Abstract
The timing of perceptual events depends on an anatomically and functionally connected network comprising basal ganglia, cerebellum, pre-frontal cortex and supplementary motor area. Recent studies demonstrate the cerebellum to be involved in absolute, duration-based timing, but not in relative timing based on a regular beat. Conversely, functional involvement of the striatum is observed in relative timing, but its role in absolute timing is unclear.
This work tests the specific role of the basal ganglia in the perceptual timing of auditory events. It aims to distinguish the hypothesised unified model of time perception (Teki, Grube, & Griffiths, 2012), in which the striatum is a mandatory component for all timing tasks, from a modular system in which they subserve relative timing, with absolute timing processed by the cerebellum.
Test groups comprised individuals with Multiple System Atrophy, a disorder in which similar pathology can produce clinical deficits associated with dysfunction of the cerebellum (MSA-C, n=8) or striatum (MSA-P, n=10), and early symptomatic Huntington's disease (HD, n=14). Individuals with chronic autoimmune peripheral neuropathy (n=11) acted as controls.
Six adaptive tasks were carried out to assess perceptual thresholds for absolute timing through duration discrimination for sub- and supra-second time intervals, and relative timing through the detection of beat-based regularity and irregularity, detection of a delay within an isochronous sequence, and the discrimination of sequences with metrical structure.
All three patient groups exhibited impairments in performance in comparison with the control group for all tasks, and severity of impairment was significantly correlated with disease progression. No differences were demonstrated between MSA-C and MSA-P, and the most severe impairments were observed in those with HD.
The data support an obligatory role for the basal ganglia in all tested timing tasks, both absolute and relative, as predicted by the unified model. The results are not compatible with models of a brain timing network based upon independent modules.
Keywords: Perceptual timing, Beat, Basal ganglia, Multiple System Atrophy, Huntington's disease
Graphical abstract
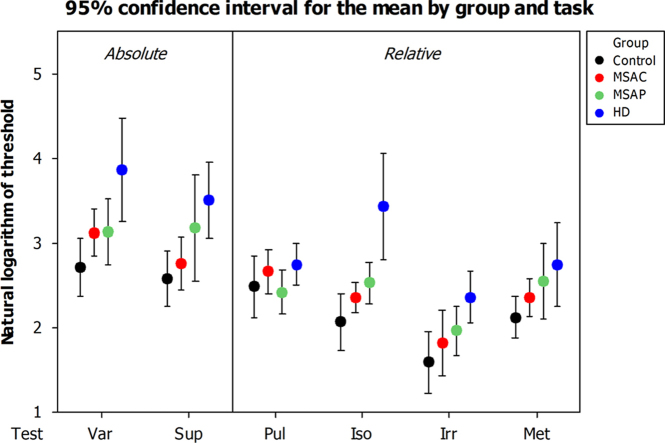
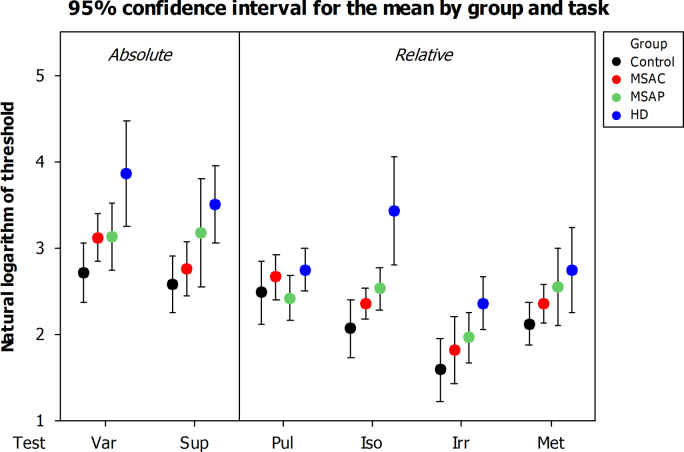
Ninety five percent confidence intervals for mean group performance by task. Var: sub-second variable-interval discrimination. Sup: supra-second variable-interval discrimination. Pul: detection of regularity (pulse or beat) within an irregular sequence. Iso: detection of deviation from isochrony. Irr: detection of irregularity within a regular sequence. Met: detection of distortion of a sequence with strong metrical structure.
Highlights
-
•
Patients with basal ganglia disease undertook a battery of perceptual timing tasks.
-
•
All patients displayed poorer performance than neurological control participants.
-
•
Performance in Huntington's disease was worse than Multiple System Atrophy.
-
•
Poorer performance was significantly correlated with disease progression.
-
•
These findings support the hypothesised unified model of time perception.
1. Introduction
The human auditory system must encode temporal features over six orders of magnitude, from the auditory information at a sub-millisecond level that subserves source localisation and pitch processing, to the level of seconds and tens of seconds for the processing of sentences and ‘streams’ of sounds (Bregman, 1990, Mauk and Buonomano, 2004).
It is generally agreed that the neuronal representation of timing information at and beyond the level of hundreds of milliseconds relies on at least one central mechanism, which is often described as an internal clock (Ivry and Schlerf, 2008, Treisman, 1963). Brain areas implicated in this mechanism include the cerebellum (Ivry, 1993, Ivry and Keele, 1989), basal ganglia (Gibbon et al., 1997, Grahn and Brett, 2007, Grahn and McAuley, 2009, Meck and Benson, 2002), supplementary motor area (Halsband et al., 1993, Macar et al., 2004, Schwartze et al., 2012) and pre-frontal cortex (Lewis and Miall, 2003, Lewis and Miall, 2006, Oshio, 2011, Oshio et al., 2008). These areas are anatomically linked (Akkal et al., 2007, Bostan et al., 2010, Bostan and Strick, 2010, Hoshi et al., 2005, Jurgens, 1984) and, during timing tasks, functionally interconnected (Chen et al., 2006, Grahn and Rowe, 2009).
While there are undoubtedly further complexities, and room for sub-division, in recent years it has become evident that there is a difference in the neural substrate responsible for the processing of absolute time, where the durations of single intervals are processed, and relative time, where comparative judgments of duration are made based on a regular beat (Keele et al., 1989, McAuley and Jones, 2003, Teki et al., 2011). In particular, there is an accumulating body of evidence that the cerebellum is required for the perceptual processing of absolute but not relative time; patients with spinocerebellar ataxia type 6, a stereotyped cerebellar degeneration, were specifically impaired relative to controls when asked to compare the absolute duration of discrete intervals (Grube, Cooper, Chinnery, & Griffiths, 2010). This impairment was especially marked when interval duration was roved between trials to minimise overall temporal context, and was absent when a regular beat was present for relative comparison. Congruent results have been obtained in a cohort of neurologically normal subjects in whom transcranial magnetic stimulation was applied to the medial cerebellum (Grube, Lee, Griffiths, Barker, & Woodruff, 2010).
A number of studies have demonstrated that perceptual timing performance in neurologically normal individuals is superior when relative, beat-based, information is present (Essens and Povel, 1985, Grube et al., 2010, Grube and Griffiths, 2009), and that the presence of beat-based information facilitates the detection of change in other auditory domains (Geiser, Notter, & Gabrieli, 2012). In functional imaging studies the basal ganglia are strongly implicated as a basis for processing relative time (Geiser et al., 2012, Grahn and Brett, 2007, Grahn and McAuley, 2009, Teki et al., 2011). A physiological mechanism for this function is suggested by the Striatal Beat Frequency model, in which a timing signal is generated by a striato-thalamo-cortical circuit (Meck et al., 2008, Oprisan and Buhusi, 2011). It is proposed that the medium spiny neurons in the dorsal striatum act as coincidence detectors for oscillatory activity (Matell and Meck, 2004, Miall, 1989) and that the periodicity of the oscillator is regulated by dopaminergic input from the substantia nigra (Allman and Meck, 2012, Buhusi and Meck, 2005, Oprisan and Buhusi, 2011).
The immediate question raised by accounts of separable neural bases for absolute and relative timing analysis is the way in which these mechanisms relate to each other. There are two distinct theories. In the first, absolute and relative time are processed by separate and independent neural modules (in the cerebellum and basal ganglia respectively), with the more precisely encoded relative mechanism taking precedence when it is available (Cope et al., 2011, Teki et al., 2011). In the second, these neural modules are not functionally independent but part of an integrated system, as elaborated in the hypothesised unified model of time perception (Meck, 2005, Teki et al., 2012). This model proposes that time intervals are encoded as a combination of multiples of a duration that is determined by the oscillatory period of the Striatal Beat Frequency, and an error-correction signal representing deviation away from this. It is hypothesised that the oscillatory period is modulated by recent temporal context, and entrains to an externally provided beat with increasing precision as more time intervals are provided, enabling this model to account for “multiple-look” models of temporal sensitivity (Drake and Botte, 1993, Miller and McAuley, 2005).
