Abstract
Ketamine is a unique anesthetic reagent known to produce various psychotic symptoms. Ketamine has recently been reported to elicit a long-lasting antidepressant effect in patients with major depression. Although recent studies provide insight into the molecular mechanisms of the effects of ketamine, the antidepressant mechanism has not been fully elucidated. To understand the involvement of the brain serotonergic system in the actions of ketamine, we performed a positron emission tomography (PET) study on non-human primates. Four rhesus monkeys underwent PET studies with two serotonin (5-HT)-related PET radioligands, [11C]AZ10419369 and [11C]DASB, which are highly selective for the 5-HT1B receptor and serotonin transporter (SERT), respectively. Voxel-based analysis using standardized brain images revealed that ketamine administration significantly increased 5-HT1B receptor binding in the nucleus accumbens and ventral pallidum, whereas it significantly reduced SERT binding in these brain regions. Fenfluramine, a 5-HT releaser, significantly decreased 5-HT1B receptor binding, but no additional effect was observed when it was administered with ketamine. Furthermore, pretreatment with 2,3-dihydroxy-6-nitro-7-sulfamoylbenzo(f)quinoxaline (NBQX), a potent antagonist of the glutamate α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid (AMPA) receptor, blocked the action of ketamine on the 5-HT1B receptor but not SERT binding. This indicates the involvement of AMPA receptor activation in ketamine-induced alterations of 5-HT1B receptor binding. Because NBQX is known to block the antidepressant effect of ketamine in rodents, alterations in the serotonergic neurotransmission, particularly upregulation of postsynaptic 5-HT1B receptors in the nucleus accumbens and ventral pallidum may be critically involved in the antidepressant action of ketamine.
Keywords: AMPA receptor, ketamine, macaque monkey, nucleus accumbens, 5-HT1B receptor, [11C]AZ10419369
Introduction
Ketamine (2-chlorphenyl-2-methylamino-cyclohexanone), a noncompetitive antagonist of the N-methyl-D-aspartic acid (NMDA) glutamate receptor, is known to induce anesthesia, analgesia, hallucinations and a dissociative state.1, 2, 3, 4 In addition, ketamine has recently been demonstrated to have an antidepressant action in patients suffering from treatment-resistant major depressive disorder; onset occurs within 2 h and the duration of the effect is several days following a single administration.5,6 Because existing antidepressants—such as selective serotonin reuptake inhibitors (SSRIs), monoamine oxidase inhibitors and tricyclic antidepressants—take several weeks to reach their full therapeutic effect, ketamine is a possible attractive novel treatment for depression.
Ketamine has also been reported to induce an antidepressant response in rodents: a decrease in immobility time during the forced swim test.7,8 Pretreatment with 2,3-dihydroxy-6-nitro-7-sulfoamoylbenzo(f)quinoxaline (NBQX), a potent blocker of the glutamate α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid (AMPA) receptor, has been reported to attenuate this ketamine-induced behavior in rodents.7, 8, 9 Although the mechanism underlying the blocking effect of NBQX remains poorly understood, AMPA receptor activation has been speculated to have a pivotal role in the antidepressant action of ketamine.10
Several lines of evidence suggest that alterations in the serotonergic component of the central nervous system underlie the pathophysiology of depression.11 Indeed, SSRIs, which increase extracellular serotonin (5-HT) concentrations and extend the duration and magnitude of 5-HT-mediated signals, are widely used as conventional antidepressants, although the onset of therapeutic benefits usually requires several weeks.12 The gap in timing between the immediate increase in the synaptic 5-HT level mediated by SSRIs and the slow symptomatic recovery has not been completely explained yet, but desensitization of presynaptic 5-HT1A and 5-HT1B receptor sub-types has been proposed. In particular, 5-HT1B receptors function as both presynaptic autoreceptors and postsynaptic heteroreceptors.13,14 5-HT1B autoreceptors are located presynaptically on serotonergic neurons and are thought to be involved in a feedback mechanism that inhibits 5-HT neuronal firing and release. In contrast, 5-HT1B heteroreceptors are located on and may exhibit inhibitory activity on glutamatergic, GABAergic, dopaminergic, noradrenergic and cholinergic neurons. In cell culture studies, ketamine inhibits monoamine transporters including the 5-HT transporter (SERT) in a dose-dependent manner.15,16 Ketamine also increases extracellular levels of 5-HT and 5-HT tissue contents in the brain of rodents.17,18 However, the effects of ketamine on the serotonergic system involving presynaptic autoreceptors and SERT in primates have not yet been elucidated.
The decreased brain levels of adaptor protein p11, which regulates the cell-surface expression of 5-HT1B receptors, has been reported in both the mouse model of depression and in patients with unipolar major depression.19 Antidepressant drugs and electroconvulsive therapy were reported to increase p11 mRNA levels in the rodent brain; in keeping with this, mice overexpressing p11 demonstrate antidepressant responses, whereas p11-null mice display a depressive phenotype.19 Recently, a neuroimaging study using positron emission tomography (PET) showed that 5-HT1B receptor binding in the ventral striatum including the nucleus accumbens and ventral pallidum is reduced in patients with major depressive disorder compared with healthy controls.20
To elucidate the involvement of the serotonergic system in the antidepressant action of ketamine in the living brain, we performed a PET imaging study using nonhuman primates and two PET radioligands [11C]AZ10419369 and 11C-labeled N,N-dimethyl-2-(2-amino-4-cyanophenylthio)benzylamine ([11C]DASB), which are highly selective for the 5-HT1B receptor and SERT, respectively. In the case of [11C]AZ10419369, we also investigated the effect of ketamine in combination with fenfluramine, a 5-HT releaser, because [11C]AZ10419369 binding to 5-HT1B receptors is reported to be affected by 5-HT release.21,22 Moreover, to clarify the involvement of the AMPA receptor, we tested whether pretreatment with NBQX blocked the action of ketamine on the binding of [11C]AZ10419369 and [11C]DASB to the 5-HT1B receptor and SERT, respectively.
