Abstract
Myelination and neurite outgrowth both occur during brain development, and their disturbance has been previously been implicated in the pathophysiology of schizophrenia. Leucine-rich repeat and immunoglobulin domain-containing protein (Lingo-1) is a potent negative regulator of axonal myelination and neurite extension. As co-factors of Lingo-1 signaling (Nogo receptor (NgR), With No Lysine (K) (WNK1) and Myelin transcription factor 1 (Myt1)) have been implicated in the genetics of schizophrenia, we explored for the first time the role of Lingo-1 signaling pathways in this disorder. Lingo-1 protein, together with its co-receptor and co-factor proteins NgR, tumor necrosis factor (TNF) receptor orphan Y (TROY), p75, WNK1 and Myt1, have never been explored in the pathogenesis of schizophrenia. We examined protein levels of Lingo-1, NgR, TROY, p75, WNK1, Myt1 and myelin basic protein (MBP) (as a marker of myelination) within the post-mortem dorsolateral prefrontal cortex (DLPFC) (37 schizophrenia patients versus 37 matched controls) and hippocampus (Cornu Ammonis, CA1 and CA3) (20 schizophrenia patients versus 20 matched controls from the same cohort). Both of these brain regions are highly disrupted in the schizophrenia pathophysiology. There were significant increases in Lingo-1 (P<0.001) and Myt1 (P=0.023) and a reduction in NgR (P<0.001) in the DLPFC in schizophrenia subjects compared with controls. There were also increases in both TROY (P=0.001) and WNK1 (P=0.011) in the CA1 of schizophrenia subjects and, in contrast to the DLPFC, there was an increase in NgR (P=0.006) in the CA3 of schizophrenia subjects compared with controls. No significant difference was reported for MBP levels (P>0.05) between the schizophrenia and control groups in the three tested regions. This is the first time that a study has shown altered Lingo-1 signaling in the schizophrenia brain. Our novel findings may present a direct application for the use of a Lingo-1 antagonist to complement current and future schizophrenia therapies.
Keywords: Lingo-1, Myt1, Nogo receptor, p75/TROY, schizophrenia, WNK1
Introduction
Schizophrenia is a severe neuropsychiatric disorder with an elusive etiology, thought to result from abnormal brain development during the first 3 decades of life.1 Dysfunction of axonal myelination has been a prominent hypothesis of schizophrenia pathophysiology, supported by gene expression,2, 3, 4 histopathology5,6 and imaging studies.7, 8, 9, 10 Interestingly, myelination peaks during late adolescence, coinciding with the onset of schizophrenia,11 and involves associative cortical regions implicated in the disorder, such as dorsolateral prefrontal cortex (DLPFC) and superior temporal gyrus.12 In normal brain development, oligodendrocytes are the cells responsible for forming myelin around neurons. Leucine-rich repeat and immunoglobulin domain-containing protein (Lingo-1), a transmembrane signal-transducing molecule selectively expressed on oligodendrocytes and neurons,13 has been reported to be a potent negative regulator of oligodendrocyte differentiation, axonal integrity and myelination.13,14 Its action notably involves the Nogo receptor (NgR) as a part of a co-receptor complex. NgR binds with either the p75 neurotrophin receptor or tumor necrosis factor (TNF) receptor orphan Y (TROY).15, 16, 17 The resulting trimolecular receptor complex Lingo-1/NgR/p75 or Lingo-1/NgR/TROY activates Ras homolog gene family, member A (RhoA), initiating a cascade of intracellular molecular events resulting in collapse of growth cones, preventing further axonal growth and inhibiting myelination.13,18 The existence of additional signaling co-factors has been suggested based on the absence of p75 and TROY on Lingo-1 containing neurons projecting to the spinal cord.19
Using the intracellular domain of Lingo-1 as bait, Zhang et.al.20 identified several candidates directly interacting with Lingo-1 including serine threonine kinase With No Lysine K (WNK1). Both suppression and overexpression of WNK1 promotes neurite extension and eliminates the inhibitory response to the Nogo ligand in cortical neurons.20 In addition, expression of the WNK1 gene has been reported to be upregulated in the PFC of schizophrenia sufferers,21 suggesting that it has a role in this disorder. Myelin transcription factor 1 (Myt1), a postmitotic neuronal specific zinc protein, and its highly conserved homolog, Myt1-like (Myt1l), are two other well-characterized direct intracellular binding partners of Lingo-1. Although disruption of the Myt1l gene was reported in a Dutch schizophrenia population,22 little is known about the implication of the Myt1 gene and protein in schizophrenia.
Functional genetic polymorphisms in the NgR gene previously associated with schizophrenia have been reported to directly affect interactions between NgR and its ligands or with its co-receptors (p75, TROY and Lingo-1) in in vitro neuronal culture.23,24 Interestingly, NgR knockout mice are now an established animal model for schizophrenia,24 linking downregulation or loss of NgR with schizophrenia; however, it is still unknown how NgR is involved in the pathophysiology of the disease.
Despite the implication of some of Lingo-1's co-factors in schizophrenia, no studies have specifically investigated a link between Lingo-1 and schizophrenia, although numerous studies have reported a dysfunctional profile of myelination gene expression in the post-mortem PFC and hippocampus in schizophrenia sufferers.2,25 As Lingo-1 is a known myelination inhibitor shown to inhibit oligodendrocyte differentiation and myelination when overexpressed,26 studying Lingo-1 in schizophrenia is a valid and innovative avenue of research. For the first time, we have examined Lingo-1 and its signaling partners in post-mortem schizophrenia DLPFC and hippocampus (CA1 and CA3 regions), regions highly disrupted in schizophrenia.27, 28, 29
Materials and methods
Human brain tissue samples
Human DLPFC and hippocampus (CA1 and CA3) samples were obtained from the New South Wales (NSW) Brain Bank Network. The sample cohort consisted of 37 schizophrenia subjects (including 7 schizoaffective disorder subjects) and 37 controls, matched for age at death, post-mortem interval (PMI), brain pH and RNA integrity (Table 1). Owing to limited availability of hippocampus tissue from this same cohort, 20 schizophrenia subjects from this cohort and their matched controls were used (Table 1). There was no significant difference in subject demographics (age at death, PMI, brain pH and RNA integrity) between DLPFC and hippocampus cohorts (P>0.05; Table 1). Further details regarding demography and clinical factors of all subjects pre- and post-mortem have been previously described30 and can be found in Supplementary Information (Supplementary Table ST1). Subjects with schizophrenia were diagnosed using Diagnostic and Statistical Manual of Mental Disorders IV criteria. All subjects with schizophrenia were prescribed antipsychotics at the time of death and a lifetime chlorpromazine equivalent was calculated for each patient. Clinical assessments, selection of schizophrenia cases and matched controls, assessment of tissue quality and preparation of tissue homogenates were all performed by the NSW Brain Bank Network and Schizophrenia Research Laboratory.30 All studies were approved by and conducted according to the guidelines of the Human Research Ethics Committees at the University of Wollongong (HE 99/222) and the University of NSW (HREC 07261).