These two models make differing predictions about the effect of basal ganglia dysfunction in perceptual timing. If there are indeed two separable mechanisms, basal ganglia pathology would result in poorer performance for relative but not absolute timing tasks. If, on the other hand, the hypothesised unified model of time perception is correct, basal ganglia pathology that decreased the precision of the Striatal Beat Frequency oscillation would result in poorer performance for both relative and absolute timing tasks.
The majority of previous neuropsychological studies of the basal ganglia's role in time perception have used Parkinson's disease as a model (see Allman and Meck, 2012, Jones and Jahanshahi, in press for recent detailed reviews). Although there is generalised neuronal degeneration, the dysfunction in Parkinson's disease is not primarily degeneration of the striatum, but rather an uneven loss of its dopaminergic input (Kish, Shannak, & Hornykiewicz, 1988).
This study aims to measure the perceptual abilities of patients with intrinsic basal ganglia dysfunction on tasks designed to test specific aspects of absolute and relative timing. Rather than a primary loss of dopaminergic input, as in Parkinson's disease, our patient groups had either predominant degeneration of the striatum (Huntington's disease) or combined degeneration of the striatum, substantia nigra and cerebellum (Multiple System Atrophy). We would predict that, if absolute and relative timing are subserved by separate neurological modules, patients with predominantly striatal pathology would be impaired only on tasks of relative timing, and consequently the statistical difference demonstrated by our paradigm would be an interaction between patient group and perceptual test. If, on the other hand, the hypothesised unified model of time perception is correct, and timing relies on a Striatal Beat Frequency oscillation with cerebellar error correction, we would predict that these patients would be impaired at both absolute and relative timing tasks, and consequently the statistical difference demonstrated by our paradigm would be a main effect of patient group.
2. Patient groups
Three groups of patient volunteers were recruited.
Multiple System Atrophy Syndrome (MSA) is a rare, degenerative neurological disorder characterised by glial cytoplasmic inclusions with associated cell loss and gliosis, primarily of the caudate, putamen, globus pallidus, substantia nigra, Purkinje cells, locus coeruleus and pons (Papp, Kahn, & Lantos, 1989). As well as by autonomic dysfunction, the condition is characterised by ataxia and Parkinsonism, with patients clinically classified as having Multiple System Atrophy with Cerebellar features (MSA-C) or Multiple System Atrophy with Parkinsonian features (MSA-P), depending on which features are predominant in the early stage. Of note, however, patients of either clinical subtype frequently have degeneration of both the cerebellum and basal ganglia (Burn & Jaros, 2001).
Huntington's disease (HD) is a genetic neurodegenerative disorder caused by expansion of a CAG triplet repeat within the Huntingtin gene located on the short arm of chromosome 4 (See Walker (2007) for a recent clinical review). It is characterised by prominent cell loss with consequent atrophy of the caudate and putamen, with a particular predominance for the medium spiny neurons implicated in the Striatal Beat Frequency model (Graveland et al., 1985, Vonsattel and DiFiglia, 1998). Individuals carrying the HD gene can become symptomatic at any age, and early basal ganglia degeneration predates clinical manifestation (Albin et al., 1992, Aylward et al., 1996, Thieben et al., 2002). Clinical features comprise psychiatric and behavioural disorders (most commonly depression and anxiety), cognitive dysfunction (especially of executive function) and motor features (typically chorea and an inability to sustain movements).
Control patients had long-term peripheral neuropathies, specifically chronic inflammatory demyelinating polyneuropathy (CIDP) and multifocal motor neuropathy with conduction block (MFMN), and were receiving regular infusions of intravenous immunoglobulin in hospital. These conditions are neurological diseases that affect peripheral nerves but spare the central nervous system. In previous studies we have recruited an age-matched, neurologically-normal control population through open advertisement but in this study, in which it was foreseen that patients might be globally impaired, we felt that we needed to match our control population not only in terms of IQ and educational achievement, but also in exposure to medical services and motivation to participate in the study to ensure that no alternate explanation for poorer performance could be implicated.
3. Methods
Approval for study conduct was gained from the local research ethics committee and the governance boards of all recruiting hospitals. All subjects provided informed consent for participation.
3.1. Subjects
Nineteen patients with Multiple System Atrophy were initially recruited, comprising 10 subjects with a clinical diagnosis of MSA-P and 9 with MSA-C. In all patients MSA was diagnosed by a consultant neurologist; 9 of the 10 patients with MSA-P and 6 of the 9 patients with MSA-C met second-consensus criteria for ‘Probable’ MSA, with the remaining 4 patients being classified by these criteria as ‘Possible’ MSA (Gilman et al. 2008). One patient who was initially recruited with MSA-C had significant concomitant cognitive impairment (Addenbrooke's Cognitive Examination – Revised edition score 61 (56 excluding fluency component)). He displayed extremely poor and highly variable performance to the extent that the researcher was not completely confident that he had understood the task requirements. This patient's data were therefore excluded from analysis.
Fourteen patients with Huntington's disease were recruited by their usual consultant from a larger recruitment pool, with the stated aim of covering a range of severity of motor disease with as little subjective cognitive involvement as possible. Unified Huntington's Disease Rating Scale Motor (UHDRS-M (Huntington Study Group, 1996)) component scores ranged from 0 to 44 out of 120, with a mean of 29 and standard deviation of 13. Thirteen of the 14 patients had genetic confirmation of diagnosis. The final patient had a family history of the disease and a typical presentation; he was accepting of the diagnosis but declined genetic testing. The group included one 43 year-old, pre-symptomatic patient with a pre-clinical genetic diagnosis.
Eleven control patients with peripheral neuropathies were recruited.
Descriptive statistics for the four groups are shown in Table 1. The control group was well matched to the MSA groups in all domains. Patients with Huntington's disease spanned a much wider age range (20–75), and were younger on average. They had more years of education, but performed less well on general cognitive testing. They also had a lower and more variable premorbid full-scale IQ as estimated by the Wechsler Test of Adult Reading, but patients with early stage Huntington's disease are known to perform more poorly than would be expected on standardised reading tests so it is likely that this difference is spurious (Crawford, Parker, & Besson, 1988).
Table 1.
Descriptive statistics for subject groups. Mean values are given with standard deviations in brackets. ACE-R denotes Addenbrooke's Cognitive Examination – Revised edition. As disorders of motor control can impair and slow speech, the fluency component of this examination was discarded to allow comparison between groups. The maximum achievable score was therefore 86. Premorbid Full-scale IQ was estimated by the Wechsler Test of Adult Reading.
|
Descriptive statistics for subject groups | ||||||||
|---|---|---|---|---|---|---|---|---|
| Group | Group size | Age | Age leaving education | Forwards digit span | Backwards digit span | ACE-R without fluency | Estimated premorbid full-scale IQ | Years since symptom onset |
| Control | 11 | 60 (8) | 17 (2.4) | 12 (2) | 8 (2) | 82 (3) | 105 (5) | N/A |
| MSA-P | 10 | 66 (8) | 16 (2.5) | 10 (2) | 6 (2) | 83 (2) | 105 (8) | 6 (3) |
| MSA-C | 8 | 68 (5) | 16 (0.8) | 10 (2) | 6 (2) | 78 (5) | 103 (7) | 4 (2) |
| HD | 14 | 47 (16) | 18 (3.5) | 9 (2) | 5 (2) | 74 (7) | 96 (12) | 3 (3) |
3.2. Stimuli
All stimuli were constructed from pure tones of 100 ms duration including 20 ms raised cosine onset and offset ramps, presented at 300 Hz and at a default volume of 75 dB SPL. If patients reported difficulty in hearing the stimuli clearly or found them uncomfortably loud, they were allowed to adjust the volume of stimulus presentation to their own preference. Stimuli were generated digitally in real time using MATLAB version 7.11 (R2010b) and presented diotically through external Edirol UA-4X soundcards and Sennheiser HD 250 headphones. Subjects were tested individually in a quiet room.