Materials and methods
Subjects
Four male rhesus monkeys (Macaca mulatta; aged 5–11 years; weight, 5–10 kg) were used. Before the PET study, all animals underwent magnetic resonance imaging (MRI) (3T, Allegra, Siemens, Erlangen, Germany) to provide information on brain anatomy. After MRI, a small head holder (20 × 28 × 19 mm3) was attached to the skull under aseptic surgical conditions for painless fixation of the monkey's head during PET. Animals were maintained and handled in accordance with the recommendations of the United States National Institutes of Health and the local Animal Experimental Committee of Kobe Institute of RIKEN (MAH18-05-15).
Experimental protocol for administration of ketamine and related drugs
A bolus injection of ketamine (30 mg per body weight, i.v.) was administered with atropine pretreatment (0.05 mg kg−1, i.m.) 100 min before the start of the PET scan. Immediately after the treatment, a continuous infusion of ketamine (7.5 mg kg−1h−1, i.v.) was initiated and maintained until the end of the PET scan. In our preliminary experiments, we examined the dose relationship of ketamine on the serotonergic system from 0.75 mg kg−1h−1, which is subanesthetic and is matched to 0.5 mg kg−1per 40 min in a human study causing antidepressive action in some patients with major depression, to 7.5 mg kg−1h−1, which can maintain animals in a sedated state. We observed dose-dependent changes in binding that were not consistently significant at 0.75 mg kg−1h−1. Therefore, we selected 7.5 mg kg−1h−1 for further experiments. Fenfluramine (5 mg kg−1, i.v.; Sigma-Aldrich, St. Louis, MO, USA) was administered 15 min before the start of PET scan. NBQX (1 mg kg−1, i.v.; Wako Pure Chemicals, Osaka, Japan) was administered 10 min before the start of the ketamine infusion. Endotracheal intubation was performed only when animals were exposed to NBQX.
Animals were subjected to [11C]AZ10419369 PET under the following conditions: administration of (1) the vehicle alone in the conscious state (Cont); (2) fenfluramine in the conscious state (Fen); (3) a combination of ketamine and vehicle (Ket); and (4) a combination of ketamine and fenfluramine (Ket+Fen). The same animals were also used to study the effects of administering a combination of ketamine and NBQX (Ket+NBQX) condition. Because 5-HT1B receptor expression in the visual cortex is sensitive to visual stimuli,23 the eyes of the animals were covered with a mask from 150 min before the start of [11C]AZ10419369 PET. [11C]DASB PET scans were performed twice in each animal under the vehicle alone in the conscious state (Cont) and ketamine-treated (Ket) conditions, with an additional scan under the Ket+NBQX condition.
PET study with [11C]AZ10419369 and [11C]DASB
According to previously described methods,24 [11C]AZ10419369 was synthesized by N-[11C]methylation of the corresponding N-methyl precursor, 8-(1-piperazinyl)-5-methylchrom-2-en-4-one-2-(4-morpholinophenyl) carboxamide, with [11C]methyl triflate. The radiochemical purity was >94%, and the specific radioactivity was 43±12 (mean±s.d.) GBq μmol−1. The injected radioactivity was 34.8±2.5 MBq kg−1. The [11C]DASB was prepared based on a previously described method,25 with radiochemical purity of >99.5% and specific radioactivity of 49±15 GBq μmol−1. The injected radioactivity was 36.0±3.2 MBq kg−1.
Before the PET scan, the saphenous vein in one of the legs was cannulated for infusion of drugs or the PET radioligand. Vital signs such as electrocardiography and oxygen saturation were continuously monitored with biomonitoring equipment (BSM-5192; NIHON KOHDEN, Tokyo, Japan). We used a small animal PET scanner, the microPET Focus220 (Siemens Medical Solutions, Knoxville, TN, USA) that has a spatial resolution of 1.35 mm in full width at half maximum at the center of the field of view. As previously described,26 while in the PET scanner, animals were fixed in a sitting position to a primate chair custom-made for rhesus monkeys. The chair was tilted at 30° (ketamine-treated condition) or 45° (ketamine-untreated condition) allowing the animals to remain more comfortable than in a supine or prone posture. A 30-min transmission scan with a 68Ga-68Ge line source was performed for attenuation correction of emission scans, and the emission PET scan was performed for 90 min for each PET radioligand. Images were acquired in the three-dimensional list mode and reconstructed with an algorithm of filtered back projection with data smoothed using the Hanning filter with a cutoff of 0.4. To obtain quantitative images, the emission images were corrected for attenuation using blank and transmission images, but they were not corrected for scatter since the correction algorithm was considered inappropriate in terms of brain tissue homogeneity. A dynamic histogram acquired during 90 min of scanning (6 × 10 s, 6 × 30 s, 11 × 60 s, 15 × 180 s and 3 × 600 s) was used for data analysis.
