Abstract
Inner ear damage leads to nerve fiber growth and synaptogenesis in the ventral cochlear nucleus (VCN). In this study, we documented the relationship between hair cell loss patterns and synaptic plasticity in the chinchilla VCN using immunolabeling of the growth associated protein-43 (GAP-43), a protein associated with axon outgrowth and modification of presynaptic endings. Unilateral round window application of carboplatin caused hair cell degeneration in which inner hair cells (IHC) were more vulnerable than outer hair cells (OHC). One month after carboplatin treatment (0.5 to 5 mg/ml), we observed varying patterns of cochlear hair cell loss and GAP-43 expression in VCN. Both IHC loss and OHC loss were strongly correlated with increased GAP-43 immunolabeling in the ipsilateral VCN. We speculate that two factors might promote the expression of GAP-43 in the VCN; one is the loss of afferent input through IHC or the associated type I auditory nerve fibers. The other occurs when the medial olivocochlear efferent neurons lose their cochlear targets, the OHC, and may as compensation increase their synapse numbers in the VCN.
Keywords: carboplatin, growth associated protein-43, cochlear nucleus, chinchilla, hearing loss, hair cells
1. Introduction
Sensorineural hearing loss causes degenerative as well as regenerative processes in the central auditory system. Cochlear ablation or auditory nerve transection leads to massive degeneration of auditory nerve fibers (Wenthold and Gulley, 1977; Hoeffding and Feldman, 1988; Morest et al., 1997) and deafferentation of neurons in the cochlear nucleus. Likewise, in rat (Michler and Illing, 2003) and chinchilla (Bilak et al., 1997; Kim et al., 1997), exposure to high intensity noise caused degeneration of hair cells, auditory nerve fibers and presynaptic endings on cochlear nucleus neurons. In addition to these well-known degenerative changes, hearing loss associated with cochlear ablation or noise trauma is frequently accompanied by compensatory neuroplastic changes such as growth of fibers and presynaptic endings in the cochlear nucleus (Benson et al., 1997; Bilak et al., 1997; Illing et al., 1997; Michler and Illing, 2002; Muly et al., 2002; Kim et al., 2004). However, the exact relationship between the type and degree of hair cell loss and the type and degree of cochlear nucleus plasticity is poorly understood.
Inner hair cells (IHC) and outer hair cells (OHC) have different functions and their associated auditory nerve fibers different innervation patterns within the cochlear nucleus. Thus, it is reasonable to assume that selective loss of IHC versus OHC might induce different neuroplastic changes in the central auditory system. IHC transduce acoustic stimuli into neuronal signals that are conducted by myelinated type I auditory nerve fibers, which comprise approximately 90–95% of the afferent input to the cochlear nucleus (Spoendlin, 1985). On the other hand, OHC appear to mainly amplify incoming signals in a frequency dependent manner through their electromotility (Mountain, 1980; Davis, 1983; Dallos and Evans, 1995). While OHC are connected to the cochlear nucleus via type II auditory nerve fibers (Brown 1987, Benson and Brown, 2004), they only comprise 5–10% of the auditory nerve fibers (Spoendlin, 1985).
The growth associated protein-43 (GAP-43) is a well-established and sensitive neuroanatomical marker for axon outgrowth and synaptic remodeling (Benowitz and Routenberg, 1997). GAP-43 is a membrane associated protein located in presynaptic endings or axonal growth cones (de Graan et al., 1985; Baetge and Hammang, 1991; Meiri et al., 1998). It is highly expressed during neurite outgrowth and synaptogenesis in the developing nervous system. In the adult nervous system, GAP-43 is expressed in fibers and presynaptic endings (Skene and Willard, 1981; Skene, 1989; Benowitz and Perrone-Bizzozero, 1991; Lin et al., 1992; Mahalik et al., 1992) and is up-regulated during axonal regeneration after damage (Skene and Willard, 1981; Schaden et al., 1994; Ng et al., 1995; Vaudano et al., 1995; Chaisuksunt et al., 2000). In the normal, adult ventral cochlear nucleus (VCN), GAP-43 is expressed at moderate levels in presynaptic endings, but is strongly increased after hearing loss caused by cochlear ablation (Illing et al., 1997; Meidinger et al., 2006), noise trauma (Michler and Illing, 2002) or treatment with ototoxic drugs (Kraus et al., 2009).
The platinum based anticancer drug carboplatin is cytotoxic to hair cells. In the chinchilla, IHC are significantly more vulnerable to carboplatin than OHC. Systemic injections as well as local application of carboplatin on the round window destroy hair cells in a dose dependent manner. Both IHC and OHC are destroyed with high doses whereas low to moderate doses selectively destroy IHC (Wake et al., 1993, 1994; Takeno et al., 1994a, b; Trautwein et al., 1996; Hofstetter et al., 1997a, b; Ding et al., 1999; Reyes et al., 2001). Previously, we have found evidence that loss of IHC and OHC through round window application of carboplatin induced synaptic growth or modifications in the VCN, but not in the dorsal cochlear nucleus (DCN; Kraus et al., 2009). Hair cell loss and VCN plasticity increased with survival time. Pronounced changes were observed one month after treatment (Kraus et al., 2009, Zhou et al., 2009) and VCN plasticity corresponded to the location of hair cell loss in a tonotopic manner (Kraus et al., 2009). The goal of the present study was to determine if VCN synaptic plasticity varied with IHC loss versus OHC loss and the degree of hair cell loss. In order to address these issues, we compared GAP-43 expression pattern in the VCN in cases with varying patterns of IHC loss but similar OHC loss as well as in cases with varying patterns of OHC loss but with similar IHC loss.
2. Methods
2.1. Animals
Eight adult chinchillas of either gender received carboplatin on the right round window in order to induce unilateral hair cell degeneration. Animals were sacrificed 31 days after treatment. Additionally, one untreated animal was used as a naive control. All animal procedures were approved by the University of Buffalo Institutional Animal Care and Use Committee (Protocol #HER05080Y).
2.2. Carboplatin application
Animals (N=8) were anesthetized with isoflurane (Webster Veterinary Supply Inc., Sterling, MA, USA; 5% induction and 2% maintenance) and positioned in a standard head holder. Before surgery, animals were given sterile saline (10 ml, 0.9%, s.q.), atropine (0.15 mg/kg, s.q.), and analgesic (buprenorphine; 0.05 mg/kg i.m.). On the right side, a 3 mm hole was made in the posterior bulla to provide access to the round window. The concentration of carboplatin was varied across animals from 0.5 mg/ml to 5.0 mg/ml in 0.9% saline. With a microsyringe a drop of the carboplatin solution (50 µl) was gently placed on the round window. The hole was closed with dental cement and the wound sutured. Post surgery animals received buprenorphine (0.05 mg/kg i.m) and carprofen (4 mg/kg i.m.). One day after surgery, animals received a second injection of carprofen (4 mg/kg i.m.).
2.3. Tissue preparation
On the day of sacrifice, animals received a lethal dose of sodium pentobarbital and were transcardially perfused with phosphate buffered saline (PBS, 0.1 M, pH 7.4; Sigma-Aldrich, St. Louis, MO, USA) for 5 minutes and then with 10% phosphate buffered formalin (Fisher Scientific, Pittsburgh, PA, USA) at room temperature for 15 minutes. Cochleae and brains were then post-fixated with 10% phosphate buffered formalin (Fisher Scientific, Pittsburgh, PA, USA) for one week. The outer ear canals and the bullae were carefully examined to rule out the possibility of ear canal obstruction, mechanical damage or infection.
2.4. Cochleograms
Our procedures for preparing cytocochleograms have been described previously (Ding et al., 1998; 1999). The organ of Corti was carefully dissected out of the cochlea, stained with Harris hematoxylin solution, mounted on glass slides in glycerin and coverslipped. Cochleograms were prepared with custom software showing percent missing hair cells as a function of percent distance from apex of the cochlea using lab norms. Specimens were examined at 400X with a compound microscope (Carl Zeiss MicroImaging, Inc., Thornwood, NY, USA) and hair cells were counted in 0.24 mm intervals along the entire length of the cochlea. Hair cells (IHC and OHC separately) were counted as present when cell body and cuticular plate were intact. Finally, the average IHC and OHC loss was calculated for the basal third, middle third and apical third of the cochlea; regions that correspond to high, mid and low frequencies respectively.
