Abstract
Low grade cervical squamous abnormalities [low grade squamous intraepithelial lesions (LSIL, CIN1)] can be confused with or followed by high grade (HSIL, CIN2/3) lesions, expending considerable resources. Recently, a cell of origin for cervical neoplasia was proposed in the squamocolumnar junction (SCJ); HSILs are almost always SCJ marker-positive (+) but LSILs include SCJ+ and negative (−) subsets. Abnormal cervical biopsies from 214 patients were classified by two experienced pathologists ("panel") as LSIL or HSIL using published criteria. SILs were scored SCJ+ and SCJ- using SCJ-specific antibodies (Keratin7, AGR2, MMP7 and GDA). Assessments of interobserver agreement, p16ink4 staining pattern, proliferative index and outcome were compared. The original diagnostician agreed with the panel diagnosis of HSIL and SCJ- LSIL in all cases (100%). However for SCJ+ LSIL, panelists disagreed with each other on 15% and with the original diagnostician on 46.2%. Comparing SCJ- and SCJ+ LSILs, 60.2% and 94.9% scored p16ink4 positive, 23% and 74.4% showed strong (full-thickness) p16ink4 staining, and 0/54 (0%) and 8/33 (24.2%) with follow-up had an HSIL outcome respectively. Some SCJ+ LSILs are more likely to both generate diagnostic disagreement and be associated with HSIL. Conversely, SCJ- LSILs generate little observer disagreement and when followed, have a very low risk of HSIL outcome. Thus, SCJ biomarkers in conjunction with histology may segregate LSILs with very low risk of HSIL outcome and conceivably could be used as a management tool to reduce excess allocation of resources to the followup of these lesions.
INTRODUCTION
Human papillomavirus (HPV) has been linked to cervical cancer and its precursors from the early 1980's1,2 and research has uncovered more than 100 HPV genotypes3 and produced a successful preventive vaccine in 20024. Despite this, the management of women with precancerous lesions has remained inefficient, largely due to the lack of clarity regarding which precursors are most likely to progress and require excisional therapy (LEEP or cone biopsy). This uncertainty has manifested largely in the classification of cervical intraepithelial neoplasia (CIN or SIL). Lesions classified as CIN1 (LSIL) or CIN3 (HSIL) are more easily separated, justifying conservative management for the former and ablation for the latter. Lesions classified as CIN2 (HSIL) present a conundrum due to the fact that they are often treated by ablation but cannot be consistently distinguished from LSIL. The latter issue has resulted in unnecessary ablation for many lesions that confer a low risk for cancer outcome. A second problem is the fact that up to 13% of LSIL biopsies are followed by a histologic diagnosis of CIN3 5–9, necessitating regular follow-up of LSIL. Thus, precursors at the lower end of the spectrum, while at low risk for malignancy during follow-up, result in considerable expense. Biomarkers such as p16ink4 - linked to high-risk HPV infection - have been proposed to aid in this separation10, but are diffusely positive in a significant percentage of LSILs due to the fact that the latter are often associated with carcinogenic HPVs11,12. HPV DNA methylation was also suggested to be a potential biomarker for cervical cancer development13. However, the procedure for analyzing the methylation status using pyrosequencing assay is complex.
Most cervical cancers arise in the region of the squamocolumnar junction (SCJ) 14,15. Recently, we discovered a discrete population of cuboidal (to low columnar) cells at the cervical SCJ that shared a unique expression profile with HSILs and cancers16,17. Moreover, SCJ-specific biomarkers (keratin 7, AGR2, MMP7 and GDA) also highlighted a subset of LSILs infected with high risk HPVs and displaying an intense p16ink4 expression16. The purpose of this study was to 1) survey a large population of cervical precursor lesions to determine precisely the distribution of these biomarkers, 2) identify SCJ-positive (+) and negative (−) LSILs, and 3) compare the two groups with respect to their expression of p16ink4, proliferating index, diagnosis and follow-up.
