Abstract
The Massachusetts Virtual Epidemiologic Network (MAVEN) was deployed in 2006 by the Massachusetts Department of Public Health, Bureau of Infectious Disease to serve as an integrated, Web-based disease surveillance and case management system. MAVEN replaced program-specific, siloed databases, which were inaccessible to local public health and unable to integrate electronic reporting. Disease events are automatically created without human intervention when a case or laboratory report is received and triaged in real time to state and local public health personnel. Events move through workflows for initial notification, case investigation, and case management. Initial development was completed within 12 months and recent state regulations mandate the use of MAVEN by all 351 jurisdictions. More than 300 local boards of health are using MAVEN, there are approximately one million events, and 70 laboratories report electronically. MAVEN has demonstrated responsiveness and flexibility to emerging diseases while also streamlining routine surveillance processes and improving timeliness of notifications and data completeness, although the long-term resource requirements are significant.
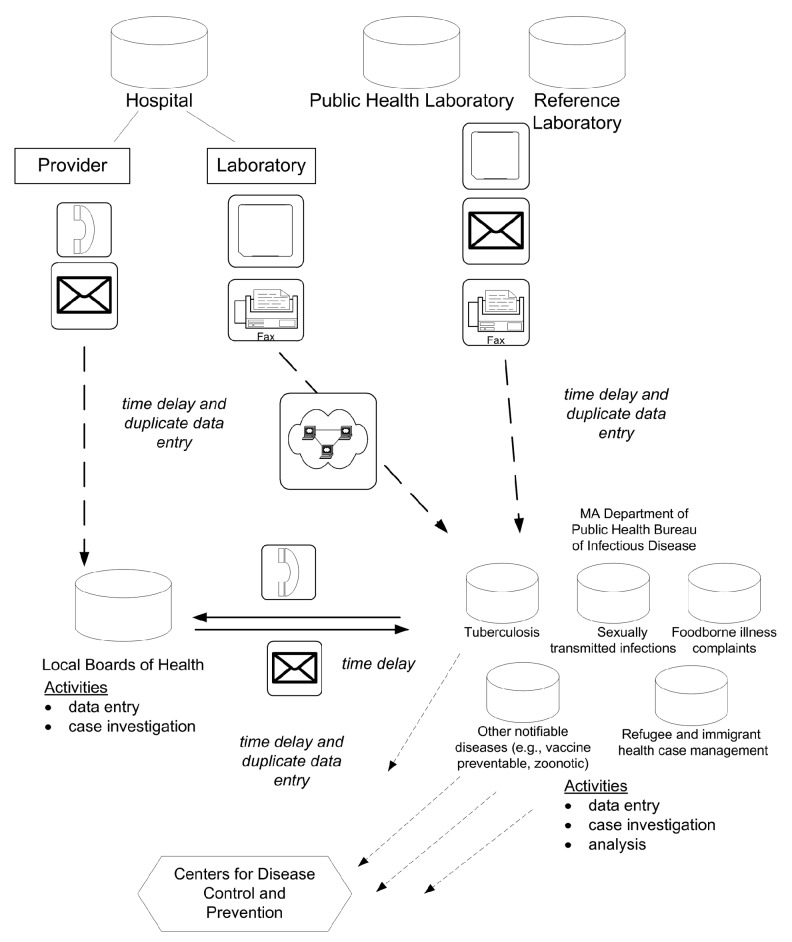
Massachusetts has nearly 90 reportable infectious diseases, resulting in approximately 150,000 disease reports annually. Until 2006, the Massachusetts Department of Public Health (MDPH) Bureau of Infectious Disease (BID) maintained program-specific, siloed databases that were inaccessible to local public health agencies (i.e., 351 city/town-specific health departments in the state) and unable to integrate with electronic laboratory reporting (ELR) (Figure 1), resulting in work redundancy and time delays.
Figure 1.
The infectious disease surveillance systems in Massachusetts in 2006a
aA depiction of the data flows for infectious disease surveillance in Massachusetts, prior to the implementation of MAVEN in 2006. Data for specific disease groups and programs were housed in siloed databases. The disparate system was heavily dependent on paper reporting, had inherent time delays, and there were no opportunities for integrated, comprehensive data management and analysis.
