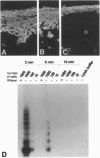
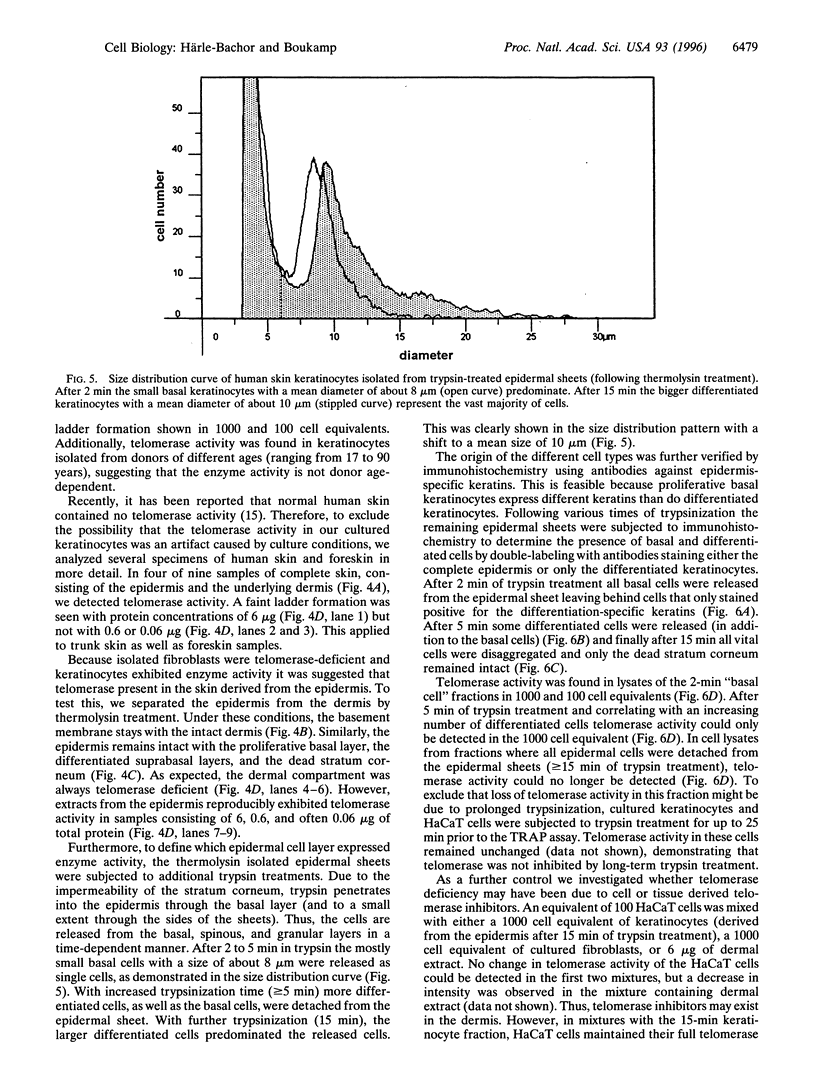
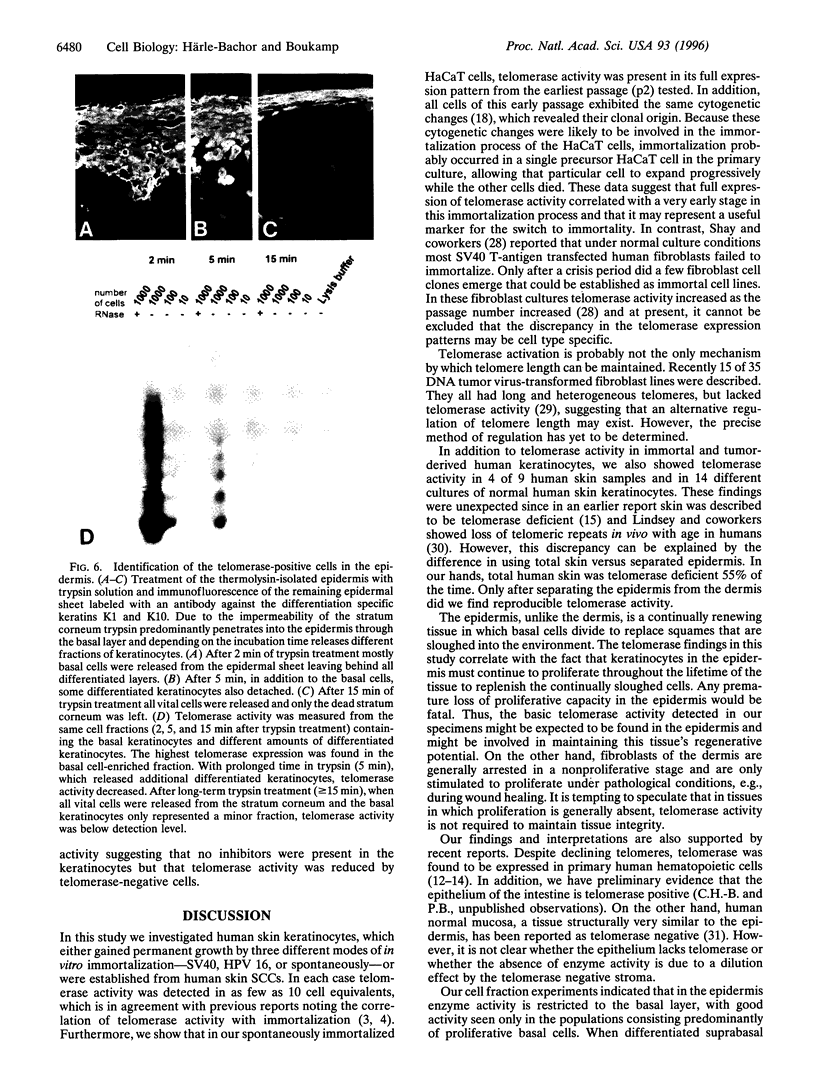
Abstract
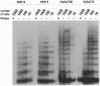
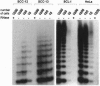
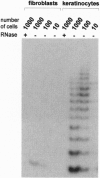
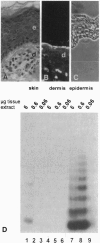
Cellular senescence is defined by the limited proliferative capacity of normal cultured cells. Immortal cells overcome this regulation and proliferate indefinitively. One step in the immortalization process may be reactivation of telomerase activity, a ribonucleoprotein complex, which, by de novo synthesized telomeric TTAGGG repeats, can prevent shortening of the telomeres. Here we show that immortal human skin keratinocytes, irrespective of whether they were immortalized by simian virus 40, human papillomavirus 16, or spontaneously, as well as cell lines established from human skin squamous cell carcinomas exhibit telomerase activity. Unexpectedly, four of nine samples of intact human skin also were telomerase positive. By dissecting the skin we could show that the dermis and cultured dermal fibroblasts were telomerase negative. The epidermis and cultured skin keratinocytes, however, reproducibly exhibited enzyme activity. By separating different cell layers of the epidermis this telomerase activity could be assigned to the proliferative basal cells. Thus, in addition to hematopoietic cells, the epidermis, another example of a permanently regenerating human tissue, provides a further exception of the hypothesis that all normal human somatic tissues are telomerase deficient. Instead, these data suggest that in addition to contributing to the permanent proliferation capacity of immortal and tumor-derived keratinocytes, telomerase activity may also play a similar role in the lifetime regenerative capacity of normal epidermis in vivo.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allsopp R. C., Harley C. B. Evidence for a critical telomere length in senescent human fibroblasts. Exp Cell Res. 1995 Jul;219(1):130–136. doi: 10.1006/excr.1995.1213. [DOI] [PubMed] [Google Scholar]
- Baden H. P., Kubilus J., Wolman S. R., Steinberg M. L., Phillips S. B., Kvedar J. C. NM1 keratinocyte line is cytogenetically and biologically stable and exhibits a unique structural protein. J Invest Dermatol. 1987 Dec;89(6):574–579. doi: 10.1111/1523-1747.ep12461235. [DOI] [PubMed] [Google Scholar]