Table 1. Subject demographics from DLPFC and hippocampus brain cohorts for schizophrenia.
| Demographics |
Control subjects |
Schizophrenia subjects |
||
|---|---|---|---|---|
| DLPFC | Hippocampus | DLPFC | Hippocampus | |
| Age at death (years) | 51.1±14.6 | 58.2±12.6 | 51.3±14.1 | 55.5±13.5 |
| Post-mortem interval (hours) | 24.8±11.0 | 26.1±12.8 | 28.8±13.8 | 28.3±10.1 |
| Brain pH | 6.7±0.3 | 6.6±0.3 | 6.6±0.3 | 6.6±0.3 |
| RNA integrity | 7.3±0.6 | 7.2±0.7 | 7.3±0.6 | 7.2±0.5 |
| Gender | 7 F, 30 M | 2 F, 18 M | 13 F, 24 M | 9 F, 11 M |
Abbreviations: DLPFC, dorsolateral prefrontal cortex; F, female; M, male.
Data are expressed as mean±s.d.
Human brain tissue preparation
Tissue from human DLPFC samples was obtained from the middle one-third (rostro-caudally) of the middle frontal gyrus from coronal slabs anterior to the genu of corpus callosum30 (corresponding to Brodmann Area 46). Tissue samples from both CA1 and CA3 regions of the hippocampus were obtained from the level of the lateral geniculate nucleus. Tissue was homogenized gently in a homogenizing buffer (50 mM Tris, pH 7.5, 50% glycerol), and diluted 1:20 (by volume) by protease inhibitor cocktail (Sigma, Australia). Protein concentrations were determined using a spectrophotometer. All samples were diluted to a concentration of 2 μg μl−1 and stored at −80 °C until required for immunoblotting, as previously detailed.31
Immunoblotting
Relative levels of all proteins were determined by immunoblot analysis. Proteins were resolved by SDS-polyacrylamide gel electrophoresis and were transferred to polyvinylidene fluoride membranes (Bio-Rad, Australia). Membranes were probed with anti-Lingo-1 (1:500; ab23631, Abcam, UK), anti-NgR (1:500; ab26291, Abcam), anti-p75 (1:500; ab8874, Abcam), anti-TROY (1:200; ab12126, Abcam), anti-WNK1 (1:500; ab128858, Abcam), anti-Myt1 (1:500; ab82844, Abcam), anti-myelin basic protein (MBP) (1:1000; ab53294, Abcam) primary polyclonal or monoclonal antibodies. Visualization and quantification of immunoblot bands was performed using the Gel Logic 2200 Pro (Carestream Molecular Imaging; Rochester, NY, USA). All samples were run in duplicate or triplicate, with samples loaded in a randomized order with a relatively even number of schizophrenia and control samples per gel to minimize the effects of gel-to-gel variability on the results. A pooled sample, combining aliquots from all 74 subjects, was loaded onto each gel within the experiment to account for any gel-to-gel variability (which was calculated to be between 9 and 13% (1.28±0.11 to 0.38±0.05), and all immunoblot bands were normalized to a β-actin (1:5000; MAB1501, Millipore, Australia) same lane loading control. Mean β-actin expression levels did not differ between schizophrenia and control groups in either DLPFC (P>0.05) or hippocampal regions (P>0.05). Levels of β-actin mRNA have been previously shown to be unaltered in this cohort.30 All experiments and quantifications were performed blind to diagnosis. A more detailed immunoblot method can be found in Supplementary Information.
Statistical analysis
Statistical analyses were performed using SPSS (version 19.0, SPSS, Chicago, IL, USA). All data were normally distributed (K-S 0.317≤P≤1.000). Outliers were identified (cases deemed to be more than two standard deviations (s.d.) from their respective group mean) and removed before statistical analyses. Analyses of schizophrenia compared with control groups were performed and reported in groups, with the outliers removed. As our schizophrenia group consisted of schizophrenia and schizoaffective subjects, analyses comparing the groups were performed both including the schizoaffective subjects and with the schizoaffective subjects excluded. Multivariate analyses of variance (MANOVA) were performed to determine whether there was a significant difference in protein levels between schizophrenia and control groups. Pearson's correlations were performed to determine the extent to which continuous variables (age at death, brain pH, RNA integrity, PMI, freezer storage time and brain weight) were associated with changes in protein expression. In cases where significant associations with independent variables were found, multivariate analyses of covariance (MANCOVA) were performed to account for continuous variables that can affect protein expression in post-mortem brain studies.32 Categorical confounds such as gender and brain hemisphere were analyzed by two-way MANOVA. Cause of death (suicide versus natural death) was also considered as a confounding factor and was analyzed within the schizophrenia group using independent t-tests. Bonferroni correction for multiple testing set the t-test significance to P<0.007. The significance for all other statistical tests was set to P<0.05. Data are expressed as mean±s.d.
Note: The term ‘schizophrenia subjects' hereafter refers to the whole group including the schizoaffective subjects unless otherwise specified. Diagnostic and Statistical Manual of Mental Disorders IV diagnosed schizophrenia subjects will be referred to as ‘schizophrenia only'.
