Abstract
Methods for imaging the molecular or cellular profile of tissue are being developed for all forms of non-invasive cardiovascular imaging. It is thought that these technologies will potentially improve patient outcomes by allowing diagnosis of disease at an early-stage, monitoring disease progression, providing important information on patient risk, and for tailoring therapy to the molecular basis of disease. Molecular imaging is also already assuming an important role in science by providing a better understanding of the molecular basis of cardiovascular pathology, for assessing response to new therapies, and for rapidly optimizing new or established therapies. Ultrasound-based molecular imaging is one of these new approaches. Contrast-enhanced ultrasound molecular imaging relies on the detection of novel site-targeted microbubbles (MB) or other acoustically active particles which are administered by intravenous injection, circulate throughout the vascular compartment, and are then retained and imaged within regions of disease by ligand-directed binding. The technique is thought to be advantageous in practical terms of cost, time, and ease of use. The aim of this review is to discuss the molecular participants of cardiovascular disease that have been targeted for ultrasound imaging, general features of site-targeted MB, imaging protocols, and potential roles of ultrasound molecular imaging in cardiovascular research and clinical medicine.
Keywords: Molecular imaging, Contrast ultrasound, Microbubbles
Introduction
Imaging techniques that allow the non-invasive assessment of disease processes at the molecular level have been developed for essentially all types of medical diagnostic imaging including ultrasound, magnetic resonance imaging, radionuclide/positron emission imaging, radiographic imaging, and optical imaging.1) The most common approach to molecular imaging has been to develop novel site-targeted contrast agents that are either activated by a disease-related process or are retained due to their adherence to disease-related molecules and are imaged after clearance of free tracer. For the diverse array of imaging technologies, there are major differences between the detectors with regards to resolution, sensitivity, speed and cost. There are also important differences between the imaging probes used for different forms of imaging based on biodistribution, in vivo kinetics, temporal resolution, safety, and cost. No single form of molecular imaging is likely to fulfill the diverse needs of clinicians and biomedical scientists. Instead, there are certain applications where one type of molecular imaging is likely to provide the most reasonable approach based on characteristics of the detector and imaging probe.
Molecular imaging with contrast-enhanced ultrasound (CEU) relies on the selective targeting and retention of encapsulated microbubbles (MB) or other acoustically-active particles to sites of disease.2) An ultrasound-based approach has several advantages and disadvantages compared to other forms of molecular imaging. One important limitation is that only events within the vascular space can be detected using targeted MBs with conventional size distribution. As is the case with all forms of molecular imaging with particle-based contrast agents, MB attachment is more complex than a 1 : 1 binding profile between imaging probe and target molecule. Instead, MB attachment depends on a wide array of factors that include but are not limited to shear forces, the ligand on the MB surface, the targeted molecule, and bond kinetics. Advantages of CEU molecular imaging are that it provides real-time information, represents a good balance between spatial resolution and sensitivity, is portable is rapid, is widely available, and does not involve non-ionizing radiation.2),3) In this review, we discuss some of the basic principles, and the most pertinent clinical applications where molecular imaging with ultrasound may have a major impact.
Molecular Targets for Cardiovascular Molecular Imaging
Ultrasound contrast agents to a large extent remain within the intravascular space.4) Accordingly, CEU molecular imaging is best suited for imaging disease processes that occur within the vascular compartment. There are several key pathobiologic processes that occur at the blood vessel-blood pool interface that are common to many cardiovascular diseases. They include: 1) endothelial cell activation and endothelial cell adhesion molecule (ECAM) expression, 2) leukocyte-endothelial interactions, and 3) thrombus formation and platelet adhesion. In this section we will discuss these targets for CEU molecular imaging that are used to provide information on disease development and progression in atherosclerosis, tissue ischemia, thrombosis, and angiogenesis.
Leukocytes and endothelial inflammatory adhesion molecules
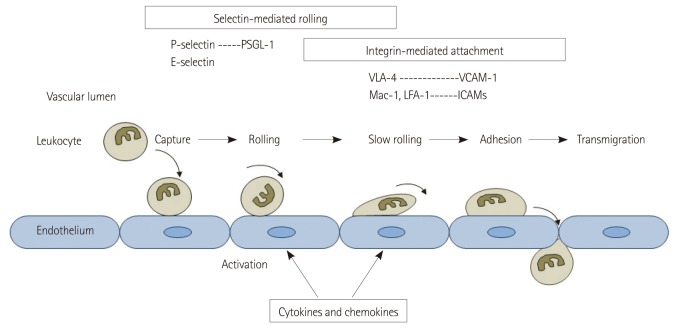
Endothelial activation and leukocyte adhesion to the activated endothelium are hallmarks of the inflammatory process in cardiovascular disease.5),6) Fig. 1 shows the orchestrated processes that result in leukocyte recruitment in inflammation which include leukocyte capture, rolling along the endothelial surface, adhesion, and transmigration across the endothelial boundary.7),8) The process of leukocyte recruitment is governed by the local generation of cytokines and chemokines and the subsequent expression of ECAMs which interact with leukocyte counterligands.7),8) One of the key ECAMs involved in the initial inflammatory response are selectins (P- and E-selectin) which are a family of long adhesion molecules that extend beyond the endothelial glycocalyx and contribute primarily to leukocyte capture and rolling by interacting with glycoprotein counterligands that are constitutively expressed on leukocytes.9) The selectin-mediated interactions can result in transient bond formation even in the face of high shear stress. The rolling of leukocytes exposes them to molecular signals that result in the expression of activated integrins which are heterodimeric molecules that interact with other ECAMs such as intercellular adhesion molecules (ICAMs) and vascular cell adhesion molecule-1 (VCAM-1). These integrin mediated interactions result in firm adhesion.7)
Fig. 1.