MA = Massachusetts
MAVEN = Massachusetts Virtual Epidemiologic Network
PURPOSE
The Massachusetts Virtual Epidemiologic Network (MAVEN) was deployed in 2006 by the BID to serve as an integrated Web-based disease surveillance and case management system. MAVEN enables BID's Office of Integrated Services and Informatics Services to distribute critical case and outbreak information rapidly, allowing MDPH and local city/town boards of health to focus on the most urgent situations. (Note that in Massachusetts, municipal boards of health are the public health jurisdictions, as Massachusetts does not have the county health department structure that exists in many other states.) In this article, we describe the mechanism and tools that MDPH has implemented to facilitate infectious disease response using MAVEN.
METHODS
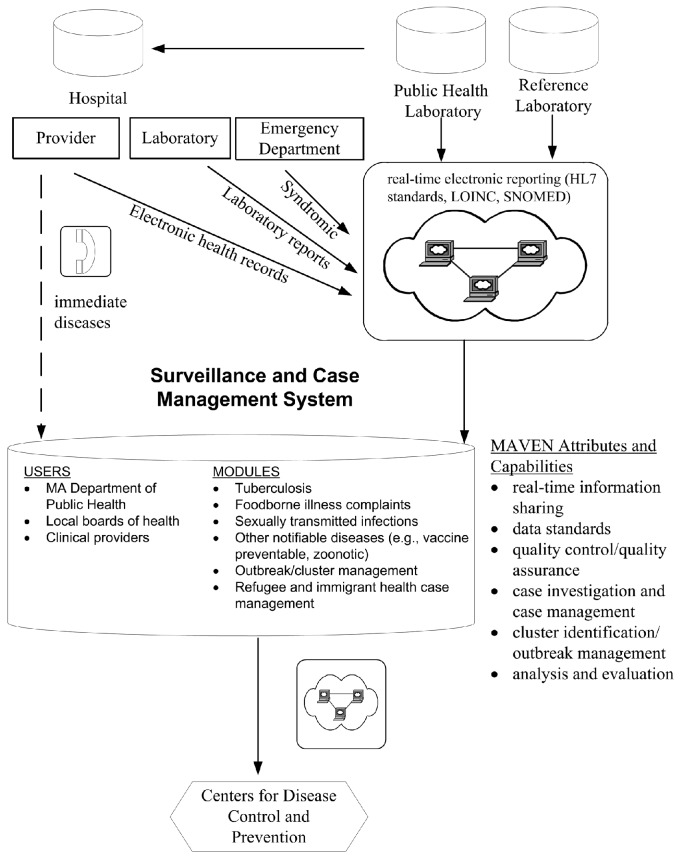
Reported suspect, probable, and confirmed notifiable diseases are referred to as disease events in MAVEN. MAVEN is a person-based, relational database: if a given person has three different disease events, that person is in MAVEN once, and the three events are linked to that individual. MAVEN is a Web-based system designed to communicate with a variety of existing applications, including ELR and electronic health record (EHR) reporting. Data extracts are sent to the Centers for Disease Control and Prevention (CDC) via the National Electronic Telecommunication System for Surveillance and the Public Health Information Network Messaging System. MAVEN is primarily modified through configuration and does not require substantial coding. The structure of MAVEN is illustrated in Figure 2.
Figure 2.
MAVEN, the current, unified infectious disease surveillance and case management system in Massachusetts, post-2006a
aA depiction of the flow of data for infectious disease surveillance and case management in Massachusetts since the implementation of MAVEN in 2006. Data for most reportable infectious diseases are housed in a common database and reports from laboratories and electronic health records are received through a single electronic infrastructure that is compliant with national standards. The integrated system allows for real-time reporting and public health response, in addition to new data analysis and program evaluation capabilities.
