Abstract
Several genes have recently been identified as risk factors for schizophrenia (SZ) by genome-wide association studies (GWAS), including ZNF804A which is thought to function in transcriptional regulation. However, the downstream pathophysiological changes that these genes confer remain to be elucidated. In 143 subjects (68 clinical high risk, first episode or chronic cases; 75 controls), we examined the association between 21 genetic markers previously identified by SZ GWAS or associated with putative intermediate phenotypes of SZ against three event-related potential (ERP) measures: mismatch negativity (MMN), amplitude of P300 during an auditory oddball task, and P300 amplitude during an auditory novelty oddball task. Controlling for age and sex, significant genetic association surpassing Bonferroni correction was detected between ZNF804A marker rs1344706 and P300 amplitude elicited by novel sounds (beta=4.38, P=1.03 × 10−4), which is thought to index orienting of attention to unexpected, salient stimuli. Subsequent analyses revealed that the association was driven by the control subjects (beta=6.35, P=9.08 × 10−5), and that the risk allele was correlated with higher novel P300b amplitude, in contrast to the significantly lower amplitude observed in cases compared to controls. Novel P300b amplitude was significantly correlated with a neurocognitive measure of auditory attention under interference conditions, suggesting a relationship between novel P300b amplitude and higher-order attentional processes. Our results suggest pleiotropic effects of ZNF804A on risk for SZ and neural mechanisms that are indexed by the novel P300b ERP component.
Introduction
The genetic contribution to developing SZ is relatively high, with heritability estimates of 81% by meta-analysis of twin studies1 and 64% by a large family-based study.2 Recent efforts to identify genetic risk loci in order to gain insight into SZ pathophysiology have involved GWAS with increasing success, and nearly 20 loci have now been associated with SZ.3 One of the first genes to be identified with genome-wide significant evidence was ZNF804A, a brain-expressed gene that encodes a zinc finger domain indicating a transcriptional regulatory function. ZNF804A was initially implicated in risk of SZ and psychosis by a GWAS, and subsequently replicated by several targeted association studies.4, 5, 6, 7, 8, 9, 10 A report of stronger association in SZ cases with higher IQ suggests that variation in ZNF804A may confer risk of a disease subtype with relatively preserved cognition.11
Impaired cognitive functioning based on neuropsychological tests, alterations in neuroanatomy and brain dysfunction as measured by structural and functional neuroimaging, and electrophysiological changes are all associated with SZ. In addition, impairments are also frequently observed in unaffected relatives, suggesting that these traits may represent heritable intermediate phenotypes that could be used to decipher disease pathology.12,13 Event-related potentials (ERPs) reflect pre- and post-synaptic activity in neurons, and index normal and pathological brain processes in real time.14 Several ERPs are considered to be intermediate phenotypes of SZ, with amplitude or latency abnormalities observed in affected individuals and unaffected relatives.15, 16, 17 This includes the novel P300 component elicited ~300 ms after complex, ‘novel' sounds in an oddball sequence,18 which is reported to have abnormal amplitude in SZ and high-risk subjects.19,20 Further, evidence of P300 deficits in healthy siblings of affected subjects21,22 provides support for the novel P300 ERP component being an intermediate phenotype of SZ.
The novel P300 is thought to represent a brain mechanism involved in the rapid orienting of attention to events that are unexpected, salient and contextually novel.23, 24, 25 Detection of novel stimuli involves, among other areas, the anterior cingulate,26 followed by engagement of other brain regions important for processing and habituation to the stimulus,27 notably the dorsolateral prefrontal cortex and the hippocampus.18,28, 29, 30 Menon and Uddin31 have proposed a model whereby the anterior cingulate response to a novel stimulus is preceded by amplification of the salient event by the insula. Both brain regions define the ‘salience network' that is important in dynamic switching between central-executive and default mode networks in processes mediating detection of salient events and capturing of attention.32
Several candidate genes have been studied within the context of association with ERP components and other putative intermediate phenotypes of SZ. Catechol-O-methyltransferase (COMT) has been associated with auditory oddball P300 amplitude in SZ subjects, unaffected relatives and healthy individuals.33,34 Neuregulin 1 (NRG1) is reported to be associated with latency of the auditory oddball P300 component,35,36 while its receptor, ERBB4, has been related to reaction time in a visual oddball task.37 Dysbindin 1 (DTNBP1) has been associated with amplitude of the P1 component in a visual ERP task.38 Other genes have been associated with cognitive domains that are impaired in SZ, including GRIN2B,39 KIBRA,40 MTHFR41 and DISC1.42 Several genes identified in recent GWAS of SZ, including ZNF804A, have also been investigated for relationships with cognitive performance, neuroanatomical features and neural connectivity that are abnormal in SZ.11,43, 44, 45
In the present study, we sought to examine the genetic contribution to ERP abnormalities as putative intermediate phenotypes of SZ to gain insight into the biological pathways that mediate brain dysfunction in this disorder. We explored associations between well-characterized electrophysiological components and genes with strong prior evidence of contributing to risk of SZ by GWAS or to variation of putative intermediate phenotypes. The primary focus of this article is P300b peak amplitude evoked during an auditory novelty oddball task (denoted hereafter as novel P300b), because it was the only ERP component displaying a genetic association surpassing statistical significance after correction for multiple tests. We also explored the relationship between the novel P300b component and experimental neurocognitive measures of auditory attention and working memory as a means of linking novel P300 to cognitive and brain functions important in SZ. Our analyses indicate that the ZNF804A gene previously implicated in risk for SZ is significantly associated with novel P300b amplitude in a pleiotropic manner that may be independent from its contribution to SZ.
Materials and methods
Subjects
Subjects were recruited through the Boston CIDAR study (www.bostoncidar.org). The sample (N=143) utilized in this study consisted of a total of 68 cases, including 27 individuals with clinical high risk (CHR) or putatively prodromal symptoms, 25 persons within the first episode of schizophrenia (FESZ), and 16 people with chronic schizophrenia (CSZ), as well as 75 healthy control participants. Patients were recruited by referrals from clinicians or through local hospitals and clinics, and controls were recruited through newspaper and website advertisements. The study was approved by the local IRB committees at Harvard Medical School, Beth Israel Deaconess Medical Center, Massachusetts General Hospital, Brigham and Women's Hospital and the Veteran Affairs Boston Healthcare System (Brockton campus). All study participants, or legal guardians for those under 18 years of age, gave written informed consent and received payment for participation.
DSM-IV diagnoses were based on interviews with the Structured Clinical Interview for DSM-IV-TR (SCID), Research Version (First et al., 2002) and information from patient medical records. All FESZ participants met DSM-IV-TR criteria for SZ, schizoaffective disorder or schizophreniform disorder, and none had more than one year of continuous antipsychotic treatment. CSZ participants met DSM-IV-TR criteria for SZ or schizoaffective disorder and had an illness duration of at least five years. Inclusion criteria for CHR subjects incorporated different approaches. The majority (N=23) were categorized with ‘late prodromal syndromes' as classified by the Criteria of Prodromal States46 based on the Scale of Prodromal Symptoms.47 The other four CHR participants were categorized with ‘early prodromal syndromes', which are more subtle and require wider criteria for definition,48 and include the so-called basic symptoms49,50 and schizotypal personality disorder.48,51 Antipsychotic medication use was estimated using chlorpromazine equivalents calculated according to Stoll.52
Controls were drawn from the same geographic bases with comparable age, gender, race and ethnicity, handedness, and parental socioeconomic status evaluated using the Hollingshead two-factor index.53 Controls were ascertained to be comparable to each of the CHR, FESZ and CSZ groups. Exclusion criteria for controls included any current or past major DSM-IV-TR Axis I disorder, developmental disorders, psychiatric hospitalizations, prodromal symptoms, schizotypal or other Cluster A personality disorders, a first degree relative with psychosis, or current or past use of antipsychotics. Sleeping aids or anxiolytic agents for occasional use were acceptable, as well as other past psychotropic medication use if not within the prior 6 months.
Exclusion criteria for all subjects included sensory-motor handicaps, neurological disorders, medical illnesses that significantly impair neurocognitive function, diagnosis of intellectual disability (IQ<70), not fluent in English, DSM-IV substance abuse within the past month or dependence within the past 3 months, current suicidality, or history of electroconvulsive therapy for controls or electroconvulsive therapy within the past 5 years for cases.
ERP tasks
Three different auditory tasks were employed: the novelty oddball, the classical oddball and the mismatch negativity (MMN). The latter two tasks were performed as detailed in McCarley et al.54 and Niznikiewicz et al., 55 respectively, and are not described here. As explained above, we have focused on the novel P300b given its association with genetic data.
