Abstract
Capnocytophaga canimorsus is a facultative Gram-negative bacillus that is typically a constituent of the oral flora of dogs and cats. It was first isolated by Bobo and Newton in 1976 from a man presenting with meningitis following a dog bite. Transmission to humans follows various animal-related injuries, which may be gross or subtle. C canimorsus can cause a spectrum of syndromes ranging from skin and soft tissue infection to invasive disease such as meningitis or endocarditis. The present article reports a case of C canimorsus meningitis in a patient with the classic risk factor of alcoholic liver cirrhosis. Clinical suspicion was confirmed by culture and genetic identification of the blood isolate. The present article reviews the Capnocytophaga genus, the clinical syndromes most commonly associated with this zoonotic organism, its laboratory identification and treatment.
Keywords: Capnocytophaga canimorsus, Dog bite, Gram-negative bacillus, Meningitis
Abstract
Le Capnocytophaga canimorsus est un bacille Gram négatif facultatif qui fait généralement partie de la flore buccale des chiens et des chats. C’est Bobo et Newton qui l’ont isolé pour la première fois en 1976, chez un homme souffrant d’une méningite après avoir été mordu par un chien. La transmission aux humains est secondaire à plusieurs blessures liées aux animaux, qui peuvent être évidentes ou discrètes. Le C canimorsus peut causer une série de syndromes, de l’infection de la peau et des tissus mous à une maladie invasive comme la méningite ou l’endocardite. Le présent article rend compte d’un cas de méningite à C canimorsus chez un patient ayant les facteurs de risque classiques de cirrhose hépatique alcoolique. La présomption clinique a été confirmée par culture et identification génétique de l’isolat sanguin. Dans le présent article, on trouve une analyse du gène Capnocytophaga, des syndromes cliniques les plus associés à cet organisme zoonotique, de son identification en laboratoire et de son traitement.
In 1976, Bobo and Newton (1) described a syndrome that would forever change mankind’s relationships with their canines. A previously undescribed, fastidious Gram-negative bacillus was isolated from blood and cerebrospinal fluid of a man who developed septicemia and meningitis approximately one week after a dog bite. In fact, these organisms had been referred to the Centers for Disease Control and Prevention (Georgia, USA) for identification since 1961, forming the ‘dysgonic fermenter type 2’ (DF-2) collection (2). Shortly thereafter, Butler et al (3) reviewed the clinical and epidemiological findings for 17 patients from whose blood DF-2 strains were isolated, demonstrating that this organism was, in fact, the cause of the syndrome described by Bobo and Newton, and confirming the precipitating contact with dogs. Given that dogs are the most common household pets in North America and that approximately one million dog bites occur annually (4), the present report highlights the pathogenicity of an uncommon zoonotic bacteria, Capnocytophaga canimorsus, and includes a literature review of the syndromes it causes and recommendations for management.
CASE PRESENTATION
A 56-year-old man initially presented to a peripheral hospital with a four-day history of fever, headache and arthralgias, with nausea and retching. His medical history was significant for mixed connective tissue disease with associated Raynaud’s phenomenon, which had caused several previous episodes of patchy, self-limited digital necrosis; he was never treated with immunosuppressants. He was also hypertensive and had benign prostatic hypertrophy. A few years previously, he was found to have a progressive mild thrombocytopenia (100×109/L) associated with mild hepatosplenomegaly; however, he was not compliant with further investigations. He regularly consumed alcohol, estimated by his wife to be six beers per day.
Soon after arrival to the hospital, the patient developed progressive obtundation requiring intubation. In the context of an altered mental status and concurrent fever, a meningitic and/or encephalitic process was suspected. A computed tomography scan of the head revealed no mass lesion or hemorrhage. Ampicillin, ceftriaxone, vancomycin and acyclovir were initiated before lumbar puncture. Dexamethasone was not administered. Cerebrospinal fluid was turbid, with 855 leukocytes/mL (97% neutrophils), 210 red blood cells/mL, elevated protein (2.7 g/dL) and low glucose (0.2 mmol/L); concomitant blood glucose was 7.04 mmol/L. No organisms were observed on Gram stain.
Because of profound neurological impairment, the patient was transferred to a tertiary care hospital. Within approximately 24 h, blood cultures from the initial hospital revealed a Gram-negative filamentous bacillus, also isolated from two sets of blood cultures at the referral centre. On careful examination, a small eschar at the base of the left index finger was noted (Figure 1). According to the patient’s wife and daughter, the recently acquired family dog had bitten the patient one week previously.
Figure 1).
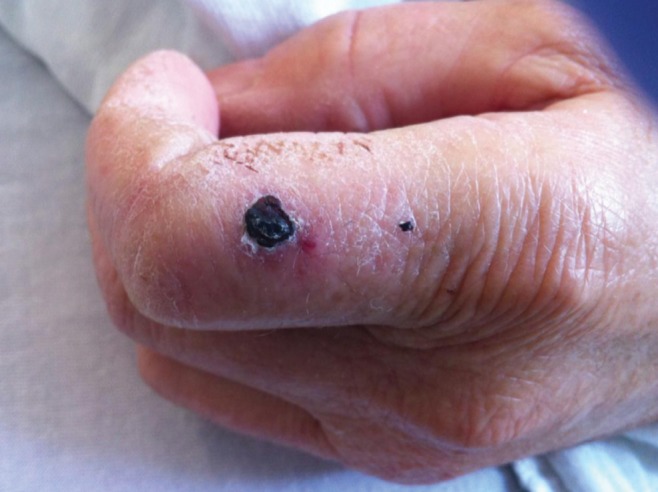
Necrotic eschar of the left index finger, raising concern for an animal bite injury
The constellation of a Gram-negative bacillary meningitis in an individual with probable cirrhosis and a dog-bite injury raised suspicion for Capnocytophaga meningitis. Other than thrombocytopenia, there was no evidence of disseminated intravascular coagulation (DIC). Antibiotics were eventually de-escalated to meropenem at a dose of 2 g every 8 h, pending speciation of the Gram-negative bacillus.
