Abstract
Aim
To evaluate the effect of incorporating the polyphenol, curcumin, into nanodisk (ND) particles on its biological activity.
Materials & methods
Curcumin-NDs formulated with different scaffold proteins were incubated with cultured glioblastoma multiforme cells.
Results
When ApoE was employed as the ND scaffold protein, enhanced curcumin uptake was observed. Furthermore, ApoE curcumin-NDs induced greater cell death than either free curcumin or ApoAI curcumin-NDs. A total of 1 h after exposure of glioblastoma multiforme cells to ApoE curcumin-NDs, significant curcumin uptake was detected while ApoE was localized at the cell surface. After 2 h, a portion of the curcumin had migrated to the nucleus, giving rise to enhanced fluorescence intensity in discrete intranuclear sites.
Conclusion
ApoE-mediated interaction of curcumin-NDs with glioblastoma multiforme cells leads to enhanced curcumin uptake and increased biological activity.
Keywords: ApoE, apoptosis, confocal fluorescence microscopy, curcumin, glioblastoma, nanodisk
In 2011, Ghosh et al. formulated the naturally occurring polyphenol, curcumin, into nanodisk (ND) particles, conferring water solubility to this compound [1]. NDs are self-assembled bio-nanoparticles composed of a disk-shaped phospholipid bilayer whose edge is stabilized by a scaffold protein, usually a member of the class of exchangeable apolipoproteins [2,3]. To date, NDs have been formulated with different hydrophobic compounds, including the polyene antibiotic, amphotericin B [4] and the isoprenoid, all-trans-retinoic acid [5]. These otherwise water-insoluble bioactive agents are solubilized by integration into the nanoparticle bilayer. In the case of curcumin, formulation into NDs enhances its growth-inhibition properties against cultured hepatocarcinoma cells [1]. Interest in curcumin has grown in recent years based on its putative pharmacologic effects, which include antioxidant, anti-inflammatory and cancer-prevention properties [6,7]. However, full exploitation of the potential benefits of curcumin has not been realized owing to its poor oral bioavailability and insolubility in aqueous media [8,9].
In vitro data indicate that curcumin elicits proapoptotic effects on cultured glioblastoma multiforme (GBM) cells [10–12]. Furthermore, intraperitoneal administration of curcumin improved the survival rate of mice with intracerebral gliomas [13]. Given the growth inhibition and proapoptotic effects of curcumin-NDs documented in cultured hepatocarcinoma and lymphoma cells [14], we sought to investigate whether NDs would facilitate delivery of curcumin to GBM cells. Results obtained reveal enhanced curcumin uptake when GBM cells were incubated with curcumin-NDs formulated with ApoE as the scaffold component. High-resolution confocal fluorescence microscopy images reveal ApoE binding to the GBM cell surface together with internalization of curcumin. The finding that the ND scaffold protein influences curcumin uptake has important implications for in vivo therapeutic applications of this biocompatible, nanoscale delivery vehicle.
Material & methods
Reagents
Curcumin was purchased from Cayman Chemical (MI, USA) and used without further purification. Dimyristoylphosphatidylcholine was obtained from Avanti Polar Lipids Inc. (AL, USA). The ND scaffold proteins, recombinant human ApoAI and human ApoE3 (N-terminal residues 1–183) were expressed in Escherichia coli and isolated as described previously [15,16]. CellTiter 96® nonradioactive cell proliferation (3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide [MTT]) assay kit was obtained from Promega (WI, USA). Alexa Fluor® 647 goat anti-mouse IgG, Hoechst 33342 and the Vybrant® Apoptosis Assay Kit were purchased from Life Technologies Corp. (CA, USA). Monoclonal antibody 1D7, directed against ApoE, was a gift of Ross Milne, University of Ottawa (ON, Canada) [17]. Heparin was obtained from Sigma-Aldrich (MO, USA), catalog #H-3149 at 177 unit/mg. A 50 mg/ml stock solution was prepared in deionized water.
Curcumin-NDs
Curcumin-NDs were formulated as described by Ghosh et al. [1]. Briefly, 10 mg dimyristoyl-phosphatidylcholine was dissolved in chloroform/ methanol (3:1 v/v) and dried under a stream of N2 gas. Following dispersal of the prepared lipids in phosphate-buffered saline (PBS; 20 mM sodium phosphate, 150 mM sodium chloride, pH 7.0) by bath sonication, 1 mg curcumin (from a 20 mg/ml stock solution in dimethylsulfoxide) was added. A total of 4 mg of scaffold protein, either ApoAI or ApoE, was added drop-wise with further bath sonication to generate the respective curcumin-NDs. NDs were then dialyzed (molecular weight cut-off: 6–8 kDa) overnight against PBS, filtered through a 0.22-µm sterile filter and stored at 4°C until use. Empty NDs, lacking curcumin, were prepared in the same manner except that curcumin was omitted from the formulation.
Quantitation of curcumin incorporation into NDs
Curcumin-ND samples were diluted 1:100 in methanol and the absorption spectra recorded on a Lambda 20 spectrophotometer (Perkin-Elmer, MA, USA). The amount of curcumin incorporated in ND preparations was calculated using a curcumin molar absorption coefficient at 428 nm of 48,000 M−1 cm−1 [18].
Cell culture
The GBM cell lines SF-763 and SF-767 were obtained from Trudy M Forte (Children’s Hospital Oakland Research Institute, CA, USA). Cells were maintained in high-glucose DMEM (Thermo Scientific Hyclone, UT, USA) supplemented with 10% fetal bovine serum, 100 U/ml penicillin and 100 µg/ml streptomycin. Cells were cultured at 37°C in a humidified atmosphere of 5% CO2 and 95% air. Experiments were conducted using cells passaged between six and ten times.
