Abstract
The current standards in radiotherapy of high-grade gliomas (HGG) are based on anatomic imaging techniques, usually computed tomography (CT) scanning and magnetic resonance imaging (MRI). The guidelines vary depending on whether the HGG is a histological grade 3 anaplastic glioma (AG) or a grade 4 glioblastoma multiforme (GBM). For AG, T2-weighted MRI sequences plus the region of contrast enhancement in T1 are considered for the delineation of the gross tumor volume (GTV), and an isotropic expansion of 15 to 20 mm is recommended for the clinical target volume (CTV). For GBM, the Radiation Therapy Oncology Group favors a two-step technique, with an initial phase (CTV1) including any T2 hyperintensity area (edema) plus a 20 mm margin treated with up to 46 Gy in 23 fractions, followed by a reduction in CTV2 to the contrast enhancement region in T1 with an additional 25 mm margin. The European Organisation of Research and Treatment of Cancer recommends a single-phase technique with a unique GTV, which comprises the T1 contrast enhancement region plus a margin of 20 to 30 mm. A total dose of 60 Gy in 30 fractions is usually delivered for GBM, and a dose of 59.4 Gy in 33 fractions is typically given for AG. As more than 85% of HGGs recur in field, dose-escalation studies have shown that 70 to 75 Gy can be delivered in 6 weeks with relevant toxicities developing in < 10% of the patients. However, the only randomized dose-escalation trial, in which the boost dose was guided by conventional MRI, did not show any survival advantage of this treatment over the reference arm. HGGs are amongst the most infiltrative and heterogeneous tumors, and it was hypothesized that the most highly aggressive areas were missed; thus, better visualization of these high-risk regions for radiation boost could decrease the recurrence rate. Innovations in imaging and linear accelerators (LINAC) could help deliver the right doses of radiation to the right subvolumes according to the dose-painting concept. Advanced imaging techniques provide functional information on cellular density (diffusion MRI), angiogenesis (perfusion MRI), metabolic activity and cellular proliferation [positron emission tomography (PET) and magnetic resonance spectroscopy (MRS)]. All of these non-invasive techniques demonstrated good association between the images and histology, with up to 40% of HGGs functionally presenting a high activity within the non-contrast-enhanced areas in T1. New LINAC technologies, such as intensity-modulated and stereotactic radiotherapy, help to deliver a simultaneous integrated boost (SIB) > 60 Gy. Trials delivering a SIB into a biological GTV showed the feasibility of this treatment, but the final results, in terms of clinical benefits for HGG patients, are still pending. Many issues have been identified: the variety of MRI and PET machines (and amino-acid tracers), the heterogeneity of the protocols used for image acquisition and post-treatment, the geometric distortion and the unreliable algorithms for co-registration of brain anatomy with functional maps, and the semi-quiescent but highly invasive HGG cells. These issues could be solved by the homogenization of the protocols and software applications, the simultaneous acquisition of anatomic and functional images (PET-MRI machines), the combination of complementary imaging tools (perfusion and diffusion MRI), and the concomitant addition of some ad hoc targeted drugs against angiogenesis and invasiveness to chemoradiotherapy. The integration of these hybrid data will construct new synthetic metrics for fully individualized treatments.
Keywords: Radiotherapy, advanced imaging, MRI, PET, high-grade gliomas
For radiation oncologists, high-grade gliomas (HGGs) are a very exciting topic, but also a frustrating one. In terms of evidence-based medicine, no significant progress has been made since the concomitant and adjuvant addition of temozolomide (TMZ) to radiotherapy. The European Organisation for Research and Treatment of Cancer-National Cancer Institute of Canada (EORTC-NCIC) randomized phase III trial[1] was published in 2005 and dealt only with glioblastoma multiforme (GBM, WHO grade 4), and not with anaplastic gliomas (AG, WHO grade 3)[2]. Since then, despite new concepts in HGG knowledge and numerous innovations in imaging and linear accelerator (LINAC) technologies, no clinically relevant progress has been made for these patients. Why does this discrepancy exist, and what are the “next steps”?
We first review the historical background that defines the current standards for radiotherapy of HGG, then present new concepts and the most promising innovations in imaging and LINAC technologies. We then describe the most relevant image-guided radiotherapy trials, identify the main bottlenecks and their potential solutions, and end with short-term perspectives.
Rationale for Two Distinct Standards in Radiotherapy of Grade 4 vs. Grade 3 HGG
The current situation: an unsatisfactory compromise between efficacy and toxicity
Within the group of HGGs, the last 2007 WHO classification distinguishes grade 4 GBMs (astrocytomas) from grade 3 AG using their highly infiltrative characteristics. In GBMs, lesions are more systematically and strongly enhanced after gadolinium (Gd) injection and are associated with central necrosis. In contrast, AG often shows non-enhanced areas, which are nevertheless clearly demonstrated in pathologic examination by multiple stereotactic biopsies, with a strong hypersignal in T2/fluid-attenuated inverse recovery (FLAIR)-weighted sequences[3].
Because of its superior spatial resolution, magnetic resonance imaging (MRI) is favored in the management of HGG, but its superiority over computed tomography (CT) scan has not been clinically demonstrated. The peritumoral environment of HGG is known to include highly variable proportions of both vasogenic edema and important populations of glioma cells. Consequently, the Radiation Therapy Oncology Group (RTOG) and the EORTC recommend considering these T2 hyperintensity regions as either a macroscopically pathologic volume [defined as the gross tumor volume (GTV)] or as a microscopically pathologic volume [defined as the clinical target volume (CTV)], depending on the glioma grade and the research groups or organizations (RTOG or EORTC).