2.5. Immunolabeling of cochlear nucleus
Brainstems containing the cochlear nuclei were immersed in 30% sucrose (Sigma-Aldrich) in 0.1M PBS pH 7.4 overnight at 4 °C for c ryoprotection. The following day brainstems were cut into 30 µm thin frontal sections on a freezing microtome at −30 °C . Free floating sections were collected and washed in 0.1 M PBS, pH 7.4, pretreated with H2O2 (Fisher Scientific) for 30 minutes at room temperature for peroxidase deactivation, then pre-incubated in blocking buffer containing 10% normal horse serum (Vector Laboratories, Burlingame, CA, USA) and 0.05% Triton X-100 (Fisher Scientific) in 0.1 M PBS, pH 7.4 for 30 minutes at room temperature. Subsequently, sections were exposed for 2 h at room temperature to an antibody against GAP-43 (MAB347; made in mouse; clone 9-1E12; Millipore, Billerika, MA), which has been shown to specifically detect and bind GAP-43 (Goslin et al., 1991; Schreyer and Skene,, 1991). GAP-43 antibody was added at a concentration of 0.2 µg/ml in 0.1 M PBS (pH 7.4) with 1.0% normal horse serum and 0.05% Triton X-100. GAP-43 was visualized through the indirect staining method utilizing a secondary antibody (rat-adsorbed anti-mouse IgG for GAP-43, 7 µl/ml; Vector Laboratories Burlingame, CA) in PBS with 0.05% Triton X-100 and 1.5% normal horse serum, then the avidin-biotin-peroxidase complex (Elite-ABC, Vector Laboratories; 20 µl of Reagent A and B respectively/ml) in PBS with 0.05% Triton X-100, and finally diaminobenzidine tetrahydrochloride (DAB, 0.05%, Sigma-Aldrich) with nickel ammonium sulfate (0.3%; Fisher Scientific) and H2O2 (0.0015%) in 0.1 M Tris buffer, pH 7.2 (Trizma-base, Sigma-Aldrich). The secondary antibody and ABC incubation steps lasted 1 hour each at room temperature. Between all incubation steps, sections were washed in 0.1 M PBS, pH 7.4 for 15 minutes and the buffer was changed 3 times. Before DAB staining, sections were washed in 0.1 M Tris buffer, pH 7.2. DAB exposure lasted until sections reached the desired staining intensity and duration ranged from 1 to 5 minutes. Care was taken to keep staining at a comparable intensity level in all animals, and not to under stain or over stain tissue in order to obtain reliable and consistent staining ratios. After staining, sections were washed in Tris buffer once, then in PBS several times, mounted on gelatin-coated slides (Fisher Superfrost plus; Fisher Scientific), then dried overnight. Finally, sections were dehydrated in increasing concentrations of ethanol, cleared in xylene and sealed with DPX (Fisher Scientific).
2.6. Photomicrographs and statistical analysis
The left and right VCN from each animal were visualized under bright field illumination (Axioskop, Carl Zeiss MicroImaging, Inc), photographed using a digital camera (SPOT Insight; Diagnostic Instruments, Inc., Sterling Heights, MI, USA) and processed with imaging software (SPOT Software, version 4.6) into 8-bit grey scale. Evaluation and assembly of images were done with Adobe Photoshop 5.5 (Adobe Systems Inc., San Jose, CA, USA). Calculations were done with Microsoft Excel (Version 2003; Microsoft Corporation, Redmond, WA, USA) and statistical evaluations were done with GraphPad Prism (Version 5.01; GraphPad Software Inc, La Jolla, CA, USA).
For evaluation of GAP-43 expression in the left versus right VCN of each animal, images of 6 VCN sections on the left and on the right side were taken under identical illumination conditions. Left and right VCN were matched with respect to location on the rostral-caudal axis. Three sections from the posterior VCN with an inter-section distance of 180 µm and three sections from the anterior VCN with an intersection-distance of 180 µm were used for this analysis. The most caudal and rostral sections were not used due to their small size, as well as the region between posterior and anterior VCN due to the large abundance of nerve fiber bundles. From each VCN image, mean grey tone values were obtained from representative fields in three subregions of the VCN: the dorsal high-frequency region, the middle mid-frequency region, and ventral low-frequency region. In each VCN image, a 0.2 × 0.2 mm square was placed at three distinct areas within each subregion of interest (dorsal, middle or ventral VCN) and the average gray scale value from these three 0.2 × 0.2 mm areas was used for further calculations. Regions at the margin of VCN, larger blood vessels, or ventral areas containing axon bundles were excluded from measurement and analysis. For each VCN image, obtained grey tone values were normalized to the image background grey which was set to 1. Normalized mean grey tone values of unstained tissue from separate unstained VCN sections plus the normalized image background grey tone values were then subtracted from the normalized grey tone values of immunolabeled sections in order to obtain net immunostaining grey tone values.
Statistical comparisons were made to determine significant correlations between IHC or OHC loss and GAP-43 expression in the VCN. The right-to-left ratio of staining intensity was calculated for each VCN subregion (dorsal high-frequency region, middle mid-frequency region and ventral low-frequency region) for each section separately. Then the average (+/−SEM) right-to-left ratio for each subregion was obtained for each animal. Correlations (Pearson, two-tailed, P < 0.05) were computed between hair cell loss and changes of VCN immunolabeling, by pairing percent hair cell loss and VCN right-to-left staining ratios from matching frequency regions (high, middle and low frequencies) from the same animal.
3. Results
3.1. Hair cell loss and GAP-43 expression in VCN
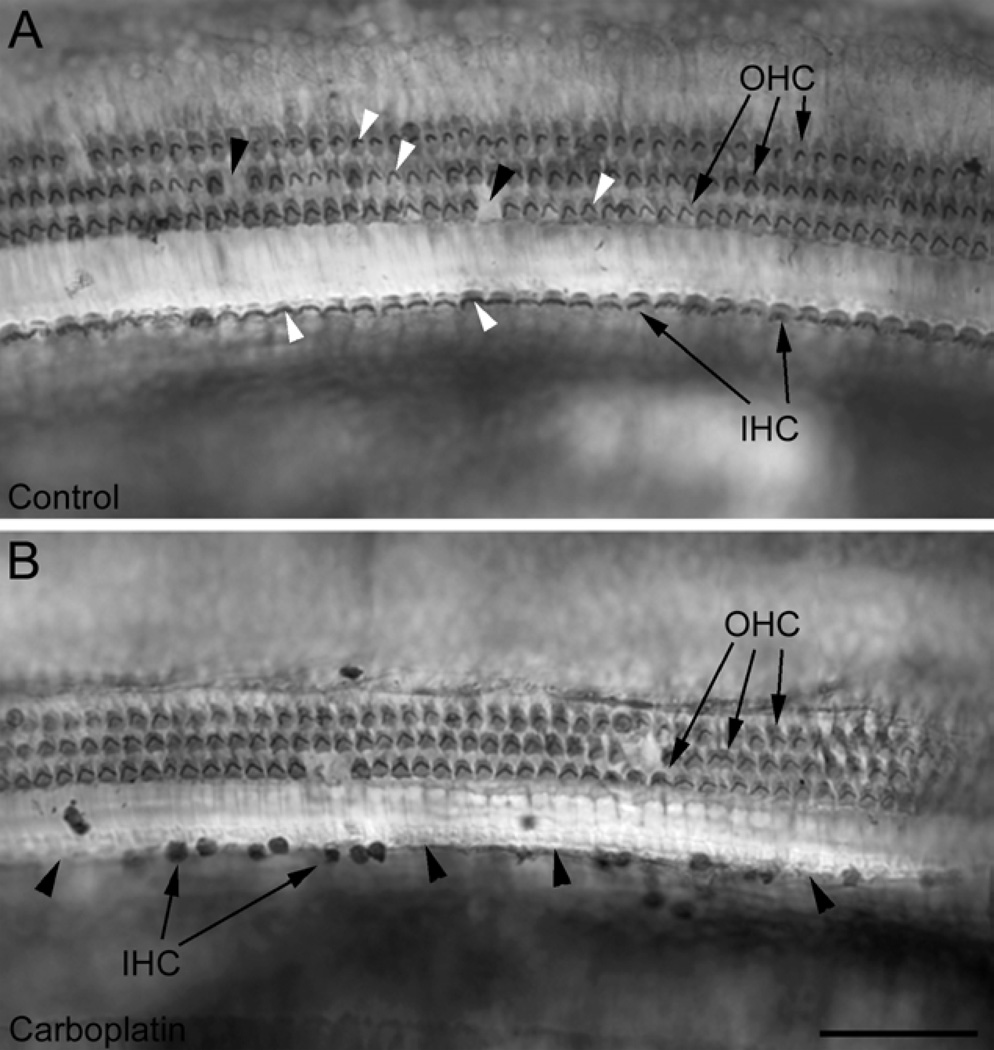
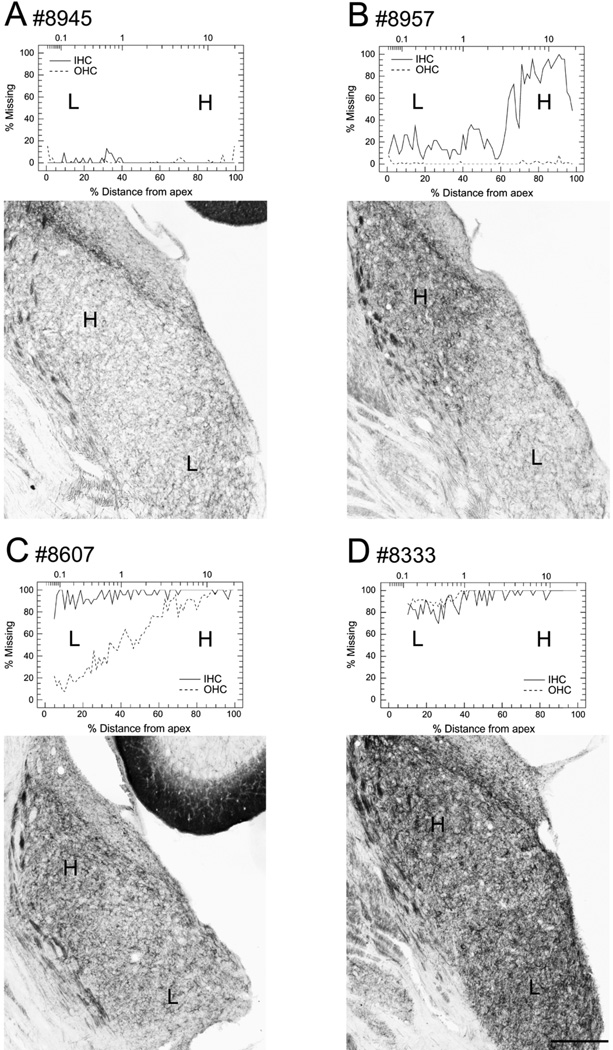
Unilateral carboplatin application on the round window resulted in hair cell loss (Fig. 1) and increased GAP-43 expression in VCN on the same side 31 days after treatment (Fig. 2, 3). The pattern of hair cell loss varied among individual animals ranging from no loss, partial hair cell loss to complete loss of all hair cells (Fig. 4, Table 1). The four cochleograms in figure 4 show the variation in the location and extent of hair cell loss in the treated ears of experimental animals; the upper x-axis presents the frequency-place map for the chinchilla (Greenwood, 1990) and the lower x-axis shows the percent distance from the apex. In most animals with partial hair cell loss, more IHC than OHC were missing (Fig. 1, 4, Table 1), consistent with earlier observations that IHC are more vulnerable than OHC to carboplatin (see Introduction).
