Abstract
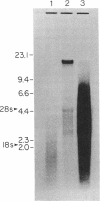
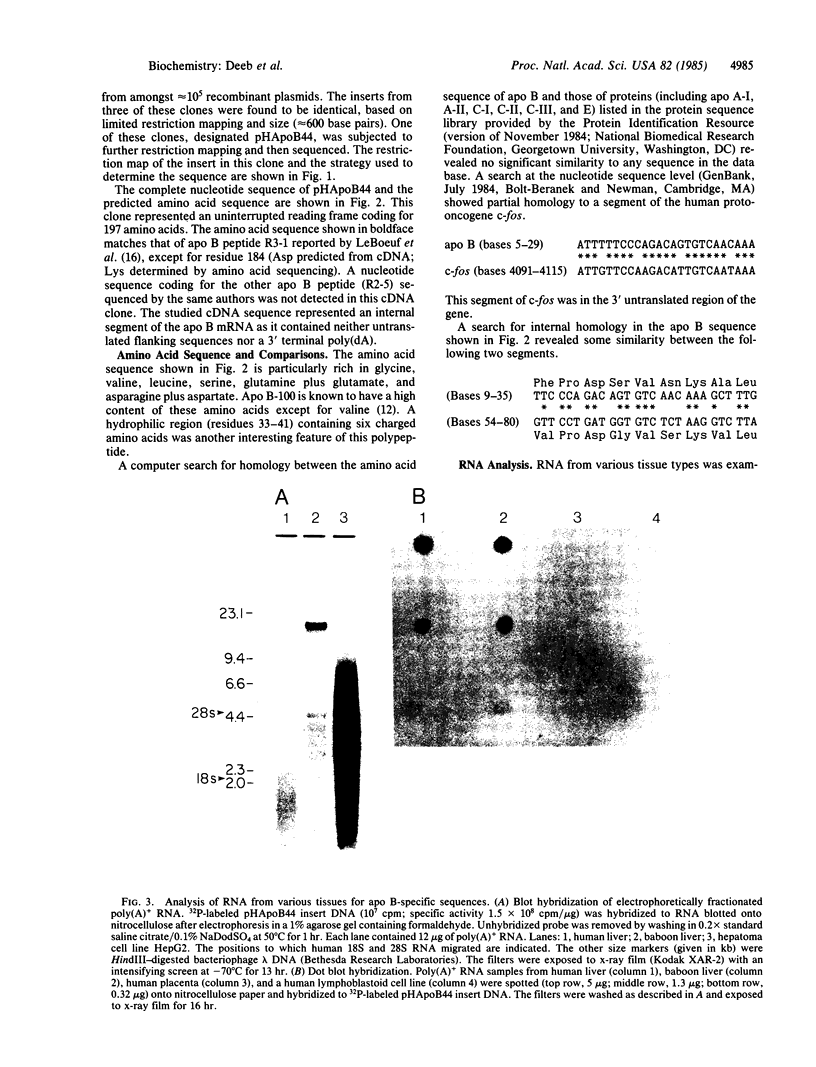
A human liver cDNA library was screened for sequences coding for apolipoprotein B (apo B), the major protein of human low density lipoproteins. A mixture of synthetic oligonucleotides (26 bases long) coding for an amino acid sequence known to exist in apo B was used as a hybridization probe. A clone was identified that had a cDNA insert of 593 base pairs and that contained sequences coding for a peptide of 24 residues that had earlier been isolated from apo B by limited proteolysis. The entire nucleotide sequence of the cDNA insert consists of one open reading frame coding for 197 amino acids. Apo B-related RNAs were found in human liver, baboon liver, and the human hepatoma cell line HepG2. None were detected in placenta, simian virus 40 (SV40)-transformed fibroblasts, and a lymphoblastoid cell line. The length of the mature apo B mRNA was estimated to be 18 kb, enough to code for a protein with a molecular weight in the neighborhood of 500,000.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradley W. A., Rohde M. F., Gotto A. M., Jr, Jackson R. L. The cyanogen bromide peptides of the apoprotein of low density lipoprotein (ApoB): its molecular weight from a chemical view. Biochem Biophys Res Commun. 1978 Apr 14;81(3):928–935. doi: 10.1016/0006-291x(78)91440-7. [DOI] [PubMed] [Google Scholar]
- Brown M. S., Goldstein J. L. Lipoprotein receptors in the liver. Control signals for plasma cholesterol traffic. J Clin Invest. 1983 Sep;72(3):743–747. doi: 10.1172/JCI111044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunzell J. D., Albers J. J., Chait A., Grundy S. M., Groszek E., McDonald G. B. Plasma lipoproteins in familial combined hyperlipidemia and monogenic familial hypertriglyceridemia. J Lipid Res. 1983 Feb;24(2):147–155. [PubMed] [Google Scholar]
- Brunzell J. D., Sniderman A. D., Albers J. J., Kwiterovich P. O., Jr Apoproteins B and A-I and coronary artery disease in humans. Arteriosclerosis. 1984 Mar-Apr;4(2):79–83. doi: 10.1161/01.atv.4.2.79. [DOI] [PubMed] [Google Scholar]
- Cardin A. D., Witt K. R., Barnhart C. L., Jackson R. L. Sulfhydryl chemistry and solubility properties of human plasma apolipoprotein B. Biochemistry. 1982 Aug 31;21(18):4503–4511. doi: 10.1021/bi00261a048. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Goldstein J. L., Brown M. S. The low-density lipoprotein pathway and its relation to atherosclerosis. Annu Rev Biochem. 1977;46:897–930. doi: 10.1146/annurev.bi.46.070177.004341. [DOI] [PubMed] [Google Scholar]
- Kane J. P. Apolipoprotein B: structural and metabolic heterogeneity. Annu Rev Physiol. 1983;45:637–650. doi: 10.1146/annurev.ph.45.030183.003225. [DOI] [PubMed] [Google Scholar]
- Kane J. P., Hardman D. A., Paulus H. E. Heterogeneity of apolipoprotein B: isolation of a new species from human chylomicrons. Proc Natl Acad Sci U S A. 1980 May;77(5):2465–2469. doi: 10.1073/pnas.77.5.2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowles B. B., Howe C. C., Aden D. P. Human hepatocellular carcinoma cell lines secrete the major plasma proteins and hepatitis B surface antigen. Science. 1980 Jul 25;209(4455):497–499. doi: 10.1126/science.6248960. [DOI] [PubMed] [Google Scholar]