Schematic illustrating mechanisms of leukocyte recruitment during inflammation. Key adhesion molecules involved in rolling and adhesion are shown. PSGL-1: P-selectin glycoprotein ligand-1, VCAM-1: vascular cell adhesion molecule-1, ICAM: intercellular adhesion molecule, VLA-4: very late antigen-4, LFA-1: lymphocyte function-associated antigen-1.
The wide array of adhesion molecules and the large library of ligands that have been created to detect or inhibit them have potentiated CEU molecular imaging of inflammation. MBs have been targeted to either ECAMs or leukocyte adhesion molecules to detect endothelial activation or leukocyte recruitment. Moreover, since different leukocyte subpopulations (neutrophils, monocyte subsets, etc.) are distinguished by specific cell surface markers, it has been possible to differentiate the type of inflammatory responses by targeting different cell surface markers.
Thrombus formation
For imaging the fibrin component of thrombus, ultrasound contrast agents have been directly targeted to fibrin.10),11) This approach has not advanced rapidly because only recently have appropriate ligands that recognize only fibrin and do not bind plasma fibrinogen been developed. MBs have also been targeted to tissue factor in regions of vascular injury,10),12) which may provide a useful target for evaluating subclinical plaque rupture since tissue factor in inflamed plaques is ordinarily not available to binding by intravascular agents. Because of the pivotal role of platelets in thrombus formation and in inflammation in cardiovascular diseases, and because of the availability of a wide array of ligands, CEU molecular imaging of thrombus has more commonly been directed at platelets or platelet-binding processes. Similar to the inflammatory response, platelet adhesion and subsequent aggregation are mediated by a series of events that involve adhesion molecules. Under normal conditions, the endothelium plays an important role in preventing platelet adhesion through multiple mechanisms.13) Under situations of vascular inflammation or injury platelets adhere either through interactions with the endothelium, to monocytes, or to subendothelial matrix in regions where endothelial integrity is lost.14) Although the potential mediators of platelet adhesion are broad (Fig. 2), CEU molecular imaging of platelet adhesion has focused on several key molecular processes. The most common approach has been to target the glycoprotein IIb/IIIa (GPIIb/IIIa) integrin that is involved in platelet adhesion and fibrinogen-mediated aggregation. Although arginine-glycine-aspartate (RGD)-containing peptides have been frequently used for targeting, the approach of using activation-specific antibodies or antibody fragments on the MB surface decreases non-specific interaction with non-activated circulating platelets in the blood pool.15),16) The platelet GP1bα receptor plays a key role in platelet recruitment and rolling by interacting with a wide variety of ligands including activated von Willebrand factor (VWF) and leukocyte integrins.17) This receptor has also been targeted by conjugating molecules representing the GP1bα-binding domain of VWF to MBs.18) Recombinant GP1bα has also been conjugated to MBs to image VWF which can become active and form ultra-large multimers either by binding to subendothelial collagen, or on the endothelial surface in regions where shear stress is high or when regulatory proteases become inactive, such as in thrombotic thrombocytopenic purpura and atherosclerosis.19)
Fig. 2.
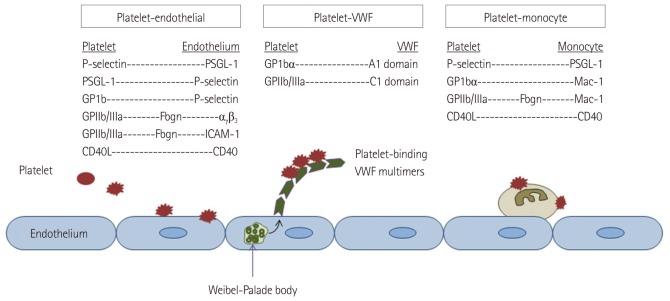
Schematic illustrating potential mechanisms of platelet adhesion in cardiovascular disease. PSGL-1: P-selectin glycoprotein ligand-1, ICAM: intercellular adhesion molecule, VWF: von Willebrand factor. GP1bα: glycoprotein Ib-alpha, GpIIb/IIIa: glycoprotein IIb/IIIa.
Imaging angiogenesis
Angiogenesis (formation of new vessels), arteriogenesis (growth and remodeling of the arteriolar network), and development of large collaterals are important processes in either the pathogenesis or adaptive response to cardiovascular disease. Ultrasound molecular imaging of vascular remodeling has relied on targeting MBs to some of the key processes that are detectable by an intravascular contrast agent. Targets have included specific endothelial integrins expressed during neovascularization, such as αv-integrins, ECAMs that are upregulated during vascular activation and remodeling, growth factor receptors such as vascular endothelial growth factor-1 (VEGF-R1), and markers for the specific subset of inflammatory cells that are important in vascular remodeling.20-26) It is important to realize that not all forms of vascular remodeling utilize the same molecular mechanisms so that imaging targets will differ for compensatory angiogenesis that occurs in ischemic tissue and for adverse neovascularization that occurs in tumors or the vasa vasorum of plaques.