Image data analysis
Reconstructed PET images were processed and analyzed using PMOD image analysis software version 3.0 (PMOD Technologies, Zurich, Switzerland). To generate the parametric images, we calculated the binding potential (BPND) by quantifying the ratio at the equilibrium of specifically bound radioligand to that of non-displaceable radioligand. Logan linear graphical analysis with a reference tissue model (Logan reference) was performed with exclusion of data recorded during approximately the first 15 min of [11C]AZ10419369.27 The k2′ value (the clearance rate constant from the reference region) was estimated by parametric analysis with a simplified reference tissue model using the cerebellum as a reference region and the occipital cortex as the 5-HT1B receptor-rich region. [11C]DASB BPND values were obtained using the multilinear reference tissue model 2 (MRTM2).28 The k2′ value was estimated by parametric analysis with MRTM using the cerebellum as a reference region and the dorsal raphe nucleus and tectum as the SERT-rich region. Analyses of the PET data were performed in two stages: generation of parametric images and statistical comparisons. For parametric image generation, a summated PET image from 0 to 90 min (sumPETI) was first created. BPND images were then transformed into a standard MRI template space, which was originally created by our institute using brain MRI data from 20 rhesus monkeys, including those used in this study, using the product of two transformation matrices: (sumPETI to individual MRI) × (individual MRI to standard MRI template). Thus, normalized parametric BPND images were generated using PMOD software, and they were spatially smoothed using a 3.5-mm Gaussian kernel of full width at half maximum. For statistical parametric mapping (SPM) analysis, we analyzed the [11C]AZ10419369 BPND images with SPM8, which enabled us to make comparisons on a voxel-wise basis. Repeated ANOVAs were performed to assess the effects of ketamine on [11C]AZ10419369 BPND both for fenfluramine- and its vehicle-treated conditions (a main effect of ketamine), to assess the effects of fenfluramine on [11C]AZ10419369 BPND both for ketamine-treated and -untreated conditions (a main effect of fenfluramine) and to compare whether fenfluramine-induced changes in [11C]AZ10419369 BPND differed between the ketamine-treated and -untreated conditions (a ketamine × fenfluramine interaction effect). Given the exploratory nature of this analysis, the threshold was set at P<0.001, uncorrected for multiple comparisons (T=4.30) and 500 or more contiguous voxels. To calculate region of interest (ROI)-based parameter values, six anatomical ROIs—the nucleus accumbens (Acb), ventral part of the globus pallidus (ventral GP), the midline nucleus reuniens of the thalamus (Tha-Re), occipital cortex (Occ), lateral geniculate nucleus (LGN) and cerebellum—were manually drawn on the template MRI images, with reference to a stereotaxic rhesus brain MRI atlas. Our initial analysis including the Acb, ventral GP and Tha-Re showed significant differences in 5-HT1B receptor binding between ketamine-treated and -untreated conditions. We therefore included additional brain regions related to the visual system, the Occ and LGN, into the ROI set. Regional BPND values were obtained by applying this ROI set to the individual parametric [11C]AZ10419369 and [11C]DASB BPND images that were normalized to the template MRI.
Statistical analysis
Post hoc analyses of a main effect of condition on [11C]AZ10419369 and [11C]DASB BPND values were performed with Dunnett's test to compare the different combinations of ketamine and NBQX conditions with the Cont condition. Comparisons of [11C]AZ10419369 BPND between the right and left sides were made using the paired t-test with Bonferroni correction. P-values <0.05 were regarded as statistically significant. All analyses were performed using the SPSS software package (Version 11.5, SPSS Inc., Chicago, IL, USA).
Results
Effect of ketamine on [11C]AZ10419369 binding to the 5-HT1B receptor
The time-activity curves of the brain levels of [11C]AZ10419369 in each condition showed high uptake in the Occ and GP and low uptake in the cerebellum (Supplementary Figure S1). In the ketamine-treated conditions (both Ket and Ket+Fen), an early peak in the curves for these brain regions was followed by a rapid washout. The maximum uptake in the fenfluramine-treated condition (both Fen and Ket+Fen) tended to be lower than that in the vehicle-treated conditions (both Cont and Ket). Although the time-activity curve patterns were similar for the Ket+NBQX and Cont conditions, the maximum uptake in the Occ in the Ket+NBQX condition tended to be lower than that in the Cont condition (Supplementary Figures S1A and S1E).
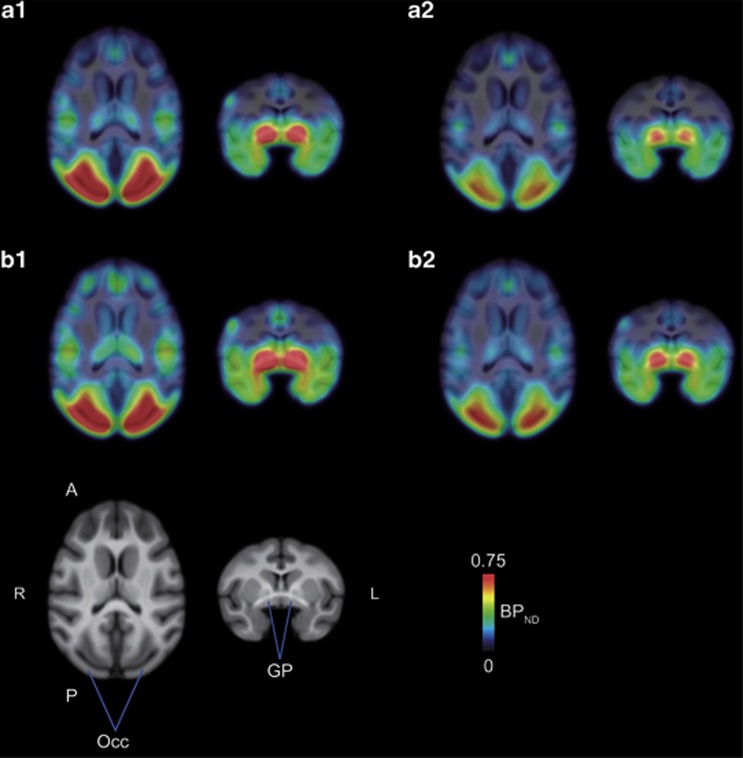
Parametric images of [11C]AZ10419369 BPND were successfully generated in all animals for each condition. The BPND images were normalized to the standard MRI template, and averaged images are shown for each condition (Figure 1). As shown in Figure 2, voxel-based statistical parametric analysis revealed two clusters of significantly increased BPND for the ketamine-treated conditions compared with the ketamine-untreated conditions (both Cont and Fen) (T=4.30, uncorrected P<0.001). The clusters were located in the Acb/ventral GP (Figure 2) and Tha-Re. No decreases in BPND in any region in the ketamine-treated conditions were observed compared with the ketamine-untreated conditions. SPM analysis also revealed two large clusters of decreased BPND for the fenfluramine-treated conditions compared with the vehicle control conditions. These large clusters extended across a range of brain areas consisting of the Acb, ventral GP, Tha-Re, substantia nigra and Occ. The distribution was nearly identical to that of binding sites observed with the in vitro autoradiography of [11C]AZ10419369 (Supplementary Figure S2). In addition, no increases in BPND in any region in the fenfluramine-treated conditions were observed compared with vehicle conditions. Finally, the SPM analysis showed no significant ketamine × fenfluramine interaction in [11C]AZ10419369 binding to the 5-HT1B receptor.
Figure 1.
