Abstract
Purpose
The objective of this analysis was to evaluate the effects of dietary B vitamin intakes on creatinine-adjusted urinary total arsenic concentration among individuals participating in the Health Effects of Arsenic Longitudinal Study (HEALS) cohort in Araihazar, Bangladesh. Arsenic exposure is a major public health problem in Bangladesh, where nearly 77 million people have been chronically exposed to arsenic through the consumption of naturally contaminated groundwater. Dietary factors influencing the metabolism of ingested arsenic may potentially be important modifiers of the health effects of arsenic in this population.
Methods
Daily average B vitamin intakes from a validated food frequency questionnaire and laboratory data on drinking water and urinary arsenic concentrations among 9,833 HEALS cohort participants were utilized. Statistical analyses were conducted using generalized estimating equations incorporating knotted spline linear regression.
Results
Increasing dietary intakes of thiamin, niacin, pantothenic acid, and pyridoxine were found to significantly increase urinary total arsenic excretion, adjusted for daily arsenic intake from drinking water and other potential confounders.
Conclusions
These results suggest that higher intakes of certain B vitamins may enhance the excretion of arsenic from the body. This study offers new insights into modifiable dietary factors that relate to arsenic excretion and thus provides potential avenues for the prevention of arsenic-related health effects.
Keywords: Arsenic, Bangladesh, B vitamins, Cross-sectional analysis
Introduction
Arsenic exposure is a major public health problem in Bangladesh, where nearly 77 million people have been chronically exposed to arsenic through the consumption of groundwater. Beginning in the 1970s, hand-pumped wells were installed in Bangladesh to provide the population with a source of pathogen-free groundwater for consumption. Since then, the number of wells, which serve as the population’s primary source of drinking water, has exponentially increased. Unfortunately, the groundwater in Bangladesh is naturally contaminated with high levels of arsenic, a phenomenon discovered only after the population had already accrued decades of exposure and an epidemic of classical arsenical skin lesions occurred [1, 2].
Many of the human health effects of arsenic have been established based on epidemiologic studies, which have demonstrated a significant association between the consumption of arsenic through drinking water and cancers of the skin, lung, bladder, liver, and kidney [3–7], neurological disease [8], cardiovascular disease [9], as well as other non-malignant diseases [10, 11].
Urinary total arsenic concentration is a commonly employed biomarker of arsenic exposure in epidemiologic research. The linear relationship between urinary total arsenic and water arsenic concentrations has been previously determined [12–15]. Urinary total arsenic concentration represents the amount of ingested arsenic that is absorbed and excreted [16]. There is known interindividual variability in the amount of arsenic excreted. B vitamins have been associated with increased arsenic excretion through various mechanisms, including methylation capacity [17, 18]; therefore, this study was designed to determine the extent to which dietary intake of these essential nutrients may be associated with increased arsenic excretion.
The one-carbon metabolism pathway is hypothesized to be involved in the metabolism of arsenic through methylation of inorganic arsenic [19]. Several B vitamins—including folate (vitamin B9), pyridoxine (vitamin B6), cobalamin (vitamin B12), and riboflavin (vitamin B2)—play a key role in one-carbon metabolism [20, 21]. The functions of thiamin (vitamin B1), niacin (vitamin B3), and pantothenic acid (vitamin B5) have been less widely examined in relation to arsenic metabolism but have been previously shown to have marginal associations with urinary arsenic species [22].
Given the public health implications for a highly arsenic-exposed population, the present study focused on potentially modifiable dietary factors that may relate to arsenic excretion. The objective of this analysis was to evaluate whether dietary intake of B vitamins is associated with creatinine-adjusted urinary total arsenic concentration among individuals participating in the Health Effects of Arsenic Longitudinal Study (HEALS). HEALS provides us an invaluable opportunity to assess this relationship with individual-level data on both arsenic exposure (daily arsenic intake based on water arsenic concentration and amount of water consumed) and arsenic excretion (creatinine-adjusted urinary total arsenic concentration) in a large study sample.
Subjects and methods
HEALS is an ongoing, population-based cohort study in Araihazar, Bangladesh, aiming to examine both the short-and long-term health effects of arsenic exposure. The selection of cohort participants, study design, and methods have been described in detail elsewhere and are briefly summarized here [23].
Study sample
The cohort participants are residents of three unions (administrative units) of Araihazar, Bangladesh—a well-defined 25-km2 rural area east of the capital city, Dhaka. Prior to subject recruitment, all wells in the area (n = 5,966) were tested for their arsenic concentration as part of a pre-cohort survey. A complete enumeration of the study area population (n = 65,876) was also conducted as part of this survey. Individuals were eligible for participation if they were married, living in their current bari (a cluster of household dwellings occupied by members of the extended family) for at least 3 years, and aged between 18 and 75. Between 2000 and 2002, a total of 11,746 men and women were enrolled into the cohort (with a response rate of 97.5%) and completed a detailed interview, including a semi-quantitative food frequency questionnaire (FFQ). The participants also underwent a detailed clinical examination by trained physicians and provided blood and urine samples. Study physicians and participants were unaware of the arsenic concentration in the drinking water at the time of the recruitment and data/sample collection.
