Abstract
Purpose
This study was designed to determine the tumorigenicity of the AS30D HCC cell line following orthotopic injection into rat liver and preliminarily characterize the tumor model by both magnetic resonance imaging (MRI) and ultrasound (US) as well as histopathology and immunohistochemistry.
Materials
AS30D cell line in vitro proliferation was assessed by using MTT assay. Female rats (N = 5) underwent injection of the AS30D cell line into one site in the liver. Rats subsequently underwent MR imaging at days 7 and 14 to assess tumor establishment and volume. One rat underwent US of the liver at day 7. Rats were euthanized at day 7 or 14 and livers were subjected to gross, histopathologic (H&E), and immunohistochemical (CD31) analysis to assess for tumor growth and neovascularization.
Results
AS30D cell line demonstrated an in vitro doubling time of 33.2 ± 5.3 h. MR imaging demonstrated hyperintense T2-weighted and hypointense T1-weighted lesions with tumor induction in five of five and three of three sites at days 7 and 14, respectively. The mean (SD) tumor volume was 126.1 ± 36.2 mm3 at day 7 (N = 5). US of the liver demonstrated a well-circumscribed, hypoechoic mass and comparison of tumor dimensions agreed well with MRI. Analysis of H&E- and CD31-stained sections demonstrated moderate-high grade epithelial tumors with minimal tumor necrosis and evidence of diffuse intratumoral and peritumoral neovascularization by day 7.
Conclusions
AS30D HCC cell line is tumorigenic following orthotopic injection into rat liver and can be used to generate an early vascularizing, slower-growing rat HCC tumor model.
Keywords: Hepatocellular carcinoma, Rat model, Magnetic resonance imaging
Introduction
Animal models are critical for both mechanistic as well as therapeutic preclinical oncology research. In the field of interventional oncology (IO), a number of animal models have been utilized for examining the safety, efficacy, and mechanisms of both percutaneous ablative and catheter-directed therapies in the liver [1–3]. More recently, there has been growing interest in the use of orthotopic rat hepatocellular carcinoma (HCC) models in the field of IO, including the Morris (McA-RH7777) and Novikoff (N1S1) HCC models, the latter has been utilized as a model in the liver for catheter-directed chemotherapy, and Y-90 administration as well as irreversible electroporation and laser ablation [4–10].
However, recent studies utilizing whole genome expression profiling and comparative functional genomics in human and murine HCC have identified different molecular subtypes with different prognostic and therapeutic significance [11–14]. Consequently, no single cell line or animal model can recapitulate the molecular or phenotypic heterogeneity of HCC. As such, there is a need for a diversity of HCC cell lines and animal models in preclinical interventional oncologic research to better understand how differential tumor biology affects therapeutic response to both percutaneous and catheter-directed therapies [1]. The AS30D HCCcell line was derived from the liver of a female Sprague-Dawley (SD) rat by induction with chemical carcinogenesis with 3-methyl-4-dimethylaminoazobenze in a distinct manner from the N1S1 cell line [15, 16]. However, unlike the N1S1 cell line, the tumorigenicity of the AS30D cell line following orthotopic injection into SD rat liver is not known. Given the cost-effectiveness and technical feasibility of developing syngeneic orthotopic rat HCC models and their broad applicability for interventional oncologic studies and oncologic liver imaging, generation of new orthotopic models with other cell lines is necessary to increase the biologic diversity of existing HCC models.
The purpose of this study was to determine the tumorigenicity of the AS30D HCC cell line following orthotopic injection into rat liver and preliminarily characterize the tumor model by both magnetic resonance imaging (MRI) and ultrasound (US) as well as histopathology and immunohistochemistry.
Materials and Methods
Rats
All studies approved by institutional animal care and use committee (IACUC). Five female SD rats (Charles River, Wilmington, MA) weighing 350–400 g were used in this pilot study. Rats were housed in individual cages in a climate controlled setting with access to food and water ad libitum and maintained on a 12-h light/dark cycle.