Spatially normalized parametric images of [11C]AZ10419369 fused onto the rhesus MRI template in each condition. Averaged BPND images (n=4) in the Cont (a1), Fen (a2), Ket (b1) and Ket+Fen (b2) conditions were superimposed on the template MRI. The BPND images are shown in color, whereas the MRIs are shown in gray scale. For each condition, transaxial (left) and coronal (right) slices are shown. A, anterior; GP, globus pallidus; L, left; Occ, occipital cortex; P, posterior; R, right.
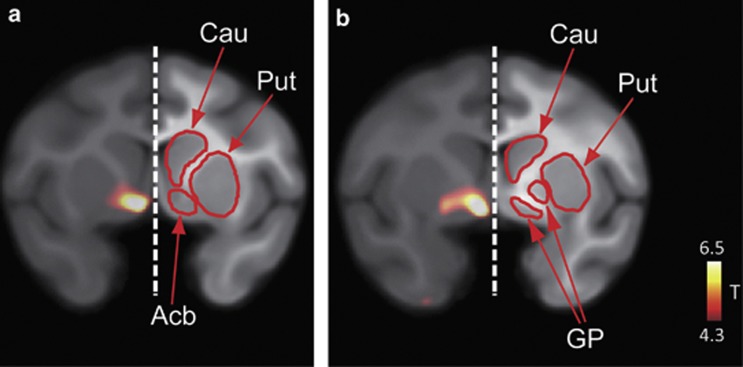
Figure 2.
Voxel-wise comparisons of [11C]AZ10419369 BPND between the ketamine-treated and untreated conditions. Coronal views of the clusters of significant increases in the ketamine-treated condition are shown. Coronal sections are shown in the level of Acb (a) and GP (b). The statistical threshold was set at P<0.001 uncorrected (T-value >4.3). Acb, nucleus accumbens; Cau, caudate nucleus; GP, globus pallidus; Put, putamen.
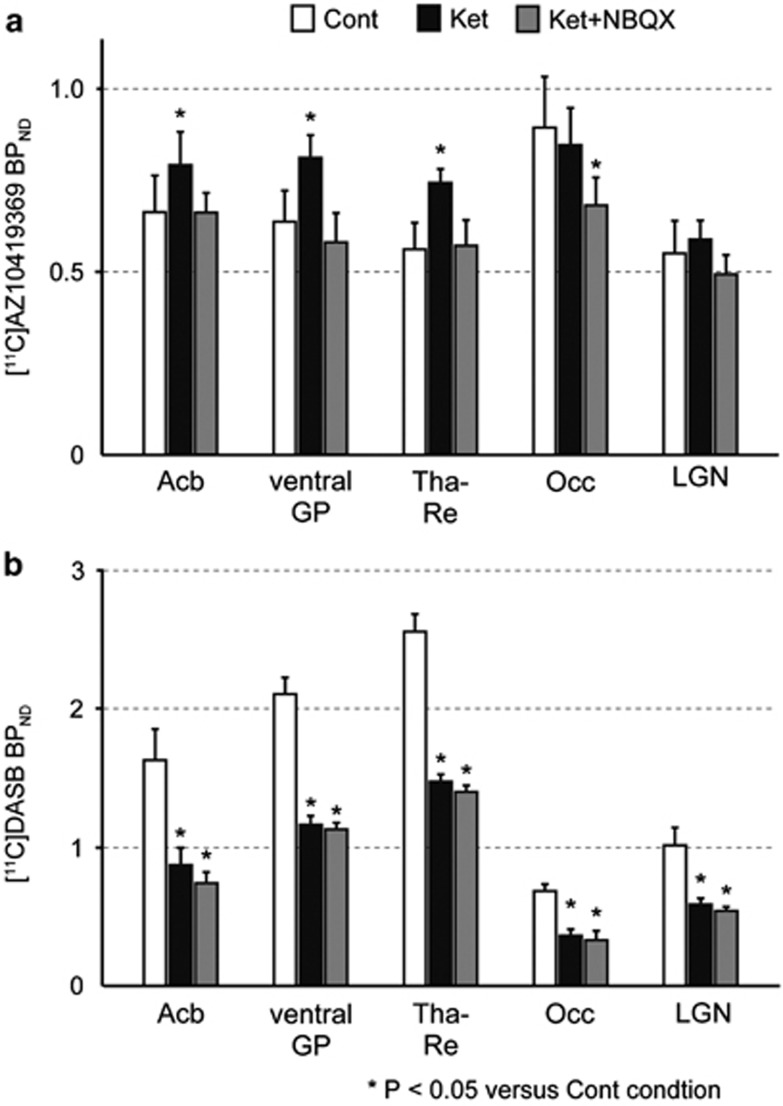
Regional BPND values obtained by applying the ROI set including the Acb, ventral GP, Tha-Re, Occ and LGN also revealed increased [11C]AZ10419369 binding by ketamine in the first three of these regions (Table 1). With the exception of the Tha-Re, no significant differences were observed between the right and left sides (P>0.05, paired t-test with Bonferroni correction). [11C]AZ10419369 binding in the Acb, ventral GP and Tha-Re was significantly higher in the Ket condition than in the Cont condition (average value of two Cont conditions obtained in the fenfluramine and NBQX experiments) (P<0.05) but did not differ between the Ket+NBQX and Cont conditions (Figure 3a). In contrast, binding in the Occ and LGN did not differ significantly between the Ket and Cont conditions. Binding in the Occ under the Ket+NBQX condition was significantly decreased compared with the Cont condition, whereas that in the LGN did not differ between these conditions.
Table 1. Ketamine- and fenfluramine-induced changes in [11C]AZ10419369 BPND.
| Region | Consciousness | Ketamine | Ketamine-induced increase | ||
|---|---|---|---|---|---|
| Vehicle | Fenfluramine | Vehicle | Fenfluramine | Peak of T-value | |
| Cont | Fen | Ket | Ket+Fen | ||
| Acb | 0.64±0.10 | 0.45±0.06 | 0.80±0.09 | 0.55±0.09 | 9.23a |
| Ventral GP | 0.63±0.09 | 0.49±0.04 | 0.82±0.06 | 0.60±0.07 | 9.38a |
| Tha-Re | 0.54±0.06 | 0.36±0.02 | 0.74±0.04 | 0.46±0.05 | 7.77a |
| Occ | 0.87±0.15 | 0.51±0.07 | 0.85±0.10 | 0.58±0.07 | 2.24 |
| LGN | 0.54±0.07 | 0.39±0.03 | 0.59±0.03 | 0.47±0.03 | 3.16 |
Abbreviations: Acb, nucleus accumbens; LGN, lateral geniculate nucleus; Occ, occipital cortex; Tha-Re, midline nucleus reuniens of the thalamus; Ventral GP, ventral part of the globus pallidus. Values are expressed as the mean ±s.e.m.