Despite significant disease revealed by magnetic resonance imaging of the brain, which demonstrated fluid levels in both ventricles with diffusion restriction suggestive of ventriculitis, likely due to protein-aceous necrotic debris such as pus (Figure 2), repeat lumbar puncture after three days of antibiotic treatment revealed resolving pleocytosis, protein and glucose concentrations, with no organism observed on Gram stain nor isolated in culture. Over the next few days, the patient’s mental and neurological status gradually improved, allowing extubation and eventual transfer to the original hospital, where he completed a total of three weeks of inpatient antibiotic therapy with meropenem.
Figure 2).
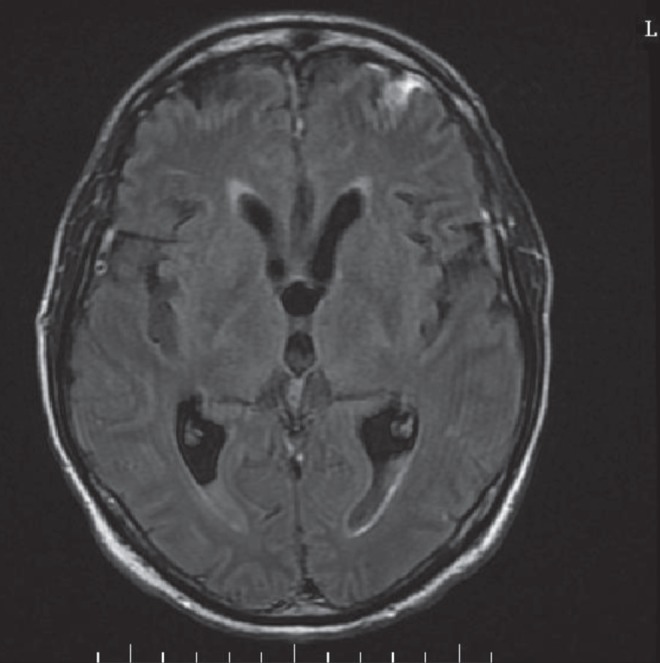
Magnetic resonance image of the brain revealing fluid levels in the ventricles bilaterally with diffusion restriction, suggestive of ventriculitis
Microbiological identification of the Gram-negative bacillus was hampered by its fastidious nature. Although the organism was detected using BACTEC blood culture bottles (Becton Dickinson, USA) after approximately two days, primary subculture onto solid media revealed only poor growth in anaerobically incubated blood agar plates. Subculture onto chocolate agar incubated in CO2 permitted sufficient growth for subsequent identification by the provincial reference laboratory (Laboratoire de Santé Publique du Québec, Sainte-Anne-de-Bellevue, Québec) via 16S ribosomal RNA gene sequencing, which confirmed the isolate to be C canimorsus seven days after the blood cultures were drawn.
DISCUSSION
The genus Capnocytophaga, proposed by Leadbetter et al (5) in 1979, includes capnophilic, facultatively anaerobic, non-spore-forming Gram-negative bacteria of the family Flavobacteriaceae. The genus is infamous for two species, C canimorsus (previously termed Centers for Disease Control and Prevention DF-2) and Capnocytophaga cynodegmi (DF-2-like). Their previous designation, ‘dysgonic fermenter’, referred to the phenotypic characteristics (ie, slow and relatively poorly growing bacterium, fermenting carbohydrates); their current species names derive from their notorious zoonotic association, in that they are transmitted primarily through dog bites (Latin canis = dog and morsus = bite; Greek kyno = dog and degmos = bite). However, the genus also includes a variety of commensal species (some of which constituted the previous DF-1 group) that can be found in the oral flora of humans: Capnocytophaga ochracea, Capnocytophaga gingivalis, Capnocytophaga granulosa, Capnocytophaga haemolytica, Capnocytophaga sputigena, Capnocytophaga leadbetteri and Capnocytophaga genospecies AHN8471 (6).
Since its discovery in 1976 (1), C canimorsus has been implicated as a pathogenic agent in a variety of severe human infections, including septicemia, endocarditis, purpura fulminans and meningitis, and is transmitted by dogs or, occasionally, cats (7). C canimorsus is a commensal organism of the oral flora of these animals; using bacterial isolation methods, it has been detected in 25.5% of dogs and 21% of cats (8), and in 74% of dogs and 57% of cats using polymerase chain reaction sequencing methods (9). While this implicates domesticated canines and felines as the most likely source of human infections, illustrating the crucial need to obtain a history of animal exposures when evaluating patients, it should be noted that no clear documented animal source is identified in approximately 10% of cases (10).