MAVEN = Massachusetts Virtual Epidemiologic Network
HL7 = Health Level Seven
LOINC = Logical Observation Identifiers Names and Codes
SNOMED = Systematized Nomenclature of Medicine
MA = Massachusetts
Disease events are created via ELR, EHR, or manual data entry. ELR allows laboratories to transmit near real-time data through the use of Logical Observation Identifiers Names and Codes (LOINC®) and Systematized Nomenclature of Medicine (SNOMED®) codes. Based on the LOINC/SNOMED combination, which indicates the laboratory test that was conducted and the test result, respectively, as well as specimen date, MAVEN creates a new disease event or appends the data to an existing one. This functionality is driven through configurable de-duplication algorithms based on person demographics and disease-specific epidemiology.
The ELR infrastructure can also support data from EHRs. A proof-of-concept project was undertaken to develop disease detection algorithms to mine EHRs of a large multi-specialty practice.1,2 Reports of specific notifiable diseases, along with relevant clinical and epidemiologic information, are sent electronically to MAVEN. This transmission allows for faster reporting, decreases the burden of reporting on providers, and relieves public health investigators of considerable follow-up.
MAVEN uses role-based security. Each user (e.g., MDPH surveillance staff, epidemiologists, and nurses, as well as local health department staff) is assigned a role, which is defined by a set of permissions. The permissions enable access to particular components and content and define which activities a user can perform. Security access can be at the variable level; for example, human immunodeficiency virus (HIV) status within a tuberculosis (TB) event is only viewable by select users. The group configuration determines which events specific to their program or local jurisdiction group members can see. Each user can have multiple roles and be part of multiple groups.
A key feature of MAVEN is its “workflows,” which are electronic folders that triage and organize events based on predetermined criteria. Events move through workflows, including those for initial notification, case investigation, and case management activities. A user can review events residing in various workflows depending on role and group membership. Responses to specific questions result in events moving into and out of workflows. Notifiable diseases are classified by MDPH BID as either “immediate” or “routine.” Immediate diseases are those diseases requiring an urgent public health response. Routine diseases are a lower priority for case investigation and follow-up by the local boards of health. MAVEN automatically recognizes newly created urgent events, sends them to a workflow monitored by an epidemiologist, and notifies the appropriate state and local authorities via text message or e-mail on a 24/7 basis. MAVEN also captures aggregate outbreak and cluster data, which can be linked to individual-level events that are part of the outbreak. The system allows comma-delimited files to be uploaded. For example, when a comma-delimited file that consists of a line list of individuals is uploaded to MAVEN, the system will create an event for each individual in the list; these new individual events will be linked to the outbreak event. New questionnaires or variables can be created for specific outbreaks and will appear in each of the outbreak-linked events in a specified question package.
MAVEN was developed to allow for disease-specific case management. The management of perinatal hepatitis B virus (HBV) infections illustrates aspects of this capacity. When a laboratory result indicating HBV infection is entered, the age and gender of the case is automatically assessed; female cases aged 14–44 years enter a workflow monitored by MDPH nurses. MDPH nurses call the ordering clinician to determine pregnancy status. If pregnancy status cannot be assessed on the first try, the case is moved into a workflow that refreshes and updates in two-week intervals, prompting additional attempts. Confirmed pregnant cases are kept in a holding workflow until delivery. When an HBV-positive woman delivers, the birthing hospital completes a one-page TeleForm® (Autonomy Cardiff, Vista, California) report with optical character recognition. The form is scanned into MAVEN, resulting in an update to the woman's event and the creation of a new event for her baby. The baby's event contains information on initial receipt of HBV vaccine and immune globulin and is monitored to ensure complete vaccination.
BID has created a collection of standardized reports that allow for efficient monitoring and analysis. Users can also extract data in Microsoft® Excel or comma-delimited files. Extracts can include any variables that are captured for the specific event, including laboratory data.
MAVEN and related surveillance activities are funded through a variety of state and federal resources, such as Centers for Disease Control and Prevention (CDC) cooperative agreements. Grant programs include, but are not limited to, Epidemiology and Laboratory Capacity for Infectious Diseases, Immunization and Vaccines for Children, Public Health Emergency Preparedness, Tuberculosis Elimination Cooperative Agreement, and Affordable Care Act funding. For the first three years, MDPH epidemiology, informatics, and information technology (IT) staff contributions were in-kind. MDPH contracted with Consilience Software in Austin, Texas, to develop the core product for public health case management and surveillance, with specific Massachusetts requirements. This contract involved an initial licensing fee. The core product is proprietary and also requires an annual maintenance fee. Currently, MDPH makes all modifications in-house, except when there are changes that affect the core product. The local health departments do not incur direct costs, except staff time for training.