Targeted Contrast Agents
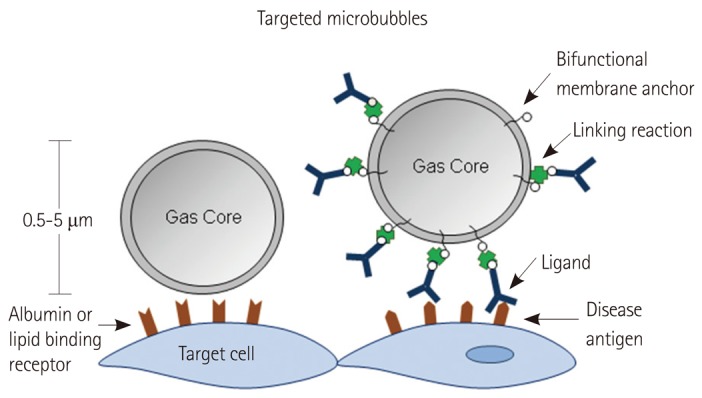
Microbubble contrast agents are gas-filled microspheres stabilized by shells comprised of proteins, lipids or polymers.27) Signal generation occurs from either stable cavitation (volumetric oscillation) or inertial cavitation (destruction and dispersion) of MBs. The size of MBs that are conventionally used for cardiovascular or radiology applications is generally in the range of 1 to 5 µm in diameter, which permits their intravenous injection and free passage through the microcirculation of the pulmonary and peripheral circulation. It should be noted that smaller non-MB contrast agents such as echogenic liposomes and liquid-core emulsion agents, some of which can undergo phase-transition to gaseous bodies, have also been developed and are being investigated for potential extravascular targeting.
Targeting of MBs have been accomplished using two general approaches (Fig. 3). One strategy relies on non-specific attachment of MBs to activated leukocytes and even endothelial cells via opsonization. This process is mediated by complement activation and deposition on the shell surface, especially for lipid-shelled MBs.28-30) Opsonization is also probably responsible in part for their clearance by the reticuloendothelial organs but can also mediate binding to cells with complement receptors. Amplification of this process can be achieved by chemical modification of lipid shell components such as adding phosphatidylserine.28-30) A more specific targeting approach involves attachment of targeting ligands (antibodies, peptides, glycoproteins, etc.) to the surface of MBs using any number of chemical conjugation strategies. This method allows characterization of the upregulation of specific adhesion molecules or recruitment of specific cellular populations. Successful site-targeted imaging relies on many considerations (Fig. 4) including the density and binding kinetics of the targeting ligands; surface concentration and availability of the target molecule; and the specificity of the ligand for the target, and the target for the disease process of interest.31)
Fig. 3.
Strategies used for targeting of microbubble contrast agents.
Fig. 4.
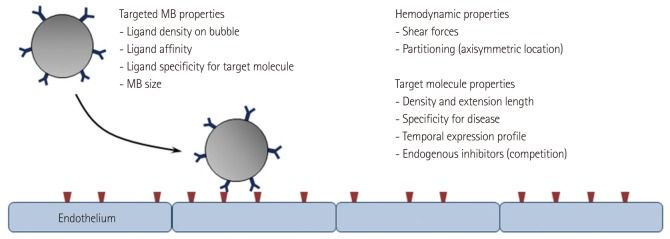
Determinants of the success of ligand-directed targeting of microbubbles contrast agents.
Molecular Imaging Protocols
The aim of CEU molecular imaging is to detect signal from targeted MBs that are retained in tissue due to their selective adherence to target molecules. The most commonly used approach, illustrated in Fig. 5, involves single bolus injection of agent and then delaying imaging of retained MBs until there is sufficient clearance of freely circulating MBs from the blood pool.30) Clearance generally requires 5-10 minutes depending on the type and dose of MB, the tissue of interest, and the animal species.29) The signal intensity in the initial frame on resumption of imaging reflects the total tissue concentration of both retained MBs and the small amount of freely circulating MBs that remain.29),30) The signal contribution from the circulating component can then be calculated by acoustic destruction of MBs and evaluating degree of signal replenishment over the course of several seconds. The difference between the replenishment signal and that on the initial frame(s) represents signal component from retained agent. It should be noted that successful molecular imaging depends on in vivo stability of MBs over the period from injection to imaging. Stability can be influenced by the size (faster removal for larger particles) and composition of the ultrasound contrast agent. In general, signal from retained MBs has been quantified as video intensity within a defined region-ofinterest. This approach works best when imaging MB retention within the microcirculation of an organ. When quantifying signal at the endothelial surface of large vessel, defining a region-of-interest is more difficult for detecting MBs due to the thin layer of contrast and specular reflection, which has created the need for innovative approaches to imaging.
Fig. 5.