The number of cases of human infections due to C canimorsus was estimated to be 0.5 to 0.7 per one million inhabitants per year in large studies performed in Denmark and the Netherlands (11,12). This rate likely varies with different social, cultural and economic habits. These estimates are also skewed on the one hand by the frequent use of empirical antibiotics in the clinical management of animal bites and, on the other, by the fastidiousness of the organism and, thus, challenges regarding its isolation and identification in routine microbiology laboratories.
Historically, infections due to C canimorsus more commonly affect men (male:female ratio ranging from 2.7:1 to 3.75:1), with the mean age varying between 50 and 60 years of age (10,12–14), likely reflecting both the demographics of exposure and, importantly, the corresponding underlying risk factors. Since its first description, hyposplenism (typically from splenectomy) and alcohol-induced liver disease have emerged as significant risk factors for severe, noncellulitis C canimorsus infection (ie, septicemia, meningitis) (1,3,10,13,15,16). Although hematological malignancy and steroid use are often cited as risk factors for Capnocytophaga species infections, these predisposing conditions are, in reality, more commonly observed with invasive infections with species endogenous to humans, rather than the zoonotic ones. Of note, up to 40% of severe C canimorsus infections have occurred in subjects in whom no underlying predisposing condition is apparent (13). Although complicated septicemia (eg, with DIC, organ failure and/or shock) tends to be more often reported in individuals with hyposplenism or alcoholic liver disease, there are cases in which neither of these conditions is present. In such cases, C canimorsus septicemia may reflect a sentinel manifestation of an acquired or congenital defect in host defenses.
Overall mortality rates for severe C canimorsus infections range from 13% to 33% (11,12,17); notably, and perhaps paradoxically, meningitis portends a lower mortality rate of 5% (13).
Laboratory identification
Capnocytophaga species appear as long, slender Gram-negative bacilli with tapered ends, typically measuring 3 μm to 6 μm in length (18), compared with 2 μm for Escherichia coli. Capnocytophaga species are slow-growing on solid media. Some isolates will grow better anaerobically on primary isolation, as in the present case but, typically, these bacteria are characterized by their requirement for 5% to 10% CO2 enrichment for optimum growth. This capnophilicity is particularly apparent on attempts at subculture. The organisms grow best at 35°C to 37°C on either blood or chocolate agar. The type of ‘blood agar’ appears to be important; heart infusion agar with 5% rabbit blood allows colonies to appear within 24 h to 72 h, whereas Trypticase soy agar base with 5% sheep blood yields poorer growth (2,10,16). The addition of a Staphylococcus aureus streak to the agar plate has been reported to enhance growth of the organism, a phenomenon not reproduced by the use of ‘X’ (hemin) and ‘V’ (nicotinamide adenine dinucleotide) paper strips (19,20); this manoeuvre was not attempted in the present case. Capnocytophaga species do not grow on MacConkey agar. Visible colonies typically require two to four days of incubation to appear and are nonhemolytic. Colonial morphology is usually flat and irregular with fingerlike projections. These fingerlike projections are attributed to ‘gliding motility’ characteristic of this genus. The zoonotic Capnocytophaga species (C canimorsus, C cynodegmi) are oxidase positive and catalase positive, whereas those constituting the human oral flora are oxidase negative and catalase negative.
16S ribosomal RNA gene sequencing has become the gold standard for the identification of bacteria with ambiguous biochemical profiles (21). This method has proved successful in distinguishing isolates from Capnocytophaga species (22), and has been widely reported in the literature as a specific and reliable diagnostic tool for C canimorsus (23–25).
Clinical syndromes
The incubation period between the microbial exposure from contact with an infected animal to development of symptoms is, on average, seven days (range one to eight days, occasionally up to 30 days) (13,26).
The zoonotic Capnocytophaga species infections result in various presentations. The most common is localized skin and soft tissue infection. Typically, patients present within hours after a dog bite; at this point, there may be little significant evidence of local inflammation. Later, infection may present with localized cellulitis, pain at the site of injury, a purulent discharge, lymphangitis and regional lymphadenopathy. However, these rarely progress to more severe disease because they occur within the context of obvious bite injuries, which are typically treated preventively with antibiotics.
On the other hand, more dramatic presentations include fulminant septicemia, peripheral gangrene or meningitis, which are not uncommonly reported in the literature. Unlike their skin and soft tissue infection counterparts, these severe manifestations are not preceded by gross injury, but often by apparently benign bites, scratches or even licks. In our case, the history of the dog bite was suggested by the presence of an eschar, which had been dismissed as the digital gangrene of a Raynaud attack.
Sporadic cases manifesting as endocarditis, pleural empyema, corneal keratitis or osteomyelitis/septic arthritis have also been reported, although these appear to be infrequent. Infections caused by endogenous human Capnocytophaga species are also uncommon but increasingly reported.
In septicemia due to C canimorsus, in addition to fever and chills, the most common initial symptoms are myalgia and gastrointestinal complaints, similar to our case (11). Occasionally, gastrointestinal bleeding manifestations (eg, hemoptysis, hematuria, melena) may be the presenting symptoms, which may be misleading and delay diagnosis (27). Cutaneous manifestations are common and pleomorphic, ranging from a petechial or purpuric rash at one extreme, to purpura fulminans causing peripheral gangrene often necessitating amputation at the other (11,28–30). Anemia and thrombocytopenia may develop in isolation or as part of DIC, the latter occurring in up to 34% of cases of C canimorsus sepsis (10).