Cell Line and Proliferation
The AS30D rat HCC cell line (DSMZ, Germany) was cultured in a 37 °C, 5 % CO2 humidified incubator and passaged three times per week per distributor instructions to maintain >90 % cell viability [15]. To determine the in vitro doubling time, the AS30D cell line was plated in 96-well plates in complete media in cell dilutions between 1,000–20,000 cells/well (N = 6 replicates) and incubated in a 37 °C, 5 % CO2 incubator for 24–96 h. At 24, 48, 72, and 96 h, MTT cell proliferation assay (ATCC, Manassas, VA) was performed per manufacturer instruction and absorbance measured on a DTX 880 microplate reader (Beckman Coulter, Brea, CA). For each cell dilution, the average absorbance (log10) was first plotted versus time to determine the linear relationship between cell number and absorbance using Prism 5.0 (GraphPad Software, Inc., La Jolla, CA) and next the doubling time (hours) was calculated using the method described by Minko et al. [17].
Model Development
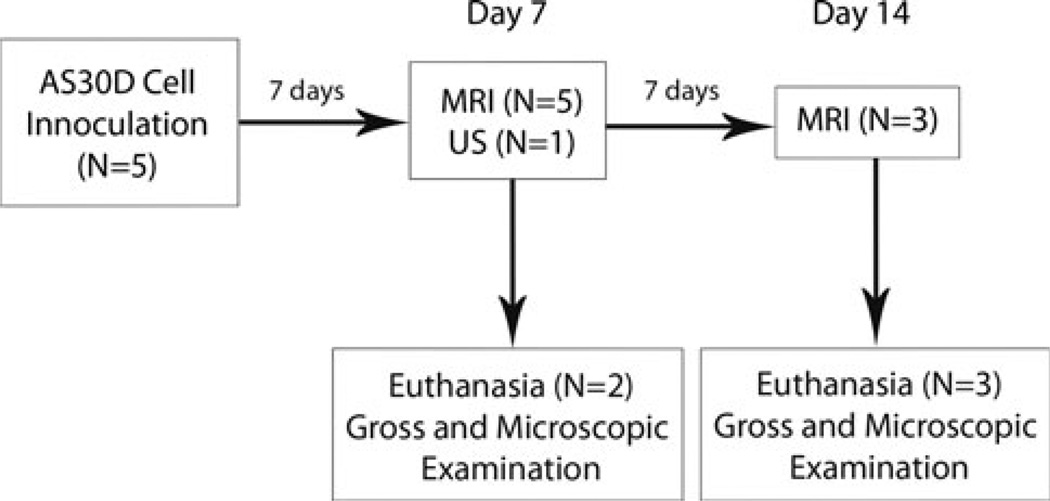
The experimental design is summarized as a flow diagram in Fig. 1. The rats were anesthetized with an intraperitoneal injection of ketamine/xylazine and a vertical midline incision was made beginning just inferior to the xyphoid process as previously described [10]. 3 × 106 cells in 150 µl of incomplete media were injected into a subcapsular location in the middle hepatic lobe of each rat (N = 5) [10]. Incision was closed with surgical suture as previously described and rats allowed to recover for 7 days [10].
Fig. 1.
Flow diagram showing the experimental design and procedures
Magnetic Resonance Imaging
The rats were anesthetized, placed in a BC-10 wrist coil manufactured by the institutional MRI research lab, and imaged using a 3.0T MR scanner (GE Medical Inc., Milwaukee, WI) with non-contrast-enhanced Fast Spin Echo (FSE) T1 and T2 in three planes at 7 (N = 5) and 14 (N = 3) days after cell injection to assess tumor induction and dimensions [10]. Tumor induction and dimensions were measured from the FSE T2 images in the axial, coronal, or sagittal planes by an MR radiologist. Tumor volumes were calculated by using the formula for an ellipsoid by multiplying the maximum longitudinal diameter of the tumor in millimeters (mm) in the superior–inferior (SI), lateral–medial (LM), and anterior–posterior (AP) planes by 0.523 as the correction factor [SI × LM × AP × 0.523] [10].