For patients with GBM, two techniques are available, during which the RTOG defines an overall larger target volume, possibly increasing acute and late toxicities[4]. The EORTC favors a single-phase technique, consisting of 30 fractions of 2 Gy. The GTV is defined as the region of enhancement (without edema) on preoperative CT/MRI for patients who underwent biopsy or the surgical tumor bed plus any residual enhancing tumor that is seen on the planning scan in patients who underwent resection. Co-registration of pre- and postoperative MRI/CT is strongly encouraged. The CTV is defined as the GTV plus an isotropic margin of 2 cm (3 cm max), but this margin can be reduced in anatomic regions where spread is unlikely, such as bony structures and adjacent normal meninges. The planning target volume (PTV) adds 0.5 to 0.7 cm, depending on the centers and LINACs. In the case of complete or large surgical removal, the position of the tumor bed may have shifted, and the CTV should take into account the new position of the abnormalities on the planning scan.
The RTOG favors a cone-down technique, which uses two different volumes. The GTV is defined in a manner identical to that of the EORTC recommendation and in the RTOG trials 0825 and 0913[5]. The CTV should include any edema shown on the CT/MRI scan (T2/FLAIR hyperintensity), and the PTV1 should include the CTV with a margin of 2.0 cm, as well as a margin to account for set up accuracy. If no edema is present, then a margin of 2.5 cm should be added. Clinical judgment may be used to adapt the PTV1 by excluding sensitive structures, such as the optic chiasm. The PTV1 is treated with a dose of 46 Gy in 23 fractions. The PTV2 should include the GTV with a margin of 2.5 cm plus set up error, and the PTV2 should be treated with 14 Gy in 7 fractions, for a total cumulative dose of 60 Gy.
For grade 3 patients, as described in the ongoing EORTC and RTOG trial 26053[6], the GTV is defined as the entire region of high signal intensity on the T2-weighted MRI images or FLAIR sequences (corresponding to the hypodense area on CT images), plus the region of enhancement on postoperative CT/MRI if available, or as the region of enhancement on preoperative CT/MRI if postoperative imaging is not available, plus the tumor resection margin. In some cases, no enhancement can be seen, and GTV is defined according to the T2 abnormality. The CTV is defined as a 1.5 to 2.0 cm volumetric expansion of the GTV, and the PTV will add 0.5 to 0.7 cm, depending on the centers and LINACs. A total dose of 59.4 Gy in 33 fractions of 1.8 Gy (single phase) is recommended.
Importantly, the EORTC guidelines strongly recommend keeping the dose to the normal brain without the PTV ideally below 60% of the prescribed dose (36 Gy). Recent studies suggest that larger irradiated volumes could be directly responsible for clinically relevant toxicities[7]. An isotropic expansion of 15 mm from the GTV to the CTV is now generally accepted, as it was in the EORTC 26062 trial for GBM patients over 65 years old. Particularly for grade 3 glioma patients, but also for grade 4 long-term survivors, the fear of excessive neurologic toxicity still justifies the recommended total dose of 59.4 to 60 Gy, with a fractionation of 1.8 to 2 Gy per day and 5 fractions per week.
The classical dose escalation concept: still a non-evidence-based challenge
Even if an improvement in local control and survival was shown in HGG when the radiation dose was escalated from 40 to 60 Gy[8], more than 85% of HGGs still recur within the field. Delivering more than 60 Gy seems logical, but the only prospective randomized trial (RTOG 93-05) failed to demonstrate any advantage in favor of an anatomic MRI–guided stereotactic dose escalation concept[9]. However, it is clear that the rationale still exists[10],[11], and a total dose of 70 to 72 Gy is clinically tolerable for selected patients, providing that advanced imaging techniques are used, with less than 10% radiologic necrosis and with less than half of these patients having clinically relevant lesions, such as sensorimotor deficit or surgery needed for necrosis[12],[13].
The real question is the efficacy endpoint. Could higher doses be beneficial for HGG patients, in terms of better local control and survival? A better knowledge of HGG natural history and advances in imaging and technology could help answer this question.
New Concepts and New Technologies Are Promising
New concepts
HGG has to be considered a continuum of heterogeneous tumors with intricate and mixed biological profiles that exhibit strong capacities to develop early resistance to treatments. The mean age for patients with grade 2 gliomas is 40 to 45 years, whereas it is 50 years for patients with grade 3 gliomas and 60 to 65 years for patients with glioblastomas. This probably depicts the natural history of these tumors, which develop an increasingly heterogeneous profile with time, as a real continuum.
HGGs are among the most heterogeneous solid tumors in oncology, and glioma cell invasion is a multistep process[4]. Within the same HGG, populations of grade 2, grade 3, and/or grade 4 glioma cells probably coexist, some with high mitotic activity and a proangiogenic profile, whereas others are mostly necrotic or quiescent; these latter cells exhibit the predominantly infiltrative characteristics with a rather low metabolic activity[14]. Consequently, the academic concept of homogenously delivering “the right dose to the right volume” seems clearly inadequate. Furthermore, the capacity of these HGGs to increase their aggressiveness, even during the very early chemo-radiation period, has been demonstrated. The invasiveness of irradiated glial cells is increased, and an angiogenic HGG with massive edema can evolve with time into a mostly infiltrating tumor after a long exposure to anti-angiogenic drugs, such as bevacizumab. Adaptive radiotherapy is one treatment strategy that should be considered for these very “clever” tumors.