Cell-uptake study
A BD FACSCalibur™ instrument (BD Biosciences, CA, USA) was used to detect cell-associated curcumin. Cells were plated at 1 × 106 cells per well in six-well plates and allowed to attach overnight. On the following day, the culture medium was replaced with reduced serum medium, Opti-MEM® (Life Technologies), supplemented with 5, 10 or 20 µM ApoAI curcumin-NDs, ApoE curcumin-NDs or free curcumin (in dimethylsulfoxide). Untreated cells and cells treated with equivalent amounts of empty NDs served as control. After 24 h, the cells were washed with PBS and trypsinized. Cells were centrifuged to remove trypsin and washed with PBS before resuspending in FACS buffer (PBS with 1% serum and 5 mM EDTA). The mean curcumin fluorescence of >95% of the cell population from the different treatment groups was determined and compared. Values reported are the mean ± standard deviation of three independent experiments.
Cell-viability assay
Cells were plated in 96-well culture plates at 3000 cells per well and allowed to attach overnight. After 24 h, the medium was replaced with Opti-MEM supplemented with specified concentrations of free curcumin, ApoAI curcumin-NDs, ApoE curcumin-NDs or empty NDs. A total of 48 h after treatment, cell proliferation assays were performed as described by the manufacturer. Briefly, cells were incubated with MTT for 2 h at 37°C followed by addition of solubilization buffer (provided by manufacturer). After 1 h of incubation at room temperature, absorbance was read at 570 nm. Values expressed are the mean ± standard deviation (n = 4) percent cell viability relative to untreated cells.
Apoptosis assay
The Vybrant Apoptosis Assay Kit was used as per the manufacturer’s guidelines to measure cellular apoptosis by flow cytometry. SF-767 GBM cells were plated at 1 × 106 cells/well in six-well plates and treated with specified concentrations of ApoAI curcumin-NDs, ApoE curcumin-NDs, free curcumin or empty NDs for 40 h. After treatment, cells were washed with ice-cold PBS, trypsinized, washed an additional time with annexin-binding buffer (kit component) and incubated with biotin-conjugated annexin V followed by Alexa Fluor® 350 (Invitrogen, CA, USA) streptavidin solution to label early apoptotic cells. In total, 1 µl of a 1 mg/ml propidium iodide stock solution was added to each sample prior to flow cytometry measurements on a BD FACSAria™ (BD Biosciences).
Confocal fluorescence microscopy
For confocal microscopy, SF-767 cells were plated at 1.5 × 105 cells/well, on poly-l-lysine-coated 12-mm round coverslips (BD Biosciences) in 12-well plates. After 24 h, the medium was replaced with serum-free medium. Cells were incubated with 20 µM curcumin (as ApoE curcumin-NDs), empty NDs or left untreated. After 1 h, cells were washed with ice-cold PBS and fixed with 4% paraformaldehyde for 10 min. Cells were permeabilized with 0.1% Triton™ X-100 (Sigma-Aldrich) in PBS followed by incubation with monoclonal antibody 1D7. For detection, an Alexa Fluor 647-labeled goat anti-mouse IgG secondary antibody was used. Hoechst 33342 was used to stain nuclei. To study the cellular itinerary of curcumin as a function of time, cells were incubated with ApoE curcumin-NDs for 30 min, 1, 2 and 4 h. At each time point, cells were washed with ice-cold PBS and fixed with 4% paraformaldehyde. Coverslips were mounted on a glass slide using VECTASHIELD® Mounting Medium (Vector Laboratories, CA, USA) and sealed. Confocal images were acquired on a Zeiss LSM710 microscope (Zeiss, NY, USA) with a 63×, 1.4 NA oil objective with a pinhole of 90 µm.
Statistical analysis
Data from cell proliferation assays are expressed as percent viable cells (viability after treatment with either free curcumin or curcumin-NDs over viability after treatment with medium only). Statistical significance between treatment groups was calculated using the two-tailed Student’s t-test (GraphPad, CA, USA). For the apoptosis assay, data are expressed as percent apoptotic cells (annexin V-positive/propidium iodide-negative plus annexin V-positive/propidium iodide-positive) over total cells. Statistical significance for data obtained from the cell-uptake study and apoptosis assay were determined by one-way analysis of variance and Newman–Keuls multiple comparison test. P-values of <0.05 were considered significant.