OUTCOMES
Initial development was completed in 12 months and deployed to BID staff in September 2006 for all infectious diseases except sexually transmitted infections (STIs), HIV/acquired immunodeficiency syndrome (AIDS), and TB. This first release included core functionalities: notifications, prompts to review event information, and basic surveillance reports. Historic data for notifiable diseases from 1988 through August 2006 were migrated from legacy systems. In February 2007, the TB module was enabled. In August 2007, BID began deployment to local boards of health in a phased approach involving comprehensive training. An outbreak module was added in August 2009. In October 2010, the Refugee and Immigrant Health module was enabled wherein a new refugee/immigrant who receives case management becomes an event. Enhancements to existing functionality have been added routinely, including new workflows, reports, and features such as coinfection alerts. More than 150 workflows and reports are currently used at the state and local level. The STI and HIV/AIDS modules are in development.
In July 2011, MDPH promulgated new regulations mandating the use of MAVEN by all 351 local jurisdictions, with the goal of achieving 95% use by the end of 2012. As of November 2013, more than 324 (92.3%) local boards of health were using the system. There are more than 750 MAVEN users and more than one million events in the system. Sixty-eight hospital clinical laboratories and two commercial laboratories, in addition to the Massachusetts Hinton State Laboratory Institute, report via ELR. A multisite physician group began reporting information to MAVEN on eight notifiable diseases via their EHRs, and additional disease algorithms for EHR reporting are under development.
The capacity and flexibility of MAVEN was demonstrated during the first wave of the 2009 influenza H1N1 pandemic when, in April 2009, heightened surveillance became necessary and BID quickly reconfigured MAVEN. Within one day, a new result code for H1N1 swine-origin influenza (H1N1) was created, allowing results to be sent via ELR from the Hinton State Laboratory (the only laboratory in Massachusetts with this testing capacity at the start of the pandemic). A unique variable for the rapid identification of H1N1 cases was created for influenza events, in addition to a workflow that identified newly created H1N1 events based on laboratory data. By the end of the first week, variables were added to capture key clinical symptoms, and a new group was created to give users access to just influenza events, expanding the number of BID staff who could assist with investigations. A report was created to extract data on H1N1 influenza events. Beginning in June 2009, when MAVEN received a new positive laboratory report for H1N1 influenza, a single-page TeleForm pre-populated with patient demographic information was sent to the ordering provider requesting additional case data. A blank version of this form was also available to clinicians for high-risk patients with influenza-like illness, allowing MDPH epidemiologists to begin investigations without laboratory confirmation.
We have previously described improved timeliness of reporting for hepatitis C virus infection following the implementation of MAVEN.3 Although data are preliminary, we have evidence suggesting the case management function for perinatal HBV has resulted in identification of at-risk newborns at a rate higher than the upper bound of the predicted number, and successful interventions to prevent vertical transmission. A recent situation regarding a newly arrived refugee baby born to a mother with chronic HBV infection highlighted the triage capability of MAVEN. The baby was reported on a Refugee Health Assessment form as HBV-positive, but a confirmatory laboratory report had not been received. This delay resulted in an automatic letter to the Refugee Health Assessment site requesting the laboratory report. Once the report was received and entered into MAVEN, the baby's event populated a workflow followed by the perinatal HBV case management team. Because the baby was also in the system as a refugee event, the nurse manager had access to data that had been reported on the form, thus immediately providing the nurse with key case information that she/he would have had to obtain otherwise (e.g., immunization status, whether the infection was perinatal in origin, and whether there were susceptible close contacts in the household).
LESSONS LEARNED
Massachusetts has demonstrated that the integration of surveillance activities is achievable. We have created a flexible system that responds to unique programmatic needs, enables prompt response to public health emergencies, and addresses state and local public health requirements. It took less than one year to implement basic functionality for MAVEN within BID. During the next six years, we brought MAVEN to more than 90% of 351 local boards of health, with regulations mandating the use of MAVEN by all jurisdictions. Some boards of health are not yet using MAVEN due to limited personnel resources at the local health department and low volume of morbidity.
