Neurol. Res. 2009;31:799–806. doi: 10.1179/016164109X12445505689526. [DOI] [PubMed] [Google Scholar]
- Hu CJ, Chen SD, Yang DI, Lin TN, Chen CM, Huang TH, et al. Promoter region methylation and reduced expression of thrombospondin-1 after oxygen-glucose deprivation in murine cerebral endothelial cells. J. Cereb. Blood Flow Metab. 2006;26:1519–1526. doi: 10.1038/sj.jcbfm.9600304. [DOI] [PubMed] [Google Scholar]
- Hullinger TG, Montgomery RL, Seto AG, Dickinson BA, Semus HM, Lynch JM, et al. Inhibition of miR-15 protects against cardiac ischemic injury. Circ. Res. 2012;110:71–81. doi: 10.1161/CIRCRESAHA.111.244442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulsmans M, De Keyzer D, Holvoet P. MicroRNAs regulating oxidative stress and inflammation in relation to obesity and atherosclerosis. FASEB J. 2011;25:2515–2527. doi: 10.1096/fj.11-181149. [DOI] [PubMed] [Google Scholar]
- Humphreys MR, Castle EP, Lohse CM, Sebo TJ, Leslie KO, Andrews PE. Renal ischemia time in laparoscopic surgery: an experimental study in a porcine model. Int. J. Urol. 2009;16:105–109. doi: 10.1111/j.1442-2042.2008.02173.x. [DOI] [PubMed] [Google Scholar]
- Ikeda H, Suzuki Y, Suzuki M, Koike M, Tamura J, Tong J, et al. Apoptosis is a major mode of cell death caused by ischaemia and ischaemia/reperfusion injury to the rat intestinal epithelium. Gut. 1998;42:530–537. doi: 10.1136/gut.42.4.530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inagaki K, Chen L, Ikeno F, Lee FH, Imahashi K, Bouley DM, et al. Inhibition of delta-protein kinase C protects against reperfusion injury of the ischemic heart in vivo. Circulation. 2003;108:2304–2307. doi: 10.1161/01.CIR.0000101682.24138.36. [DOI] [PubMed] [Google Scholar]
- Ioannou A, Dalle Lucca J, Tsokos GC. Immunopathogenesis of ischemia/ reperfusion-associated tissue damage. Clin. Immunol. 2011;141:3–14. doi: 10.1016/j.clim.2011.07.001. [DOI] [PubMed] [Google Scholar]
- Ishikawa M, Cooper D, Arumugam TV, Zhang JH, Nanda A, Granger DN. Platelet-leukocyte-endothelial cell interactions after middle cerebral artery occlusion and reperfusion. J. Cereb. Blood Flow Metab. 2004;24:907–915. doi: 10.1097/01.WCB.0000132690.96836.7F. [DOI] [PubMed] [Google Scholar]
- Jaeschke H, Farhood A, Smith CW. Neutrophils contribute to ischemiareperfusion injury in rat liver in vivo. FASEB J. 1990;4:3355–3359. [PubMed] [Google Scholar]
- Jaeschke H, Bautista AP, Spolarics Z, Spitzer JJ. Superoxide generation by neutrophils and Kupffer cells during in vivo reperfusion after hepatic ischemia in rats. J. Leukoc. Biol. 1992;52:377–382. doi: 10.1002/jlb.52.4.377. [DOI] [PubMed] [Google Scholar]
- Jaeschke H, Bautista AP, Spolarics Z, Farhood A, Spitzer JJ. Proceedings of the 5th International Congress on Oxygen Radicals. Amsterdam: Elsevier Science Publishers; 1993. Enhanced generation of superoxide by complement-stimulated Kupffer cells and priming of neutrophils during the initial reperfusion phase after hepatic ischemia. [Google Scholar]
- Jennings RB, Reimer KA. The cell biology of acute myocardial ischemia. Annu. Rev. Med. 1991;42:225–246. doi: 10.1146/annurev.me.42.020191.001301. [DOI] [PubMed] [Google Scholar]
- Jennings RB, Sommers HM, Smyth GA, Flack HA, Linn H. Myocardial necrosis induced by temporary occlusion of a coronary artery in the dog. Arch. Pathol. 1960;70:68–78. [PubMed] [Google Scholar]
- Jeyaseelan K, Lim KY, Armugam A. MicroRNA expression in the blood and brain of rats subjected to transient focal ischemia by middle cerebral artery occlusion. Stroke. 2008;39:959–966. doi: 10.1161/STROKEAHA.107.500736. [DOI] [PubMed] [Google Scholar]
- Ji X, Luo Y, Ling F, Stetler RA, Lan J, Cao G, et al. Mild hypothermia diminishes oxidative DNA damage and pro-death signaling events after cerebral ischemia: a mechanism for neuroprotection. Front. Biosci. 2007;12:1737–1747. doi: 10.2741/2185. [DOI] [PubMed] [Google Scholar]
- Jiang M, Liu K, Luo J, Dong Z. Autophagy is a renoprotective mechanism during in vitro hypoxia and in vivo ischemia-reperfusion injury. Am. J. Pathol. 2010;176:1181–1192. doi: 10.2353/ajpath.2010.090594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang M, Zhang Y, Dusting GJ. NADPH oxidase-mediated redox signaling: roles in cellular stress response, stress tolerance, and tissue repair. Pharmacol. Rev. 2011;63:218–242. doi: 10.1124/pr.110.002980. [DOI] [PubMed] [Google Scholar]
- Jin Y, Silverman AJ, Vannucci SJ. Mast cell stabilization limits hypoxic-ischemic brain damage in the immature rat. Dev. Neurosci. 2007a;9:373–384. doi: 10.1159/000105478. [DOI] [PubMed] [Google Scholar]
- Jin Y, Silverman AJ, Vannucci SJ. Mast cells are early responders after hypoxiaischemia in immature rat brain. Stroke. 2009;40:3107–3112. doi: 10.1161/STROKEAHA.109.549691. [DOI] [PubMed] [Google Scholar]
- Jones AW, Durante W, Korthuis RJ. Heme oxygenase-1 deficiency leads to alteration of soluble guanylate cylcase redox regulation. J. Pharmacol. Exp. Ther. 2010;335:85–91. doi: 10.1124/jpet.110.169755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jong HR, Ko GJ, Wasowska BA, Rabb H. The interaction between ischemiareperfusion and immune responses in the kidney. J. Mol. Med. 2009;87:859–864. doi: 10.1007/s00109-009-0491-y. [DOI] [PubMed] [Google Scholar]
- Julia PL, Kofsky ER, Buckberg GD, Young HH, Bugyi HI. Studies of myocardial protection in the immature heart. I. Enhanced tolerance of immature versus adult myocardium to global ischemia with reference to metabolic differences. J. Thorac. Cardiovasc. Surg. 1990;100:879–887. [PubMed] [Google Scholar]
- Kahle KT, Simard JM, Staley KJ, Nahed BV, Jones PS, Sun D. Molecular mechanisms of ischemic cerebral edema: role of electroneutral ion transport. Physiology (Bethesda) 2009;24:257–265. doi: 10.1152/physiol.00015.2009. [DOI] [PubMed] [Google Scholar]
- Kaiser RA, Bueno OF, Lips DJ, Doevendans PA, Jones F, Kimball TF, et al. Targeted inhibition of p38 mitogen-activated protein kinase antagonizes cardiac injury and cell death following ischemia-reperfusion in vivo. J. Biol. Chem. 2004;279:15524–15530. doi: 10.1074/jbc.M313717200. [DOI] [PubMed] [Google Scholar]
- Kaiser RA, Liang Q, Bueno O, Huang Y, Lackey T, Klevitsky R, et al. Genetic inhibition or activation of JNK1/2 protects the myocardium from ischemiareperfusion-induced cell death in vivo. J. Biol. Chem. 2005;280:32602–32608. doi: 10.1074/jbc.M500684200. [DOI] [PubMed] [Google Scholar]
- Kaludercic N, Takimoto E, Nagayama T, Lai E, Chen K, Shih JC, et al. Lack of monoamine oxidase A and B activity prevents heart failure in pressure-overloaded mice. J. Mol. Cell. Cardiol. 2010;48:pS13. [Google Scholar]
- Karp CL. Links between innate and adaptive immunity. In: Serhan CN, Ward PA, Gilroy DW, editors. Fundamentals of Inflammation. New York, NY,: Cambridge University Press; 2010. pp. 28–37. [Google Scholar]
- Karra L, Berent-Maoz B, Ben-Zaimra M, Levi-Schaffer F. Are we ready to downregulate mast cells? Curr. Opin. Immunol. 2009;21:708–714. doi: 10.1016/j.coi.2009.09.010. [DOI] [PubMed] [Google Scholar]
- Kassahun WT, Schulz T, Richter O, Hauss J. Unchanged high mortality rates from acute occlusive intestinal ischemia: six year review. Langenbecks Arch. Surg. 2008;393:163–171. doi: 10.1007/s00423-007-0263-5. [DOI] [PubMed] [Google Scholar]
- Kelton JG, Warkentin TE, Hayward CP, Murphy WG, Moore JC. Calpain activity in patients with thrombotic thrombocytopenic purpura is associated with platelet microparticles. Blood. 1992;80:2246–2251. [PubMed] [Google Scholar]
- Khalil PN, Neuhof C, Huss R, Pollhammer M, Khalil MN, Neuhof H, et al. Calpain inhibition reduces infarct size and improves global hemodynamics and left ventricular contractility in a porcine myocardial ischemia/reperfusion model. Eur. J. Pharmacol. 2005;528:124–131. doi: 10.1016/j.ejphar.2005.10.032. [DOI] [PubMed] [Google Scholar]
- Khandoga A, Biberthaler P, Enders G, Axmann S, Hatter J, Messmer K, et al. Platelet adhesion mediated by fibrinogen-intercellular adhesion molecule-1 binding induces tissue injury in the postischemic liver in vivo. Tansplantation. 2002;74:681–688. doi: 10.1097/00007890-200209150-00016. [DOI] [PubMed] [Google Scholar]
- Khandoga A, Hanschen M, Kessler JS, Kronbach F. CD4+ T cells contribute to postischemic liver injury in mice by interacting with sinusoidal endothelium and platelets. Hepatology. 2006;43:306–315. doi: 10.1002/hep.21017. [DOI] [PubMed] [Google Scholar]
- Kim HJ, Rowe M, Ren M, Hong JS, Chen PS, Chuang DM. Histone deacetylase inhibitors exhibit anti-inflammatory and neuroprotective effects in a rat permanent ischemic model of stroke: multiple mechanisms of action. J. Pharmacol. Exp. Ther. 2007;321:892–901. doi: 10.1124/jpet.107.120188. [DOI] [PubMed] [Google Scholar]
- Kim J, Kim DS, Park MJ, Cho HJ, Zervos AS, Bonventre JV, et al. Omi/ HtrA2 protease is associated with tubular cell apoptosis and fibrosis induced by unilateral ureteral obstruction. Am. J. Physiol. Renal Physiol. 2010;298:F1332–F1340. doi: 10.1152/ajprenal.00737.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King LA, Toledo AH, Rivera-Chavez FA, Toledo-Pereyra LH. Role of p38 and JNK in liver ischemia and reperfusion. J. Hepatobiliary Pancreat.Surg. 2009;16(6):763–770. doi: 10.1007/s00534-009-0155-x. [DOI] [PubMed] [Google Scholar]
- Kinross J, Warren O, Basson S, Holmes E, Silk D, Darzi A, et al. Intestinal ischemia/reperfusion injury: defining the role of the gut microbiome. Biomark. Med. 2009;3:175–192. doi: 10.2217/bmm.09.11. [DOI] [PubMed] [Google Scholar]
