Abstract
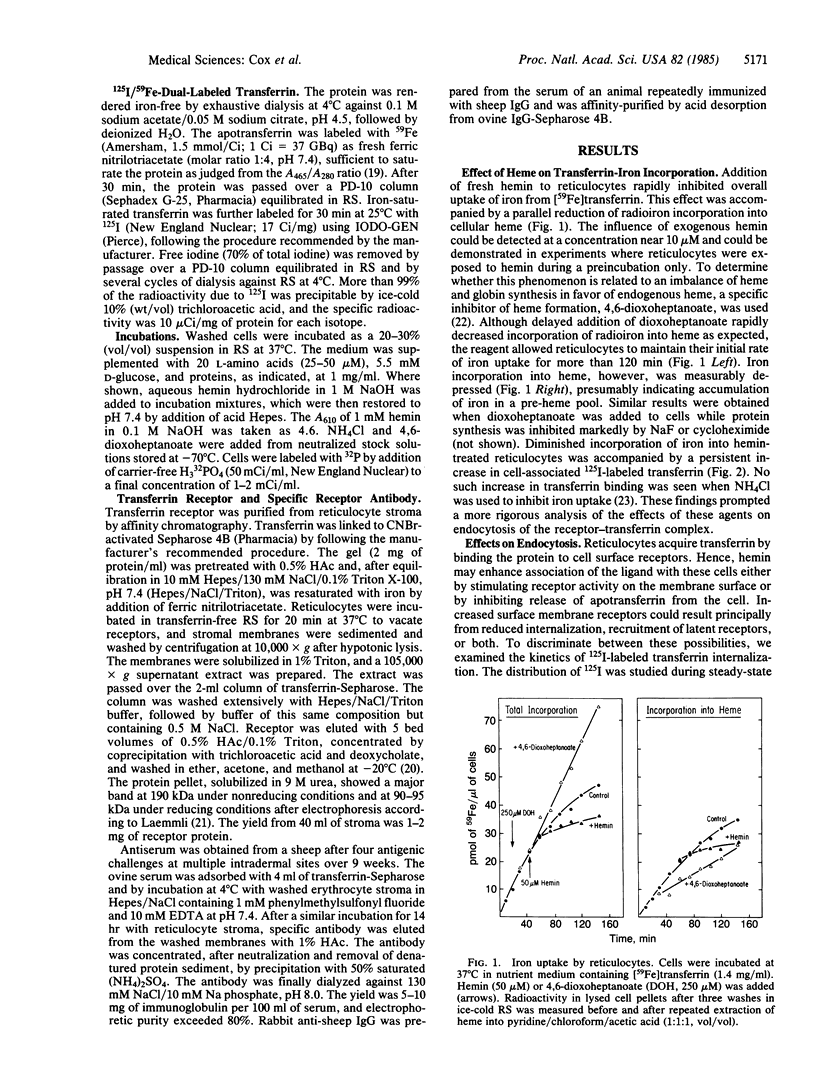
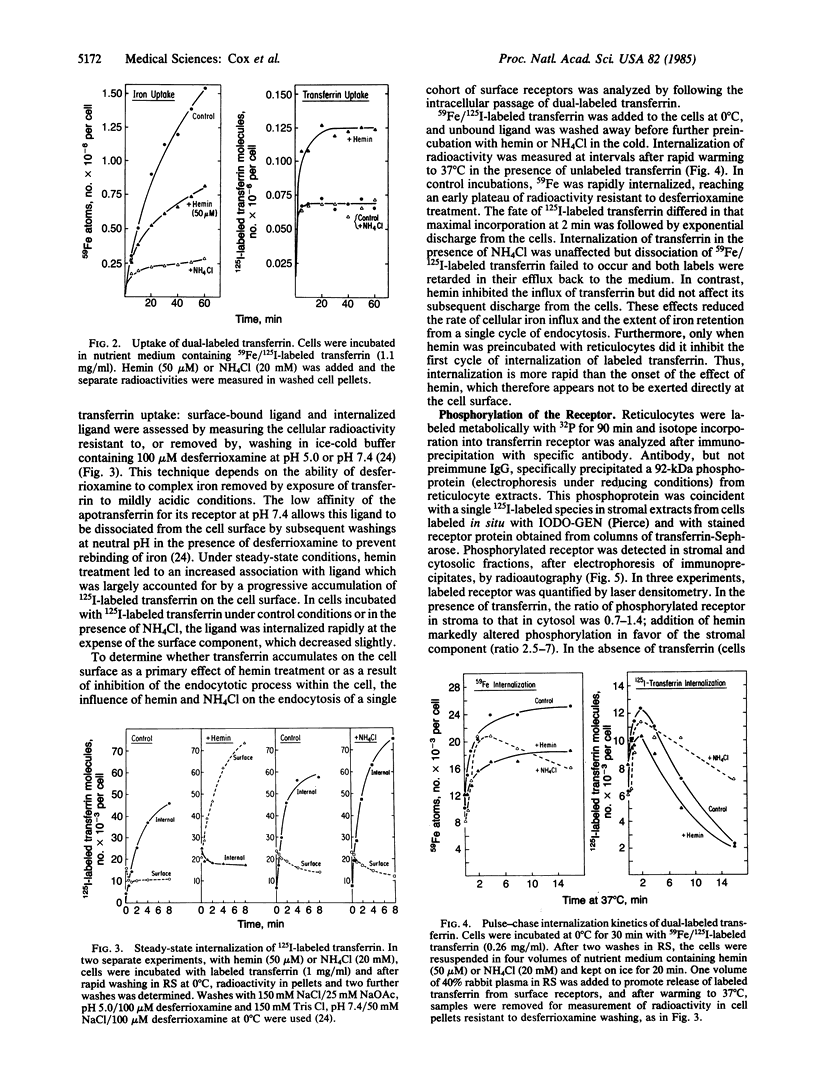
Addition of hemin to reticulocytes inhibits incorporation of iron from transferrin [Ponka, P. & Neuwirt, J. (1969) Blood 33, 609-707]. Heme also regulates protein synthesis in immature erythroid cells through its effects on phosphorylation of the initiation factor eIF-2. We have therefore examined its effects on endocytosis of iron-transferrin and phosphorylation of the transferrin receptor. Hemin (10-50 microM) reduced iron transport but increased cell-associated transferrin. When intracellular iron delivery was inhibited by NH4Cl, no such increase in cell-associated transferrin was seen. During uptake of 125I-labeled transferrin in the steady state, the use of a washing technique to dissociate bound transferrin on the cell membrane showed that radioligand accumulated on the surface of hemin-treated cells. Hemin reduced the initial influx of transferrin, thereby diminishing incorporation of iron. Receptor phosphorylation was investigated by immunoprecipitation of reticulocyte extracts after metabolic labeling with [32P]Pi. In the absence of ligand, phosphorylated receptor was chiefly localized on cell stroma. Exposure to transferrin increased cytosolic phosphorylated receptor from 15-30% to approximately 50% of the total, an effect overcome by hemin treatment. Addition of hemin in the presence of transferrin enhanced net phosphorylated receptor in the reticulocyte in association with a redistribution of phosphorylated receptor to stromal membranes. The findings suggest a possible relationship of phosphorylation to endocytosis of the transferrin receptor in reticulocytes.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aoki Y., Wada O., Urata G., Takaku F., Nakao K. Purification and some properties of delta-aminolevulinate (ALA) synthetase in rabbit reticulocytes. Biochem Biophys Res Commun. 1971 Feb 5;42(3):568–575. doi: 10.1016/0006-291x(71)90409-8. [DOI] [PubMed] [Google Scholar]
- Baker E., Shaw D. C., Morgan E. H. Isolation and characterization of rabbit serum and milk transferrins. Evidence for difference in sialic acid content only. Biochemistry. 1968 Apr;7(4):1371–1378. doi: 10.1021/bi00844a019. [DOI] [PubMed] [Google Scholar]
