Abstract
Major depressive disorder (MDD) is one of the leading causes of global disability. It is a risk factor for noncompliance with medical treatment, with about 40% of patients not responding to currently used antidepressant drugs. The identification and clinical implementation of biomarkers that can indicate the likelihood of treatment response are needed in order to predict which patients will benefit from an antidepressant drug. While analyzing the blood plasma proteome collected from MDD patients before the initiation of antidepressant medication, we observed different fibrinogen alpha (FGA) levels between drug responders and nonresponders. These results were replicated in a second set of patients. Our findings lend further support to a recently identified association between MDD and fibrinogen levels from a large-scale study.
Keywords: antidepressant, biomarkers, drug response, fibrinogen, proteomics
Introduction
Major depressive disorder (MDD) is a leading cause of global disability and a risk factor for noncompliance with medical treatment.1 As a multivariate disorder presenting a wide range of symptoms, MDD treatment success varies among patients. About 40% of MDD patients do not respond to current treatments. Moreover, there is a high rate of relapse and treatment resistance,2 and MDD patients have a high risk of attempting suicide.3
The identification and clinical implementation of biomarkers that can indicate the likelihood of treatment response are needed to improve this situation.4 In a recent article, Wium-Andersen et al.5 reported an association of elevated plasma fibrinogen levels and MDD. In the present study, we have asked the question whether fibrinogen levels can also serve as a predictive marker for the antidepressant treatment response. We used a mass spectrometry-based proteomics screen of blood plasma samples from MDD patients before antidepressant treatment. We found altered levels of fibrinogen alpha chain (FGA) in the plasma from drug responders compared to nonresponders. These differences were quantified and compared by western blot.
Materials and methods
Subjects
Initially, we analyzed the proteome of plasma specimens from 25 inpatients suffering from MDD, who participated in the Munich Antidepressant Response Signature (MARS) project.6 A first round of validation was performed in 17 patients—8 responders and 9 nonresponders, followed by a second round of validation in a separate set of 24 patients—16 responders and 8 nonresponders (Table 1). The two cohorts were built based on the time of sample collection in the clinic. While the first round of experiments was performed, samples from the second cohort were collected with no bias. Details of the study were explained to patients and a written informed consent was obtained. The study was approved by the ethics committee of the Medical Faculty at the Ludwig-Maximilians University Munich. Patients were diagnosed by trained psychiatrists according to the DSM-IV criteria. Antidepressant treatment outcome was weekly evaluated with the 21 item version of the Hamilton Rating Scale for Depression (HAM-D). Response was defined as a 50% or larger reduction of the HAM-D score after 6 weeks of treatment compared with the HAM-D score on admission to the hospital of the Max Planck Institute of Psychiatry. Two patients were discharged with no HAM-D ratings at T6. Their weekly ratings at an earlier time point were used to classify them as responder or nonresponder. The mean age for responders and nonresponders was 45 and 47 years, respectively. No significant differences were observed in this regard between the two groups (P=0.6398, Mann–Whitney). Significant differences between the two groups were also not observed regarding body mass index (BMI: P=0.2239, Mann–Whitney), cholesterol levels (P=0.6883, Mann–Whitney) and gender (P=1.000, two-sided Fisher's exact test). None of the study subjects had diabetes. When admitted to the clinic, the majority of patients were not taking any anti-inflammatory or immunosuppresant medications. Only three patients were using nonsteroidal anti-inflammatory drugs (Supplementary Table 1).
Table 1. Patients' clinical data including gender, age, BMI, plasma cholesterol levels and HAM-D.