Results
Protein expression in the DLPFC and hippocampus in schizophrenia versus control subjects
Lingo-1
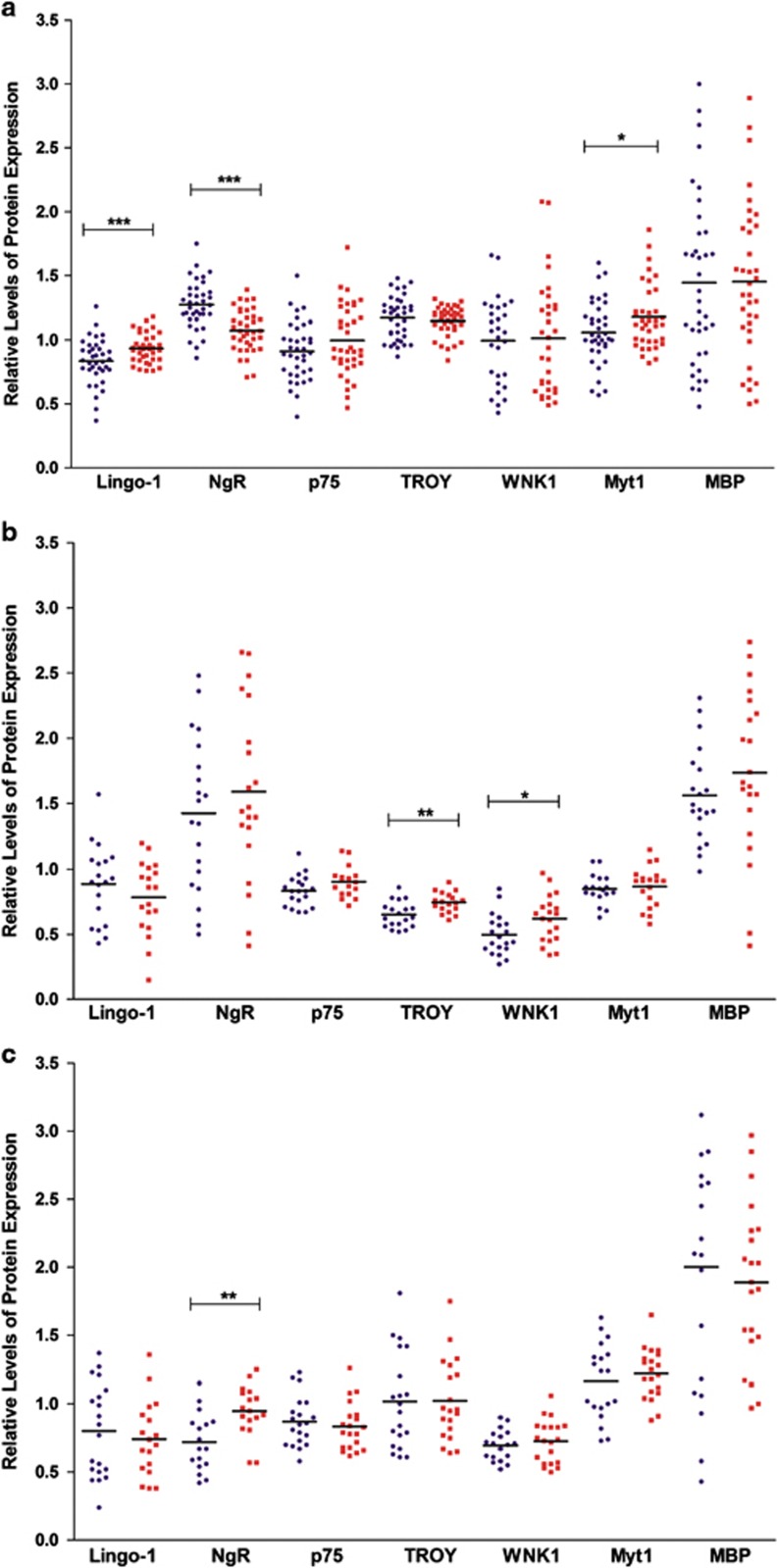
Analysis of the DLPFC showed a statistically significant 12% increase in levels of Lingo-1 protein in schizophrenia subjects compared with controls (F1,56=14.419; P<0.001; Figure 1a). Following the removal of the schizoaffective subjects, this difference increased to a 20% increase in Lingo-1 expression in the schizophrenia-only group compared with controls (F1,51=15.229; P<0.001; Supplementary Figure SF1A). In contrast, hippocampal results showed no significant difference in Lingo-1 expression between schizophrenia subjects and controls in neither CA1 (F1,30=0.664; P=0.422; Figure 1b) nor CA3 regions (F1,29=0.066; P=0.800; Figure 1c). Similarly, following the removal of the schizoaffective subjects, there was no significant difference in Lingo-1 expression in the CA1 (F1,27=0.508; P=0.482; Supplementary Figure SF1B) nor CA3 (F1,26=0.063; P=0.804; Supplementary Figure SF1C).
Figure 1.
Relative protein expression in the (a) dorsolateral prefrontal cortex (DLPFC), (b) CA1 and (c) CA3 in control (blue circles) and schizophrenia, including schizoaffective (red squares) subjects. *P<0.05, **P<0.01 and ***P<0.001.
NgR
In contrast to Lingo-1, NgR protein levels showed a statistically significant 16% reduction in schizophrenia subjects compared with controls in the DLPFC (F1,56=27.229; P<0.001; Figure 1a). When the schizoaffective subjects were removed, this difference increased further to an 18% reduction in NgR expression in the schizophrenia-only group compared with controls (F1,51=26.277; P<0.001; Supplementary Figure SF1A). There was no significant change in NgR expression in the CA1 hippocampal region either including (F1,30=0.224; P=0.639; Figure 1b) or excluding the schizoaffective subjects (F1,27=0.218; P=0.644; Supplementary Figure SF1B). However, there was a highly significant 31.5% increase in NgR expression between schizophrenia and control subjects (F1,29=8.900; P=0.006; Figure 1c) in the CA3. When schizoaffective subjects were removed, a 25% increase was still observed in the schizophrenia-only group compared with controls (F1,26=6.237; P=0.019; Supplementary Figure SF1C).
p75 and TROY
Interestingly, neither p75 nor its homolog TROY showed any significant difference in levels of protein expression in schizophrenia subjects compared with controls in the DLPFC either before (F1,56=2.012; P=0.162 and F1,56=0.745; P=0.392; Figure 1a) or after the removal of the schizoaffective subjects (F1,51=1.174; P=0.284 and F1,51=1.135; P=0.292; Supplementary Figure SF1A). p75 remained unaltered in both CA1 and CA3 regions of the hippocampus when including (F1,30=2.481; P=0.126; Figure 1b and F1,29=1.166; P=0.289; Figure 1c) and excluding the schizoaffective subjects (F1,27=3.436; P=0.075;Supplementary Figure SF1B and F1,26=0.814; P=0.375; Supplementary Figure SF1C). However, its homolog TROY showed a significant 14.5% increase in the CA1 (F1,30=13.039; P=0.001; Figure 1b) in schizophrenia that was increased to an 18% increase following the removal of the schizoaffective subjects (F1,27=12.037; P=0.002; Supplementary Figure SF1B). The level of TROY expression remained unchanged in the CA3 region both including (F1,29=0.080; P=0.779; Figure 1c) and excluding the schizoaffective subjects (F1,26=0.126; P=0.726; Supplementary Figure SF1C).
WNK1
Analysis of WNK1 revealed no statistically significant difference in expression in the DLPFC between schizophrenia and control subjects (F1,56=0.172; P=0.864; Figure 1a), nor when the schizoaffective subjects were removed (F1,51=0.162; P=0.689; Supplementary Figure SF1A). However, a 25.5% increase in WNK1 expression was observed in the CA1 of schizophrenia subjects compared with controls (F1,30=7.284; P=0.011; Figure 1b) that was increased to a significant 30% increase in WNK1 expression when the schizoaffective subjects were removed (F1,27=6.030; P=0.021; Supplementary Figure SF1B). No significant alterations in WNK1 expression were observed in the CA3 either before (F1,29=1.223; P=0.278; Figure 1c) or after the removal of the schizoaffective subjects (F1,26=1.255; P=0.273; Supplementary Figure SF1C).
Myt1
Within the DLPFC, a significant 11.5% increase in Myt1 expression was observed in schizophrenia compared with control groups (F1,56=5.494; P=0.023; Figure 1a). When the schizoaffective subjects were removed, this difference increased to a 14.5% increase in Myt1 expression in the schizophrenia-only group compared with controls (F1,51=5.041; P=0.029; Supplementary Figure SF1A). There were no significant differences in Myt1 expression levels in either CA1 nor CA3 hippocampal regions either before (F1,30=0.996; P=0.326; Figure 1b and F1,29=0.036; P=0.852; Figure 1c) or after the removal of the schizoaffective subjects (F1,27=0.528; P=0.474; Supplementary Figure SF1B and F1,26=0.025; P=0.875; Supplementary Figure SF1C).