In C canimorsus meningitis, other than a possible bite wound on examination (as in the present case), the clinical picture and cerebrospinal fluid findings are indistinguishable from bacterial meningitis caused by typical organisms. There is the suggestion that, compared with typical bacterial meningitides, some cases of C canimorsus meningitis may demonstrate a lesser degree of pleocytosis (<1000 white blood cells/mL) along with a high percentage (≥30%) of lymphocytes (31). These cases may be confused with meningitis due to Listeria monocytogenes or, perhaps, viruses; confusion with the latter may lead to premature cessation of antibiotics, which may be disastrous. An interesting sequela of C canimorsus meningitis appears to be permanent sensorineural hearing loss, which has even been described as a primary complaint (31–33). Whether C canimorsus meningitis causes deafness more frequently than other causes of meningitis remains to be demonstrated. Radiological findings are rarely reported in the literature. Generally, computed tomography scanning of the head in affected patients has been denoted as being unchanged from baseline. In the present case, diffusion-weighted magnetic resonance imaging enabled us to appreciate the gravity and devastating pathogenicity of C canimorsus. To our knowledge, there are no reports of purulent ventriculitis or cerebritis caused by this organism described to date.
C canimorsus can also cause endocarditis. One-third of cases occur in individuals with pre-existing cardiac lesions (34). Given its fastidious nature, C canimorsus may be difficult to isolate as the causative organism, prompting one author to suggest it be added to the list of HACEK (Haemophilus species, Aggregatibacter actinomycetemcomitans, Cardiobacterium hominis, Eikenella corrodens and Kingella kingae) group organisms (35). Although aortic and tricuspid valves have been commonly involved in reported cases (34), there are no distinguishing clinical or echocardiogenic features specific to C canimorsus. Similar to other infectious endocarditides, C canimorsus endocarditis may be complicated by paravalvular abscess (36), fistula (34), myo/pericarditis (11) and myocardial infarction (37,38). Surgical intervention was required in up to 47% of cases (34).
Pathogenesis
The pathogenic mechanisms underlying Capnocytophaga infections are not well understood but appear to segregate with the ‘dysgonic fermenter’ group. Infections due to DF-1 and related endogenous Capnocytophaga species are primarily reported in patients following mucosal injury (eg, from mucositis and neutropenia associated with cytoreductive chemotherapy [39–41], periodontitis [42] or chorioamnionitis [43,44]). In such cases, an intact mucosal epithelium appears to be a critical factor in host defense.
Infections due to zoonotic Capnocytophaga appear to occur by a different process. The classic risk factors for severe infection with C canimorsus are hyposplenism and liver disease/cirrhosis, typically from alcohol use (as in the present case). Hyposplenism may confer susceptibility to C canimorsus because of impaired antibody-mediated opsonophagocytosis; this hypothesis is supported by increased phagocytosis and killing of the bacteria, at least by murine macrophages following opsonization with specific antibody, but not with complement (45). The basis for susceptibility in cirrhotic patients may involve the unique enzyme complex of C canimorsus that enables it to harvest amino sugars of glycan chains from host glycoproteins; in particular, this complex targets N-acetylglucosamine residues (46). In liver disease/cirrhosis, there are alterations in the glycosylation of numerous serum glycoproteins, including liver-derived proteins as well as immunoglobulins. Interestingly, as liver disease progresses, an increase in N-acetylglucosamine-containing glycoproteins is measurable (47). It is attractive to speculate, therefore, that the selective susceptibility to C canimorsus in liver disease may reflect the unique opportunism of this species to feed on exactly those substrates that become more readily available.
Treatment
The fastidious nature of Capnocytophaga species, the lack of validated susceptibility testing methods and the lack of interpretive criteria makes standardized data on the in vitro antimicrobial susceptibility patterns of this genus difficult to collect. However, a general pattern describing Capnocytophaga species antimicrobial susceptibility proposed by Jolivet-Gougeon et al (17) is as follows: they are typically considered to be susceptible to penicillins, third-generation cephalosporins, carbapenems, clindamycin, doxycycline and chloramphenicol. Most isolates are also susceptible to macrolides, rifampin and fluoroquinolones. Susceptibility to vancomycin, metronidazole and aminoglycosides demonstrates significant variability among studies, likely reflecting differences in methodology. Most isolates are considered to be resistant to aztreonam, trimethoprim, fosfomycin and aminoglycosides. Although Capnocytophaga species are increasingly recognized to produce β-lactamase, detectable by the routine chromogenic nitrocefin test, review of the literature reveals that this resistance is observed more commonly in human endogenous Capnocytophaga species isolates (40,48–51) rather than zoonotic ones (34,52). Fortunately, β-lactamase-producing isolates remain susceptible to β-lactamase-resistant β-lactams or β-lactam/β-lactamase inhibitor combinations. Given the unclear correlation between in vitro antimicrobial susceptibility testing and clinical outcome, no firm recommendations can be given regarding antimicrobial selection for the various C canimorsus syndromes, particularly for patients demonstrating type I (immunogobulin E-mediated) allergic reactions to β-lactams. Nonetheless, recommendations for targeted therapy of the different Capnocytophaga species syndromes, once the organism has been isolated, are proposed in Table 1.
TABLE 1.