We have demonstrated that statewide, real-time data exchange via ELR and systems such as MAVEN is feasible. Traditional disease notification methods can result in limitations in timeliness, completeness, and information exchange between state and local public health. ELR has been shown to improve the completeness and timeliness of disease surveillance.4,5 While BID uses MAVEN for activities related to infectious disease surveillance, reporting, and investigation, other jurisdictions use the MAVEN functionalities for non-infectious disease programmatic needs, such as vaccine management and prescription drug monitoring.
Although we have shown that leveraging resources from multiple cooperative agreements allows a state health department to create a stable, yet nimble surveillance infrastructure, resource investment in implementing and maintaining MAVEN is substantial. Massachusetts developed and manages the system in-house enabling rapid response, although limited IT resources have delayed many planned modifications. Initially, the system was supported by three full-time IT specialists and five informaticians/epidemiologists, who were also responsible for surveillance activities. As the system has grown, however, the number of staff required to support MAVEN has increased. Now, there are seven BID staff and five IT developers, in addition to a small number of centralized IT hosting and database management staff who support MAVEN.
An early analysis of the impact of MAVEN and other system modifications on hepatitis C virus infection reporting showed a decrease in median reporting time to MDPH BID (from 454 days in 2004 to 26 days in 2008).3 At the five-year mark, BID began a full evaluation of the surveillance system, following guidance put forth by CDC.6,7 Preliminary findings indicate improved timeliness of notification to BID and from BID to local boards of health. Further, MAVEN functionality (e.g., data extraction capability and capacity of the system to amend individual events over time and document the additions with auditing functionality) allows BID to readily examine the timeliness and completeness of reporting. MAVEN has allowed BID to streamline infectious diseases surveillance processes, but further development and integration has been slowed due to limited resources.
Footnotes
The authors acknowledge the significant and ongoing contributions of current and former epidemiologists, informaticians, and information technology staff in the development of the Massachusetts Virtual Epidemiologic Network (MAVEN).
REFERENCES
- 1.Automated detection and reporting of notifiable diseases using electronic medical records versus passive surveillance-Massachusetts, June 2006–July 2007. MMWR Morb Mortal Wkly Rep. 2008;57(14):373–6. [PubMed] [Google Scholar]
- 2.Klompas M, Lazarus R, Daniel J, Haney GA, Campion FX, Kruskal BA, et al. Electronic medical record Support for Public health (ESP): automated detection and reporting of statutory notifiable diseases to public health authorities. Adv Dis Surv. 2007;3:1–5. [Google Scholar]
- 3.Heisey-Grove D, Church DR, Haney GA, DeMaria A., Jr Enhancing surveillance for hepatitis C through public health informatics. Public Health Rep. 2011;126:13–8. doi: 10.1177/003335491112600105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Doyle TJ, Glynn MK, Groseclose SL. Completeness of notifiable infectious disease reporting in the United States: an analytical literature review. Am J Epidemiol. 2002;155:866–74. doi: 10.1093/aje/155.9.866. [DOI] [PubMed] [Google Scholar]
- 5.Overhage JM, Grannis S, McDonald CJ. A comparison of the completeness and timeliness of automated electronic laboratory reporting and spontaneous reporting of notifiable conditions. Am J Public Health. 2008;98:344–50. doi: 10.2105/AJPH.2006.092700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.German RR, Lee LM, Horan JM, Milstein RL, Pertowski CA, Waller MN. Updated guidelines for evaluating public health surveillance systems: recommendations from the Guidelines Working Group. MMWR Recomm Rep. 2001;50(RR-13):1–35. [PubMed] [Google Scholar]
- 7.Buehler JW, Hopkins RS, Overhage JM, Sosin DM, Tong V. Framework for evaluating public health surveillance systems for early detection of outbreaks: recommendations from the CDC Working Group. MMWR Recomm Rep. 2004;53(RR-5):1–11. [PubMed] [Google Scholar]