MBP
MBP levels in the DLPFC revealed no significant alteration between the schizophrenia and control groups (F1,56=0.001; P=0.980; Figure 1a), nor was there any change following the removal of the schizoaffective subjects (F1,51=0.021; P=0.886; Supplementary Figure SF1A). Analysis of hippocampal regions showed no significant alterations in MBP expression in either CA1 (F1,30=1.043; P=0.315; Figure 1b) or CA3 regions (F1,29=0.006; P=0.940; Figure 1c). Similarly, there was no change in either the CA1 (F1,27=0.592; P=0.448; Supplementary Figure SF1B) nor in the CA3 (F1,26=0.068; P=0.796; Supplementary Figure SF1C) following the exclusion of the schizoaffective subjects.
Confounding factors had no impact on levels of protein expression
Peri-mortem and demographic variables can im pact on protein expression in post-mortem human studies.32 A number of continuous variables were found to be significantly correlated with levels of protein expression in our study (Supplementary Tables ST2-ST4). MANCOVAs were conducted to account for those continuous variables (age at death, brain pH, PMI, freezer storage time and brain weight) that did correlate with levels of protein expression, which might have an impact on our data. Importantly, when MANCOVAs were conducted, the significant difference in protein expression between schizophrenia and control subjects was retained within Lingo-1 data (F1,50=10.376; P=0.002), NgR data (F1,50=29.250; P<0.001) and Myt1 data (F1,50=8.612; P=0.005) in the DLPFC. Significance was also retained in TROY data (F1,24=12.329; P=0.002) and WNK1 data (F1,24=10.333; P=0.004) in the CA1, as well as in NgR data (F1,23=7.250; P=0.013) in the CA3. All nonsignificant data for remaining proteins in their respective brain regions remained nonsignificant following the MANCOVAs (P>0.05).
Gender was ana lyzed as a categorical confound by two-way MANOVA. It was revealed that there was a significant diagnosis by gender interaction for Lingo-1 levels in the DLPFC (F1,55=4.362; P=0.041). A statistically significant 19.5% increase in Lingo-1 expression was found in schizophrenia males compared with control males (P<0.001). There was no significant diagnosis by gender interaction for Lingo-1 either within the CA1 (F1,28=1.543; P=0.225) or CA3 (F1,27=4.119; P=0.052) hippocampal regions. In addition, there were no significant diagnosis by gender interactions within the DLPFC, CA1 or CA3 for any of the remaining proteins (Supplementary Table ST5). Brain hemisphere was also analyzed as a categorical confound by two-way MANOVA. There were no significant diagnosis by hemisphere interactions for any of the tested proteins in any of the tested brain regions (Supplementary Table ST5).
Finally, we assessed whether our proteins of interest varied with incidence of suicide compared with natural death among our schizophrenia subjects. Independent t-tests revealed that there was a slight decrease in Lingo-1 expression in the DLPFC of schizophrenia subjects who committed suicide compared with the schizophrenia subjects who died of natural causes (t1,26.440=−2.159; P=0.040). In contrast, there was an increase in both NgR (t1,35=2.471; P=0.018) and Myt1 (t1,10.900=2.577; P=0.026) expression in the DLPFC of schizophrenia subjects who committed suicide compared with those who died of natural causes. However, Bonferroni correction for multiple testing resulted in a significance level of P<0.007; therefore, all significance related to the incidence of suicide was lost. All other proteins in all brain regions were found to have no significance relating to the incidence of suicide in the schizophrenia subjects.
Discussion
This is the first study to examine the expression profile of Lingo-1 and its signaling partner proteins in schizophrenia, identifying alterations of these pathways in the DLPFC and hippocampus (CA1 and CA3)—brain regions highly relevant to schizophrenia pathophysiology providing highly sought after, novel directions for therapeutic strategies.
First evidence for the implication of Lingo-1 in schizophrenia
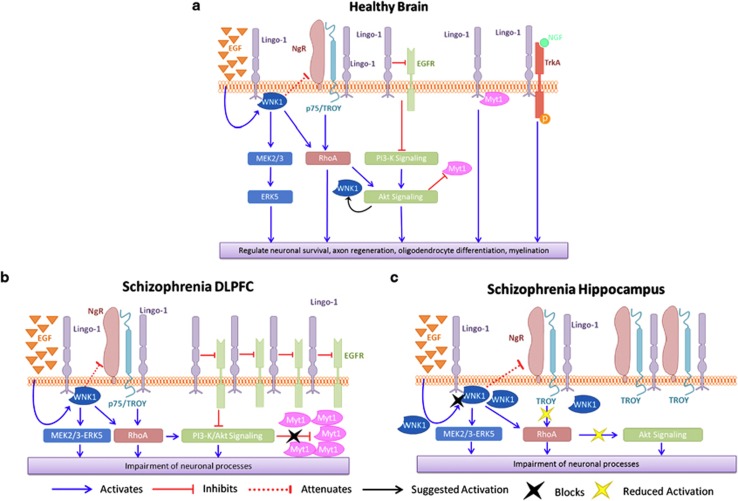
Neuronal sprouting and myelination are developmental milestones shown to be disrupted in schizophrenia and are an integral part of the neurodevelopmental hypothesis for schizophrenia.33, 34, 35 Accordingly, Lingo-1 has been shown to be expressed early in embryonic development (E14 in rodents) in both oligodendrocytes and neurons,36 and has been shown to inhibit axonal outgrowth and myelination in the brain.13,37 These significant parallels between Lingo-1 and neural development support the idea that Lingo-1 may be a contributing factor to schizophrenia pathogenesis, but will require additional studies to elucidate this role. Our study reports, for the first time, levels of Lingo-1 protein expression in post-mortem DLPFC, CA1 and CA3 in schizophrenia. A significantly higher level of Lingo-1 protein was found in the DLPFC from schizophrenia compared with control subjects (P<0.001) but not in the hippocampus (P>0.05). Downregulation of epidermal growth factor (EGF) receptor (EGFR) induced by Lingo-1's inhibitory action may be partly involved in the regional specificity of Lingo-1 expression in DLPFC compared with hippocampus in schizophrenia brains. Lingo-1 can directly inhibit EGFR independently of EGF activation (Figure 2a), leading to inhibition of PI3/Akt signaling pathways.38,39 Owing to limited quantities of post-mortem tissue available from this schizophrenia brain cohort, it was not possible to measure levels of EGFR in DLPFC and hippocampus of this cohort. However, previous post-mortem studies have reported higher levels of EGFR protein in DLPFC (Brodmann area 46) but not hippocampal regions in schizophrenia versus control brains.40 Although it is unclear how Lingo-1 inhibits EGFR expression and function, higher levels of Lingo-1 observed only in DLPFC and not in hippocampus of schizophrenia brains in our study seem pertinent. In the context of schizophrenia pathophysiology, we postulate that greater levels of EGFR in the DLPFC may have a role in Lingo-1 upregulation observed in this region, leading to inhibition of PI3/Akt signaling pathways in schizophrenia (Figure 2b).
Figure 2.