Recommendations for the management of various Capnocytophaga species infections
| Infection | Recommended first-line therapy | Alternative therapies | Comments |
|---|---|---|---|
| Dog/cat bite-related injury | Amoxicillin/clavulanic acid, 875 mg/125 mg orally twice per day or 500 mg/125 mg orally three times per day (10)
|
For patients with non-type I (immunoglobulin E-mediated) penicillin allergies, consider second- or third-generation cephalosporin + anti-anaerobic agent (metronidazole or clindamycin) For patients with type I (immunoglobulin E-mediated) penicillin allergy, consider either doxycycline 100 mg orally twice per day or fluoroquinolone + anti-anaerobic agent (metronidazole or clindamycin) |
Dog bites include crush, lacerating and puncture wounds. Cat bites include deep puncture wounds and scratches. Only 15% to 20% of dog bite wounds become infected. Crush injuries, puncture wounds and hand wounds are more likely to become infected than scratches. Licks of pre-existing wounds may also become infected. Although antibiotics reduce the incidence of infection, 14 patients may have to be treated to prevent one infection (48) Note that dog and cat bite-related infections are polymicrobial. Therapy should be directed toward canine/feline oral anaerobes/facultative anaerobes (eg, Pasteurella, Capnocytophaga), Staphylococcus aureus, Streptococcus species Proper management includes (mnemonic: WATeR):
|
| Brain abscess/meningitis | If no β-lactamase is detected, consider penicillins (high dose) If β-lactamase-producing isolate, consider third-generation cephalosporin or carbapenem |
No clinical reports in the literature to guide alternative treatment options For patients with β-lactam allergy, consider desensitization |
Treatment duration: 14 to 21 days |
| Bacteremia, including endocarditis | If no β-lactamase is detected, consider penicillins (high dose) If β-lactamase-producing isolate, consider β-lactam/β-lactamase inhibitor or carbapenem The role for combination therapy (eg, with fluoroquinolones) has been reported but is not defined |
No clinical reports in the literature to guide alternative treatment options For patients with β-lactam allergy, consider desensitization, or perhaps the use of one or more of the following agents (note: these are bacteriostatic)
|
Rare (<20 cases), with no distinguishing features on patient history, physical examination or echocardiography Valve involvement: aortic > tricuspid > mitral (29) Because of its fastidious nature, may be a cause of ‘culture-negative’ endocarditis As with other endocarditides, monitor for need for surgical intervention |
| Postsplenectomy sepsis | β-lactam/β-lactamase inhibitor or carbapenem | Respiratory fluoroquinolone + vancomycin | Organisms that typically cause postsplenectomy sepsis: Streptococcus pneumoniae, Neisseria meningitidis and Haemophilus influenzae; therefore, empirical antimicrobial therapy should include coverage of these organisms |
For penetrating dog or cat bites, the polymicrobial nature of the wound necessitates proper wound management, including copious irrigation with normal saline and determining the extent of the wound. Antibiotics should be considered, particularly for a patient at risk for complications. The spectrum of the selected antibiotic must also cover more common pathogens, including anaerobes, Staphylococcus species, Streptococcus species and Pasteurella species. In patients who present within 8 h of the bite and if there is no evidence of infection, a ‘prophylaxis’ approach may be considered. This approach appears to reduce the risk of wound infection by nearly one-half, although 14 patients need to be treated to prevent one infection (53). In the presence of infection, the depth of infection needs to be determined (eg, tenosynovitis, osteomyelitis, septic arthritis), with appropriate surgical consultation as necessary, and an appropriate duration of antimicrobial therapy provided. Additionally, tetanus and rabies prophylaxis need to be considered.
For the different Capnocytophaga species syndromes, targeted therapy ultimately depends on whether the isolate produces β-lactamase. For isolates that do not, penicillins and aminopenicillins are appropriate. For those that produce a β-lactamase, therapeutic options include a β-lactam/β-lactamase inhibitor combination, a β-lactamase-resistant β-lactam or a carbapenem. Clindamycin, a respiratory fluoroquinolone or doxycycline may be appropriate alternatives, although reports of clinical efficacy in the literature are scant. Combination therapy may be theoretically advantageous, but again, the absolute benefit has not been investigated.
The duration of antimicrobial therapy for treatment of Capnocytophaga species infections is hampered by the absence of randomized studies. As such, extrapolations from other similar infections provide the basis for clinical management. For C canimorsus meningitis, the recommended length of treatment varies between 14 and 21 days or longer, as for other Gram-negative meningitis infections (13). For endocarditis, the duration of treatment is at least four to six weeks, with monitoring need for surgical intervention, while at least six to eight weeks may be required for bone/joint involvement.
CONCLUSION
C canimorsus can cause a variety of syndromes. Hyposplenism and alcoholic liver disease are the main risk factors for severe infection; these conditions should raise suspicion for this organism. Its fastidious nature may delay or prevent isolation and appropriate speciation. Antimicrobial susceptibility testing is not standardized. However, clues to its presence should be sought by thorough review of history and physical examination. With appropriate, duly initiated therapy, C canimorsus meningitis, a potentially fatal infection, has a relatively favourable prognosis compared with other more common bacterial meningitides.
Acknowledgments
The authors thank the Laboratoire de santé publique du Québec for their collaboration in identifying C canimorsus, the causative pathogen in this case. Dr Popiel was the winner of the Canadian Journal of Infectious Diseases and Medical Microbiology Trainee Review Article Award, presented at the Association of Medical Microbiology and Infectious Canada 2013 Annual Conference, April 4 to 6, Quebec City, Quebec.
