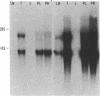
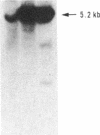
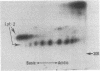
Abstract
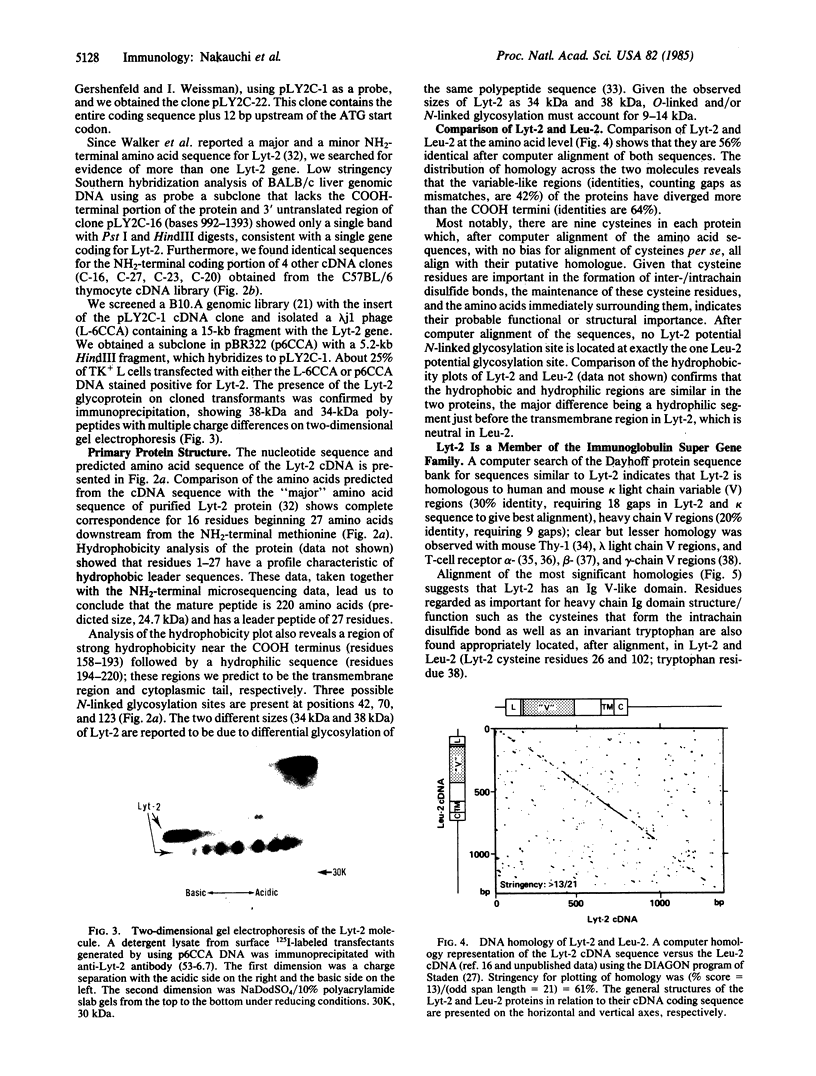
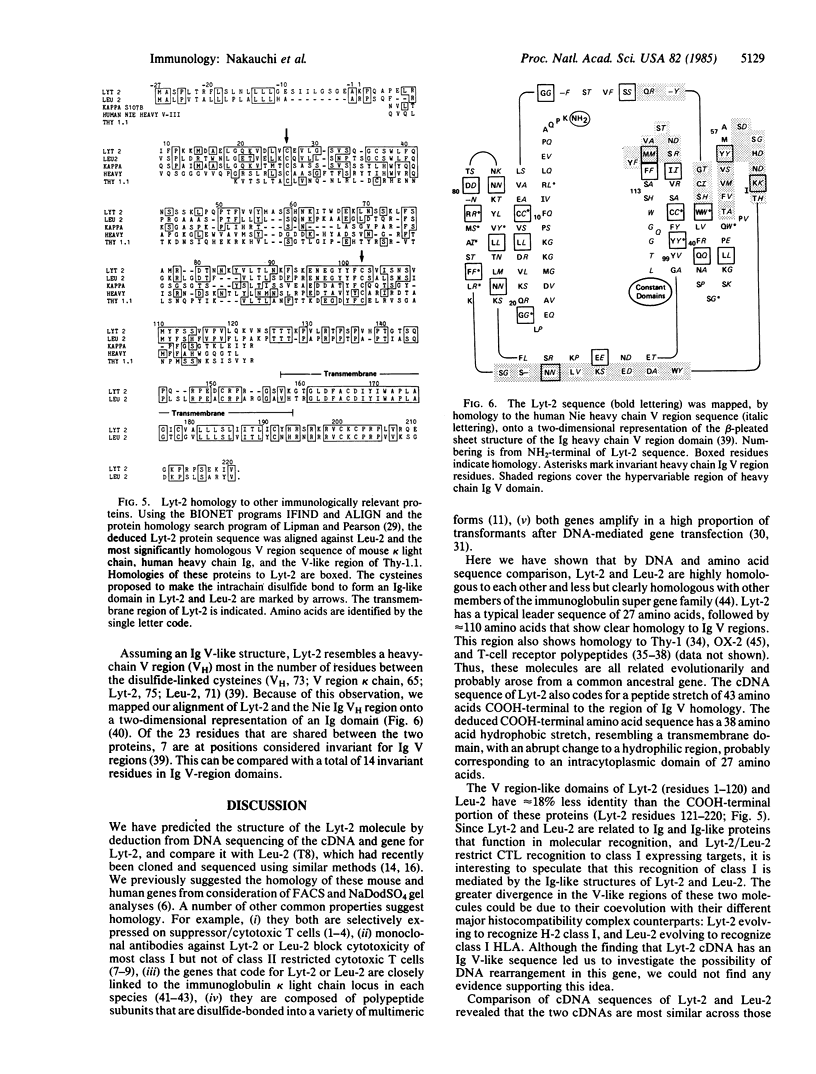
The sequence of Lyt-2 cDNA shows that it is a new member of the immunoglobulin super gene family. Analysis of the predicted amino acid sequence indicates that the Lyt-2 polypeptide is synthesized with a 27-amino acid leader, and that the mature protein has an immunoglobulin variable region (Ig V)-related sequence of approximately 100 amino acids, an extracellular spacer of 43, a transmembrane region of 38, and an intracytoplasmic region of 27 amino acids. Lyt-2 and its human analogue Leu-2 are 56% homologous; analysis indicates that the Ig V-related domains of the two molecules have evolved away from each other faster than the carboxyl-terminal half of the proteins.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cantor H., Boyse E. A. Functional subclasses of T-lymphocytes bearing different Ly antigens. I. The generation of functionally distinct T-cell subclasses is a differentiative process independent of antigen. J Exp Med. 1975 Jun 1;141(6):1376–1389. doi: 10.1084/jem.141.6.1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantor H., Boyse E. A. Lymphocytes as models for the study of mammalian cellular differentiation. Immunol Rev. 1977 Jan;33:105–124. doi: 10.1111/j.1600-065x.1977.tb00364.x. [DOI] [PubMed] [Google Scholar]
- Chien Y. H., Gascoigne N. R., Kavaler J., Lee N. E., Davis M. M. Somatic recombination in a murine T-cell receptor gene. Nature. 1984 May 24;309(5966):322–326. doi: 10.1038/309322a0. [DOI] [PubMed] [Google Scholar]
- Chien Y., Becker D. M., Lindsten T., Okamura M., Cohen D. I., Davis M. M. A third type of murine T-cell receptor gene. Nature. 1984 Nov 1;312(5989):31–35. doi: 10.1038/312031a0. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Clark M. J., Gagnon J., Williams A. F., Barclay A. N. MRC OX-2 antigen: a lymphoid/neuronal membrane glycoprotein with a structure like a single immunoglobulin light chain. EMBO J. 1985 Jan;4(1):113–118. doi: 10.1002/j.1460-2075.1985.tb02324.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dialynas D. P., Loken M. R., Glasebrook A. L., Fitch F. W. Lyt-2-/Lyt 3- variants of a cloned cytolytic T cell line lack an antigen receptor functional in cytolysis. J Exp Med. 1981 Mar 1;153(3):595–604. doi: 10.1084/jem.153.3.