Results
Curcumin uptake by cultured GBM cells
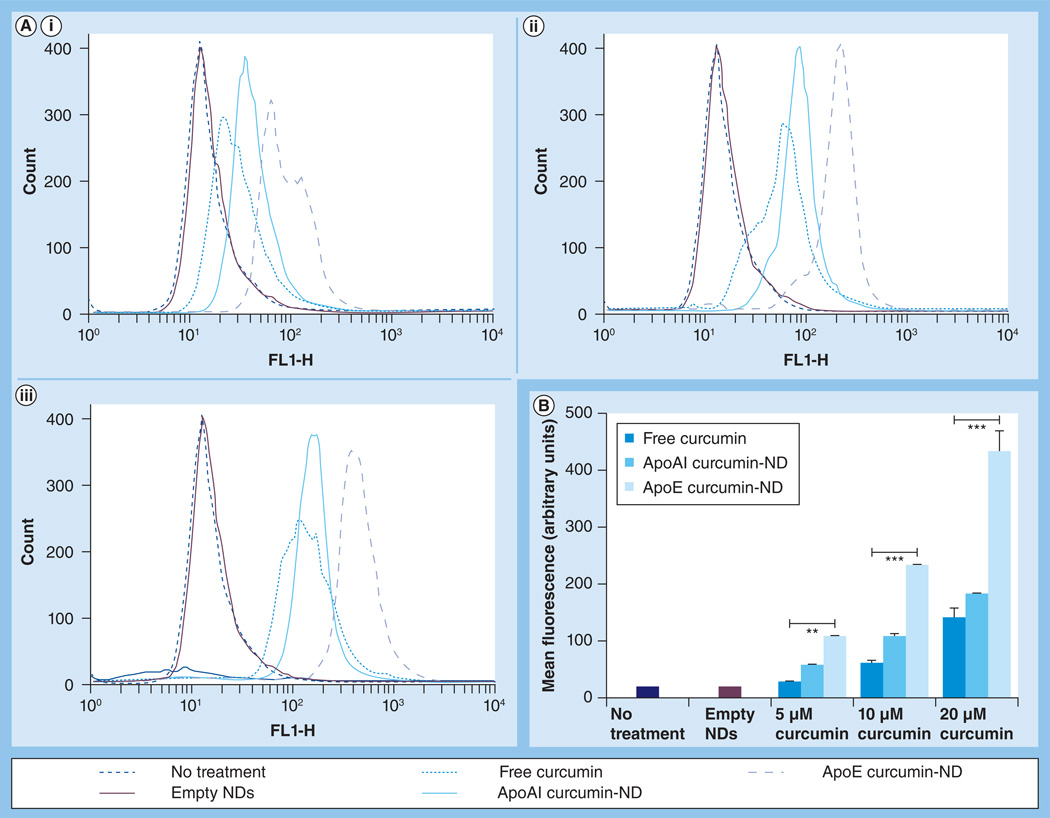
To examine curcumin uptake by GBM cells, the intrinsic fluorescence of this polyphenol [1] was monitored by flow cytometry. Figure 1 depicts curcumin accumulation in cultured SF-767 cells following incubation with free curcumin or curcumin-NDs. Untreated cells and cells incubated with empty NDs lacking curcumin gave rise to a single population that served as a reference control. Incubation of the cells with free curcumin induced a concentration-dependent shift in cellular fluorescence. Mean cellular curcumin fluorescence intensity was higher for curcumin-NDs formulated with ApoAI compared with free curcumin, although this was not statistically significant. By contrast, mean cellular curcumin fluorescence intensity was significantly increased at all concentrations examined when curcumin was formulated into NDs with ApoE as the scaffold protein. When the same experiment was performed in a second GBM cell line, SF-763, similar results were obtained (data not shown).
Figure 1. Effect of nanodisks on curcumin internalization by glioblastoma multiforme cells.
Following incubation for 24 h, cells were washed and analyzed by flow cytometry. (A) Representative data from three independent experiments, where ‘Count’ refers to the cell number. Each plot represents curcumin fluorescence detected for a cell population after incubation with (A,i) 5 µM, (Aii) 10 µM and (A,iii) 20 µM curcumin. (B) Mean curcumin fluorescence for >95% of the cell population as a function of curcumin concentration and formulation. The FL1 channel was used to detect curcumin fluorescence.
**p < 0.01.
***p < 0.001.
FL1-H: Height of the histogram that represents the mean curcumin fluorescence intensity detected by channel 1; ND: Nanodisk.
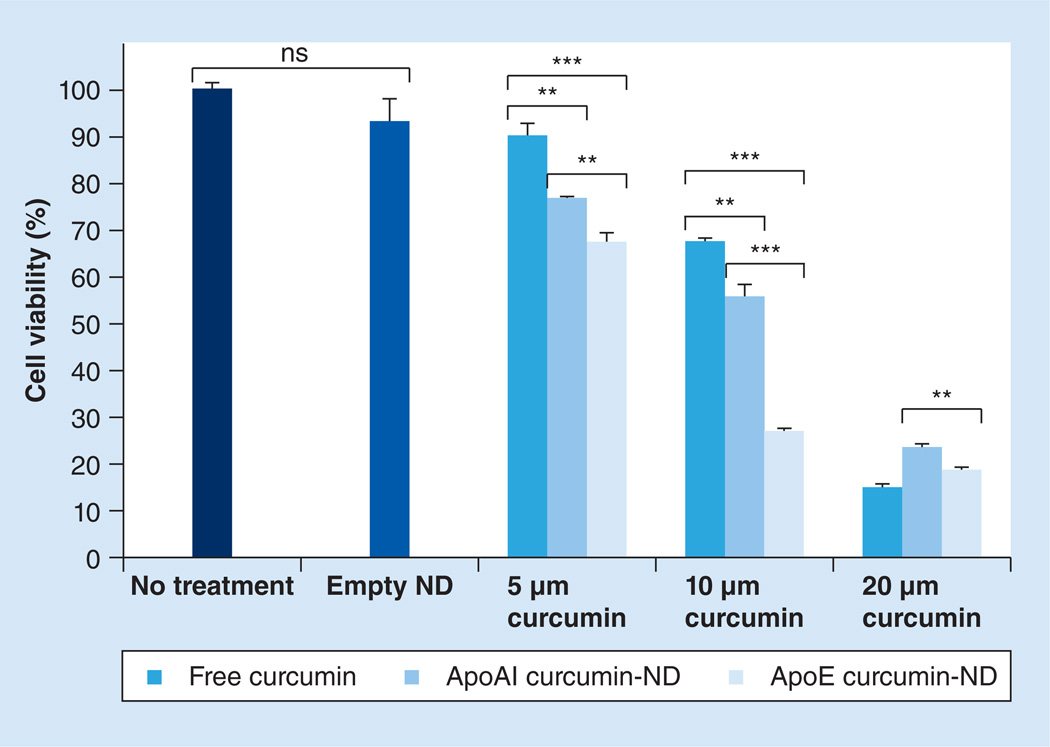
Effect of curcumin on GBM cell viability
SF-767 GBM cells were incubated with free curcumin or curcumin-NDs for 48 h, followed by determination of cell viability (Figure 2). Compared with free curcumin, ApoAI curcumin-NDs and ApoE curcumin-NDs showed a greater induction of cell death at 5 and 10 µM curcumin. At 20 µM curcumin, cell viability was <25% in all cases, indicating that under these conditions this concentration is too high to discern differences among these formulations. Interestingly, however, at all curcumin concentrations, ApoE curcumin-NDs had a greater effect on GBM cell viability than ApoAI curcumin-NDs. Similar results were obtained with SF-763 cells (data not shown) and are consistent with the increased curcumin fluorescence intensity of cells incubated with ApoE curcumin-NDs.