Footnotes
DISCLOSURES: Dr Vinh has received speaker honoraria and research support from CSL Behring Canada, as well as research support from Astellas Pharma Canada. These associations do not have direct or indirect relations to this article.
REFERENCES
- 1.Bobo RA, Newton EJ. A previously undescribed gram-negative bacillus causing septicemia and meningitis. Am J Clin Pathol. 1976;65:564–9. doi: 10.1093/ajcp/65.4.564. [DOI] [PubMed] [Google Scholar]
- 2.Brenner DJ, Hollis DG, Fanning GR, Weaver RE. Capnocytophaga canimorsus sp. nov. (formerly CDC group DF-2), a cause of septicemia following dog bite, and C. cynodegmi sp. nov., a cause of localized wound infection following dog bite. J Clin Microbiol. 1989;27:231–5. doi: 10.1128/jcm.27.2.231-235.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Butler T, Weaver RE, Ramani TK, et al. Unidentified Gram-negative rod infection. A new disease of man. Ann Intern Med. 1977;86:1–5. doi: 10.7326/0003-4819-86-1-1. [DOI] [PubMed] [Google Scholar]
- 4.Janda JM, Graves MH, Lindquist D, Probert WS. Diagnosing Capnocytophaga canimorsus infections. Emerg Infect Dis. 2006;12:340–2. doi: 10.3201/eid1202.050783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Leadbetter ER, Holt SC, Socransky SS. Capnocytophaga: New genus of gram-negative gliding bacteria. I. General characteristics, taxonomic considerations and significance. Arch Microbiol. 1979;122:9–16. doi: 10.1007/BF00408040. [DOI] [PubMed] [Google Scholar]
- 6.Frandsen EV, Poulsen K, Kononen E, Kilian M. Diversity of Capnocytophaga species in children and description of Capnocytophaga leadbetteri sp. nov. and Capnocytophaga genospecies AHN8471. Int J Syst Evol Microbiol. 2008;58(Pt 2):324–36. doi: 10.1099/ijs.0.65373-0. [DOI] [PubMed] [Google Scholar]
- 7.Valtonen M, Lauhio A, Carlson P, et al. Capnocytophaga canimorsus septicemia: Fifth report of a cat-associated infection and five other cases. Eur J Clin Microbiol Infect Dis. 1995;14:520–3. doi: 10.1007/BF02113430. [DOI] [PubMed] [Google Scholar]
- 8.Blanche P, Bloch E, Sicard D. Capnocytophaga canimorsus in the oral flora of dogs and cats. J Infect. 1998;36:134. doi: 10.1016/s0163-4453(98)93918-4. [DOI] [PubMed] [Google Scholar]
- 9.Suzuki M, Kimura M, Imaoka K, Yamada A. Prevalence of Capnocytophaga canimorsus and Capnocytophaga cynodegmi in dogs and cats determined by using a newly established species-specific PCR. Vet Microbiol. 2010;144:172–6. doi: 10.1016/j.vetmic.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 10.Lion C, Escande F, Burdin JC. Capnocytophaga canimorsus infections in human: Review of the literature and cases report. Eur J Epidemiol. 1996;12:521–33. doi: 10.1007/BF00144007. [DOI] [PubMed] [Google Scholar]
- 11.Pers C, Gahrn-Hansen B, Frederiksen W. Capnocytophaga canimorsus septicemia in Denmark, 1982–1995: Review of 39 cases. Clin Infect Dis. 1996;23:71–5. doi: 10.1093/clinids/23.1.71. [DOI] [PubMed] [Google Scholar]
- 12.van Dam AP, Jansz A. Capnocytophaga canimorsus infections in the Netherlands: A nationwide survey. Clin Microbiol Infect. 2011;17:312–5. doi: 10.1111/j.1469-0691.2010.03195.x. [DOI] [PubMed] [Google Scholar]
- 13.Le Moal G, Landron C, Grollier G, Robert R, Burucoa C. Meningitis due to Capnocytophaga canimorsus after receipt of a dog bite: Case report and review of the literature. Clin Infect Dis. 2003;36:e42–6. doi: 10.1086/345477. [DOI] [PubMed] [Google Scholar]
- 14.de Boer MG, Lambregts PC, van Dam AP, van’t Wout JW. Meningitis caused by Capnocytophaga canimorsus: When to expect the unexpected. Clin Neurol Neurosurg. 2007;109:393–8. doi: 10.1016/j.clineuro.2007.02.010. [DOI] [PubMed] [Google Scholar]
- 15.Hicklin H, Verghese A, Alvarez S. Dysgonic fermenter 2 septicemia. Rev Infect Dis. 1987;9:884–90. doi: 10.1093/clinids/9.5.884. [DOI] [PubMed] [Google Scholar]
- 16.Rubin SJ. DF-2: A fastidious fermentative Gram-negative rod. Eur J Clin Microbiol. 1984;3:253–7. doi: 10.1007/BF02014896. [DOI] [PubMed] [Google Scholar]
- 17.Jolivet-Gougeon A, Sixou JL, Tamanai-Shacoori Z, Bonnaure-Mallet M. Antimicrobial treatment of Capnocytophaga infections. Int J Antimicrob Agents. 2007;29:367–73. doi: 10.1016/j.ijantimicag.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 18.Engelkirk PG, Duben-Engelkirk J. Laboratory Diagnosis of Infectious Diseases: Essentials of Diagnostic Microbiology. Baltimore: Lippincott Williams & Wilkins; 2008. [Google Scholar]