MATERIALS AND METHODS
Case material and tissue classification
The study was approved by the institutional review board at Brigham and Women's Hospital (Boston, MA). 214 formalin-fixed paraffin-embedded cervical biopsies accessioned between 2005 and 2011 were retrieved from archival material in the Women’s and Perinatal Pathology Division. The original histological diagnoses were re-examined by two experienced pathologists (MRN and CPC; the "panel") without any pathologic or clinical information and classified as LSIL or HSIL using published criteria.18 Disagreements were resolved by group review comprised of the two panel pathologists. All cases were subdivided into SCJ+ and SCJ- based on both the staining pattern for SCJ-specific markers (positive vs negative) (see below) and the location (the location confirmed the immunostaining profile). When a HPV-related lesion is located in the SCJ, the (pre)neoplastic epithelium displays a positive staining for all SCJ-specific markers. The majority of cases analyzed in this study were stained with all SCJ-specific antibodies. However, staining for only one of these markers is sufficient to classify a lesion as of SCJ type. To avoid misclassification of benign epithelial abnormalities, each biopsy was stained for p16ink4 (a surrogate biomarker for carcinogenic HPV infection) and Ki67 (a proliferation marker up-regulated in SILs) 12, 19, 20.
For all LSIL diagnoses, pathology records of subsequent cytology (Pap smear) and follow-up diagnoses (from biopsy or excisional procedure) were reviewed when available. For each recorded follow-up diagnoses of HSIL, the pathology slides were retrieved, reviewed individually by the panel members and the diagnosis compared to the original. For these follow-up biopsies or excisional specimens, a panel diagnosis of HSIL required an independent diagnosis of CIN2/3 by both panel members. If either member did not make a HSIL diagnosis, the follow-up biopsy was not classified as HSIL. The purpose of this strategy was to maximize the rigor with which a follow-up diagnosis of HSIL was confirmed, given that it would imply an upgrade (HSIL) in an outcome sample.
Immunohistochemistry
Immunohistochemical analyses were performed as previous described17, 21 and included the following antibodies: anti-p16ink4 (Santa Cruz Biotechnology, Santa Cruz, CA, USA) and anti-Ki67 (Abcam, Cambridge, MA, USA) were used to confirm the presence of a preneoplastic lesion and assess proliferative activity. Anti-keratin7 (Clone RCK 105, Thermo Scientific, Waltham, MA, USA), anti-keratin 7 (Clone OV-TL12/30, Dako, Glostrup, Denmark), anti-AGR2 (Proteintech, Chicago, IL, USA), anti-GDA (Sigma-Aldrich, Saint-Louis, MO, USA) and anti-MMP7 (R and D systems, Minneapolis, MN, USA) were used to distinguish SCJ+ and SCJ- CINs. Both keratin 7 antibodies (clones RCK 105 and OV-TL12/30) displayed a similar staining pattern (an optimal dilution of primary antibodies should however be determined precisely) (data not shown). Mouse, rabbit and goat control IgG (Santa Cruz Biotechnology) were used as negative control.
Immunostaining assessment
p16ink4 and Ki67 immunolabeling was evaluated by using a semi-quantitative score based on the intensity and distribution of staining. Scoring of p16ink4 included both nuclear and cytoplasmic staining, and was graded as 0 (negative), 1 (rare dispersed positive cells), 2 (continuous over one third but no more than two thirds of the epithelial thickness), and 3 (strong and diffuse staining, uniform from basal layer to epithelial surface). Staining classified as 2 or 3 conforms to what is conventionally termed "positive" p16ink4 staining. Scoring of Ki67 was based on nuclear staining in the upper third of the epithelium; 1, 2, 3, 4 represented samples in which positive cells were detectable, respectively, in 1–25%, 26–50%, 51–75% and >75% of the designated epithelial region. Regarding AGR2, MMP7 and GDA, these SCJ-specific markers were classified as positive when intense diffuse cytoplasmic immunoreactivity of the entire cervical squamous preneoplastic epithelium was observed. Keratin 7 was either expressed by the suprabasal and/or apical cell layers (positive staining) or were not expressed (negative).