Figure 2. Effect of curcumin on glioblastoma multiforme cell viability.
SF-767 cells were incubated with empty nanodisk or the indicated concentrations of free curcumin, ApoAI curcumin-ND and ApoE curcumin-ND. After 48 h, cell viability was determined using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay according to the manufacturer’s instructions.
**p < 0.01.
***p < 0.001.
ND: Nanodisk; ns: Not significant.
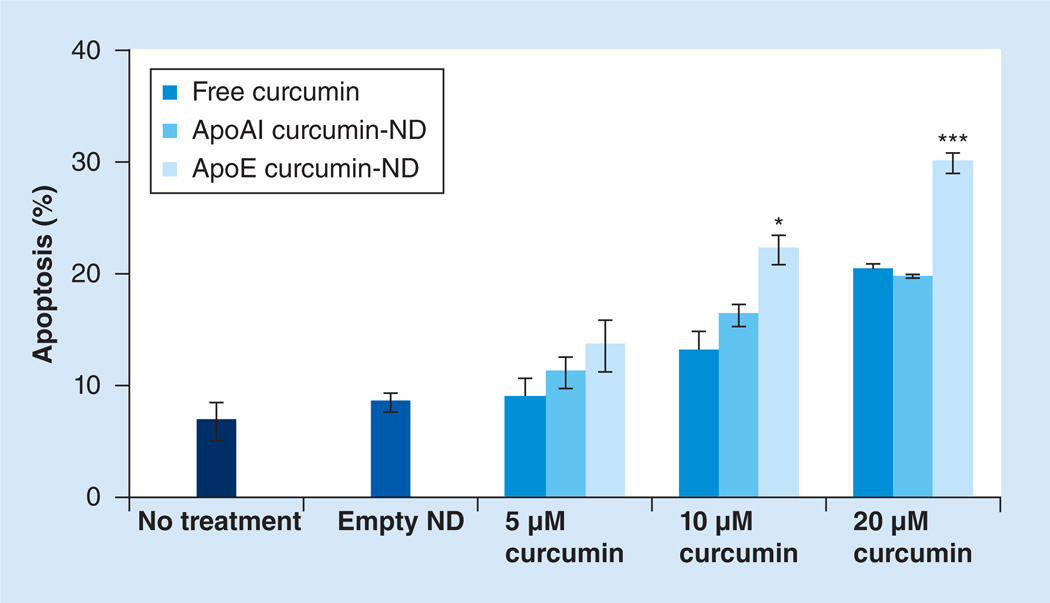
Curcumin-ND-induced GBM cell apoptosis
The relative ability of free curcumin, ApoAI curcumin-NDs or ApoE curcumin-NDs to induce apoptosis in cultured SF-767 cells is shown in Supplementary Figure 1 (see online at www.futuremedicine.com/doi/suppl/10.2217/NNM.13.35). Compared with untreated cells, empty NDs had no effect on the percentage of apoptotic cells. By contrast, compared with either ApoAI curcumin-NDs or free curcumin, ApoE curcumin-NDs enhanced the percentage of apoptotic cells at all concentrations tested with the difference reaching statistical significance at 10 and 20 µM curcumin (Figure 3).
Figure 3. Effect of curcumin on glioblastoma multiforme apoptosis.
SF-767 cells were incubated with 5, 10 and 20 µM free curcumin, ApoAI curcumin-ND and ApoE curcumin-ND. After 40 h, the percentage apoptotic cells was assessed by flow cytometry following annexin V and propidium iodide staining. Values are the mean ± standard deviation (n = 3).
*p < 0.05.
***p < 0.001.
ND: Nanodisk.