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans R. L., Wall D. W., Platsoucas C. D., Siegal F. P., Fikrig S. M., Testa C. M., Good R. A. Thymus-dependent membrane antigens in man: inhibition of cell-mediated lympholysis by monoclonal antibodies to TH2 antigen. Proc Natl Acad Sci U S A. 1981 Jan;78(1):544–548. doi: 10.1073/pnas.78.1.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottlieb P. D. Genetic correlation of a mouse light chain variable region marker with a thymocyte surface antigen. J Exp Med. 1974 Nov 1;140(5):1432–1437. doi: 10.1084/jem.140.5.1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedrick S. M., Cohen D. I., Nielsen E. A., Davis M. M. Isolation of cDNA clones encoding T cell-specific membrane-associated proteins. Nature. 1984 Mar 8;308(5955):149–153. doi: 10.1038/308149a0. [DOI] [PubMed] [Google Scholar]
- Kavathas P., Herzenberg L. A. Amplification of a gene coding for human T-cell differentiation antigen. Nature. 1983 Nov 24;306(5941):385–387. doi: 10.1038/306385a0. [DOI] [PubMed] [Google Scholar]
- Kavathas P., Herzenberg L. A. Stable transformation of mouse L cells for human membrane T-cell differentiation antigens, HLA and beta 2-microglobulin: selection by fluorescence-activated cell sorting. Proc Natl Acad Sci U S A. 1983 Jan;80(2):524–528. doi: 10.1073/pnas.80.2.524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavathas P., Sukhatme V. P., Herzenberg L. A., Parnes J. R. Isolation of the gene encoding the human T-lymphocyte differentiation antigen Leu-2 (T8) by gene transfer and cDNA subtraction. Proc Natl Acad Sci U S A. 1984 Dec;81(24):7688–7692. doi: 10.1073/pnas.81.24.7688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landegren U., Ramstedt U., Axberg I., Ullberg M., Jondal M., Wigzell H. Selective inhibition of human T cell cytotoxicity at levels of target recognition or initiation of lysis by monoclonal OKT3 and Leu-2a antibodies. J Exp Med. 1982 May 1;155(5):1579–1584. doi: 10.1084/jem.155.5.1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledbetter J. A., Evans R. L., Lipinski M., Cunningham-Rundles C., Good R. A., Herzenberg L. A. Evolutionary conservation of surface molecules that distinguish T lymphocyte helper/inducer and cytotoxic/suppressor subpopulations in mouse and man. J Exp Med. 1981 Feb 1;153(2):310–323. doi: 10.1084/jem.153.2.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledbetter J. A., Herzenberg L. A. Xenogeneic monoclonal antibodies to mouse lymphoid differentiation antigens. Immunol Rev. 1979;47:63–90. doi: 10.1111/j.1600-065x.1979.tb00289.x. [DOI] [PubMed] [Google Scholar]