Figure 1.
Photomicrographs of surface preparations of the chinchilla inner ear showing a single row of inner hair cells (IHC, arrows) and three rows of outer hair cells (OHC, arrows). (A) In the untreated control ear IHC and OHC could be clearly recognized with their stereocilia (white arrowheads). Black arrowheads point to a few missing OHC. (B) Thirty-one days after application of a moderate-dose of carboplatin, there was a pronounced loss of hair cells, typically more IHC than OHC. Arrowheads point to empty spaces between remaining IHC. Scale bar = 100 urn.
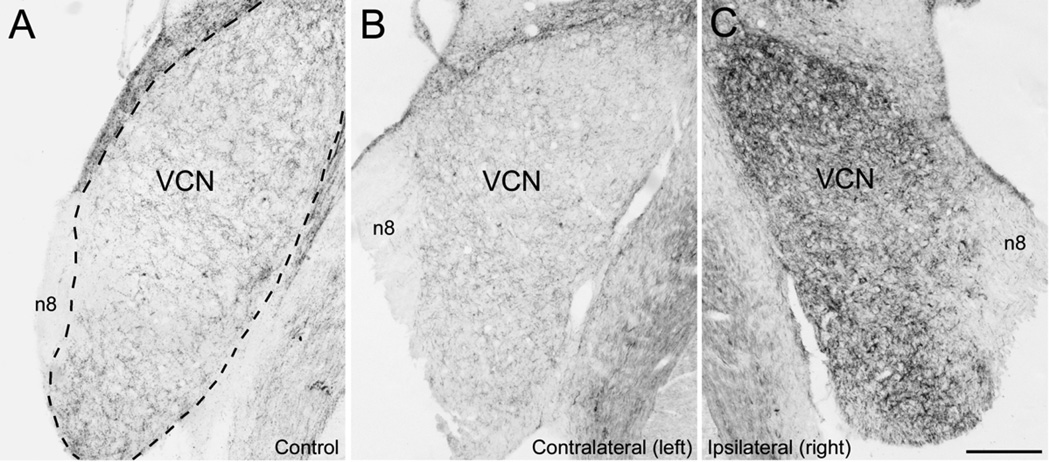
Figure 2.
Expression of GAP-43 in VCN of a naive control animal (A) and in the left and right VCN (contralateraly and ipsilaterally to treatment respectively) of one experimental animal with loss of all hair cells (#8959) 31 days after carboplatin application on right round window (B, C): In the naive control (A), GAP-43 was expressed throughout the VCN at a moderate level in bouton-like structures and some fibers. In contrast, GAP-43 was largely undetectable in the auditory nerve (n8). After carboplatin application on the right round window, which resulted in massive loss of hair cells in the right cochlea, the VCN as well as the auditory nerve showed strong GAP-43 immunoreactivity on the right side, ipsilaterally to treatment (C) while GAP-43 staining on the left side, contralaterally to treatment (B) remained similar to the naive control animal (A). Dashed line in (A) indicates the outline of the VCN. n8, 8th nerve, VCN, ventral cochlear nucleus. Scale bar = 250 urn.
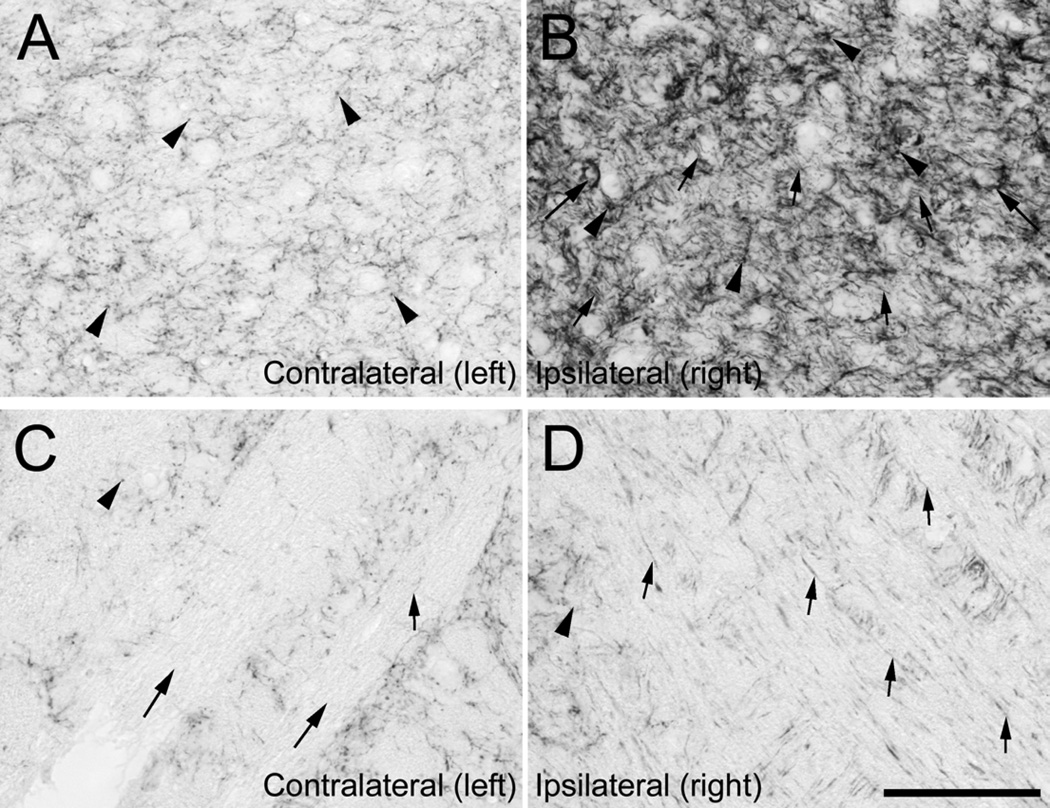
Figure 3.
Expression of GAP-43 in the ventral cochlear nucleus (A, B) and auditory nerve (C, D) at high magnification, in one experimental animal (# 8959) 31 days after receiving carboplatin on the right round window, which resulted in loss of IHC and OHC in the treated ear: In VCN on the left side, contralaterally to treatment (A), GAP-43 was present at moderate levels in bouton-like structures (arrowheads). In VCN on the right side, ipsilaterally to treatment (B) GAP-43 was highly expressed in bouton-like structures (arrowheads), in fibers (small arrows) and in ring-like structures (large arrows). Auditory nerve fiber bundles on the left side (C, large arrows) did not contain GAP-43 except for single fibers (small arrow). In contrast, auditory nerve fiber bundles on the right side (D) contained many fibers positive for GAP-43. Scale bar = 100 µm.
Figure 4.