Novelty oddball task
Subjects performed a novelty, 3-stimulus auditory oddball task over 4 minutes. Stimuli (N=180) included brief tones (82 ms duration, 75 dB sound pressure level), with 20% (36) infrequent target tones (1.5 kHz) and 60% (108) frequent standard tones (1 kHz), as well as 20% (36) infrequent non-target novel stimuli (300–320 ms duration, 75 dB sound pressure level) consisting of six different complex environmental sounds (for example, dog bark, door slamming).18 The inter-stimulus interval was 976 ms (onset-to-onset). Subjects were instructed to silently count the target tones.
EEG recording and processing
The EEG was recorded with a Biosemi Active-Two system using sintered Ag/AgCl electrodes in an electrode cap at 64 standard scalp sites (DC-100 Hz bandpass filter, 512 Hz digitization rate) with DC offsets kept below 25 mV. During data acquisition, all channels were referred to the system's internal loop (CMS/DRL sensors located in the parietal region). The bipolar vertical electro-oculogram was derived from electrode Fp1 and an electrode below the left eye. The horizontal electro-oculogram was derived from electrodes on the left and right outer canthi.
The EEG data were processed using the BrainVision Analyzer package, version 2.0 (Brain Products GmbH, Munich, Germany). The continuous EEG recordings were segmented from −100 to 900 ms relative to stimulus onset and re-referenced to the algebraic sum of the left and right mastoids. Ocular correction was performed using the method of Gratton et al.56 Epochs with artifacts exceeding +/−100 μV with a maximal allowed difference of 200 μV were excluded. Average ERPs were computed from the artifact-free epochs for each subject separately for each type of stimulus and baseline corrected with a pre-stimulus baseline of −100 to 0 ms following digital filtering (0–16 Hz). The number of epochs included in the average novel P300b ERP across all subjects was 33.6±2.99 (cases, 33.4±3.2; controls, 34.1±2.5; F(1,141)=2.1, P=0.147).
ERP analyses
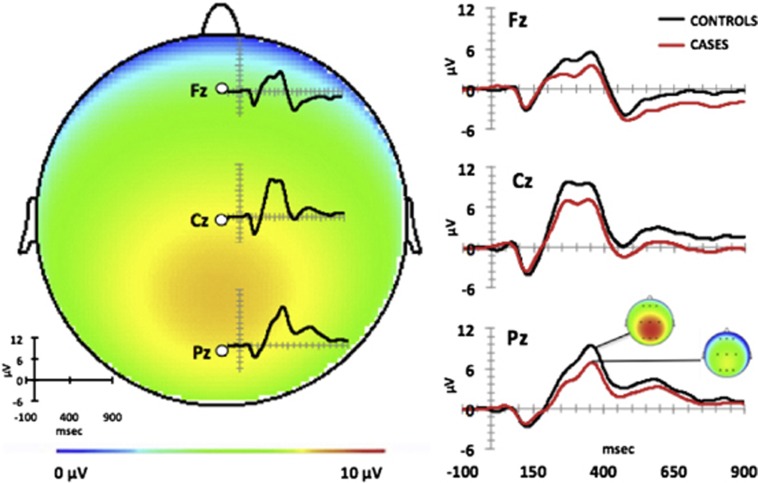
In the novelty oddball task, the peak amplitude of the novel P300 peak elicited by novel sounds was measured at electrode sites F1/Fz/F2 (frontal), C1/Cz/C2 (central), and P1/Pz/P2 (parietal). The latency range for selecting the peak in individual subject data was determined by visual inspection of the grand average ERPs, and all electrodes of interest were inspected to ensure that the same component was selected at each site. The novel P300 waveform in these data was biphasic (Figure 1), with an early peak, novel P300a, occurring 215–290 ms post stimulus, and a later peak, that we designate as novel P300b, between 315–390 ms. Amplitude of the P300a peak was highest at central sites, while the P300b peak was highest at central and parietal sites (Figure 1). Thus, novel P300b amplitude at the Pz electrode was chosen for genetic analyses to avoid overlap with the scalp distribution of novel P300a amplitude.
Figure 1.
Novel P300 waveforms. Left: topographic map illustrating the central−parietal scalp distribution of novel P300b amplitude measured at latency between 315−390 ms and waveforms at the midline electrodes (Fz, Cz and Pz) in controls and cases combined (N=143). Right: novelty P300b oddball task grand average waveforms in controls (N=75) and in cases (N=68) at the midline electrodes (Fz, Cz and Pz). Color distribution of topographical maps corresponds to novel P300b amplitude between 0 and 10 μV.
Neuropsychological tests
Subjects were administered a large neurocognitive test battery including tests estimating premorbid IQ (the Reading subtest from the Wide Range Achievement Test-4 (WRAT-4);57 and current IQ (the Vocabulary and Block Design T-scores of the Wechsler Abbreviated Scale of Intelligence (WASI).58 The ‘vigilance', ‘working memory' and ‘working memory plus interference' measures from the Seidman auditory continuous performance test battery (ACPT)59,60 were utilized to assess different components of auditory attention. All three auditory measures utilize letters of the alphabet presented on an audiotape at a rate of one per second for 90 seconds, with three blocks for vigilance and two blocks each for the two working memory conditions (10.5 min total). The vigilance measure taps sustained auditory attention/vigilance by requiring a rapid response (tapping a pencil) each time a letter ‘A' immediately follows a letter ‘Q' (QA vigilance). The auditory working memory measure involves rapidly responding each time a letter ‘A' follows four letters after a letter ‘Q' (Q3A memory). The more complex and higher load working memory plus interference condition (Q3A memory-interference) places increased demands on working memory by invoking auditory working memory when confronted with distracting/interfering information (that is, embedding distracting combinations of ‘Q', ‘A' or ‘QA' in between the cue ‘Q' letters and the target ‘A's that follow four letters after, thus dividing attention, preventing counting and stressing the capacity to perform dual tracking). The hit rate (% of correctly responding to the target letter ‘A's) was determined for each of the three conditions.59,60
Statistical analyses of ERP data
Statistical analyses of ERP data were performed with SPSS v.19 (IBM, Armonk, NY, USA). Novel P300b peak amplitude was analyzed using MANOVA with group (all cases versus all controls) as a between factor, and within factors of region (frontal/central/parietal) and site (F1/Fz/F2, C1/Cz/C2, P1/Pz/P2). We also assessed differences between diagnostic subgroups by comparing CHR, FESZ, and their matched controls, and comparing CSZ and their matched controls, using the MANOVA design described above. As IQ and pSES were significantly different between cases versus controls, the MANOVA was carried out with and without IQ and pSES as covariates; however, results did not differ, therefore only results without covarying for IQ and pSES are reported. Bonferroni-corrected P-values are reported.
Spearman's rho correlations were calculated between novel P300b amplitude at the Pz electrode and three measures from the Seidman ACPT, ‘vigilance', ‘working memory' and ‘working memory plus interference'. The contributions of IQ and higher-order cognitive processes to novel P300b amplitude were further examined by entering current IQ and ‘working memory with interference' performance as independent variables in a multiple regression model with novel P300b amplitude as the dependent variable.
Genotyping and quality control
Blood or, in some instances, saliva was collected from all subjects at the time of recruitment. DNA was extracted, quantified using Invitrogen's Quant-iT (TM) dsDNA Assay Kit (Life Technologies, Carlsbad, CA, USA) and stored at −80°C. Genotyping was conducted at the Broad Institute of Massachusetts Institute of Technology and Harvard using the Illumina OmniExpress array (Illumina, Inc., San Diego, CA, USA) containing 733 202 single-nucleotide polymorphisms (SNPs). Quality control steps were performed on the full data using PLINK61 as follows: SNPs were removed if they had <1% minor allele frequency, <95% genotyping rate, deviation from Hardy−Weinberg equilibrium (P<1 × 10−6), or differential missingness between cases and controls at the SNP or haplotype level. Three subjects with low genotyping rates (<90%) and/or high heterozygosity values (>34%) were removed. SNPs not directly genotyped were imputed with Beagle software62 using HapMap3 reference panels containing >1.2 million markers.63 All imputed SNPs included in association analyses were imputed with high confidence (Info >0.85).