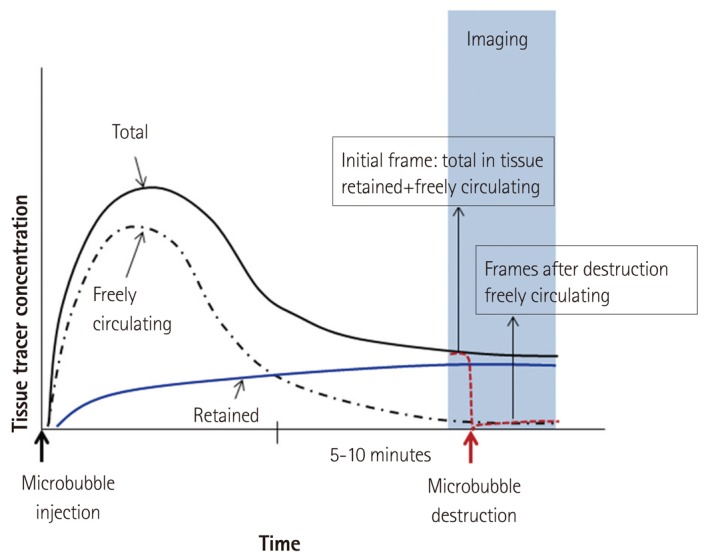
Schematic illustrating a common algorithm for molecular imaging with targeted MB contrast agents.
Cardiovascular Applications for Ultrasound Molecular Imaging
Atherosclerosis
The pathophysiology of atherosclerosis is diverse, complex, and changes over the decades between disease inception and clinical events. Although the changing molecular environment provides many potential targets for molecular imaging, the selection of the target must be guided by the stage of disease and intended clinical use. For example, evaluation for a "vulnerable plaque" or "vulnerable patient" defined as heightened susceptibility to acute atherothrombotic complication is best accomplished by imaging the specific processes that lead to plaque rupture or thrombotic occlusion. These events may include platelet adhesion, endothelial activation, macrophage activity, or protease activity.32) In contrast, assessing very early disease is likely to rely on imaging the accumulation of oxidized lipids or ECAM expression. Ultrasound molecular imaging has been used to primarily detect the processes that occur at the endothelial-blood pool interface.
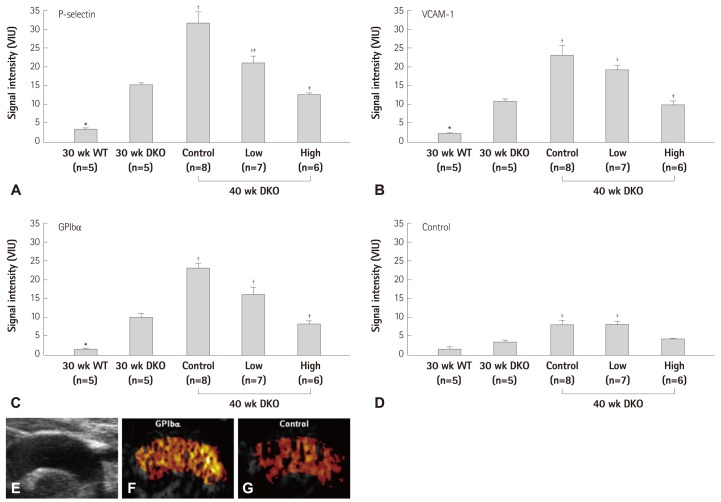
For in vivo molecular imaging of endothelial activation, MBs targeted to adhesion molecules such as P-selectin, ICAM-1, and VCAM-1 have been applied. CEU imaging with these agents have been used in murine models to not only detect advanced high-risk plaques, but also the earliest stages of endothelial activation even before development of significant neo-intimal formation.33-35) In murine models, imaging of VCAM-1 has also been shown to provide readout of diet-induced changes in the degree of plaque inflammatory activity.35) More recently, targeted imaging of VCAM-1 and P-selectin have successfully been performed in non-human primate models of atherosclerosis.36) In these studies, early endothelial activation associated with obesity and insulin resistance could be detected about a year before any significant increase in intima-medial thickness. Several studies have also suggested that very high-risk atherosclerotic disease may not need sophisticated targeting strategies. Non-specific retention of lipid and albumin MBs, presumably through interactions with the activated vascular endothelium, has been used to detect atherosclerotic disease in animal models.37) With regards to its application in pre-clinical drug development, CEU molecular imaging of ECAM expression in atherosclerosis has also been used to evaluate the therapeutic impact of emerging therapies. For example, CEU molecular imaging has been used to evaluate how new anti-oxidants that inhibit nicotinamide adenine dinucleotide phosphate oxidase can reduce plaque inflammation and thrombosis (Fig. 6).18)
Fig. 6.
CEU molecular imaging of endothelial activation and platelet adhesion in the aorta in a murine model of age-dependent atherosclerosis (LDL-/-, ApoBec-1-/-), and the effect of anti-oxidant therapy with the NADPH-oxidase-inhibitor apocynin at high or low dose. A-D: molecular imaging signal of P-selectin, VCAM-1, GP1bα, and control MBs in mice at 30 weeks of age and at 40 weeks of age with and without treatment with apocynin. Thoracic aorta images from a non-treated 40 weeks mouse are shown with high-frequency 2-D ultrasound (E) and CEU molecular imaging with GP1bα-targeted and control MBs (F and G).18) CEU: contrast-enhanced ultrasoud, NADPH: nicotinamide adenine dinucleotide phosphate, MB: microbubble, VCAM-1: vascular cell adhesion molecule-1, WT: wild-type mice, DKO: double knockout mice, LDL: low-density lipoprotein, 2-D: 2-dimensional.