For the purposes of this analysis, participants with incomplete FFQ data or implausible FFQ data—caloric intake less than 500 or more than 3,500 kcal/day—were excluded (n = 735). Individuals with missing data on urinary total arsenic concentration (n = 298) or water arsenic intake (n = 16) were excluded. Additionally, participants with the presence of arsenical skin lesions at enrollment (or missing skin lesion assessments) were also excluded (n = 864), since there is evidence that the presence of skin lesions may alter the urinary arsenic excretion profile [24]. The resulting sample size for this analysis was 9,833.
The study protocol was approved by the Institutional Review Boards of The University of Chicago, Columbia University, and the Bangladesh Medical Research Council. Informed consent was obtained from all participants prior to the interview.
Urinary total arsenic concentration
Spot urine samples were collected in 50-ml acid-washed tubes during the interview and kept in portable coolers with ice packs until storage at −20 °C at the end of the day. All samples were kept frozen until shipment on dry ice to the Trace Metal Facility Core Laboratory at Columbia University. Urinary total arsenic concentration was analyzed by graphite furnace atomic absorption spectrometry according to the method of Nixon et al. [25], with a detection limit of 2 μg/L. Urinary creatinine was measured by a colorimetric Sigma Diagnostics Kit (Sigma, St. Louis, MO), and creatinine-adjusted urinary total arsenic concentration was subsequently expressed as micrograms per gram creatinine. Quality assurance was taken by participation in an inter-laboratory program for urinary arsenic with the Institut de Sante Publique du Quebec. The interclass correlation coefficient for urinary total arsenic over the study period (2000–2002) showed excellent agreement with the control target values. The average intraassay coefficient of variation (CV) and interassay CV for the control urines run daily for this period were 5.9 and 3.5%, respectively.
Dietary B vitamins intake
Dietary intake was assessed with an interviewer-administered FFQ, which recorded how often on average each of a comprehensive list of 39 culturally appropriate potential food items was consumed in the previous year and the usual amount consumed per meal. Briefly, participants were asked how many months of the year, how many days per week, and how many times per day they consumed each food item. They were also asked the usual portion size (measured as spoonfuls, cupfuls, or bowlfuls), with locally used serving items available for reference. The frequency of intake was multiplied by usual portion size to obtain average grams per day for each food item. Detailed information on this semi-quantitative FFQ, developed and validated in this target population, is described elsewhere [26]. Average daily nutrient intake was estimated based on the United States Department of Agriculture (USDA) Nutrient Database for Standard Reference [27] and an Indian food nutrient database [28]. Since the Indian food nutrient database did not contain all the nutrients of interest, USDA-derived values were used and presented here. For nutrients that were available from both sources, analyses were also conducted using the Indian-derived values, and since results were not appreciably different they were not shown. Average daily intakes of thiamin, niacin, pantothenic acid, pyridoxine, cobalamin, and folate were evaluated in the present analysis. For the purpose of this analysis, nutrient intake was divided by body weight and expressed as daily unit intake of nutrient per kg.
Covariate assessment
Sociodemographic factors included sex, age in years, years of education, and television ownership (a measure of socioeconomic status). Smoking history was also obtained.
Well water was analyzed for arsenic concentration by graphite furnace atomic absorption spectrometry, with a detection limit of 5 μg/L [29]. Water samples found to have <5 μg/L of arsenic were subsequently reanalyzed by inductively coupled plasma-mass spectrometry (ICP-MS), with a detection limit of 0.1 μg/L [30]. Exposure to arsenic through drinking water was assessed based on well water arsenic concentration, well usage history, and water consumption patterns. Daily arsenic intake (μg/kg/day) was calculated by multiplying (well water arsenic concentration of the current well) × (1/body weight) × (self-reported daily amount of water consumed from that well). If participants drank from a secondary well in addition to their primary well, information was also collected on that well and was included in the daily arsenic intake computation.
Statistical analyses
Participants were categorized into quartiles of nutrient intake according to the distribution among the total sample eligible for analysis (n = 9,833). The lowest quartile was used as the reference category.
Based on the empirical dose–response relationship between the logarithm of creatinine-adjusted urinary total arsenic concentration and the logarithm of daily arsenic intake (Fig. 1), we fit a linear spline regression model with knots specified at −2 and +2 taking the form:
where Y is the logarithm of creatinine-adjusted urinary total arsenic concentration, b0 is the intercept, b1 is the slope representing the overall effect of the logarithm of daily arsenic intake, b2 is the additional effect for the range −2 to 2 of the daily arsenic intake slope, b3 is the additional effect for the range greater than 2 of the daily arsenic intake slope, and b4 – b6 are the effects for nutrient intakes coded as dummy variables with the lowest quartile as the reference category. Additional covariates were added as potential confounders.
Fig. 1.