- Kinross JM, Darzi AW, Nicholson JK. Gut microbiome-host interactions in health and disease. Genome Med. 2011;3:14–000. doi: 10.1186/gm228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinsey GR, Sharma R, Huang L, Li L, Vergis AL, Ye H, et al. Regulatory T cells suppress innate immunity in kidney ischemia-reperfusion injury. J. Am. Soc. Nephrol. 2009;20:1744–1753. doi: 10.1681/ASN.2008111160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinsey GR, Huang L, Vergis AL, Li L, Okusa MD. Regulatory T cells contribute to the protective effect of ischemic preconditioning in the kidney. Kidney Int. 2010;77:771–780. doi: 10.1038/ki.2010.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiray M, Bagriyanik HA, Pekcetin C, Ergur BU, Uysal N. Protective effects of deprenyl in transient cerebral ischemia in rats. Chin. J. Physiol. 2008;51:275–281. [PubMed] [Google Scholar]
- Kobayashi A, Imamura H, Isobe M, Matsuyama Y, Soeda J, Matsunaga K, et al. Mac-1 (CD11b/CD18) and intercellular adhesion molecule-1 in ischemiareperfusion injury of rat liver. Am. J. Physiol. Gastrointest. Liver Physiol. 2001;281:G577–G585. doi: 10.1152/ajpgi.2001.281.2.G577. [DOI] [PubMed] [Google Scholar]
- Kohda Y, Gemba M. Cephaloridine induces translocation of protein kinase C delta into mitochondria and enhances mitochondrial generation of free radicals in the kidney cortex of rats causing renal dysfunction. J. Pharmacol. Sci. 2005;98:49–57. doi: 10.1254/jphs.fp0040926. [DOI] [PubMed] [Google Scholar]
- Kohli V, Madden JF, Bentley RC, Clavien PA. Calpain mediates ischemic injury of the liver through modulation of apoptosis and necrosis. Gastroenterology. 1999;116:168–178. doi: 10.1016/s0016-5085(99)70241-6. [DOI] [PubMed] [Google Scholar]
- Kokura S, Wolf RE, Yoshikawa T, Ichikawa H, Granger DN, Aw TY. Endothelial cells exposed to anoxia/reoxygenation are hyperadhesive to T-lymphocytes: kinetics and molecular mechanisms. Microcirculation. 2000;7:13–23. [PubMed] [Google Scholar]
- Koponen S, Goldsteins G, Keinänen R, Koistinaho J. Induction of protein kinase Cd subspecies in neurons and microglia after transient global brain ischemia. J. Cereb. Blood Flow Metab. 2000;20:93–102. doi: 10.1097/00004647-200001000-00013. [DOI] [PubMed] [Google Scholar]
- Kristián T. Metabolic stages, mitochondria and calcium in hypoxic/ischemic brain damage. Cell Calcium. 2004;36:221–233. doi: 10.1016/j.ceca.2004.02.016. [DOI] [PubMed] [Google Scholar]
- Kristiansen M, Graverson JH, Jacobsen C, Sonne O, Hoffman HJ, Law SK, et al. Identification of the haemoglobin scavenger receptor. Nature. 2001;409:198–201. doi: 10.1038/35051594. [DOI] [PubMed] [Google Scholar]
- Kroemer G, Galluzzi L, Brenner C. Mitochondrial membrane permeabilization in cell death. Physiol. Rev. 2007;87:99–163. doi: 10.1152/physrev.00013.2006. [DOI] [PubMed] [Google Scholar]
- Kroemer G, Galluzzi L, Vandenabeele P, Abrams J, Alnemri ES, Baehrecke EH, et al. Classification of cell death: recommendations of the Nomenclature Committee on Cell Death 2009. Cell Death Differ. 2009;16:3–11. doi: 10.1038/cdd.2008.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krutzfeldt J, Rajewsky N, Braich R, Rajeev KG, Tuschl T, Manoharan M, et al. Silencing of microRNAs in vivo with ‘antagomirs’. Nature. 2005;438:685–689. doi: 10.1038/nature04303. [DOI] [PubMed] [Google Scholar]
- Kuan CY, Whitmarsh AJ, Yang DD, Liao G, Schloemer AJ, Dong C, et al. A critical role of neural-specific JNK3 for ischemic apoptosis. Proc. Natl. Acad. Sci. U.S.A. 2003;100:15184–15189. doi: 10.1073/pnas.2336254100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubes P, Suzuki M, Granger DN. Nitric oxide: an endogenous modulator of leukocyte adhesion. Proc. Natl. Acad. Sci. U.S.A. 1991;88:4651–4655. doi: 10.1073/pnas.88.11.4651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuboki S, Sakai N, Tschöp J, Edwards MJ, Lentsch AB, Caldwell CC. Distinct contributions of CD4+ T cell subsets in hepatic ischemia/reperfusion injury. Am. J. Physiol. 2009;296:G1054–G1059. doi: 10.1152/ajpgi.90464.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kukreja RC, Yin C, Salloum FN. MicroRNAs: new players in cardiac injury and protection. Mol. Pharmacol. 2011;80:558–564. doi: 10.1124/mol.111.073528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar P, Shen Q, Pivetti CD, Lee ES, Wu MH, Yuan SY. Molecular mechanisms of endothelial hyperpermeability: implications in inflammation. Expert Rev. Mol. Med. 2009;11:e19. doi: 10.1017/S1462399409001112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kvietys PR, Granger DN. Role of reactive oxygen and nitrogen species in the vascular responses to inflammation. Free Radic. Biol. Med. 2012;52:556–592. doi: 10.1016/j.freeradbiomed.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam FW, Burns AR, Smith CW, Rumbaut RE. Platelets enhance neutrophil transendothelial migration via P-selectin glycoprotein ligand-1. Am. J. Physiol. Heart Circ. Physiol. 2011;300:H468–H475. doi: 10.1152/ajpheart.00491.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam V, Su J, Koprowski S, Hsu A, Tweddell JS, Rafiee P, et al. Intestinal microbiota determine severity of myocardial infarction in rats. FASEB J. 2012;26:1727–1735. doi: 10.1096/fj.11-197921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langley-Evans SC, McMullen S. Developmental origins of adult disease. Med. Princ. Pract. 2010;19:87–98. doi: 10.1159/000273066. [DOI] [PubMed] [Google Scholar]
- Lappas CM, Day YJ, Marshall MA, Engelhardt VH, Linden J. Adenosine A2A receptor activation reduces hepatic ischemia reperfusion injury by inhibiting CD1d-dependent NKT activation. J. Exp. Med. 2006;203:2639–2648. doi: 10.1084/jem.20061097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LATE_Study_Group. Late Assessment of Thrombolytic Efficacy (LATE) study with alteplase 6–24 hours after onset of acute myocardial infarction. Lancet. 1993;342:759–766. [PubMed] [Google Scholar]
- Lazarus B, Messina A, Barker JE, Hurley JV, Romeo R, Morrison WA, et al. The role of mast cells in schaemia-reperfusion injury in murine skeletal muscle. J. Pathol. 2000;191:443–448. doi: 10.1002/1096-9896(2000)9999:9999<::AID-PATH666>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Le DA, Wu Y, Huang Z, Matsushita K, Plesnila N, Augustinack JC, et al. Caspase activation and neuroprotection in caspase-3- deficient mice after in vivo cerebral ischemia and in vitro oxygen glucose deprivation. Proc. Natl. Acad. Sci. U.S.A. 2002;99:15188–15193. doi: 10.1073/pnas.232473399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JM, Grabb MC, Zipfel GJ, Choi DW. Brain tissue responses to ischemia. J. Clin. Invest. 2000;106:723–731. doi: 10.1172/JCI11003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KH, Kim SE, Lee YS. SP600125, a selective JNK inhibitor, aggravates hepatic ischemia-reperfusion injury. Exp. Mol. Med. 2006;38:408–416. doi: 10.1038/emm.2006.48. [DOI] [PubMed] [Google Scholar]
- Lee H, Green D, Lai L, Hou YJ, Jensenius JC, Liu D, et al. Early complement factors in the local tissue immunocomplex generated during intestinal ischemia/reperfusion injury. Mol. Immunol. 2010a;47:972–981. doi: 10.1016/j.molimm.2009.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HA, Hong SH, Kim JW, Jang IS. Possible involvement of DNA methylation in NKCC1 gene expression during postnatal development and in response to ischemia. J. Neurochem. 2010b;114:520–529. doi: 10.1111/j.1471-4159.2010.06772.x. [DOI] [PubMed] [Google Scholar]
- Lee H-L, Chen C-L, Yeh ST, Zweier JL, Chen Y-R. Biphasic modulation of the mitochondrial electron transport chain in myocardial ischemia and reperfusion. Am. J. Physiol. 2012;302:H1410–H1422. doi: 10.1152/ajpheart.00731.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei K, Davis RJ. JNK phosphorylation of Bim-related members of the Bcl2 family induces Bax-dependent apoptosis. Proc. Natl. Acad. Sci. U.S.A. 2003;100:2432–2437. doi: 10.1073/pnas.0438011100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemay S, Rabb H, Postler G, Singh AK. Prominent and sustained up-regulation of gp130-signaling cytokines and the chemokine MIP-2 in murine renal ischemiareperfusion injury. Transplantation. 2000;69:959–963. doi: 10.1097/00007890-200003150-00049. [DOI] [PubMed] [Google Scholar]
- Leroyer AS, Anfosso F, Lacroix R, Sabatier F, Simoncini S, Njock SM, et al. Endothelial-derived microparticles: biological conveyors at the crossroad of inflammation, thrombosis and angiogenesis. Thromb. Haemost. 2010;104:456–463. doi: 10.1160/TH10-02-0111. [DOI] [PubMed] [Google Scholar]
- Levine B, Kroemer G. Autophagy in the pathogenesis of disease. Cell. 2008;132:27–42. doi: 10.1016/j.cell.2007.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewen A, Matz P, Chan PH. Free radical pathways in CNS injury. J. Neurotrauma. 2000;17:871–890. doi: 10.1089/neu.2000.17.871. [DOI] [PubMed] [Google Scholar]
- Li R, Ding T, Liu X, Li C. Influence of SB203580 on cell apoptosis and P38MAPK in renal ischemia/reperfusion injury. J. Huazhong Univ. Sci. Technolog. Med. Sci. 2006;26:50–52. doi: 10.1007/BF02828037. [DOI] [PubMed] [Google Scholar]
- Li JY, Gu X, Zhang WH, Jia S, Zhou Y. GdCl3 abates hepatic ischemiareperfusion injury by inhibiting apoptosis in rats. Hepatobiliary Panceat. Dis. Int. 2009;8:518–523. [PubMed] [Google Scholar]
- Liao L, Harris NR, Granger DN. Oxidized low-density lipoproteins and microvascular responses to ischemia-reperfusion. Am. J. Physiol. 1996;271:H2508–H2514. doi: 10.1152/ajpheart.1996.271.6.H2508. [DOI] [PubMed] [Google Scholar]