Imaging of thrombus, activated platelets, and von Willebrand factor
Thrombosis plays a major role in many pathophysiologic processes in cardiovascular disease. Accordingly, there are is interest in using targeted imaging to enhance the detection of large thrombi within vessels or the cardiac chamber, microvascular thrombosis such as that which occurs with microvascular no-reflow, or a pro-thrombotic environment such in the region of a high-risk atherosclerotic plaque. It should also be noted that sonothrombolysis, the acoustic disruption of clot, is accelerated by MB cavitation and that thrombus-targeted MBs can further improve this therapy.38)
Imaging of the pro-thrombotic environment has been achieved by targeting MBs to a wide variety of ligands. Echogenic liposomes have been targeted to both tissue factor and fibrin in animal models of atherosclerosis.10),12) A more common approach has been to image platelets which play a major role in acute coronary syndromes by targeting the GPIIb/IIIa receptor through surface conjugation of either RGD-peptides or antibodies (whole or fragments), or the platelet GP1bα receptor by surface conjugation of a peptide representing the A1 binding domain of VWF.15),16),18) By targeting GPIIb/IIIa it is has been possible to use CEU molecular imaging to monitor thrombolysis in animal models of carotid thrombosis.16) By targeting GP1bα it has been possible to image the low level of platelet-endothelial interactions that contribute to the conversion of a plaque to unstable phenotype.18) Recently, CEU molecular imaging with MBs bearing recombinant GP1bα has been used to definitively demonstrate the presence of dysregulated VWF on the surface of atherosclerotic plaques.19) These data are important since they have provided insight into new mechanisms by which oxidative stress leads to a pro-atherothrombotic environment.
Tissue ischemia
Ischemic memory imaging refers to non-invasive visualization of molecular events that occur with ischemia but that can persist for hours after restoration of normal perfusion. This approach is clinically important because it allows after-the-fact recognition of ischemic insult even in the absence of infarction. This approach may also be particularly useful for diagnosing active ischemia in patients who have preexisting wall motion abnormalities and perfusion defects from prior events.39) An ultrasound approach to ischemic memory imaging is attractive because of the speed and portable nature of CEU molecular imaging.
Molecular imaging of ischemia was first achieved with anionic MBs bearing phosphatidylserine which allowed detection of postischemic inflammatory cell recruitment.40) More recently, selectin targeting has been used involving MBs bearing either mAb against P-selectin, sialyl lewis-X residues, or a recombinant form of the endogenous selectin counterligand P-selectin glycoprotein ligand-1 (PSGL-1).33),41),42) P-selectin is an ideal target since it is prestored within endothelial Weibel-Palade bodies, is released very rapidly after ischemia, and persists for hours after resolution of ischemia. By using PSGL-1 as a targeting moiety, it has been possible to also image later phase E-selectin expression which may help extend the post-ischemic window for successful CEU ischemic memory imaging.41) Imaging of selectin expression has been shown in murine models and in non-human primates to detect myocardial ischemic insult without infarction long after post-ischemic stunning has resolved (Fig. 7).41),42)
Fig. 7.
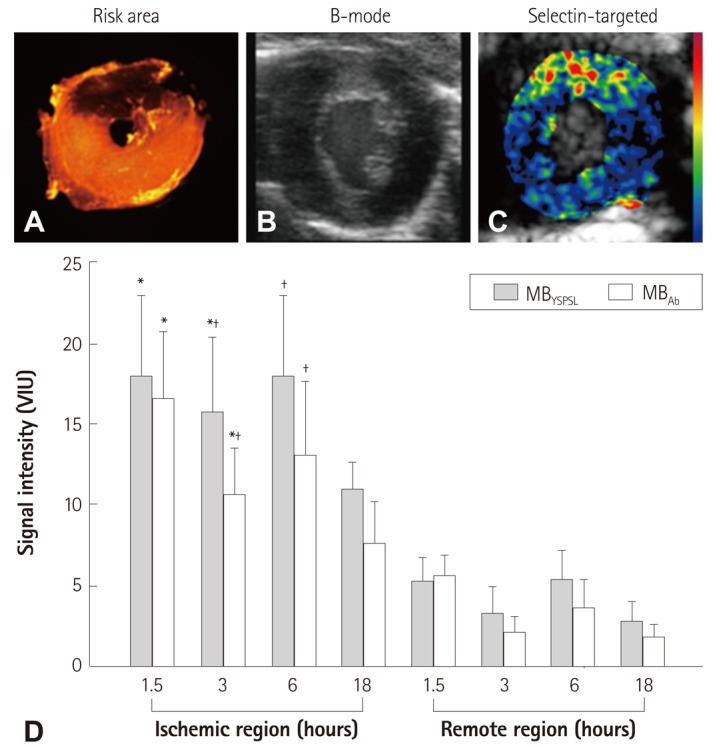
Ischemic memory imaging with CEU molecular imaging of P-selectin in mice. A: post-mortem microsphere-defined risk area during LAD occlusion. B and C: B-mode and P-selectin-targeted imaging several hours after producing brief (10 minutes) of transient ischemia of the LAD territory. D: P-selectin molecular imaging data using MBs bearing either PSGL-1 (MBYSPSL) or anti-P-selectin antibody (MBAb) from either the previously ischemic risk area or remote area. Data are shown for various reperfusion time intervals after 10 minutes of ischemia.*p<0.05 vs. remote region. Adapted from Davidson et al. J Am Coll Cardiol 2012;60:1690-7.41) MB: microbubble, CEU: contrast-enhanced ultrasoud, PSGL-1: P-selectin glycoprotein ligand-1.