Schematization of Lingo-1signaling pathways in the (a) healthy brain, (b) dorsolateral prefrontal cortex (DLPFC) region from schizophrenia sufferers and (c) hippocampus region from schizophrenia sufferers. Akt, protein kinase B (PKB); EGF, epidermal growth factor; EGFR, epidermal growth factor receptor; ERK, extracellular signal-reduced kinase; Lingo-1, leucine-rich repeat and immunoglobulin domain-containing protein; MEK, mitogen-activated protein kinase; NGF, nerve growth factor; NgR, Nogo receptor; P75, P75 neurotrophin receptor; PI3-K, phosphatidylinositide 3-kinase; RhoA, Ras homolog gene family, member A; TrkA, transforming tyrosine kinase protein receptor A; TROY (TNFRSF19), tumor necrosis factor receptor super family, member 19; WNK1, With No Lysine (K).
Similar to the expression profile of Lingo-1, we found a significantly higher level of Myt1 protein in the DLPFC (P=0.023) of schizophrenia compared with control subjects, but similar to Lingo-1, levels of Myt1 were not significantly altered in CA1 or CA3 (P>0.05). As Lingo-1 is also upregulated in the DLPFC in the schizophrenia group, it can be considered that inhibition of Akt signaling pathways by Lingo-1 through its different signaling partners (Figure 2b) may reduce negative regulation of Myt1 by Akt intracellularly, resulting in higher levels of Myt1 in this region. Considering the role of Lingo-1 and Myt1 in oligodendrocyte and myelin dysfunction, it was not surprising to find an increase in levels of both these proteins in the DLPFC. Although these changes may be associated with alterations in oligodendrocyte and myelin function, we did not observe any changes in MBP levels, as an indication of myelination, between groups. A reduction in MBP expression was previously reported by a smaller study in the enthorhinal cortex between schizophrenia and control groups;41 however, it is difficult to compare MBP expression between studies, as many factors can influence myelination analysis such as age at death, cause of death between cohorts and antipsychotic treatment (Supplementary Table ST4).42 Further, post-mortem and imaging studies are required to address the question of how the differential Lingo-1 expression profile may affect myelination processes in schizophrenia brains.
Regulation of Lingo-1 pathways in schizophrenia: brain structure-specific relationship?
Unlike Lingo-1 expression, our study found NgR expression levels to be decreased in the DLPFC of schizophrenia compared with control subjects (P<0.001). This finding in schizophrenia subjects is supported by an animal study, suggesting that NgR and Lingo-1 may be functionally coupled, following a demonstration of inverse regulation of NgR and Lingo-1 mRNA expression in the cortex as a result of drug-induced brain activity.43 However, we reported no change in NgR levels in CA1 (P>0.05), whereas a significantly higher level of NgR expression was found in CA3 (P=0.006) from schizophrenia compared with control subjects. These findings highlight brain structure-specific regulation of NgR in schizophrenia; however, it remains unclear how these opposing NgR changes contribute to schizophrenia pathophysiology.
We observed a significant increase in levels of WNK1 in CA1 from schizophrenia compared with control subjects (P=0.011), but not in DLPFC or CA3 (P>0.05). As illustrated in Figure 2a and as reported previously,20 overexpression of WNK1 reduces Nogo-induced inhibition of neurite extension, and thus inhibits activation of RhoA.20 Considering the higher level of WNK1 that is present in the CA1 and not in the CA3 of schizophrenia compared with control subjects, together with the inhibitory potential on NgR by WNK1, we speculate that WNK1 may normalize CA1 levels of NgR resulting in a similar level of NgR expression in this brain region in schizophrenia compared with controls (Figure 2c). Further investigations are necessary to define how this process occurs in vivo. To our knowledge, NgR protein expression levels in post-mortem tissue have only ever been reported in hippocampus in a cohort for Alzheimer's Disease,44 where increased NgR expression was found in Alzheimer's patients, suggesting a common role of NgR in a larger scope of brain disorders.
With regard to schizophrenia, few studies have reported levels of expression of p75 in the post-mortem schizophrenia brain, despite brain-derived neurotrophic factor (another widely distributed central nervous system neurotrophin) levels being extensively studied in schizophrenia in serum and post-mortem brains owing to its key regulatory role in synaptic plasticity and neuronal a ctivity.45 Similar to our results in the hippocampus and DLPFC, Dunham et. al.46 reported no change in p75 in the hippocampus of their schizophrenia group compared with controls in the Stanley consortium brains. Given that expression of p75 is higher in the developing brain compared with the adult brain, where its expression is limited to certain neuronal subpopulations,17 examination of TROY (functional homolog of p75 in the Lingo-1/NgR complex; Figure 2a) levels in the central nervous system was pertinent in the context of this study. No difference in expression of TROY was found in the DLPFC or in the CA3 from the schizophrenia group compared with controls (P>0.05); however, a significantly higher level of TROY was observed in the CA1 of schizophrenia patients compared with controls (P=0.001). As TROY is a major partner of Lingo-1/NgR/TROY complex, responsible for downstream cellular transduction, and considering the upregulation of NgR we have reported in the CA3, we can ask if there was a causal relationship between our hippocampal results. However, TROY levels were not significantly different in the CA3, suggesting additional mechanisms may be involved in the regulation of this protein that will need to be further investigated.
Similar to TROY, WNK1 (a negative regulator of NgR) was found to be elevated in schizophrenia compared with control brains within CA1 (P=0.011) but not CA3 or DLPFC (P>0.05). This last result observed in our study was in accordance with literature reporting a similar level of WNK1 gene expression in DLPFC post-mortem tissue from schizophrenia sufferers compared with controls.21 However, it is unclear why WNK1 is overexpressed only in the CA1 region in our schizophrenia group. Interestingly, overexpression of WNK1 has also been reported to significantly reduce the interaction between endogenous WNK1 and Lingo-1, suggesting that it may serve as a binding platform for Lingo-1, however, its kinase activity may not be necessary for Lingo-1 signaling.20 Furthermore, disruption of the WNK1 gene in mice leads to death of the embryo at day 13,47,48 suggesting an essential role of WNK1 in embryonic and neural development, which is a critical period implicated in schizophrenia.
A structure-specific regulation for Lingo-1 pathways seems to apply in the context of schizophrenia pathophysiology (Figure 2). Interestingly, differences in the myelination profile of DLPFC and hippocampus have been reported, showing an abundance of myelination in early development in the hippocampus, whereas levels were reported to be low at birth in the PFC, increasing throughout different stages of life.1,49 This differential and regionally specific myelination profile throughout development might support a brain region-specific regulation of the various pathways involved in myelination processes throughout life.
Our results show alterations in Lingo-1 signaling pathways in both the schizophrenia-only group and the entire schizophrenia group including schizoaffective subjects compared with controls. This suggests that the variations in these pathways are applicable to the schizophrenia spectrum. Nevertheless, as the magnitude of change in our results were generally increased in the schizophrenia-only group compared with controls, it seems that the changes in Lingo-1 signaling pathways reported in this study may be more specific to schizophrenia than schizoaffective disorder. Interestingly, a study that examined myelination genes in the Stanley consortium brains (15 schizophrenia and 15 bipolar disorder patients compared with controls) showed that both diagnostic groups had a differential expression profile of myelination-related genes compared with controls. The differential expression profile of the genes was not the same across schizophrenia and bipolar disorder subjects; however, there was a significant overlap.3 As schizoaffective disorder is characterized by both the psychotic symptoms of schizophrenia and the mood swing symptoms of bipolar disorder, it seems likely that myelination disturbances related to Lingo-1 pathways are more specific to schizophrenia subjects only. However, further investigations are necessary using a cohort that includes a wider number of schizoaffective patients compared with schizophrenia and controls to confirm this hypothesis.