- Liesz AL, Suri-Payer E, Veltkamp C, Doerr H, Sommer C, Rivest S, et al. Regulatory T cells are key cerebroprotective immunomodulators in acute experimental stroke. Nat. Med. 2009;15:192–199. doi: 10.1038/nm.1927. [DOI] [PubMed] [Google Scholar]
- Lima B, Forrester MT, Hess DT, Stamler JS. S-nitrosylation in cardiovascular signaling. Circ. Res. 2010;106:633–646. doi: 10.1161/CIRCRESAHA.109.207381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindemann S, Klingel B, Fisch A, Meyer J, Darius H. Increased platelet sensitivity toward platelet inhibitors during physical exercise in patients with coronary artery disease. Thromb. Res. 1999;93:51–59. doi: 10.1016/s0049-3848(98)00155-8. [DOI] [PubMed] [Google Scholar]
- Linfert D, Austen WG, Jr, Rabb H. Lymphocytes and ischemia-reperfusion injury. Transplant. Rev. 2009;23:1–10. doi: 10.1016/j.trre.2008.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Kubes P. Molecular mechanisms of leukocyte recruitment: organ-specific mechanisms of action. Thromb. Haemost. 2003;89:213–220. [PubMed] [Google Scholar]
- Liu P, McGuire PM, Fisher MA, Farhood A, Smith CW, Jaeschke H. Activation of Kupffer cells and neutrophils for reactive oxygen formation is responsible for endotoxin-enhanced liver injury after hepatic ischemia. Shock. 1995;3:56–62. [PubMed] [Google Scholar]
- Liu M, Chien CC, Grigoryev DN, Gandalfo MT, Colvin RB, Rabb H. Effect of T cells on vascular permeability in early ischemic acute kidney injury in mice. Microvasc. Res. 2009;77:340–347. doi: 10.1016/j.mvr.2009.01.011. [DOI] [PubMed] [Google Scholar]
- Loi P, Paular F, Pajak B, Nagy N, Salmon I, Moser M, et al. The fate of dendritic cells in a mouse model of liver ischemia/reperfusion injury. Transplant. Proc. 2004;36:1275–1279. doi: 10.1016/j.transproceed.2004.05.052. [DOI] [PubMed] [Google Scholar]
- Lucchesi BR, Kilgore KS. Complement inhibitors in myocardial ischemia/reperfusion injury. Immunopharmacology. 1997;38:27–42. doi: 10.1016/s0162-3109(97)00060-x. [DOI] [PubMed] [Google Scholar]
- Ma X, Becker Buscaglia LE, Barker JR, Li Y. MicroRNAs in NF-kappaB signaling. J. Mol. Cell Biol. 2011;3:159–166. doi: 10.1093/jmcb/mjr007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maekawa A, Lee JK, Nagaya T, Kamiya K, Yasui K, Horiba M, et al. Overexpression of calpastatin by gene transfer prevents troponin I degradation and ameliorates contractile dysfunction in rat hearts subjected to ischemia/reperfusion. J. Mol. Cell. Cardiol. 2003;35:1277–1284. doi: 10.1016/s0022-2828(03)00238-4. [DOI] [PubMed] [Google Scholar]
- Mao XR, Crowder CM. Protein misfolding induces hypoxic preconditioning via a subset of the unfolded protein response machinery. Mol. Cell. Biol. 2010;30:5033–5042. doi: 10.1128/MCB.00922-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin M, Mory C, Piescher A, Wittekind C, Fiedler M, Uhlmann D. Protective effects of early CD4+ T cell reduction in hepatic ischemia/reperfusion injury. J. Gastrointest. Surg. 2010;14:511–519. doi: 10.1007/s11605-009-1104-3. [DOI] [PubMed] [Google Scholar]
- Martindale JJ, Fernandez R, Thuerauf D, Whittaker R, Gude N, Sussman MA, et al. Endoplasmic reticulum stress gene induction and protection from ischemia/ reperfusion injury in the hearts of transgenic mice with a tamoxifen-regulated form of ATF6. Circ. Res. 2006;98:1186–1193. doi: 10.1161/01.RES.0000220643.65941.8d. [DOI] [PubMed] [Google Scholar]
- Marzocco S, Di Paola R, Autore G, Mazzon E, Pinto A, Caputi AP, et al. Calpain inhibitor I reduces intestinal ischemia-reperfusion injury in the rat. Shock. 2004;21:38–44. doi: 10.1097/01.shk.0000095056.62263.b2. [DOI] [PubMed] [Google Scholar]
- Massberg S, Enders G, Matos FC, Tomic LI, Leiderer R, Eisenmenger S, et al. Fibrinogen deposition at the postischemic vessel wall promotes platelet adhesion during ischemia-reperfusion in vivo. Blood. 1999;94:3829–3838. [PubMed] [Google Scholar]
- Matzinger P. The danger model: a renewed sense of self. Science. 2002;296:301–305. doi: 10.1126/science.1071059. [DOI] [PubMed] [Google Scholar]
- Maurer M, Wedemeyer J, Metz M, Piliponsky AM, Weller K, Chatterjea D, et al. Mast cells promote homeostasis by limiting endothelin-1-induced toxicity. Nature. 2004;432:512–516. doi: 10.1038/nature03085. [DOI] [PubMed] [Google Scholar]
- Mause SF, Weber C. Microparticles: protagonists of a novel communication network for intercellular information exchange. Circ. Res. 2010;107:1047–1057. doi: 10.1161/CIRCRESAHA.110.226456. [DOI] [PubMed] [Google Scholar]
- McCall CE, El Gazzar M, Liu T, Vachharajani V, Yoza B. Epigenetics, bioenergetics, and microRNA coordinate gene-specific reprogramming during acute systemic inflammation. J. Leukoc. Biol. 2011;90:439–446. doi: 10.1189/jlb.0211075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald B, Pittman K, Menezes GB, Hirota SA, Slaba I, Waterhouse CCM, et al. Intravascular danger signals guide neutrophils to sites of sterile inflammation. Science. 2010;330:362–366. doi: 10.1126/science.1195491. [DOI] [PubMed] [Google Scholar]
- McDougal WS. Renal perfusion/reperfusion injuries. J. Urol. 1988;140:1325–1330. doi: 10.1016/s0022-5347(17)42037-4. [DOI] [PubMed] [Google Scholar]
- Mehta D, Malik AB. Signaling mechanisms regulating endothelial permeability. Physiol. Rev. 2006;86:279–367. doi: 10.1152/physrev.00012.2005. [DOI] [PubMed] [Google Scholar]
- Menini S, Amadio L, Oddi G, Ricci C, Pesci C, Pugliese F, et al. Deletion of p66Shc longevity gene protects against experimental diabetic glomerulopathy by preventing diabetes-induced oxidative stress. Diabetes. 2006;55:1642–1650. doi: 10.2337/db05-1477. [DOI] [PubMed] [Google Scholar]
- Meszaros AJ, Reichner JS, Albina JE. Macrophage-induced neutrophil apoptosis. J. Immunol. 2000;165:435–441. doi: 10.4049/jimmunol.165.1.435. [DOI] [PubMed] [Google Scholar]
- Metukuri MR, Beer-Stolz D, Namas RA, Dhupar R, Torres A, Loughran PA, et al. Expression and subcellular localization of BNIP3 in hypoxic hepatocytes and liver stress. Am. J. Physiol. Gastrointest. Liver Physiol. 2009;296:G499–G509. doi: 10.1152/ajpgi.90526.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metz M, Maurer M. Mast cells–key effector cells in immune responses. Trends Immunol. 2007;28:234–241. doi: 10.1016/j.it.2007.03.003. [DOI] [PubMed] [Google Scholar]
- Meyer K, Zhang H, Zhang L. Direct effect of cocaine on epigenetic regulation of PKCe gene repression in the fetal rat heart. J. Mol. Cell. Cardiol. 2009;47:504–511. doi: 10.1016/j.yjmcc.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minamino T, Komuro I, Kitakaze M. Endoplasmic reticulum stress as a therapeutic target in cardiovascular disease. Circ. Res. 2010;107:1071–1082. doi: 10.1161/CIRCRESAHA.110.227819. [DOI] [PubMed] [Google Scholar]
- Moquin D, Chan FK. The molecular regulation of programmed necrotic cell injury. Trends Biochem. Sci. 2010;35:434–441. doi: 10.1016/j.tibs.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan MJ, Kim YS, Liu ZG. TNFa and reactive oxygen species in necrotic cell death. Cell Res. 2008;18:343–349. doi: 10.1038/cr.2008.31. [DOI] [PubMed] [Google Scholar]
- Moritz KM, De Matteo R, Dodic M, Jefferies AJ, Arena D, Wintour EM, et al. Prenatal glucocorticoid exposure in the sheep alters renal development in utero: implications for adult renal function and blood pressure control. Am. J. Physiol. Regul. Integr. Comp Physiol. 2011;301:R500–R509. doi: 10.1152/ajpregu.00818.2010. [DOI] [PubMed] [Google Scholar]
- Muramatsu Y, Furuichi Y, Tojo N, Moriguchi A, Maemoto T, Nakada H, et al. Neuroprotective efficacy of FR901459, a novel derivative of cyclosporin A, in in vitro mitochondrial damage and in vivo transient cerebral ischemia models. Brain Res. 2007;1149:181–190. doi: 10.1016/j.brainres.2007.02.036. [DOI] [PubMed] [Google Scholar]
- Murayama T, Tanabe M, Matsuda S, Shimazu M, Kamei S, Wakabayashi G, et al. JNK (c-Jun NH2 terminal kinase) and p38 during ischemia reperfusion injury in the small intestine. Transplantation. 2006;81:1325–1330. doi: 10.1097/01.tp.0000209167.48030.6b. [DOI] [PubMed] [Google Scholar]
- Murphy E, Steenbergen C. Ion transport and energetics during cell death and protection. Physiology (Bethesda) 2008;23:115–123. doi: 10.1152/physiol.00044.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murriel CL, Churchill E, Inagaki K, Szweda LI, Mochly-Rosen D. Protein kinase Cd activation induces apoptosis in response to cardiac ischemia and reperfusion damage: a mechanism involving BAD and the mitochondria. J. Biol. Chem. 2004;279:47985–47991. doi: 10.1074/jbc.M405071200. [DOI] [PubMed] [Google Scholar]
- Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation. 1986;74:1124–1136. doi: 10.1161/01.cir.74.5.1124. [DOI] [PubMed] [Google Scholar]
- Muschen M, Warskulat U, Peters-Regehr T, Bode JG, Kubitz R, Haussinger D. Involvement of CD95 (Apo-1/Fas) ligand expressed by rat Kupffer cells in hepatic immunoregulation. Gastroenterology. 1999;116:666–677. doi: 10.1016/s0016-5085(99)70189-7. [DOI] [PubMed] [Google Scholar]
- Muthusamy V, Bosenberg M, Wajapeyee N. Redefining regulation of DNA methylation by RNA interference. Genomics. 2010;96:191–198. doi: 10.1016/j.ygeno.2010.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano Y, Kondo T, Matsuo R, Hashimoto I, Kawasaki T, Kohno K, et al. Platelet dynamics in the early phase of postischemic liver in vivo. J. Surg. Res. 2008;149:192–198. doi: 10.1016/j.jss.2007.09.016. [DOI] [PubMed] [Google Scholar]