The new paradigm of unhomogenously delivering and adapting the right dose to the right volume seems clearly more adequate than the “homogeneity” principle, but more sophisticated tools are needed, namely advanced imaging with new algorithms, ad hoc softwares for reliable co-registrations[15],[16], high-tech LINACs, and new mathematic modeling of tumor progression for dynamic prediction of tumor growth[17],[18].
As an early step in radiotherapy is tumor delineation, there is an urgent need for better dynamic visualization of these new “biological targets,” beyond the conventional CT scanner and the anatomic MRI, which disclose only static captures of a HGG at a single moment.
New technologies: advanced imaging techniques and high-tech LINACs are available
Advanced imaging: magnetic resonance spectroscopy (MRS), perfusion and diffusion MRI, and positron emission tomography (PET)
The potential imaging of biomarkers of tumor invasion is highly interesting[4]. Even the high soft tissue resolution of MRI has failed to improve the direct visualization of the real tumor margins, and HGG cells extend widely and in a non-isotropic way beyond the limits of the T2 hypersignal, up to 25 mm in most cases[3]. The need for more biological imaging is evident, as this will simultaneously spare more normal brain volume and will better target the HGG's minimal extension into the brain parenchyma.
These advanced imaging techniques provide so-called functional information on cellularity (diffusion MRI), angiogenesis (perfusion MRI), metabolic activity (PET and MRS), and cellular proliferation (PET and MRS). For each of these techniques, direct association between the images and the histological reality has been demonstrated by image-guided stereotactic biopsies[19]–[21].
MRS can yield proton (1H) spectra from selected areas within the brain, from which levels of cellular metabolites can be derived. Among these metabolites, choline-containing compounds (Cho) and N-acetylaspartate (NAA) could act as potential biomarkers for tumor activity. Cho is a membrane component that is increased in viable tumors, whereas NAA is a neuronal marker that is decreased in tumors due to neuronal loss. Gliomas show a marked high resonance in the spectral region of Cho and/or a low NAA resonance, implying increases in the Cho/NAA ratio[4],[16]. Pirzkall et al.[22] developed an abnormality Cho/NAA index (CNI) and showed that a metabolically active tumor (defined as CNI > 2) was observed outside of the MRI-defined volumes in a non-isotropic manner.
Overall, 30% to 50% of the T2 hyperintense lesions outside of the contrast-enhanced area had a CNI > 2.5. In another study, using the Cho/creatinine (Cr) ratio as an alternative marker of metabolic activity[23], MR anatomic post-contrast T1 sequences overestimated the GTV (defined by Cho/Cr ratio of > 3) by 40%, and, at the same time, T2-based imaging overestimated the CTV (defined by the Cho/Cr ratio of > 1) by 30% in half of the patients, suggesting the overtreatment of normal brain tissue.
However, many limitations for the routine use of MRS persist, as the presence of clips, clotted blood, and skull bone highly degrade its quality and reliability. Moreover, the smallest voxel size is 10 mm × 10 mm × 10 mm, which is much larger than the voxel size of the CT and MRI used for radiotherapy planning (1 mm in-plane resolution). Decreasing the size of each voxel to improve the spatial resolution can notably increase the scan time and decrease the signal-to-noise ratio; consequently, the use of 3T MRI could be a partial answer.
Perfusion-weighted (PWI) MRI measures the temporal changes of T1 or T2* signal intensity caused by the passage of intravascular contrast agent (usually gadolinium) delivered to the capillary bed of the tissues. Using pharmacokinetic models with an artery input function allows for the evaluation of the association between vascular perfusion and permeability, which is increased in the region of interest in gliomas of higher histological grades. Dynamic susceptibility contrast-enhanced MRI uses T2*-weighted gradient echo-planar imaging (EPI) sequences, which are acquired during the first pass of the Gd injection, to characterize the cerebral blood volume (CBV) at the tissue level. On the other hand, dynamic contrast-enhanced MRI exploits the T1 contrast phase to monitor tracer dynamics and provides a reliable semi-quantitative measurement of microvascular permeability. It was shown that relative CBV and permeability could have independent prognostic values in unfavorable grade 2 gliomas and in HGG[24],[25]. A threshold of 1.75 was used as a cut-off value for grading, and perfusion/permeability maps were also shown to be useful in differentiating active glioma from post-radiation necrosis[26],[27]. As HGG usually presents with anarchic angiogenesis, an abnormal permeability could be a surrogate marker for tumor aggressiveness, whereas perfusion could be an indicator of HGG volume. Therefore, it could be of interest to exploit these rapidly acquired T2* sequences, which only add a few minutes of treatment time, and this non-invasive tool for better radiation delineation of highly active areas of the HGG (Figures 1 and 2).
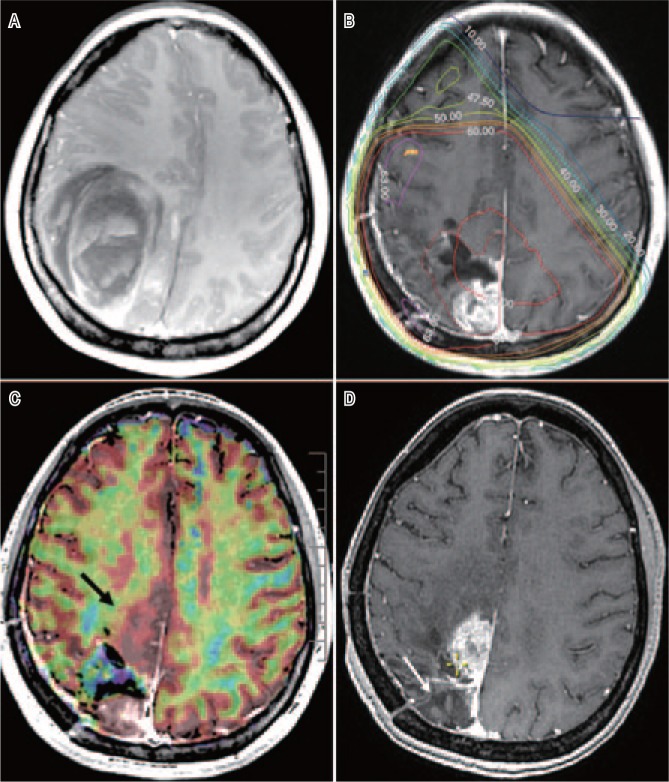
Figure 1. Magnetic resonance imaging (MRI) of a right-handed woman with glioblastoma multiforme (GBM).