Genetic markers
Thirteen SNPs conferring risk for SZ identified by published GWAS64, 65, 66, 67 and surpassing a genome-wide significance threshold68 of P<5 × 10−8 were selected. SNPs meeting this criterion but with minor allele frequencies <10% were excluded due to too few minor allele carriers in this sample. In addition, eight SNPs in candidate genes were included that have prior evidence from at least two studies for association with SZ intermediate phenotypes, including ERP abnormalities (COMT33), neuropsychological impairments (ERBB4,37,69,70 MTHFR,71,72 DISC1,42 COMT,69,73 KIBRA,40 NRG1,69 GRIN2B69), and structural and functional neuroimaging measures (DTNBP1,74,75 DISC1,42,76 NRG1,76 GRIN2B,77 ERBB4,37 COMT,78 KIBRA79). We identified a total of 21 SNPs meeting these selection criteria (Supplementary Table 1).
Genetic association analyses
Genetic association analyses were performed using linear regression in PLINK with covariates accounting for sex and age, as well as population substructure captured by the first five multidimensional scaling components. Diagnostic subgroup (CHR, FESZ, CSZ and control) likely impacts true performance differences, but it is also a potential confounding variable since medication and other environmental conditions may differ by group. Accordingly, main analyses were conducted without diagnostic subgroup as a covariate, and results were subsequently confirmed in analyses with diagnostic subgroup as a covariate in the model. The following ERP components were tested: MMN at the Fz electrode, oddball P300 amplitude at the Cz electrode, and novel P300b amplitude at the Pz electrode. Bonferroni correction was applied to the genetic association results, not accounting for potential correlations between the ERP variables, to yield a study-wide significance threshold of P<7.9 × 10−4 for the three ERP variables and 21 SNPs tested. As current IQ and pSES differed significantly by ZNF804A genotype, novel P300b amplitude was analyzed using MANCOVA with genotype as a between factor and pSES and current IQ as covariates. Post hoc analyses exploring the strength of ZNF804A association were conducted separately by diagnostic subgroup, sex and genetically defined Caucasian ancestry (the largest ancestral population within this sample, N=87). Genetic association tests between ZNF804A and current IQ and a measure of auditory attention (Seidman ACPT ‘working memory plus interference') were also conducted.
Results
Subject characteristics
Sample demographic data are summarized in Table 1. Most variables did not differ between controls and cases (combined CHR, FESZ and CSZ), with the exception of current IQ (F(1,141)=9.5; P<0.01), years of formal education (F(1,141)=4.6; P<0.05), parental socioeconomic status (F(1,141)=4.35, P<0.05) and global assessment of functioning (GAF) score (F(1,136)=485, P<0.01). As the cases were a composite of three diagnostic groups, Supplementary Table 2 reports demographic data for CHR and FESZ cases both matched to their control group, and for CSZ cases matched to their control group.
Table 1. Sociodemographic and clinical information.
| Controls (N=75) | Cases (N=68) | P-value |
rs1344706 |
||||
|---|---|---|---|---|---|---|---|
| AA (N=66) | AC (N=54) | CC (N=23) | P-value | ||||
| Age | 26.1 (10.5) | 26.4 (12.0) | NS | 27.0 (11.8) | 25.3 (10.6) | 26.2 (11.4) | NS |
| Male/Female | 46/29 | 49/19 | NS | 40/26 | 39/15 | 16/7 | NS |
| Premorbid IQ | 114.6 (15.4) | 110.9 (16.3) | NS | 111.0 (15.1) | 115.6 (15.4) | 111.8 (18.7) | NS |
| Current IQ | 117.8 (14.0) | 110.3 (15.0) | <0.01 | 110.8 (15.5) | 118.4 (12.4) | 114.4 (16.9) | <0.05 |
| Education | 13.9 (2.7) | 12.9 (2.7) | <0.05 | 13.5 (2.7) | 13.4 (2.8) | 13.4 (2.7) | NS |
| pSES | 1.8 (0.9) | 2.1 (1.0) | <0.05 | 2.2 (1.1) | 1.7 (0.77) | 1.91 (0.9) | <0.05 |
| GAF | 84.5 (7.7) | 49.2 (11.0) | <0.01 | 66.0 (20.5) | 70.9 (19) | 62.7 (20.9) | NS |
| CPZ equivalents | NA | 248.8 (214.7) | NA | 343.1 (269.6) | 160.2 (118.8) | 189.3 (123.6) | NS |
Abbreviations: CPZ, chlorpromazine; GAF, global assessment of functioning; NA, not applicable; NS, not significant; pSES, parental socioeconomic status. CPZ equivalents were calculated for subjects on antipsychotic medication (N=36, including 9 CHR, 14 FESZ and 13 CSZ subjects). Values are mean (s.d.). Fisher's Exact Test was used for categorical variables and ANOVA for continuous variables (P>0.05).
Novel P300b amplitude is significantly reduced in SZ cases
MANOVA revealed a main effect of group (all cases versus all controls) on novel P300b amplitude (F(1,141)=26.2; P<0.001), which was significantly lower in cases than controls (Figure 1; Supplementary Table 3). There was also a main effect of brain region (F(2,140)=74.0, P<0.001), but no interaction of region by group (F(2,140)=0.02, P=0.98). Post hoc analysis revealed higher P300b amplitude at parietal and central regions (both P<0.001) relative to frontal (Figure 1), therefore, genetic association analyses utilized novel P300b amplitude data at the Pz electrode. In analyses of the diagnostic subgroups (Supplementary Figure 1), a main effect of group was also found in the MANOVA comparing CHR, FESZ and their matched controls (F(2,107)=7.0, P<0.01). Post hoc analysis revealed no difference in amplitude between CHR and FESZ groups (P=0.99), with both groups significantly lower than controls (P<0.01). The lack of amplitude differences between CHR and FESZ justified inclusion of CHR subjects as cases in all analyses. MANOVA of CSZ and matched controls also revealed a main effect of group (F(1,31)=15.5; P<0.001).
Relationship between novel P300b and attention to auditory stimuli
In order to better understand the neural substrates of novel P300b, we examined its relationship with higher-order cognitive processes. As the auditory novel P300 paradigm is thought to index salience detection, we investigated performance in the Seidman ACPT task that indexes sustained and selective attention to auditory stimuli. We examined three measures, ‘vigilance', ‘working memory' and ‘working memory plus interference'. Performance on all three tasks was significantly impaired in cases relative to controls (Supplementary Table 3). While novel P300b amplitude was not significantly correlated with the vigilance or working memory measures (rho=0.17, P=0.21 and rho=0.21, P=0.06, respectively) a significant correlation was found for the ‘working memory plus interference' measure (rho=0.4, P<0.001; Supplementary Figure 2).
To further examine this relationship, ‘working memory with interference' performance and current IQ were entered as independent variables in a multiple regression model with novel P300b amplitude as the dependent variable. The model was statistically significant (F(2,133)=12.3, P=0.0005), explaining 14.4% of the variance in novel P300b amplitude. Only ‘working memory with interference' significantly contributed to the model (beta=0.3; P=0.001), suggesting a relationship between novel P300b amplitude and higher-order attentional processes. IQ did not significantly contribute to the model (beta=0.113; P=0.24), therefore it was not included as a covariate in subsequent genetic association analyses.
Significant association between ZNF804A and novel P300b amplitude
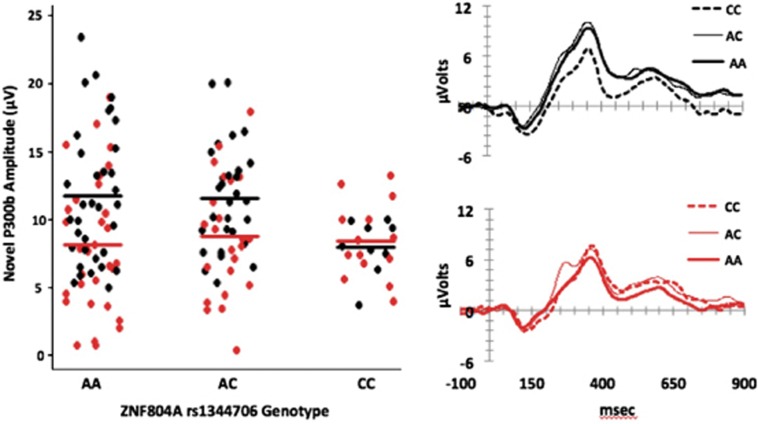
Genetic association analyses of 21 SNPs conferring risk for SZ or impacting putative intermediate phenotypes were performed for each of the three ERP variables, MMN (Fz), oddball P300 amplitude (Cz), and novel P300b amplitude (Pz) (Supplementary Table 4). The only result to attain study-wide significance (that is, P<7.9 × 10−4) was between the ZNF804A marker, rs1344706, and novel P300b amplitude (beta=4.38, P=1.03 × 10−4; Table 2), explaining 7.2% of the total variance. The ‘A' allele for this A/C SNP, which has previously been associated with increased risk for SZ and psychosis, was associated with higher novel P300b amplitude (Figure 2). Since diagnostic group could be a potentially confounding variable, analyses including group (CHR, FESZ, CSZ and controls) as a covariate were also performed, but this had a negligible effect on the results (beta=4.21, P=1.38 × 10−4). Therefore, subsequent post hoc analyses did not include diagnostic group as a covariate. In addition, MANCOVA of P300b amplitude with genotype as a between factor and pSES and current IQ as covariates revealed a main effect of genotype (F(2,138)=3.64, P=0.029) and no interaction effect of the covariates, indicating that these variables are not confounding the ZNF804A association. Amplitude was significantly lower in CC genotype than AA (P<0.05), while amplitude of AA and AC did not differ (P=0.99).