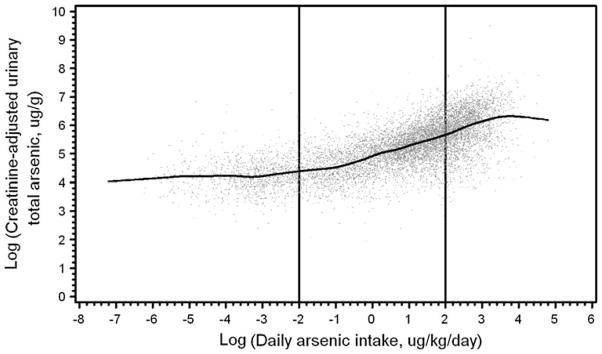
Scatterplot of logarithms of creatinine-adjusted urinary total arsenic versus daily arsenic intake with individual values plotted as gray points and cubic smoothing spline indicated in black line. The vertical lines at −2 and +2 indicate location of knots for linear spline regression model used in subsequent analyses
Generalized estimating equations (GEE), clustered on primary drinking well, were used to estimate regression coefficients and 95% confidence intervals of log-transformed creatinine-adjusted urinary total arsenic concentration by quartile of nutrient intake. These estimates were adjusted for logarithm daily arsenic intake (linear spline with knots at −2, +2), daily total water consumption (ml/day), sex, age (y), total energy (kcal/day), formal education attainment (yes, no), education (y), TV ownership (yes, no), and smoking status (never vs. former, never vs. current). The percent increase of creatinine-adjusted urinary total arsenic concentration in each quartile of dietary intake was presented by exponentiating regression coefficients. To test for linear trend across quartiles of nutrient intake, an ordinal variable coded for nutrient intake quartiles was included in the regression model and the associated P value for the coefficient was interpreted as the P value for trend.
Statistical analyses were performed using the Statistical Analysis System, including the procedure GENMOD for GEE analyses, release 9.1.3 (SAS Institute, Inc., Cary, North Carolina).
Results
Participant characteristics are shown in Table 1. There were 9,833 individuals from the HEALS cohort included in this study who did not have evidence of arsenical skin lesions. The study sample was predominantly female, aged 18–40, of low socioeconomic status (no formal education or television ownership), and never smokers. The median (± interquartile range) concentration of well water arsenic was 57.0 ± 131.0 μg/L. The median daily arsenic intake was 3.2 ± 7.7 μg/kg/day, median creatinine-adjusted urinary total arsenic was 194.0 ± 239.0 μg/g, and median energy intake was 2,200.1 ± 786.8 kcal/day.
Table 1.
Selected characteristics of the HEALS study sample
| Characteristic | Study sample (N = 9,833)
|
|
|---|---|---|
| N | % | |
| Sex | ||
| Male | 3,873 | 39.4 |
| Female | 5,960 | 60.6 |
| Age (years) | ||
| 18–30 | 3,200 | 32.5 |
| 31–40 | 3,546 | 36.1 |
| 41–50 | 2,205 | 22.4 |
| 51–75 | 882 | 9.0 |
| Education (years) | ||
| 0 | 4,310 | 43.8 |
| 1–5 | 2,927 | 29.8 |
| 6+ | 2,591 | 26.4 |
| TV ownership | ||
| Yes | 3,463 | 35.2 |
| No | 6,370 | 64.8 |
| Cigarette smoking | ||
| Never | 6,619 | 67.3 |
| Former | 581 | 5.9 |
| Current | 2,633 | 26.8 |
| Mean (SD) | Median (IQR) | |
|---|---|---|
| Well water arsenic (μg/L) | 96.1 (109.8) | 57.0 (131.0) |
| Daily arsenic intake (μg/kg/day) | 6.0 (7.8) | 3.2 (7.7) |
| Creatinine-adjusted urinary total arsenic (μg/g) | 273.4 (306.2) | 194.0 (239.0) |
| Energy intake (kcal/day) | 2,229.4 (532.1) | 2,200.1 (786.8) |
SD, standard deviation
IQR, interquartile range
The distributions of nutrients, creatinine-adjusted urinary total arsenic concentration and daily arsenic intake are shown in Table 2 by quartile of nutrient intake. Increasing quartiles (Q1 lowest intakes) of nutrient intakes for thiamin, niacin, pantothenic acid, pyridoxine, cobalamin, and folate were associated with a significant increasing trend in creatinine-adjusted urinary total arsenic concentration. Except for cobalamin, increased intake of all B vitamins was associated with increased daily arsenic intake; increased intake of cobalamin was associated with significant decreased daily arsenic intake. Table 3 shows the Pearson correlation coefficients for each pair of nutrients, which ranged from 0.15 to 0.98. These results suggest that a moderate range of the variability in dietary intakes of B vitamins can be explained by food items in which these nutrients occur together.
Table 2.