| Patient ID | Gender | Age | BMI | Cholesterol | HAM-D T0 | HAM-D T6 | Western blot relative signal |
|---|---|---|---|---|---|---|---|
| First experiment | |||||||
| Nonresponders | |||||||
| 1 | M | 48 | 25.15 | 207 | 29 | 22 | 67.37 |
| 2 | M | 51 | 25.39 | 187 | 27 | 22 | 49.30 |
| 3 | M | 28 | 28.28 | 187 | 26 | 14 | 58.03 |
| 4 | M | 58 | 27.58 | 215 | 36 | 35 | 60.41 |
| 5 | F | 33 | 20 | 203 | 24 | 18 | 64.59 |
| 6 | F | 53 | 24.49 | 211 | 28 | 22 | 59.27 |
| 7 | F | 54 | 19.61 | 192 | 27 | 17 | 68.12 |
| 8 | F | 39 | 26.76 | 219 | 35 | 28 | 60.20 |
| Responders | |||||||
| 9 | F | 70 | 24.73 | 208 | 35 | 11 | 52.99 |
| 10 | M | 31 | 25 | 214 | 21 | 0 | 46.30 |
| 11 | M | 68 | 28.08 | 215 | 32 | 3 | 52.82 |
| 12 | F | 36 | 19.72 | 194 | 20 | 6 | 57.45 |
| 13 | F | 25 | 18.71 | 154 | 28 | 1 | 55.81 |
| 14 | M | 67 | 23.12 | 171 | 38 | 3 | 50.86 |
| 15 | F | 21 | 18.59 | 114 | 33 | 0 | 36.01 |
| 16 | F | 41 | 20 | 175 | 30 | 7 | 36.88 |
| 17 | M | 33 | 28.41 | 123 | 33 | 13 | 31.39 |
| Second experiment | |||||||
| Nonresponders | |||||||
| 18 | M | 75 | 23.80 | 172 | 28 | 16 | 65.07 |
| 19 | F | 36 | 24.80 | 135 | 21 | 14 | 66.15 |
| 20 | M | 53 | 28.04 | 162 | 30 | 20 | 53.03 |
| 21 | F | 22 | 20.25 | 188 | 33 | 23 | 63.51 |
| 22 | M | 22 | 25.54 | 190 | 22 | 25 | 56.01 |
| 23 | F | 43 | 24.10 | 190 | 18 | NA | 51.74 |
| 24 | F | 68 | 24.51 | 185 | 24 | 24 | 57.75 |
| 25 | M | 72 | 27.28 | 190 | 29 | 25 | 56.11 |
| Responders | |||||||
| 26 | F | 49 | 24.98 | 211 | 21 | 2 | 32.83 |
| 27 | F | 25 | 20.20 | 208 | 34 | 10 | 59.28 |
| 28 | M | 43 | 24.89 | 181 | 29 | 10 | 51.37 |
| 29 | M | 34 | 20.05 | 132 | 22 | 0 | 55.71 |
| 30 | M | 87 | 26.07 | 155 | 27 | 4 | 63.12 |
| 31 | F | 39 | 22.28 | 136 | 21 | 9 | 64.87 |
| 32 | F | 24 | 18.75 | 209 | 39 | 14 | 44.64 |
| 33 | M | 68 | 24.88 | 157 | 21 | 10 | 55.68 |
| 34 | M | 58 | 28.09 | 210 | 34 | 8 | 50.45 |
| 35 | M | 31 | 23.80 | 207 | 41 | 20 | 49.50 |
| 36 | M | 44 | 26.94 | 211 | 25 | 0 | 37.13 |
| 37 | M | 49 | 24.30 | 200 | 25 | 9 | 28.55 |
| 38 | M | 62 | 24.38 | 207 | 30 | 10 | 32.66 |
| 39 | F | 56 | 24.54 | 185 | 28 | NA | 51.11 |
| 40 | F | 39 | 27.18 | 215 | 25 | 6 | 51.10 |
| 41 | F | 35 | 19.96 | 124 | 30 | 12 | 51.14 |
Abbreviations: BMI, body mass index; HAM-D, Hamilton Rating Scale for Depression; F, female; M, male; NA, not applicable.
Blood plasma samples
Blood samples were collected shortly after admission to the hospital in sample tubes containing potassium EDTA. Plasma was separated from blood using an Accuspin System HystopaqueTM-1077 (Sigma Aldrich, Taufkirchen, Germany) according to the manufacturer's protocol and protein concentrations determined by Bradford assay.
Western blot
Twenty micrograms of total protein extracts from each blood plasma sample was run individually on a 12% SDS minigel (BioRad, Hercules, CA, USA). Proteins were transferred to Immobilon PVDF membrane (Millipore, Bedford, MA, USA) at 100 V for 1 h using a cooling system. Membranes were treated with 5% Carnation Instant Nonfat Dry Milk in TBS-T for 4 h, rinsed in TBS-T three times for 20 min and incubated with an FGA antibody (Abcam, Cambridge, UK) at a 1:1000 dilution in TBS-T overnight at 4 °C. After the incubation, membranes were washed twice for 15min with TBS-T. Next, membranes were incubated with an anti-c-MYC-peroxidase antibody (GE Healthcare, Uppsala, Sweden) for 40 min at RT, washed with water and TBS-T, and incubated with ECL solution (GE Healthcare) for 1 min. Membranes were subsequently scanned using a Gel Doc XR+ System (BioRad). The optical densities of the FGA band were assessed using Quantity One software (BioRad). Afterwards, the Immobilon PVDF membrane was stained with Coomassie Blue to ascertain equal protein loading in each gel lane. In addition, the Coomassie Blue-stained serum albumin protein band was used as an internal control for equal protein loading.