The statistical threshold is set at Po0.001, uncorrected for multiple comparisons (T =4.30).
Figure 3.
Effects of ketamine (with and without NBQX pretreatment) on binding to the 5-HT1B receptor and SERT. Binding potential (BPND) of [11C]AZ10419369 to 5-HT1B receptor (a) and BPND of [11C]DASB to SERT (b) in five brain regions are shown in three conditions. Asterisks (*) indicate significant differences compared with the Cont condition. Statistical analyses were performed the Dunnett's test. The following regions are shown: the nucleus accumbens (Acb), ventral part of the globus pallidus (ventral GP), midline nucleus reuniens of the thalamus (Tha-Re), lateral geniculate nucleus (LGN) and occipital cortex (Occ). The bar graphs show the mean±s.e.m.
Effect of ketamine on [11C]DASB binding to the SERT
The time-activity brain uptake curves of [11C]DASB for each condition showed high uptake in the Acb, Occ, GP and thalamus (Supplementary Figure S3). As was the case for PET with [11C]AZ10419369, an early peak in the curves for these brain regions was followed by a rapid washout in the Ket conditions (Supplementary Figures S3A and S3B). Moreover, the Ket+NBQX condition showed a similar time-activity curve as the Ket condition (Supplementary Figures S3B and S3C). Ketamine significantly decreased [11C]DASB binding in almost all brain regions, including the Acb, ventral GP, Tha-Re, Occ and LGN compared with the Cont condition (P<0.05, Figure 3b and Supplementary Figure S4). Pretreatment with NBQX did not change the decrease in [11C]DASB binding in any regions.
Discussion
We performed a PET study using [11C]AZ10419369 and [11C]DASB to elucidate the involvement of the serotonergic system in the antidepressant actions of ketamine in the living brain. Using conscious rhesus monkeys, we obtained the following results, which strongly suggest a critical role for the 5-HT1B receptor in the Acb, ventral GP and Tha-Re: (i) ketamine administration significantly increased 5-HT1B receptor binding in three brain regions, the Acb, ventral GP and Tha-Re, where SERT binding was significantly decreased and (ii) pretreatment with NBQX blocked the ketamine-induced increases in 5-HT1B receptor binding in the Acb, ventral GP and Tha-Re but not in the LGN and Occ, whereas it did not affect ketamine-induced decreases in SERT binding.
Studies in both cell culture and rats have revealed that ketamine inhibits reuptake of 5-HT and increases tissue contents and extracellular levels of 5-HT in the brain.15, 16, 17, 18 In the present study, we also observed a reduction in SERT binding with ketamine administration. [11C]AZ10419369 has been shown to be sensitive to the release of brain 5-HT levels induced by fenfluramine administration in nonhuman primates.22 Intravenous administration of 5 mg kg−1 fenfluramine, which was previously demonstrated to produce a 20-fold increase in brain 5-HT levels in macaque monkeys,29 decreased BPND by 31% and 29% in conscious and ketamine-treated rhesus monkeys, respectively, indicating that fenfluramine did not induce any changes in 5-HT1B receptor binding in conscious vs ketamine-treated monkeys. These results indicate that no changes of functionality of SERT were induced by ketamine regarding the release and reuptake of 5-HT in vivo. Ketamine likely does not increase 5-HT release by reducing the functionality of SERT. However, it is also possible that the amount of 5-HT released that is induced by ketamine through a reduction in SERT is quite small and thus difficult to detect by PET with [11C]AZ10419369 in vivo. Further studies such as microdialysis measurement of 5-HT directly from the Acb in monkeys are needed to determine the mechanism of ketamine on the release of 5-HT. Although the importance of the ketamine-induced decrease in SERT binding remains unclear, our data strongly indicate that ketamine administration may have little effect on 5-HT release in the nonhuman primate brain. Moreover, ketamine administration increased 5-HT1B receptor binding in specific brain regions but did not decrease binding in any region. Therefore, ketamine-induced increases in 5-HT1B receptor binding may be caused by upregulation of postsynaptic receptors but not by direct competition with released extracellular 5-HT.
Pretreatment with NBQX is known to block the antidepressant actions of ketamine in rodents.7, 8, 9 Our results showed that administration of NBQX completely blocked ketamine-induced increases in 5-HT1B receptor binding but did not affect ketamine-induced decreases in SERT binding. A number of AMPA receptor potentiators have shown antidepressant actions in preclinical studies and are anticipated to have medicinal application in the treatment of depression.30 More recently, AMPA itself has been shown to have antidepressant actions in the rat model of depression, inducing increases in synapsin I levels and activation of mammalian target of rapamycin in the hippocampus.31 Most of fast excitatory synaptic transmission in the mammalian central nervous system is mediated by the AMPA receptor, whose function is critical for synaptic plasticity.32 Ketamine increases the spine density, synaptic activity and levels of synaptic proteins including both synapsin I and GluR1, which is an AMPA receptor subtype.10 Ketamine has also been reported to induce glutamate release in the prefrontal cortex of the conscious rat.33,34 Although a direct association of our finding of a sedative dose of ketamine and the antidepressive action reported by a low dose is difficult, selective inhibition by NBQX of upregulation of 5-HT1B strongly implicates its relevance to the antidepressive action mediated by AMPA receptors. Given these findings, we propose that the effect of ketamine on the 5-HT1B receptor, but not the SERT, through activation of the glutamate AMPA receptor, is most likely to contribute to its antidepressant action.