Cochleograms showing the degree of hair cell loss in the right ear and photomicrographs showing GAP-43 expression in the right VCN 31 days after carboplatin application on the right round window in four experimental animals: Cochleograms show percent loss of IHC (solid line) and OHC (dashed line), the lower horizontal axis shows the percent distance from the apex; the upper horizontal axis the frequency-place map for the chinchilla cochlea expressed in kHz. (A) Animals with little or no hair cell loss (#8945) showed only moderate GAP-43 expression in VCN, comparable to the naive control. Animals with pronounced hair cell loss showed strong GAP-43 expression in VCN on the same side. (B, C) GAP-43 labeling in the VCN followed a tonotopic manner such that high frequency cochlear lesions resulted in strong GAP-43 labeling in high-frequency regions of the VCN. In animals with partial hair cell loss, there were typically more IHC missing than OHC (#8957 and #8607). These animals also showed a gradient of hair cell loss with more hair cells missing in the basal high frequency region than in the apical low frequency region, as well as stronger GAP-43 expression in the dorsal high frequency VCN than in the ventral low frequency VCN. (D) Animals with total or almost total hair cell loss (#8333) along the entire cochlea showed strong GAP-43 expression along the entire dorsal-to-ventral gradient of the VCN. H, high frequencies; L, low frequencies. Scale bar = 250 µm.
Table 1.
Analysis of hair cell loss (IHC and OHC) and immunoreactivity in VCN (i:c ratios of GAP-43) in matching frequency regions from eight experimental animals with carboplatin applied to the right round window.
| Animal | Frequency | Cochlear Region |
IHC loss (%) |
OHC loss (%) |
VCN Region |
VCN, GAP-43 i:c ratio |
n8 GAP-43 |
|---|---|---|---|---|---|---|---|
| # 8945 | High | Basal turn | 0.00 | 1.43 | Dorsal | 0.8261824 | l: − |
| Middle | Middle turn | 1.04 | 0.14 | Middle | 0.8391482 | r: − | |
| Low | Apical turn | 1.61 | 1.12 | Ventral | 0.9041731 | ||
| # 8958 | High | Basal turn | 2.50 | 11.20 | Dorsal | 1.056180 | l: − |
| Middle | Middle turn | 1.50 | 0.64 | Middle | 0.9992841 | r: − | |
| Low | Apical turn | 0.18 | 1.62 | Ventral | 0.9167453 | ||
| # 8961 | High | Basal turn | 21.04 | 0.38 | Dorsal | 0.8899869 | l: − |
| Middle | Middle turn | 26.16 | 0.84 | Middle | 0.9131013 | r: − | |
| Low | Apical turn | 10.97 | 1.32 | Ventral | 0.9267626 | ||
| # 8957 | High | Basal turn | 79.00 | 1.00 | Dorsal | 1.731345 | l: − |
| Middle | Middle turn | 22.00 | 0.00 | Middle | 1.146468 | r: + | |
| Low | Apical turn | 16.00 | 1.00 | Ventral | 0.9815341 | ||
| # 8607 | High | Basal turn | 99.12 | 93.19 | Dorsal | 1.512054 | [No data] |
| Middle | Middle turn | 96.94 | 62.77 | Middle | 1.459321 | ||
| Low | Apical turn | 92.43 | 23.64 | Ventral | 1.213368 | ||
| # 8333 | High | Basal turn | 98.95 | 100.00 | Dorsal | 2.332905 | l: − |
| Middle | Middle turn | 94.61 | 98.91 | Middle | 3.596729 | r: + | |
| Low | Apical turn | 82.87 | 89.54 | Ventral | 2.725628 | ||
| # 8959 | High | Basal turn | 100.00 | 100.00 | Dorsal | 3.219907 | l: − |
| Middle | Middle turn | 100.00 | 100.00 | Middle | 3.187501 | r: + | |
| Low | Apical turn | 100.00 | 100.00 | Ventral | 2.782275 | ||
| # 8608 | High | Basal turn | 100.00 | 100.00 | Dorsal | 3.550979 | l: − |
| Middle | Middle turn | 100.00 | 100.00 | Middle | 3.372276 | r: + | |
| Low | Apical turn | 100.00 | 100.00 | Ventral | 3.025038 | ||
Qualitative data on GAP-43 expression in auditory nerve fibers, “−” presents no difference from the untreated naive control with no GAP-43 expression in the auditory except for single individual fibers, “+” presents GAP-43 expression in numerous fibers. GAP-43, growth-associated protein-43; IHC, inner hair cells; OHC, outer hair cells; VCN, ventral cochlear nucleus.
In the naïve, control animal GAP-43 was expressed at a moderate level throughout the VCN (Fig 2A); labeling appeared mainly in bouton-like structures which have previously been identified as presynaptic endings in the rat (Meidinger et al., 2006). GAP-43 expression could also be seen in a few nerve fibers in the VCN but not in cell bodies. Within the auditory nerve (n8), almost no nerve fibers contained GAP-43. In experimental animals, GAP-43 expression in VCN as well as in auditory nerve fibers on the untreated (left) side appeared similar to the naive control; moderate immunoreactivity was present in bouton-like structures and in a few fibers in the VCN, but almost no immunoreactivity was evident within the auditory nerve (n8) (Fig. 2B, 3A,C).
The VCN and auditory nerve fibers on the treated (right) side showed varying patterns of GAP-43 staining depending on degree of hair cell loss (Fig. 2C, 3B,D, 4; Table 1). In animals with little or no hair cell loss (#8945, #8958, #8961) there was no recognizable difference from the left, untreated side, or from the naive control. In these cases, there was moderate GAP-43 expression in the VCN and almost no expression in the auditory nerve. In contrast, in animals with extensive hair cell loss (#8957, #8959, #8607, #8333 and #8608) GAP-43 staining was conspicuously darker on the right, treated side than on the left, untreated side or in the naive control. The increased immunoreactivity was restricted to morphologically distinct structures (Fig. 3B). Compared to the naive control and the left, untreated side in experimental animals, staining on the right, treated side was strongly increased in bouton-like structures. Additionally, there was increased expression of GAP-43 in ring-like structures and fiber fascicles. Moreover, in the auditory nerve numerous fibers were present and many of those immunopositive for GAP-43 (Fig. 3D).
3.2. Correlation analysis
Since a strong increase in immunostaining was seen in the right VCN (ipsilaterally to treatment), but little or no increase was observed in the left VCN (contralaterally to treatment) compared to the naive control, the right-to-left ratio (ipsilateral-to-contralateral; i:c) of VCN staining intensity was used to assess the observed differences. Ipsilateral-to-contralateral (i:c) ratio values > 1 represent increased expression in the right VCN on the treated side whereas values near 1 represent no difference in staining between the two sides.
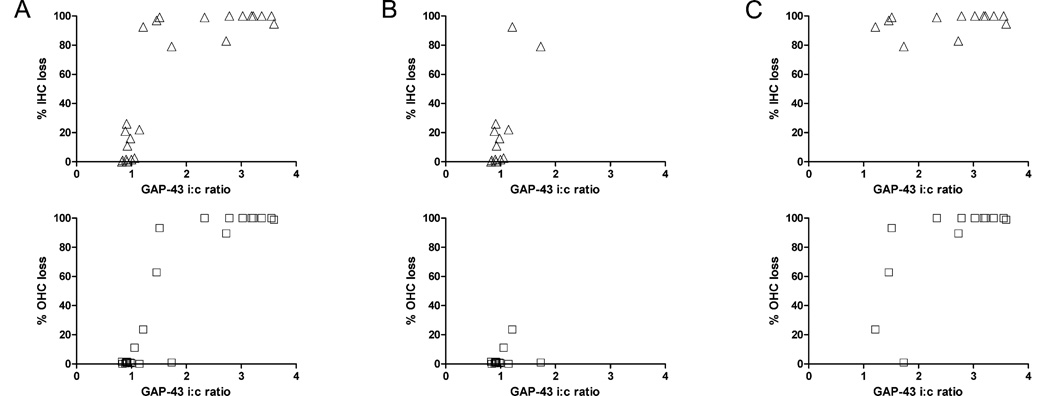
Table 1 shows the i:c staining ratios in the dorsal (high frequencies), middle (mid-frequencies) and ventral (low-frequencies) regions of the VCN versus the degree of IHC loss and OHC loss in the basal (high-frequencies), middle (mid-frequencies) and apical (low frequencies) thirds of the cochlea in each individual animal. Figure 4 shows cochlear and VCN regions of four of the experimental animals. Figure 5A shows scattergrams of IHC (top row) and OHC (bottom row) loss values versus the GAP-43 i:c ratio of VCN staining intensity for each animal using data from the low-, mid- and high-frequency regions. GAP-43 staining ratios increased significantly with increases in IHC and OHC losses (N=24; P<0.0001 for IHC; P<0.0001 for OHC). In order to determine the specific contribution of IHC loss, GAP-43 staining ratios were evaluated further in a subset of data (N=13) from animals (#8945, #8958, #8961, #8957, #8607) in which there were regions with little or no OHC loss (Fig. 5B, bottom row) but highly varying IHC losses (Fig. 5B, top row). In this subgroup, GAP-43 staining increased and was strongly correlated with IHC loss (P=0.0019) but not with OHC loss (P=0.4477). To evaluate the contribution of OHC loss, another subset of data (N=13) were obtained from animals (#8957, #8607, #8333, #8608, #8959) in which there were regions with all or almost all IHC missing (Fig. 5C, top row) but highly varying OHC loss (Fig. 5C, bottom row). In this subgroup GAP-43 staining increased and was strongly correlated with OHC loss (P=0.0071), but not with IHC loss (P=0.2313).