Statistics
All analyses were carried out using SPSS statistical software (IBM, Armonk, NY, USA). According to the D'Agostino & Pearson normality test, the two groups evaluated here presented a normal distribution. For the first group, the P-value for nonresponders is 0.6100 and for responders 0.3716; for the second group, the P-value for nonresponders is 0.7213 and for responders 0.5509. Considering that 95% of the Gaussian distributed samples should be within two s.d. of the mean, values that differed more than two s.d. from the mean were excluded from the analysis (Chauvenet's Criterion). Differences between the groups were assessed using analysis of covariance (ANCOVA) considering BMI and age as covariates. P<0.05 was considered significant.
Results and discussion
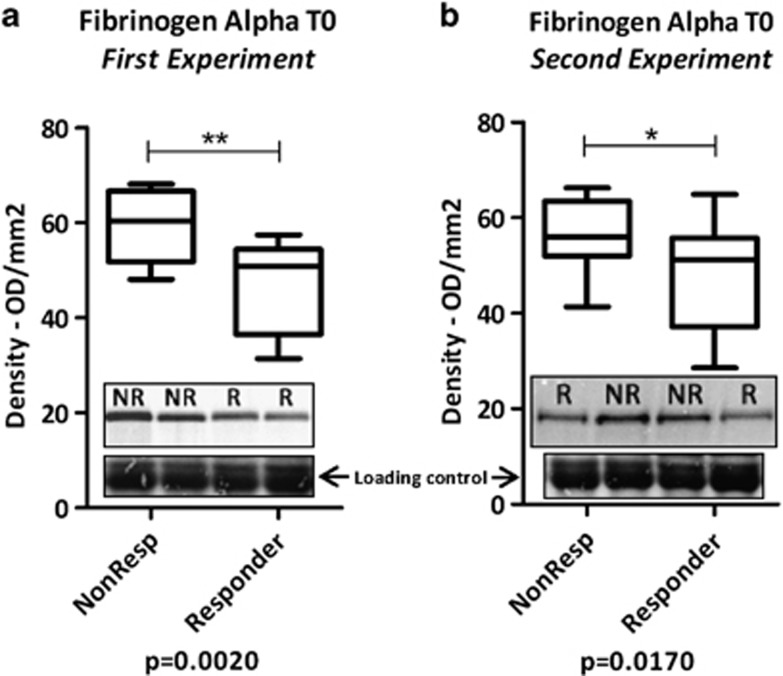
The plasma proteome analyses by mass spectrometry of antidepressant responders and nonresponders revealed altered FGA levels at baseline (data not shown). In the present study, we confirmed this result by western blot analysis of crude plasma samples from two separate patient groups (Table 1 and Figure 1). As our aim was to find a generic antidepressant response biomarker, we did not take into account the type of antidepressant used by each patient. Consistent with the results from our initial mass spectrometry analysis, we found higher FGA levels in nonresponders compared with responders in both patient groups (Figures 1a and b). BMI and age were considered as covariates and did not influence the results (Figure 1).
Figure 1.
Western blot FGA protein band densities of nonresponders and responders before treatment. (a) First patient validation set, with BMI (P=0.149) and age (P=0.114) as covariates; (b) second patient validation set with BMI (P=0.174) and age (P=0.452) as covariates. Insets show representative western blot images. *P<0.05; **P<0.01.
Our findings add further support to recently published data on an association between elevated plasma fibrinogen levels and depression in 73 367 subjects.5,7 Other studies have also shown an association of acute phase proteins including fibrinogen with MDD.8,9 A meta-analysis of plasma cytokine levels in MDD patients has found elevated TNF-alpha and IL-6 levels in depressed patients compared with healthy controls.10 Also, C-reactive protein, IL-1 and IL-6 have been associated with depression.11 All these results suggest an activated inflammatory status in MDD12 that may affect serotonergic neurotransmission.13,14 Other studies suggest an association of fibrinogen with MDD pathogenesis. Patients with coronary heart disease presenting depression symptoms tended to have elevated fibrinogen levels.15 Also, male MDD patients with hypertension had lower fibrinogen levels.16 Our data add one more piece to this puzzle, suggesting that baseline plasma fibrinogen levels can serve as a biomarker to gauge the success of antidepressant treatment response.