Distribution of nutrient intakes and arsenic measures
| Quartile of nutrient intake
|
P for trenda | ||||
|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | ||
| Thiamin | |||||
| N | 2,458 | 2,458 | 2,459 | 2,458 | |
| Median intake (mg/kg/day) | 0.031 | 0.040 | 0.047 | 0.058 | |
| Range | <0.036 | 0.036–0.043 | 0.044–0.051 | >0.051 | |
| Mean daily arsenic intake (μg/kg/day)b | 4.8 (6.4)c | 5.5 (7.1) | 6.3 (7.8) | 7.3 (9.2) | 0.0001 |
| Mean urinary total arsenic (μg/g)d | 241.6 (228.8) | 273.5 (412.4) | 276.6 (265.6) | 301.6 (283.6) | 0.0001 |
| Riboflavin | |||||
| N | 2,458 | 2,458 | 2,459 | 2,458 | |
| Median intake (mg/kg/day) | 0.014 | 0.017 | 0.021 | 0.027 | |
| Range | <0.016 | 0.016–0.019 | 0.020–0.023 | >0.023 | |
| Mean daily arsenic intake (μg/kg/day) | 5.4 (6.8) | 5.6 (6.9) | 6.1 (7.8) | 6.9 (9.2) | 0.0001 |
| Mean urinary total arsenic (μg/g) | 270.0 (262.5) | 274.9 (268. 9) | 269.5 (387.4) | 279.0 (289.5) | 0.69 |
| Niacin | |||||
| N | 2,458 | 2,458 | 2,459 | 2,458 | |
| Median intake (mg/kg/day) | 0.455 | 0.592 | 0.710 | 0.868 | |
| Range | <0.529 | 0.529–0.651 | 0.652–0.774 | >0.774 | |
| Mean daily arsenic intake (μg/kg/day) | 4.9 (6.6) | 5.7 (7.2) | 6.0 (7.5) | 7.3 (9.3) | 0.0001 |
| Mean urinary total arsenic (μg/g) | 241.4 (232.9) | 273.8 (412.5) | 272.9 (257.9) | 305.3 (286.8) | 0.0001 |
| Pantothenic acid | |||||
| N | 2,458 | 2,458 | 2,459 | 2,458 | |
| Median intake (mg/kg/day) | 0.105 | 0.134 | 0.157 | 0.192 | |
| Range | <0.121 | 0.121–0.145 | 0.146–0.171 | >0.171 | |
| Mean daily arsenic intake (μg/kg/day) | 4.9 (6.5) | 5.5 (6.9) | 6.2 (7.8) | 7.3 (9.4) | 0.0001 |
| Mean urinary total arsenic (μg/g) | 246.3 (236.2) | 265.5 (305.8) | 281.9 (377.1) | 299.7 (286.4) | 0.0001 |
| Pyridoxine | |||||
| N | 2,458 | 2,458 | 2,459 | 2,458 | |
| Median intake (mg/kg/day) | 0.052 | 0.067 | 0.079 | 0.097 | |
| Range | <0.059 | 0.059–0.072 | 0.073–0.086 | >0.086 | |
| Mean daily arsenic intake (μg/kg/day) | 4.9 (6.5) | 5.5 (6.9) | 6.1 (7.7) | 7.5 (9.4) | 0.0001 |
| Mean urinary total arsenic (μg/g) | 244.7 (232.4) | 273.2 (412.5) | 271.0 (249.5) | 304.5 (294.8) | 0.0001 |
| Cobalamin | |||||
| N | 2,458 | 2,458 | 2,459 | 2,458 | |
| Median intake (μg/kg/day) | 0.014 | 0.027 | 0.040 | 0.064 | |
| Range | <0.021 | 0.021–0.033 | 0.034–0.048 | >0.048 | |
| Mean daily arsenic intake (μg/kg/day) | 6.7 (8.3) | 5.7 (7.5) | 5.7 (7.2) | 5.8 (8.0) | 0.0001 |
| Mean urinary total arsenic (μg/g) | 299.7 (288.8) | 265.9 (253.1) | 260.7 (231.9) | 267.2 (415.9) | 0.0001 |
| Folate | |||||
| N | 2,458 | 2,458 | 2,459 | 2,458 | |
| Median intake (μg/kg/day) | 3.770 | 5.013 | 6.225 | 8.525 | |
| Range | <4.438 | 4.438–5.580 | 5.581–7.091 | >7.091 | |
| Mean daily arsenic intake (μg/kg/day) | 5.2 (6.5) | 5.4 (7.1) | 6.0 (7.5) | 7.4 (9.4) | 0.0001 |
| Mean urinary total arsenic (μg/g) | 266.5 (252.4) | 272.3 (405.4) | 267.0 (246.2) | 287.5 (293.6) | 0.06 |
P for trend calculated from linear regression coefficient based on the ordinal value for each quartile
Crude mean values, unadjusted for covariates
Mean (SD), all such values
Creatinine adjusted
Table 3.
Pearson correlation coefficients of weight-standardized nutrients (n = 9,833)
| Variable | Thiamin | Riboflavin | Niacin | Pantothenic acid | Pyridoxine | Cobalamin | Folate |
|---|---|---|---|---|---|---|---|
| Thiamin | 1.00 | 0.65 | 0.98 | 0.96 | 0.94 | 0.15 | 0.57 |
| Riboflavin | 1.00 | 0.59 | 0.75 | 0.72 | 0.60 | 0.82 | |
| Niacin | 1.00 | 0.94 | 0.92 | 0.19 | 0.46 | ||
| Pantothenic acid | 1.00 | 0.96 | 0.31 | 0.63 | |||
| Pyridoxine | 1.00 | 0.25 | 0.62 | ||||
| Cobalamin | 1.00 | 0.37 | |||||
| Folate | 1.00 |
Based on evidence of a nonlinear dose–response relationship of the logarithms of creatinine-adjusted urinary total arsenic to daily arsenic intake (Fig. 1), daily arsenic intake was modeled using a linear spline. The slopes of the segments are shown in Table 4. For example, for the slope between −2 and +2, the coefficient of 0.3356 means that a difference between 2 subjects of 10% in daily arsenic intake is associated with a mean difference of 3.356% in creatinine-adjusted urinary arsenic concentration. The relationship between creatinine-adjusted urinary total arsenic and well water arsenic concentration was also examined and the shape of the dose–response relationship between the logarithms of both continuous measures was not appreciably different than that with daily arsenic intake (analyses not shown).