A, preo-perative MRI, T1-weighted after gadolinium (Gd) injection, of a right-handed woman shows a right-sided parieto-occipital grade 4 GBM. B, at 4 weeks after resection, before 3D conformal radiotherapy (60 Gy) and temozolomide treatment, T1-Gd MRI shows the operative bed and an early posterior reevolution. C, T1-Gd MRI co-registered with the perfusion map (in red) acquired at the same time. The posterior part of the macroscopically active GBM is clearly hyper-perfused and enhanced, unlike the anterior non-enhanced area (black arrow), which is also highly hyper-perfused and included in the 60 Gy isodose. D, at 14 months after treatment, the posterior part is controlled (white arrow), and the area of recurrence (yellow cross) is clearly shown in the non-enhanced but hyper-perfused zone.
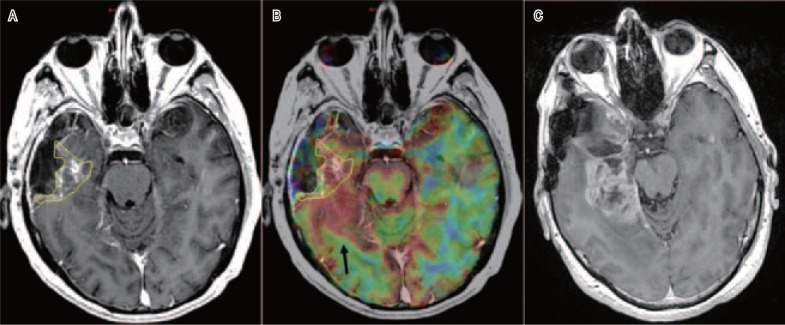
Figure 2. A “radically resected” anaplastic glioma with a highly hyper-perfused but non-enhanced active tumor.
A, postoperative T1-Gd MRI in 4 weeks after a radical resection of the anaplastic glioma shows a posterior residual enhancement (yellow line). B, T1-Gd MRI co-registered with the perfusion map (in red) acquired at the same time. The posterior part is non-enhanced but highly hyper-perfused (black arrow). C, posterior recurrence in 8 months at radiotherapy (59.4 Gy).
Diffusion-weighted imaging (DWI) and diffusion tensor imaging (DTI) are MRI methods that produce images to help identify tumor cellularity and boundaries. In DWI, a loss of image intensity of each voxel reflects the rate of microscopic water diffusion (apparent diffusion coefficient). DTI evaluates the preferred direction of HGG infiltration by different tensor metrics, some of which are robust, such as fractional anisotropy or the mean diffusivity index, and some use new mathematic algorithms for better sensitivity and specificity[28]. As HGG preferentially spreads along the white matter tracks, this ability to “image” tissue infiltration could be complementary to the perfusion/permeability metrics, which visualize only angiogenesis.
In PET, measuring the uptake of amino acids by using either 11C-methionine (MET) or 18F-fluoroethyl-L-tyrosine (FET) has been shown to be a more reliable method of visualizing brain tumor metabolism than using 18F-fluorodeoxyglucose (FDG)[21],[29]. Studies that fuse MRI and MET-PET images have shown that the volume of increased MET uptake is greater than the volume of Gd enhancement on T1-weighted MRI, and, although it is smaller than the volume of T2 hypersignal, it extends beyond T2 in most cases. Authors have shown that high MET uptake was visible 0.8 to 3 cm beyond the T1 contrast enhancement in 69% of cases[12]. However, a main issue concerning MET-PET is that the tracer exhibits a very short half-life and is hardly available in routine clinics; however, alternative tracers have been proposed.
18F-fluorothymidine (FLT), which is known to be actively taken up into dividing cells, has an excellent contrast-to-background ratio[30], and studies measuring FLT uptake have shown that it associates well with tissue markers of proliferation. However, even if areas of abnormality are larger than those visualized on MRI when using image-guided biopsies, it has also been shown that FLT could underestimate the extent of the tumors in half of the cases[31].
FET-PET and MRI data of GTV were compared. It was found that the size and geometric location of the GTVs, as defined by the biological tumor volumes, differed in a majority of the 17 evaluated patients[32]. Recently, other authors have proposed to integrate FET-PET for target volume definition in the contouring process to avoid larger incongruence between anatomic and biological imaging techniques[33].
Biopsy validation of PET with another amino acid tracer, 18F-dopamine (DOPA), and its biodistribution in gliomas for radiation target delineation were recently published in a series of 10 patients and 23 biopsy specimens. A maximum standardized uptake value (SUVmax)-based threshold was proposed to define the high-grade portions of the gliomas when delineating the radiation boost volumes[21].
Notably for each technique, the authors claim that their imaging biomarker has an independent prognostic value, such as the ratio of cerebral blood volume (rCBV), the Cho/NAA ratio in MRS, and the ratio of SUVmax in PET. Consequently, these markers could be useful in three situations: 1) to help radiotherapy in target delineation; 2) to better predict outcome; and 3) to monitor therapeutic response[34]–[38].