- Boukamp P., Petrussevska R. T., Breitkreutz D., Hornung J., Markham A., Fusenig N. E. Normal keratinization in a spontaneously immortalized aneuploid human keratinocyte cell line. J Cell Biol. 1988 Mar;106(3):761–771. doi: 10.1083/jcb.106.3.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boukamp P., Tilgen W., Dzarlieva R. T., Breitkreutz D., Haag D., Riehl R. K., Bohnert A., Fusenig N. E. Phenotypic and genotypic characteristics of a cell line from a squamous cell carcinoma of human skin. J Natl Cancer Inst. 1982 Mar;68(3):415–427. [PubMed] [Google Scholar]
- Broccoli D., Young J. W., de Lange T. Telomerase activity in normal and malignant hematopoietic cells. Proc Natl Acad Sci U S A. 1995 Sep 26;92(20):9082–9086. doi: 10.1073/pnas.92.20.9082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryan T. M., Englezou A., Gupta J., Bacchetti S., Reddel R. R. Telomere elongation in immortal human cells without detectable telomerase activity. EMBO J. 1995 Sep 1;14(17):4240–4248. doi: 10.1002/j.1460-2075.1995.tb00098.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cairns J. Mutation selection and the natural history of cancer. Nature. 1975 May 15;255(5505):197–200. doi: 10.1038/255197a0. [DOI] [PubMed] [Google Scholar]
- Chadeneau C., Hay K., Hirte H. W., Gallinger S., Bacchetti S. Telomerase activity associated with acquisition of malignancy in human colorectal cancer. Cancer Res. 1995 Jun 15;55(12):2533–2536. [PubMed] [Google Scholar]
- Counter C. M., Avilion A. A., LeFeuvre C. E., Stewart N. G., Greider C. W., Harley C. B., Bacchetti S. Telomere shortening associated with chromosome instability is arrested in immortal cells which express telomerase activity. EMBO J. 1992 May;11(5):1921–1929. doi: 10.1002/j.1460-2075.1992.tb05245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Counter C. M., Gupta J., Harley C. B., Leber B., Bacchetti S. Telomerase activity in normal leukocytes and in hematologic malignancies. Blood. 1995 May 1;85(9):2315–2320. [PubMed] [Google Scholar]
