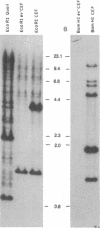
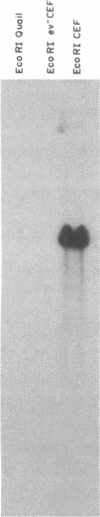
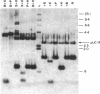
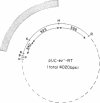
Abstract
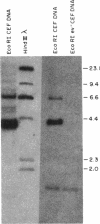
Using a molecularly cloned viral DNA probe representing the entire avian sarcoma virus (ASV) reverse transcriptase (pol) gene, we have detected related sequences in DNA preparations from two avain species, ev- chickens and Japanese quail, previously demonstrated to lack all endogenous avain leukosis viruses. Nucleotide sequence homology was detected only when hybridization conditions, which allowed the formation of stable duplexes with as much as 30% base mismatch, were used. No sequence homology could be detected when stringent hybridization conditions were used. Nucleotide sequence analysis of a clone representing the major pol-specific EcoRI restriction fragment from ev- chicken embryo fibroblasts revealed DNA homology as high as 72% and implied amino acid homology as high as 82% when compared to the sequence of the ASV strain Prague C pol gene. These data reveal the presence of retroviral pol gene sequences in avian cell lines that lack endogenous retrovirus sequences, suggesting that a reverse transcriptase-related gene exists in these cells as either part of a more distantly evolved retrovirus or a cellular gene.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Astrin S. M., Buss E. G., Haywards W. S. Endogenous viral genes are non-essential in the chicken. Nature. 1979 Nov 15;282(5736):339–341. doi: 10.1038/282339a0. [DOI] [PubMed] [Google Scholar]
- Bauer G., Hofschneider P. H. An RNA-dependent DNA polymerase, different from the known viral reverse transcriptases, in the chicken system. Proc Natl Acad Sci U S A. 1976 Sep;73(9):3025–3029. doi: 10.1073/pnas.73.9.3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein L. B., Mount S. M., Weiner A. M. Pseudogenes for human small nuclear RNA U3 appear to arise by integration of self-primed reverse transcripts of the RNA into new chromosomal sites. Cell. 1983 Feb;32(2):461–472. doi: 10.1016/0092-8674(83)90466-x. [DOI] [PubMed] [Google Scholar]
- Breathnach R., Chambon P. Organization and expression of eucaryotic split genes coding for proteins. Annu Rev Biochem. 1981;50:349–383. doi: 10.1146/annurev.bi.50.070181.002025. [DOI] [PubMed] [Google Scholar]
- Chen E. Y., Howley P. M., Levinson A. D., Seeburg P. H. The primary structure and genetic organization of the bovine papillomavirus type 1 genome. Nature. 1982 Oct 7;299(5883):529–534. doi: 10.1038/299529a0. [DOI] [PubMed] [Google Scholar]
- Danos O., Katinka M., Yaniv M. Human papillomavirus 1a complete DNA sequence: a novel type of genome organization among papovaviridae. EMBO J. 1982;1(2):231–236. doi: 10.1002/j.1460-2075.1982.tb01152.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denison R. A., Weiner A. M. Human U1 RNA pseudogenes may be generated by both DNA- and RNA-mediated mechanisms. Mol Cell Biol. 1982 Jul;2(7):815–828. doi: 10.1128/mcb.2.7.815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollis G. F., Hieter P. A., McBride O. W., Swan D., Leder P. Processed genes: a dispersed human immunoglobulin gene bearing evidence of RNA-type processing. Nature. 1982 Mar 25;296(5855):321–325. doi: 10.1038/296321a0. [DOI] [PubMed] [Google Scholar]
- Howley P. M., Israel M. A., Law M. F., Martin M. A. A rapid method for detecting and mapping homology between heterologous DNAs. Evaluation of polyomavirus genomes. J Biol Chem. 1979 Jun 10;254(11):4876–4883. [PubMed] [Google Scholar]
- Jaenisch R. Endogenous retroviruses. Cell. 1983 Jan;32(1):5–6. doi: 10.1016/0092-8674(83)90491-9. [DOI] [PubMed] [Google Scholar]