Finally, all of these functional imaging techniques probably identify similar, more active target areas[39]. However, it is also possible that dividing and infiltrating cells appear to represent clusters of distinct tumor phenotypes and that most invasive cells will not be highly dividing[14],[40].
Once the individual biological GTVs and organs at risk (OaR) are delineated, these volumes need to be transferred through the ad hoc treatment planning system (TPS) into the LINACs, using the most robust and reliable mathematic models, algorithms, and techniques.
High-tech LINACs: stereotactic and/or intensity-modulated radiotherapy (IMRT)
Progress has been made in LINACS technologies to better shape the final PTV. Micromultileaf collimators of a few mm thickness, which are highly reliable and non-invasive immobilization devices, enable sophisticated stereotactic radiation delivery and are usually associated with IMRT, making simultaneous integrated boost (SIB) feasible, even for very small or large complex-shaped targets. Sparing OaR, such as the cochleas, brainstem, and hippocampus, together with lowering the integral dose to the normal brain is possible, even when delivering high doses to large target volumes[41]–[43].
However, if integration of MRI/CT images into the TPS is routine in the clinics, combining advanced imaging techniques with these high-tech LINACs remains a challenge. It requires adapting or creating new algorithms and software applications to reliably co-register these functional or metabolic maps with brain anatomy. These essential tools are not yet largely or commercially available, and only a few image-guided dose-escalating radiation protocols have been reported, mainly in monocenter phase II series.
Positive Results from the Dose Escala-tion Image-guided Radiotherapy Trials Are Still Expected
Tsien et al.[12] determined the maximum tolerated dose in 38 consecutive patients treated with a dose escalation scheme consisting of concurrent TMZ delivery and 66 to 81 Gy over 6 weeks. Radiation boost was based only on contrast-enhancing T1-Gd MRI, using IMRT for SIB. The initial CTV was defined as the T1-Gd contrast enhancement area plus 1.5 cm, and the smaller one (CTV2) had a 5 mm expansion. Pre-radiation 11C-MET-PET was co-registered for correlations with sites of failure only retrospectively. With a median follow-up of 54 months, the median overall survival was 20 months, and the authors showed that patients could safely undergo concomitant TMZ treatment with 75 Gy in 30 fractions. Additionally, MET-PET appeared to predict the regions of high risk of recurrence that were not defined by MRI.
In a prospective phase II study, Piroth et al.[13] also used a SIB IMRT technique to deliver a boost dose of 72 Gy guided by FET-PET. The CTV-FET 72 Gy was defined from the postoperative PET imaging and covered the volume within a tumor-to-normal brain ratio (TBR) cut off value of FET uptake > 1.6. The initial CTV was the T1-Gd contrast enhancement area plus a 1.5 cm margin. With a median follow-up of 15 months for a total of 22 patients with GBM, the authors reported a median survival of 14.8 months, which was not different from that of the EORTC-NCIC trial. All local relapses were detected within the 95% dose volume of the initial MRI-defined PTV (60 Gy). Because of relevant modifications of brain structures with time, the exact geometric relationship between FET-PET relapse areas and the FET-base boost is difficult to determine.
Einstein et al.[44] reported a phase II study defining high-risk tumor volumes using a monovoxel MRS to deliver a stereotactic radiosurgical boost, with one session of 15 to 24 Gy (depending on the volume of the high-risk area) performed 2 weeks before conformal radiotherapy. Feasibility was shown in a series of 35 GBM patients, with a median survival of 15.8 months for the entire cohort and 20.8 months for those who underwent concurrent TMZ treatment. The median follow-up was not reported, and local control was not specifically analyzed.
Finally, Ken et al.[45] published the most advanced study (NCT01507506), an ongoing prospective and randomized phase II trial that integrates multivoxel 3D MRS images into the TPS for GBM dose-painting to guide SIB using IMRT. The reference arm delivers 60 Gy, while the experimental arm delivers 72 Gy within the high-risk sub-volumes (Cho/NAA ratio > 2). The authors have presented their methodology in detail and have shown that it is possible to integrate MRS images into the TPS, delivering high doses without increasing the doses to the OaR. This trial (“Spectro-glio”) is set to include 220 patients in 3 years.
Issues: Radioresistance, Dynamic Heterogeneity, and a Variety of Imaging Techniques
The dynamic heterogeneity of each HGG phenotype reflects the complexity of the underlying genotype, which changes over time, beginning very early in response to chemo-radiation[46],[47]. Delivering boost doses exclusively to the highly aggressive areas of HGG is one way to potentially overcome radio-resistance. However, as conventional radiotherapy planning is anatomically defined and remains quite identical during all 6 weeks of the treatment, re-evaluating the radiotherapy plan between the third and fourth weeks of chemo-radiation scheme could also be a challenging goal. Incorporation of these functional imaging techniques, such as MRS, PWI, and PET, into the TPS poses serious technical issues.
Basic co-registration of CT and MRI scans uses mutual anatomic information. In contrast, anatomic localization and tissue differentiation are highly degraded in advanced imaging modalities, and external markers or anatomic landmarks, such as osseous structures, cannot be easily identified. Moreover, accurate inter-modality spatial alignment is difficult for EPI-based images, such as perfusion and diffusion maps, which suffer from nonlinear geometric distortion due to magnetic susceptibility variations. In the end, the accuracy of hybrid image co-registration using conventional methods is usually low, and approximations derived from different imaging modalities could be added, resulting in errors in detecting “true” relationships between the structural and functional alterations at a very local level.