Ultrasound
One rat underwent US of the liver at 7 days postinjection to assess the feasibility of US for tumor visualization and measurement of tumor dimensions for comparison with MR images. Briefly, the rat was anesthetized with inhalation isoflurane and the abdomen shaved and prepped with depilatory cream to remove excess fur. The rat was secured to a warmed stage and underwent US of the liver using the Vevo770™ small animal US system (Visualsonics, Toronto, Canada) with a 30 MHz transducer (RMV-707B). The maximum longitudinal diameter of the tumor in millimeters (mm) in the SI, LM, and AP planes was measured from the US images for comparison of the three corresponding tumor dimensions measured from MRI.
Gross and Microscopic Pathology
Rats were euthanized with CO2 inhalation at days 7 (N = 2) and 14 (N = 3), and liver and tumor tissue harvested for gross and microscopic analysis from all hepatic lobes in all rats as well as lungs for examination of micrometastases. The specimens were placed in 10 % neutral buffered formalin (Fisher Scientific/Acros, Waltham, MA), embedded in paraffin, sectioned with a microtome, and stained with hematoxylin–eosin (H&E) to assess cytologic features and evidence for tumor necrosis and local and vascular invasion. In addition, to assess for evidence of early tumor neovascularization, sections underwent immunohistochemical staining for CD31 [18]. Briefly, slides were dewaxed in xylene and rehydrated through graded alcohols. Antigen retrieval was performed by heat treatment in citrate buffer for 30 min, using a vegetable steamer. Sections were incubated with rabbit polyclonal CD31 antibody (1:50; Abcam #ab28364). Rabbit Envision Plus HRP labeled polymer (DakoCytomation) was used for the secondary antibody. Color development was performed using diaminobenzidine (DAB) liquid substrate (Sigma, St. Louis, MO) followed by hematoxylin counterstain. Slides were dehydrated through graded alcohols, cleared with xylene, and coverslipped using Permount mounting media (Fisher Scientific). All slides were evaluated for cytologic features, tumor growth, local and vascular invasion, necrosis, and neovascularization by light microscopy by an experienced pathologist (21 years).
Statistical Analysis
All statistical analyses were performed using JMP 9.0 (SAS, Cary, NC). Tumor volume (mm3) was compared between days 7 and 14 by using the exact Mann–Whitney test. The agreement in measured tumor dimensions (mm) between MRI and US was analyzed by using the Bland–Altman method [19]. P < 0.05 was the level for statistical significance.
Results
Proliferation Index
AS30D cell line demonstrated an in vitro doubling time of approximately 33.2 ± 5.3 h.
Magnetic Resonance Imaging
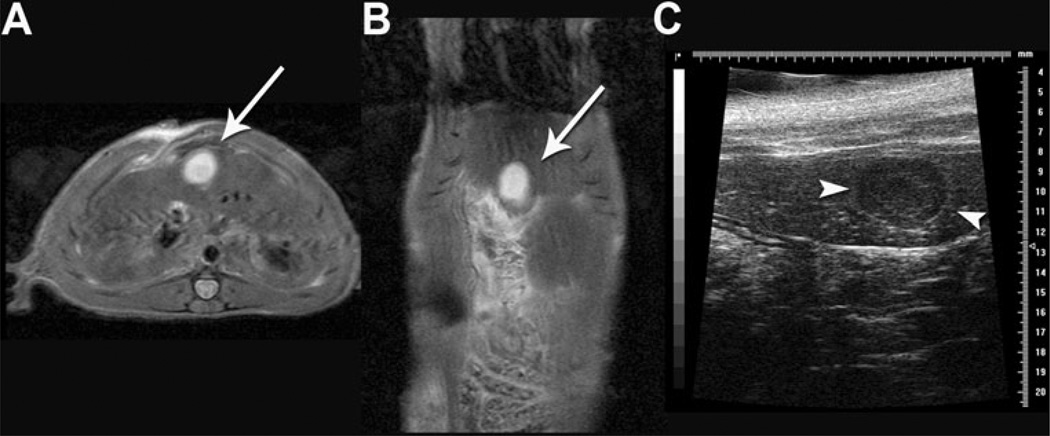
3.0T MR imaging at day 7 demonstrated hyperintense T2-weighted (Figs. 2A, B) and hypointense T1-weighted (data not shown) liver lesions in the rats. MR imaging revealed solitary liver lesions at five of five sites (100 %) and three of three sites (100 %) in female rats at days 7 and 14, respectively. The mean (SD) tumor volume was 126.1 ± 36.2 mm3 at day 7 (N = 5) but did not significantly change from day 7 to day 14 in the three rats still under study (107.2 ± 35.2 mm3 vs. 79.3 ± 29.7 mm3, respectively; P > 0.05).