Table 2. Association analyses for ZNF804A SNP rs1344706 and novel P300b amplitude.
| Group | N | Beta | P-value |
|---|---|---|---|
| All subjects | 143 | 4.38 | 1.03 × 10−4 |
| Combined cases | 68 | 1.97 | 0.21 |
| CHR | 27 | 2.75 | 0.33 |
| FESZ | 25 | 0.88 | 0.74 |
| CSZ | 16 | 3.21 | 0.61 |
| Controls | 75 | 6.35 | 9.08 × 10−5 |
Abbreviations: CHR, clinical high risk; CSZ, chronic schizophrenia; FESZ, first episode of schizophrenia.
Figure 2.
ZNF804A rs1344706 association with novel P300b amplitude. Left: scatterplot of novel P300b amplitude at the Pz electrode by rs1344706 genotype in controls (black dots) and cases (red dots). Horizontal lines depict average amplitude in controls or cases. Right: novelty oddball task grand average waveforms at the Pz electrode by genotype in controls (top panel; black lines) and in cases (bottom panel; red lines).
Association analyses of only cases or only controls revealed that the ZNF804A association was strongly driven by the control subjects (beta=6.35, P=9.08 × 10−5), with little contribution from the cases when CHR, FESZ and CSZ diagnostic groups were analyzed separately or together (Table 2). Association results were similar between males and females. Restricting analyses to participants of Caucasian ancestry (N=87), the largest ancestry group represented in this study, yielded a relatively similar effect size (beta=3.34; P=0.012) as in the entire study population (beta=4.38).
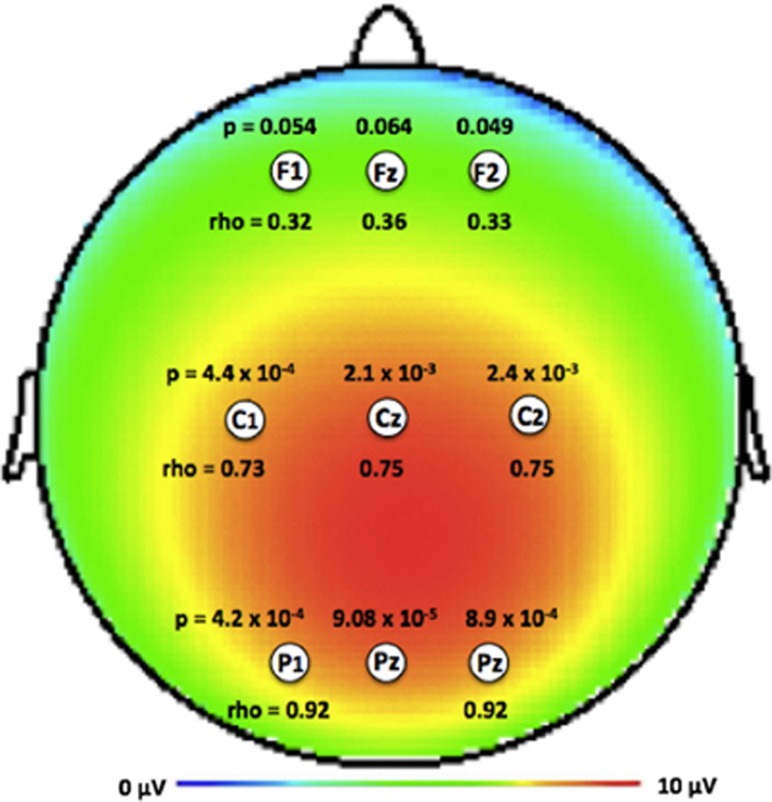
We also examined whether the association detected between ZNF804A and novel P300b amplitude at the Pz electrode was supported by analyses of other electrodes. Associations were detected with novel P300b amplitude at other parietal, central and frontal electrodes (4.2 × 10−4<P<0.064; Figure 3), although the results were less significant than for the Pz electrode. The strength of the ZNF804A association with novel P300b amplitude decreased from parietal sites (P1, Pz, P2) to central sites (C1, Cz, C2) to frontal sites (F1, Fz, F2), paralleling the stepwise decrease in the correlations between novel P300b amplitude at the Pz electrode and amplitudes at central and frontal electrode sites (Figure 3). These results suggest that the ZNF804A association may be more specific for mechanisms indexed by novel P300b amplitude at parietal sites.
Figure 3.
Distribution of ZNF804A rs1344706 association with novel P300b amplitude in controls. Rs1344706 association results with novel P300b amplitude at latency between 315−390 msec are shown for frontal, central and parietal electrodes (uncorrected P-values above electrode labels). Correlation between novel P300b amplitude at the Pz electrode and amplitudes at other electrodes (Spearman's rho values below electrode labels) decreases stepwise from parietal to central to frontal sites as the strength of rs1344706 association with novel P300b amplitude decreases (that is, less significant P-values). All Spearman's rho correlations significant at P<0.01.
Discussion
ZNF804A was among the first genes associated with risk of SZ by GWAS with statistical evidence surpassing genome-wide significance.4,7 The ‘A' allele of SNP rs1344706 in ZNF804A was associated with increased risk of psychosis, with an odds ratio of 1.11 in a combined analysis of SZ and bipolar disorder.4 Subsequent studies focused on ZNF804A strongly supported association with SZ.5, 6, 7, 8, 9, 10, 11 ZNF804A is expressed in the brain, and the risk allele of rs1344706 has been associated with decreased mRNA expression during fetal brain development.80 Knockdown of ZNF804A in neural progenitor cells changes expression of genes pertaining to neuronal outgrowth and migration, synapse formation, and cell adhesion, all of which have been implicated in the pathophysiology of SZ.81 Animal and cell culture studies have shown that ZNF804A is associated with expression of COMT, an enzyme that degrades catecholamines, and the DRD2 dopamine receptor.82 These data provide a possible link between novel P300, ZNF804A and dopaminergic signaling, as there is evidence that the novel P300 ERP component is mediated by catecholaminergic activity.83 In silico studies have also suggested possible involvement of ZNF804A in regulation of oligodendrocyte proliferation and differentiation.84 This might impact neural connectivity, and in fact ZNF804A has been associated with connectivity between brain regions that are important in processing of auditory stimuli.18,27, 28, 29, 30,85
The present investigation detected significant association between ZNF804A and amplitude of the P300b electrophysiological component elicited by a novelty oddball task. The novel P300 component represents a brain mechanism dedicated to re-orienting attention to salient and novel events in the environment.24,25 Novel P300 is of particular interest since SZ is characterized by core cognitive deficits, notably, general difficulty in concentrating attention on relevant stimuli while ignoring unimportant stimuli,86 including during the CHR period.87,88 Indeed, the cases in our study exhibited lower P300b amplitude compared to controls, which is supported by prior reports of high-risk, recent onset, and chronic SZ subjects.19,20,89,90 Interestingly, we found that the risk-associated allele of rs1344706 was related to increased novel P300b amplitude, in contrast to the lower amplitude observed in SZ cases. This result implies that ZNF804A has pleiotropic effects on ERPs and SZ, in that the allele associated with increased risk of SZ is also related to higher P300b amplitude, opposite to that observed in SZ. Previous studies have also suggested that genes implicated in SZ etiology may exhibit pleiotropic effects on intermediate phenotypes including ERPs, gray matter volume, cognitive performance and sensorimotor gating.11,44,69,91 While this study is limited by a small sample size and mixed population that may increase the likelihood of false positive results, our findings are supported by those of O'Donoghue et al.92 (this issue), who found a remarkably similar relationship between the rs13444706 risk allele and increased P300 amplitude elicited by an auditory oddball task. Although O'Donoghue et al. did not utilize the same novel oddball task, both oddball P300 and novel P300 are elicited in response to rare, deviant and salient stimuli.26,93,94 Therefore, their results strongly support our findings and provide independent evidence for pleiotropic effects of ZNF804A on SZ and regulation of brain processes mediating ERPs.