- Cox T. M., O'Donnell M. W., Camilleri M., Burghes A. H. Isolation and characterization of a mutant liver aldolase in adult hereditary fructose intolerance. Identification of the enzyme variant by radioassay in tissue biopsy specimens. J Clin Invest. 1983 Jul;72(1):201–213. doi: 10.1172/JCI110958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dautry-Varsat A., Ciechanover A., Lodish H. F. pH and the recycling of transferrin during receptor-mediated endocytosis. Proc Natl Acad Sci U S A. 1983 Apr;80(8):2258–2262. doi: 10.1073/pnas.80.8.2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebert P. S., Hess R. A., Frykholm B. C., Tschudy D. P. Succinylacetone, a potent inhibitor of heme biosynthesis: effect on cell growth, heme content and delta-aminolevulinic acid dehydratase activity of malignant murine erythroleukemia cells. Biochem Biophys Res Commun. 1979 Jun 27;88(4):1382–1390. doi: 10.1016/0006-291x(79)91133-1. [DOI] [PubMed] [Google Scholar]
- Gallo R. C. The inhibitory effect of heme on heme formation in vivo: possible mechanism for the regulation of hemoglobin synthesis. J Clin Invest. 1967 Jan;46(1):124–132. doi: 10.1172/JCI105505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grayzel A. I., Hörchner P., London I. M. The stimulation of globin synthesis by heme. Proc Natl Acad Sci U S A. 1966 Mar;55(3):650–655. doi: 10.1073/pnas.55.3.650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris D. C., Aisen P. Iron-donating properties of transferrin. Biochemistry. 1975 Jan 28;14(2):262–268. doi: 10.1021/bi00673a011. [DOI] [PubMed] [Google Scholar]
- Hemmaplardh D., Morgan E. H. The role of endocytosis in transferrin uptake by reticulocytes and bone marrow cells. Br J Haematol. 1977 May;36(1):85–96. doi: 10.1111/j.1365-2141.1977.tb05758.x. [DOI] [PubMed] [Google Scholar]
- Hunt R. C., Ruffin R., Yang Y. S. Alterations in the transferrin receptor of human erythroleukemic cells after induction of hemoglobin synthesis. J Biol Chem. 1984 Aug 10;259(15):9944–9952. [PubMed] [Google Scholar]
- Iacopetta B., Morgan E. Heme inhibits transferrin endocytosis in immature erythroid cells. Biochim Biophys Acta. 1984 Oct 12;805(2):211–216. doi: 10.1016/0167-4889(84)90170-8. [DOI] [PubMed] [Google Scholar]