Table 4.
Slopes between log water arsenic intake and log creatinine-adjusted urinary total arsenic concentration from GEE analysis
| Segment | Multivariate estimatea
|
|
|---|---|---|
| β coefficient | 95% CI | |
| <−2 | 0.0232 | −0.0069, 0.0534 |
| −2 to +2 | 0.3356 | 0.3220, 0.3491 |
| >+2 | 0.4888 | 0.4366, 0.5411 |
Adjusted for water total, sex, age, total energy, formal education, education years, TV ownership, and smoking status (never vs. former, never vs. current)
The associations between nutrient intakes and creatinine-adjusted urinary total arsenic concentration are shown in Table 5. After adjustment for potential confounders, thiamin (P for trend = 0.017), niacin (P for trend = 0.001), pantothenic acid (P for trend = 0.055) and pyridoxine (P for trend = 0.015) were associated with increased creatinine-adjusted urinary total arsenic concentration. Significant associations were not observed for dietary riboflavin, cobalamin, or folate.
Table 5.
Associations between nutrient intake and log creatinine-adjusted urinary total arsenic concentration from GEE analysis
| Nutrient | Multivariate estimatea
|
|
|---|---|---|
| % increase in urinary total arsenic concentration | 95% CI | |
| Thiamin | ||
| Q1 | Reference | |
| Q2 | 3.8 | 0.6, 7.0 |
| Q3 | 4.4 | 0.8, 8.1 |
| Q4 | 5.8 | 1.3, 10.5 |
| P for trendb | 0.017 | |
| Riboflavin | ||
| Q1 | Reference | |
| Q2 | 1.1 | −1.7, 4.0 |
| Q3 | −1.4 | −4.4, 1.7 |
| Q4 | −1.2 | −4.5, 2.3 |
| P for trend | 0.27 | |
| Niacin | ||
| Q1 | Reference | |
| Q2 | 3.3 | 0.04, 6.6 |
| Q3 | 5.7 | 1.9, 9.6 |
| Q4 | 7.8 | 3.0, 12.9 |
| P for trend | 0.001 | |
| Pantothenic acid | ||
| Q1 | Reference | |
| Q2 | 3.2 | 0.05, 6.5 |
| Q3 | 3.3 | −0.2, 6.8 |
| Q4 | 4.6 | 0.4, 9.1 |
| P for trend | 0.055 | |
| Pyridoxine | ||
| Q1 | Reference | |
| Q2 | 3.6 | 0.4, 6.9 |
| Q3 | 3.5 | −0.02, 7.1 |
| Q4 | 6.0 | 1.6, 10.5 |
| P for trend | 0.015 | |
| Cobalamin | ||
| Q1 | Reference | |
| Q2 | −0.3 | −3.1, 2.6 |
| Q3 | −0.7 | −3.8, 2.3 |
| Q4 | −1.2 | −4.4, 2.0 |
| P for trend | 0.42 | |
| Folate | ||
| Q1 | Reference | |
| Q2 | −0.9 | −3.7, 1.9 |
| Q3 | −0.8 | −3.7, 2.1 |
| Q4 | −1.6 | −4.8, 1.7 |
| P for trend | 0.39 | |
Adjusted for logarithm daily arsenic dose (linear spline with knots at −2, +2), water total, sex, age, total energy, formal education, education years, TV ownership, and smoking status (never vs. former, never vs. current)
P for trend calculated from linear regression coefficient based on the ordinal value for each quartile
Discussion
In this cross-sectional analysis, there was an overall difference in creatinine-adjusted urinary total arsenic concentration with increased nutrient intakes of thiamin, niacin, pantothenic acid, and pyridoxine. These findings are consistent with our hypothesis that for a given level of water arsenic intake, higher intakes of B vitamins enhance elimination of arsenic, as measured by increased urinary total arsenic concentration. Thus, individuals with lower intakes of certain B vitamins may have higher retention or increased tissue storage of arsenic, which may increase their risk of arsenic-related disease.
The findings from this analysis further support previous research from this cohort showing that for a given level of arsenic exposure, increased intakes of pyridoxine significantly and thiamin marginally were associated with a lower prevalence of arsenical skin lesions [31]. Together, these results suggest that increased retention of arsenic may play a role in carcinogenesis risk.