3.3. Procedure
Performance data were recorded using a paradigm that measured perceptual ability independently of any deficit in motor response with unlimited response time. A two alternative forced-choice paradigm was employed with an adaptive 2-down 1-up tracking algorithm (see Supplementary Fig. 1 for a typical trace from a subject with HD, demonstrating a reliable threshold measurement despite poor performance). While this paradigm is known to be slightly less efficient in the estimation of threshold than alternatives involving a three alternative forced-choice or 3-down 1-up tracking algorithms (Kollmeier et al., 1988, Leek, 2001), this consideration is outweighed in this study by the reduced working memory requirement when one less interval is compared and the reduction in minimum run length with the simpler adaptive algorithm. Each test comprised 48 trials, and was preceded by a minimum of 5 practice trials until the subject demonstrated consistent performance and was comfortable with the demands of the task. Each trial contained one target and one reference stimulus in a fixed randomised order and separated by an inter-stimulus interval of 1500 ms, and the subject was asked to indicate whether the target was first or second. Subjects indicated their choice either by using a keyboard, a custom-built button box, or by pointing to a large number, printed within a circle on a piece of paper. Once the response had been indicated, feedback (correct or incorrect) was given and the next trial followed after an inter-trial interval of 1500 ms. The difference between target and reference was initially easily discernible, decreased after two consecutive correct responses and increased after each incorrect one. A larger step size was used up to the fourth turn-point (change between increase and decrease) and after that a smaller one. Thresholds were calculated as the average of the final six turn-points. This allowed the estimation of the 70.7% correct point on the psychometric function (Levitt, 1971). Each test was performed twice. If the estimated thresholds between two tests runs were within the sum of their standard deviations, overall performance was calculated as the mean of the two measured thresholds. Otherwise, a third test run was performed and overall performance was calculated as the mean of the two better threshold measurements.
3.3.1. Tasks of absolute, duration-based timing
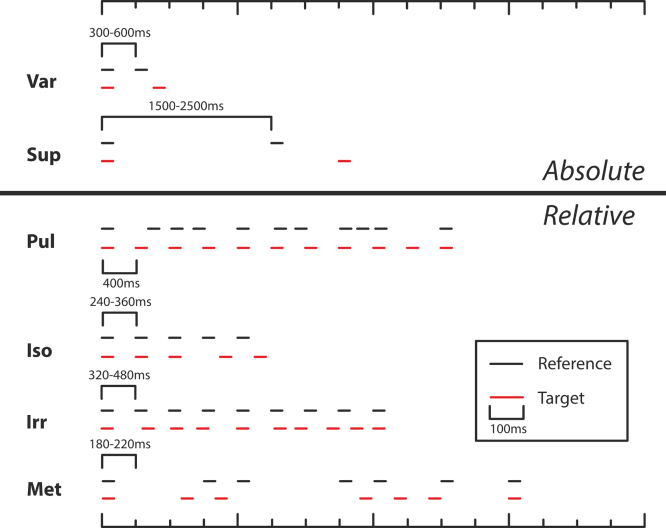
Variable interval timing (Var) (Fig. 1a): in this task subjects were presented with two inter-onset intervals (IOI) in the subsecond range, delineated by pairs of tones and asked to discriminate the longer target from the shorter reference. The reference IOI was varied from 300 to 600 ms in 60 ms steps, with all durations occurring equally frequently in each test run and in pseudorandomised order, fixed across subjects. This task was based upon that used by Grube, Cooper et al. (2010), but differed in that the IOI rather than the silent interval between tones was adaptively adjusted. During practice runs the target IOI was 70% longer than the reference IOI. This difference was then adaptively adjusted by the paradigm in steps of 10% and then 5% of the reference IOI. If subjects found this difference difficult to distinguish, this difference was increased to 140% then 210% until consistent performance and subjective comfort were demonstrated. In these cases, step sizes were proportionally increased to 20%/10% and 30%/15%.
Fig. 1.
Schematic illustration of stimuli for the tasks of perceptual timing. Horizontal lines depict tones (300 Hz, 100 ms). In each case the reference stimulus is shown along with the initial target stimulus. As tasks progressed, the target stimulus was adaptively controlled to be increasingly similar to the reference stimulus, dependant on participant performance. Var: sub-second variable-interval discrimination. Sup: supra-second variable-interval discrimination. Pul: detection of regularity (pulse or beat) within an irregular sequence. Iso: detection of deviation from isochrony. Irr: detection of irregularity within a regular sequence. Met: detection of distortion of a sequence with strong metrical structure.
Suprasecond variable interval timing (Sup) (Fig. 1b): this task was identical to task Var except that the reference IOI was in the suprasecond range; varying from 1500 ms to 2500 ms in 200 ms steps.
3.3.2. Tasks of relative, beat-based timing
Pulse, or regularity, detection (Pul) (Fig. 1c): in this task subjects were presented with an irregular reference sequence of 11 tones and asked to detect a target sequence displaying a greater degree of regularity. The reference was based upon an isochronous sequence with an IOI of 400 ms, but each inter-onset interval was randomly shortened or lengthened by a random amount between 15%, and 45%, resulting in an average jitter of 30% that rendered the underlying regular beat imperceptible (Madison & Merker, 2002). During practice runs the target was isochronous, and average jitter was adaptively introduced in steps of 4% and then 2.5%. This task was based upon task Reg in Grube, Cooper et al. (2010), but with additional constraints placed upon randomisation: the total amount of jitter could never be larger in the target than the reference and was always between 80% and 120% of the intended jitter difference; sequences could not contain more than three shortened or lengthened intervals in a row, or be comprised of primarily shortened or lengthened intervals.
Detection of deviation from isochrony (Iso) (Fig. 1d): in this task subjects were presented with a reference isochronous sequence comprising five tones with an IOI of 300 ms ±20% and asked to detect a target sequence in which the third IOI was lengthened. This task was based upon that used by Grube, Lee et al. (2010), but differed in that the periodicity of the isochronous sequence was randomly roved from 240 ms to 360 ms and the duration of the IOI rather than the silent interval between tones was adaptively adjusted. The initial difference in the length of the target sequence IOI was 20%, and this was adjusted in steps of 2% and then 0.67%. If subjects found this difference difficult to distinguish, this difference was increased up to a maximum of 100% in 20% increments until consistent performance and subjective comfort were demonstrated. In these cases, step sizes were increased proportionally.
Detection of irregularity (Irr) (Fig. 1f): this task was similar to Pul except that subjects were asked to distinguish a completely isochronous reference sequence from a target with a varying amount of jitter. The reference sequence had a fixed IOI of 400 ms ±20%, and comprised 9 tones. The target began with 20% jitter, at which level anisochrony was easily detected, and this was adjusted in step sizes of 3% and then 1%.
Detection of deviation from a metrical pattern (Met) (Fig. 1e): in this task subjects were presented with a rhythmic sequence of 7 tones with an underlying metrical beat based on two levels of periodicity (Grube & Griffiths, 2009). The lower-level periodicity was randomly roved between 180 and 220 ms in 4 ms steps. They were then asked to distinguish a reference, which differed in periodicity from the initial sequence but had an identical metrical structure, from the target, which differed in periodicity and was disrupted in metrical structure. This disruption comprised a lengthening or shortening of the duration of medium and long duration silent intervals such that the length of these intervals was no longer a multiple of the underlying lower-level periodicity. This task was based upon that used by Grube, Cooper et al. (2010), but again differed in that duration of the IOI rather than the silent interval between tones was adaptively adjusted. The initial distortion was 65%, and this was adaptively adjusted in steps of 12% and 6%.
3.4. Order of testing
Tasks were performed by all participants in the following stereotyped order: Var, Pul, Iso, Irr, Sup, Met. Frequent breaks were provided between tasks, and at the half-way stage cognitive testing was undertaken. This testing comprised the Wechsler Test of Adult Reading, an assessment of forwards and backwards digit span, and a Revised Addenbrooke's Cognitive Examination (Mioshi, Dawson, Mitchell, Arnold, & Hodges, 2006), from which the fluency component was discarded due to its lack of utility in individuals with Parkinsonian symptoms or disturbances of speech. The HD group undertook additional tests known to be sensitive to early cognitive decline in Huntington's disease, namely the Stroop colour-word test (Stroop, 1935), symbol digit substitution (Smith, 1982), verbal category and alphabetical fluency, and trail making A and B (Reitan, 1958).
3.5. Statistical analysis
Distribution of the data was assessed in IBM SPSS Version 19 by the Kolmogorov–Smirnov test for normality with Lillefors significance correction. Data for both the control and MSA groups strongly violated the assumption of normality in their raw form, but were normally distributed when their natural logarithms were analysed. Data from the HD group continued to violate the assumption of normality as natural logarithms, but the test statistic was reduced from 0.270 to 0.113. Mauchly's test of sphericity was employed to test the overall suitability of data for the application of the general linear model. Raw data violated the assumption of equal variances, but the natural logarithms of data did not. For these reasons, all further analyses were carried out on the natural logarithm of test thresholds.