- 19.Schoen RT, Wohlgelernter D, Barden GE, Swartz TJ. Infection with CDC group DF-2 gram-negative rod: Report of two cases. Arch Intern Med. 1980;140:657–8. [PubMed] [Google Scholar]
- 20.Chan PC, Fonseca K. Septicaemia and meningitis caused by dysgonic fermenter-2 (DF-2) J Clin Pathol. 1986;39:1021–4. doi: 10.1136/jcp.39.9.1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Woo PC, Ng KH, Lau SK, et al. Usefulness of the MicroSeq 500 16S ribosomal DNA-based bacterial identification system for identification of clinically significant bacterial isolates with ambiguous biochemical profiles. J Clin Microbiol. 2003;41:1996–2001. doi: 10.1128/JCM.41.5.1996-2001.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ciantar M, Newman HN, Wilson M, Spratt DA. Molecular identification of Capnocytophaga spp. via 16S rRNA PCR-restriction fragment length polymorphism analysis. J Clin Microbiol. 2005;43:1894–901. doi: 10.1128/JCM.43.4.1894-1901.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Le Meur A, Albert JD, Gautier P, et al. Acute tenosynovitis of the ankle due to Capnocytophaga cynodegmi/canimorsus as identified by 16S rRNA gene sequencing. Joint Bone Spine. 2008;75:749–51. doi: 10.1016/j.jbspin.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 24.Meybeck A, Aoun N, Granados D, Pease S, Yeni P. Meningitis due to Capnocytophaga canimorsus: Contribution of 16S RNA ribosomal sequencing for species identification. Scand J Infect Dis. 2006;38:375–7. doi: 10.1080/00365540500488873. [DOI] [PubMed] [Google Scholar]
- 25.Risum M, Ellekvist P. [Capnocytophaga canimorsus meningitis diagnosed by means of a 16S rRNA analysis] Ugeskrift for laeger. 2012;174:280–1. [PubMed] [Google Scholar]
- 26.Gaastra W, Lipman LJ. Capnocytophaga canimorsus. Vet Microbiol. 2010;140:339–46. doi: 10.1016/j.vetmic.2009.01.040. [DOI] [PubMed] [Google Scholar]
- 27.Biedermann P, Deligne D. [Meningitis due to Capnocytophaga canimorsus with misleading initial digestive symptom] Ann Biol Clin (Paris) 2004;62:110–4. [PubMed] [Google Scholar]
- 28.Kullberg BJ, Westendorp RG, van ‘t Wout JW, Meinders AE. Purpura fulminans and symmetrical peripheral gangrene caused by Capnocytophaga canimorsus (formerly DF-2) septicemia – a complication of dog bite. Medicine (Baltimore) 1991;70:287–92. doi: 10.1097/00005792-199109000-00001. [DOI] [PubMed] [Google Scholar]
- 29.Deshmukh PM, Camp CJ, Rose FB, Narayanan S. Capnocytophaga canimorsus sepsis with purpura fulminans and symmetrical gangrene following a dog bite in a shelter employee. Am J Med Sci. 2004;327:369–72. doi: 10.1097/00000441-200406000-00015. [DOI] [PubMed] [Google Scholar]
- 30.Linton DM, Potgieter PD, Roditi D, et al. Fatal Capnocytophaga canimorsus (DF-2) septicaemia. A case report. S Afr Med J. 1994;84:857–60. [PubMed] [Google Scholar]
- 31.Gasch O, Fernandez N, Armisen A, Verdaguer R, Fernandez P. Community-acquired Capnocytophaga canimorsus meningitis in adults: Report of one case with a subacute course and deafness, and literature review. Enferm Infecc Microbiol Clin. 2009;27:33–6. doi: 10.1016/j.eimc.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 32.Widlund M, Duberg AS. [Permanent hearing loss following dog bite. Capnocytophaga canimorsus caused severe infection with sepsis] Lakartidningen. 2010;107:1771–3. [PubMed] [Google Scholar]
- 33.Monrad RN, Hansen DS. Three cases of Capnocytophaga canimorsus meningitis seen at a regional hospital in one year. Scand J Infect Dis. 2012;44:320–4. doi: 10.3109/00365548.2011.635314. [DOI] [PubMed] [Google Scholar]
- 34.Hayani O, Higginson LA, Toye B, Burwash IG. Man’s best friend? Infective endocarditis due to Capnocytophaga canimorsus. Can J Cardiol. 2009;25:e130–2. doi: 10.1016/s0828-282x(09)70076-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sandoe JA. Capnocytophaga canimorsus endocarditis. J Med Microbiol. 2004;53(Pt 3):245–8. doi: 10.1099/jmm.0.05274-0. [DOI] [PubMed] [Google Scholar]
- 36.Ngaage DL, Kotidis KN, Sandoe JA, Unnikrishnan Nair R. Do not snog the dog: Infective endocarditis due to Capnocytophaga canimorsus. Eur J Cardiothorac Surg. 1999;16:362–3. doi: 10.1016/s1010-7940(99)00131-1. [DOI] [PubMed] [Google Scholar]