- Ledbetter J. A., Seaman W. E., Tsu T. T., Herzenberg L. A. Lyt-2 and lyt-3 antigens are on two different polypeptide subunits linked by disulfide bonds. Relationship of subunits to T cell cytolytic activity. J Exp Med. 1981 Jun 1;153(6):1503–1516. doi: 10.1084/jem.153.6.1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipman D. J., Pearson W. R. Rapid and sensitive protein similarity searches. Science. 1985 Mar 22;227(4693):1435–1441. doi: 10.1126/science.2983426. [DOI] [PubMed] [Google Scholar]
- Littman D. R., Thomas Y., Maddon P. J., Chess L., Axel R. The isolation and sequence of the gene encoding T8: a molecule defining functional classes of T lymphocytes. Cell. 1985 Feb;40(2):237–246. doi: 10.1016/0092-8674(85)90138-2. [DOI] [PubMed] [Google Scholar]
- Naim H. Y., Luescher B., Corradin G., Bron C. The mouse Lyt-2/3 antigen complex--II. Structural analysis of the subunits. Mol Immunol. 1984 Apr;21(4):337–341. doi: 10.1016/0161-5890(84)90105-6. [DOI] [PubMed] [Google Scholar]
- Nakayama E., Shiku H., Stockert E., Oettgen H. F., Old L. J. Cytotoxic T cells: Lyt phenotype and blocking of killing activity by Lyt antisera. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1977–1981. doi: 10.1073/pnas.76.4.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poljak R. J., Amzel L. M., Chen B. L., Phizackerley R. P., Saul F. The three-dimensional structure of the fab' fragment of a human myeloma immunoglobulin at 2.0-angstrom resolution. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3440–3444. doi: 10.1073/pnas.71.9.3440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinherz E. L., Schlossman S. F. The differentiation and function of human T lymphocytes. Cell. 1980 Apr;19(4):821–827. doi: 10.1016/0092-8674(80)90072-0. [DOI] [PubMed] [Google Scholar]
- Saito H., Kranz D. M., Takagaki Y., Hayday A. C., Eisen H. N., Tonegawa S. A third rearranged and expressed gene in a clone of cytotoxic T lymphocytes. Nature. 1984 Nov 1;312(5989):36–40. doi: 10.1038/312036a0. [DOI] [PubMed] [Google Scholar]