Fig. 2.
3.0T magnetic resonance (MR) and US imaging of rat liver 7 days postsurgery with AS30D HCC cell injection at the time of surgery. Axial (A) and coronal Fast Spin Echo (FSE) (B) T2-weighted MR images demonstrate a 0.5-cm hyperintense liver lesion. C Corresponding US demonstrates a circumscribed, hypoechoic lesion (white arrowheads) within the liver lobe
Ultrasound
US of one tumor-bearing rat demonstrated a circumscribed, hypoechoic lesion within the liver (Fig. 2C). Comparison of the maximum longitudinal diameter of the tumor in millimeters (mm) in the superior–inferior (SI), lateral–medial (LM), and anterior–posterior (AP) planes measured from MRI and US demonstrated good agreement by Bland–Altman analysis with a bias of 0.2 ± 0.4 mm (−1.0 to 0.6 mm, 95 % limits of agreement; Table 1).
Table 1.
Comparison of AS30D tumor dimension (mm) measurements by MRI and US
| Tumor dimension (mm) | MRI | US |
|---|---|---|
| Anterior–posterior | 5.1 | 5.0 |
| Width | 6.1 | 6.8 |
| Superior–inferior | 5.7 | 5.8 |
Pathology
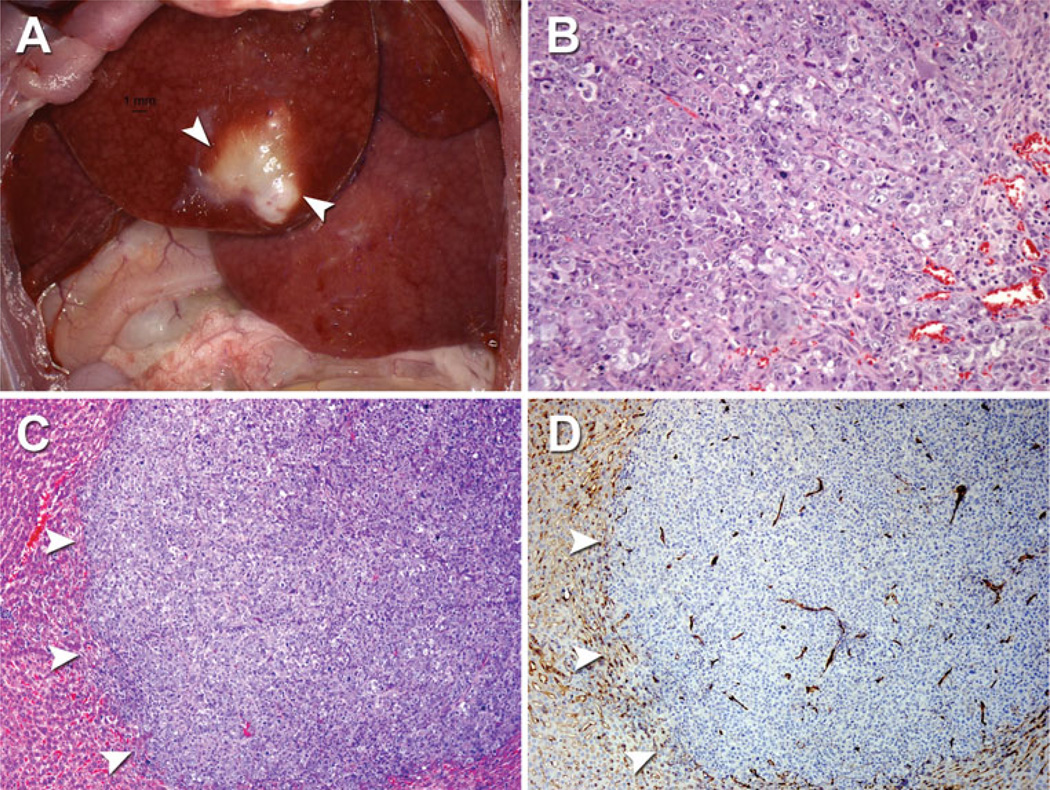
In the rats, gross analysis demonstrated a solid, yellow-white mass growing within the liver parenchyma at day 7 (N = 2) and day 14 (N = 3; Fig. 3A). Analysis of H&E-stained sections revealed a moderate–high grade, well-circumscribed, expansive epithelial-shaped tumor demonstrating cellular and nuclear pleomorphism, mitotic figures, intracellular vacuoles, intratumoral vessel formation, and minimal areas of central tumor necrosis at days 7 and 14 but without evidence of local or vascular invasion (Fig. 3B, C). In addition, immunohistochemical analysis of CD31 stained sections demonstrated diffuse intratumoral and peritumoral-positive CD31 staining confirming tumor neovascularization as early as day 7 after cell injection (Fig. 3D). Microscopic examination of the lungs at days 7 and 14 did not reveal evidence of micrometastases (data not shown).