- Counter C. M., Hirte H. W., Bacchetti S., Harley C. B. Telomerase activity in human ovarian carcinoma. Proc Natl Acad Sci U S A. 1994 Apr 12;91(8):2900–2904. doi: 10.1073/pnas.91.8.2900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dürst M., Dzarlieva-Petrusevska R. T., Boukamp P., Fusenig N. E., Gissmann L. Molecular and cytogenetic analysis of immortalized human primary keratinocytes obtained after transfection with human papillomavirus type 16 DNA. Oncogene. 1987;1(3):251–256. [PubMed] [Google Scholar]
- Dürst M., Gallahan D., Jay G., Rhim J. S. Glucocorticoid-enhanced neoplastic transformation of human keratinocytes by human papillomavirus type 16 and an activated ras oncogene. Virology. 1989 Dec;173(2):767–771. doi: 10.1016/0042-6822(89)90595-3. [DOI] [PubMed] [Google Scholar]
- Greider C. W., Blackburn E. H. A telomeric sequence in the RNA of Tetrahymena telomerase required for telomere repeat synthesis. Nature. 1989 Jan 26;337(6205):331–337. doi: 10.1038/337331a0. [DOI] [PubMed] [Google Scholar]
- Greider C. W., Blackburn E. H. Identification of a specific telomere terminal transferase activity in Tetrahymena extracts. Cell. 1985 Dec;43(2 Pt 1):405–413. doi: 10.1016/0092-8674(85)90170-9. [DOI] [PubMed] [Google Scholar]
- Harley C. B. Telomere loss: mitotic clock or genetic time bomb? Mutat Res. 1991 Mar-Nov;256(2-6):271–282. doi: 10.1016/0921-8734(91)90018-7. [DOI] [PubMed] [Google Scholar]
- Harley C. B., Vaziri H., Counter C. M., Allsopp R. C. The telomere hypothesis of cellular aging. Exp Gerontol. 1992 Jul-Aug;27(4):375–382. doi: 10.1016/0531-5565(92)90068-b. [DOI] [PubMed] [Google Scholar]
- Hiyama K., Hirai Y., Kyoizumi S., Akiyama M., Hiyama E., Piatyszek M. A., Shay J. W., Ishioka S., Yamakido M. Activation of telomerase in human lymphocytes and hematopoietic progenitor cells. J Immunol. 1995 Oct 15;155(8):3711–3715. [PubMed] [Google Scholar]
- Jones P. H., Harper S., Watt F. M. Stem cell patterning and fate in human epidermis. Cell. 1995 Jan 13;80(1):83–93. doi: 10.1016/0092-8674(95)90453-0. [DOI] [PubMed] [Google Scholar]
- Kim N. W., Piatyszek M. A., Prowse K. R., Harley C. B., West M. D., Ho P. L., Coviello G. M., Wright W. E., Weinrich S. L., Shay J. W. Specific association of human telomerase activity with immortal cells and cancer. Science. 1994 Dec 23;266(5193):2011–2015. doi: 10.1126/science.7605428. [DOI] [PubMed] [Google Scholar]