- Netticadan T, Temsah R, Osada M, Dhalla NS. Status of Ca2+/calmodulin protein kinase phosphorylation of cardiac SR proteins in ischemia-reperfusion. Am. J. Physiol. 1999;277:C384–C391. doi: 10.1152/ajpcell.1999.277.3.C384. [DOI] [PubMed] [Google Scholar]
- Niccoli G, Burzotta F, Galiuto L, Crea F. Myocardial no-reflow in humans. J. Am. Coll. Cardiol. 2009;54:281–292. doi: 10.1016/j.jacc.2009.03.054. [DOI] [PubMed] [Google Scholar]
- Niccoli G, Cosentino N, Spaziani C, Minelli S, Fracassi F, Crea F. New strategies for the management of no-reflow after primary percutaneous coronary intervention. Expert Rev. Cardiovasc. Ther. 2011;9:615–630. doi: 10.1586/erc.11.49. [DOI] [PubMed] [Google Scholar]
- Nielsen M, Zimmer J, Diemer NH. Endonuclease G expression in thalamic reticular nucleus after global cerebral ischemia. Exp. Brain Res. 2008;190:81–89. doi: 10.1007/s00221-008-1452-3. [DOI] [PubMed] [Google Scholar]
- Nieswandt B, Pleines I, Bender M. Platelet adhesion and activation mechanisms in arterial thrombosis and ischaemic stroke. J. Thromb. Haemost. 2011;9(Suppl. 1):92–104. doi: 10.1111/j.1538-7836.2011.04361.x. [DOI] [PubMed] [Google Scholar]
- Nilakantan V, Liang HL, Rajesh S, Mortensen J, Chandran K. Time-dependant protective effects of mangenese(III) tetrakis (1-methyl-4-pyridyl) porphyrin on mitochondrial function following renal ischemia-reperfusion injury. Free Radic. Res. 2010;44:773–782. doi: 10.3109/10715761003786164. [DOI] [PubMed] [Google Scholar]
- Nourshargh S, Krombach F, Dejana E. The role of JAM-A and PECAM-1 in modulating leukocyte infiltration in inflamed and ischemic tissues. J. Leukoc. Biol. 2006;80:714–718. doi: 10.1189/jlb.1105645. [DOI] [PubMed] [Google Scholar]
- Nourshargh S, Hordijk PL, Sixt M. Breaching multiple barriers: leukocyte motility through venular walls and the interstitium. Nat. Rev. Mol. Cell Biol. 2010;11:366–378. doi: 10.1038/nrm2889. [DOI] [PubMed] [Google Scholar]
- O’Donnell CJ, Nabel EG. Genomics of cardiovascular disease. N. Engl. J. Med. 2011;365:2098–2109. doi: 10.1056/NEJMra1105239. [DOI] [PubMed] [Google Scholar]
- Okuno S, Saito A, Hayashi T, Chan PH. The c-Jun N-terminal protein kinase signaling pathway mediates Bax activation and subsequent neuronal apoptosis through interaction with Bim after transient focal cerebral ischemia. J. Neurosci. 2004;24:7879–7887. doi: 10.1523/JNEUROSCI.1745-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong SB, Gustafsson AB. New roles for mitochondria in cell death in the reperfused myocardium. Cardiovasc. Res. 2012;94:190–196. doi: 10.1093/cvr/cvr312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong SB, Subrayan S, Lim SY, Yellon DM, Davidson SM, Hausenloy DJ. Inhibiting mitochondrial fission protects the heart against ischemia/reperfusion injury. Circulation. 2010;121:2012–2022. doi: 10.1161/CIRCULATIONAHA.109.906610. [DOI] [PubMed] [Google Scholar]
- Ordy JM, Wengenack TM, Bialobok P, Coleman PD, Rodier P, Baggs RB, et al. Selective vulnerability and early progression of hippocampal CA1 pyramidal cell degeneration and GFAP-positive astrocyte reactivity in the rat four-vessel occlusion model of transient global ischemia. Exp. Neurol. 1993;119:128–139. doi: 10.1006/exnr.1993.1014. [DOI] [PubMed] [Google Scholar]
- Orsini F, Moroni M, Contursi C, Yano M, Pelicci P, Giorgio M, et al. Regulatory effects of the mitochondrial energetic status on mitochondrial p66Shc. Biol. Chem. 2006;387:1405–1410. doi: 10.1515/BC.2006.176. [DOI] [PubMed] [Google Scholar]
- Osman M, Russell J, Granger DN. Lymphocyte-derived interferon-γ mediates ischemia-reperfusion-induced leukocyte and platelet adhesion in intestinal microcirculation. Am. J. Physiol. 2009;296:G659–G663. doi: 10.1152/ajpgi.90495.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacher P, Beckman JS, Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiol. Rev. 2007;87:315–424. doi: 10.1152/physrev.00029.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park PO, Haglund U. Regeneration of small bowel mucosa after intestinal ischemia. Crit. Care Med. 1992;20:135–139. doi: 10.1097/00003246-199201000-00026. [DOI] [PubMed] [Google Scholar]
- Park P, Haas M, Cunningham PN, Bao L, Alexander JJ, Quigg RJ. Injury in renal ischemia-reperfusion is independent from immunoglobulins and T-lymphocytes. Am. J. Physiol. 2002;282:F352–F357. doi: 10.1152/ajprenal.00160.2001. [DOI] [PubMed] [Google Scholar]
- Park S, Kondo T, Nakano Y, Murata S, Fukunaga K, Oda T, et al. Platelet adhesion in the sinusoid caused hepatic injury by neutrophils after hepatic ischemia reperfusion. Platelets. 2010;21:282–288. doi: 10.3109/09537101003637265. [DOI] [PubMed] [Google Scholar]
- Parks DA, Granger DN. Xanthine oxidase: biochemistry distribution and physiology. Acta Physiol. Scand. 1986;548:87–99. [PubMed] [Google Scholar]
- Patel B, Fisher M. Therapeutic advances in myocardial microvascular resistance: unravelling the enigma. Pharmacol. Ther. 2010;127:131–147. doi: 10.1016/j.pharmthera.2010.04.014. [DOI] [PubMed] [Google Scholar]
- Patterson AJ, Chen M, Xue Q, Xiao D, Zhang L. Chronic prenatal hypoxia induces epigenetic programming of PKC{epsilon} gene repression in rat hearts. Circ. Res. 2010;107:365–373. doi: 10.1161/CIRCRESAHA.110.221259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng S, Kuang Z, Zhang Y, Xu H, Cheng Q. The protective effects and potential mechanism of Calpain inhibitor Calpeptin against focal cerebral ischemiareperfusion injury in rats. Mol. Biol. Rep. 2011;38:905–912. doi: 10.1007/s11033-010-0183-2. [DOI] [PubMed] [Google Scholar]
- Peppiatt CM, Howarth C, Mobbs P, Attwell D. Bidirectional control of CNS capillary diameter by pericytes. Nature. 2006;443:700–704. doi: 10.1038/nature05193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrelli M-G, Pagliaro P, Penna C. Ischemia/reperfusion injury and cardioprotective mechanisms: role of mitochondria and reactive oxygen species. World J. Cardiol. 2011;3:186–200. doi: 10.4330/wjc.v3.i6.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters MJ, Dixon G, Kotowicz KT, Hatch DJ, Heyderman RS, Klein NJ. Circulating platelet-neutrophil complexes represent a subpopulation of activated neutrophils primed for adhesion phagocytosis and intracellular killing. Br. J. Haematol. 1999;106:391–399. doi: 10.1046/j.1365-2141.1999.01553.x. [DOI] [PubMed] [Google Scholar]
- Phillis JW, O’Regan MH. The role of phospholipases, cyclooxygenases and lipoxygenases in cerebral ischemic/traumatic injuries. Crit. Rev. Neurobiol. 2003;15:61–90. doi: 10.1615/critrevneurobiol.v15.i1.30. [DOI] [PubMed] [Google Scholar]
- Piao CS, Kim JB, Han PL, Lee JK. Administration of the p38 MAPK inhibitor SB203580 affords brain protection with a wide therapeutic window against focal ischemic insult. J. Neurosci. Res. 2003;73:537–544. doi: 10.1002/jnr.10671. [DOI] [PubMed] [Google Scholar]
- Pigazzi A, Heydrick S, Folli F, Benoit S, Michelson A, Loscalzo J. Nitric oxide inhibits thrombin receptor-activating peptide-induced phosphoinositide 3-kinase activity in human platelets. J. Biol. Chem. 1999;274:14368–14375. doi: 10.1074/jbc.274.20.14368. [DOI] [PubMed] [Google Scholar]
- Premen AJ, Banchs V, Womack WA, Kvietys PR, Granger DN. Importance of collateral circulation in the vascularly occluded feline intestine. Gastroenterology. 1987;92:1215–1219. doi: 10.1016/s0016-5085(87)91080-8. [DOI] [PubMed] [Google Scholar]
- Proebstl D, Voisin MB, Woodfin A, Whiteford J, D’Acquisto F, Jones GE, et al. Pericytes support neutrophil subendothelial cell crawling and breaching of venular walls in vivo. J. Exp. Med. 2012;209:1219–1234. doi: 10.1084/jem.20111622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puglisi RN, Strande L, Santos M, Schulte G, Hewitt CW, Whalen TV. Beneficial effects of cyclosporine and rapamycin in small bowel ischemic injury. J. Surg. Res. 1996;65:115–118. doi: 10.1006/jsre.1996.0352. [DOI] [PubMed] [Google Scholar]
- Qin B, Yang H, Xiao B. Role of microRNAs in endothelial inflammation and senescence. Mol. Biol. Rep. 2012;39:4509–4518. doi: 10.1007/s11033-011-1241-0. [DOI] [PubMed] [Google Scholar]
- Quartara L, Maggi CA. The tachykinin NK1 receptor. Part II: Distribution and pathophysiological roles. Neuropeptides. 1998;32:1–49. doi: 10.1016/s0143-4179(98)90015-4. [DOI] [PubMed] [Google Scholar]
- Raedschelders K, Ansley DM, Chen DDY. The cellular and molecular origin of reactive oxygen species generation during myocardial ischemia and reperfusion. Pharmacol. Ther. 2012;133:230–255. doi: 10.1016/j.pharmthera.2011.11.004. [DOI] [PubMed] [Google Scholar]
- Rautou PE, Vion AC, Amabile N, Chironi G, Simon A, Tedgui A, et al. Microparticles, vascular function, and atherothrombosis. Circ. Res. 2011;109:593–606. doi: 10.1161/CIRCRESAHA.110.233163. [DOI] [PubMed] [Google Scholar]
- Ren M, Leng Y, Jeong M, Leeds PR, Chuang DM. Valproic acid reduces brain damage induced by transient focal cerebral ischemia in rats: potential roles of histone deacetylase inhibition and heat shock protein induction. J. Neurochem. 2004;89:1358–1367. doi: 10.1111/j.1471-4159.2004.02406.x. [DOI] [PubMed] [Google Scholar]
- Ren XP, Wu J, Wang X, Sartor MA, Qian J, Jones K, et al. MicroRNA-320 is involved in the regulation of cardiac ischemia/reperfusion injury by targeting heatshock protein 20. Circulation. 2009;119:2357–2366. doi: 10.1161/CIRCULATIONAHA.108.814145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds RM. Corticosteroid-mediated programming and the pathogenesis of obesity and diabetes. J. Steroid Biochem. Mol. Biol. 2010;122:3–9. doi: 10.1016/j.jsbmb.2010.01.009. [DOI] [PubMed] [Google Scholar]