Dedicated algorithms for threshold-based segmentation (either for CBV or SUV quantification) and reliable co-registration of these dynamic functional maps on static MRI/CT anatomy are still rare, do not share a real common language, and are very operator-dependent. Solutions exist for each of these advanced techniques, such as acquiring anatomic MRI during the same imaging session with similar technique (e.g., T2-weighted for PWI) and tissue contrast, the use of non-linear registration methods to better match the anatomy of the functional and anatomic images, and registering the reference image set (the CT image) in the same scanning position as the functional image set (for PET and MRS).
Finally, the heterogeneity in methodology, imaging techniques (either acquisition of the sequences or postprocessing steps), and dedicated software applications are also evident in the various protocols and softwares available for neuro-radiologists. As the European Neuro-oncologic Imaging Platform at EORTC[48], we will unify our techniques by including full details of the whole workflow of perfusion MRI protocol for all HGGs, similar to the ongoing prospective 26053 and 26101 side-studies.
Next Steps: Combination, Homogeni-zation, Adaptation, and Integration
The current trend is to combine imaging as an integrated multi-modality technique. Each of these techniques has the potential to improve delineation for radiotherapy planning over an individual modality. For HGG patients, it will become increasingly feasible to integrate a high-tech, preradiation imaging planning, such as 18F-DOPA-PET plus, in the same week or ideally simultaneous with PWI and DTI MRI data acquisition, complementary to conventional T2 and T1-Gd sequences.
Hybrid visualization of HGG presenting angiogenic characteristics vs. other mainly infiltrative characteristics will guide the boost for dose-painting and will potentially determine whether concomitant treatment with antiangiogenic and/or antiinvasiveness drugs are needed[49]. Of note, the first PET/MRI machines are available now, and HGG could be a good model[50]. It will be a challenge to adequately determine the timing of the very early evaluations, even per-therapeutically.
Defining new synthetic semi-quantitative metrics, with complete integration and careful interpretation of all these “hybrid” data and fancy images, will be the final “next step”!
References
- 1.Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 2.Louis DN, Ohgaki H, Wiestler OD, et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114:97–109. doi: 10.1007/s00401-007-0243-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kelly PJ, Daumas-Duport C, Kispert DB, et al. Imaging-based stereotaxic serial biopsies in untreated intracranial glial neoplasms. J Neurosurg. 1987;66:865–874. doi: 10.3171/jns.1987.66.6.0865. [DOI] [PubMed] [Google Scholar]
- 4.Price SJ, Gillard JH. Imaging biomarkers of brain tumour margin and tumour invasion. Br J Radiol. 2011;84:S159–S167. doi: 10.1259/bjr/26838774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chinnaiyan P, Won M, Wen PY, et al. RTOG 0913: a phase 1 study of daily everolimus (RAD001) in combination with radiation therapy and temozolomide in patients with newly diagnosed glioblastoma. Int J Radiat Oncol Biol Phys. 2013;86:880–884. doi: 10.1016/j.ijrobp.2013.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.EORTC trials: groups.eortc.be/brain/html/trials.html
- 7.Minniti G, Amelio D, Amichetti M, et al. Patterns of failure and comparison of different target volume delineations in patients with glioblastoma treated with conformal radiotherapy plus concomitant and adjuvant temozolomide. Radiother Oncol. 2010;97:377–381. doi: 10.1016/j.radonc.2010.08.020. [DOI] [PubMed] [Google Scholar]
- 8.Bleehen NM, Stenning SP. A Medical Research Council trial of two radiotherapy doses in the treatment of grades 3 and 4 astrocytoma. The Medical Research Council Brain Tumour Working Party. Br J Cancer. 1991;64:769–774. doi: 10.1038/bjc.1991.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Souhami L, Seiferheld W, Brachman D, et al. Randomized comparison of stereotactic radiosurgery followed by conventional radiotherapy with carmustine to conventional radiotherapy with carmustine for patients with glioblastoma multiforme: report of Radiation Therapy Oncology Group 93-05 protocol. Int J Radiat Oncol Biol Phys. 2004;60:853–860. doi: 10.1016/j.ijrobp.2004.04.011. [DOI] [PubMed] [Google Scholar]
- 10.Nieder C, Mehta MP. Advances in translational research provide a rationale for clinical re-evaluation of high-dose radiotherapy for glioblastoma. Med Hypotheses. 2011;76:410–413. doi: 10.1016/j.mehy.2010.11.005. [DOI] [PubMed] [Google Scholar]
- 11.Petrecca K, Guiot MC, Panet-Raymond V, et al. Failure pattern following complete resection plus radiotherapy and temozolomide is at the resection margin in patients with glioblastoma. J Neurooncol. 2013;111:19–23. doi: 10.1007/s11060-012-0983-4. [DOI] [PubMed] [Google Scholar]