A general feature of G-protein-coupled receptors is the existence of complex intracellular regulatory mechanisms that modulate receptor responsiveness, and 5-HT1B receptor-trafficking regulation has been demonstrated in a cell line system using recombinant fusion proteins.35 Ketamine may influence receptor-trafficking regulation. A cell culture study showed that p11, a 5-HT1B receptor binding protein, increased the localization of the 5-HT1B receptor at the cell surface.19 Recently, p11 knockout mice have been demonstrated to show depression-like behavior (increased immobility time in the tail suspension test), which was normalized by restoration of p11 expression within the Acb using gene therapy.36 p11 increases the localization of the 5-HT1B receptor at the cell surface and its mRNA is increased by electroconvulsive therapy, which may be closely associated with synaptic potentiation. Synaptic potentiation, such as that observed following ketamine administration and mediated by AMPA receptors, is thought to be involved in antidepressive action.37 Trafficking of 5-HT receptor proteins is controlled by association with several specific adaptor proteins including p11.38 5-HT1B receptors are co-localized with AMPA receptors in postsynaptic dendrites in the hippocampus.39 Therefore, the acute effect of ketamine on the increase in 5-HT1B-selective binding in the Acb may be caused by receptor translocation to cell surface that is mediated by AMPA receptors, which may function by increasing p11 or by increasing the association between p11 and 5-HT1B receptors. Further studies are needed to clarify the molecular mechanism.
The Acb is a component of the neural circuit that regulates motivation, reward responses and mood.40,41 Imaging studies show an absence of the usual increase in Acb activity in depressed patients in response to positive stimuli.42 Furthermore, deep brain stimulation to the Acb improves the symptoms of depression.43 Several neuroanatomical and neuroimaging studies have suggested that the cortico-striato-pallido-thalamic loop is implicated in mood disorders.44 This loop includes the ventral GP and medial thalamus as well as the Acb. For example, patients with depression show increases in glucose metabolism and cerebral blood flow in the medial thalamus and decreases in 5-HT1B receptor binding in the ventral GP.20 Recently, an electrophysiological study in nonhuman primates demonstrated that the ventral GP provides expected reward value signals that are used to facilitate or inhibit motor actions.45 The present study found that ketamine administration significantly increased 5-HT1B receptor binding in the ventral GP and Tha-Re, suggesting that the modulation of serotonergic synaptic transmission in these regions of the cortico-striato-pallido-thalamic loop may be critical in the progression of depressive symptoms. Taken together, the present results strongly suggest that the 5-HT1B receptor in the Acb and the ventral GP in the cortico-striato-pallido-thalamic loop may be critically involved in the antidepressant action of ketamine.
Our results suggest that 5-HT1B receptor imaging will yield important information about depression. The binding potential of [11C]P943, another 5-HT1B receptor-selective PET radioligand, was recently demonstrated to be significantly decreased in the ventral pallidum/ventral striatum in patients with major depression compared with healthy controls.20 PET imaging studies of 5-HT1B receptor binding has also been reported in some psychiatric disorders, such as post-traumatic stress disorder (PTSD), alcohol dependence and pathological gambling.46, 47, 48 In particular, 5-HT1B receptor binding is increased in the ventral GP and ventral striatum (including the Acb) of patients with alcohol dependence and is decreased in the amygdala, anterior cingulate cortex and caudate nucleus in PTSD. The bidirectional changes in 5-HT1B receptor binding observed in the same brain regions, including the Acb and ventral GP, imply that the availability of 5-HT1B receptor imaging of these regions has important implications for understanding the pathophysiological mechanisms in major depression and related psychiatric diseases.
In conclusion, upregulation of postsynaptic 5-HT1B receptors in the Acb and ventral GP may be critically involved in the antidepressant action of ketamine, and PET imaging studies of 5-HT1B receptor binding may be useful in the diagnosis of major depression as well as in the development of novel antidepressants.
Acknowledgments
This work was supported by the Japan Society for the Promotion of Science (JSPS) KAKENHI Grant number 24300196. We thank Chiho Takeda for technical assistance with the PET study; Tomoko Mori and Masahiro Kurahashi for supplying the radiotracers and cyclotron operation; and Yasuhiro Wada, Emi Hayashinaka and Kayo Onoe for technical support in analysis of PET data. We also thank Masahiro Ohno for the excellent technical assistance with this study.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Translational Psychiatry website (http://www.nature.com/tp)
Supplementary Material
References
- Giannini AJ, Underwood NA, Condon M. Acute ketamine intoxication treated by haloperidol: a preliminary study. Am J Ther. 2000;7:389–391. doi: 10.1097/00045391-200007060-00008. [DOI] [PubMed] [Google Scholar]
- Morgan CJ, Curran HV. Ketamine use: a review. Addiction. 2012;107:27–38. doi: 10.1111/j.1360-0443.2011.03576.x. [DOI] [PubMed] [Google Scholar]
- Peck TE, Hill SA, Williams M.Pharmacology for anaesthesia and intensive care3rd edn., Cambridge University Press: Cambridge; 2008 [Google Scholar]
- Quibell R, Prommer EE, Mihalyo M, Twycross R, Wilcock A. Ketamine*. J Pain Symptom Manage. 2011;41:640–649. doi: 10.1016/j.jpainsymman.2011.01.001. [DOI] [PubMed] [Google Scholar]