The limitations of this study brought about by the utilization of post-mortem brain tissues have to be considered. As reported in the results section and in Supplementary Tables ST2-ST4, expression levels of the tested proteins remain significant after accounting for confounding factors from our brain cohort demographics. Both age at death (r=-0.397; P=0.015) and duration of illness (r=-0.377; P=0.021) were significantly correlated with NgR protein expression in the DLPFC of schizophrenia subjects, suggesting an age-related effect on NgR protein levels in schizophrenia. It has previously been shown that age at death is inextricably linked to duration of illness in this schizophrenia brain cohort.30 The longer a patient lives with schizophrenia, the longer time frame they have to accumulate detrimental neural changes, thus it cannot be determined whether NgR protein levels are correlated with age at death or the length of time they lived with the disorder.
We can see from the correlations shown by our study that the longer the duration of illness, or the older the patients were, the lower the NgR levels in the DLPFC. Interestingly, EGF levels have been previously reported to be positively correlated with duration of illness in the same brain region within another post-mortem schizophrenia cohort.40 As EGF levels activate WNK1, (Figure 2a), and considering the elevated EGF levels in long-term schizophrenia, a downregulation of NgR according to duration of illness seems consistent within the context of our results and hypothesis. Furthermore, a positive correlation between WNK1 levels and antipsychotic treatment was also shown in the CA1 hippocampal region within the schizophrenia group (r=0.466; P=0.039). As reported by previous primate, rodent and cell line studies, antipsychotic treatment (notably second generation antipsychotics) can induce modifications in major intracellular cascades (such as extracellular signal-reduced kinase and Akt signaling pathways) leading to changes in neuronal plasticity and survival.49 As the majority of schizophrenia patients in our study were treated with second generation antipsychotic drugs (Supplementary Table ST1) and because of the suggested activation of WNK1 by Akt signaling pathways (Figure 2a), it appears that the significant difference in WNK1 levels reported in the CA1 of schizophrenia patients compared with controls might be influenced by psychotropic treatment. However, the majority of reports studying molecular mechanisms of neuroprotective effects of antipsychotic drugs were performed in the PFC, where we did not report any significant alterations in WNK1 levels in schizophrenia compared with control groups.
In summary, we provide the first report investigating the implication of Lingo-1 and its signaling partner proteins in schizophrenia pathophysiology. We show for the first time a brain structure-specific alteration of Lingo-1 signaling pathways in schizophrenia with: (1) significantly elevated levels of Lingo-1 and Myt1 proteins, and a decreased level of NgR protein in the DLPFC; (2) a significant increase of downstream Lingo-1 co-factors TROY and WNK1 in the CA1; and (3) significantly elevated levels of the Lingo-1 co-receptor NgR in the CA3. As illustrated in Figure 2 in the context of schizophrenia pathophysiology, two groups of Lingo-1 pathways appear to have predominant brain-specific roles: EGFR/Lingo-1/Myt1 in the PFC and WNK/NgR/TROY in the hippocampus. Even if some of these signaling partners have been previously involved in schizophrenia at the protein level (EGFR40 and Akt50) or genetic level (NgR23,24 WNK121 and Myt1l22 genes), the integration of these protein synergies in the context of schizophrenia is completely novel. However, further analysis will be required to characterize these interactions at a molecular- and cell-specific level (neurons versus oligodendrocytes). Nevertheless, the relevance of our finding immediately offers a direct application to schizophrenia therapy. Owing to the role of the many Lingo-1 pathways as a negative regulator of myelination and neurite outgrowth, and considering the implication of both of these central processes in cognitive performance, antagonists of Lingo-1 appear to be a new potential candidate in schizophrenia therapy. We suspect that pharmacological blockade of Lingo-1 will notably improve treatment for cognitive symptoms of schizophrenia, which are currently not appropriately treated with antipsychotic medications available at present. We are currently exploring this hypothesis by investigating the potential for an anti-Lingo-1 drug (one of which is being currently evaluated in clinical trials for multiple sclerosis) in the treatment of schizophrenia. By characterizing novel molecular mechanisms implicated in schizophrenia, our results offer a new avenue of research into the pathophysiology of schizophrenia and may facilitate improvements to current therapies for treating this devastating disease.
Acknowledgments
This work was supported by an Illawarra Health and Medical Research Institute Program Grant and by the Schizophrenia Research Institute, using infrastructure funding from the NSW Ministry of Health. Tissues were received from the NSW Tissue Resource Centre at the University of Sydney and the Sydney Brain Bank, which is supported by the National Health and Medical Research Council of Australia, The University of NSW, Neuroscience Research Australia, the Schizophrenia Research Institute and the National Institute of Alcohol Abuse and Alcoholism (NIH (NIAAA) R24AA012725). Tissues were prepared by the Schizophrenia Research Laboratory, a joint initiative of the Schizophrenia Research Institute, University of NSW, Neuroscience Research Australia and the Macquarie Group Foundation. CP was supported by a National Health and Medical Research Council (NHMRC) Senior Principal Research Fellowship (ID: 628386) and National Alliance for Research on Schizophrenia and Depression (NARSAD) Distinguished Investigator Award. FF-E designed and supervised the experiments and wrote the manuscript; JLA performed the experiments and wrote the manuscript; KAN participated in writing the manuscript and performing the experiments; CP and XFH suggested changes to the final version of the manuscript utilizing their knowledge of schizophrenia. JLA is supported by an Ian Scott Scholarship from Australian Rotary Health.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Translational Psychiatry website (http://www.nature.com/tp)
Supplementary Material
References