Figure 5.
A) Scattergrams showing hair cell loss (IHC, triangles; OHC, squares) plotted against the ipsi-to-contralateral (i:c) GAP-43 staining ratios in the VCN for matching frequencies and animals: Data pairs (N=24) were obtained separately from low-frequency regions (ventral VCN and apical third of cochlea), middle-frequency regions, (middle VCN and middle third of the cochlea) and high-frequency regions (dorsal VCN and basal third of the cochlea). Ratios >1 indicate that GAP-43 expression is greater on the right, treated side relative to the left, untreated side. GAP-43 increased significantly with increases in IHC and OHC losses. (B) Scattergrams for a subset of the data (N=13) covering all cases with little or no loss of OHC (0–24%), but strongly varying amount of IHC loss (0–92%). In this subgroup, GAP-43 expression was correlated with IHC loss but not OHC loss. (C) Scattergrams for a subset of data points (N=13) covering all cases with a high degree of IHC loss (triangles, 79–100%) and strongly varying amounts of OHC loss (1–100%). In this subgroup, the increase in GAP-43 expression was correlated with OHC loss but not IHC loss.
4. Discussion
4.1. Summary results
The main finding of the current study is that GAP-43 upregulation in the VCN was strongly correlated with carboplatin-induced IHC and OHC loss. In cases where there was minimal OHC loss (OHC constant) but varying patterns of IHC loss, GAP-43 expression in the VCN increased with the degree of IHC loss. Likewise, in cases where there was nearly total loss of IHC (IHC constant) but varying patterns of OHC loss, GAP-43 expression increased with the degree of OHC loss.
4.2. GAP-43 expression in auditory nerve
Earlier studies have shown that systemic carboplatin injection causes significant degeneration of spiral ganglion neurons and auditory nerve fibers, which begins prior to hair cell death (Ding et al., 1998; Takeno et al., 1998; Ding et al., 1999; Wang et al., 2003). These very early neuropathologies may be due to excitotoxicity caused by excess glutamate release from malfunctioning IHC (Wang et al., 2003). Lin et al (2011) found degeneration of synaptic ribbons on IHC and subsequent spiral ganglion loss after noise exposure, likely due to excitotoxicity and even though the IHC themselves survived.
In the current study, numerous auditory nerve fibers were still present 31 days after carboplatin treatment even after loss of all hair cells. This is consistent with observations that spiral ganglion neurons are able to survive despite lack of hair cells, presumably due to survival factors provided by support cells (Sugawara et al., 2005; Zilberstein et al., 2012). Moreover, type II spiral ganglion cells may be particularly resistant against carboplatin-induced degeneration. While they receive excitatory glutamatergic input from OHC they may only respond to strong acoustic stimulation (Weisz et al., 2009). This suggests that type II neurons may be resistant to excitotoxicity consistent with previous studies in which kainic acid was infused into the cochlea (Juiz et al., 1989). Type II neurons were also found to be more resistant to neomycin ototoxicity than type I neurons (Leake and Hradek, 1988).
The up-regulation of GAP-43 in auditory nerve fibers on the treated side is indicative of mechanisms associated with regeneration or remodeling. On the other hand, surviving neurons showing markers for regeneration or remodeling such as GAP-43 up-regulation may nevertheless degenerate at a later time point (Schmitt et al., 1999; Kraus and Illing, 2005). In addition to rapid degeneration, Takeno et al. (1998) showed slow progressive degeneration of spiral ganglion neurons between 2 and 12 weeks after carboplatin treatment, where spiral ganglion cell death and IHC loss matched in extent as well as in location on the cochlear axis. Similarly, removal of all hair cells within 24 h by ethacrynic acid and gentamicin caused progressive degeneration of almost all spiral ganglion neurons with less than 30% remaining after one month and less than 1% remaining after four months (McFadden et al., 2004). Sugawara and colleagues showed a particularly slow, progressive degeneration of the auditory nerve over more than five years after carboplatin treatment (Sugawara et al., 2005); the slow degeneration of auditory nerve fibers may be related to neurotrophic factors that promote neuronal survival. Numbers of surviving hair cells, support cells (Sugawara et al., 2005) or olivocochlear fibers (McFadden et al., 2004) may determine the extent as well as the duration of progressive auditory nerve fiber degeneration. All together, the findings in the present and earlier studies demonstrate that ototoxic drug treatment causes loss of sensory input to VCN cells due to hair cell loss as well as progressive spiral ganglion cell and auditory nerve degeneration, but where a subset may possibly survive. Up-regulation of GAP-43 indicates a capability of structural remodeling of auditory nerve synapses on VCN cells, but it remains unclear if or what kind of long-lasting alterations occur.
4.3. Increased GAP-43 expression in VCN
Severe hair cell loss in the right cochlea increased the GAP-43 right-to-left ratio in VCN; this increase was mainly due to elevated GAP-43 expression in the right VCN since GAP-43 expression on the left, undamaged side was similar to the naive control. The observed ipsilateral increase is consistent with previous observations in other models of hearing loss involving unilateral cochlear ablation (Illing et al., 1997) or unilateral noise trauma (Michler and Illing, 2002; Kraus et al., 2011) showing strong up-regulation of GAP-43 in the ipsilateral VCN. While changes in GAP-43 expression may also have occurred on the contralateral side (as seen after noise trauma, Michler et al., 2002) to a smaller extent, such changes were not detected in our material.
4.3.1. Origin of GAP-43
The carboplatin-induced increase in GAP-43 expression in the ipsilateral VCN (Fig. 2, 3) could conceivably originate in auditory nerve fibers coursing through the VCN and in their presynaptic endings terminating on VCN neurons. Evidence in support of this hypothesis comes from observations showing the presence of GAP-43 in auditory nerve fibers as well as in fiber fascicles and ring-like structures (i.e., presumably end bulbs of Held; Ryugo and Fekete, 1982) in the VCN (Fig. 2C). This suggests that type I fibers may be one source contributing to the GAP-43 increase in VCN after carboplatin treatment. Another source may be the surviving type II fibers, which synapse on multipolar, bushy cells and small cells in the central VCN (Benson and Brown, 2004) in addition to the peripheral granule cell regions (Ryugo, 1992). However, other data suggest that auditory nerve fibers are not the major source of GAP-43 upregulation. If GAP-43 was being upregulated in auditory nerve fibers coursing through the cochlea nucleus, then GAP-43 should also increase in DCN since both type I and type II auditory nerve fibers also terminate in the DCN as well as the VCN. Failure to observe a major increase in GAP-43 in the DCN suggests that their endings are unlikely to be a major source for GAP-43 increase in the VCN (Kraus et al., 2009). Therefore, other structures are more likely to play a prominent role in GAP-43 up-regulation.
GAP-43 expression was strongly increased in numerous bouton-like structures in the VCN. Previous studies have provided evidence that these originate in medial olivocochlear (MOC) neurons following cochlear ablation (Kraus and Illing, 2004; Meidinger et al., 2006): When most MOC neurons were removed, there was little up-regulation of GAP-43 in the VCN after cochlear destruction compared to animals with intact MOC neurons (Kraus et al., 2004). Besides projecting to the OHC, MOC neurons send excitatory axonal collaterals to the VCN which may serve to counterbalance the inhibitory feedback at the level of the cochlea (Benson and Brown, 1990; Warr, 1992). With reduced cochlear input to the VCN following cochlear ablation or hair cell loss, there may be a greater opportunity for MOC neurons to form excitatory synapses on VCN neurons that have been deprived of most of their afferent inputs. Functionally, increased excitatory input from MOC neurons may compensate for the reduced input from the auditory nerve resulting from cochlear damage, and may thus play a role in rebalancing binaural inputs as we have discussed earlier (Kraus et al., 2004; Kraus et al., 2009) or for suppressing aberrant neural activity that gives rise to tinnitus (Kraus et al., 2011). There is also evidence for cross-modal plasticity originating in somatosensory systems. Projections from the spinal trigeminal nucleus and the cuneate nucleus to the cochlear nucleus, including the magnocellular region in the VCN, increase after deafening as reflected by increased expression of vesicular glutamate transporters in the guinea pig (Zeng et al., 2012). These projections may be responsible for increasing spontaneous activity in the cochlear nucleus and may therefore play a role in tinnitus (Shore et al., 2007; Dehmel et al., 2008; Zeng et al., 2012).