Curcumin uptake follows separation from ApoE NDs
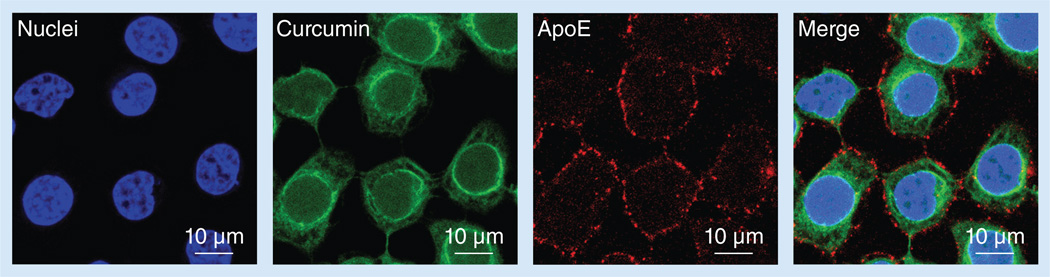
To investigate curcumin uptake by GBM cells following incubation with ApoE curcumin-NDs, confocal fluorescence microscopy was performed (Figure 4). The bulk of the ApoE (and presumably the ND particles) were localized at the cell surface 1 h after incubation of SF-767 cells with ApoE curcumin-NDs. On the other hand, curcumin showed strong intracellular fluorescence intensity. Therefore, it is evident that ApoE curcumin-NDs interaction with the surface of GBM cells is followed by curcumin separation from NDs and entry to the cell interior with minimal ApoE internalization.
Figure 4. Cellular localization of curcumin and ApoE.
SF-767 cells were incubated with 20 µM ApoE curcumin-nanodisk for 1 h at 37°C and washed to remove unbound nanodisks. Cells were fixed and confocal fluorescence microscopy used to detect curcumin fluorescence. ApoE was detected with monoclonal antibody 1D7 and an Alexa Fluor® 647-labeled (Life Technologies Corp., CA, USA) anti-mouse secondary antibody. Hoechst 33342 (Life Technologies Corp.) was used to stain the cell nuclei.
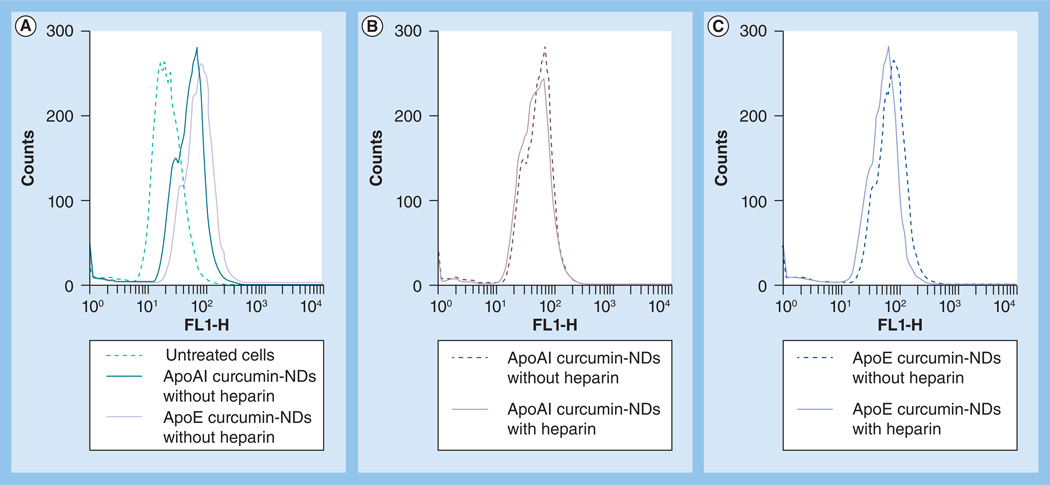
Effect of heparin on curcumin uptake from NDs
The enhancement in cellular curcumin uptake observed when ApoE was employed as the ND scaffold protein suggests a specific cell surface binding interaction may be involved. To address this, curcumin-NDs were incubated with SF-767 cells for 1 h in the presence and absence of heparin. After washing to remove heparin and unbound NDs, the cells were incubated further for 24 h and cellular fluorescence analyzed by flow cytometry (Figure 5). As expected, in control incubations lacking heparin, cellular curcumin fluorescence accumulation was greater with ApoE curcumin-NDs compared with ApoAI curcumin-NDs. The presence of heparin in cell incubations had no effect on cell-associated curcumin fluorescence from ApoAI curcumin-NDs. By contrast, heparin interfered with the enhancement in cellular curcumin fluorescence observed when these cells were incubated with ApoE curcumin-NDs.
Figure 5. Effect of heparin on nanodisk-mediated curcumin uptake by SF-767 cells.
Cells were seeded at 1 × 106 cells/well and incubated with curcumin NDs (20 µM curcumin) in the presence and absence of 50 µg heparin. After 1 h, cells were washed with phosphate-buffered saline and fresh medium was added. Cells were incubated further for 24 h and subjected to flow cytometry to detect internalized curcumin. (A) Untreated cells, ApoAI curcumin NDs without heparin and ApoE curcumin NDs without heparin. (B) Cells incubated with ApoAI curcumin NDs in the asbence and presence of heparin. (C) Cells incubated with ApoE curcumin NDs in the absence or presence of heparin. Data are representative of an experiment that was performed on two separate occasions. FL1-H: Height of the histogram that represents the mean curcumin fluorescence intensity detected by channel 1; ND: Nanodisk.
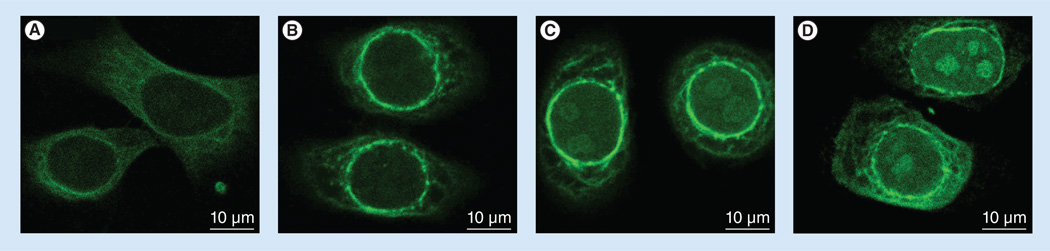
The intracellular itinerary of curcumin
To investigate the fate of internalized curcumin as a function of time, confocal microscopy was used to monitor curcumin fluorescence in SF-767 GBM cells (Figure 6). After 30 min of incubation with ApoE curcumin-NDs, curcumin displayed a diffuse cytosolic distribution. At 1 h, distinct perinuclear enrichment in curcumin fluorescence appears. After 2 h, the perinuclear fluorescence remains but is complemented by noticeable curcumin fluorescence within the nucleus. At 4 h, the intranuclear fluorescence signal was increased and appeared to concentrate at distinct intranuclear sites.