Group differences were assessed in Minitab 16 with repeated-measures, mixed-methods, nested general linear model comparisons (Laird & Ware, 1982), with the individual identifier of ‘subject number’ included as a random and nesting factor. The main effects of test, group, subject number and run number were assessed, as well as the test×group interaction.
Correlations were assessed in the control and MSA groups in Minitab 16 using the General Regression function, which allows the construction and assessment of a single regression containing both continuous and categorical variables. This was not possible for the HD group, as there was a significant degree of collinearity between potential regression factors. Individual regression analyses are therefore discussed below.
4. Results
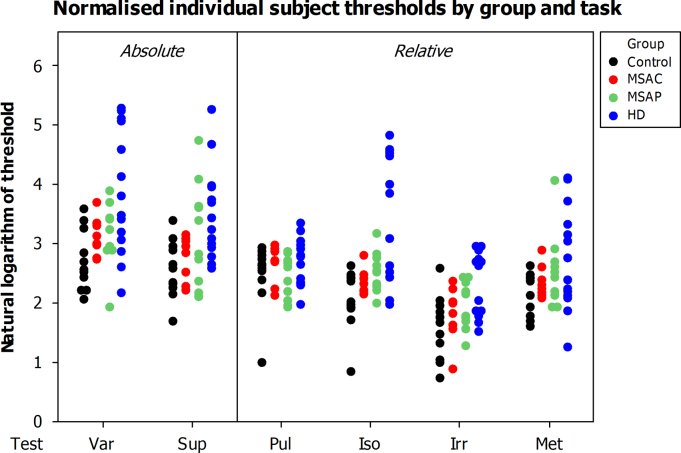
Threshold performance was measured by adaptive tracking of thresholds (Supplementary Fig. 1) for all six tasks. Individual subject thresholds are shown in Fig. 2, and the 95% confidence intervals for group means are shown in Fig. 3. Multiple between-group comparisons were performed and results are shown in Table 2.
Fig. 2.
Measured, normalised thresholds for individual subjects for each task.
Fig. 3.
Ninety five percent confidence intervals for mean group performance by task.
Table 2.
Results of the repeated-measures, mixed-methods, nested general linear model comparison between groups (p values; italics, significant effect).
|
General linear model comparison between groups | |||||
|---|---|---|---|---|---|
| Comparison | Test | Group | Test×Group | Subject number | Run number |
| MSA-C vs control | <0.001 | 0.083 | 0.927 | <0.001 | 0.776 |
| MSA-P vs control | <0.001 | 0.035 | 0.028 | <0.001 | 0.964 |
| HD vs control | <0.001 | 0.001 | <0.001 | <0.001 | 0.839 |
| MSA-C vs MSA-P | <0.001 | 0.414 | 0.059 | <0.001 | 0.882 |
| HD vs all MSA | <0.001 | 0.006 | <0.001 | <0.001 | 0.897 |
| All MSA vs control | <0.001 | 0.045 | 0.077 | <0.001 | 0.878 |
| All patients vs control | <0.001 | 0.001 | <0.001 | <0.001 | 0.940 |
Patients with basal ganglia disease performed significantly less well overall than control subjects. Those with HD and MSA-P had significantly higher thresholds than controls, but this difference only approached significance for those with MSA-C (p=0.083). Patients with MSA-P and MSA-C did not significantly differ in their performance (p=0.414), and as a combined group patients with MSA performed significantly more poorly than controls but significantly better than patients with HD.
There was a clear main effect of task (p<0.001) for all comparisons. As expected (see Sanabria, Capizzi, and Correa (2011) for a review), all groups were able to use the information provided by a regular context to improve their ability to discriminate interval durations; control participants' mean threshold was 17.0% difference in inter-onset interval for simple duration discrimination and 8.6% for the detection of deviation from isochrony. Although the thresholds are higher overall, perhaps reflecting our use of elderly patient controls rather than undergraduate students, the relative threshold reduction of 49% is very similar to that demonstrated by Miller and McAuley (2005) in their systematic examination of tempo sensitivity in isochronous sequences. Patients with Multiple System Atrophy performed less well on both tasks, but were able to derive benefit of a similar magnitude from the presence of a regular context; mean thresholds improved from 25.8% to 13.3% for patients with MSA-P (a 48.6% reduction), and from 23.8% to 10.7% for those with MSA-C (a 54.8% reduction). Patients with HD performed much more poorly overall, and demonstrated less improvement in the presence of an isochronous beat: from 76.5% to 49.3% (a 35.5% reduction).
Some comparisons resulted in a group×test interaction, but this was not due to a discernible difference in relative performance between tasks of absolute and relative timing. Fig. 3 illustrates that in all tasks except the detection of regularity (Pul) mean group thresholds followed the same pattern of progressively poorer performance from control to MSA-C to MSA-P to HD.
In the light of previous literature supporting a relatively increased role for the cerebellum at sub-second durations and the basal ganglia at supra-second durations (Koch et al., 2007, Lewis and Miall, 2003), we had an a-priori hypothesis that patients with MSA-P would be relatively more impaired at absolute timing at the supra-second level, and those with MSA-C at the sub-second level. This was assessed with a two-tailed, paired t-test and the difference approached, but did not reach, statistical significance (p=0.07).
There was no significant effect at the p<0.10 level in any comparison of whether runs were performed first or second – this implies that there were no significant effects of practice or fatigue at the group level.
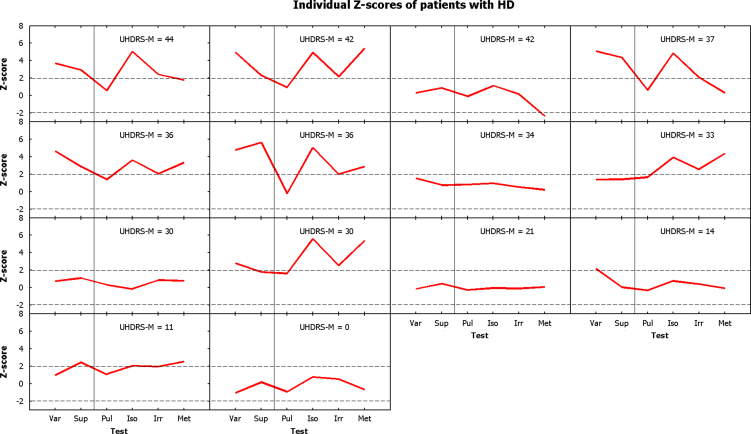
It is notable in Fig. 2 that there was considerable inter-individual variability in subject performance. All groups demonstrated a significant main effect of subject number at the p<0.001 level, indicating that this was in part because some subjects performed more poorly on all tasks than other subjects. Fig. 4 displays the individual performance profiles of the patients with HD, arranged by severity of motor symptoms, compared to the control participants. This figure illustrates that group differences were not the result of isolated and variable poor performances, but rather that some individuals were performing more poorly than others at all tasks. Similar data are displayed for patients with MSA-P and MSA-C, arranged by years of symptoms, in Supplementary Figs. 2 and 3 respectively. We carried out regression analyses to explore the factors underlying these differences.
Fig. 4.
Individual performance profiles of patients with HD, expressed as Z-scores compared to the control population, and arranged by severity of motor symptoms. Higher scores correspond to poorer performance. Dashed lines at ±1.96 illustrate the bounds of a 95% confidence interval for individual comparisons (i.e. values above the upper line would be statistically significantly poorer performance than would be expected from an individual taken from the control population if an individual comparison were made, while those below the lower line would be significantly better).
For control and MSA groups, the significance of factors in a general regression model was calculated as reported in Supplementary Table 1. For the control group, the only significant predictor of performance was age (p=0.018), with older individuals performing slightly more poorly than younger individuals. For the group with MSA, there were additional significant effects of forwards digit span (p=0.026) and years since symptom onset (p=0.004). Individuals with shorter digit spans and who had a longer duration of illness performed significantly more poorly overall. Analysis of collinearity demonstrated that in both groups the only co-correlated factors were performance on an ACE-R, estimated premorbid full-scale IQ and, in the control group, backwards digit span. None of these factors contributed significantly to the model, so the analysis is valid. Notably, there was no correlation between these cognitive measures and years since symptom onset in the MSA group.