- Berman RM, Cappiello A, Anand A, Oren DA, Heninger GR, Charney DS, et al. Antidepressant effects of ketamine in depressed patients. Biol Psychiatry. 2000;47:351–354. doi: 10.1016/s0006-3223(99)00230-9. [DOI] [PubMed] [Google Scholar]
- Zarate CA, Jr, Singh JB, Carlson PJ, Brutsche NE, Ameli R, Luckenbaugh DA, et al. A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry. 2006;63:856–864. doi: 10.1001/archpsyc.63.8.856. [DOI] [PubMed] [Google Scholar]
- Autry AE, Adachi M, Nosyreva E, Na ES, Los MF, Cheng PF, et al. NMDA receptor blockade at rest triggers rapid behavioural antidepressant responses. Nature. 2011;475:91–95. doi: 10.1038/nature10130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeng S, Zarate CA, Jr, Du J, Schloesser RJ, McCammon J, Chen G, et al. Cellular mechanisms underlying the antidepressant effects of ketamine: role of alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptors. Biol Psychiatry. 2008;63:349–352. doi: 10.1016/j.biopsych.2007.05.028. [DOI] [PubMed] [Google Scholar]
- Koike H, Iijima M, Chaki S. Involvement of AMPA receptor in both the rapid and sustained antidepressant-like effects of ketamine in animal models of depression. Behav Brain Res. 2011;224:107–111. doi: 10.1016/j.bbr.2011.05.035. [DOI] [PubMed] [Google Scholar]
- Li N, Lee B, Liu RJ, Banasr M, Dwyer JM, Iwata M, et al. mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science. 2010;329:959–964. doi: 10.1126/science.1190287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann JJ. Role of the serotonergic system in the pathogenesis of major depression and suicidal behavior. Neuropsychopharmacology. 1999;21 ((2 Suppl:99S–105S. doi: 10.1016/S0893-133X(99)00040-8. [DOI] [PubMed] [Google Scholar]
- Blier P, de Montigny C, Chaput Y. Modifications of the serotonin system by antidepressant treatments: implications for the therapeutic response in major depression. J Clin Psychopharmacol. 1987;7 ((6 Suppl:24S–35S. [PubMed] [Google Scholar]
- Pytliak M, Vargova V, Mechirova V, Felsoci M. Serotonin receptors—from molecular biology to clinical applications. Physiol Res. 2011;60:15–25. doi: 10.33549/physiolres.931903. [DOI] [PubMed] [Google Scholar]
- Ruf BM, Bhagwagar Z. The 5-HT1B receptor: a novel target for the pathophysiology of depression. Curr Drug Targets. 2009;10:1118–1138. doi: 10.2174/138945009789735192. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Sun L. Antidepressants modulate the in vitro inhibitory effects of propofol and ketamine on norepinephrine and serotonin transporter function. J Clin Neurosci. 2008;15:1264–1269. doi: 10.1016/j.jocn.2007.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura M, Sato K, Okada T, Yoshiya I, Schloss P, Shimada S, et al. Ketamine inhibits monoamine transporters expressed in human embryonic kidney 293 cells. Anesthesiology. 1998;88:768–774. doi: 10.1097/00000542-199803000-00029. [DOI] [PubMed] [Google Scholar]
- Chatterjee M, Verma R, Ganguly S, Palit G. Neurochemical and molecular characterization of ketamine-induced experimental psychosis model in mice. Neuropharmacology. 2012;63:1161–1171. doi: 10.1016/j.neuropharm.2012.05.041. [DOI] [PubMed] [Google Scholar]
- Tao R, Auerbach SB. Anesthetics block morphine-induced increases in serotonin release in rat CNS. Synapse. 1994;18:307–314. doi: 10.1002/syn.890180406. [DOI] [PubMed] [Google Scholar]
- Svenningsson P, Chergui K, Rachleff I, Flajolet M, Zhang X, El Yacoubi M, et al. Alterations in 5-HT1B receptor function by p11 in depression-like states. Science. 2006;311:77–80. doi: 10.1126/science.1117571. [DOI] [PubMed] [Google Scholar]
- Murrough JW, Henry S, Hu J, Gallezot JD, Planeta-Wilson B, Neumaier JF, et al. Reduced ventral striatal/ventral pallidal serotonin1B receptor binding potential in major depressive disorder Psychopharmacology 2011213547–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finnema SJ, Varrone A, Hwang TJ, Gulyas B, Pierson ME, Halldin C, et al. Fenfluramine-induced serotonin release decreases [ 11 C]AZ10419369 binding to 5-HT1B-receptors in the primate brain. Synapse. 2010;64:573–577. doi: 10.1002/syn.20780. [DOI] [PubMed] [Google Scholar]
- Finnema SJ, Varrone A, Hwang TJ, Halldin C, Farde L. Confirmation of fenfluramine effect on 5-HT(1B) receptor binding of [ 11 C]AZ10419369 using an equilibrium approach. J Cereb Blood Flow Metab. 2012;32:685–695. doi: 10.1038/jcbfm.2011.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watakabe A, Komatsu Y, Sadakane O, Shimegi S, Takahata T, Higo N, et al. Enriched expression of serotonin 1B and 2A receptor genes in macaque visual cortex and their bidirectional modulatory effects on neuronal responses. Cereb Cortex. 2009;19:1915–1928. doi: 10.1093/cercor/bhn219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierson ME, Andersson J, Nyberg S, McCarthy DJ, Finnema SJ, Varnas K, et al. [ 11 C]AZ10419369: a selective 5-HT1B receptor radioligand suitable for positron emission tomography (PET). Characterization in the primate brain. Neuroimage. 2008;41:1075–1085. doi: 10.1016/j.neuroimage.2008.02.063. [DOI] [PubMed] [Google Scholar]
- Wilson AA, Ginovart N, Schmidt M, Meyer JH, Threlkeld PG, Houle S.Novel Radiotracers for Imaging the Serotonin Transporter by Positron Emission Tomography: Synthesis, Radiosynthesis, and in Vitro and ex Vivo Evaluation of 11 C-Labeled 2-(Phenylthio)araalkylamines J Med Chem 2000433103–3110. [DOI] [PubMed] [Google Scholar]
- Yokoyama C, Yamanaka H, Onoe K, Kawasaki A, Nagata H, Shirakami K, et al. Mapping of serotonin transporters by positron emission tomography with [ 11 C]DASB in conscious common marmosets: comparison with rhesus monkeys. Synapse. 2010;64:594–601. doi: 10.1002/syn.20766. [DOI] [PubMed] [Google Scholar]