- Rodriguez SF, Granger DN. Role of blood cells in ischemia-reperfusion-induced endothelial barrier failure. Cardiovasc. Res. 2010;87:291–299. doi: 10.1093/cvr/cvq090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roe ND, Ren J. Nitric oxide synthase uncoupling: a therapeutic target in cardiovascular diseases. Vascul. Pharmacol. 2012 doi: 10.1016/j.vph.2012.02.004. epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Romagnani S. Regulation of the T cell response. Clin. Exp. Allergy. 2006;36:1357–1366. doi: 10.1111/j.1365-2222.2006.02606.x. [DOI] [PubMed] [Google Scholar]
- Rosenbaum DM, Degterev A, David J, Rosenbaum PS, Roth S, Grotta JC, et al. Necroptosis, a novel form of caspase-independent cell death, contributes to neuronal damage in a retinal ischemia-reperfusion injury model. J. Neurosci. Res. 2010;88:1569–1576. doi: 10.1002/jnr.22314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossen RD, Michael LH, Hawkins HK, Youker K, Dreyer WJ, Baughn RE, et al. Cardiolipin-protein complexes and initiation of complement activation after coronary artery occlusion. Circ. Res. 1994;75:546–555. doi: 10.1161/01.res.75.3.546. [DOI] [PubMed] [Google Scholar]
- Roy S, Khanna S, Hussain SR, Biswas S, Azad A, Rink C, et al. MicroRNA expression in response to murine myocardial infarction: miR-21 regulates fibroblast metalloprotease-2 via phosphatase and tensin homologue. Cardiovasc. Res. 2009;82:21–29. doi: 10.1093/cvr/cvp015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell J, Cooper D, Tailor A, Stokes KY, Ganger DN. Low venular shear rates promote leukocyte-dependent recruitment of adherent platelets. Am. J. Physiol. 2003;284:G123–G129. doi: 10.1152/ajpgi.00303.2002. [DOI] [PubMed] [Google Scholar]
- Ryter SW, Alam J, Choi AMK. Heme oxygenase-1/carbon monoxide: from basic science to therapeutic applications. Physiol. Rev. 2006;86:583–650. doi: 10.1152/physrev.00011.2005. [DOI] [PubMed] [Google Scholar]
- Salter JW, Krieglstein CF, Isserkutz AC, Granger DN. Platelets modulate ischemia/reperfusion-induced leukocyte recruitment in the mesenteric circulation. Am. J. Physiol. 2001;281:G1432–G1439. doi: 10.1152/ajpgi.2001.281.6.G1432. [DOI] [PubMed] [Google Scholar]
- Sanada S, Komuro I, Kitakaze M. Pathophysiology of myocardial reperfusion injury: preconditioning, postconditioning and translational aspects of protective measures. Am. J. Physiol. 2011;301:H1723–H1741. doi: 10.1152/ajpheart.00553.2011. [DOI] [PubMed] [Google Scholar]
- Santen S, Wang Y, Menger MD, Jeppsson B, Thorlacius H. Mast-cell-dependent secretion of CXC chemokines regulates ischemia-reperfusion-induced leukocyte recruitment in the colon. Int. J. Colorectal Dis. 2008;23:527–534. doi: 10.1007/s00384-007-0436-2. [DOI] [PubMed] [Google Scholar]
- Santén S, Mihaescu A, Laschke MW, Menger MD, Wang Y, Jeppsson B, et al. p38 MAPK regulates ischemia-reperfusion-induced recruitment of leukocytes in the colon. Surgery. 2009;145:303–312. doi: 10.1016/j.surg.2008.10.011. [DOI] [PubMed] [Google Scholar]
- Santora RJ, Lie ML, Grigoryev DN, Nasir O, Moore FA, Hassoun HT. Therapeutic distant organ effects of regional hypothermia during mesenteric ischemiareperfusion injury. J. Vasc. Surg. 2010;52:1003–1014. doi: 10.1016/j.jvs.2010.05.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapega AA, Heppenstall RB, Chance B, Park YS, Sokolow D. Optimizing tourniquet application and release times in extremity surgery. A biochemical and ultrastructural study. J. Bone Joint Surg. Am. 1985;67:303–314. [PubMed] [Google Scholar]
- Saxton NE, Barclay JL, Clouston AD, Fawcett J. Cyclosporin A pretreatment in a rat model of warm ischaemia/reperfusion injury. J. Hepatol. 2002;36:241–247. doi: 10.1016/s0168-8278(01)00248-3. [DOI] [PubMed] [Google Scholar]
- Sayed D, Abdellatif M. MicroRNAs in development and disease. Physiol. Rev. 2011;91:827–887. doi: 10.1152/physrev.00006.2010. [DOI] [PubMed] [Google Scholar]
- Saztpute SR, Park JM, Jang HR, Agreda P, Liu M, Gandalfo MT, et al. The role for T cell repertoire/antigen-specific interactions in experimental kidney ischemia reperfusion injury. J. Immunol. 2009;183:984–992. doi: 10.4049/jimmunol.0801928. [DOI] [PubMed] [Google Scholar]
- Schinzel AC, Takeuchi O, Huang Z, Fisher JK, Zhou Z, Rubens J, et al. Cyclophilin D is a component of mitochondrial permeability transition and mediates neuronal cell death after focal cerebral ischemia. Proc. Natl. Acad. Sci. U.S.A. 2005;102:12005–12010. doi: 10.1073/pnas.0505294102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroen B, Heymans S. Small but smart—microRNAs in the centre of inflammatory processes during cardiovascular diseases, the metabolic syndrome and ageing. Cardiovasc. Res. 2012;93:605–613. doi: 10.1093/cvr/cvr268. [DOI] [PubMed] [Google Scholar]
- Schwartz BG, Kloner RA. Coronary no reflow. J. Mol. Cell. Cardiol. 2012;52:873–882. doi: 10.1016/j.yjmcc.2011.06.009. [DOI] [PubMed] [Google Scholar]
- Scorrano L, Oakes SA, Opferman JT, Cheng EH, Sorcinelli MD, Pozzan T, et al. BAX and BAK regulation of endoplasmic reticulum Ca2+: a control point for apoptosis. Science. 2003;300:135–139. doi: 10.1126/science.1081208. [DOI] [PubMed] [Google Scholar]
- Schroen B, Heymans S. Small but smart-microRNAs in the centre of inflammatory processes during cardiovascular diseases, the metabolic syndromeand ageing. Cardiovasc. Res. 2012;93:605–613. doi: 10.1093/cvr/cvr268. [DOI] [PubMed] [Google Scholar]
- Sepramaniam S, Armugam A, Lim KY, Karolina DS, Swaminathan P, Tan JR, et al. MicroRNA 320a functions as a novel endogenous modulator of aquaporins 1 and 4 as well as a potential therapeutic target in cerebral ischemia. J. Biol. Chem. 2010;285:29223–29230. doi: 10.1074/jbc.M110.144576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sesti C, Simkhovich BZ, Kalvinsh I, Kloner RA. Mildronate, a novel fatty acid oxidation inhibitor and antianginal agent, reduces myocardial infarct size without affecting hemodynamics. J. Cardiovasc. Pharmacol. 2006;47:493–499. doi: 10.1097/01.fjc.0000211732.76668.d2. [DOI] [PubMed] [Google Scholar]
- Settergren M, Böhm F, Malmström RE, Chancon KM, Pernow J. L-arginine and tetrahydrobiopterin protects against ischemia/reperfusion-induced endothelial dysfunction in patients with type 2 diabetes mellitis and coronary artery disease. Atherosclerosis. 2009;204:73–78. doi: 10.1016/j.atherosclerosis.2008.08.034. [DOI] [PubMed] [Google Scholar]
- Sharma AK, Laubach VE, Ramos SI, Zhao Y, Stukenborg G, Linden J, et al. Adenosine A2A receptor activation on CD4+ T lymphocytes and neutrophils attenuates lung ischemia-reperfusion injury. J. Thorac. Cardiovasc. Surg. 2010;139:474–482. doi: 10.1016/j.jtcvs.2009.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen XD, Ke B, Zhai Y, Gao F, Anselmo D, Lassman CR, et al. Stat4 and Stat6 signaling in hepatic ischemia/reperfusion injury in mice: HO-1 dependence of Stat4 disruption-mediated cytoprotection. Hepatology. 2003;37:269–303. doi: 10.1053/jhep.2003.50066. [DOI] [PubMed] [Google Scholar]
- Shen X, Wang Y, Gao F, Ren F, Busuttil RW, Kupiec-Weglinski JW, et al. CD4+ T cells promote tissue inflammation via CD40 signaling without de novo activation in a murine model of liver ischemia/reperfusion injury. Hepatology. 2009;50:1537–1546. doi: 10.1002/hep.23153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi J, Fujiedo H, Kokubo Y, Wake K. Apoptosis of neutrophils and their elimination by Kupffer cells in rat liver. Hepatology. 1996;24:1256–1263. doi: 10.1053/jhep.1996.v24.pm0008903407. [DOI] [PubMed] [Google Scholar]
- Shi Y, Melnikov VY, Schrier RW, Edelstein CL. Downregulation of the calpain inhibitor protein calpastatin by caspases during renal ischemia-reperfusion. Am. J. Physiol. Renal Physiol. 2000;279:F509–F517. doi: 10.1152/ajprenal.2000.279.3.F509. [DOI] [PubMed] [Google Scholar]
- Shi J, Gilbert GE, Kokubo Y, Ohashi T. Role of the liver in regulating numbers of circulating neutrophils. Blood. 2001;98:1226–1230. doi: 10.1182/blood.v98.4.1226. [DOI] [PubMed] [Google Scholar]
- Shichita T, Sugiyama Y, Ooboshi H, Sugimori H, Nakagawa R, Takeda I, et al. Pivotal role of cerebral interleukin-17-producing γδT cells in the delayed phase of ischemic brain injury. Nat. Med. 2009;15:946–951. doi: 10.1038/nm.1999. [DOI] [PubMed] [Google Scholar]
- Shimamura K, Kawamura H, Nagara T, Kato T, Naito T, Kameyama H, et al. Association of NKT cells and granulocytes with liver injury after reperfusion of the portal vein. Cell. Immunol. 2005;234:31–38. doi: 10.1016/j.cellimm.2005.04.022. [DOI] [PubMed] [Google Scholar]
- Shiva S, Sack MN, Greer JJ, Duranski M, Ringwoood LA, Burwell L, et al. Nitrite augments tolerance to ischemia/reperfusion injury via the modulation of mitochondrial electron transfer. J. Exp. Med. 2007;204:2089–2102. doi: 10.1084/jem.20070198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sies H. Oxidative stress: introductory remarks. In: Sies H, editor. Oxidative Stress. London: Academic press; 1985. pp. 1–8. [Google Scholar]
- Singh D, Chander V, Chopra K. Cyclosporine protects against ischemia/reperfusion injury in rat kidneys. Toxicology. 2005;207:339–347. doi: 10.1016/j.tox.2004.09.018. [DOI] [PubMed] [Google Scholar]
- Sitailo LA, Tibudan SS, Denning MF. Bax activation and induction of apoptosis in human keratinocytes by the protein kinase C delta catalytic domain. J. Invest. Dermatol. 2004;123:434–443. doi: 10.1111/j.0022-202X.2004.23403.x. [DOI] [PubMed] [Google Scholar]