The expression of fibrinogen in hepatocytes depends on acute phase inflammatory stimuli.17 Our results show that high plasma fibrinogen levels are associated with a poor antidepressant response and indicate that nonresponders present an elevated inflammatory status compared with responders. Indeed, other studies also suggest that MDD patients with increased inflammatory protein levels tend to be treatment resistant.18 An increased production of acute phase proteins, such as fibrinogen, causes the activation of the indoleamine 2,3 dioxygenase, which catabolizes tryptophan, thus decreasing serotonin availability.19 High levels of circulating fibrinogen are also associated with and targeted in the treatment of cardiovascular disease.20 Similarly, in the case of antidepressant resistance, MDD patients could be subjected to medication that modulates fibrinogen levels21,22 before drug treatment in order to increase the likelihood of response. The administration of aspirin for example is currently used in the treatment of cardiovascular diseases. Aspirin promotes fibrinogen acetylation, which alters its clotting properties.23 Acetylated fibrinogen is a less active acute phase protein24, which might affect serotonin levels.
Although preliminary, our data obtained from a naturalistic set of samples imply that plasma FGA levels are not only of value as a biomarker for depression5 but can also serve as a predictive antidepressant response marker. This could have potential for clinical implementation following validation in larger sample cohorts.
Acknowledgments
We thank Gertrud Ernst-Jansen, Karin Hofer, Elisabeth Kappelmann, Maria Lebar and Christiane Rewerts for excellent technical assistance with clinical samples.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Translational Psychiatry website (http://www.nature.com/tp)
Supplementary Material
References
- DiMatteo MR, Lepper HS, Croghan TW. Depression is a risk factor for noncompliance with medical treatment: meta-analysis of the effects of anxiety and depression on patient adherence. Arch Intern Med. 2000;160:2101–2107. doi: 10.1001/archinte.160.14.2101. [DOI] [PubMed] [Google Scholar]
- Rosenzweig-Lipson S, Beyer CE, Hughes ZA, Khawaja X, Rajarao SJ, Malberg JE, et al. Differentiating antidepressants of the future: efficacy and safety. Pharmacol Ther. 2007;113:134–153. doi: 10.1016/j.pharmthera.2006.07.002. [DOI] [PubMed] [Google Scholar]
- Nrugham L, Holen A, Sund AM. Suicide attempters and repeaters: depression and coping: a prospective study of early adolescents followed up as young adults. J Nerv Ment Dis. 2012;200:197–203. doi: 10.1097/NMD.0b013e318247c914. [DOI] [PubMed] [Google Scholar]
- Martins-de-Souza D, Harris LW, Guest PC, Turck CW, Bahn S. The role of proteomics in depression research. Eur Arch Psy Clin Neurosci. 2010;260:499–506. doi: 10.1007/s00406-009-0093-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wium-Andersen MK, Orsted DD, Nordestgaard BG. Association between elevated plasma fibrinogen and psychological distress, and depression in 73 367 individuals from the general population. Mol Psychiatry. 2012;18:854–855. doi: 10.1038/mp.2012.129. [DOI] [PubMed] [Google Scholar]
- Hennings JM, Owashi T, Binder EB, Horstmann S, Menke A, Kloiber S, et al. Clinical characteristics and treatment outcome in a representative sample of depressed inpatients—findings from the Munich Antidepressant Response Signature (MARS) project. J Psychiatr Res. 2009;43:215–229. doi: 10.1016/j.jpsychires.2008.05.002. [DOI] [PubMed] [Google Scholar]
- Wium-Andersen MK, Orsted DD, Nordestgaard BG. Elevated plasma fibrinogen, psychological distress, antidepressant use, and hospitalization with depression: Two large population-based studies. Psychoneuroendocrinology. 2012;38:638–647. doi: 10.1016/j.psyneuen.2012.08.006. [DOI] [PubMed] [Google Scholar]