- LeBoeuf R. C., Miller C., Shively J. E., Schumaker V. N., Balla M. A., Lusis A. J. Human apolipoprotein B: partial amino acid sequence. FEBS Lett. 1984 May 7;170(1):105–108. doi: 10.1016/0014-5793(84)81378-2. [DOI] [PubMed] [Google Scholar]
- Lee D. M., Valente A. J., Kuo W. H., Maeda H. Properties of apolipoprotein B in urea and in aqueous buffers. The use of glutathione and nitrogen in its solubilization. Biochim Biophys Acta. 1981 Oct 23;666(1):133–146. doi: 10.1016/0005-2760(81)90099-0. [DOI] [PubMed] [Google Scholar]
- Mahley R. W., Innerarity T. L., Rall S. C., Jr, Weisgraber K. H. Plasma lipoproteins: apolipoprotein structure and function. J Lipid Res. 1984 Dec 1;25(12):1277–1294. [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Milne R. W., Marcel Y. L. Monoclonal antibodies against human low density lipoprotein. Stoichiometric binding studies using Fab fragments. FEBS Lett. 1982 Sep 6;146(1):97–100. doi: 10.1016/0014-5793(82)80712-6. [DOI] [PubMed] [Google Scholar]
- Pollard H., Scanu A. M., Taylor E. W. On the geometrical arrangement of the protein subunits of human serum low-density lipoprotein: evidence for a dodecahedral model. Proc Natl Acad Sci U S A. 1969 Sep;64(1):304–310. doi: 10.1073/pnas.64.1.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith R., Dawson J. R., Tanford C. The size and number of polypeptide chains in human serum low density lipoprotein. J Biol Chem. 1972 Jun 10;247(11):3376–3381. [PubMed] [Google Scholar]
- Socorro L., Camejo G. Preparation and properties of soluble, immunoreactive apoLDL. J Lipid Res. 1979 Jul;20(5):631–638. [PubMed] [Google Scholar]
- Steele J. C., Jr, Reynolds J. A. Characterization of the apolipoprotein B polypeptide of human plasma low density lipoprotein in detergent and denaturation solutions. J Biol Chem. 1979 Mar 10;254(5):1633–1638. [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA transferred or dotted nitrocellulose paper. Methods Enzymol. 1983;100:255–266. doi: 10.1016/0076-6879(83)00060-9. [DOI] [PubMed] [Google Scholar]
- Wallace R. B., Johnson M. J., Hirose T., Miyake T., Kawashima E. H., Itakura K. The use of synthetic oligonucleotides as hybridization probes. II. Hybridization of oligonucleotides of mixed sequence to rabbit beta-globin DNA. Nucleic Acids Res. 1981 Feb 25;9(4):879–894. doi: 10.1093/nar/9.4.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace R. B., Shaffer J., Murphy R. F., Bonner J., Hirose T., Itakura K. Hybridization of synthetic oligodeoxyribonucleotides to phi chi 174 DNA: the effect of single base pair mismatch. Nucleic Acids Res. 1979 Aug 10;6(11):3543–3557. doi: 10.1093/nar/6.11.3543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zannis V. I., Breslow J. L., SanGiacomo T. R., Aden D. P., Knowles B. B. Characterization of the major apolipoproteins secreted by two human hepatoma cell lines. Biochemistry. 1981 Dec 8;20(25):7089–7096. doi: 10.1021/bi00528a006. [DOI] [PubMed] [Google Scholar]