DNA isolation and HPV16/18 detection
DNA was extracted from paraffin sections using the QIAamp FFPE tissue kit (Qiagen, Valencia, CA, USA) according to the manufacturer’s instructions. For PCR reactions, primer sequences were as follows: HPV16 forward, 5’-AGC TGT CAT TTA ATT GCT CAT AAC AGT A-3’; HPV16 reverse, 5’-TGT GTC CTG AAG AAA AGC AAA GAC-3’; HPV18 forward, 5’-CGA ACC ACA ACG TCA CAC AAT-3’; HPV18 reverse, 5’-GCT TAC TGC TGG GAT GCA CA-3’. Forty cycles, including denaturation at 95°C for 45 sec, annealing for 45 sec and extension at 72°C for 1 min, were used for the analysis. The human β-globin control primer set (PC03-04) was used as a experimental control. Samples were run on 1.8% agarose gels containing ethidium bromide and visualized with an UV transilluminator.
Statistical analysis
Statistical analysis was performed with the Instat 3 software (Graph-Pad Software, San Diego, CA, USA). The Pearson χ2 test was performed to compare the significance of p16ink4a and Ki67 expression in SCJ+ and SCJ- CINs. Differences were considered as statistically significant when P values were less than 0.05.
RESULTS
Panel agreement and expression of SCJ-specific biomarkers in cervical preneoplastic lesions
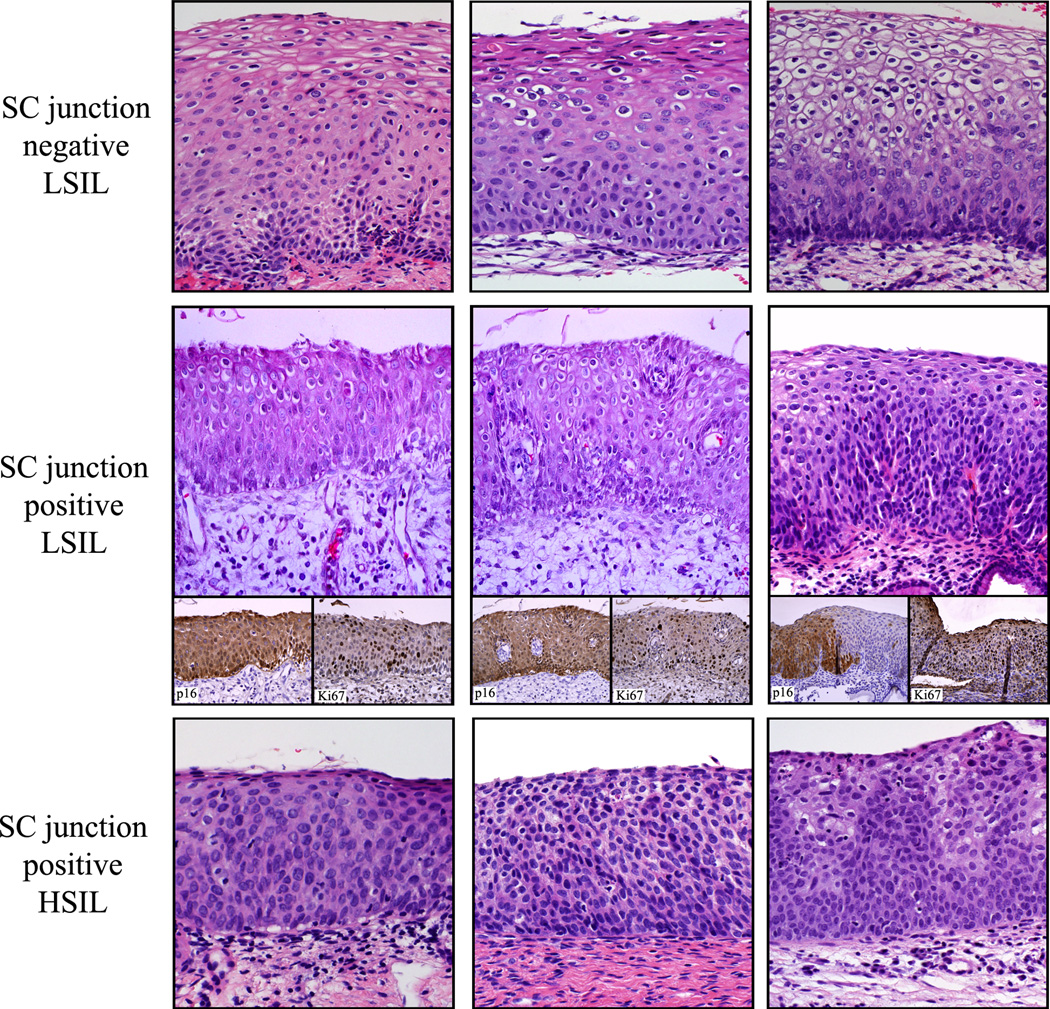
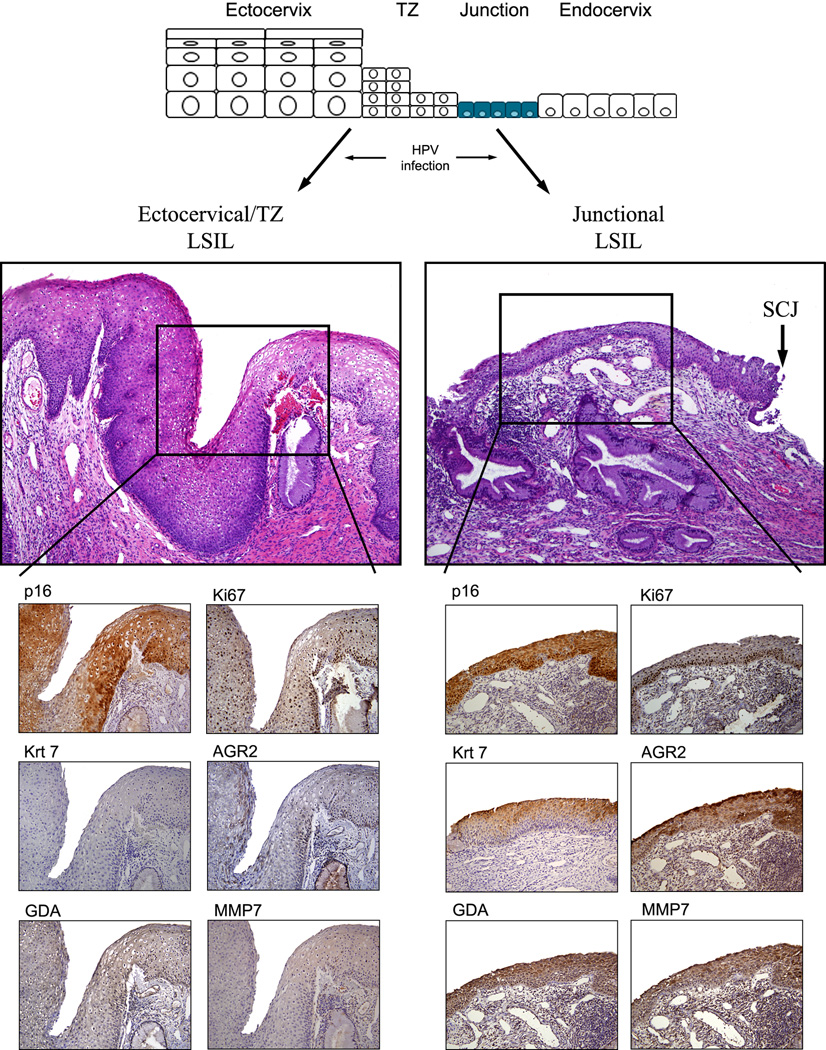
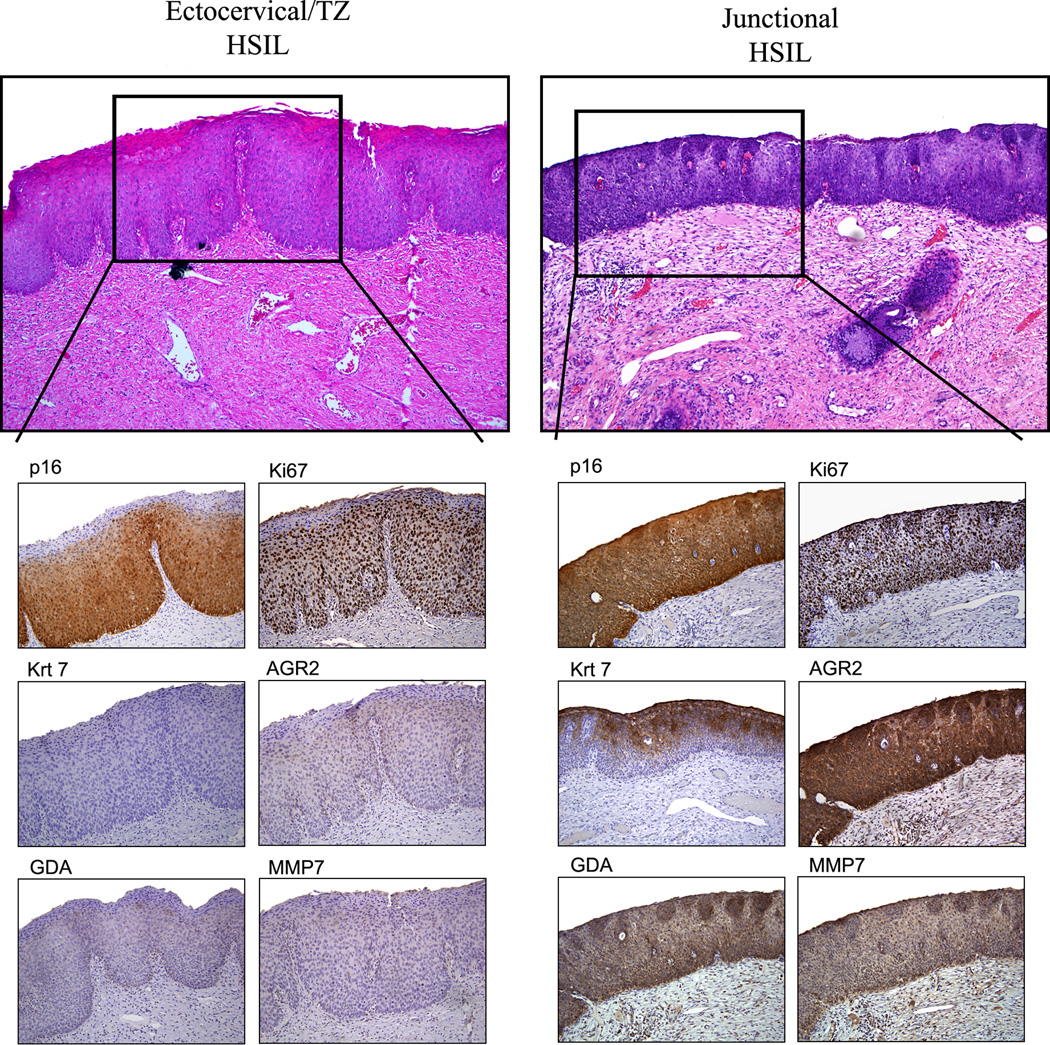
Reviewers agreed on a diagnosis of HSIL and LSIL in 96/97 (98.9%) and 111/117 (94.8%) cases, respectively. There was disagreement on 7 (3.3%) biopsies. These cases were resolved by group review comprised of the two panel pathologists. As previously shown in native cervical epithelium, neither ectocervix/transformation zone (TZ) nor endocervical epithelium reacted with the antibodies to keratin 7, AGR2, GDA and MMP7. 16 SCJ markers were detected in 39/117 (33.3%) LSILs and 89/97 (91.8%) HSILs. Examples of lesions studied (hematoxylin eosin) are illustrated in Figure 1. The immunostaining results are shown in Figures 2 and 3 and summarized in Table 1. When located in the SCJ, preneoplastic lesions displayed a diffuse full thickness AGR2, GDA and MMP7 immunoreactivity. Regarding keratin 7, this protein was mainly expressed in suprabasal and apical cell layers (Figure 2 and 3).