- Klingelhutz A. J., Barber S. A., Smith P. P., Dyer K., McDougall J. K. Restoration of telomeres in human papillomavirus-immortalized human anogenital epithelial cells. Mol Cell Biol. 1994 Feb;14(2):961–969. doi: 10.1128/mcb.14.2.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsey J., McGill N. I., Lindsey L. A., Green D. K., Cooke H. J. In vivo loss of telomeric repeats with age in humans. Mutat Res. 1991 Jan;256(1):45–48. doi: 10.1016/0921-8734(91)90032-7. [DOI] [PubMed] [Google Scholar]
- Morin G. B. The human telomere terminal transferase enzyme is a ribonucleoprotein that synthesizes TTAGGG repeats. Cell. 1989 Nov 3;59(3):521–529. doi: 10.1016/0092-8674(89)90035-4. [DOI] [PubMed] [Google Scholar]
- Nilsson P., Mehle C., Remes K., Roos G. Telomerase activity in vivo in human malignant hematopoietic cells. Oncogene. 1994 Oct;9(10):3043–3048. [PubMed] [Google Scholar]
- Pereira-Smith O. M., Smith J. R. Evidence for the recessive nature of cellular immortality. Science. 1983 Sep 2;221(4614):964–966. doi: 10.1126/science.6879195. [DOI] [PubMed] [Google Scholar]
- Rheinwald J. G., Beckett M. A. Tumorigenic keratinocyte lines requiring anchorage and fibroblast support cultured from human squamous cell carcinomas. Cancer Res. 1981 May;41(5):1657–1663. [PubMed] [Google Scholar]
- Rhyu M. S. Telomeres, telomerase, and immortality. J Natl Cancer Inst. 1995 Jun 21;87(12):884–894. doi: 10.1093/jnci/87.12.884. [DOI] [PubMed] [Google Scholar]
- Shay J. W., Tomlinson G., Piatyszek M. A., Gollahon L. S. Spontaneous in vitro immortalization of breast epithelial cells from a patient with Li-Fraumeni syndrome. Mol Cell Biol. 1995 Jan;15(1):425–432. doi: 10.1128/mcb.15.1.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor-Papadimitriou J., Purkis P., Lane E. B., McKay I. A., Chang S. E. Effects of SV40 transformation on the cytoskeleton and behavioural properties of human keratinocytes. Cell Differ. 1982 May;11(3):169–180. doi: 10.1016/0045-6039(82)90008-2. [DOI] [PubMed] [Google Scholar]
- Tilgen W., Boukamp P., Breitkreutz D., Dzarlieva R. T., Engstner M., Haag D., Fusenig N. E. Preservation of morphological, functional, and karyotypic traits during long-term culture and in vivo passage of two human skin squamous cell carcinomas. Cancer Res. 1983 Dec;43(12 Pt 1):5995–6011. [PubMed] [Google Scholar]