Fig. 3.
Gross and microscopic pathology of AS30D tumor model. A Gross pathology of AS30D tumor in the right segment of the median hepatic lobe (white arrowheads) 7 days after cell injection. Photomicrographs: B AS30D tumor with evidence of cellular and nuclear pleomorphism, mitotic figures, intracellular vacuoles and blood vessel formation (H&E 100×). C AS30D tumor (H&E, 50×) and D corresponding immunohistochemistry demonstrating diffuse intratumoral and peritumoral CD31 staining outlining neovascularization (CD31, 50×). White arrowheads in (C) and (D) denote tumor margin
Discussion
In this study, an orthotopic, syngeneic immunocompetent rat HCC model was developed using the AS30D rat HCC cell line and preliminarily characterized by imaging (MRI, US) and microscopic pathology (H&E, CD31). Given the need for cost-effective, biologically diverse small animal HCC models in preclinical tumor biology and IO research, the AS30D model may serve as an early, vascularizing HCC model for both mechanistic as well as therapeutic studies in HCC [1, 2].
Successful technical development of orthotopic tumor models is affected by both biological variables, including the tumorigenicity of the cell line, and technical variables, including cell number, injection medium and volume, needle gauge, and injection speed [4, 5, 7, 8, 10, 20–22]. Because the AS30D cell line was derived from female rats, the study protocol included female SD rats. Guo et al. [4] reported successful generation of both the N1S1 tumor model in SD rats and the Morris (McA-RH7777) model in Buffalo rats using rats with the corresponding sex from which each HCC cell line was derived. Moreover, previous studies have reported a range of cell numbers from 1 × 106 to 6 × 106 cells per orthotopic injection using the N1S1 cell line [4, 5, 7, 8, 10, 20–22]. Preliminary experiments injecting 1 × 106 AS30D cells failed to result in tumor growth (data not shown). Injection of 3 × 106 into female SD rat livers resulted in 100 % tumor induction by day 7 confirmed by MRI and pathology. These findings demonstrate the importance of cell linespecific dose response for tumor induction. Additionally, tumor growth was maintained in three of three rats by day 14 as confirmed by MRI and both gross and microscopic pathology, but the tumor volume was not significantly different than day 7. The AS30D cell line demonstrates a relatively slow doubling time of approximately 33 h in vitro compared with N1S1 cell line with a doubling times of approximately 18 h in vitro (data not shown). This slower proliferation rate for the AS30D cell line in vitro may explain in part the slower tumor growth in vivo. Taken together, these data demonstrate proof of concept that the AS30D cell line is tumorigenic following orthotopic injection into the liver of immunocompetent SD rats but suggest that that injection of greater than 3 × 106 cells may be necessary for larger or sustained tumor growth.
Regarding imaging characterization, FSE MR imaging demonstrated hyperintense T2-weighted liver lesions and hypointense T1-weighted liver lesions. Although MRI provides high-resolution images for tumor visualization, US is more widely available and easier to use. Therefore, US was utilized in one tumor-bearing rat using the Vevo770™ small animal system with inhalation anesthesia to compare tumor visualization by US to corresponding MRI [23]. US demonstrated a circumscribed, hypoechoic lesion within the liver. The tumor morphology and dimensions measured by US agreed well with the MRI data. Taken together, US is technically feasible and congruent with MRI findings and therefore may provide an alternative imaging modality for assessing tumor growth in this model.