Dietary folate, cobalamin, and riboflavin were not associated with creatinine-adjusted urinary total arsenic concentration, after adjustment for important predictors and confounders. These nutrients are known to be important cofactors in one-carbon metabolism, which plays a role in the detoxification of arsenic through methylation. It has been demonstrated that dimethyl arsenic (DMA) is more readily eliminated than inorganic arsenic and monomethyl arsenic (MMA), signifying that individuals with a better methylation capacity would have higher urinary total arsenic concentrations because a larger proportion of the ingested inorganic arsenic would be methylated and excreted [32]. Although in a previous analysis of urinary arsenic species among a subset of the HEALS cohort, no association of dietary folate with urinary arsenic species was observed, and only marginal associations with riboflavin and cobalamin were detected [22]. Conversely, a 12-week folate supplementation intervention in a subset of HEALS participants with low plasma concentrations of folate showed a significant reduction in blood MMA and total arsenic concentration and an increase in urinary DMA [33]. Additionally, this intervention was shown to decrease the proportion of arsenic excreted as MMA and inorganic arsenic but no treatment effect was seen on urinary total arsenic concentration subsequent to the 12-week intervention—although urinary total arsenic concentration was not a primary analytic endpoint of this intervention [34]. While an effect of folate was seen on urinary arsenic metabolites, it is possible that the effect on total urinary arsenic concentration was missed due to interval of urine collection [34]. A possible explanation for the null findings with respect to dietary folate in this study is that the FFQ measure may be an over-estimate of folate since foods are typically prepared by prolonged cooking in this population, thus degrading the amount of folate [35]. Additionally, it is possible that there is not enough meaningful variability of these nutrients within our study population to detect their biological effect on arsenic metabolism. While we see a reasonable range in the distribution of FFQ-derived nutrient values, the underlying distribution of actual nutritional status may not be as variable due to measurement error in the FFQ instrument, which performs well to rank participants but not to quantify the actual nutritional intake. Alternatively, individuals may have been sufficiently nourished for particular nutrients resulting in our inability to detect their biological effect.
There are several strengths of this study. First, we used a validated FFQ. Our study instrument contains the food items most commonly consumed by our study population based on comparison with food diaries in this population [26] and captures the major variability in diet. While the actual nutrient intakes may not be accurately estimated by the FFQ within our study population, it is likely that it does rank participants reasonably well into quartiles of nutrient intakes. Second, arsenic exposure has been measured at the individual-level based on concentrations in the well water and urine. In addition to arsenic concentration in the water, we were able to construct a measure of daily arsenic intake by integrating information on the amount of water consumed per day, as well as exposure from major secondary sources of well water. Third, because we do not expect nutritional effects to be particularly large, the large sample size enhances our ability to detect associations.
A limitation of this study is that the FFQ measures average diet; the actual nutritional status of the individual at the time of interview and urine sample collection may have varied from the reported average diet due to seasonal variability or fluctuations in household income. While this is a potential source of misclassification, our FFQ validation study found that seasonal variability in the reported intake of B vitamins showed the least variability [26]. Therefore, measurement error due to seasonality for these particular nutrients may be minimal. Another possible limitation of this study is that the contribution of arsenic intake from food, including water for food preparation, has not been measured. We have comprehensively captured arsenic intake through drinking water; however, dietary intake of arsenic is much more complex and has not been measured in our study population. In order for dietary arsenic intake to bias the associations observed in this analysis, food items rich in certain B vitamins would have to systematically also contain higher arsenic concentrations. We deem this to be an unlikely bias operating in this analysis. In fact, in a separate analysis, we have evaluated intake of rice, the most abundant and highest water-containing food item in the Bangladeshi diet and found that it is unrelated to urinary arsenic after holding water arsenic constant (unpublished data).
Possible directions of future research include investigations with dietary intake measured based on biochemical indicators, which would allow for the interpretation of the nutrient doses critical to arsenic metabolism. Additionally, assessment in contexts where B vitamins are given as nutritional interventions in arsenic-exposed populations could also help to further elucidate their role in arsenic metabolism and prevention of adverse health effects.
In conclusion, we found that intakes of thiamin, niacin, pantothenic acid, and pyridoxine were associated with increased creatinine-adjusted urinary total arsenic concentration, adjusted for daily arsenic intake from drinking water and other potential confounders. This study highlights the importance of further clarifying the role of diet in arsenic toxicity and for possible promotion of dietary interventions in arsenic-exposed populations.
Acknowledgments
Supported by grants P42ES010349 from the National Institute of Environmental Health Sciences and R01CA107431 and R01CA102484 from the National Cancer Institute.
Contributor Information
Maria Argos, Department of Health Studies, The University of Chicago, 5841 S. Maryland Avenue, MC2007, Chicago, IL 60637, USA.
Paul J. Rathouz, Department of Health Studies, The University of Chicago, 5841 S. Maryland Avenue, MC2007, Chicago, IL 60637, USA
Brandon L. Pierce, Department of Health Studies, The University of Chicago, 5841 S. Maryland Avenue, MC2007, Chicago, IL 60637, USA
Tara Kalra, Department of Health Studies, The University of Chicago, 5841 S. Maryland Avenue, MC2007, Chicago, IL 60637, USA.
Faruque Parvez, Department of Environmental Health Sciences, Mailman School of Public Health, Columbia University, New York, NY, USA.
Vesna Slavkovich, Department of Environmental Health Sciences, Mailman School of Public Health, Columbia University, New York, NY, USA.
Alauddin Ahmed, Columbia University and The University of Chicago Research, Office in Bangladesh, Mohakhali, Dhaka, Bangladesh.
Yu Chen, Department of Environmental Medicine, New York University, School of Medicine, New York, NY, USA.
Habibul Ahsan, Email: habib@uchicago.edu, Department of Health Studies, The University of Chicago, 5841 S. Maryland Avenue, MC2007, Chicago, IL 60637, USA.