For the HD group, performance on cognitive tests (ACE-R with and without fluency, pre-morbid full-scale IQ estimate, Stroop, symbol-digit substitution, verbal fluency, trail making, and forwards and backwards digit span) were highly collinear, and were in turn significantly correlated with motor severity as assessed by the UHDRS-Motor score (taken together, the cognitive tests could account for 73% of the variance in UHDRS-M; the strongest single predictor was symbol-digit substitution, which accounted for 55% of the variance). Best subsets regression analysis revealed that, amongst the cognitive tests, the strongest single predictor of timing performance was the total Revised Addenbrooke's Cognitive Examination score, which resulted in an adjusted r2 of 0.30. This score was in turn highly negatively correlated with the Unified Huntington's Disease Rating Scale Motor score, with an adjusted r2 of 0.38 (p<0.001) (see Supplementary Fig. 4 for regression plot; regression equation: ACE-R=96.8−0.471 UHDRS-M). It is therefore not valid to undertake a general regression analysis containing both ACE-R and UHDRS-M scores as factors. Two separate general regression analyses were performed containing only the categorical factor of ‘task’ and the continuous factor ‘age’ plus either ACE-R or UHDRS-M. ‘Age’ was not a significant factor in either analysis, but ‘task’, ACE-R and UHDRS-M were all significant with p<0.0001. The model based upon UHDRS-M accounted for 46% of the observed variance, while that based upon the ACE-R was able to account for 62% of the observed variance. This general tendency for patients with more advanced (but still moderate) disease to perform more poorly than those who were pre-symptomatic or mildly affected can be appreciated from Fig. 4, which is arranged by severity of motor symptoms.
5. Discussion
The primary findings of the analyses were that: patients with HD performed more poorly than those with MSA, who in turn performed more poorly than control subjects; there was no difference in performance between subjects with MSA-C and MSA-P; there was no difference in performance patterns between the groups for absolute and relative timing tasks; there were significant individual differences in performance within the groups, and these differences could be accounted for in part by duration of illness (a surrogate marker of disease progression) in MSA and degree of cognitive or motor impairment in HD.
5.1. Cognition and time perception
Before discussing the implications of these findings, it is important to consider whether poorer timing performance in more severe Huntington's disease might be a direct effect of a greater degree of cognitive involvement. It has been proposed by some authors that, in early Huntington's disease, motor symptoms often exist in the absence of cognitive impairment (de Boo et al., 1997). Conversely other authors have demonstrated subtle cognitive decline in pre-symptomatic HD gene carriers (Hahn-Barma et al., 1998, Lawrence et al., 1998). In this study the recruitment aims were explicitly to identify individuals with motor symptoms in the absence of subjective cognitive impairment but, even in this context, detailed neuropsychometric testing demonstrated significant cognitive deficits that were strongly correlated with motor involvement. It appears, therefore, that motor and cognitive decline are inextricably linked in the early stages of HD, during which basal ganglia degeneration is predominant (Albin et al., 1992, Aylward et al., 1996). In the control and MSA groups the results of cognitive testing did not correlate significantly with perceptual timing performance. However these groups were less cognitively impaired than those with HD and their results had a smaller spread, so it is possible that timing performance is not impacted until individuals reach a ‘cognitive threshold’. Against this hypothesis is the observation that group differences did not significantly vary between tasks, despite significantly differing task demands. The task with the lowest inter-group difference was that of regularity detection (Pul). This could be considered the most cognitively demanding task as it requires the grasp of an abstract concept (differing degrees of irregularity) and the comparison of two relatively long sequences. In contrast, the HD group demonstrated the most striking deficits in the detection of deviation from isochrony (Iso), which could be argued to be the least cognitively demanding task as it does not critically require the comparison of a target to a reference sequence but can be accomplished by the comparison of interval lengths within the sequence. Further, there was no difference in impairment between the absolute timing tasks with sub-second and supra-second interval lengths, despite the increased working memory and concentration requirements of the latter. We would therefore conclude that cognitive impairment is a surrogate marker of overall severity in HD, and correlates with timing performance as a function of this, rather than because of a direct impact upon subjects' ability to perform the tasks.
5.2. Basal ganglia disorders and time perception
The majority of previous studies of the basal ganglia's role in time perception have used Parkinson's disease as a model (Allman & Meck, 2012). Where a distinction has been made, and motor control confounds eliminated, these patients have been found to be impaired on tests based on a regular beat, but not on those without (Grahn & Brett, 2009). In general, the findings of these studies can be attributed to a decrease in the speed of the internal clock (Harrington, Haaland, & Hermanowicz, 1998), especially when medication was withdrawn (Artieda et al., 1992, Jones et al., 2008, Pastor et al., 1992). Although there is generalised neuronal degeneration, the dysfunction in Parkinson's disease is not primarily degeneration of the striatum, but rather an uneven loss of its dopaminergic input (Kish et al., 1988). Similar decreases in internal clock speed can be demonstrated in both rats and humans exposed to dopamine blockade, while increases occur with exposure to dopamine agonists (Buhusi and Meck, 2002, Cheng et al., 2007, Coull et al., 2011, MacDonald and Meck, 2005, Meck, 1983, Meck, 1986, Meck, 1996). Taken together, these studies therefore support the view that dopaminergic input regulates the accuracy of the Striatal Beat Frequency oscillation through the introduction of a consistent bias, but does not otherwise affect its precision. In this context, both the modular and unified models of time perception would predict the findings of impairment in relative but not absolute timing performance, as the additional bias-related error induced by the change in clock speed is constant across stimuli. Recent demonstrations of alterations in the functional connectivity of striatum and frontal cortex according to dopaminergic state are also likely to contribute to these findings, and might account for the variability in magnitude of impairment in PD according to task demands (Jahanshahi et al., 2010).
Few studies have looked at patients with intrinsic dysfunction of the basal ganglia, typically using beat-based motor or duration-based perceptual tasks. Ten patients with unilateral basal ganglia lesions due to stroke were found to be poorer than controls at detecting and tracking tempo changes in a guided finger tapping task (Schwartze, Keller, Patel, & Kotz, 2011). In contrast, other authors have failed to find a deficit in finger tapping precision in a group of three patients with unilateral ischaemic basal ganglia lesions (Aparicio, Diedrichsen, & Ivry, 2005). Coslett and colleagues tested two patients with bilateral basal ganglia lesions, the first from a prolonged hypoxic encephalopathy secondary to cardiac arrhythmia and the second due to bilateral ischaemic infarction. These patients were less accurate and more variable in the reproduction of isochronous finger taps, but were not found to be significantly impaired on a psychophysical task of absolute timing (Coslett, Wiener, & Chatterjee, 2010). No psychophysical tasks of relative perceptual timing were employed in any of these studies.
5.3. The nature of perceptual timing deficits in striatal disorder
A number of the between-group comparisons displayed in Table 2 revealed a group×task interaction. Examination of Figs. 2 and 3 reveals that this can be accounted for by a much lower between-group difference in performance in the task of regularity detection (Pul) than in the other tasks. The other tasks of relative timing employed in this study rely upon the detection of deviations from expected tone onset time within a beat-based sequence. In contrast, the task of regularity or pulse detection measures subjects' ability to detect subtle regularity within a predominantly irregular sequence and as such has no strong underlying periodicity on which to base expectations. Recent functional imaging work in healthy individuals has demonstrated greater activation of the putamen during tasks that require internal continuation or prediction within a beat sequence than during the detection of regularity (Grahn & Rowe, 2012), and that this activation reflects the stability of synchronisation between an internally generated rhythm and external cues (Hove, Fairhurst, Kotz, & Keller, 2013). Similarly, Geiser and Kaelin-Lang (2011) demonstrated no impairment of beat detection in a cohort of patients with early Parkinson's disease, regardless of whether they were tested before or after administration of dopaminergic medication (this changed only reaction time). Other psychophysical studies have demonstrated that healthy individuals are unable to prevent themselves from entraining to a rhythmic visual cue (Rohenkohl, Coull, & Nobre, 2011), or to fully adapt their predictions of tone onset time to account for changing temporal context (Cope, Grube, & Griffiths, 2012). Taken together with the present findings, the available data suggest that temporal prediction within a beat-based context crucially relies upon the striatum, and that executive control of this process is limited. Further, they imply that the detection of regularity within an irregular sequence, or “beat finding”, is a task of relative timing that is not dependant on the basal ganglia.
Some authors argue for the existence of separate clock mechanisms, with distinct neural substrates, for the analysis of intervals above and below one second in duration. Neuroimaging studies have demonstrated selectively increased activation of the cerebellum for the discrimination of sub-second intervals, and of the basal ganglia for supra-second intervals (Lewis & Miall, 2003) (although this finding is not universal, see Wiener, Turkeltaub, & Coslett (2010) for a review). This is supported by the observation that transcranial magnetic stimulation of the cerebellum impairs subjects' ability to reproduce sub-second but not supra-second time intervals (Koch et al., 2007). Our own data demonstrate a corresponding trend; patients with MSA-C were relatively more impaired in the discrimination of sub-second intervals, and patients with MSA-P more impaired in the discrimination of supra-second intervals (p=0.07). While this dissociation could be taken as evidence for the existence of separated modules (Koch, Oliveri, & Caltagirone, 2009), these data can alternatively be explained by the hypothesised unified model of time perception as increasing durations include a greater number of periods of the Striatal Beat Frequency, resulting in a relative increase in the weighting of the precision of this mechanism (Meck et al., 2008).