- Pantelis C, Yücel M, Wood SJ, Velakoulis D, Sun D, Berger G, et al. Structural brain imaging evidence for multiple pathological processes at different stages of brain development in schizophrenia. Schizophr Bull. 2005;31:672–696. doi: 10.1093/schbul/sbi034. [DOI] [PubMed] [Google Scholar]
- Hakak Y, Walker JR, Li C, Wong WH, Davis KL, Buxbaum JD, et al. Genome-wide expression analysis reveals dysregulation of myelination-related genes in chronic schizophrenia. Proc Natl Acad Sci USA. 2001;98:4746–4751. doi: 10.1073/pnas.081071198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tkachev D, Mimmack ML, Ryan MM, Wayland M, Freeman T, Jones PB, et al. Oligodendrocyte dysfunction in schizophrenia and bipolar disorder. Lancet. 2003;362:798–805. doi: 10.1016/S0140-6736(03)14289-4. [DOI] [PubMed] [Google Scholar]
- Barley K, Dracheva S, Byne W. Subcortical oligodendrocyte- and astrocyte-associated gene expression in subjects with schizophrenia, major depression and bipolar disorder. Schizophr Res. 2009;112:54–64. doi: 10.1016/j.schres.2009.04.019. [DOI] [PubMed] [Google Scholar]
- Uranova N, Orlovskaya D, Vikhreva O, Zimina I, Kolomeets N, Vostrikov V, et al. Electron microscopy of oligodendroglia in severe mental illness. Brain Res Bull. 2001;55:597–610. doi: 10.1016/s0361-9230(01)00528-7. [DOI] [PubMed] [Google Scholar]
- Schmitt A, Wilczek K, Blennow K, Maras A, Jatzko A, Petroianu G, et al. Altered thalamic membrane phospholipids in schizophrenia: a postmortem study. Biol Psychiatry. 2004;56:41–45. doi: 10.1016/j.biopsych.2004.03.019. [DOI] [PubMed] [Google Scholar]
- Shenton ME, Dickey CC, Frumin M, McCarley RW. A review of MRI findings in schizophrenia. Schizophr Res. 2001;49:1–52. doi: 10.1016/s0920-9964(01)00163-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartzokis G, Nuechterlein KH, Lu PH, Gitlin M, Rogers S, Mintz J. Dysregulated brain development in adult men with schizophrenia: a magnetic resonance imaging study. Biol Psychiatry. 2003;53:412–421. doi: 10.1016/s0006-3223(02)01835-8. [DOI] [PubMed] [Google Scholar]
- Walterfang M, Wood SJ, Velakoulis D, Neuropathological Pantelis C. neurogenetic and neuroimaging evidence for white matter pathology in schizophrenia. Neurosci Biobehav Rev. 2006;30:918–948. doi: 10.1016/j.neubiorev.2006.02.001. [DOI] [PubMed] [Google Scholar]
- Walterfang M, Velakoulis D, Whitford TJ, Pantelis C. Understanding aberrant white matter development in schizophrenia: an avenue for therapy. Exp Rev Neurother. 2011;11:971–987. doi: 10.1586/ern.11.76. [DOI] [PubMed] [Google Scholar]
- Takahashi N, Nielsen KS, Aleksic B, Petersen S, Ikeda M, Kushima I, et al. Loss of function studies in mice and genetic association link receptor protein tyrosine phosphatase α to schizophrenia. Biol Psychiatry. 2011;70:626–635. doi: 10.1016/j.biopsych.2011.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearlson GD, Petty RG, Ross CA, Tien AY. Schizophrenia: a disease of heteromodal association cortex. Neuropsychopharmacology. 1996;14:1–17. doi: 10.1016/S0893-133X(96)80054-6. [DOI] [PubMed] [Google Scholar]
- Mi S, Miller RH, Lee X, Scott ML, Shulag-Morskaya S, Shao Z, et al. LINGO-1 negatively regulates myelination by oligodendrocytes. Nat Neurosci. 2005;8:745–751. doi: 10.1038/nn1460. [DOI] [PubMed] [Google Scholar]
- Rudick RA, Mi S, Sandrock AW., Jr LINGO-1 antagonists as therapy for multiple sclerosis: in vitro and in vivo evidence. Exp Opin Biol Ther. 2008;8:1561–1570. doi: 10.1517/14712598.8.10.1561. [DOI] [PubMed] [Google Scholar]
- Yamashita T, Higuchi H, Tohyama M. The p75 receptor transduces the signal from myelin-associated glycoprotein to Rho. J Cell Biol. 2002;157:565–570. doi: 10.1083/jcb.200202010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang KC, Kim JA, Sivasankaran R, Segal R, He Z. P75 interacts with the Nogo receptor as a co-receptor for Nogo, MAG and OMgp. Nature. 2002;420:74–78. doi: 10.1038/nature01176. [DOI] [PubMed] [Google Scholar]
- Shao Z, Browning JL, Lee X, Scott ML, Shulga-Morskaya S, Allaire N, et al. TAJ/TROY, an orphan TNF receptor family member, binds Nogo-66 receptor 1 and regulates axonal regeneration. Neuron. 2005;45:353–359. doi: 10.1016/j.neuron.2004.12.050. [DOI] [PubMed] [Google Scholar]
- Hu F, Strittmatter SM. Regulating axon growth within the postnatal central nervous system. Semin Perinatol. 2004;28:371–378. doi: 10.1053/j.semperi.2004.10.001. [DOI] [PubMed] [Google Scholar]
- Barrette B, Vallières N, Dubé M, Lacroix S. Expression profile of receptors for myelin-associated inhibitors of axonal regeneration in the intact and injured mouse central nervous system. Mol Cell Neurosci. 2007;34:519–538. doi: 10.1016/j.mcn.2006.12.004. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Xu X, Zhang Y, Zhou J, Yu Z, He C. LINGO-1 interacts with WNK1 to regulate nogo-induced inhibition of neurite extension. J Biol Chem. 2009;284:15717–15728. doi: 10.1074/jbc.M808751200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maycox PR, Kelly F, Taylor A, Bates S, Reid J, Logendra R, et al. Analysis of gene expression in two large schizophrenia cohorts identifies multiple changes associated with nerve terminal function. Mol Psychiatry. 2009;14:1083–1094. doi: 10.1038/mp.2009.18. [DOI] [PubMed] [Google Scholar]
- Vrijenhoek T, Buizer-Voskamp JE, van der Stelt I, Strengman E, Sabatti C, Geurts van Kessel A, et al. Recurrent CNVs disrupt three candidate genes in schizophrenia patients. Am J Hum Genet. 2008;83:504–510. doi: 10.1016/j.ajhg.2008.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinibaldi L, De Luca A, Bellacchio E, Conti E, Pasini A, Paloscia C, et al. Mutations of the Nogo-66 receptor (RTN4R) gene in schizophrenia. Hum Mutat. 2004;24:534–535. doi: 10.1002/humu.9292. [DOI] [PubMed] [Google Scholar]
- Budel S, Padukkavidana T, Liu BP, Feng Z, Hu F, Johnson S, et al. Genetic variants of Nogo-66 receptor with possible association to schizophrenia block myelin inhibition of axon growth. J Neurosci. 2008;28:13161–13172. doi: 10.1523/JNEUROSCI.3828-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dracheva S, Davis KL, Chin B, Woo DA, Schmeidler J, Haroutunian V. Myelin-associated mRNA and protein expression deficits in the anterior cingulate cortex and hippocampus in elderly schizophrenia patients. Neurobiol Dis. 2006;21:531–540. doi: 10.1016/j.nbd.2005.08.012. [DOI] [PubMed] [Google Scholar]
- Mi S, Lee X, Shao Z, Thill G, Ji B, Relton J, et al. LINGO-1 is a component of the Nogo-66 receptor/p75 signaling complex. Nat Neurosci. 2004;7:221–228. doi: 10.1038/nn1188. [DOI] [PubMed] [Google Scholar]