Figure 1.
Examples of SCJ- LSIL, SCJ+ LSIL and HSIL analyzed in this study (H&E). Note the relative immaturity and the higher cellularity of the SCJ+ LSILs relative to the SCJ- LSILs.
Figure 2.
LSILs (CIN1s) stained for p16ink4, Ki67 and the SCJ-specific markers. A schematic showing proposed origin is at the top. Note the absence of staining of the ectocervical/TZ LSILs and uniform staining of junctional LSILs.
Figure 3.
HSILs (CIN2/3) stained for p16ink4, Ki67 and the SCJ-specific markers. The ratio of SCJ+ to SCJ- HSILs is over 10:1 in this study in keeping with the epidemiological ratio of cervix to vaginal squamous neoplasia.
Table 1.
Pattern of SC junction marker, p16ink4 and Ki67 expression in preneoplastic cervical biopsies.
| p16ink4a staining | Ki67 staining | |||||
|---|---|---|---|---|---|---|
| Cervical biopsies |
Number of cases (%) |
Negative | Patchy | Diffuse basal |
Diffuse full thickness |
Mean Ki67 score (±SD) |
| LSIL (CIN1) | 117 | |||||
| SC junction-specific gene expression | ||||||
| Negative | 78 (66.7%) | 6 (7.7%) | 25 (32.1%) | 29 (37.2%) | 18 (23%) | 1.72 (0.88) |
| Positive | 39 (33.3%) | 0 (0%) | 2 (5.1%) | 8 (20.5%) | 29 (74.4%) | 1.62 (0.64) |
| HSIL (CIN2/3) | 97 | |||||
| SC junction-specific gene expression | ||||||
| Negative | 8 (8.2%) | 0 (0%) | 0 (0%) | 2 (25%) | 6 (75%) | 2.86 (0.98) |
| Positive | 89 (91.8%) | 0 (0%) | 0 (0%) | 4 (4.5%) | 85 (95.5%) | 3.13 (0.85) |
The distribution of the staining patterns of p16ink4 between SC junction positive and negative LSIL were significantly different (p<0.001).
The mean Ki67 score in LSIL was statistically lower than that observed in HSIL (p<0.01). No statistical difference was observed between SC junction positive and negative SIL.
Original diagnosis, SCJ marker status and reviewer agreement
Of 97 cases classified as HSIL by the panel, all were diagnosed as HSIL by the original pathologist. Eighteen of 117 LSILs (15.4%) were originally classified as HSIL. However, when the SCJ+ and SCJ- LSILs were evaluated separately, SCJ+ LSILs were significantly more likely to have been classified as HSIL by the original pathologist (18 of 39 (46.2%) versus 0 of 78 (0%); p<0.001) (Table 2). Figure 1 show representative example of preneoplastic lesions (LSIL/HSIL) analyzed in the present study. Despite the higher cellularity of the SCJ+ LSILs relative to the SCJ- LSILs, the weak proliferative index compared to HSIL (Figure 1 and listed in Table 1) and the relatively weak number of overlapping nuclei and mitotic figures support a diagnosis of LSIL.
Table 2.
Number of SC junction positive and negative LSIL, original diagnosis and number of outcome events during the study follow-up period.