Pathologic characterization of the AS30D tumor model demonstrated a solid mass with gross evidence of vascularization that was confirmed by both H&E and CD31 immunostaining, suggesting that the AS30D tumor exhibits neoangiogenesis early during tumorigenesis. The vascular features of the AS30D tumor may be more similar to those reported by Guo et al. [4] in the Morris (McA-RH7777) HCC model than the N1S1 model by CD34 immunostaining.H&E analysis demonstrated morphologic tumor growth in a sheet like pattern similar to the N1S1 tumor [4, 10]. Microscopic analysis did not reveal evidence of local or vascular tumor invasion within the liver or micrometastases in the lungs at days 7 and 14. However, given the slow proliferation rate in vitro, a longer study period may be necessary to better characterize the metastatic potential of this model.
Our study had a number of limitations. Extended AS30D tumor growth was examined in a small number of rats for up to 14 days in this pilot study. The studies outlined warrant further investigation in a larger sample size of rats for a longer duration to determine further the optimal tumor growth kinetics and to assess better the metastatic potential of this model. Additionally, contrast-enhanced MRI was not performed in these rats. The early neovascularization of the AS30D tumor was not determined until after euthanasia at pathologic analysis. However, given the vascular nature of the tumor, contrast-enhanced imaging should be explored. Moreover, US was technically feasible and showed good correspondence with MRI measurements performed in only one animal but warrants further investigation to characterize more completely the ultrasonographic features of the AS30D tumor. Furthermore, the present model utilized inoculation of HCC cells into normal liver. However, given the critical role of cirrhosis and tumor microenvironment in the clinical pathogenesis of HCC, the AS30D model warrants further investigation in the setting of background liver disease similar to the recently described N1S1 common bile duct ligation (CBDL) model to recapitulate more closely the HCC tumor microenvironment in the setting of background liver disease [10]. Finally, given the phenotypic and genotypic variation in HCC cell lines used in preclinical research, further work is needed to characterize the genetic and molecular mechanisms driving the AS30D cell line to phenotype this model relative to other murine, rat, and human HCC cell lines and is the subject of ongoing work [11–13].
In this pilot study, we demonstrated that the AS30D HCC cell line is tumorigenic following orthotopic injection into the liver of immunocompetent SD rats to generate an early vascularizing HCC tumor model and demonstrated the feasibility of noninvasive imaging monitoring of tumor growth by both MRI and US. As evidenced by growing interest in preclinical development of synergistic anticancer therapies in IO, the need for a diversity of small animal, disease-specific models is critical to advance the science and clinical translation of these novel therapeutics [24–26]. As such, the AS30D model may serve as an additional orthotopic HCC model to previously reported N1S1 and Morris (McA-RH7777) models for preclinical HCC tumor biology and IO research.
Acknowledgments
Infrastructure support provided by NIH construction grant NIH C06 RR018898. This publication was made possible by CTSA Grant Number UL1 TR000135 from the National Center for Advancing Translational Sciences (NCATS), a component of the National Institutes of Health (NIH). Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NIH. Additional research support provided in part by SIR Foundation Allied Scientist Training Grant (Mr. Scott Thompson) and RSNA Research Scholar Grant (Dr. David Woodrum).
Disclosures Regarding disclosures, Mr. Scott Thompson has received a research grant from the SIR Foundation (SIR Allied Scientist Training Grant); Dr. Matthew Callstrom has received research grants from Siemens Medical Systems and Endocare, Inc.; Dr. Lewis Roberts has received research grants from Bristol Myers-Squibb, Merck, Nordion and Bayer; Dr. David Woodrum has received a research Grant from Visualase, Inc., and an RSNA Research Scholar Grant.
Footnotes
Conflict of interest The authors report no conflicts of interest and have conducted this research in compliance with institutional ethical and regulatory guidelines.
Contributor Information
Scott M. Thompson, Medical Scientist Training Program, Mayo Clinic, Rochester, MN, USA
Matthew R. Callstrom, Department of Radiology, Mayo Clinic, 200 First Street Southwest, Rochester, MN 55905, USA
Bruce Knudsen, Department of Laboratory Medicine and Pathology, Mayo Clinic, Rochester, MN, USA.