4.3.2. Possible triggers
The increase of GAP-43 in the VCN was associated with severe hair cell loss in tonotopically matching areas. A tonotopic up-regulation of GAP-43 was previously observed after partial ablation of the rat cochlea where IHC as well as OHC and spiral ganglion were removed (Illing et al., 2005) as well as after carboplatin treatment of the chinchilla ear (Kraus et al., 2009). The results of the current study in which the degree of IHC and OHC was manipulated indicate that both IHC loss and OHC loss contribute to the increase in GAP-43 expression in the VCN. In cases with little or no OHC loss, GAP-43 expression in the ipsilateral VCN increased with increasing loss of IHC. Similarly, in cases where virtually all IHC were missing, GAP-43 expression continued to increase when the OHC were destroyed. One interpretation of these results is that both IHC and OHC loss modulate the expression of GAP-43 in the VCN. Correlation of GAP-43 increase with IHC loss might suggest that the increase may be directly caused by the IHC loss itself, which reduces the input on VCN cells. Alternatively, the input on VCN cells can be reduced by loss of the associated type I auditory nerve fibers, which has been found to match the loss of IHC (Takeno et al., 1998). Third, degeneration of IHC synaptic ribbons interrupts the transmission from IHC to the auditory nerve and VCN. Since synaptic ribbons degeneration precedes IHC loss after carboplatin treatment (Wang et al., 2003), it appears a more likely trigger than IHC loss itself. Synaptic ribbon degeneration has also been found to occur on surviving IHC after noise exposure, thereby reducing input to VCN despite IHC survival (Lin et al., 2011). Assuming that synaptic ribbon degeneration is the major cause for GAP-43 increase in VCN, instead of IHC or auditory nerve fiber loss, our results suggest that most synapse degeneration occurs in regions with destroyed IHC, and only little in regions with intact IHC, otherwise the GAP-43 increase would not match IHC loss patterns. In further support of this, after noise exposure, regions with synapse degenerations were the regions with slow spiral ganglion cell death (Lin et al., 2011), which matches patterns of IHC loss after carboplatin (Takeno et al., 1998). Together, IHC death, synaptic ribbon degeneration or type I auditory nerve fiber loss all result in reduced neural input to the VCN, thereby decreasing primary sensory input to VCN cells which increases expression of GAP-43.
Similarly, OHC loss or degeneration of type II auditory nerve fibers may result in reduced signaling to the VCN thereby increasing GAP-43. However, this would most likely only play a minor role since type II neurons comprise only 5–10% of the auditory nerve. Second, while type II neurons project to central regions of VCN where they share postsynaptic targets with type I fibers (Benson and Brown, 2004), their synaptic inputs are considerably smaller than the type I fiber endings. Third, because of their suggested high resistance to excitotoxicity, type II fiber degeneration after carboplatin seems unlikely. Another mechanism by which OHC loss can reduce input to VCN is the resulting loss of the cochlear amplifier (Dallos and Harris, 1978) which reduces IHC/type I fiber signaling to the VCN. However, in cases where all IHC were destroyed in this study, loss of OHC cochlear amplification is unlikely responsible for increased GAP-43 because afferent signaling via the IHC/type I fibers would already be absent due to loss of IHC. Thus, it is somewhat puzzling that OHC loss correlated with a strong up-regulation of GAP-43 in the VCN.
But OHC loss may increase GAP-43 expression in VCN indirectly via its effect on MOC-neurons. A major source of sprouting synapses in the VCN after hearing loss apparently comes from cholinergic MOC neurons, as observed after cochlear ablation (Kraus and Illing, 2004; Meidinger et al., 2006). These MOC neurons mainly project to OHC (White and Warr, 1983; Warr, 1992; Azeredo et al., 1999) and only a few project to IHC (Warr and Boche, 2003; Sobkowicz et al., 2004). Since OHC loss results in the loss of the post-synaptic targets of the MOC neurons, this may stimulate compensatory regenerative mechanisms in MOC neurons causing them to establish new synapses in the VCN. However, Brown and Vetter (2008) found no increase of MOC innervation to the VCN despite nonfunctional MOC-synapses on OHC in the α9 knock-out mouse, making the hypothesis of central MOC synapse sprouting as a compensation mechanism for lost cochlear innervation less attractive. Thus, OHC loss alone appears unlikely to trigger synapse sprouting in VCN, but may require additional conditions to occur. One such requirement may be deafferentation of postsynaptic cells in VCN caused by auditory nerve fiber degeneration, which may cause a demand for growth of new synapses on these cells. More speculatively, GAP-43 increase is not triggered by OHC loss, but by type I fiber degeneration which may follow a similar topographic pattern as OHC loss after carboplatin treatment at higher doses.
In summary, IHC loss as well as OHC loss correlates with increased GAP-43 expression in the VCN ipsilateral to the lesion. Suggested mechanisms for increased GAP-43 expression are reduced sensory input to VCN cells by loss of hair cells, synaptic ribbons or auditory nerve fibers, and possibly by loss of MOC neuron targets.
Carboplatin application on the chinchilla round window results in hair cell degeneration
Hair cell loss causes GAP-43 increase in the ventral cochlear nucleus ipsilaterally
GAP-43 increase correlates with degree of inner hair cell loss as well as outer hair cell loss
Acknowledgements
Supported by NOHR Grant 1068911 and by NIH grant R01DC009091 and R01DC009219.
List of Abbreviations
- DCN
dorsal cochlear nucleus
- GAP-43
growth associated protein-43
- i:c
ipsi-to-contralateral
- IHC
inner hair cells
- MOC neurons
medial olivocochlear neurons
- OHC
outer hair cells
- VCN
ventral cochlear nucleus
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
KS Kraus, Email: skkraus@buffalo.edu.
D Ding, Email: dding@buffalo.edu.
H Jiang, Email: hj5@buffalo.edu.
MH Kermany, Email: Mohammad.HabibyKermany@roswellpark.org.
S Mitra, Email: sucharita.mitra@gmail.com.
RJ Salvi, Email: salvi@buffalo.edu.
References
- Azeredo WJ, Kliment ML, Morley BJ, Relkin E, Slepecky NB, Sterns A, Warr WB, Weekly JM, Woods CI. Olivocochlear neurons in the chinchilla: a retrograde fluorescent labelling study. Hear. Res. 1999;134:57–70. doi: 10.1016/s0378-5955(99)00069-6. [DOI] [PubMed] [Google Scholar]
- Baetge EE, Hammang JP. Neurite outgrowth in PC12 cells deficient in GAP-43. Neuron. 1991;6:21–30. doi: 10.1016/0896-6273(91)90118-j. [DOI] [PubMed] [Google Scholar]
- Benowitz LI, Perrone-Bizzozero NI. The expression of GAP-43 in relation to neuronal growth and plasticity: when, where, how, and why. Prog. Brain. Res. 1991;89:69–87. doi: 10.1016/s0079-6123(08)61716-1. [DOI] [PubMed] [Google Scholar]
- Benowitz LI, Routtenberg A. GAP-43: an intrinsic determinant of neuronal development and plasticity. Trends Neurosci. 1997;20:84–91. doi: 10.1016/s0166-2236(96)10072-2. [DOI] [PubMed] [Google Scholar]
- Benson CG, Gross JS, Suneja SK, Potashner SJ. Synaptophysin immunoreactivity in the cochlear nucleus after unilateral cochlear or ossicular removal. Synapse. 1997;25:243–257. doi: 10.1002/(SICI)1098-2396(199703)25:3<243::AID-SYN3>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Benson TE, Brown MC. Synapses formed by olivocochlear axon branches in the mouse cochlear nucleus. J. Comp. Neurol. 1990;295:52–70. doi: 10.1002/cne.902950106. [DOI] [PubMed] [Google Scholar]
- Benson TE, Brown MC. Postsynaptic targets of type II auditory nerve fibers in the cochlear nucleus. J. Assoc. Res. Otolaryngol. 2004;5:111–125. doi: 10.1007/s10162-003-4012-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilak M, Kim J, Potashner SJ, Bohne BA, Morest DK. New growth of axons in the cochlear nucleus of adult chinchillas after acoustic trauma. Exp. Neurol. 1997;147:256–268. doi: 10.1006/exnr.1997.6636. [DOI] [PubMed] [Google Scholar]