- Kang C. Y., Temin H. M. Endogenous RNA-directed DNA polymerase activity in uninfected chicken embryos. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1550–1554. doi: 10.1073/pnas.69.6.1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karin M., Richards R. I. Human metallothionein genes--primary structure of the metallothionein-II gene and a related processed gene. Nature. 1982 Oct 28;299(5886):797–802. doi: 10.1038/299797a0. [DOI] [PubMed] [Google Scholar]
- Krzyzek R. A., Watts S. L., Anderson D. L., Faras A. J., Pass F. Anogenital warts contain several distinct species of human papillomavirus. J Virol. 1980 Oct;36(1):236–244. doi: 10.1128/jvi.36.1.236-244.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law M. F., Lancaster W. D., Howley P. M. Conserved polynucleotide sequences among the genomes of papillomaviruses. J Virol. 1979 Oct;32(1):199–207. doi: 10.1128/jvi.32.1.199-207.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemischka I., Sharp P. A. The sequences of an expressed rat alpha-tubulin gene and a pseudogene with an inserted repetitive element. Nature. 1982 Nov 25;300(5890):330–335. doi: 10.1038/300330a0. [DOI] [PubMed] [Google Scholar]
- Livingston D. M., Serxner L. E., Howk D. J., Hudson J., Todaro G. J. Characterization of a new murine cellular DNA polymerase. Proc Natl Acad Sci U S A. 1974 Jan;71(1):57–62. doi: 10.1073/pnas.71.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Mondal H., Gallagher R. E., Gallo R. C. RNA-directed DNA polymerase from human leukemic blood cells and from primate type-C virus-producing cells: high- and low-molecular-weight forms with variant biochemical and immunological properties. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1194–1198. doi: 10.1073/pnas.72.3.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mondal H. Occurrence of particle-bound reverse transcriptase in human amniotic fluid. Biochem Biophys Res Commun. 1977 Nov 7;79(1):67–75. doi: 10.1016/0006-291x(77)90061-4. [DOI] [PubMed] [Google Scholar]
- Nelson J., Leong J. A., Levy J. A. Normal human placentas contain RNA-directed DNA polymerase activity like that in viruses. Proc Natl Acad Sci U S A. 1978 Dec;75(12):6263–6267. doi: 10.1073/pnas.75.12.6263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Scarpulla R. C., Wu R. Nonallelic members of the cytochrome c multigene family of the rat may arise through different messenger RNAs. Cell. 1983 Feb;32(2):473–482. doi: 10.1016/0092-8674(83)90467-1. [DOI] [PubMed] [Google Scholar]
- Scolnick E. M., Aaronson S. A., Todaro G. J., Parks W. P. RNA dependent DNA polymerase activity in mammalian cells. Nature. 1971 Jan 29;229(5283):318–321. doi: 10.1038/229318a0. [DOI] [PubMed] [Google Scholar]
- Shalloway D., Zelenetz A. D., Cooper G. M. Molecular cloning and characterization of the chicken gene homologous to the transforming gene of Rous sarcoma virus. Cell. 1981 May;24(2):531–541. doi: 10.1016/0092-8674(81)90344-5. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Temin H. M. On the origin of RNA tumor viruses. Annu Rev Genet. 1974;8:155–177. doi: 10.1146/annurev.ge.08.120174.001103. [DOI] [PubMed] [Google Scholar]
- Van Arsdell S. W., Denison R. A., Bernstein L. B., Weiner A. M., Manser T., Gesteland R. F. Direct repeats flank three small nuclear RNA pseudogenes in the human genome. Cell. 1981 Oct;26(1 Pt 1):11–17. doi: 10.1016/0092-8674(81)90028-3. [DOI] [PubMed] [Google Scholar]
- Wilde C. D., Crowther C. E., Cowan N. J. Diverse mechanisms in the generation of human beta-tubulin pseudogenes. Science. 1982 Aug 6;217(4559):549–549. doi: 10.1126/science.6178164. [DOI] [PubMed] [Google Scholar]
- Wilde C. D., Crowther C. E., Cripe T. P., Gwo-Shu Lee M., Cowan N. J. Evidence that a human beta-tubulin pseudogene is derived from its corresponding mRNA. Nature. 1982 May 6;297(5861):83–84. doi: 10.1038/297083a0. [DOI] [PubMed] [Google Scholar]