- Ibrahim N. G., Gruenspecht N. R., Freedman M. L. Hemin feedback inhibition at reticulocyte delta-aminolevulinic acid synthetase and delta-aminolevulinic acid dehydratase. Biochem Biophys Res Commun. 1978 Feb 28;80(4):722–728. doi: 10.1016/0006-291x(78)91304-9. [DOI] [PubMed] [Google Scholar]
- Johnstone R. M., Adam M., Turbide C., Larrick J. Phosphorylation of the transferrin receptor in isolated sheep reticulocyte plasma membranes. Can J Biochem Cell Biol. 1984 Sep;62(9):927–934. doi: 10.1139/o84-119. [DOI] [PubMed] [Google Scholar]
- KARIBIAN D., LONDON I. M. CONTROL OF HEME SYNTHESIS BY FEEDBACK INHIBITION. Biochem Biophys Res Commun. 1965 Jan 18;18:243–249. doi: 10.1016/0006-291x(65)90747-3. [DOI] [PubMed] [Google Scholar]
- Klausner R. D., Ashwell G., van Renswoude J., Harford J. B., Bridges K. R. Binding of apotransferrin to K562 cells: explanation of the transferrin cycle. Proc Natl Acad Sci U S A. 1983 Apr;80(8):2263–2266. doi: 10.1073/pnas.80.8.2263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klausner R. D., Harford J., van Renswoude J. Rapid internalization of the transferrin receptor in K562 cells is triggered by ligand binding or treatment with a phorbol ester. Proc Natl Acad Sci U S A. 1984 May;81(10):3005–3009. doi: 10.1073/pnas.81.10.3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klausner R. D., Van Renswoude J., Ashwell G., Kempf C., Schechter A. N., Dean A., Bridges K. R. Receptor-mediated endocytosis of transferrin in K562 cells. J Biol Chem. 1983 Apr 25;258(8):4715–4724. [PubMed] [Google Scholar]
- LONDON I. M., BRUNS G. P., KARIBIAN D. THE REGULATION OF HEMOGLOBIN SYNTHESIS AND THE PATHOGENESIS OF SOME HYPOCHROMIC ANEMIAS. Medicine (Baltimore) 1964 Nov;43:789–802. doi: 10.1097/00005792-196411000-00024. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Levin D., Ranu R. S., Ernst V., London I. M. Regulation of protein synthesis in reticulocyte lysates: phosphorylation of methionyl-tRNAf binding factor by protein kinase activity of translational inhibitor isolated from hemedeficient lysates. Proc Natl Acad Sci U S A. 1976 Sep;73(9):3112–3116. doi: 10.1073/pnas.73.9.3112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matts R. L., Levin D. H., London I. M. Effect of phosphorylation of the alpha-subunit of eukaryotic initiation factor 2 on the function of reversing factor in the initiation of protein synthesis. Proc Natl Acad Sci U S