Our finding of association with novel P300b amplitude in controls but not in cases, which was also observed by O'Donoghue et al.,92 could be due to several factors. One potential scenario is that the brain pathology present in individuals at high risk or diagnosed with SZ may disrupt the ZNF804A effect on P300 amplitude and thereby mask the association with elevated P300 amplitude in cases. It is also possible that other genetic, epigenetic and environmental risk factors that are highly loaded in cases but not controls influence ERPs such that the ZNF804A effect is obscured. Alternatively, the small case- and control-only samples had low statistical power, so failure to detect an association in some analyses is not surprising. Additional studies are needed to confirm our findings, elucidate the function of ZNF804A in SZ and brain function, and clarify its interaction with other risk factors.
Investigation of the functional significance of ZNF804A association with SZ has focused on brain structure and connectivity between neural circuits during performance of cognitive or emotional tasks, domains particularly affected in SZ.95,96 The risk allele of rs1344706 is hypothesized to modulate connectivity between the dorsolateral prefrontal cortex and hippocampus, specifically during working memory tasks, in both healthy controls43,85 and SZ subjects.97 These studies are pertinent to our results, as they indicate that ZNF804A may modulate specific neural circuitry that has been implicated in the dynamics of novel P300.18,27, 28, 29, 30 As novel P300 indexes a complex dynamic process in which detection of the novel event occurs via the anterior cingulate,26 among other regions, and habituation to the novel event via recruitment of working memory through the dorsolateral prefrontal cortex and hippocampus,18,27, 28, 29 our genetic association results with novel P300b are in line with published studies demonstrating ZNF804A association with modulation of connectivity between these brain regions.85,97,98 Thus, the present investigation supports the notion that ZNF804A regulates specific and dynamic brain processes, possibly at the interface between the salience and executive networks.32,99
On reviewing the extant literature, some studies suggest that SZ subjects carrying the rs1344706 risk allele may have milder impairments than non-carriers. For example, SZ risk allele carriers have larger regional gray matter volume and relatively intact cognitive performance,11 indicating that ZNF804A may delineate a subtype of SZ with preserved brain function in some domains. However, other studies have found greater impairment in SZ subjects carrying the risk allele, including heightened psychotic symptoms.100 In healthy controls, risk allele carriers appear to be more impaired, with impaired attention,91 deleterious effects on gray matter volume and cortical thickness and neural activity reported in default network areas45,91 and the Theory of Mind network.101 However, the latter studies only evaluated controls, therefore it is unknown whether these deleterious effects would also be observed in SZ subjects. It should also be kept in mind that different approaches have been used across studies (for example, grouping subjects of different genotypes) and that correction for multiple testing has not consistently been performed, which could result in inconsistent or spurious results. Future studies of both SZ and healthy subjects across multiple domains will be important in elucidating the role of ZNF804A in brain function and risk of SZ.
In conclusion, our finding of significant association between ZNF804A and novel P300b amplitude concurs with prior literature suggesting a specific role of this gene in modulating brain circuits that are neurobiological substrates of this ERP component.
Acknowledgments
This research was supported by the National Institute of Mental Health of the National Institutes of Health under Award Numbers 1P50 MH080272 (RWM), U01 MH081928 (LJS) and 1R01 MH092380 (TLP). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Genotyping was funded by a Massachusetts General Hospital Executive Committee on Research Interim Support Fund award (TLP). SEB is partially supported by NARSAD Young Investigator Grant 19753. We thank all subjects for their participation in the study. We also thank the clinical and data management staff from the Boston CIDAR study and the Commonwealth Research Center, including: Matcheri Keshavan, Joanne Wojcik, Ann Cousins, Michelle Friedman-Yakoobian, Anthony J Giuliano, Andrea Gnong Granato, Lauren Gibson, Sarah Hornbach, Julia Schutt, Kristy Klein, Maria Hiraldo, Grace Francis, Corin Pilo, Rachael Serur, Grace Min, Alison Thomas, and Molly Franz. We also thank Tamara Tasoff, Beril Yaffe, and Danbee Kim for electrophysiology assistance (Harvard), and Kimberly Chambert (Broad Institute of MIT and Harvard), Patience Gallagher, Steve Haddad, Brian Galloway, and Jenna Tarasoff (Massachusetts General Hospital) for genotyping assistance.
All authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Translational Psychiatry website (http://www.nature.com/tp)
Supplementary Material
References
- Sullivan PF, Kendler KS, Neale MC. Schizophrenia as a complex trait: evidence from a meta-analysis of twin studies. Arch Gen Psychiatry. 2003;60:1187–1192. doi: 10.1001/archpsyc.60.12.1187. [DOI] [PubMed] [Google Scholar]
- Lichtenstein P, Yip BH, Bjork C, Pawitan Y, Cannon TD, Sullivan PF, et al. Common genetic determinants of schizophrenia and bipolar disorder in Swedish families: a population-based study. Lancet. 2009;373:234–239. doi: 10.1016/S0140-6736(09)60072-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergen SE, Petryshen TL. Genome-wide association studies of schizophrenia: does bigger lead to better results. Curr Opin Psychiatry. 2012;25:76–82. doi: 10.1097/YCO.0b013e32835035dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Donovan MC, Craddock N, Norton N, Williams H, Peirce T, Moskvina V, et al. Identification of loci associated with schizophrenia by genome-wide association and follow-up. Nat Genet. 2008;40:1053–1055. doi: 10.1038/ng.201. [DOI] [PubMed] [Google Scholar]
- Stefansson H, Ophoff RA, Steinberg S, Andreassen OA, Cichon S, Rujescu D, et al. Common variants conferring risk of schizophrenia. Nature. 2009;460:744–747. doi: 10.1038/nature08186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg S, Mors O, Borglum AD, Gustafsson O, Werge T, Mortensen PB, et al. Expanding the range of ZNF804A variants conferring risk of psychosis. Mol Psychiatry. 2011;16:59–66. doi: 10.1038/mp.2009.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams HJ, Norton N, Dwyer S, Moskvina V, Nikolov I, Carroll L, et al. Fine mapping of ZNF804A and genome-wide significant evidence for its involvement in schizophrenia and bipolar disorder. Mol Psychiatry. 2011;16:429–441. doi: 10.1038/mp.2010.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwab SG, Kusumawardhani AA, Dai N, Qin W, Wildenauer MD, Agiananda F, et al. Association of rs1344706 in the ZNF804A gene with schizophrenia in a case/control sample from Indonesia. Schizophr Res. 2013;147:46–52. doi: 10.1016/j.schres.2013.03.022. [DOI] [PubMed] [Google Scholar]
- Zhang R, Yan JD, Valenzuela RK, Lu SM, Du XY, Zhong B, et al. Further evidence for the association of genetic variants of ZNF804A with schizophrenia and a meta-analysis for genome-wide significance variant rs1344706. Schizophr Res. 2012;141:40–47. doi: 10.1016/j.schres.2012.07.013. [DOI] [PubMed] [Google Scholar]
- Zhang R, Valenzuela RK, Lu S, Meng L, Guo T, Du X, et al. Is the conserved mammalian region of ZNF804A locus associated with schizophrenia? A population-based genetics analysis. Schizophr Res. 2011;133:159–164. doi: 10.1016/j.schres.2011.09.012. [DOI] [PubMed] [Google Scholar]