Jill L. Anderson, Division of Physiology and Bioengineering, Mayo Clinic, Rochester, MN 55905, USA
Kim A. Butters, Department of Laboratory Medicine and Pathology, Mayo Clinic, Rochester, MN, USA
Joseph P. Grande, Department of Laboratory Medicine and Pathology, Mayo Clinic, Rochester, MN, USA
Lewis R. Roberts, Division of Gastroenterology and Hepatology, Mayo Clinic, Rochester, MN, USA
David A. Woodrum, Email: woodrum.david@mayo.edu, Department of Radiology, Mayo Clinic, 200 First Street Southwest, Rochester, MN 55905, USA.
References
- 1.Aravalli RN, Steer CJ, Sahin MB, Cressman EN. Stem cell origins and animal models of hepatocellular carcinoma. Dig Dis Sci. 2010;55(5):1241–1250. doi: 10.1007/s10620-009-0861-x. [DOI] [PubMed] [Google Scholar]
- 2.Aravalli RN, Golzarian J, Cressman EN. Animal models of cancer in interventional radiology. Eur Radiol. 2009;19(5):1049–1053. doi: 10.1007/s00330-008-1263-8. [DOI] [PubMed] [Google Scholar]
- 3.Burke CT, Cullen JM, State A, et al. Development of an animal model for radiofrequency ablation of primary, virally induced hepatocellular carcinoma in the woodchuck. J Vasc Interv Radiol. 2011;22(11):1613–1618. doi: 10.1016/j.jvir.2011.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guo Y, Klein R, Omary RA, Yang GY, Larson AC. Highly malignant intra-hepatic metastatic hepatocellular carcinoma in rats. Am J Transl Res. 2010;3(1):114–120. [PMC free article] [PubMed] [Google Scholar]
- 5.Ju S, McLennan G, Bennett SL, et al. Technical aspects of imaging and transfemoral arterial treatment of N1-S1 tumors in rats: an appropriate model to test the biology and therapeutic response to transarterial treatments of liver cancers. J Vasc Interv Radiol. 2009;20(3):410–414. doi: 10.1016/j.jvir.2008.12.408. [DOI] [PubMed] [Google Scholar]
- 6.Lin WY, Tsai SC, Hsieh JF, Wang SJ. Effects of 90Y-microspheres on liver tumors: comparison of intratumoral injection method and intra-arterial injection method. J Nucl Med. 2000;41(11):1892–1897. [PubMed] [Google Scholar]
- 7.Guo Y, Zhang Y, Klein R, et al. Irreversible electroporation therapy in the liver: longitudinal efficacy studies in a rat model of hepatocellular carcinoma. Cancer Res. 2010;70(4):1555–1563. doi: 10.1158/0008-5472.CAN-09-3067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chan HH, Chu TH, Chien HF, et al. Rapid induction of orthotopic hepatocellular carcinoma in immune-competent rats by non-invasive ultrasound-guided cells implantation. BMC Gastroenterol. 2010;10:83. doi: 10.1186/1471-230X-10-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McLennan G, Bennett SL, Ju S, et al. Tumor response and apoptosis of N1-S1 rodent hepatomas in response to intra-arterial and intravenous benzamide riboside. Cardiovasc Intervent Radiol. 2012;35(3):645–652. doi: 10.1007/s00270-011-0140-z. [DOI] [PubMed] [Google Scholar]
- 10.Thompson SM, Callstrom MR, Knudsen B, et al. Development and preliminary testing of a translational model of hepatocellular carcinoma for MR imaging and interventional oncologic investigations. J Vasc Interv Radiol. 2012;23(3):385–395. doi: 10.1016/j.jvir.2011.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee JS, Chu IS, Mikaelyan A, et al. Application of comparative functional genomics to identify best-fit mouse models to study human cancer. Nat Genet. 2004;36(12):1306–1311. doi: 10.1038/ng1481. [DOI] [PubMed] [Google Scholar]
- 12.Lee JS, Thorgeirsson SS. Comparative and integrative functional genomics of HCC. Oncogene. 2006;25(27):3801–3809. doi: 10.1038/sj.onc.1209561. [DOI] [PubMed] [Google Scholar]