A. 1983 May;80(9):2559–2563. doi: 10.1073/pnas.80.9.2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May W. S., Jacobs S., Cuatrecasas P. Association of phorbol ester-induced hyperphosphorylation and reversible regulation of transferrin membrane receptors in HL60 cells. Proc Natl Acad Sci U S A. 1984 Apr;81(7):2016–2020. doi: 10.1073/pnas.81.7.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan E. H. Inhibition of reticulocyte iron uptake by NH4Cl and CH3NH2. Biochim Biophys Acta. 1981 Mar 20;642(1):119–134. doi: 10.1016/0005-2736(81)90143-7. [DOI] [PubMed] [Google Scholar]
- Ponka P., Neuwirt J. Regulation of iron entry into reticulocytes. I. Feedback inhibitory effect of heme on iron entry into reticulocytes and on heme synthesis. Blood. 1969 May;33(5):690–707. [PubMed] [Google Scholar]
- Ponka P., Neuwirt J. Regulation of iron entry into reticulocytes. II. Relationship between hemoglobin synthesis and entry of iron into reticulocytes. Biochim Biophys Acta. 1971 Feb 23;230(2):381–392. [PubMed] [Google Scholar]
- Rutherford T. R., Clegg J. B., Weatherall D. J. K562 human leukaemic cells synthesise embryonic haemoglobin in response to haemin. Nature. 1979 Jul 12;280(5718):164–165. doi: 10.1038/280164a0. [DOI] [PubMed] [Google Scholar]
- Schneider C., Sutherland R., Newman R., Greaves M. Structural features of the cell surface receptor for transferrin that is recognized by the monoclonal antibody OKT9. J Biol Chem. 1982 Jul 25;257(14):8516–8522. [PubMed] [Google Scholar]
- Schulman H. M., Wilczynska A., Ponka P. Transferrin and iron uptake by human lymphoblastoid and K-562 cells. Biochem Biophys Res Commun. 1981 Jun;100(4):1523–1530. doi: 10.1016/0006-291x(81)90691-4. [DOI] [PubMed] [Google Scholar]
- Zucker W. V., Schulman H. M. Stimulation of globin-chain initiation by hemin in the reticulocyte cell-free system. Proc Natl Acad Sci U S A. 1968 Feb;59(2):582–589. doi: 10.1073/pnas.59.2.582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Renswoude J., Bridges K. R., Harford J. B., Klausner R. D. Receptor-mediated endocytosis of transferrin and the uptake of fe in K562 cells: identification of a nonlysosomal acidic compartment. Proc Natl Acad Sci U S A. 1982 Oct;79(20):6186–6190. doi: 10.1073/pnas.79.20.6186. [DOI] [PMC free article] [PubMed] [Google Scholar]