- Brown MC. Morphology of labeled afferent fibers in the guinea pig cochlea. J. Comp. Neurol. 1987;260:591–604. doi: 10.1002/cne.902600411. [DOI] [PubMed] [Google Scholar]
- Brown MC, Vetter DE. Olivocochlear neuron central anatomy is normal in α9 knockout mice. J. Assoc. Res. Otolaryngol. 2008;10:64–75. doi: 10.1007/s10162-008-0144-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaisuksunt V, Zhang Y, Anderson PN, Campbell G, Vaudano E, Schachner M, Liberman AR. Axonal regeneration from CNS neurons in the cerebellum and brainstem of adult rats: correlation with the patterns of expression and distribution of messenger RNAs for L1, CHL1, c-Jun and growth-associated protein-43. Neuroscience. 2000;100:87–108. doi: 10.1016/s0306-4522(00)00254-2. [DOI] [PubMed] [Google Scholar]
- Dallos P, Evans BN. High-frequency motility of outer hair cells and the cochlear amplifier. Science. 1995;267:2006–2009. doi: 10.1126/science.7701325. [DOI] [PubMed] [Google Scholar]
- Dallos P, Harris D. Properties of auditory nerve responses in absence of outer hair cells. J. Neurophysiol. 1978;41:365–383. doi: 10.1152/jn.1978.41.2.365. [DOI] [PubMed] [Google Scholar]
- Davis H. An active process in cochlear mechanics. Hear. Res. 1983;9:79–90. doi: 10.1016/0378-5955(83)90136-3. [DOI] [PubMed] [Google Scholar]
- de Graan PN, van Hooff CO, Tilly BC, Oestreicher AB, Schotman P, Gispen WH. Phosphoprotein B-50 in nerve growth cones from fetal rat brain. Neurosci. Lett. 1985;61:235–241. doi: 10.1016/0304-3940(85)90470-7. [DOI] [PubMed] [Google Scholar]
- Dehmel S, Cui YL, Shore SE. Cross-modal interactions of auditory and somatic inputs in the brainstem and midbrain and their imbalance in tinnitus and deafness. Am. J. Audiol. 2008;17:193–209. doi: 10.1044/1059-0889(2008/07-0045). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding D, Wang J, Salvi R, Henderson D, Hu BH, McFadden SL. Selective loss of inner hair cells and type-I spiral ganglion neurons in carboplatin-treated chinchillas. Mechanisms of damage and protection. Ann. N. Y. Acad. Sci. 1999;884:152–170. doi: 10.1111/j.1749-6632.1999.tb08640.x. [DOI] [PubMed] [Google Scholar]
- Ding D, Wang J, Zheng X-Y, Salvi RJ. Early damage of spiral ganglion caused by carboplatin in chinchilla. Journal of Audiology and Speech Pathology. 1998;6:65–67. [Google Scholar]
- Goslin K, Schreyer DJ, Skene JH, Banker G. Changes in distribution of GAP-43 during the development of neuronal polarity. J. Neurosci. 1991;10:588–602. doi: 10.1523/JNEUROSCI.10-02-00588.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood DD. A cochlear frequency-position function for several species--29 years later. J. Acoust. Soc. Am. 1990;87:2592–2604. doi: 10.1121/1.399052. [DOI] [PubMed] [Google Scholar]
- Hoeffding V, Feldman ML. Degeneration in the cochlear nerve of the rat following cochlear lesions. Brain Res. 1988;449:104–115. doi: 10.1016/0006-8993(88)91029-3. [DOI] [PubMed] [Google Scholar]
- Hofstetter P, Ding D, Powers N, Salvi RJ. Quantitative relationship of carboplatin dose to magnitude of inner and outer hair cell loss and the reduction in distortion product otoacoustic emission amplitude in chinchillas. Hear. Res. 1997a;112:199–215. doi: 10.1016/s0378-5955(97)00123-8. [DOI] [PubMed] [Google Scholar]
- Hofstetter P, Ding D, Salvi RJ. Magnitude and pattern of inner and outer hair cell loss in chinchilla as function of carboplatin dose. Audiology. 1997b;36:301–311. doi: 10.3109/00206099709071981. [DOI] [PubMed] [Google Scholar]
- Illing RB, Horváth M, Laszig R. Plasticity of the auditory brainstem: effects of cochlear ablation on GAP-43 immunoreactivity in the rat. J. Comp. Neurol. 1997;382:116–138. doi: 10.1002/(sici)1096-9861(19970526)382:1<116::aid-cne8>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Illing RB, Kraus KS, Meidinger MA. Reconnecting neuronal networks in the auditory brainstem following unilateral deafening. Hear. Res. 2005;206:185–199. doi: 10.1016/j.heares.2005.01.016. [DOI] [PubMed] [Google Scholar]
- Juiz JM, Rueda J, Merchan JA, Sala ML. The effects of kainic acid on the cochlear ganglion of the rat. Hear. Res. 1989;40:65–74. doi: 10.1016/0378-5955(89)90100-7. [DOI] [PubMed] [Google Scholar]
- Kim JJ, Gross J, Morest DK, Potashner SJ. Quantitative study of degeneration and new growth of axons and synaptic endings in the chinchilla cochlear nucleus after acoustic overstimulation. J. Neurosci. Res. 2004;77:829–842. doi: 10.1002/jnr.20211. [DOI] [PubMed] [Google Scholar]
- Kim JJ, Morest DK, Bohne BA. Degeneration of axons in the brainstem of the chinchilla after auditory overstimulation. Hear. Res. 1997;103:169–191. doi: 10.1016/s0378-5955(96)00173-6. [DOI] [PubMed] [Google Scholar]
- Kraus KS, Ding D, Jiang H, Lobarinas E, Sun W, Salvi RJ. Relationship between noise-induced hearing-loss, persistent tinnitus and growth-associated protein-43 expression in the rat cochlear nucleus: Does synaptic plasticity in ventral cochlear nucleus suppress tinnitus. NeuroScience. 2011;194:309–325. doi: 10.1016/j.neuroscience.2011.07.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraus KS, Ding D, Zhou Y, Salvi RJ. Central auditory plasticity after carboplatin-induced unilateral inner ear damage in the chinchilla: Up-regulation of GAP-43 in the ventral cochlear nucleus. Hear. Res. 2009;255:33–43. doi: 10.1016/j.heares.2009.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraus KS, Illing RB. Superior olivary contributions to auditory system plasticity: medial but not lateral olivocochlear neurons are the source of cochleotomy-induced GAP-43 expression in the ventral cochlear nucleus. J. Comp. Neurol. 2004;475:169–191. doi: 10.1002/cne.20180. [DOI] [PubMed] [Google Scholar]
- Kraus KS, Illing RB. Cell death or survival: Molecular and connectional conditions for olivocochlear neurons after axotomy. NeuroScience. 2005;134:467–481. doi: 10.1016/j.neuroscience.2005.04.037. [DOI] [PubMed] [Google Scholar]
- Leake PA, Hradek GT. Cochlear pathology of long term neomycin induced deafness in cats. Hear. Res. 1988;33:11–33. doi: 10.1016/0378-5955(88)90018-4. [DOI] [PubMed] [Google Scholar]
- Lin LH, Bock S, Carpenter K, Rose M, Norden JJ. Synthesis and transport of GAP-43 in entorhinal cortex neurons and perforant pathway during lesion-induced sprouting and reactive synaptogenesis. Mol. Brain Res. 1992;14:147–153. doi: 10.1016/0169-328x(92)90024-6. [DOI] [PubMed] [Google Scholar]
- Lin HW, Furman AC, Kujawa SG, Liberman MC. Primary neural degeneration in the guinea pig cochlea after reversible noise-induced threshold shift. J. Assoc. Res. Otolaryngol. 2011;12:605–616. doi: 10.1007/s10162-011-0277-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahalik TJ, Carrier A, Owens GP, Clayton G. The expression of GAP-43 mRNA during late embryonic and early postnatal development of the CNS of the rat: an in situ hybridization study. Dev. Brain Res. 1992;67:75–83. doi: 10.1016/0165-3806(92)90027-t. [DOI] [PubMed] [Google Scholar]
- McFadden SL, Ding D, Jiang H, Salvi RJ. Time course of efferent fiber and spiral ganglion cell degeneration following complete hair cell loss in the chinchilla. Brain Res. 2004;997:40–51. doi: 10.1016/j.brainres.2003.10.031. [DOI] [PubMed] [Google Scholar]
- Meidinger MA, Hildebrandt-Schoenfeld H, Illing RB. Cochlear damage induces GAP-43 expression in cholinergic synapses of the cochlear nucleus in the adult rat: a light and electron microscopy study. Eur. J. Neurosci. 2006;23:3187–3199. doi: 10.1111/j.1460-9568.2006.04853.x. [DOI] [PubMed] [Google Scholar]