- 37.Ehrbar HU, Gubler J, Harbarth S, Hirschel B. Capnocytophaga canimorsus sepsis complicated by myocardial infarction in two patients with normal coronary arteries. Clin Infect Dis. 1996;23:335–6. doi: 10.1093/clinids/23.2.335. [DOI] [PubMed] [Google Scholar]
- 38.Mitchell I, McNeillis N, Bowden FJ, Nikolic G. Electrocardiographic myocardial infarction pattern in overwhelming post-splenectomy sepsis due to Capnocytophaga canimorsus. Intern Med J. 2002;32:415–8. doi: 10.1046/j.1445-5994.2002.00236.x. [DOI] [PubMed] [Google Scholar]
- 39.Bilgrami S, Bergstrom SK, Peterson DE, et al. Capnocytophaga bacteremia in a patient with Hodgkin’s disease following bone marrow transplantation: Case report and review. Clin Infect Dis. 1992;14:1045–9. doi: 10.1093/clinids/14.5.1045. [DOI] [PubMed] [Google Scholar]
- 40.Maury S, Leblanc T, Rousselot P, Legrand P, Arlet G, Cordonnier C. Bacteremia due to Capnocytophaga species in patients with neutropenia: High frequency of beta-lactamase-producing strains. Clin Infect Dis. 1999;28:1172–4. doi: 10.1086/517772. [DOI] [PubMed] [Google Scholar]
- 41.Bonatti H, Rossboth DW, Nachbaur D, et al. A series of infections due to Capnocytophaga spp in immunosuppressed and immunocompetent patients. Clin Microbiol Infect. 2003;9:380–7. doi: 10.1046/j.1469-0691.2003.00538.x. [DOI] [PubMed] [Google Scholar]
- 42.Duong M, Besancenot JF, Neuwirth C, Buisson M, Chavanet P, Portier H. Vertebral osteomyelitis due to Capnocytophaga species in immunocompetent patients: Report of two cases and review. Clin Infect Dis. 1996;22:1099–101. doi: 10.1093/clinids/22.6.1099. [DOI] [PubMed] [Google Scholar]
- 43.Iralu JV, Roberts D, Kazanjian PH. Chorioamnionitis caused by Capnocytophaga: Case report and review. Clin Infect Dis. 1993;17:457–61. doi: 10.1093/clinids/17.3.457. [DOI] [PubMed] [Google Scholar]
- 44.Mekouar H, Voortman G, Bernard P, Hutchings G, Boeras A, Rodriguez-Villalobos H. Capnocytophaga species and perinatal infections: Case report and review of the literature. Acta Clin Belg. 2012;67:42–5. doi: 10.2143/ACB.67.1.2062626. [DOI] [PubMed] [Google Scholar]
- 45.Meyer S, Shin H, Cornelis GR. Capnocytophaga canimorsus resists phagocytosis by macrophages and blocks the ability of macrophages to kill other bacteria. Immunobiology. 2008;213:805–14. doi: 10.1016/j.imbio.2008.07.019. [DOI] [PubMed] [Google Scholar]
- 46.Renzi F, Manfredi P, Mally M, Moes S, Jeno P, Cornelis GR. The N-glycan glycoprotein deglycosylation complex (Gpd) from Capnocytophaga canimorsus deglycosylates human IgG. PLoS Pathog. 2011;7:e1002118. doi: 10.1371/journal.ppat.1002118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Morelle W, Flahaut C, Michalski JC, Louvet A, Mathurin P, Klein A. Mass spectrometric approach for screening modifications of total serum N-glycome in human diseases: Application to cirrhosis. Glycobiology. 2006;16:281–93. doi: 10.1093/glycob/cwj067. [DOI] [PubMed] [Google Scholar]
- 48.Arlet G, Sanson-Le Pors MJ, Castaigne S, Perol Y. Isolation of a strain of beta-lactamase-producing Capnocytophaga ochracea. J Infect Dis. 1987;155:1346. doi: 10.1093/infdis/155.6.1346. [DOI] [PubMed] [Google Scholar]
- 49.Roscoe DL, Zemcov SJ, Thornber D, Wise R, Clarke AM. Antimicrobial susceptibilities and beta-lactamase characterization of Capnocytophaga species. Antimicrob Agents Chemother. 1992;36:2197–200. doi: 10.1128/aac.36.10.2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jolivet-Gougeon A, Buffet A, Dupuy C, et al. In vitro susceptibilities of Capnocytophaga isolates to beta-lactam antibiotics and beta-lactamase inhibitors. Antimicrob Agents Chemother. 2000;44:3186–8. doi: 10.1128/aac.44.11.3186-3188.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jolivet-Gougeon A, Guerin J, Tamanai-Shacoori Z, Gandemer V, Sixou JL, Bonnaure-Mallet M. Influence of previous antimicrobial therapy on oral carriage of beta-lactamase producing Capnocytophaga isolates. Acta Paediatr. 2008;97:964–7. doi: 10.1111/j.1651-2227.2008.00824.x. [DOI] [PubMed] [Google Scholar]
- 52.Roscoe D, Clarke A. Resistance of Capnocytophaga species to beta-lactam antibiotics. Clin Infect Dis. 1993;17:284–5. doi: 10.1093/clinids/17.2.284. [DOI] [PubMed] [Google Scholar]
- 53.Cummings P. Antibiotics to prevent infection in patients with dog bite wounds: A meta-analysis of randomized trials. Ann Emerg Med. 1994;23:535–40. doi: 10.1016/s0196-0644(94)70073-7. [DOI] [PubMed] [Google Scholar]