Figure 6. Localization of internalized curcumin as a function of time.
SF-767 cells were incubated with ApoE curcumin-nanodisk (20 µM curcumin) for (A) 30 min, (B) 1 h, (C) 2h and (D) 4 h, and curcumin localization was monitored by confocal fluorescence microscopy.
Discussion & conclusion
The naturally occurring polyphenol, curcumin, elicits antiproliferative and proapoptotic effects [19]. Studies in animal models of GBM revealed that intraperitoneal injection of curcumin leads to significant reductions in tumor xenograft size and increased time of survival [12,13]. Whereas these results indicate a therapeutic benefit of curcumin against GBM in vivo, neither the route of administration nor the vehicle used to administer curcumin are suitable for use in humans. To address this, we evaluated the biological effects of curcumin-NDs in two GBM cell lines. These self-assembled nanoparticles are capable of entrapping and solubilizing significant quantities of curcumin. Ease of formulation, intrinsic stability and interchangeability of the ND scaffold protein represent key advantages of this delivery vehicle [2]. The fact that formulation of curcumin into ND confers water solubility to this compound, with no loss of biological activity [1,14], suggests that therapeutic benefit may result.
An ideal curcumin delivery vehicle would display tissue-targeting capability and facilitated uptake, with minimal modification or degradation of the bioactive agent. In a previous study with curcumin-NDs, it was shown that curcumin is able to migrate from one ND particle to another, indicating its association with NDs is not irreversible [1]. In the present study, evidence for release of curcumin from NDs was obtained by confocal fluorescence microscopy. Remarkably, when ApoE curcumin-NDs were incubated with GBM cells, curcumin was taken up by the cells while the ApoE scaffold remained localized at the cell surface. Insofar as curcumin uptake by GBM cells was greater when ApoE was used as the ND scaffold component, it is conceivable that specific binding of ApoE to the GBM cell surface brings curcumin into close cell contact, such that off-loading from the ND particle, and uptake by the cell, is facilitated. It is note worthy that GBM cells express increased amounts of heparan sulfate proteoglycans (HSPGs) [20] and have abundant endocytic receptors of the low-density lipo protein receptor family [21]. ApoE is well known as a ligand for members of the low-density lipoprotein receptor family [22] and binds avidly to HSPGs [23]. The observation that incubation of ApoE curcumin-NDs with GBM cells in the presence of heparin attenuates the enhancement in cellular curcumin fluorescence accumulation observed with this ND scaffold indicates that ApoE interaction with one or more cell-surface components promotes curcumin uptake. While further experiments are required to elucidate the mechanisms involved, the fact that ApoE and curcumin do not colocalize within the cell and most ApoE remains at the cell surface after curcumin is internalized, suggests that ND whole-particle internalization is not involved. Therefore, it is plausible that ApoE-dependent ND–cell surface interactions, perhaps via HSPGs, promote curcumin off-loading and transit to the cell interior in the absence of ND particle internalization. This proposed mode of delivery is supported by the observed perinuclear localization of curcumin 1 h after incubation of ApoE curcumin-NDs with GBM cells. Had the entire ND undergone receptor-mediated endocytosis, it may be anticipated that curcumin would be directed to lysosomes where degradative enzymes may have an adverse effect on its biological activity. While studying cellular uptake of curcumin as a function of time, we observed enhanced curcumin fluorescence in distinct sites within the nucleus. Following reports that curcumin interacts directly with the minor groove of DNA [24–26], it is conceivable that nuclear localization may be related to its mechanism of action. Whereas the nature of the intranuclear sites of enhanced curcumin fluorescence is not clear at present, further study of curcumin localization to the nucleus, and correlation with progression toward apoptosis, may provide insight.
In summary, we demonstrated that enhanced uptake of curcumin from ApoE NDs correlates with increased cytotoxic and apoptotic effects in cultured GBM cells. The results suggest that ApoE may be a preferred ND scaffold protein for delivery of curcumin to GBM cells. The apparent off-loading of curcumin from NDs in the absence of ApoE internalization, combined with the potent biological activity of this formulation, suggests that ApoE curcumin-NDs have therapeutic potential. This is important because GBM is the most prevalent type of primary brain tumor in adults [27–29]. Current standard therapy involves surgical resection of the tumor followed by chemotherapy and radiotherapy. Even with this aggressive line of treatment, median survival for patients diagnosed with high-grade stage IV GBM is <1 year. By exploiting the cell-binding properties of ApoE NDs, targeted delivery of curcumin to GBM or other cancer cells in vivo may be possible.
Future perspective
Although curcumin has shown potent anticancer effects in several model systems, its development as a therapeutic is hampered by water insolubility and poor bioavailability. Progress towards greater use of curcumin relies on the generation of a delivery vehicle that surmounts these obstacles. Given their ease of formulation, versatility in component composition and intrinsic stability, curcumin-NDs offer a path forward for human clinical trials. It is envisioned that these biocompatible nanoparticles may provide a feasible strategy for targeted delivery of curcumin to tissues such that significant clinical benefit will be realized.