- Walters JT, Corvin A, Owen MJ, Williams H, Dragovic M, Quinn EM, et al. Psychosis susceptibility gene ZNF804A and cognitive performance in schizophrenia. Arch Gen Psychiatry. 2010;67:692–700. doi: 10.1001/archgenpsychiatry.2010.81. [DOI] [PubMed] [Google Scholar]
- Agnew-Blais J, Seidman LJ. Neurocognition in youth and young adults under age 30 at familial risk for schizophrenia: a quantitative and qualitative review. Cogn Neuropsychiatry. 2013;18:44–82. doi: 10.1080/13546805.2012.676309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thermenos HW, , , Keshavan MS, , , Juelich RJ, , , Molokotos E, , , Whitfield-Gabrieli S, , , Brent BK.et al. A review of neuroimaging studies of young relatives of individuals with schizophrenia: a developmental perspective from schizotaxia to schizophrenia. Am J Med Genet B Neuropsychiatr Genet 2013; 162: 604–635. [DOI] [PubMed] [Google Scholar]
- McCarley RW, Nakamura M, Shenton ME, Salisbury DF. Combining ERP and structural MRI information in first episode schizophrenia and bipolar disorder. Clin EEG Neurosci. 2008;39:57–60. doi: 10.1177/155005940803900206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michie PT, Innes-Brown H, Todd J, Jablensky AV. Duration mismatch negativity in biological relatives of patients with schizophrenia spectrum disorders. Biol Psychiatry. 2002;52:749–758. doi: 10.1016/s0006-3223(02)01379-3. [DOI] [PubMed] [Google Scholar]
- Price GW, Michie PT, Johnston J, Innes-Brown H, Kent A, Clissa P, et al. A multivariate electrophysiological endophenotype, from a unitary cohort, shows greater research utility than any single feature in the Western Australian family study of schizophrenia. Biol Psychiatry. 2006;60:1–10. doi: 10.1016/j.biopsych.2005.09.010. [DOI] [PubMed] [Google Scholar]
- Hall MH, Schulze K, Rijsdijk F, Kalidindi S, McDonald C, Bramon E, et al. Are auditory P300 and duration MMN heritable and putative endophenotypes of psychotic bipolar disorder? A Maudsley Bipolar Twin and Family Study. Psychol Med. 2009;39:1277–1287. doi: 10.1017/S0033291709005261. [DOI] [PubMed] [Google Scholar]
- Knight RT. Decreased response to novel stimuli after prefrontal lesions in man. Electroencephalogr Clin Neurophysiol. 1984;59:9–20. doi: 10.1016/0168-5597(84)90016-9. [DOI] [PubMed] [Google Scholar]
- Mathalon DH, Hoffman RE, Watson TD, Miller RM, Roach BJ, Ford JM. Neurophysiological Distinction between Schizophrenia and Schizoaffective Disorder. Front Hum Neurosci. 2010;3:70. doi: 10.3389/neuro.09.070.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mondragon-Maya A, Solis-Vivanco R, Leon-Ortiz P, Rodriguez-Agudelo Y, Yanez-Tellez G, Bernal-Hernandez J, et al. Reduced P3a amplitudes in antipsychotic naive first-episode psychosis patients and individuals at clinical high-risk for psychosis. J Psychiatr Res. 2013;47:755–761. doi: 10.1016/j.jpsychires.2012.12.017. [DOI] [PubMed] [Google Scholar]
- Turetsky BI, Cannon TD, Gur RE. P300 subcomponent abnormalities in schizophrenia: III. Deficits In unaffected siblings of schizophrenic probands. Biol Psychiatry. 2000;47:380–390. doi: 10.1016/s0006-3223(99)00290-5. [DOI] [PubMed] [Google Scholar]
- Turetsky BI, Calkins ME, Light GA, Olincy A, Radant AD, Swerdlow NR. Neurophysiological endophenotypes of schizophrenia: the viability of selected candidate measures. Schizophr Bull. 2007;33:69–94. doi: 10.1093/schbul/sbl060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squires NK, Squires KC, Hillyard SA. Two varieties of long-latency positive waves evoked by unpredictable auditory stimuli in man. Electroencephalogr Clin Neurophysiol. 1975;38:387–401. doi: 10.1016/0013-4694(75)90263-1. [DOI] [PubMed] [Google Scholar]
- Ranganath C, Rainer G. Neural mechanisms for detecting and remembering novel events. Nat Rev Neurosci. 2003;4:193–202. doi: 10.1038/nrn1052. [DOI] [PubMed] [Google Scholar]
- Polich J. Updating P300: an integrative theory of P3a and P3b. Clin Neurophysiol. 2007;118:2128–2148. doi: 10.1016/j.clinph.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dien J, Spencer KM, Donchin E. Localization of the event-related potential novelty response as defined by principal components analysis. Brain Res Cogn Brain Res. 2003;17:637–650. doi: 10.1016/s0926-6410(03)00188-5. [DOI] [PubMed] [Google Scholar]
- Sokolov EN. Higher nervous functions; the orienting reflex. Annu Rev Physiol. 1963;25:545–580. doi: 10.1146/annurev.ph.25.030163.002553. [DOI] [PubMed] [Google Scholar]
- Knight R. Contribution of human hippocampal region to novelty detection. Nature. 1996;383:256–259. doi: 10.1038/383256a0. [DOI] [PubMed] [Google Scholar]
- Yamaguchi S, Hale LA, D'Esposito M, Knight RT. Rapid prefrontal-hippocampal habituation to novel events. J Neurosci. 2004;24:5356–5363. doi: 10.1523/JNEUROSCI.4587-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axmacher N, Cohen MX, Fell J, Haupt S, Dumpelmann M, Elger CE, et al. Intracranial EEG correlates of expectancy and memory formation in the human hippocampus and nucleus accumbens. Neuron. 2010;65:541–549. doi: 10.1016/j.neuron.2010.02.006. [DOI] [PubMed] [Google Scholar]
- Menon V, Uddin LQ. Saliency, switching, attention and control: a network model of insula function. Brain Struct Funct. 2010;214:655–667. doi: 10.1007/s00429-010-0262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bressler SL, Menon V. Large-scale brain networks in cognition: emerging methods and principles. Trends Cogn Sci. 2010;14:277–290. doi: 10.1016/j.tics.2010.04.004. [DOI] [PubMed] [Google Scholar]
- Gallinat J, Bajbouj M, Sander T, Schlattmann P, Xu K, Ferro EF, et al. Association of the G1947A COMT (Val(108/158)Met) gene polymorphism with prefrontal P300 during information processing. Biol Psychiatry. 2003;54:40–48. doi: 10.1016/s0006-3223(02)01973-x. [DOI] [PubMed] [Google Scholar]
- Golimbet V, Gritsenko I, Alfimova M, Lebedeva I, Lezheiko T, Abramova L, et al. Association study of COMT gene Val158Met polymorphism with auditory P300 and performance on neurocognitive tests in patients with schizophrenia and their relatives. World J Biol Psychiatry. 2006;7:238–245. doi: 10.1080/15622970600670970. [DOI] [PubMed] [Google Scholar]
- Bramon E, Shaikh M, Broome M, Lappin J, Berge D, Day F, et al. Abnormal P300 in people with high risk of developing psychosis. Neuroimage. 2008;41:553–560. doi: 10.1016/j.neuroimage.2007.12.038. [DOI] [PubMed] [Google Scholar]
- Kang C, Yang X, Xu X, Liu H, Su P, Yang J. Association study of neuregulin 1 gene polymorphisms with auditory P300 in schizophrenia. Am J Med Genet B Neuropsychiatr Genet. 2012;159B:422–428. doi: 10.1002/ajmg.b.32045. [DOI] [PubMed] [Google Scholar]
- Konrad A, Vucurevic G, Musso F, Stoeter P, Dahmen N, Winterer G. ErbB4 genotype predicts left frontotemporal structural connectivity in human brain. Neuropsychopharmacology. 2009;34:641–650. doi: 10.1038/npp.2008.112. [DOI] [PubMed] [Google Scholar]
- Donohoe G, Morris DW, De Sanctis P, Magno E, Montesi JL, Garavan HP, et al. Early visual processing deficits in dysbindin-associated schizophrenia. Biol Psychiatry. 2008;63:484–489. doi: 10.1016/j.biopsych.2007.07.022. [DOI] [PubMed] [Google Scholar]
- Jablensky A, Morar B, Wiltshire S, Carter K, Dragovic M, Badcock JC, et al. Polymorphisms associated with normal memory variation also affect memory impairment in schizophrenia. Genes Brain Behav. 2011;10:410–417. doi: 10.1111/j.1601-183X.2011.00679.x. [DOI] [PubMed] [Google Scholar]
- Papassotiropoulos A, Stephan DA, Huentelman MJ, Hoerndli FJ, Craig DW, Pearson JV, et al. Common Kibra alleles are associated with human memory performance. Science. 2006;314:475–478. doi: 10.1126/science.1129837. [DOI] [PubMed] [Google Scholar]