References
- 1.Chowdhury UK, Biswas BK, Chowdhury TR, Samanta G, Mandal BK, Basu GC, Chanda CR, Lodh D, Saha KC, Mukherjee SK, Roy S, Kabir S, Quamruzzaman Q, Chakraborti D. Groundwater arsenic contamination in Bangladesh and West Bengal, India. Environ Health Perspect. 2000;108:393–397. doi: 10.1289/ehp.00108393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tondel M, Rahman M, Magnuson A, Chowdhury IA, Faruquee MH, Ahmad SA. The relationship of arsenic levels in drinking water and the prevalence rate of skin lesions in Bangladesh. Environ Health Perspect. 1999;107:727–729. doi: 10.1289/ehp.99107727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Smith AH, Goycolea M, Haque R, Biggs ML. Marked increase in bladder and lung cancer mortality in a region of Northern Chile due to arsenic in drinking water. Am J Epidemiol. 1998;147:660–669. doi: 10.1093/oxfordjournals.aje.a009507. [DOI] [PubMed] [Google Scholar]
- 4.Chen CJ, Chen CW, Wu MM, Kuo TL. Cancer potential in liver, lung, bladder and kidney due to ingested inorganic arsenic in drinking water. Br J Cancer. 1992;66:888–892. doi: 10.1038/bjc.1992.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hopenhayn-Rich C, Biggs ML, Fuchs A, Bergoglio R, Tello EE, Nicolli H, Smith AH. Bladder cancer mortality associated with arsenic in drinking water in Argentina. Epidemiology. 1996;7:117–124. doi: 10.1097/00001648-199603000-00003. [DOI] [PubMed] [Google Scholar]
- 6.Hopenhayn-Rich C, Biggs ML, Smith AH. Lung and kidney cancer mortality associated with arsenic in drinking water in Cordoba, Argentina. Int J Epidemiol. 1998;27:561–569. doi: 10.1093/ije/27.4.561. [DOI] [PubMed] [Google Scholar]
- 7.Tseng WP. Effects and dose–response relationships of skin cancer and blackfoot disease with arsenic. Environ Health Perspect. 1977;19:109–119. doi: 10.1289/ehp.7719109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brouwer OF, Onkenhout W, Edelbroek PM, de Kom JF, de Wolff FA, Peters AC. Increased neurotoxicity of arsenic in methylene tetrahydrofolate reductase deficiency. Clin Neurol Neurosurg. 1992;94:307–310. doi: 10.1016/0303-8467(92)90179-7. [DOI] [PubMed] [Google Scholar]
- 9.Wu MM, Kuo TL, Hwang YH, Chen CJ. Dose-response relation between arsenic concentration in well water and mortality from cancers and vascular diseases. Am J Epidemiol. 1989;130:1123–1132. doi: 10.1093/oxfordjournals.aje.a115439. [DOI] [PubMed] [Google Scholar]
- 10.Rahman M, Tondel M, Ahmad SA, Chowdhury IA, Faruquee MH, Axelson O. Hypertension and arsenic exposure in Bangladesh. Hypertension. 1999;33:74–78. doi: 10.1161/01.hyp.33.1.74. [DOI] [PubMed] [Google Scholar]
- 11.Rahman M, Tondel M, Ahmad SA, Axelson O. Diabetes mellitus associated with arsenic exposure in Bangladesh. Am J Epidemiol. 1998;148:198–203. doi: 10.1093/oxfordjournals.aje.a009624. [DOI] [PubMed] [Google Scholar]
- 12.Calderon RL, Hudgens E, Le XC, Schreinemachers D, Thomas DJ. Excretion of arsenic in urine as a function of exposure to arsenic in drinking water. Environ Health Perspect. 1999;107:663–667. doi: 10.1289/ehp.99107663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Caceres DD, Pino P, Montesinos N, Atalah E, Amigo H, Loomis D. Exposure to inorganic arsenic in drinking water and total urinary arsenic concentration in a Chilean population. Environ Res. 2005;98:151–159. doi: 10.1016/j.envres.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 14.Biggs ML, Kalman DA, Moore LE, Hopenhayn-Rich C, Smith MT, Smith AH. Relationship of urinary arsenic to intake estimates and a biomarker of effect, bladder cell micronuclei. Mutat Res. 1997;386:185–195. doi: 10.1016/s1383-5742(97)00012-4. [DOI] [PubMed] [Google Scholar]
- 15.Meza MM, Kopplin MJ, Burgess JL, Gandolfi AJ. Arsenic drinking water exposure and urinary excretion among adults in the Yaqui Valley, Sonora, Mexico. Environ Res. 2004;96:119–126. doi: 10.1016/j.envres.2003.08.010. [DOI] [PubMed] [Google Scholar]
- 16.Hughes MF. Biomarkers of exposure: a case study with inorganic arsenic. Environ Health Perspect. 2006;114:1790–1796. doi: 10.1289/ehp.9058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vahter M. Methylation of inorganic arsenic in different mammalian species and population groups. Sci Prog. 1999;82(Pt 1):69–88. doi: 10.1177/003685049908200104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zheng Y, Wu J, Ng JC, Wang G, Lian W. The absorption and excretion of fluoride and arsenic in humans. Toxicol Lett. 2002;133:77–82. doi: 10.1016/s0378-4274(02)00082-6. [DOI] [PubMed] [Google Scholar]