Supplementary Material
Executive summary.
Background
-
▪
Nanodisks (NDs) are self-assembled nanoscale phospholipid/apolipoprotein particles that can be loaded with high amounts of the polyphenol curcumin, a phytochemical that has emerged as an anticancer agent with potential therapeutic use in primary, malignant brain tumor glioblastoma multiforme (GBM).
Materials & methods
-
▪
To address challenges regarding curcumin’s bioavailability, curcumin-NDs were formulated with two different apolipoprotein scaffolds, ApoAI and ApoE, and the ability of these formulations to deliver curcumin and elicit biological effects were evaluated in cultured GBM cells.
Results
-
▪
Flow cytometry revealed enhanced curcumin uptake by GBM cells incubated with ApoE curcumin-NDs compared with either ApoAI curcumin-NDs or free curcumin.
-
▪
Enhanced uptake translated into higher antiproliferative and apoptotic effects for the ApoE curcumin-ND formulation.
-
▪
Confocal microscopy showed that the ApoE scaffold bound to GBM cell surface with off-loading of the curcumin cargo over time followed by accumulation of curcumin in discrete intranuclear sites.
Discussion & conclusion
-
▪
GBM cells express high amounts of heparan sulfate proteoglycans and receptors of the low-density lipoprotein receptor family for which ApoE is a known ligand. Evidence of ApoE ND-scaffold binding to the GBM cell surface in conjunction with enhanced curcumin delivery suggests that ApoE curcumin-NDs may facilitate curcumin delivery to GBM cells in vivo.
-
▪
The biocompatible nature of NDs together with the apparent targeting capability of its scaffold component creates an attractive delivery vehicle for curcumin.
Aknowledgement
The authors would like to thank A Johl for technical assistance.
This work was supported by a grant from the NIH (HL-64159) and an American Heart Association Western States Affiliate Predoctoral Fellowship (#10PRE3600031).
Footnotes
Financial & competing interests disclosure
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Ethical conduct of research
The authors state that they have obtained appropriate institutional review board approval or have followed the principles outlined in the Declaration of Helsinki for all human or animal experimental investigations.
References
Papers of special note have been highlighted as:
▪ of interest
- 1. Ghosh M, Singh ATK, Xu W, Sulchek T, Gordon LI, Ryan RO. Curcumin nanodisks: formulation and characterization. Nanomedicine. 2011;7(2):162–167. doi: 10.1016/j.nano.2010.08.002. ▪ Contains details regarding the curcumin nanodisk (ND) formulation.
- 2. Ryan RO. Nanodisks: hydrophobic drug delivery vehicle. Expert Opin. Drug Deliv. 2008;5(3):343–351. doi: 10.1517/17425247.5.3.343. ▪ Reviews nanodisks as a drug-delivery platform. The concept of using NDs for targeted drug delivery is described.
- 3.Ryan RO. Nanobiotechnology applications of reconstituted high density lipoprotein. J. Nanobiotechnology. 2010;8:28. doi: 10.1186/1477-3155-8-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Oda MN, Hargreaves P, Beckstead JA, Redmond KA, van Antwerpen R, Ryan RO. Reconstituted high-density lipoprotein enriched with the polyene antibiotic, amphotericin B. J. Lipid Res. 2006;47(2):260–267. doi: 10.1194/jlr.D500033-JLR200. [DOI] [PubMed] [Google Scholar]
- 5.Redmond KA, Nguyen TS, Ryan RO. All-trans retinoic acid nanodisks. Int. J. Pharm. 2007;339(1–2):246–250. doi: 10.1016/j.ijpharm.2007.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Epstein J, Sanderson IR, Macdonald TT. Curcumin as a therapeutic agent: the evidence from in vitro, animal and human studies. Br. J. Nutr. 2010;103(11):1545–1557. doi: 10.1017/S0007114509993667. [DOI] [PubMed] [Google Scholar]
- 7.Spiller SE, Logsdon NJ, Deckard LA, Sontheimer H. Inhibition of nuclear factor kappa-B signaling reduces growth in medulloblastoma in vivo . BMC Cancer. 2011;11:136. doi: 10.1186/1471-2407-11-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Anand P, Kunnumakkara AB, Newman RA, Aggarwal BB. Bioavailability of curcumin: problems and promises. Mol. Pharm. 2007;4(6):807–818. doi: 10.1021/mp700113r. ▪ Discusses curcumin’s poor bioavailability and how this has hindered its therapeutic evaluation.
- 9.Schaffer M, Schaffer PM, Zidan J, Bar-Sela G. Curcuma as a functional food in the control of cancer and inflammation. Curr Opin. Clin. Nutr. Metab. Care. 2011;14(6):588–597. doi: 10.1097/MCO.0b013e32834bfe94. [DOI] [PubMed] [Google Scholar]
- 10.Huang TY, Tsai TH, Hsu CW, Hsu YC. Curcuminoids suppress the growth and induce apoptosis through caspase-3-dependent pathways in glioblastoma multiforme (GBM) 8401 cells. J. Agric. Food Chem. 2010;58(19):10639–10645. doi: 10.1021/jf1016303. [DOI] [PubMed] [Google Scholar]
- 11.Senft C, Polacin M, Priester M, Seifert V, Kögel D, Weissenberger J. The nontoxic natural compound curcumin exerts antiproliferative, anti-migratory, and anti-invasive properties against malignant gliomas. BMC Cancer. 2010;10:491. doi: 10.1186/1471-2407-10-491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zanotto-Filho A, Braganhol E, Edelweiss MI, et al. The curry spice curcumin selectively inhibits cancer cells growth in vitro and in preclinical model of glioblastoma. J. Nutr. Biochem. 2012;23(6):591–601. doi: 10.1016/j.jnutbio.2011.02.015. ▪ Evaluates the benefits of curcumin in a preclinical model of glioblastoma. Results suggest that curcumin might be a potential therapeutic agent for treating glioblastoma multiforme. However, in vivo studies were performed with curcumin administered intraperitoneally, dissolved in dimethyl sulfoxide, a mode of administration that is not suitable for clinical use of curcumin.