5.4. The pathology of timing deficits in striatal disorder
The most important finding of this work is that both absolute and relative timing performance are similarly impaired in MSA and HD, and that these deficits correlate strongly with disease progression. The clinical distinction between MSA-C and MSA-P did not significantly impact upon timing performance, which we suggest reflects the similar underlying pathophysiology affecting both striatum and cerebellum in these patients (Burn & Jaros, 2001) resulting in similar measured impairments. Strikingly, patients with HD, who have primary degeneration of the basal ganglia, were more impaired than those with MSA, who have degeneration of both the basal ganglia and cerebellum, at all durations and even on specific tests of absolute timing. It is possible that this reflects the known early loss in HD of striatal medium spiny neurons (Graveland et al., 1985, Vonsattel and DiFiglia, 1998), thought to be essential to the Striatal Beat Frequency model, but might simply reflect more severe general involvement of the basal ganglia.
6. Conclusion
Overall, the present findings lend strong support to the view of the basal ganglia as a mandatory component for absolute, duration-based as well as relative, beat-based timing as proposed in the hypothesised unified model of time perception (Teki et al., 2012), but could not be explained by separate neural modules in cerebellum and basal ganglia respectively subserving absolute and relative timing.
Acknowledgements
This work was supported by a Wellcome Trust Senior Fellowship awarded to TDG (reference 091681). We are grateful to Dr. Kirstie Anderson, Dr. Mark Baker, Ms. Joanne Brown, Dr. Uma Nath, Dr. Andrew Schaefer, and Dr. Ian Schofield for their assistance with patient identification and recruitment.
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial-No Derivative Works License, which permits non-commercial use, distribution, and reproduction in any medium, provided the original author and source are credited.
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.neuropsychologia.2013.09.039.
Appendix A. Supporting materials
Supplementary data
A typical adaptive track from a single test run on task Sup by a subject with Huntington's Disease.
Supplementary data
Supplementary data
Supplementary data
References
- Akkal D., Dum R.P., Strick P.L. Supplementary motor area and presupplementary motor area: Targets of basal ganglia and cerebellar output. Journal of Neuroscience. 2007;27:10659–10673. doi: 10.1523/JNEUROSCI.3134-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albin R.L., Reiner A., Anderson K.D., Dure L.S. t., Handelin B., Balfour R. Preferential loss of striato-external pallidal projection neurons in presymptomatic Huntington's disease. Annals of Neurology. 1992;31:425–430. doi: 10.1002/ana.410310412. [DOI] [PubMed] [Google Scholar]
- Allman M.J., Meck W.H. Pathophysiological distortions in time perception and timed performance. Brain. 2012;135:656–677. doi: 10.1093/brain/awr210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aparicio P., Diedrichsen J., Ivry R.B. Effects of focal basal ganglia lesions on timing and force control. Brain and Cognition. 2005;58:62–74. doi: 10.1016/j.bandc.2004.09.009. [DOI] [PubMed] [Google Scholar]
- Artieda J., Pastor M.A., Lacruz F., Obeso J.A. Temporal discrimination is abnormal in Parkinson's disease. Brain. 1992;115(Pt 1):199–210. doi: 10.1093/brain/115.1.199. [DOI] [PubMed] [Google Scholar]
- Aylward E.H., Codori A.M., Barta P.E., Pearlson G.D., Harris G.J., Brandt J. Basal ganglia volume and proximity to onset in presymptomatic Huntington disease. Archives of Neurology. 1996;53:1293–1296. doi: 10.1001/archneur.1996.00550120105023. [DOI] [PubMed] [Google Scholar]
- Bostan A.C., Dum R.P., Strick P.L. The basal ganglia communicate with the cerebellum. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:8452–8456. doi: 10.1073/pnas.1000496107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bostan A.C., Strick P.L. The cerebellum and basal ganglia are interconnected. Neuropsychology Review. 2010;20:261–270. doi: 10.1007/s11065-010-9143-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bregman A.S. MIT Press; Cambridge, MA: 1990. Auditory scene analysis. [Google Scholar]
- Buhusi C.V., Meck W.H. Differential effects of methamphetamine and haloperidol on the control of an internal clock. Behavioral Neuroscience. 2002;116:291–297. doi: 10.1037//0735-7044.116.2.291. [DOI] [PubMed] [Google Scholar]
- Buhusi C.V., Meck W.H. What makes us tick? Functional and neural mechanisms of interval timing. Nature Reviews Neuroscience. 2005;6:755–765. doi: 10.1038/nrn1764. [DOI] [PubMed] [Google Scholar]
- Burn D.J., Jaros E. Multiple system atrophy: Cellular and molecular pathology. Molecular Pathology. 2001;54:419–426. [PMC free article] [PubMed] [Google Scholar]
- Chen J.L., Zatorre R.J., Penhune V.B. Interactions between auditory and dorsal premotor cortex during synchronization to musical rhythms. NeuroImage. 2006;32:1771–1781. doi: 10.1016/j.neuroimage.2006.04.207. [DOI] [PubMed] [Google Scholar]
- Cheng R.K., Hakak O.L., Meck W.H. Habit formation and the loss of control of an internal clock: Inverse relationship between the level of baseline training and the clock-speed enhancing effects of methamphetamine. Psychopharmacology (Berl) 2007;193:351–362. doi: 10.1007/s00213-007-0783-2. [DOI] [PubMed] [Google Scholar]
- Cope T.E., Grube M., Griffiths T.D. Temporal predictions based on a gradual change in tempo. Journal of the Acoustical Society of America. 2012;131:4013–4022. doi: 10.1121/1.3699266. [DOI] [PubMed] [Google Scholar]
- Cope T.E., Sedley W., Griffiths T.D. Timing and the auditory brain. Advances in Clinical Neuroscience and Rehabilitation. 2011;10:10–13. [Google Scholar]
- Coslett H.B., Wiener M., Chatterjee A. Dissociable neural systems for timing: Evidence from subjects with basal ganglia lesions. PLoS One. 2010;5:e10324. doi: 10.1371/journal.pone.0010324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coull J.T., Cheng R.K., Meck W.H. Neuroanatomical and neurochemical substrates of timing. Neuropsychopharmacology. 2011;36:3–25. doi: 10.1038/npp.2010.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford J.R., Parker D.M., Besson J.A. Estimation of premorbid intelligence in organic conditions. British Journal of Psychiatry. 1988;153:178–181. doi: 10.1192/bjp.153.2.178. [DOI] [PubMed] [Google Scholar]
- de Boo G.M., Tibben A., Lanser J.B., Jennekens-Schinkel A., Hermans J., Maat-Kievit A. Early cognitive and motor symptoms in identified carriers of the gene for Huntington disease. Archives of Neurology. 1997;54:1353–1357. doi: 10.1001/archneur.1997.00550230030012. [DOI] [PubMed] [Google Scholar]
- Drake C., Botte M.C. Tempo sensitivity in auditory sequences: Evidence for a multiple-look model. Perception & Psychophysics. 1993;54:277–286. doi: 10.3758/bf03205262. [DOI] [PubMed] [Google Scholar]
- Essens P.J., Povel D.-J. Metrical and nonmetrical representations of temporal patterns. Perception & Psychophysics. 1985;37:1–7. doi: 10.3758/bf03207132. [DOI] [PubMed] [Google Scholar]
- Geiser E., Kaelin-Lang A. The function of dopaminergic neural signal transmission in auditory pulse perception: Evidence from dopaminergic treatment in Parkinson's patients. Behavioural Brain Research. 2011;225:270–275. doi: 10.1016/j.bbr.2011.07.019. [DOI] [PubMed] [Google Scholar]
- Geiser E., Notter M., Gabrieli J.D.E. A corticostriatal neural system enhances auditory perception through temporal context processing. Journal of Neuroscience. 2012;32:6177–6182. doi: 10.1523/JNEUROSCI.5153-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbon J., Malapani C., Dale C.L., Gallistel C.R. Toward a neurobiology of temporal cognition: Advances and challenges. Current Opinion in Neurobiology. 1997;7:170–184. doi: 10.1016/s0959-4388(97)80005-0. [DOI] [PubMed] [Google Scholar]