- Roffman JL, Weiss AP, Deckersbach T, Freudenreich O, Henderson DC, Purcell S, et al. Effects of the methylenetetrahydrofolate reductase (MTHFR) C677T polymorphism on executive function in schizophrenia. Schizophr Res. 2007;92:181–188. doi: 10.1016/j.schres.2007.01.003. [DOI] [PubMed] [Google Scholar]
- Carless MA, Glahn DC, Johnson MP, Curran JE, Bozaoglu K, Dyer TD, et al. Impact of DISC1 variation on neuroanatomical and neurocognitive phenotypes. Mol Psychiatry. 2011;16:1096–1104, 1063. doi: 10.1038/mp.2011.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esslinger C, Kirsch P, Haddad L, Mier D, Sauer C, Erk S, et al. Cognitive state and connectivity effects of the genome-wide significant psychosis variant in ZNF804A. Neuroimage. 2011;54:2514–2523. doi: 10.1016/j.neuroimage.2010.10.012. [DOI] [PubMed] [Google Scholar]
- Donohoe G, Rose E, Frodl T, Morris D, Spoletini I, Adriano F, et al. ZNF804A risk allele is associated with relatively intact gray matter volume in patients with schizophrenia. Neuroimage. 2011;54:2132–2137. doi: 10.1016/j.neuroimage.2010.09.089. [DOI] [PubMed] [Google Scholar]
- Lencz T, Szeszko PR, DeRosse P, Burdick KE, Bromet EJ, Bilder RM, et al. A schizophrenia risk gene, ZNF804A, influences neuroanatomical and neurocognitive phenotypes. Neuropsychopharmacology. 2010;35:2284–2291. doi: 10.1038/npp.2010.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods SW, Miller TJ, McGlashan TH. The ‘prodromal' patient: both symptomatic and at-risk. CNS Spectr. 2001;6:223–232. doi: 10.1017/s1092852900008609. [DOI] [PubMed] [Google Scholar]
- Miller TJ, McGlashan TH, Woods SW, Stein K, Driesen N, Corcoran CM, et al. Symptom assessment in schizophrenic prodromal states. Psychiatr Q. 1999;70:273–287. doi: 10.1023/a:1022034115078. [DOI] [PubMed] [Google Scholar]
- Olsen KA, Rosenbaum B. Prospective investigations of the prodromal state of schizophrenia: review of studies. Acta Psychiatr Scand. 2006;113:247–272. doi: 10.1111/j.1600-0447.2005.00697.x. [DOI] [PubMed] [Google Scholar]
- Klosterkotter J, Hellmich M, Steinmeyer EM, Schultze-Lutter F. Diagnosing schizophrenia in the initial prodromal phase. Arch Gen Psychiatry. 2001;58:158–164. doi: 10.1001/archpsyc.58.2.158. [DOI] [PubMed] [Google Scholar]
- Schultze-Lutter F, Klosterkotter J, Picker H, Steinmeyer E-M, Ruhrmann S. Predicting first-episode psychosis by basic symptom criteria. Clinical Neuropsychiatry. 2007;4:11–22. [Google Scholar]
- Addington J, Cornblatt BA, Cadenhead KS, Cannon TD, McGlashan TH, Perkins DO, et al. At clinical high risk for psychosis: outcome for nonconverters. Am J Psychiatry. 2011;168:800–805. doi: 10.1176/appi.ajp.2011.10081191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoll AL.The Psychopharmacology Reference Card McLean Hospital: Belmont, MA, USA; 2001 [Google Scholar]
- Hollingshead AB. Two Factor Index of Social Position. Yale University Press: New Haven, CT, USA; 1975. [Google Scholar]
- McCarley RW, Shenton ME, O'Donnell BF, Faux SF, Kikinis R, Nestor PG, et al. Auditory P300 abnormalities and left posterior superior temporal gyrus volume reduction in schizophrenia. Arch Gen Psychiatry. 1993;50:190–197. doi: 10.1001/archpsyc.1993.01820150036003. [DOI] [PubMed] [Google Scholar]
- Niznikiewicz MA, Spencer KM, Dickey C, Voglmaier M, Seidman LJ, Shenton ME, et al. Abnormal pitch mismatch negativity in individuals with schizotypal personality disorder. Schizophr Res. 2009;110:188–193. doi: 10.1016/j.schres.2008.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratton G, Coles MG, Donchin E. A new method for off-line removal of ocular artifact. Electroencephalogr Clin Neurophysiol. 1983;55:468–484. doi: 10.1016/0013-4694(83)90135-9. [DOI] [PubMed] [Google Scholar]
- Wilkinson GS, Robertson GJ. Wide Range Achievement Test-Fourth Edition. Psychological Assessment Resources: Lutz, FL, USA; 2006. [Google Scholar]
- Wechsler D. Wechsler Abbreviated Scale of Intelligence. Psychological Corporation, Harcourt Brace; New York, NY, USA; 1999. [Google Scholar]
- Seidman LJ, Breiter HC, Goodman JM, Goldstein JM, Woodruff PW, O'Craven K, et al. A functional magnetic resonance imaging study of auditory vigilance with low and high information processing demands. Neuropsychology. 1998;12:505–518. doi: 10.1037//0894-4105.12.4.505. [DOI] [PubMed] [Google Scholar]
- Seidman LJ, Meyer EC, Giuliano AJ, Breiter HC, Goldstein JM, Kremen WS, et al. Auditory working memory impairments in individuals at familial high risk for schizophrenia. Neuropsychology. 2012;26:288–303. doi: 10.1037/a0027970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MA, Bender D, et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet. 2007;81:559–575. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browning SR, Browning BL. Rapid and accurate haplotype phasing and missing-data inference for whole-genome association studies by use of localized haplotype clustering. Am J Hum Genet. 2007;81:1084–1097. doi: 10.1086/521987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- International HapMap Consortium International HapMap Consortium. Altshuler DM, International HapMap Consortium. Gibbs RA, International HapMap Consortium. Peltonen L, International HapMap Consortium. Altshuler DM, International HapMap Consortium. Gibbs RA, et al. Integrating common and rare genetic variation in diverse human populations. Nature. 2010;467:52–58. doi: 10.1038/nature09298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schizophrenia Psychiatric Genome-Wide Association Study Consortium Genome-wide association study identifies five new schizophrenia loci. Nat Genet. 2011;43:969–976. doi: 10.1038/ng.940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg S, de Jong S, Irish Schizophrenia Genomics Consortium, Andreassen OA, Werge T, Borglum AD, et al. Common variants at VRK2 and TCF4 conferring risk of schizophrenia. Hum Mol Genet. 2011;20:4076–4081. doi: 10.1093/hmg/ddr325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- International Schizophrenia Consortium, Purcell SM, Wray NR, Stone JL, Visscher PM, O'Donovan MC, et al. Common polygenic variation contributes to risk of schizophrenia and bipolar disorder. Nature. 2009;460:748–752. doi: 10.1038/nature08185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Psychiatric GWAS Consortium Bipolar Disorder Working Group Large-scale genome-wide association analysis of bipolar disorder identifies a new susceptibility locus near ODZ4. Nat Genet. 2011;43:977–983. doi: 10.1038/ng.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pe'er I, Yelensky R, Altshuler D, Daly MJ. Estimation of the multiple testing burden for genomewide association studies of nearly all common variants. Genet Epidemiol. 2008;32:381–385. doi: 10.1002/gepi.20303. [DOI] [PubMed] [Google Scholar]
- Greenwood TA, Lazzeroni LC, Murray SS, Cadenhead KS, Calkins ME, Dobie DJ, et al. Analysis of 94 candidate genes and 12 endophenotypes for schizophrenia from the Consortium on the Genetics of Schizophrenia. Am J Psychiatry. 2011;168:930–946. doi: 10.1176/appi.ajp.2011.10050723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicodemus KK, Luna A, Vakkalanka R, Goldberg T, Egan M, Straub RE, et al. Further evidence for association between ErbB4 and schizophrenia and influence on cognitive intermediate phenotypes in healthy controls. Mol Psychiatry. 2006;11:1062–1065. doi: 10.1038/sj.mp.4001878. [DOI] [PubMed] [Google Scholar]
- Roffman JL, Weiss AP, Purcell S, Caffalette CA, Freudenreich O, Henderson DC, et al. Contribution of methylenetetrahydrofolate reductase (MTHFR) polymorphisms to negative symptoms in schizophrenia. Biol Psychiatry. 2008;63:42–48. doi: 10.1016/j.biopsych.2006.12.017. [DOI] [PubMed] [Google Scholar]