- Slezak J, Tribulova N, Okruhlicova L, Dhingra R, Bajaj A, Freed D, et al. Hibernating myocardium: pathophysiology, diagnosis, and treatment. Can. J. Physiol. Pharmacol. 2009;87:252–265. doi: 10.1139/Y09-011. [DOI] [PubMed] [Google Scholar]
- Smith VA, Johnson T. Evaluation of an animal product-free variant of MegaCell MEM as a storage medium for corneas destined for transplantation. Ophthalmic Res. 2010;43:33–42. doi: 10.1159/000246576. [DOI] [PubMed] [Google Scholar]
- Smith CC, Yellon DM. Necroptosis, necrostatins and tissue injury. J. Cell. Mol. Med. 2011;15:1797–1806. doi: 10.1111/j.1582-4934.2011.01341.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CC, Davidson SM, Lim SY, Simpkin JC, Hothersall JS, Yellon DM. Necrostatin: a potentially novel cardioprotective agent? Cardiovasc. Drugs Ther. 2007;21:227–233. doi: 10.1007/s10557-007-6035-1. [DOI] [PubMed] [Google Scholar]
- Solaini G, Harris DA. Biochemical dysfunction in heart mitochondria exposed to ischaemia and reperfusion. Biochem. J. 2005;390:377–394. doi: 10.1042/BJ20042006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorimachi Y, Harada K, Saido TC, Ono T, Kawashima S, Yoshida K. Downregulation of calpastatin in rat heart after brief ischemia and reperfusion. J. Biochem. 1997;122:743–748. doi: 10.1093/oxfordjournals.jbchem.a021818. [DOI] [PubMed] [Google Scholar]
- Sorkine P, Szold O, Halpern P, Gutman M, Greemland M, Rudick V, et al. Gut decontamination reduces bowel ischemia-induced lung injury in rats. Chest. 1997;112:491–495. doi: 10.1378/chest.112.2.491. [DOI] [PubMed] [Google Scholar]
- Souza DG, Mendonça VA, de A Castro MS, Poole S, Teixeira MM. Role of tachykinin NK receptors on the local and remote injuries following ischaemia and reperfusion of the superior mesenteric artery in the rat. Br. J. Pharmacol. 2002;135:303–312. doi: 10.1038/sj.bjp.0704464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souza DG, Vieira AT, Soares AC, Pinho V, Nicoli JR, Vieira LQ, et al. The essential role of the intestinal microbiota in facilitating acute inflammatory responses. J. Immunol. 2004;173:4137–4146. doi: 10.4049/jimmunol.173.6.4137. [DOI] [PubMed] [Google Scholar]
- Stahl GL, Xu Y, Hao L, Miller M, Buras JA, Fung M, et al. Role for the alternative complement pathway in ischemia/reperfusion injury. Am. J. Pathol. 2003;162:449–455. doi: 10.1016/S0002-9440(10)63839-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stallion A, Kou TD, Latfi SQ, Miller KA, Dahms BB, Dudgeon DL, et al. Ischemia/reperfusion: a clinically relevant model of intestinal injury yielding systemic inflammation. J. Pediatr. Surg. 2005;40:470–477. doi: 10.1016/j.jpedsurg.2004.11.045. [DOI] [PubMed] [Google Scholar]
- Stefanidakis M, Newton G, Lee WY, Parkos CA, Luscinskas FW. Endothelial CD47 interaction with SIRPγ is required for human T-cell transendothelial migration under shear flow conditions in vitro. Blood. 2008;112:1280–1289. doi: 10.1182/blood-2008-01-134429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stocker R, Yamamoto Y, McDonagh A, Glazer A, Ames BN. Bilirubin is an antioxidant of possible physiological importance. Science. 1987;235:1043–1045. doi: 10.1126/science.3029864. [DOI] [PubMed] [Google Scholar]
- Stowe DF, Camara KS. Mitochondrial reactive oxygen species production in excitable cells: modulators of mitochondrial and cell function. Antioxid. Redox Signal. 2009;11:1373–1414. doi: 10.1089/ars.2008.2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strasser RH, Simonis G, Schön SP, Braun MU, Ihl-Vahl R, Weinbrenner C, et al. Two distinct mechanisms mediate a differential regulation of protein kinase C isozymes in acute and prolonged myocardial ischemia. Circ. Res. 1999;85:77–87. doi: 10.1161/01.res.85.1.77. [DOI] [PubMed] [Google Scholar]
- Strbian D, Karialainen-Lindsberg ML, Tatlisumak T, Lindsberg PJ. Cerebral mast cells regulate early ischemic brain swelling and neutrophil accumulation. J. Cereb. Blood Flow Metab. 2006;26:605–612. doi: 10.1038/sj.jcbfm.9600228. [DOI] [PubMed] [Google Scholar]
- Strbian D, Karailainen-Lindsberg ML, Kovanen PT, Tatlisumak T, Lindsberg PJ. Mast cell stabilization reduces hemorrhage formation and mortality after administration of thrombolytics in experimental ischemic stroke. Circulation. 2007;116:411–418. doi: 10.1161/CIRCULATIONAHA.106.655423. [DOI] [PubMed] [Google Scholar]
- Strbian D, Kovanen PT, Karajalainen-Lindsberg ML, Tatlisumak T, Lindsberg PJ. An emerging role of mast cells in cerebral ischemia and hemorrhage. Ann. Med. 2009;41:438–450. doi: 10.1080/07853890902887303. [DOI] [PubMed] [Google Scholar]
- Suematsu M, Ishimura Y. The heme oxygenase-carbon monoxide system: a regulator of hepatobiliary functions. Hepatology. 2000;31:3–6. doi: 10.1002/hep.510310102. [DOI] [PubMed] [Google Scholar]
- Sun J, Murphy E. Protein s-nitrosylation and cardioprotection. Circ. Res. 2010;106:285–296. doi: 10.1161/CIRCRESAHA.109.209452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Symonds ME, Pope M, Sharkey D, Budge H. Adipose tissue and fetal programming. Diabetologia. 2012;55:1597–1606. doi: 10.1007/s00125-012-2505-5. [DOI] [PubMed] [Google Scholar]
- Syrjälä SO, Keränen MA, Tuuminen R, Nykänen AI, Tammi M, Krebs R, et al. Increased Th17 rather than Th1 alloimmune response is associated with cardiac allograft vasculopathy after hypothermic presentation in the rat. J. Heart Lung Transplant. 2010;29:1047–1057. doi: 10.1016/j.healun.2010.04.012. [DOI] [PubMed] [Google Scholar]
- Szydlowska K, Tymianski M. Calcium ischemia and excitotoxicity. Cell Calcium. 2010;47:122–129. doi: 10.1016/j.ceca.2010.01.003. [DOI] [PubMed] [Google Scholar]
- Tadimalla A, Belmont PJ, Thuerauf DJ, Glassy MS, Martindale JJ, Gude N, et al. Mesencephalic astrocyte-derived neurotrophic factor is an ischemia-inducible secreted endoplasmic reticulum stress response protein in the heart. Circ. Res. 2008;103:1249–1258. doi: 10.1161/CIRCRESAHA.108.180679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tailor A, Cooper D, Granger DN. Platelet-vessel wall interactions in the microcirculation. Microcirculation. 2005;12:275–285. doi: 10.1080/10739680590925691. [DOI] [PubMed] [Google Scholar]
- Takagi Y, Nozaki K, Sugino T, Hattori I, Hashimoto N. Phosphorylation of c-Jun NH(2)-terminal kinase and p38 mitogen-activated protein kinase after transient forebrain ischemia in mice. Neurosci. Lett. 2000;294:117–120. doi: 10.1016/s0304-3940(00)01552-4. [DOI] [PubMed] [Google Scholar]
- Takagi H, Matsui Y, Hirotani S, Sakoda H, Asano T, Sadoshima J. AMPK mediates autophagy during myocardial ischemia in vivo. Autophagy. 2007;3:405–407. doi: 10.4161/auto.4281. [DOI] [PubMed] [Google Scholar]
- Talukder MA, Zweier JL, Periasamy M. Targeting calcium transport in ischaemic heart disease. Cardiovasc. Res. 2009;84:345–352. doi: 10.1093/cvr/cvp264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan JR, Koo YX, Kaur P, Liu F, Armugam A, Wong PT, et al. micro-RNAs in stroke pathogenesis. Curr. Mol. Med. 2011;11:76–92. doi: 10.2174/156652411794859232. [DOI] [PubMed] [Google Scholar]
- Taylor PB, Young BG. Effect of myocardial ischemia on uridine incorporation and histone acetylation. Can. J. Physiol. Pharmacol. 1982;60:313–318. doi: 10.1139/y82-043. [DOI] [PubMed] [Google Scholar]
- Terai K, Hiramoto Y, Masaki M, Sugiyama S, Kuroda T, Hori M, et al. AMP-activated protein kinase protects cardiomyocytes against hypoxic injury through attenuation of endoplasmic reticulum stress. Mol. Cell. Biol. 2005;25:9554–9575. doi: 10.1128/MCB.25.21.9554-9575.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terao S, Yilmaz G, Stokes KY, Russell J, Ishikawa M, Kawase T, et al. Blood cell-derived RANTES mediates cerebral microvascular dysfunction, inflammation, and tissue injury after focal ischemia-reperfusion. Stroke. 2008;39:2560–2570. doi: 10.1161/STROKEAHA.107.513150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theoharidis TC, Kempuraj D, Tagen M, Conti P, Kalogeromitros D. Differential relased mast cell mediators and the pathogenesis of inflammation. Immunol. Rev. 2007;217:65–78. doi: 10.1111/j.1600-065X.2007.00519.x. [DOI] [PubMed] [Google Scholar]
- Theruvath TP, Snoddy MC, Zhong Z, Lemasters JJ. Mitochondrial permeability transition in liver ischemia and reperfusion: role of c-Jun N-terminal kinase 2. Transplantation. 2008;85:1500–1504. doi: 10.1097/TP.0b013e31816fefb5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas WS, Mori E, Copeland BR, Yu JQ, Morrissey JH, del Zappo GJ. Tissue factor contributes to microvascular defects following cerebral ischemia. Stroke. 1993;24:847–853. doi: 10.1161/01.str.24.6.847. [DOI] [PubMed] [Google Scholar]
- Thomas A, Lenglet S, Chaurand P, Deglon J, Mangin P, Mach F, et al. Mass spectrometry for the evaluation of cardiovascular diseases based on proteomics and lipidomics. Thromb. Haemost. 2011;106:20–33. doi: 10.1160/TH10-12-0812. [DOI] [PubMed] [Google Scholar]
- Thon L, Möhlig H, Mathieu S, Lange A, Bulanova E, Winoto-Morbach S, et al. Ceramide mediates caspase-independent programmed cell death. FASEB J. 2005;19:1945–1956. doi: 10.1096/fj.05-3726com. [DOI] [PubMed] [Google Scholar]
- Thornburg KL, O’Tierney PF, Louey S. Review: the placenta is a programming agent for cardiovascular disease. Placenta. 2010;31(Suppl.):S54–S59. doi: 10.1016/j.placenta.2010.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiefenbacher CP, Chilian WM, Mitchell M, Defily DV. Restoration of endothelium-dependent vasodilation after reperfusion injury by tetrahydrobiopterin. Circulation. 1996;94:1423–1429. doi: 10.1161/01.cir.94.6.1423. [DOI] [PubMed] [Google Scholar]