- Logan J, Fowler JS, Volkow ND, Wang GJ, Ding YS, Alexoff DL. Distribution volume ratios without blood sampling from graphical analysis of PET data. J Cereb Blood Flow Metab. 1996;16:834–840. doi: 10.1097/00004647-199609000-00008. [DOI] [PubMed] [Google Scholar]
- Ichise M, Liow JS, Lu JQ, Takano A, Model K, Toyama H, et al. Linearized reference tissue parametric imaging methods: application to [ 11 C]DASB positron emission tomography studies of the serotonin transporter in human brain. J Cereb Blood Flow Metab. 2003;23:1096–1112. doi: 10.1097/01.WCB.0000085441.37552.CA. [DOI] [PubMed] [Google Scholar]
- Udo de Haes JI, Harada N, Elsinga PH, Maguire RP, Tsukada H. Effect of fenfluramine-induced increases in serotonin release on [ 18 F]MPPF binding: a continuous infusion PET study in conscious monkeys. Synapse. 2006;59:18–26. doi: 10.1002/syn.20209. [DOI] [PubMed] [Google Scholar]
- Sanacora G, Zarate CA, Krystal JH, Manji HK. Targeting the glutamatergic system to develop novel, improved therapeutics for mood disorders. Nat Rev Drug Discov. 2008;7:426–437. doi: 10.1038/nrd2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akinfiresoye L, Tizabi Y. Antidepressant effects of AMPA and ketamine combination: role of hippocampal BDNF, synapsin, and mTOR. Psychopharmacology. 2013;230:291–298. doi: 10.1007/s00213-013-3153-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traynelis SF, Wollmuth LP, McBain CJ, Menniti FS, Vance KM, Ogden KK, et al. Glutamate receptor ion channels: structure, regulation, and function. Pharmacol Rev. 2010;62:405–496. doi: 10.1124/pr.109.002451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorrain DS, Baccei CS, Bristow LJ, Anderson JJ, Varney MA. Effects of ketamine and N-methyl-D-aspartate on glutamate and dopamine release in the rat prefrontal cortex: modulation by a group II selective metabotropic glutamate receptor agonist LY379268. Neuroscience. 2003;117:697–706. doi: 10.1016/s0306-4522(02)00652-8. [DOI] [PubMed] [Google Scholar]
- Moghaddam B, Adams B, Verma A, Daly D. Activation of glutamatergic neurotransmission by ketamine: a novel step in the pathway from NMDA receptor blockade to dopaminergic and cognitive disruptions associated with the prefrontal cortex. J Neurosci. 1997;17:2921–2927. doi: 10.1523/JNEUROSCI.17-08-02921.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janoshazi A, Deraet M, Callebert J, Setola V, Guenther S, Saubamea B, et al. Modified receptor internalization upon coexpression of 5-HT1B receptor and 5-HT2B receptors. Mol Pharmacol. 2007;71:1463–1474. doi: 10.1124/mol.106.032656. [DOI] [PubMed] [Google Scholar]
- Alexander B, Warner-Schmidt J, Eriksson T, Tamminga C, Arango-Lievano M, Ghose S, et al. Reversal of depressed behaviors in mice by p11 gene therapy in the nucleus accumbens. Sci Transl Med. 2010;2:54ra76. doi: 10.1126/scitranslmed.3001079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machado-Vieira R, Salvadore G, Diazgranados N, Zarate CA., Jr Ketamine and the next generation of antidepressants with a rapid onset of action. Pharmacol Ther. 2009;123:143–150. doi: 10.1016/j.pharmthera.2009.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marin P, Becamel C, Dumuis A, Bockaert J. 5-HT receptor-associated protein networks: new targets for drug discovery in psychiatric disorders. Curr Drug Targets. 2012;13:28–52. doi: 10.2174/138945012798868498. [DOI] [PubMed] [Google Scholar]
- Peddie CJ, Davies HA, Colyer FM, Stewart MG, Rodriguez JJ. A subpopulation of serotonin 1B receptors colocalize with the AMPA receptor subunit GluR2 in the hippocampal dentate gyrus. Neurosci Lett. 2010;485:251–255. doi: 10.1016/j.neulet.2010.09.024. [DOI] [PubMed] [Google Scholar]
- Carelli RM. The nucleus accumbens and reward: neurophysiological investigations in behaving animals. Behav Cogn Neurosci Rev. 2002;1:281–296. doi: 10.1177/1534582302238338. [DOI] [PubMed] [Google Scholar]
- Rolls ET, Burton MJ, Mora F. Neurophysiological analysis of brain-stimulation reward in the monkey. Brain Res. 1980;194:339–357. doi: 10.1016/0006-8993(80)91216-0. [DOI] [PubMed] [Google Scholar]
- Epstein J, Pan H, Kocsis JH, Yang Y, Butler T, Chusid J, et al. Lack of ventral striatal response to positive stimuli in depressed versus normal subjects. Am J Psychiatry. 2006;163:1784–1790. doi: 10.1176/ajp.2006.163.10.1784. [DOI] [PubMed] [Google Scholar]
- Schlaepfer TE, Cohen MX, Frick C, Kosel M, Brodesser D, Axmacher N, et al. Deep brain stimulation to reward circuitry alleviates anhedonia in refractory major depression. Neuropsychopharmacology. 2008;33:368–377. doi: 10.1038/sj.npp.1301408. [DOI] [PubMed] [Google Scholar]
- Price JL, Drevets WC. Neurocircuitry of mood disorders. Neuropsychopharmacology. 2010;35:192–216. doi: 10.1038/npp.2009.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tachibana Y, Hikosaka O. The primate ventral pallidum encodes expected reward value and regulates motor action. Neuron. 2012;76:826–837. doi: 10.1016/j.neuron.2012.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu J, Henry S, Gallezot JD, Ropchan J, Neumaier JF, Potenza MN, et al. Serotonin 1B receptor imaging in alcohol dependence. Biol Psychiatry. 2010;67:800–803. doi: 10.1016/j.biopsych.2009.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potenza MN, Walderhaug E, Henry S, Gallezot JD, Planeta-Wilson B, Ropchan J, et al. Serotonin 1B receptor imaging in pathological gambling. World J Biol Psychiatry. 2013;14:139–145. doi: 10.3109/15622975.2011.598559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murrough JW, Czermak C, Henry S, Nabulsi N, Gallezot JD, Gueorguieva R, et al. The effect of early trauma exposure on serotonin type 1B receptor expression revealed by reduced selective radioligand binding. Arch Gen Psychiatry. 2011;68:892–900. doi: 10.1001/archgenpsychiatry.2011.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.