- 12.Tsien CI, Brown D, Normolle D, et al. Concurrent temozolomide and dose-escalated intensity-modulated radiation therapy in newly diagnosed glioblastoma. Clin Cancer Res. 2012;18:273–279. doi: 10.1158/1078-0432.CCR-11-2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Piroth MD, Pinkawa M, Holy R, et al. Integrated boost IMRT with FET-PET-adapted local dose escalation in glioblastomas. Results of a prospective phase II study. Strahlenther Onkol. 2012;188:334–339. doi: 10.1007/s00066-011-0060-5. [DOI] [PubMed] [Google Scholar]
- 14.Viel T, Talasila KM, Monfared P, et al. Analysis of the growth dynamics of angiogenesis-dependent and -independent experi-mental glioblastomas by multimodal small-animal PET and MRI. J Nucl Med. 2012;53:1135–1145. doi: 10.2967/jnumed.111.101659. [DOI] [PubMed] [Google Scholar]
- 15.Isambert A, Dhermain F, Bidault F, et al. Evaluation of an atlas-based automatic segmentation software for the delineation of brain organs at risk in a radiation therapy clinical context. Radiother Oncol. 2008;87:93–99. doi: 10.1016/j.radonc.2007.11.030. [DOI] [PubMed] [Google Scholar]
- 16.Metcalfe P, Liney GP, Holloway L, et al. The potential for an enhanced role for MRI in radiation-therapy treatment planning. Technol Cancer Res Treat. 2013;12:429–446. doi: 10.7785/tcrt.2012.500342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Holdsworth CH, Corwin D, Stewart RD, et al. Adaptive IMRT using a multiobjective evolutionary algorithm integrated with a diffusion-invasion model of glioblastoma. Phys Med Biol. 2012;57:8271–8283. doi: 10.1088/0031-9155/57/24/8271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Neal ML, Trister AD, Ahn S, et al. Response classification based on a minimal model of glioblastoma growth is prognostic for clinical outcomes and distinguishes progression from pseudoprogression. Cancer Res. 2013;73:2976–2986. doi: 10.1158/0008-5472.CAN-12-3588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sadeghi N, Salmon I, Decaestecker C, et al. Stereotactic com-parison among cerebral blood volume, methionine uptake, and histopathology in brain glioma. AJNR Am J Neuroradiol. 2007;28:455–461. [PMC free article] [PubMed] [Google Scholar]
- 20.McKnight TR, Lamborn KR, Love TD, et al. Correlation of magnetic resonance spectroscopic and growth characteristics within Grades II and III gliomas. J Neurosurg. 2007;106:660–666. doi: 10.3171/jns.2007.106.4.660. [DOI] [PubMed] [Google Scholar]
- 21.Pafundi DH, Laack NN, Youland RS, et al. Biopsy validation of 18F-DOPA PET and biodistribution in gliomas for neurosurgical planning and radiotherapy target delineation: results of a prospective pilot study. Neuro Oncol. 2013;15:1058–1067. doi: 10.1093/neuonc/not002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pirzkall A, McKnight TR, Graves EE, et al. MR-spectroscopy guided target delineation for high-grade gliomas. Int J Radiat Oncol Biol Phys. 2001;50:915–928. doi: 10.1016/s0360-3016(01)01548-6. [DOI] [PubMed] [Google Scholar]
- 23.Narayana A, Chang J, Thakur S, et al. Use of MR spectroscopy and functional imaging in the treatment planning of gliomas. Br J Radiol. 2007;80:347–354. doi: 10.1259/bjr/65349468. [DOI] [PubMed] [Google Scholar]
- 24.Dhermain F, Saliou G, Parker F, et al. Microvascular leakage and contrast enhancement as prognostic factors for recurrence in unfavorable low-grade gliomas. J Neurooncol. 2010;97:81–88. doi: 10.1007/s11060-009-9992-3. [DOI] [PubMed] [Google Scholar]
- 25.Jain R, Narang J, Griffith B, et al. Prognostic vascular imaging biomarkers in high-grade gliomas: tumor permeability as an adjunct to blood volume estimates. Acad Radiol. 2013;20:478–485. doi: 10.1016/j.acra.2012.11.011. [DOI] [PubMed] [Google Scholar]
- 26.Hu LS, Eschbacher JM, Heiserman JE, et al. Reevaluating the imaging definition of tumor progression: perfusion MRI quantifies recurrent glioblastoma tumor fraction, pseudoprogression, and radiation necrosis to predict survival. Neuro Oncol. 2012;14:919–930. doi: 10.1093/neuonc/nos112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Meyzer C, Dhermain F, Ducreux D, et al. A case report of pseudoprogression followed by complete remission after proton-beam irradiation for a low-grade glioma in a teenager: the value of dynamic contrast-enhanced MRI. Radiat Oncol. 2010;5:9. doi: 10.1186/1748-717X-5-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ducreux D. Statistical analysis of multi b factor diffusion weighted images can help to distinguish between vasogenic and tumor-infiltrated edema. J MRI. 2013 Nov 4; doi: 10.1002/jmri.24399. [DOI] [PubMed] [Google Scholar]
- 29.Matsuo M, Miwa K, Tanaka O, et al. Impact of [11C] methionine positron emission tomography for target definition of glioblastoma multiforme in radiation therapy planning. Int J Radiat Oncol Biol Phys. 2012;82:83–89. doi: 10.1016/j.ijrobp.2010.09.020. [DOI] [PubMed] [Google Scholar]
- 30.Jacobs AH, Thomas A, Kracht LW, et al. 18F-fluoro-L-thymidine and 11C-methylmethionine as markers of increased transport and proliferation in brain tumors. J Nucl Med. 2005;46:1948–1958. [PubMed] [Google Scholar]
- 31.Price SJ, Fryer TD, Cleij MC, et al. Imaging regional variation of cellular proliferation in gliomas using 3′-deoxy-3′-[18F]fluoro-thymidine positron-emission tomography: an image-guided biopsy study. Clin Radiol. 2009;64:52–63. doi: 10.1016/j.crad.2008.01.016. [DOI] [PubMed] [Google Scholar]