Molecular imaging of vascular remodeling
Vascular remodeling in the form vasculoneogenesis plays a detrimental role in atherosclerosis; and in the form of angiogenesis and arteriogenesis plays a compensatory role to prevent ischemic complications in chronic severe coronary artery disease and peripheral arterial disease.43) Vascular remodeling is a complex process involving multiple different biologic triggers, signaling pathways, and cellular responses.44),45) Perfusion imaging alone is an attractive method for ultimately determining the end-result of adaptive vascular remodeling in ischemic disease. However, molecular imaging is thought to be an important research tool for understanding the biologic processes and possibly for rapid evaluation of the effect of new pro-angiogenic therapies. The endothelial molecular targets for CEU molecular imaging have been tailored for the specific cells and processes involved. CEU molecular imaging of endothelial activation specific to angiogenesis has involved imaging the αv-integrin family of adhesion molecules.20-22) Using this approach in a model of hindlimb ischemia, it has been possible to detect vascular remodeling that occurs even prior to detectable changes in perfusion. Because of the link between inflammation and angiogenesis, molecular imaging of the cellular inflammatory response can also be used to detect angiogenic processes. Recently, CEU molecular imaging has been used to evaluate the paracrine effects of stem cell therapy in ischemic limb disease.25) These studies demonstrated that certain adult progenitor cells act through endothelial activation and recruitment of a specific population of pro-angiogenic monocytes. Although targeted MBs to VEGF receptors have been developed for tumor microvascular imaging, they have not been extensively used to examine ischemia-related remodeling.46)
Future Perspectives
Techniques for molecular imaging with ultrasound have been developed on the premise that these techniques will positively impact patient outcomes. They may do so by providing earlier diagnosis of disease, by providing a more definitive diagnosis of disease, or by guiding appropriate therapy by defining the active molecular processes in any given patients (so called "personalized treatment"). With these lofty goals in mind, most of the progress that has occurred in the field has been limited to the creation of new targeted MB agents and testing their performance in animal models of disease. The transition to using these agents in humans is predicated on several key steps. The most important step is to identify applications where molecular imaging will provide the most incremental benefit to existing care paradigms. For any CEU molecular imaging application, it is then necessary to identify the best ligand for selective targeting and to define the most appropriate conjugation chemistry for MB surface coupling with regards to safety and ligand density. Finally, after safety and proof of concept studies are performed, there needs to be confirmation that application of molecular imaging technology can substantially improve outcomes and/or reduce healthcare costs. Although we are far off from agency approval of any ultrasound-based molecular imaging agent, it is reassuring that even today CEU molecular imaging is having a positive impact through its use in cardiovascular research to discover new pathophysiologic processes and as a biologic readout to test new therapeutic agents.
Acknowledgments
Dr. Lindner is supported by grants R01-HL-078610 and R01-HL-111969 from the National Institutes of Health, Bethesda, Maryland.
Footnotes
The authors have no financial conflicts of interest.
References
- 1.Sinusas AJ, Bengel F, Nahrendorf M, et al. Multimodality cardiovascular molecular imaging, part I. Circ Cardiovasc Imaging. 2008;1:244–256. doi: 10.1161/CIRCIMAGING.108.824359. [DOI] [PubMed] [Google Scholar]
- 2.Inaba Y, Lindner JR. Molecular imaging of disease with targeted contrast ultrasound imaging. Transl Res. 2012;159:140–148. doi: 10.1016/j.trsl.2011.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gessner R, Dayton PA. Advances in molecular imaging with ultrasound. Mol Imaging. 2010;9:117–127. [PMC free article] [PubMed] [Google Scholar]
- 4.Lindner JR, Song J, Jayaweera AR, Sklenar J, Kaul S. Microvascular rheology of Definity microbubbles after intra-arterial and intravenous administration. J Am Soc Echocardiogr. 2002;15:396–403. doi: 10.1067/mje.2002.117290. [DOI] [PubMed] [Google Scholar]
- 5.Ross R. Atherosclerosis--an inflammatory disease. N Engl J Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 6.Cines DB, Pollak ES, Buck CA, et al. Endothelial cells in physiology and in the pathophysiology of vascular disorders. Blood. 1998;91:3527–3561. [PubMed] [Google Scholar]
- 7.Springer TA. Adhesion receptors of the immune system. Nature. 1990;346:425–434. doi: 10.1038/346425a0. [DOI] [PubMed] [Google Scholar]