- Toko H, Takahashi H, Kayama Y, Okada S, Minamino T, Terasaki F, et al. ATF6 is important under both pathological and physiological states in the heart. J. Mol. Cell. Cardiol. 2010;49:113–120. doi: 10.1016/j.yjmcc.2010.03.020. [DOI] [PubMed] [Google Scholar]
- Tomiyama K, Ikeda A, Ueki S, Nakao A, Stolz DB, Koike Y, et al. Inhibition of Kupffer cell-mediated early proinflammatory response with carbon monoxide in transplant-induced hepatic ischemia/reperfusion injury in rats. Hepatology. 2008;48:1608–1620. doi: 10.1002/hep.22482. [DOI] [PubMed] [Google Scholar]
- Tsubokawa T, Yamaguchi-Okada M, Calvert JW, Solaroglu I, Shimamura N, Yata K, et al. Neurovascular and neuronal protection by E64d after focal cerebral ischemia in rats. J. Neurosci. Res. 2006;84:832–840. doi: 10.1002/jnr.20977. [DOI] [PubMed] [Google Scholar]
- Tsung A, Hoffman RA, Izuishi K, Critchlow ND, Nakao A, Chan MH, et al. Hepatic ischemia-reperfusion involves functional TLR4 signaling in nonparenchymal cells. J. Immunol. 2005a;175:7661–7668. doi: 10.4049/jimmunol.175.11.7661. [DOI] [PubMed] [Google Scholar]
- Tsung A, Sahai R, Tanaka H, Takao A, Fink MP, Lotze MT, et al. The nuclear factor HMGB1 mediates hepatic injury after liver ischemia-reperfusion. J. Exp. Med. 2005b;201:1135–1143. doi: 10.1084/jem.20042614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turer AT, Hill JA. Pathogenesis of myocardial ischemia-reperfusion injury and rationale for therapy. Am. J. Cardiol. 2010;106:360–368. doi: 10.1016/j.amjcard.2010.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida Y, Ke B, Freitas MCS, Ji H, Zhao D, Benjamin ER, et al. The emerging role of T cell immunoglobulin mucin-1 in the mechanism of liver ischemia and reperfusion injury in the mouse. Hepatology. 2010;51:1363–1372. doi: 10.1002/hep.23442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uemura A, Naito Y, Matsubara T. Dynamics of Ca(2+)/calmodulin-dependent protein kinase II following acute myocardial ischemia-translocation and autophosphorylation. Biochem. Biophys. Res. Commun. 2002;297:997–1002. doi: 10.1016/s0006-291x(02)02279-9. [DOI] [PubMed] [Google Scholar]
- Urbich C, Kuehbacher A, Dimmeler S. Role of microRNAs in vascular diseases, inflammation, and angiogenesis. Cardiovasc. Res. 2008;79:581–588. doi: 10.1093/cvr/cvn156. [DOI] [PubMed] [Google Scholar]
- Vaghasiya JD, Sheth NR, Bhalodia YS, Jivani NP. Exaggerated liver injury produced by renal ischemia reperfusion in diabetes: effect of exenatide. Saudi J. Gastroenterol. 2010;16:174–180. doi: 10.4103/1319-3767.65187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valko M, Leibfritz D, Moncol J, Cronin MTD, Mazur M, Telser J. Free radicals and antioxidants in normal functions and human disease. Int. J. Biochem. Cell Biol. 2007;39:44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- Van der Vusse GJ, Reneman RS, van Bilsen M. Accumulation of arachidonic acid in ischemic/reperfused cardiac tissue: possible causes and consequences. Prostaglandins Leukot. Essent. Fatty Acids. 1997;57:85–93. doi: 10.1016/s0952-3278(97)90497-x. [DOI] [PubMed] [Google Scholar]
- Vandenabeele P, Declercq W, Van Herreweghe F, Vanden Berghe T. The role of the kinases RIP1 and RIP3 in TNF-induced necrosis. Sci. Signal. 2010;3:re4. doi: 10.1126/scisignal.3115re4. [DOI] [PubMed] [Google Scholar]
- Vega VL, Mardones L, Maldonado M, Nicovani S, Manriquez V, Roa J, et al. Xanthine oxidase released from reperfused hind limbs mediate kupffer cell activation, neutrophil sequestration, and hepatic oxidative stress in rats subjected to tourniquet shock. Shock. 2000;14:565–571. doi: 10.1097/00024382-200014050-00012. [DOI] [PubMed] [Google Scholar]
- Vila-Petroff M, Salas MA, Said M, Valverde CA, Sapia L, Portiansky E, et al. CaMKII inhibition protects against necrosis and apoptosis in irreversible ischemiareperfusion injury. Cardiovasc. Res. 2007;73:689–698. doi: 10.1016/j.cardiores.2006.12.003. [DOI] [PubMed] [Google Scholar]
- Wagers AJ, Conboy IM. Cellular and molecular signatures of muscle regeneration: current concepts and controversies in adult myogenesis. Cell. 2005;122:659–667. doi: 10.1016/j.cell.2005.08.021. [DOI] [PubMed] [Google Scholar]
- Wagner S, Dybkova N, Rasenack EC, Jacobshagen C, Fabritz L, Kirchhof P, et al. Ca2+/calmodulin-dependent protein kinase II regulates cardiac Na+ channels. J. Clin. Invest. 2006;116:3127–3138. doi: 10.1172/JCI26620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh SR, Boyle JR, Tang TY, Sadat U, Cooper DG, Lapsley M, et al. Remote ischemic preconditioning for renal and cardiac protection during endovascular aneurysmrepair: a randomized controlled trial. J. Endovasc. Ther. 2009;16:680–689. doi: 10.1583/09-2817.1. [DOI] [PubMed] [Google Scholar]
- Wang Y, Ji HX, Xing SH, Pei DS, Guan QH. SP600125, a selective JNK inhibitor, protects ischemic renal injury via suppressing the extrinsic pathways of apoptosis. Life Sci. 2007;80:2067–2075. doi: 10.1016/j.lfs.2007.03.010. [DOI] [PubMed] [Google Scholar]
- Wang Y, Dawson VL, Dawson TM. Poly(ADP-ribose) signals to mitochondrial AIF: a key event in parthanatos. Exp. Neurol. 2009;218:193–202. doi: 10.1016/j.expneurol.2009.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Liu C, Zhu F, Liu F, Zhang P, Guo C, et al. Reoxygenation of hypoxia-differentiated dentritic cells induces Th1 and Th17 cell differentiation. Mol. Immunol. 2010;47:922–931. doi: 10.1016/j.molimm.2009.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B, Huang Q, Zhang W, Li N, Li J. Lactobacillus plantarum prevents bacterial translocation in rats following ischemia and reperfusion injury. Dig. Dis. Sci. 2011a;56:3187–3194. doi: 10.1007/s10620-011-1747-2. [DOI] [PubMed] [Google Scholar]
- Wang Z, Klipfell E, Bennett BJ, Koeth R, Levison BS, Dugar B, et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature. 2011b;472:57–63. doi: 10.1038/nature09922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegener S, Gottschalk B, Jovanovic V, Knab R, Fiebach JB, Schellinger PD, et al. Transient ischemic attacks before ischemic stroke: preconditioning the human brain? A multicenter magnetic resonance imaging study. Stroke. 2004b;35:616–621. doi: 10.1161/01.STR.0000115767.17923.6A. [DOI] [PubMed] [Google Scholar]
- Wehrens XH, Lehnart SE, Reiken SR, Marks AR. Ca2+/calmodulindependent protein kinase II phosphorylation regulates the cardiac ryanodine receptor. Circ. Res. 2004;94:e61–e70. doi: 10.1161/01.RES.0000125626.33738.E2. [DOI] [PubMed] [Google Scholar]
- Wei Q, Yin XM, Wang MH, Dong Z. Bid deficiency ameliorates ischemic renal failure and delays animal death in C57BL/6 mice. Am. J. Physiol. Renal Physiol. 2006;290:F35–F42. doi: 10.1152/ajprenal.00184.2005. [DOI] [PubMed] [Google Scholar]
- Weisman HF, Bartow T, Leppo MK, Boyle MP, Marsh HC, Jr, Carson GR, et al. Recombinant soluble CR1 suppressed complement activation, inflammation, and necrosis associated with reperfusion of ischemic myocardium. Trans. Assoc. Am. Physicians. 1990;103:64–72. [PubMed] [Google Scholar]
- Wershil BK, Wang ZS, Gordon JR, Galli SJ. Recruitment of neutrophils during IgE-dependent cutaneous late phase reactions in the mouse is mast celldependent. Partial inhibition of the reaction with antiserum against tumor necrosis factor-alpha. J. Clin. Invest. 1991;87:446–453. doi: 10.1172/JCI115016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whelan RS, Kaplinskiy V, Kitsis RN. Cell death in the pathogenesis of heart disease: mechanisms and significance. Annu. Rev. Physiol. 2010;72:19–44. doi: 10.1146/annurev.physiol.010908.163111. [DOI] [PubMed] [Google Scholar]
- Willems IE, Havenith MG, DeMey JG, Chaponnier C, Brown RA. The alpha-smooth muscle actin-positive cells in healing human myocardial scars. Am. J. Pathol. 1994;145:868–875. [PMC free article] [PubMed] [Google Scholar]
- Williams JP, Pechet TT, Weiser MR, Reid R, Kobzik L, Moore FD, Jr, et al. Intestinal reperfusion injury is mediated by IgM and complement. J. Appl. Physiol. 1997;86:938–942. doi: 10.1152/jappl.1999.86.3.938. [DOI] [PubMed] [Google Scholar]
- Winquist RJ, Kerr S. Cerebral ischemia-reperfusion injury and adhesion. Neurology. 1997;49:S23–S26. doi: 10.1212/wnl.49.5_suppl_4.s23. [DOI] [PubMed] [Google Scholar]
- Wittnich C. Age-related differences in myocardial metabolism affects response to ischemia. Age in heart tolerance to ischemia. Am. J. Cardiovasc. Pathol. 1992;4:175–180. [PubMed] [Google Scholar]
- Wolf PS, Merry HE, Farivar AS, McCourtie AS, Mulligan MS. Stressactivated protein kinase inhibition to ameliorate lung ischemia reperfusion injury. J. Thorac. Cardiovasc. Surg. 2008;135:656–665. doi: 10.1016/j.jtcvs.2007.11.026. [DOI] [PubMed] [Google Scholar]
- Woodfin A, Voisin MB, Beyrau M, Colom B, Caille D, Diapouli FM, et al. The junctional adhesion molecule JAM-C regulates polarized transendothelial migration of neutrophils in vivo. Nat. Immunol. 2011;12:761–769. doi: 10.1038/ni.2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu B, Qiu W, Wang P, Yu H, Cheng T, Zambetti GP, et al. p53 independent induction of PUMA mediates intestinal apoptosis in response to ischaemiareperfusion. Gut. 2007;56:645–654. doi: 10.1136/gut.2006.101683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, Manivannan A, Jiang HR, Liversidge J, Shazrp PF, Forester JV, et al. Recruitment of IFN-γ-producing (Th1-like) cells into the inflamed retina in vivo is preferentially regulated by p-selectin glycoprotein ligand 1: P/E-selectin interactions. J. Immunol. 2004;172:3215–3224. doi: 10.4049/jimmunol.172.5.3215. [DOI] [PubMed] [Google Scholar]