- Chambers JS, Perrone-Bizzozero NI. Altered myelination of the hippocampal formation in subjects with schizophrenia and bipolar disorder. Neurochem Res. 2004;29:2293–2302. doi: 10.1007/s11064-004-7039-x. [DOI] [PubMed] [Google Scholar]
- Uranova NA, Vikhreva OV, Rachmanova VI, Orlovskaya DD. Ultrastructural alterations of myelinated fibers and oligodendrocytes in the prefrontal cortex in schizophrenia: a postmortem morphometric study. Schizophr Res Treatment. 2011;2011:325789. doi: 10.1155/2011/325789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velakoulis D, Wood SJ, Wong MTH, McGorry PD, Yung A, Phillips L, et al. Hippocampal and amygdala volumes according to psychosis stage and diagnosis: a magnetic resonance imaging study of chronic schizophrenia, first-episode psychosis, and ultra-high-risk individuals. Arch Gen Psychiatry. 2006;63:139–149. doi: 10.1001/archpsyc.63.2.139. [DOI] [PubMed] [Google Scholar]
- Weickert CS, Sheedy D, Rothmond DA, Dedova I, Fung S, Garrick T, et al. Selection of reference gene expression in a schizophrenia brain cohort. Aust N Z J Psychiatry. 2010;44:59–70. doi: 10.3109/00048670903393662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fung SJ, Webster MJ, Sivagnanasundaram S, Duncan C, Elashoff M, Weickert CS. Expression of interneuron markers in the dorsolateral prefrontal cortex of the developing human and in schizophrenia. Am J Psychiatry. 2010;167:1479–1488. doi: 10.1176/appi.ajp.2010.09060784. [DOI] [PubMed] [Google Scholar]
- Ferrer I, Santpere G, Arzberger T, Bell J, Blanco R, Boluda S, et al. Brain protein preservation largely depends on the postmortem storage temperature: implications for study of proteins in human neurologic diseases and management of brain banks: a BrainNet Europe Study. J Neuropathol Exp Neurol. 2007;66:35–46. doi: 10.1097/nen.0b013e31802c3e7d. [DOI] [PubMed] [Google Scholar]
- Zalesky A, Fornito A, Seal ML, Cocchi L, Westin CF, Bullmore ET, et al. Disrupted axonal fiber connectivity in schizophrenia. Biol Psychiatry. 2011;69:80–89. doi: 10.1016/j.biopsych.2010.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makinodan M, Yamauchi T, Tatsumi K, Okuda H, Takeda T, Kiuchi K, et al. Demyelination in the juvenile period, but not in adulthood, leads to long-lasting cognitive impairment and deficient social interaction in mice. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:978–985. doi: 10.1016/j.pnpbp.2009.05.006. [DOI] [PubMed] [Google Scholar]
- Kato T, Abe Y, Sotoyama H, Kakita A, Kominami R, Hirokawa S, et al. Transient exposure of neonatal mice to neuregulin-1 results in hyperdopaminergic states in adulthood: implication in neurodevelopmental hypothesis for schizophrenia. Mol Psychiatry. 2011;16:307–320. doi: 10.1038/mp.2010.10. [DOI] [PubMed] [Google Scholar]
- Loov C, Fernqvist M, Walmsley A, Marklund N, Erlandsson A. Neutralization of LINGO-1 during In Vitro Differentiation of Neural Stem Cells Results in Proliferation of Immature Neurons. PLoS ONE. 2012;7:e29771. doi: 10.1371/journal.pone.0029771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji B, Li M, Wu WT, Yick LW, Lee X, Shao Z, et al. LINGO-1 antagonist promotes functional recovery and axonal sprouting after spinal cord injury. Mol Cell Neurosci. 2006;33:311–320. doi: 10.1016/j.mcn.2006.08.003. [DOI] [PubMed] [Google Scholar]
- Gur G, Rubin C, Katz M, Amit I, Citri A, Nilsson J, et al. LRIG1 restricts growth factor signaling by enhancing receptor ubiquitylation and degradation. EMBO J. 2004;23:3270–3281. doi: 10.1038/sj.emboj.7600342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldoni S, Iozzo RA, Kay P, Campbell S, McQuillan A, Agnew C, et al. A soluble ectodomain of LRIG1 inhibits cancer cell growth by attenuating basal and ligand-dependent EGFR activity. Oncogene. 2007;26:368–381. doi: 10.1038/sj.onc.1209803. [DOI] [PubMed] [Google Scholar]
- Futamura T, Toyooka K, Iritani S, Niizato K, Nakamura R, Tsuchiya K, et al. Abnormal expression of epidermal growth factor and its receptor in the forebrain and serum of schizophrenic patients. Mol Psychiatry. 2002;7:673–682. doi: 10.1038/sj.mp.4001081. [DOI] [PubMed] [Google Scholar]
- Parlapani E, Schmitt A, Erdmann A, Bernstein H-G, Breunig B, Gruber O, et al. Association between myelin basic protein expression and left entorhinal cortex pre-alpha cell layer disorganization in schizophrenia. Brain Res. 2009;1301:126–134. doi: 10.1016/j.brainres.2009.09.007. [DOI] [PubMed] [Google Scholar]
- Thomas EA. Molecular profiling of antipsychotic drug function. Mol Neurobiol. 2006;34:109–128. doi: 10.1385/MN:34:2:109. [DOI] [PubMed] [Google Scholar]
- Trifunovski A, Josephson A, Ringman A, Brene S, Spenger C, Olson L. Neuronal activity-induced regulation of Lingo-1. Neuroreport. 2004;15:2397–2400. doi: 10.1097/00001756-200410250-00019. [DOI] [PubMed] [Google Scholar]
- Zhu H-Y, Guo H-F, Hou H-L, Liu Y-J, Sheng S-L, Zhou J-N. Increased expression of the Nogo receptor in the hippocampus and its relation to the neuropathology in Alzheimer's disease. Hum Pathol. 2007;38:426–434. doi: 10.1016/j.humpath.2006.09.010. [DOI] [PubMed] [Google Scholar]
- Lu B, Martinowich K. Cell biology of BDNF and its relevance to schizophrenia. Novartis Found Symp. 2008;289:119–195. doi: 10.1002/9780470751251.ch10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunham JS, Deakin JFW, Miyajima F, Payton A, Toro CT. Expression of hippocampal brain-derived neurotrophic factor and its receptors in Stanley consortium brains. J Psychiatr Res. 2009;43:1175–1184. doi: 10.1016/j.jpsychires.2009.03.008. [DOI] [PubMed] [Google Scholar]
- Zambrowicz BP, Abuin A, Ramirez-Solis R, Richter LJ, Piggott J, BeltrandelRio H, et al. Wnk1 kinase deficiency lowers blood pressure in mice: a gene-trap screen to identify potential targets for therapeutic intervention. Proc Natl Acad Sci USA. 2003;100:14109–14114. doi: 10.1073/pnas.2336103100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benes FM. Myelination of cortical-hippocampal relays during late adolescence. Schizophr Bull. 1989;15:585–593. doi: 10.1093/schbul/15.4.585. [DOI] [PubMed] [Google Scholar]
- Hunsberger J, Austin DR, Henter ID, Chen G. The neurotrophic and neuroprotective effects of psychotropic agents. Dialogues Clin Neurosci. 2009;11:333–348. doi: 10.31887/DCNS.2009.11.3/jhunsberger. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emamian ES. AKT/GSK3 signaling pathway and schizophreni a. Front Mol Neurosci. 2012;5:33. doi: 10.3389/fnmol.2012.00033. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.