- 8.Ley K. Molecular mechanisms of leukocyte recruitment in the inflammatory process. Cardiovasc Res. 1996;32:733–742. [PubMed] [Google Scholar]
- 9.Ley K. The role of selectins in inflammation and disease. Trends Mol Med. 2003;9:263–268. doi: 10.1016/s1471-4914(03)00071-6. [DOI] [PubMed] [Google Scholar]
- 10.Hamilton AJ, Huang SL, Warnick D, et al. Intravascular ultrasound molecular imaging of atheroma components in vivo. J Am Coll Cardiol. 2004;43:453–460. doi: 10.1016/j.jacc.2003.07.048. [DOI] [PubMed] [Google Scholar]
- 11.Lanza GM, Wallace KD, Scott MJ, et al. A novel site-targeted ultrasonic contrast agent with broad biomedical application. Circulation. 1996;94:3334–3340. doi: 10.1161/01.cir.94.12.3334. [DOI] [PubMed] [Google Scholar]
- 12.Lanza GM, Abendschein DR, Hall CS, et al. In vivo molecular imaging of stretch-induced tissue factor in carotid arteries with ligand-targeted nanoparticles. J Am Soc Echocardiogr. 2000;13:608–614. doi: 10.1067/mje.2000.105840. [DOI] [PubMed] [Google Scholar]
- 13.Bassenge E. Antiplatelet effects of endothelium-derived relaxing factor and nitric oxide donors. Eur Heart J. 1991;12(Suppl E):12–15. doi: 10.1093/eurheartj/12.suppl_e.12. [DOI] [PubMed] [Google Scholar]
- 14.Davignon J, Ganz P. Role of endothelial dysfunction in atherosclerosis. Circulation. 2004;109(23 Suppl 1):III27–III32. doi: 10.1161/01.CIR.0000131515.03336.f8. [DOI] [PubMed] [Google Scholar]
- 15.Alonso A, Della Martina A, Stroick M, et al. Molecular imaging of human thrombus with novel abciximab immunobubbles and ultrasound. Stroke. 2007;38:1508–1514. doi: 10.1161/STROKEAHA.106.471391. [DOI] [PubMed] [Google Scholar]
- 16.Wang X, Hagemeyer CE, Hohmann JD, et al. Novel single-chain antibody-targeted microbubbles for molecular ultrasound imaging of thrombosis: validation of a unique noninvasive method for rapid and sensitive detection of thrombi and monitoring of success or failure of thrombolysis in mice. Circulation. 2012;125:3117–3126. doi: 10.1161/CIRCULATIONAHA.111.030312. [DOI] [PubMed] [Google Scholar]
- 17.Ruggeri ZM. Von Willebrand factor, platelets and endothelial cell interactions. J Thromb Haemost. 2003;1:1335–1342. doi: 10.1046/j.1538-7836.2003.00260.x. [DOI] [PubMed] [Google Scholar]
- 18.Liu Y, Davidson BP, Yue Q, et al. Molecular imaging of inflammation and platelet adhesion in advanced atherosclerosis effects of antioxidant therapy with NADPH oxidase inhibition. Circ Cardiovasc Imaging. 2013;6:74–82. doi: 10.1161/CIRCIMAGING.112.975193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McCarty OJ, Conley RB, Shentu W, et al. Molecular imaging of activated von Willebrand factor to detect high-risk atherosclerotic phenotype. JACC Cardiovasc Imaging. 2010;3:947–955. doi: 10.1016/j.jcmg.2010.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leong-Poi H, Christiansen J, Klibanov AL, Kaul S, Lindner JR. Noninvasive assessment of angiogenesis by ultrasound and microbubbles targeted to alpha(v)-integrins. Circulation. 2003;107:455–460. doi: 10.1161/01.cir.0000044916.05919.8b. [DOI] [PubMed] [Google Scholar]
- 21.Leong-Poi H, Christiansen J, Heppner P, et al. Assessment of endogenous and therapeutic arteriogenesis by contrast ultrasound molecular imaging of integrin expression. Circulation. 2005;111:3248–3254. doi: 10.1161/CIRCULATIONAHA.104.481515. [DOI] [PubMed] [Google Scholar]
- 22.Dayton PA, Pearson D, Clark J, et al. Ultrasonic analysis of peptide- and antibody-targeted microbubble contrast agents for molecular imaging of alphavbeta3-expressing cells. Mol Imaging. 2004;3:125–134. doi: 10.1162/1535350041464883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Palmowski M, Huppert J, Ladewig G, et al. Molecular profiling of angiogenesis with targeted ultrasound imaging: early assessment of antiangiogenic therapy effects. Mol Cancer Ther. 2008;7:101–109. doi: 10.1158/1535-7163.MCT-07-0409. [DOI] [PubMed] [Google Scholar]
- 24.Behm CZ, Kaufmann BA, Carr C, et al. Molecular imaging of endothelial vascular cell adhesion molecule-1 expression and inflammatory cell recruitment during vasculogenesis and ischemia-mediated arteriogenesis. Circulation. 2008;117:2902–2911. doi: 10.1161/CIRCULATIONAHA.107.744037. [DOI] [PubMed] [Google Scholar]
- 25.Ryu JC, Davidson BP, Xie A, et al. Molecular imaging of the paracrine proangiogenic effects of progenitor cell therapy in limb ischemia. Circulation. 2013;127:710–719. doi: 10.1161/CIRCULATIONAHA.112.116103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Winter PM, Morawski AM, Caruthers SD, et al. Molecular imaging of angiogenesis in early-stage atherosclerosis with alpha(v)beta3-integrin-targeted nanoparticles. Circulation. 2003;108:2270–2274. doi: 10.1161/01.CIR.0000093185.16083.95. [DOI] [PubMed] [Google Scholar]