- Gilman S., Wenning G.K., Low P.A., Brooks D.J., Mathias C.J., Trojanowski J.Q. Second consensus statement on the diagnosis of multiple system atrophy. Neurology. 2008;71:670–676. doi: 10.1212/01.wnl.0000324625.00404.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grahn J.A., Brett M. Rhythm and beat perception in motor areas of the brain. Journal of Cognitive Neuroscience. 2007;19:893–906. doi: 10.1162/jocn.2007.19.5.893. [DOI] [PubMed] [Google Scholar]
- Grahn J.A., Brett M. Impairment of beat-based rhythm discrimination in Parkinson's disease. Cortex. 2009;45:54–61. doi: 10.1016/j.cortex.2008.01.005. [DOI] [PubMed] [Google Scholar]
- Grahn J.A., McAuley J.D. Neural bases of individual differences in beat perception. NeuroImage. 2009;47:1894–1903. doi: 10.1016/j.neuroimage.2009.04.039. [DOI] [PubMed] [Google Scholar]
- Grahn J.A., Rowe J.B. Feeling the beat: Premotor and striatal interactions in musicians and nonmusicians during beat perception. Journal of Neuroscience. 2009;29:7540–7548. doi: 10.1523/JNEUROSCI.2018-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grahn J.A., Rowe J.B. Finding and feeling the musical beat: Striatal dissociations between detection and prediction of regularity. Cerebral Cortex. 2012;23(4):913–921. doi: 10.1093/cercor/bhs083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graveland G.A., Williams R.S., DiFiglia M. Evidence for degenerative and regenerative changes in neostriatal spiny neurons in Huntington's disease. Science. 1985;227:770–773. doi: 10.1126/science.3155875. [DOI] [PubMed] [Google Scholar]
- Grube M., Cooper F.E., Chinnery P.F., Griffiths T.D. Dissociation of duration-based and beat-based auditory timing in cerebellar degeneration. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:11597–11601. doi: 10.1073/pnas.0910473107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grube M., Griffiths T.D. Metricality-enhanced temporal encoding and the subjective perception of rhythmic sequences. Cortex. 2009;45:72–79. doi: 10.1016/j.cortex.2008.01.006. [DOI] [PubMed] [Google Scholar]
- Grube M., Lee K.H., Griffiths T.D., Barker A.T., Woodruff P.W. Transcranial magnetic theta-burst stimulation of the human cerebellum distinguishes absolute, duration-based from relative, beat-based perception of subsecond time intervals. Frontiers in Psychology. 2010;1:171. doi: 10.3389/fpsyg.2010.00171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn-Barma V., Deweer B., Dürr A., Dodé C., Feingold J., Pillon B. Are cognitive changes the first symptoms of Huntington's disease? A study of gene carriers. Journal of Neurology, Neurosurgery & Psychiatry. 1998;64:172–177. doi: 10.1136/jnnp.64.2.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halsband U., Ito N., Tanji J., Freund H.J. The role of premotor cortex and the supplementary motor area in the temporal control of movement in man. Brain. 1993;116(Pt 1):243–266. doi: 10.1093/brain/116.1.243. [DOI] [PubMed] [Google Scholar]
- Harrington D.L., Haaland K.Y., Hermanowicz N. Temporal processing in the basal ganglia. Neuropsychology. 1998;12:3–12. doi: 10.1037//0894-4105.12.1.3. [DOI] [PubMed] [Google Scholar]
- Hoshi E., Tremblay L., Feger J., Carras P.L., Strick P.L. The cerebellum communicates with the basal ganglia. Nature Neuroscience. 2005;8:1491–1493. doi: 10.1038/nn1544. [DOI] [PubMed] [Google Scholar]
- Hove M.J., Fairhurst M.T., Kotz S.A., Keller P.E. Synchronizing with auditory and visual rhythms: An fMRI assessment of modality differences and modality appropriateness. NeuroImage. 2013;67:313–321. doi: 10.1016/j.neuroimage.2012.11.032. [DOI] [PubMed] [Google Scholar]
- Laird N.M., Ware J.H. Random-effects models for longitudinal data. Biometrics. 1982;38:963–974. [PubMed] [Google Scholar]
- Huntington Study Group Unified Huntington's disease rating scale: reliability and consistency. Movement Disorders. 1996;11:136–142. doi: 10.1002/mds.870110204. [DOI] [PubMed] [Google Scholar]
- Ivry R. Cerebellar involvement in the explicit representation of temporal information. Annals of the New York Academy of Sciences. 1993;682:214–230. doi: 10.1111/j.1749-6632.1993.tb22970.x. [DOI] [PubMed] [Google Scholar]
- Ivry R.B., Keele S.W. Timing functions of the cerebellum. Journal of Cognitive Neuroscience. 1989;1:136–152. doi: 10.1162/jocn.1989.1.2.136. [DOI] [PubMed] [Google Scholar]
- Ivry R.B., Schlerf J.E. Dedicated and intrinsic models of time perception. Trends in Cognitive Sciences. 2008;12:273–280. doi: 10.1016/j.tics.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahanshahi M., Jones C.R.G., Zijlmans J., Katzenschlager R., Lee L., Quinn N. Dopaminergic modulation of striato-frontal connectivity during motor timing in Parkinson's disease. Brain. 2010;133:727–745. doi: 10.1093/brain/awq012. [DOI] [PubMed] [Google Scholar]
- Jones, C.R., & Jahanshahi, M. Contributions of the basal ganglia to temporal processing: evidence from Parkinson's disease. Timing and Time Perception, 10.1163/22134468-00002009, in press. [DOI]
- Jones C.R., Malone T.J., Dirnberger G., Edwards M., Jahanshahi M. Basal ganglia, dopamine and temporal processing: Performance on three timing tasks on and off medication in Parkinson's disease. Brain and Cognition. 2008;68:30–41. doi: 10.1016/j.bandc.2008.02.121. [DOI] [PubMed] [Google Scholar]
- Jurgens U. The efferent and afferent connections of the supplementary motor area. Brain Research. 1984;300:63–81. doi: 10.1016/0006-8993(84)91341-6. [DOI] [PubMed] [Google Scholar]
- Keele S.W., Nicoletti R., Ivry R.I., Pokorny R.A. Mechanisms of perceptual timing: Beat-based or interval-based judgements? Psychological Research. 1989;50:251–256. [Google Scholar]
- Kish S.J., Shannak K., Hornykiewicz O. Uneven pattern of dopamine loss in the striatum of patients with idiopathic Parkinson's disease. Pathophysiologic and clinical implications. New England Journal of Medicine. 1988;318:876–880. doi: 10.1056/NEJM198804073181402. [DOI] [PubMed] [Google Scholar]
- Koch G., Oliveri M., Caltagirone C. Neural networks engaged in milliseconds and seconds time processing: Evidence from transcranial magnetic stimulation and patients with cortical or subcortical dysfunction. Philosophical Transactions of the Royal Society B: Biological Sciences. 2009;364:1907–1918. doi: 10.1098/rstb.2009.0018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch G., Oliveri M., Torriero S., Salerno S., Lo Gerfo E., Caltagirone C. Repetitive TMS of cerebellum interferes with millisecond time processing. Experimental Brain Research. 2007;179:291–299. doi: 10.1007/s00221-006-0791-1. [DOI] [PubMed] [Google Scholar]
- Kollmeier B., Gilkey R.H., Sieben U.K. Adaptive staircase techniques in psychoacoustics: A comparison of human data and a mathematical model. Journal of the Acoustical Society of America. 1988;83:1852–1862. doi: 10.1121/1.396521. [DOI] [PubMed] [Google Scholar]
- Lawrence A.D., Hodges J.R., Rosser A.E., Kershaw A., ffrench-Constant C., Rubinsztein D.C. Evidence for specific cognitive deficits in preclinical Huntington's disease. Brain. 1998;121:1329–1341. doi: 10.1093/brain/121.7.1329. [DOI] [PubMed] [Google Scholar]
- Leek M.R. Adaptive procedures in psychophysical research. Perception and Psychophysics. 2001;63:1279–1292. doi: 10.3758/bf03194543. [DOI] [PubMed] [Google Scholar]
- Levitt H. Transformed up–down methods in psychoacoustics. Journal of the Acoustical Society America. 1971;49(Suppl 2):467+. [PubMed] [Google Scholar]
- Lewis P.A., Miall R.C. Brain activation patterns during measurement of sub- and supra-second intervals. Neuropsychologia. 2003;41:1583–1592. doi: 10.1016/s0028-3932(03)00118-0. [DOI] [PubMed] [Google Scholar]