- Panagiotakos DB, Pitsavos C, Chrysohoou C, Tsetsekou E, Papageorgiou C, Christodoulou G, et al. Inflammation, coagulation, and depressive symptomatology in cardiovascular disease-free people; the ATTICA study. Eur Heart J. 2004;25:492–499. doi: 10.1016/j.ehj.2004.01.018. [DOI] [PubMed] [Google Scholar]
- Maes M, Delange J, Ranjan R, Meltzer HY, Desnyder R, Cooremans W, et al. Acute phase proteins in schizophrenia, mania and major depression: modulation by psychotropic drugs. Psychiatry Res. 1997;66:1–11. doi: 10.1016/s0165-1781(96)02915-0. [DOI] [PubMed] [Google Scholar]
- Dowlati Y, Herrmann N, Swardfager W, Liu H, Sham L, Reim EK, et al. A meta-analysis of cytokines in major depression. Biol Psychiatry. 2010;67:446–457. doi: 10.1016/j.biopsych.2009.09.033. [DOI] [PubMed] [Google Scholar]
- Howren MB, Lamkin DM, Suls J. Associations of depression with C-reactive protein, IL-1, and IL-6: a meta-analysis. Psychosom Med. 2009;71:171–186. doi: 10.1097/PSY.0b013e3181907c1b. [DOI] [PubMed] [Google Scholar]
- Krishnadas R, Cavanagh J. Depression: an inflammatory illness. J Neurol Neurosurg Psychiatry. 2012;83:495–502. doi: 10.1136/jnnp-2011-301779. [DOI] [PubMed] [Google Scholar]
- Muller N, Myint AM, Schwarz MJ. Inflammatory biomarkers and depression. Neurotox Res. 2011;19:308–318. doi: 10.1007/s12640-010-9210-2. [DOI] [PubMed] [Google Scholar]
- Dantzer R, O'Connor JC, Lawson MA, Kelley KW. Inflammation-associated depression: from serotonin to kynurenine. Psychoneuroendocrinology. 2011;36:426–436. doi: 10.1016/j.psyneuen.2010.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duivis HE, de Jonge P, Penninx BW, Na BY, Cohen BE, Whooley MA. Depressive symptoms, health behaviors, and subsequent inflammation in patients with coronary heart disease: prospective findings from the heart and soul study. Am J Psychiatry. 2011;168:913–920. doi: 10.1176/appi.ajp.2011.10081163. [DOI] [PubMed] [Google Scholar]
- Baune BT, Neuhauser H, Ellert U, Berger K. The role of the inflammatory markers ferritin, transferrin and fibrinogen in the relationship between major depression and cardiovascular disorders—The German Health Interview and Examination Survey. Acta Psychiatr Scand. 2010;121:135–142. doi: 10.1111/j.1600-0447.2009.01435.x. [DOI] [PubMed] [Google Scholar]
- Vasse M, Paysant J, Soria J, Collet JP, Vannier JP, Soria C. Regulation of fibrinogen biosynthesis by cytokines, consequences on the vascular risk. Haemostasis. 1996;26 ((Suppl 4:331–339. doi: 10.1159/000217313. [DOI] [PubMed] [Google Scholar]
- Miller AH, Maletic V, Raison CL. Inflammation and its discontents: the role of cytokines in the pathophysiology of major depression. Biol Psychiatry. 2009;65:732–741. doi: 10.1016/j.biopsych.2008.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catena-Dell'Osso M, Rotella F, Dell'Osso A, Fagiolini A, Marazziti D. Inflammation, serotonin and major depression. Curr Drug Targets. 2013;14:571–577. doi: 10.2174/13894501113149990154. [DOI] [PubMed] [Google Scholar]
- Fish RJ, Neerman-Arbez M. Fibrinogen gene regulation. Thromb Haemost. 2012;108:419–426. doi: 10.1160/TH12-04-0273. [DOI] [PubMed] [Google Scholar]
- Yusuf S, Lessem J, Jha P, Lonn E. Primary and secondary prevention of myocardial infarction and strokes: an update of randomly allocated, controlled trials. J Hypertens Suppl. 1993;11:S61–S73. [PubMed] [Google Scholar]
- Meade TW. Haemostatic function and arterial disease. Br Med Bull. 1994;50:755–775. doi: 10.1093/oxfordjournals.bmb.a072924. [DOI] [PubMed] [Google Scholar]
- He S, Blomback M, Yoo G, Sinha R, Henschen-Edman AH. Modified clotting properties of fibrinogen in the presence of acetylsalicylic acid in a purified system. Ann N Y Acad Sci. 2001;936:531–535. doi: 10.1111/j.1749-6632.2001.tb03540.x. [DOI] [PubMed] [Google Scholar]
- Caspary EA. Studies on the acetylation of human fibrinogen. Biochem J. 1956;62:507–512. doi: 10.1042/bj0620507. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.