- 27.Lindner JR. Microbubbles in medical imaging: current applications and future directions. Nat Rev Drug Discov. 2004;3:527–532. doi: 10.1038/nrd1417. [DOI] [PubMed] [Google Scholar]
- 28.Lindner JR, Coggins MP, Kaul S, Klibanov AL, Brandenburger GH, Ley K. Microbubble persistence in the microcirculation during ischemia/reperfusion and inflammation is caused by integrin- and complement-mediated adherence to activated leukocytes. Circulation. 2000;101:668–675. doi: 10.1161/01.cir.101.6.668. [DOI] [PubMed] [Google Scholar]
- 29.Lindner JR, Dayton PA, Coggins MP, et al. Noninvasive imaging of inflammation by ultrasound detection of phagocytosed microbubbles. Circulation. 2000;102:531–538. doi: 10.1161/01.cir.102.5.531. [DOI] [PubMed] [Google Scholar]
- 30.Lindner JR, Song J, Xu F, et al. Noninvasive ultrasound imaging of inflammation using microbubbles targeted to activated leukocytes. Circulation. 2000;102:2745–2750. doi: 10.1161/01.cir.102.22.2745. [DOI] [PubMed] [Google Scholar]
- 31.Behm CZ, Lindner JR. Cellular and molecular imaging with targeted contrast ultrasound. Ultrasound Q. 2006;22:67–72. [PubMed] [Google Scholar]
- 32.Quillard T, Libby P. Molecular imaging of atherosclerosis for improving diagnostic and therapeutic development. Circ Res. 2012;111:231–244. doi: 10.1161/CIRCRESAHA.112.268144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kaufmann BA, Carr CL, Belcik JT, et al. Molecular imaging of the initial inflammatory response in atherosclerosis: implications for early detection of disease. Arterioscler Thromb Vasc Biol. 2010;30:54–59. doi: 10.1161/ATVBAHA.109.196386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Villanueva FS, Jankowski RJ, Klibanov S, et al. Microbubbles targeted to intercellular adhesion molecule-1 bind to activated coronary artery endothelial cells. Circulation. 1998;98:1–5. doi: 10.1161/01.cir.98.1.1. [DOI] [PubMed] [Google Scholar]
- 35.Kaufmann BA, Sanders JM, Davis C, et al. Molecular imaging of inflammation in atherosclerosis with targeted ultrasound detection of vascular cell adhesion molecule-1. Circulation. 2007;116:276–284. doi: 10.1161/CIRCULATIONAHA.106.684738. [DOI] [PubMed] [Google Scholar]
- 36.Chadderdon SM, Belcik JT, Bader L, et al. Pro-Inflammatory Endothelial Activation Detected by Molecular Imaging in Obese Non-Human Primates Coincides with the Onset of Insulin Resistance and Progressively Increases with Duration of Insulin Resistance. Circulation. 2013 doi: 10.1161/CIRCULATIONAHA.113.003645. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Anderson DR, Tsutsui JM, Xie F, Radio SJ, Porter TR. The role of complement in the adherence of microbubbles to dysfunctional arterial endothelium and atherosclerotic plaque. Cardiovasc Res. 2007;73:597–606. doi: 10.1016/j.cardiores.2006.11.029. [DOI] [PubMed] [Google Scholar]
- 38.Xie F, Lof J, Matsunaga T, Zutshi R, Porter TR. Diagnostic ultrasound combined with glycoprotein IIb/IIIa-targeted microbubbles improves microvascular recovery after acute coronary thrombotic occlusions. Circulation. 2009;119:1378–1385. doi: 10.1161/CIRCULATIONAHA.108.825067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Davidson BP, Lindner JR. Future applications of contrast echocardiography. Heart. 2012;98:246–253. doi: 10.1136/heartjnl-2011-300737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Christiansen JP, French BA, Klibanov AL, Kaul S, Lindner JR. Targeted tissue transfection with ultrasound destruction of plasmid-bearing cationic microbubbles. Ultrasound Med Biol. 2003;29:1759–1767. doi: 10.1016/s0301-5629(03)00976-1. [DOI] [PubMed] [Google Scholar]
- 41.Davidson BP, Kaufmann BA, Belcik JT, Xie A, Qi Y, Lindner JR. Detection of antecedent myocardial ischemia with multiselectin molecular imaging. J Am Coll Cardiol. 2012;60:1690–1697. doi: 10.1016/j.jacc.2012.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Villanueva FS, Lu E, Bowry S, et al. Myocardial ischemic memory imaging with molecular echocardiography. Circulation. 2007;115:345–352. doi: 10.1161/CIRCULATIONAHA.106.633917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Leong-Poi H. Molecular imaging using contrast-enhanced ultrasound: evaluation of angiogenesis and cell therapy. Cardiovasc Res. 2009;84:190–200. doi: 10.1093/cvr/cvp248. [DOI] [PubMed] [Google Scholar]
- 44.Sang QX. Complex role of matrix metalloproteinases in angiogenesis. Cell Res. 1998;8:171–177. doi: 10.1038/cr.1998.17. [DOI] [PubMed] [Google Scholar]
- 45.Brooks PC, Clark RA, Cheresh DA. Requirement of vascular integrin alpha v beta 3 for angiogenesis. Science. 1994;264:569–571. doi: 10.1126/science.7512751. [DOI] [PubMed] [Google Scholar]
- 46.Pysz MA, Guracar I, Tian L, Willmann JK. Fast microbubble dwell-time based ultrasonic molecular imaging approach for quantification and monitoring of angiogenesis in cancer. Quant Imaging Med Surg. 2012;2:68–80. doi: 10.3978/j.issn.2223-4292.2012.06.05. [DOI] [PMC free article] [PubMed] [Google Scholar]