- Roffman JL, Weiss AP, Deckersbach T, Freudenreich O, Henderson DC, Wong DH, et al. Interactive effects of COMT Val108/158Met and MTHFR C677T on executive function in schizophrenia. Am J Med Genet B Neuropsychiatr Genet. 2008;147B:990–995. doi: 10.1002/ajmg.b.30684. [DOI] [PubMed] [Google Scholar]
- Tsai SJ, Yu YW, Chen TJ, Chen JY, Liou YJ, Chen MC, et al. Association study of a functional catechol-O-methyltransferase-gene polymorphism and cognitive function in healthy females. Neurosci Lett. 2003;338:123–126. doi: 10.1016/s0304-3940(02)01396-4. [DOI] [PubMed] [Google Scholar]
- Donohoe G, Frodl T, Morris D, Spoletini I, Cannon DM, Cherubini A, et al. Reduced occipital and prefrontal brain volumes in dysbindin-associated schizophrenia. Neuropsychopharmacology. 2010;35:368–373. doi: 10.1038/npp.2009.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narr KL, Szeszko PR, Lencz T, Woods RP, Hamilton LS, Phillips O, et al. DTNBP1 is associated with imaging phenotypes in schizophrenia. Hum Brain Mapp. 2009;30:3783–3794. doi: 10.1002/hbm.20806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mata I, Perez-Iglesias R, Roiz-Santianez R, Tordesillas-Gutierrez D, Gonzalez-Mandly A, Berja A, et al. Additive effect of NRG1 and DISC1 genes on lateral ventricle enlargement in first episode schizophrenia. Neuroimage. 2010;53:1016–1022. doi: 10.1016/j.neuroimage.2009.11.010. [DOI] [PubMed] [Google Scholar]
- Kohannim O, Hibar DP, Jahanshad N, Stein JL, Hua X, Toga AW, et al. Predicting temporal lobe volume on MRI from genotypes using L(1)-L(2) regularized regression. Proc IEEE Int Symp Biomed Imaging. 2012. pp. 1160–1163. [DOI] [PMC free article] [PubMed]
- Pomarol-Clotet E, Fatjo-Vilas M, McKenna PJ, Monte GC, Sarro S, Ortiz-Gil J, et al. COMT Val158Met polymorphism in relation to activation and de-activation in the prefrontal cortex: a study in patients with schizophrenia and healthy subjects. Neuroimage. 2010;53:899–907. doi: 10.1016/j.neuroimage.2010.04.018. [DOI] [PubMed] [Google Scholar]
- Wang D, Liu B, Qin W, Wang J, Zhang Y, Jiang T, et al. KIBRA gene variants are associated with synchronization within the default-mode and executive control networks. Neuroimage. 2013;69:213–222. doi: 10.1016/j.neuroimage.2012.12.022. [DOI] [PubMed] [Google Scholar]
- Hill MJ, Bray NJ. Evidence that schizophrenia risk variation in the ZNF804A gene exerts its effects during fetal brain development. Am J Psychiatry. 2012;169:1301–1308. doi: 10.1176/appi.ajp.2012.11121845. [DOI] [PubMed] [Google Scholar]
- Hill MJ, Jeffries AR, Dobson RJ, Price J, Bray NJ. Knockdown of the psychosis susceptibility gene ZNF804A alters expression of genes involved in cell adhesion. Hum Mol Genet. 2012;21:1018–1024. doi: 10.1093/hmg/ddr532. [DOI] [PubMed] [Google Scholar]
- Girgenti MJ, LoTurco JJ, Maher BJ. ZNF804a regulates expression of the schizophrenia-associated genes PRSS16, COMT, PDE4B, and DRD2. PLoS One. 2012;7:e32404. doi: 10.1371/journal.pone.0032404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polich J, Criado JR. Neuropsychology and neuropharmacology of P3a and P3b. Int J Psychophysiol. 2006;60:172–185. doi: 10.1016/j.ijpsycho.2005.12.012. [DOI] [PubMed] [Google Scholar]
- Riley B, Thiselton D, Maher BS, Bigdeli T, Wormley B, McMichael GO, et al. Replication of association between schizophrenia and ZNF804A in the Irish case-control study of schizophrenia sample. Mol Psychiatry. 2010;15:29–37. doi: 10.1038/mp.2009.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esslinger C, Walter H, Kirsch P, Erk S, Schnell K, Arnold C, et al. Neural mechanisms of a genome-wide supported psychosis variant. Science. 2009;324:605. doi: 10.1126/science.1167768. [DOI] [PubMed] [Google Scholar]
- Grillon C, Courchesne E, Ameli R, Geyer MA, Braff DL. Increased distractibility in schizophrenic patients. Electrophysiologic and behavioral evidence. Arch Gen Psychiatry. 1990;47:171–179. doi: 10.1001/archpsyc.1990.01810140071010. [DOI] [PubMed] [Google Scholar]
- Giuliano AJ, Li H, Mesholam-Gately RI, Sorenson SM, Woodberry KA, Seidman LJ. Neurocognition in the psychosis risk syndrome: a quantitative and qualitative review. Curr Pharm Des. 2012;18:399–415. doi: 10.2174/138161212799316019. [DOI] [PubMed] [Google Scholar]
- Mesholam-Gately RI, Giuliano AJ, Goff KP, Faraone SV, Seidman LJ. Neurocognition in first-episode schizophrenia: a meta-analytic review. Neuropsychology. 2009;23:315–336. doi: 10.1037/a0014708. [DOI] [PubMed] [Google Scholar]
- Jahshan C, Cadenhead KS, Rissling AJ, Kirihara K, Braff DL, Light GA. Automatic sensory information processing abnormalities across the illness course of schizophrenia. Psychol Med. 2012;42:85–97. doi: 10.1017/S0033291711001061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devrim-Ucok M, Keskin-Ergen HY, Ucok A. Novelty P3 and P3b in first-episode schizophrenia and chronic schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2006;30:1426–1434. doi: 10.1016/j.pnpbp.2006.05.019. [DOI] [PubMed] [Google Scholar]
- Voineskos AN, Lerch JP, Felsky D, Tiwari A, Rajji TK, Miranda D, et al. The ZNF804A gene: characterization of a novel neural risk mechanism for the major psychoses. Neuropsychopharmacology. 2011;36:1871–1878. doi: 10.1038/npp.2011.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Donoghue T, Morris DW, Fahey C, Costa AD, Moore S, Cummings E, et al. Effects of ZNF804A on auditory P300 response in schizophrenia Transl Psychiatry 2014(this issue). [DOI] [PMC free article] [PubMed]
- Spencer KM, Dien J, Donchin E. Spatiotemporal analysis of the late ERP responses to deviant stimuli. Psychophysiology. 2001;38:343–358. [PubMed] [Google Scholar]
- Spencer KM, Dien J, Donchin E. A componential analysis of the ERP elicited by novel events using a dense electrode array. Psychophysiology. 1999;36:409–414. doi: 10.1017/s0048577299981180. [DOI] [PubMed] [Google Scholar]
- Shenton ME, Dickey CC, Frumin M, McCarley RW. A review of MRI findings in schizophrenia. Schizophr Res. 2001;49:1–52. doi: 10.1016/s0920-9964(01)00163-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fornito A, Bullmore ET. Connectomic intermediate phenotypes for psychiatric disorders. Front Psychiatry. 2012;3:32. doi: 10.3389/fpsyt.2012.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasetti R, Sambataro F, Chen Q, Callicott JH, Mattay VS, Weinberger DR. Altered cortical network dynamics: a potential intermediate phenotype for schizophrenia and association with ZNF804A. Arch Gen Psychiatry. 2011;68:1207–1217. doi: 10.1001/archgenpsychiatry.2011.103. [DOI] [PubMed] [Google Scholar]
- Meyer-Lindenberg AS, Olsen RK, Kohn PD, Brown T, Egan MF, Weinberger DR, et al. Regionally specific disturbance of dorsolateral prefrontal-hippocampal functional connectivity in schizophrenia. Arch Gen Psychiatry. 2005;62:379–386. doi: 10.1001/archpsyc.62.4.379. [DOI] [PubMed] [Google Scholar]
- Allman JM, Hakeem A, Erwin JM, Nimchinsky E, Hof P. The anterior cingulate cortex. The evolution of an interface between emotion and cognition. Ann N Y Acad Sci. 2001;935:107–117. [PubMed] [Google Scholar]
- Wassink TH, Epping EA, Rudd D, Axelsen M, Ziebell S, Fleming FW, et al. Influence of ZNF804a on brain structure volumes and symptom severity in individuals with schizophrenia. Arch Gen Psychiatry. 2012;69:885–892. doi: 10.1001/archgenpsychiatry.2011.2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter H, Schnell K, Erk S, Arnold C, Kirsch P, Esslinger C, et al. Effects of a genome-wide supported psychosis risk variant on neural activation during a theory-of-mind task. Mol Psychiatry. 2011;16:462–470. doi: 10.1038/mp.2010.18. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.