- Saito H., Kranz D. M., Takagaki Y., Hayday A. C., Eisen H. N., Tonegawa S. Complete primary structure of a heterodimeric T-cell receptor deduced from cDNA sequences. 1984 Jun 28-Jul 4Nature. 309(5971):757–762. doi: 10.1038/309757a0. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinohara N., Sachs D. H. Mouse alloantibodies capable of blocking cytotoxic T-cell function. I. Relationship between the antigen reactive with blocking antibodies and the Lyt-2 locus. J Exp Med. 1979 Sep 19;150(3):432–444. doi: 10.1084/jem.150.3.432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snow P., Spits H., De Vries J., Terhorst C. Comparison of target antigens of monoclonal reagents OKT5, OKT8, and Leu2A, which inhibit effector function of human cytotoxic T lymphocytes. Hybridoma. 1983;2(2):187–199. doi: 10.1089/hyb.1983.2.187. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Staden R. An interactive graphics program for comparing and aligning nucleic acid and amino acid sequences. Nucleic Acids Res. 1982 May 11;10(9):2951–2961. doi: 10.1093/nar/10.9.2951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staden R. Automation of the computer handling of gel reading data produced by the shotgun method of DNA sequencing. Nucleic Acids Res. 1982 Aug 11;10(15):4731–4751. doi: 10.1093/nar/10.15.4731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staden R., McLachlan A. D. Codon preference and its use in identifying protein coding regions in long DNA sequences. Nucleic Acids Res. 1982 Jan 11;10(1):141–156. doi: 10.1093/nar/10.1.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sukhatme V. P., Vollmer A. C., Erikson J., Isobe M., Croce C., Parnes J. R. Gene for the human T cell differentiation antigen Leu-2/T8 is closely linked to the kappa light chain locus on chromosome 2. J Exp Med. 1985 Feb 1;161(2):429–434. doi: 10.1084/jem.161.2.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker I. D., Hogarth P. M., Murray B. J., Lovering K. E., Classon B. J., Chambers G. W., McKenzie I. F. Ly antigens associated with T cell recognition and effector function. Immunol Rev. 1984 Dec;82:47–77. doi: 10.1111/j.1600-065x.1984.tb01117.x. [DOI] [PubMed] [Google Scholar]
- Wigler M., Sweet R., Sim G. K., Wold B., Pellicer A., Lacy E., Maniatis T., Silverstein S., Axel R. Transformation of mammalian cells with genes from procaryotes and eucaryotes. Cell. 1979 Apr;16(4):777–785. doi: 10.1016/0092-8674(79)90093-x. [DOI] [PubMed] [Google Scholar]
- Williams A. F., Gagnon J. Neuronal cell Thy-1 glycoprotein: homology with immunoglobulin. Science. 1982 May 14;216(4547):696–703. doi: 10.1126/science.6177036. [DOI] [PubMed] [Google Scholar]
- Williams A. F. The immunoglobulin superfamily takes shape. Nature. 1984 Mar 1;308(5954):12–13. doi: 10.1038/308012a0. [DOI] [PubMed] [Google Scholar]