- 32.Niyazi M, Geisler J, Siefert A, et al. FET-PET for malignant glioma treatment planning. Radiother Oncol. 2011;99:44–48. doi: 10.1016/j.radonc.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 33.Rieken S, Habermehl D, Giesel FL, et al. Analysis of FET-PET imaging for target volume definition in patients with gliomas treated with conformal radiotherapy. Radiother Oncol. 2013;109:487–492. doi: 10.1016/j.radonc.2013.06.043. [DOI] [PubMed] [Google Scholar]
- 34.Jain R, Poisson L, Narang J, et al. Genomic mapping and survival prediction in glioblastoma: molecular subclassification strengthened by hemodynamic imaging biomarkers. Radiology. 2013;267:212–220. doi: 10.1148/radiol.12120846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Galbán CJ, Chenevert TL, Meyer CR, et al. Prospective analysis of parametric response map-derived MRI biomarkers: identification of early and distinct glioma response patterns not predicted by standard radiographic assessment. Clin Cancer Res. 2011;17:4751–4760. doi: 10.1158/1078-0432.CCR-10-2098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee IH, Piert M, Gomez-Hassan D, et al. Association of 11C- methionine PET uptake with site of failure after concurrent temozolomide and radiation for primary glioblastoma multiforme. Int J Radiat Oncol Biol Phys. 2009;73:479–485. doi: 10.1016/j.ijrobp.2008.04.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Piroth MD, Holy R, Pinkawa M, et al. Prognostic impact of posto-perative, pre-irradiation (18)F-fluoroethyl-l-tyrosine uptake in glioblastoma patients treated with radiochemotherapy. Radiother Oncol. 2011;99:218–224. doi: 10.1016/j.radonc.2011.03.006. [DOI] [PubMed] [Google Scholar]
- 38.Dhermain FG, Hau P, Lanfermann H, et al. Advanced MRI and PET imaging for assessment of treatment response in patients with gliomas. Lancet Neurol. 2010;9:906–920. doi: 10.1016/S1474-4422(10)70181-2. [DOI] [PubMed] [Google Scholar]
- 39.Weber MA, Henze M, Tüttenberg J, et al. Biopsy targeting gliomas: do functional imaging techniques identify similar target areas? Invest Radiol. 2010;45:755–768. doi: 10.1097/RLI.0b013e3181ec9db0. [DOI] [PubMed] [Google Scholar]
- 40.Narayana A, Kunnakkat SD, Medabalmi P, et al. Change in pattern of relapse after antiangiogenic therapy in high-grade glioma. Int J Radiat Oncol Biol Phys. 2012;82:77–82. doi: 10.1016/j.ijrobp.2010.10.038. [DOI] [PubMed] [Google Scholar]
- 41.Gevaert T, Verellen D, Tournel K, et al. Setup accuracy of the Novalis ExacTrac 6DOF system for frameless radiosurgery. Int J Radiat Oncol Biol Phys. 2012;82:1627–1635. doi: 10.1016/j.ijrobp.2011.01.052. [DOI] [PubMed] [Google Scholar]
- 42.Marsh JC, Godbole R, Diaz AZ, et al. Sparing of the hippocampus, limbic circuit and neural stem cell compartment during partial brain radiotherapy for glioma: a dosimetric feasibility study. J Med Imaging Radiat Oncol. 2011;55:442–449. doi: 10.1111/j.1754-9485.2011.02282.x. [DOI] [PubMed] [Google Scholar]
- 43.Marsh JC, Ziel GE, Diaz AZ, et al. Integral dose delivered to normal brain with conventional intensity-modulated radiotherapy (IMRT) and helical tomotherapy IMRT during partial brain radiotherapy for high-grade gliomas with and without selective sparing of the hippocampus, limbic circuit and neural stem cell compartment. J Med Imaging Radiat Oncol. 2013;57:378–383. doi: 10.1111/1754-9485.12048. [DOI] [PubMed] [Google Scholar]
- 44.Einstein DB, Wessels B, Bangert B, et al. Phase II trial of radiosurgery to magnetic resonance spectroscopy-defined high-risk tumor volumes in patients with glioblastoma multiforme. Int J Radiat Oncol Biol Phys. 2012;84:668–674. doi: 10.1016/j.ijrobp.2012.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ken S, Vieillevigne L, Franceries X, et al. Integration method of 3D MR spectroscopy into treatment planning system for glioblastoma IMRT dose painting with integrated simultaneous boost. Radiat Oncol. 2013;8:1. doi: 10.1186/1748-717X-8-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Narayana A, Perretta D, Kunnakkat S, et al. Invasion is not an independent prognostic factor in high-grade glioma. J Cancer Res Ther. 2011;7:331–335. doi: 10.4103/0973-1482.87039. [DOI] [PubMed] [Google Scholar]
- 47.Kil WJ, Tofilon PJ, Camphausen K. Post-radiation increase in VEGF enhances glioma cell motility in vitro. Radiat Oncol. 2012;7:25. doi: 10.1186/1748-717X-7-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bean J, Flament J, Ruyskart P, et al. The European Organisation for Research and Treatment of Cancer Imaging Programme. Eur Oncol. 2010;6:92–95. [Google Scholar]
- 49.Weiler M, Pfenning PN, Thiepold AL, et al. Suppression of proinvasive RGS4 by mTOR inhibition optimizes glioma treatment. Oncogene. 2013;32:1099–1109. doi: 10.1038/onc.2012.137. [DOI] [PubMed] [Google Scholar]
- 50.Shah NJ, Oros-Peusquens AM, Arrubla J, et al. Advances in multimodal neuroimaging: hybrid MR-PET and MR-PET-EEG at 3 T and 9.4 T. J Magn Reson. 2013;229:101–115. doi: 10.1016/j.jmr.2012.11.027. [DOI] [PubMed] [Google Scholar]