- Meiri KF, Saffell JL, Walsh FS, Doherty P. Neurite outgrowth stimulated by neural cell adhesion molecules requires growth associated protein-43 (GAP-43) function and is associated with GAP-43 phosphorylation in growth cones. J. Neurosci. 1998;18:10429–10437. doi: 10.1523/JNEUROSCI.18-24-10429.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michler SA, Illing RB. Acoustic trauma induces reemergence of the growth- and plasticity-associated protein GAP-43 in the rat auditory brainstem. J. Comp. Neurol. 2002;451:250–266. doi: 10.1002/cne.10348. [DOI] [PubMed] [Google Scholar]
- Michler SA, Illing RB. Molecular plasticity in the rat auditory brainstem: modulation of expression and distribution of phosphoserine, phospho-CREB and TrkB after noise trauma. Audiol Neurootol. 2003;8:190–206. doi: 10.1159/000071060. [DOI] [PubMed] [Google Scholar]
- Morest DK, Kim J, Bohne BA. Neuronal and transneuronal degeneration of auditory axons in the brainstem after cochlear lesions in the chinchilla: cochleotopic and non-cochleotopic patterns. Hear. Res. 1997;103:151–168. doi: 10.1016/s0378-5955(96)00172-4. [DOI] [PubMed] [Google Scholar]
- Mountain DC. Changes in endolymphatic potential and crossed olivocochlear bundle stimulation alter cochlear mechanics. Science. 1980;210:71–72. doi: 10.1126/science.7414321. [DOI] [PubMed] [Google Scholar]
- Muly SM, Gross JS, Morest DK, Potashner SJ. Synaptophysin in the cochlear nucles following acoustic trauma. Exp. Neurol. 2002;177:202–221. doi: 10.1006/exnr.2002.7963. [DOI] [PubMed] [Google Scholar]
- Ng TF, So KF, Chung SK. Influence of peripheral nerve grafts on the expression of GAP-43 in regenerating retinal ganglion cells in adult hamsters. J. Neurocytol. 1995;24:487–496. doi: 10.1007/BF01179974. [DOI] [PubMed] [Google Scholar]
- Reyes S, Ding D, Sun W, Salvi R. Effect of inner and outer hair cell lesions on electrically evoked otoacoustic emissions. Hear. Res. 2001;158:139–150. doi: 10.1016/s0378-5955(01)00309-4. [DOI] [PubMed] [Google Scholar]
- Ryugo DK. The auditory nerve: Peripheral innervation, cell body morphology, and central projections. In: Webster DB, Popper AN, Fay RR, editors. The mammalian auditory pathway: Neuroanatomy. Springer Verlag; New York: 1992. pp. 23–65. [Google Scholar]
- Ryugo DK, Fekete DM. Morphology of primary axosomatic endings in the anteroventral cochlear nucleus of the cat: a study of the endbulbs of Held. J. Comp. Neurol. 1982;210:239–257. doi: 10.1002/cne.902100304. [DOI] [PubMed] [Google Scholar]
- Schaden H, Stuermer CAO, Bähr M. GAP-43 immunoreactivity and axon regeneration in retinal ganglion cells of rat. J. Neurobiol. 1994;25:1570–1578. doi: 10.1002/neu.480251209. [DOI] [PubMed] [Google Scholar]
- Schmitt AB, Breuer S, Voell M, Schwaiger FW, Spitzer C, Pech K, Brook GA, Noth J, Kreutzberg GW, Nacimiento W. GAP-43 (B-50) and C-Jun are up-regulated in axotomized neurons of Clarke's nucleus after spinal cord injury in the adult rat. Neurobiol. Dis. 1999;6:122–130. doi: 10.1006/nbdi.1998.0231. [DOI] [PubMed] [Google Scholar]
- Schreyer DJ, Skene JH. Fate of GAP-43 in ascending spinal axons of DRG neurons after peripheral nerve injury: delayed accumulation and correlation with regenerative potential. J. Neurosci. 1991;11:3738–3751. doi: 10.1523/JNEUROSCI.11-12-03738.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shore S, Zhou J, Koehler S. Neural mechanisms underlying somatic tinnitus. Prog. Brain. Res. 2007;166:107–123. doi: 10.1016/S0079-6123(07)66010-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skene JH. Axonal growth-associated proteins. Annu. Rev. Neurosci. 1989;12:127–156. doi: 10.1146/annurev.ne.12.030189.001015. [DOI] [PubMed] [Google Scholar]
- Skene JH, Willard M. Characteristics of growth-associated polypeptides in regenerating toad retinal ganglion cell axons. J. Neurosci. 1981;1:419–426. doi: 10.1523/JNEUROSCI.01-04-00419.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobkowicz HM, August BK, Slapnick SM. Synaptic arrangements between inner hair cells and tunnel fibers in the mouse cochlea. Synapse. 2004;52:299–315. doi: 10.1002/syn.20026. [DOI] [PubMed] [Google Scholar]
- Spoendlin H. Anatomy of cochlear innervation. Am. J. Otolaryngol. 1985;6:453–467. doi: 10.1016/s0196-0709(85)80026-0. [DOI] [PubMed] [Google Scholar]
- Sugawara M, Corfas G, Liberman MC. Influence of supporting cells on neuronal degeneration after hair cell loss. J. Assoc. Res. Otolaryngol. 2005;6:136–147. doi: 10.1007/s10162-004-5050-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeno S, Harrison RV, Ibrahim D, Wake M, Mount RJ. Cochlear function after selective inner hair cell degeneration induced by carboplatin. Hear. Res. 1994a;75:93–102. doi: 10.1016/0378-5955(94)90060-4. [DOI] [PubMed] [Google Scholar]
- Takeno S, Harrison RV, Mount RJ, Wake M, Harada Y. Induction of selective hair cell damage by carboplatin. Scanning Microsc. 1994b;8:97–106. [PubMed] [Google Scholar]
- Takeno S, Wake M, Mount RJ, Harrison RV. Degeneration of spiral ganglion cells in the chinchilla after inner hair cell loss induced by carboplatin. Audiol. Neurootol. 1998;3:281–290. doi: 10.1159/000013800. [DOI] [PubMed] [Google Scholar]
- Trautwein P, Hofstetter P, Wang J, Salvi R, Nostrant A. Selective inner hair cell loss does not alter distortion product otoacoustic emissions. Hear. Res. 1996;96:71–82. doi: 10.1016/0378-5955(96)00040-8. [DOI] [PubMed] [Google Scholar]
- Vaudano E, Campbell G, Anderson PN, Davies AP, Woolhead C, Schreyer DJ, Lieberman AR. The effects of a lesion or a peripheral nerve graft on GAP-43 upregulation in the adult rat brain: an in situ hybridization and immunocytochemical study. J. Neurosci. 1995;15:3594–3611. doi: 10.1523/JNEUROSCI.15-05-03594.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wake M, Takeno S, Ibrahim D, Harrison R. Selective inner hair cell ototoxicity induced by carboplatin. Laryngoscope. 1994;104:488–493. doi: 10.1288/00005537-199404000-00016. [DOI] [PubMed] [Google Scholar]
- Wake M, Takeno S, Ibrahim D, Harrison R, Mount R. Carboplatin ototoxicity: an animal model. J. Laryngol. Otol. 1993;107:585–589. doi: 10.1017/s0022215100123771. [DOI] [PubMed] [Google Scholar]
- Wang J, Ding D, Salvi RJ. Carboplatin-induced early cochlear lesion in chinchillas. Hear. Res. 2003;181:65–72. doi: 10.1016/s0378-5955(03)00176-x. [DOI] [PubMed] [Google Scholar]
- Warr WB. Organization of olivocochlear efferent systems in mammals. In: Webster DB, Popper AN, Fay RR, editors. The mammalian auditory pathway: neuroanatomy. New York: Springer Verlag; 1992. pp. 410–448. [Google Scholar]
- Warr WB, Boche JE. Diversity of axonal ramifications belonging to single lateral and medial olivocochlear neurons. Exp. Brain Res. 2003;153:499–513. doi: 10.1007/s00221-003-1682-3. [DOI] [PubMed] [Google Scholar]
- Weisz C, Glowatzki E, Fuchs P. The postsynaptic function of Type II cochlear afferents. Nature. 2009;461:1126–1129. doi: 10.1038/nature08487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenthold RJ, Gulley RL. Aspartic acid and glutamic acid levels in the cochlear nucleus after auditory nerve lesion. Brain Res. 1977;138:111–123. doi: 10.1016/0006-8993(77)90787-9. [DOI] [PubMed] [Google Scholar]
- White JS, Warr WB. The dual origins of the olivocochlear bundle in the albino rat. J Comp. Neurol. 1983;219:203–214. doi: 10.1002/cne.902190206. [DOI] [PubMed] [Google Scholar]
- Zeng C, Yang Z, Shreve L, Bledsoe S, Shore S. Somatosensory projections to cochlear nucleus are upregulated after unilateral deafness. J. Neurosci. 2012;32:15791–15801. doi: 10.1523/JNEUROSCI.2598-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Ding D, Kraus KS, Yu D, Salvi RJ. Functional and structural changes in the chinchilla cochlea and vestibular system following round window application of carboplatin. Audiol. Med. 2009;7:189–199. doi: 10.3109/16513860903335795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zilberstein Y, Liberman MC, Corfas G. Inner hair cells are not required for survival of spiral ganglion neurons in the adult cochlea. J. Neurosci. 2012;32:404–410. doi: 10.1523/JNEUROSCI.4678-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]