- 13.Perry MC, Demeule M, Regina A, Moumdjian R, Beliveau R. Curcumin inhibits tumor growth and angiogenesis in glioblastoma xenografts. Mol. Nutr. Food Res. 2010;54(8):1192–1201. doi: 10.1002/mnfr.200900277. [DOI] [PubMed] [Google Scholar]
- 14. Singh ATK, Ghosh M, Forte TM, Ryan RO, Gordon LI. Curcumin nanodisk-induced apoptosis in mantle cell lymphoma. Leuk. Lymphoma. 2011;52(8):1537–1543. doi: 10.3109/10428194.2011.584253. ▪ Documents the proapoptotic effects of curcumin-NDs in cultured lymphoma cells.
- 15.Ryan RO, Forte TM, Oda MN. Optimized bacterial expression of human apolipoprotein A–I. Protein Expr. Purif. 2003;27(1):98–103. doi: 10.1016/s1046-5928(02)00568-5. [DOI] [PubMed] [Google Scholar]
- 16.Fisher CA, Wang J, Francis GA, Sykes BD, Kay CM, Ryan RO. Bacterial overexpression, isotope enrichment and NMR analysis of the N-terminal domain of human apolipoprotein E. Biochem. Cell Biol. 1997;75(1):45–53. [PubMed] [Google Scholar]
- 17.Weisgraber KH, Rall SC, Jr, Mahley RW, Milne RW, Marcel YL, Sparrow JT. Human apolipoprotein Determination of the heparin binding sites of apolipoprotein E3. J. Biol. Chem. 1986;261(5):2068–2076. [PubMed] [Google Scholar]
- 18.Kunwar A, Barik A, Pandey R, Priyadarsini KI. Transport of liposomal and albumin loaded curcumin to living cells: an absorption and fluorescence spectroscopic study. Biochim. Biophys. Acta. 2006;1760(10):1513–1520. doi: 10.1016/j.bbagen.2006.06.012. [DOI] [PubMed] [Google Scholar]
- 19.Agrawal DK, Mishra PK. Curcumin and its analogues: potential anticancer agents. Med. Res. Rev. 2010;30(5):818–860. doi: 10.1002/med.20188. [DOI] [PubMed] [Google Scholar]
- 20.Steck PA, Moser RP, Bruner JM, et al. Altered expression and distribution of heparin sulfate proteoglycans in human gliomas. Cancer Res. 1989;49(8):2096–2103. [PubMed] [Google Scholar]
- 21.Maletínská L, Blakely EA, Bjornstad KA, Deen DF, Knoff LJ, Forte TM. Human glioblastoma cell lines: levels of low density lipoprotein receptor and low density lipoprotein receptor-related protein. Cancer Res. 2000;60(8):2300–2303. [PubMed] [Google Scholar]
- 22. Hauser PS, Narayanaswami V, Ryan RO. Apolipoprotein E: from lipid transport to neurobiology. Prog. Lipid Res. 2011;50(1):62–74. doi: 10.1016/j.plipres.2010.09.001. ▪ Contains information on the ND scaffold protein ApoE.
- 23.Weisgraber KH, Mahley RW. Human apolipoprotein E: the Alzheimer’s disease connection. FASEB J. 1996;10(13):1485–1494. doi: 10.1096/fasebj.10.13.8940294. [DOI] [PubMed] [Google Scholar]
- 24.Zsila F, Bikadi Z, Simonyi M. Circular dichroism spectroscopic studies reveal pH dependent binding of curcumin in the minor groove of natural and synthetic nucleic acids. Org. Biomol. Chem. 2004;2(20):2902–2910. doi: 10.1039/B409724F. [DOI] [PubMed] [Google Scholar]
- 25.Nafsi S, Adelzadeh M, Norouzi Z, Sarbolouki MN. Curcumin binding to DNA and RNA. DNA Cell Biol. 2009;28(4):201–208. doi: 10.1089/dna.2008.0840. [DOI] [PubMed] [Google Scholar]
- 26.Reuter S, Gupta SC, Park B, Goel A, Aggarwal BB. Epigenetic changes induced by curcumin and other natural compounds. Genes Nutr. 2011;6(2):93–108. doi: 10.1007/s12263-011-0222-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stupp R, Mason WP, Van Den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N. Engl. J. Med. 2005;352(10):987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 28.Dougherty JD, Fomchenko EI, Akuffo AA, et al. Candidate pathways for promoting differentiation or quiescence in oligodendrocyte progenitor-like cells in glioma. Cancer Res. 2012;72(18):4856–4868. doi: 10.1158/0008-5472.CAN-11-2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lima FR, Kahn SA, Soletti RC, et al. Glioblastoma: therapeutic challenges, what lies ahead. Biochim. Biophys. Acta. 2012;1826(2):338–349. doi: 10.1016/j.bbcan.2012.05.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.