- Lewis P.A., Miall R.C. Remembering the time: A continuous clock. Trends in Cognitive Sciences. 2006;10:401–406. doi: 10.1016/j.tics.2006.07.006. [DOI] [PubMed] [Google Scholar]
- Macar F., Anton J.L., Bonnet M., Vidal F. Timing functions of the supplementary motor area: An event-related fMRI study. Brain Research: Cognitive Brain Research. 2004;21:206–215. doi: 10.1016/j.cogbrainres.2004.01.005. [DOI] [PubMed] [Google Scholar]
- MacDonald C.J., Meck W.H. Differential effects of clozapine and haloperidol on interval timing in the supraseconds range. Psychopharmacology (Berl) 2005;182:232–244. doi: 10.1007/s00213-005-0074-8. [DOI] [PubMed] [Google Scholar]
- Madison G., Merker B. On the limits of anisochrony in pulse attribution. Psychology Research. 2002;66:201–207. doi: 10.1007/s00426-001-0085-y. [DOI] [PubMed] [Google Scholar]
- Matell M.S., Meck W.H. Cortico-striatal circuits and interval timing: Coincidence detection of oscillatory processes. Brain Research: Cognitive Brain Research. 2004;21:139–170. doi: 10.1016/j.cogbrainres.2004.06.012. [DOI] [PubMed] [Google Scholar]
- Mauk M.D., Buonomano D.V. The neural basis of temporal processing. Annual Review of Neuroscience. 2004;27:307–340. doi: 10.1146/annurev.neuro.27.070203.144247. [DOI] [PubMed] [Google Scholar]
- McAuley J.D., Jones M.R. Modeling effects of rhythmic context on perceived duration: A comparison of interval and entrainment approaches to short-interval timing. Journal of Experimental Psychology: Human Perception and Performance. 2003;29:1102–1125. doi: 10.1037/0096-1523.29.6.1102. [DOI] [PubMed] [Google Scholar]
- Meck W.H. Selective adjustment of the speed of internal clock and memory processes. Journal of Experimental Psychology: Animal Behavior and Processes. 1983;9:171–201. [PubMed] [Google Scholar]
- Meck W.H. Affinity for the dopamine D2 receptor predicts neuroleptic potency in decreasing the speed of an internal clock. Pharmacology Biochemistry and Behavior. 1986;25:1185–1189. doi: 10.1016/0091-3057(86)90109-7. [DOI] [PubMed] [Google Scholar]
- Meck W.H. Neuropharmacology of timing and time perception. Cognitive Brain Research. 1996;3:227–242. doi: 10.1016/0926-6410(96)00009-2. [DOI] [PubMed] [Google Scholar]
- Meck W.H. Neuropsychology of timing and time perception. Brain Cogn. 2005;58:1–8. doi: 10.1016/j.bandc.2004.09.004. [DOI] [PubMed] [Google Scholar]
- Meck W.H., Benson A.M. Dissecting the brain's internal clock: how frontal-striatal circuitry keeps time and shifts attention. Brain and Cognition. 2002;48:195–211. doi: 10.1006/brcg.2001.1313. [DOI] [PubMed] [Google Scholar]
- Meck W.H., Penney T.B., Pouthas V. Cortico-striatal representation of time in animals and humans. Current Opinion in Neurobiology. 2008;18:145–152. doi: 10.1016/j.conb.2008.08.002. [DOI] [PubMed] [Google Scholar]
- Miall C. The storage of time intervals using oscillating neurons. Neural Computation. 1989;1:359–371. [Google Scholar]
- Miller N.S., McAuley J.D. Tempo sensitivity in isochronous tone sequences: The multiple-look model revisited. Perception and Psychophysics. 2005;67:1150–1160. doi: 10.3758/bf03193548. [DOI] [PubMed] [Google Scholar]
- Mioshi E., Dawson K., Mitchell J., Arnold R., Hodges J.R. The Addenbrooke's Cognitive Examination Revised (ACE-R): A brief cognitive test battery for dementia screening. International Journal of Geriatric Psychiatry. 2006;21:1078–1085. doi: 10.1002/gps.1610. [DOI] [PubMed] [Google Scholar]
- Oprisan S.A., Buhusi C.V. Modeling pharmacological clock and memory patterns of interval timing in a striatal beat-frequency model with realistic, noisy neurons. Frontiers in Integrative Neuroscience. 2011;5:52. doi: 10.3389/fnint.2011.00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oshio K. Possible functions of prefrontal cortical neurons in duration discrimination. Frontiers in Integrative Neuroscience. 2011;5:25. doi: 10.3389/fnint.2011.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oshio K., Chiba A., Inase M. Temporal filtering by prefrontal neurons in duration discrimination. European Journal of Neuroscience. 2008;28:2333–2343. doi: 10.1111/j.1460-9568.2008.06509.x. [DOI] [PubMed] [Google Scholar]
- Papp M.I., Kahn J.E., Lantos P.L. Glial cytoplasmic inclusions in the CNS of patients with multiple system atrophy (striatonigral degeneration, olivopontocerebellar atrophy and Shy-Drager syndrome) Journal of Neurological Sciences. 1989;94:79–100. doi: 10.1016/0022-510x(89)90219-0. [DOI] [PubMed] [Google Scholar]
- Pastor M.A., Artieda J., Jahanshahi M., Obeso J.A. Time estimation and reproduction is abnormal in Parkinson's disease. Brain. 1992;115(Pt 1):211–225. doi: 10.1093/brain/115.1.211. [DOI] [PubMed] [Google Scholar]
- Reitan R.M. Validity of the trail making test as an indicator of organic brain damage. Perceptual and Motor Skills. 1958;8:271–276. [Google Scholar]
- Rohenkohl G., Coull J.T., Nobre A.C. Behavioural dissociation between exogenous and endogenous temporal orienting of attention. PLoS One. 2011;6:e14620. doi: 10.1371/journal.pone.0014620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanabria D., Capizzi M., Correa A. Rhythms that speed you up. Journal of Experimental Psychology-Human Perception and Performance. 2011;37:236–244. doi: 10.1037/a0019956. [DOI] [PubMed] [Google Scholar]
- Schwartze M., Keller P.E., Patel A.D., Kotz S.A. The impact of basal ganglia lesions on sensorimotor synchronization, spontaneous motor tempo, and the detection of tempo changes. Behavioural Brain Research. 2011;216:685–691. doi: 10.1016/j.bbr.2010.09.015. [DOI] [PubMed] [Google Scholar]
- Schwartze M., Rothermich K., Kotz S.A. Functional dissociation of pre-SMA and SMA-proper in temporal processing. NeuroImage. 2012;60:290–298. doi: 10.1016/j.neuroimage.2011.11.089. [DOI] [PubMed] [Google Scholar]
- Smith A. Western Psychological Services; Los Angeles, Ca: 1982. Symbol-Digit Modalities Test (SDMT) manual. [Google Scholar]
- Stroop J.R. Studies of interference in serial verbal reactions. Journal of Experimental Psychology. 1935;18:643–662. [Google Scholar]
- Teki S., Grube M., Griffiths T.D. A unified model of time perception accounts for duration-based and beat-based timing mechanisms. Frontiers in Integrative Neuroscience. 2012;5:90. doi: 10.3389/fnint.2011.00090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teki S., Grube M., Kumar S., Griffiths T.D. Distinct neural substrates of duration-based and beat-based auditory timing. Journal of Neuroscience. 2011;31:3805–3812. doi: 10.1523/JNEUROSCI.5561-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thieben M.J., Duggins A.J., Good C.D., Gomes L., Mahant N., Richards F. The distribution of structural neuropathology in pre-clinical Huntington's disease. Brain. 2002;125:1815–1828. doi: 10.1093/brain/awf179. [DOI] [PubMed] [Google Scholar]
- Treisman M. Temporal discrimination and the indifference interval. Implications for a model of the “internal clock”. Psychological Monographs. 1963;77:1–31. doi: 10.1037/h0093864. [DOI] [PubMed] [Google Scholar]
- Vonsattel J.P., DiFiglia M. Huntington disease. Journal of Neuropathology and Experimental Neurology. 1998;57:369–384. doi: 10.1097/00005072-199805000-00001. [DOI] [PubMed] [Google Scholar]
- Walker F.O. Huntington's disease. Lancet. 2007;369:218–228. doi: 10.1016/S0140-6736(07)60111-1. [DOI] [PubMed] [Google Scholar]
- Wiener M., Turkeltaub P., Coslett H.B. The image of time: A voxel-wise meta-analysis. NeuroImage. 2010;49:1728–1740. doi: 10.1016/j.neuroimage.2009.09.064. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data
A typical adaptive track from a single test run on task Sup by a subject with Huntington's Disease.
Supplementary data
Supplementary data
Supplementary data