- 13.Andersen JB, Factor VM, Marquardt JU, et al. An integrated genomic and epigenomic approach predicts therapeutic response to zebularine in human liver cancer. Sci Transl Med. 2010;2(54):54ra77. doi: 10.1126/scitranslmed.3001338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chi JT, Thrall DE, Jiang C, et al. Comparison of genomics and functional imaging from canine sarcomas treated with thermoradiotherapy predicts therapeutic response and identifies combination therapeutics. Clin Cancer Res. 2011;17(8):2549–2560. doi: 10.1158/1078-0432.CCR-10-2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Smith DF, Walborg EF, Jr, Chang JP. Establishment of a transplantable ascites variant of a rat hepatoma induced by 3′-methyl-4-dimethylaminoazobenzene. Cancer Res. 1970;30(9):2306–2309. [PubMed] [Google Scholar]
- 16.Hotchin JE. The cultivation of Novikoff rat hepatoma cells in vitro. Cancer Res. 1957;17(7):682–687. [PubMed] [Google Scholar]
- 17.Minko T, Kopeckova P, Kopecek J. Chronic exposure to HPMA copolymer-bound adriamycin does not induce multidrug resistance in a human ovarian carcinoma cell line. J Control Release. 1999;59(2):133–148. doi: 10.1016/s0168-3659(98)00186-2. [DOI] [PubMed] [Google Scholar]
- 18.Giatromanolaki A, Koukourakis MI, Theodossiou D, et al. Comparative evaluation of angiogenesis assessment with anti-factor-VIII and anti-CD31 immunostaining in non-small cell lung cancer. Clin Cancer Res. 1997;3(12 Pt 1):2485–2492. [PubMed] [Google Scholar]
- 19.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1(8476):307–310. [PubMed] [Google Scholar]
- 20.Tsujimoto T, Kuriyama S, Yamazaki M, et al. Augmented hepatocellular carcinoma progression and depressed Kupffer cell activity in rat cirrhotic livers. Int J Oncol. 2001;18(1):41–47. doi: 10.3892/ijo.18.1.41. [DOI] [PubMed] [Google Scholar]
- 21.Garin E, Denizot B, Roux J, et al. Description and technical pitfalls of a hepatoma model and of intra-arterial injection of radiolabelled lipiodol in the rat. Lab Anim. 2005;39(3):314–320. doi: 10.1258/0023677054307051. [DOI] [PubMed] [Google Scholar]
- 22.Lui WY, Chi CW, Hsieh CC, et al. Establishment of in vivo hepatoma models in rat and mouse from rodent hepatoma cell lines. Zhonghua Yi Xue Za Zhi (Taipei) 1995;55(5):353–360. [PubMed] [Google Scholar]
- 23.Chen JY, Chen HL, Wu SH, et al. Application of high-frequency ultrasound for the detection of surgical anatomy in the rodent abdomen. Vet J. 2011;191(2):246–252. doi: 10.1016/j.tvjl.2010.12.024. [DOI] [PubMed] [Google Scholar]
- 24.Soundararajan A, Dodd GD, 3rd, Bao A, et al. Chemoradionuclide therapy with 186Re-labeled liposomal doxorubicin in combination with radiofrequency ablation for effective treatment of head and neck cancer in a nude rat tumor xenograft model. Radiology. 2011;261(3):813–823. doi: 10.1148/radiol.11110361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang W, Ahmed M, Tasawwar B, et al. Radiofrequency ablation combined with liposomal quercetin to increase tumour destruction by modulation of heat shock protein production in a small animal model. Int J Hyperthermia. 2011;27(6):527–538. doi: 10.3109/02656736.2011.582474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ganapathy-Kanniappan S, Kunjithapatham R, Torbenson MS, et al. Human hepatocellular carcinoma in a mouse model: assessment of tumor response to percutaneous ablation by using glyceraldehyde-3-phosphate dehydrogenase antagonists. Radiology. 2012;262(3):834–845. doi: 10.1148/radiol.11111569. [DOI] [PMC free article] [PubMed] [Google Scholar]