- 19.Gamble MV, Liu X, Ahsan H, Pilsner R, Ilievski V, Slavkovich V, Parvez F, Levy D, Factor-Litvak P, Graziano JH. Folate, homocysteine, and arsenic metabolism in arsenic-exposed individuals in Bangladesh. Environ Health Perspect. 2005;113:1683–1688. doi: 10.1289/ehp.8084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Davis CD, Uthus EO. DNA methylation, cancer susceptibility, and nutrient interactions. Exp Biol Med (Maywood) 2004;229:988–995. doi: 10.1177/153537020422901002. [DOI] [PubMed] [Google Scholar]
- 21.Ulrey CL, Liu L, Andrews LG, Tollefsbol TO. The impact of metabolism on DNA methylation. Hum Mol Genet. 2005;14(Spec No 1):R139–147. doi: 10.1093/hmg/ddi100. [DOI] [PubMed] [Google Scholar]
- 22.Heck JE, Gamble MV, Chen Y, Graziano JH, Slavkovich V, Parvez F, Baron JA, Howe GR, Ahsan H. Consumption of folate-related nutrients and metabolism of arsenic in Bangladesh. Am J Clin Nutr. 2007;85:1367–1374. doi: 10.1093/ajcn/85.5.1367. [DOI] [PubMed] [Google Scholar]
- 23.Ahsan H, Chen Y, Parvez F, Argos M, Hussain AI, Momotaj H, Levy D, van Geen A, Howe G, Graziano J. Health Effects of Arsenic Longitudinal Study (HEALS): Description of a multidisciplinary epidemiologic investigation. J Expo Sci Environ Epidemiol. 2006;16:191–205. doi: 10.1038/sj.jea.7500449. [DOI] [PubMed] [Google Scholar]
- 24.Del Razo LM, Garcia-Vargas GG, Vargas H, Albores A, Gonsebatt ME, Montero R, Ostrosky-Wegman P, Kelsh M, Cebrian ME. Altered profile of urinary arsenic metabolites in adults with chronic arsenicism. A pilot study. Arch Toxicol. 1997;71:211–217. doi: 10.1007/s002040050378. [DOI] [PubMed] [Google Scholar]
- 25.Nixon DE, Mussmann GV, Eckdahl SJ, Moyer TP. Total arsenic in urine: palladium-persulfate vs nickel as a matrix modifier for graphite furnace atomic absorption spectrophotometry. Clin Chem. 1991;37:1575–1579. [PubMed] [Google Scholar]
- 26.Chen Y, Ahsan H, Parvez F, Howe GR. Validity of a food-frequency questionnaire for a large prospective cohort study in Bangladesh. Br J Nutr. 2004;92:851–859. doi: 10.1079/bjn20041277. [DOI] [PubMed] [Google Scholar]
- 27.United States Department of Agriculture. USDA Nutrient Database for Standard Reference, Release 15. 2002. Agricultural Research Service Nutrient Data Laboratory Home Page. [Google Scholar]
- 28.Gopalan C, Rama Sastri B, Balasubramanian S. Indian Council of Medical Research. National Institute of Nutrition; Hyderabad: 1989. Nutritive Value of Indian Foods. [Google Scholar]
- 29.van Geen A, Zheng Y, Versteeg R, Stute M, Horneman A, Dhar R, Steckler M, Gelman A, Small C, Ahsan H, Graziano J, Hussain I, Ahmed K. Spatial variability of arsenic in 6000 tube wells in a 25 km2 area of Bangladesh. Water Resour Res. 2003;39:1140–1155. [Google Scholar]
- 30.Cheng Z, Zheng Y, Mortlock R, Van Geen A. Rapid multielement analysis of groundwater by high-resolution inductively coupled plasma mass spectrometry. Anal Bioanal Chem. 2004;379:512–518. doi: 10.1007/s00216-004-2618-x. [DOI] [PubMed] [Google Scholar]
- 31.Zablotska LB, Chen Y, Graziano JH, Parvez F, van Geen A, Howe GR, Ahsan H. Protective effects of B vitamins and antioxidants on the risk of arsenic-related skin lesions in Bangladesh. Environ Health Perspect. 2008;116:1056–1062. doi: 10.1289/ehp.10707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vahter M. Mechanisms of arsenic biotransformation. Toxicology. 2002;181–182:211–217. doi: 10.1016/s0300-483x(02)00285-8. [DOI] [PubMed] [Google Scholar]
- 33.Gamble MV, Liu X, Slavkovich V, Pilsner JR, Ilievski V, Factor-Litvak P, Levy D, Alam S, Islam M, Parvez F, Ahsan H, Graziano JH. Folic acid supplementation lowers blood arsenic. Am J Clin Nutr. 2007;86:1202–1209. doi: 10.1093/ajcn/86.4.1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gamble MV, Liu X, Ahsan H, Pilsner JR, Ilievski V, Slavkovich V, Parvez F, Chen Y, Levy D, Factor-Litvak P, Graziano JH. Folate and arsenic metabolism: a double-blind, placebo-controlled folic acid-supplementation trial in Bangladesh. Am J Clin Nutr. 2006;84:1093–1101. doi: 10.1093/ajcn/84.5.1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McKillop DJ, Pentieva K, Daly D, McPartlin JM, Hughes J, Strain JJ, Scott JM, McNulty H. The effect of different cooking methods on folate retention in various foods that are amongst the major contributors to folate intake in the UK diet. Br J Nutr. 2002;88:681–688. doi: 10.1079/BJN2002733. [DOI] [PubMed] [Google Scholar]