- Xu Y, Huang S, Liu ZG, Han J. Poly(ADP-ribose) polymerase-1 signaling to mitochondria in necrotic cell death requires RIP1/TRAF2-mediated JNK1 activation. J. Biol. Chem. 2006;281:8788–8795. doi: 10.1074/jbc.M508135200. [DOI] [PubMed] [Google Scholar]
- Xu CF, Yu CH, Li YM. Regulation of hepatic microRNA expression in response to ischemic preconditioning following ischemia/reperfusion injury in mice. OMICS. 2009;13:513–520. doi: 10.1089/omi.2009.0035. [DOI] [PubMed] [Google Scholar]
- Xu Z, Zhang J, David KK, Yang ZJ, Li X, Dawson TM, et al. Endonuclease G does not play an obligatory role in poly(ADP-ribose) polymerase-dependent cell death after transient focal cerebral ischemia. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2010;299:R215–R221. doi: 10.1152/ajpregu.00747.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Zhao J, Evan G, Xiao C, Cheng Y, Xiao J. Circulating microRNAs: novel biomarkers for cardiovascular diseases. J. Mol. Med. (Berl) 2011a doi: 10.1007/s00109-011-0840-5. [DOI] [PubMed] [Google Scholar]
- Xu X, Zhang XA, Wang DW. The role of CYP450 epoxygenases and metabolites, epoxysatrienoic acids, cardiovascular and malignant diseases. Adv. Drug Deliv. Rev. 2011b;63:597–608. doi: 10.1016/j.addr.2011.03.006. [DOI] [PubMed] [Google Scholar]
- Yamashiro S, Noguchi K, Kuniyoshi Y, Koja K, Sakanashi M. Role of tetrahydrobiopterin on ischemia-reperfusion injury in isolated injury in isolated perfused rat hearts. J. Cardiovasc. Surg. (Torino) 2003;44:37–49. [PubMed] [Google Scholar]
- Yang JS, Lai EC. Alternative miRNA biogenesis pathways and the interpretation of core miRNA pathway mutants. Mol. Cell. 2011;43:892–903. doi: 10.1016/j.molcel.2011.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang W, Guastella J, Huang JC, Wang Y, Zhang L, Xue D, et al. MX1013, a dipeptide caspase inhibitor with potent in vivo antiapoptotic activity. Br. J. Pharmacol. 2003;140:402–412. doi: 10.1038/sj.bjp.0705450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z, Sharma AK, Linden J, Kron IL, Laubach VE. CD4+ T lymphocytes mediate acute pulmonary ischemia-reperfusion injury. J. Thorac. Cardiovasc. Surg. 2009;137:695–702. doi: 10.1016/j.jtcvs.2008.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang ZJ, Carter EL, Kibler KK, Kwansa H, Crafa DA, Martin LJ, et al. Attenuation of neonatal ischemic brain damage using a 20-HETE synthesis inhibitor. J. Neurochem. 2012;121:168–179. doi: 10.1111/j.1471-4159.2012.07666.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaoita H, Ogawa K, Maehara K, Maruyama Y. Attenuation of ischemia/reperfusion injury in rats by a caspase inhibitor. Circulation. 1998;97:276–281. doi: 10.1161/01.cir.97.3.276. [DOI] [PubMed] [Google Scholar]
- Yasojima K, Kilgore KS, Washington RA, Lucchesi BR, McGeer PL. Complement gene expression by rabbit heart: upregulation by ischemia and reperfusion. Circ. Res. 1998;82:1224–1230. doi: 10.1161/01.res.82.11.1224. [DOI] [PubMed] [Google Scholar]
- Ye Y, Hu Z, Lin Y, Zhang C, Perez-Polo JR. Downregulation of microRNA-29 by antisense inhibitors and a PPAR-gamma agonist protects against myocardial ischaemia-reperfusion injury. Cardiovasc. Res. 2010;87:535–544. doi: 10.1093/cvr/cvq053. [DOI] [PubMed] [Google Scholar]
- Ye Y, Perez-Polo JR, Aguilar D, Birnbaum Y. The potential effects of anti-diabetic medications on myocardial ischemia-reperfusion injury. Basic Res. Cardiol. 2011a;106:925–952. doi: 10.1007/s00395-011-0216-6. [DOI] [PubMed] [Google Scholar]
- Ye Y, Perez-Polo JR, Qian J, Birnbaum Y. The role of microRNA in modulating myocardial ischemia-reperfusion injury. Physiol. Genomics. 2011b;43:534–542. doi: 10.1152/physiolgenomics.00130.2010. [DOI] [PubMed] [Google Scholar]
- Yellon DM, Hausenloy DJ. Myocardial reperfusion injury. N. Engl. J. Med. 2007;357:1121–1135. doi: 10.1056/NEJMra071667. [DOI] [PubMed] [Google Scholar]
- Yemisci M, Gursoy-Ozdemir Y, Vural A, Can A, Topalkara K, Dalkara T. Pericyte contraction induced by oxidative-nitrosative stress impairs capillary reflow despite successful opening of an occluded cerebral artery. Nat. Med. 2009;15:1031–1038. doi: 10.1038/nm.2022. [DOI] [PubMed] [Google Scholar]
- Yilmaz G, Granger DN. Cell adhesion molecules and ischemic stroke. Neurol. Res. 2008;30:783–793. doi: 10.1179/174313208X341085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yilmaz G, Granger DN. Leukocyte recruitment and ischemic brain injury. Neuromolecular Med. 2010;12:193–204. doi: 10.1007/s12017-009-8074-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin KJ, Deng Z, Huang H, Hamblin M, Xie C, Zhang J, et al. miR-497 regulates neuronal death in mouse brain after transient focal cerebral ischemia. Neurobiol. Dis. 2010;38:17–26. doi: 10.1016/j.nbd.2009.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokota N, Burne-Taney M, Racusen L, Rabb H. Contrasting roles for STAT4 and STAT6 signal transduction pathways in murine renal ischemia-reperfusion injury. Am. J. Physiol. 2003;285:F319–F325. doi: 10.1152/ajprenal.00432.2002. [DOI] [PubMed] [Google Scholar]
- Yoshiya K, Lapchak PH, Thai TH, Kannan L, Rani P, Lucca JJ, et al. Depletion of gut commensal bacteria attenuates intestinal ischemia/reperfusion injury. Am. J. Physiol. Gastrointest. Liver Physiol. 2011;301:G1020–G1030. doi: 10.1152/ajpgi.00239.2011. [DOI] [PubMed] [Google Scholar]
- Young GW, Wang Y, Ping P. Understanding proteasome assembly and regulation: importance to cardiovascular medicine. Trends Cardiovasc. Med. 2008;18:93–98. doi: 10.1016/j.tcm.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X, Kem DC. Proteasome inhibition during myocardial infarction. Cardiovasc. Res. 2010;85:312–320. doi: 10.1093/cvr/cvp309. [DOI] [PubMed] [Google Scholar]
- Yuan Y, Wang JY, Xu LY, Cai R, Chen Z, Luo BY. MicroRNA expression changes in the hippocampi of rats subjected to global ischemia. J. Clin. Neurosci. 2010;17:774–778. doi: 10.1016/j.jocn.2009.10.009. [DOI] [PubMed] [Google Scholar]
- Yusof M, Kamada K, Kalogeris T, Gaskin FS, Korthuis RJ. Hydrogen sulfide triggers late-phase preconditioning in postischemic small intestine by an NOand p38 MAPK-dependent mechanism. Am. J. Physiol. Heart Circ. Physiol. 2009;296:H868–H876. doi: 10.1152/ajpheart.01111.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaccagnini G, Martelli F, Fasanaro P, Magenta A, Gaetano C, Di Carlo A, et al. p66ShcA modulates tissue response to hindlimb ischemia. Circulation. 2004;109:2917–2923. doi: 10.1161/01.CIR.0000129309.58874.0F. [DOI] [PubMed] [Google Scholar]
- Zeng Z, Huang HF, Chen MQ, Song F, Zhang YJ. Heme oxygenase-1 protects donor livers from ischemia/reperfusion injury: the role of Kupffer cells. World J. Gastroenterol. 2010;16:1285–1292. doi: 10.3748/wjg.v16.i10.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M, Carroll MC. Natural antibody mediated innate autoimmune response. Mol. Immunol. 2007;44:103–110. doi: 10.1016/j.molimm.2006.06.022. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Ramos BF, Jakschik BA. Neutrophil recruitment by tumor necrosis factor from mast cells in immune complex peritonitis. Science. 1992;258:1957–1959. doi: 10.1126/science.1470922. [DOI] [PubMed] [Google Scholar]
- Zhang M, Alicot EM, Carroll MC. Human natural IgM can induce ischemia/ reperfusion injury in a murine intestinal model. Mol. Immunol. 2008;44:103–110. doi: 10.1016/j.molimm.2008.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Wa J, Xu X, Potter BJ, Gao X. Direct relationship between levels of TNFα expression and endothelial dysfunction in reperfusion injury. Basic Res. Cardiol. 2010;105:453–464. doi: 10.1007/s00395-010-0083-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao ZQ, Nakamura M, Wang NP, Velez DA, Hewan-Lowe KO, Guyton RA, et al. Dynamic progression of contractile and endothelial dysfunction and infarct extension in the late phase of reperfusion. J. Surg. Res. 2000a;94:133–144. doi: 10.1006/jsre.2000.6029. [DOI] [PubMed] [Google Scholar]
- Zhao ZQ, Nakamura M, Wang NP, Wilcox JN, Shearer S, Ronson RS, et al. Reperfusion induces myocardial apoptotic cell death. Cardiovasc. Res. 2000b;45:651–660. doi: 10.1016/s0008-6363(99)00354-5. [DOI] [PubMed] [Google Scholar]
- Zhao BQ, Chauhan AK, Canau HM, Patten IS, Yang JJ, Dockal M, et al. Von Willebrand factor-cleaving protease ADAMTS13 reduces ischemic brain injury in experimental stroke. Blood. 2009;114:3329–3334. doi: 10.1182/blood-2009-03-213264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H, Ren C, Chen X, Shen J. From rapid to delayed and remote postconditioning: the evolving concept of ischemic postconditioning in brain ischemia. Curr. Drug Targets. 2012;13:173–187. doi: 10.2174/138945012799201621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Q, Lam PY, Han D, Cadenas E. c-Jun N-terminal kinase regulates mitochondrial bioenergetics by modulating pyruvate dehydrogenase activity in primary cortical neurons. J. Neurochem. 2008;104:325–335. doi: 10.1111/j.1471-4159.2007.04957.x. [DOI] [PubMed] [Google Scholar]
- Zhu Q, Wani AA. Histone modifications: crucial elements for damage response and chromatin restoration. J. Cell. Physiol. 2010;223:283–288. doi: 10.1002/jcp.22060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang S, Demirs JT, Kochevar IE. p38 mitogen-activated protein kinase mediates bid cleavage, mitochondrial dysfunction, and caspase-3 activation during apoptosis induced by singlet oxygen but not by hydrogen peroxide. J. Biol. Chem. 2000;275:25939–25948. doi: 10.1074/jbc.M001185200. [DOI] [PubMed] [Google Scholar]