| Panel Diagnosis | SCJ- LSIL (CIN1) | SCJ+ LSIL (CIN10 | |
|---|---|---|---|
| Number | 78 | 39 | |
| Original Diagnosis/No (%) | LSIL /78 (100) | LSIL/21 (53.8) | HSIL/18 (46.2) |
| Conization | 2 | 14 | |
| HSIL (consensus) | 0 | 0 | 8 (57%) |
| Observed without Conization | 51* | 13 | |
| Average (months) FU | 33.8 | 28.2 | |
| Range (months) | 6 to 84 | 4 to 72 | |
| No with at least one Bx/ECC | 28 (51.9%) | 7 (53.8%) | |
| HSIL report (Bx/Cyt) | 0 | 0 | |
6 cases had a prior HSIL treated by cone preceding the index biopsy
HPV genotypes, p16ink4 and Ki67 staining in SCJ+ and SCJ− CINs
The interactions between HPV viral oncoproteins and host regulatory proteins are integral to carcinogenic HPV-mediated tumorigenesis. HPV E6 and E7 proteins ultimately lead to deregulation of cell proliferation and this is reflected in abnormal expression of cell cycle-associated proteins such as p16ink4 6,19,20. We evaluated p16ink4 overexpression in our series of cervical biopsies and representative images are displayed in Figures 2 and 3. As shown in Table 1, 60.2 and 94.9% of SCJ- and SCJ+ LSILs exhibited continuous linear staining of the epithelium (i.e. positive). However, the proportion of cases with full-thickness staining was 23 and 74.4% respectively, with the SCJ+ LSILs more closely resembling HSIL (CIN2/3) in p16ink4 staining pattern. This difference in p16ink4 staining and semiquantitative scoring between the SCJ+ and SCJ- LSILs was statistically significant (P<0.01).
Figures 2 and 3 show the Ki67 distribution in SILs and the data are summarized in Table 1. Abnormal immunolabeling for this proliferative marker, with more than 75 percent positive cells in the upper third of the preneoplastic epithelium (score 4) was detected in 2 (2.8%) SCJ- LSIL, 0 (0%) SCJ+ LSIL, 3 (37.5%) SCJ- HSIL and 37 (41.6%) SCJ+ HSIL. In the group of LSIL, the distribution of Ki67 expression (measured as mean of the Ki67 semiquantitative score) was statistically lower than that observed in HSIL (p<0.01) cases. However, no statistical difference was observed between SCJ+ and SCJ- SILs.
In order to evaluate HPV16/18 infection status in SCJ+ LSILs, DNA samples were subjected to PCR amplification with HPV type-specific primers targeting these two carcinogenic HPVs. Of 39 SCJ+ LSILs, 21 (53.8%) were infected with HPV16 and 1 (2.6%) with HPV18. Ten of 18 (55.5%) cases classified as HSIL by the original pathologist scored positive for HPV16. Similarly, 11 of 21 (52.4%) cases classified by all observers as LSIL were HPV16 positive.
Clinical outcomes of SCJ+ and SCJ− LSIL
Table 2 summarizes the outcome data obtained from the patient records. The parameters recorded were follow-up cervical cytology, biopsies and LEEPs, when available. Outcome was influenced by interventions (excisional procedure), thus it was not uniform between SCJ- and SCJ+ LSILs. Of 78 SCJ- LSILs, followup information was available for 54 (69.2%) cases. The follow-up interval ranged from 6 months to 7 years. The majority of the remaining cases was obtained within a year prior to the study and had not yet recorded a follow-up sample. In all, the panel pathologists agreed on the diagnosis of LSIL. Only two of the 54 underwent an excisional procedure. Fifty one percent had at least one follow-up biopsy or endocervical curettage. No HSIL diagnoses were recorded on any test. Interestingly, six patients had undergone an excisional procedure prior to the index biopsy for HSIL. Of 39 SCJ+ LSILs (by the panel), 18 (46.2%) had been classified as HSIL by the original pathologist. Twenty underwent an excisional procedure, most of which had an original diagnosis of HSIL. Fifteen cases were originally diagnosed as HSIL on a follow-up conization; of the fourteen (57%) reviewed by the panel pathologists 8 were classified by both as HSIL. Of thirteen cases with no excisional procedure, classified by both panel and original pathologist as LSIL, seven (53.8%) had follow-up biopsy or endocervical curetting. No HSIL diagnoses (on cytology or histology) were recorded for this group. The average follow-up period for SCJ+ LSILs was 28.2 months (range, 4–72 months). In summary, of cases classified as LSIL by the panel, eight of 33 (24.2%) SCJ+ LSILs had a corroborated HSIL outcome, all based on an excisional procedure. In contrast, none of 54 SCJ- LSILs (0%) were followed by an HSIL diagnosis. These differences indicate that SCJ+ LSILs as a group are more heterogeneous than SCJ- LSILs, and a subset is more likely to be classified as HSIL by others, and thus more likely to have an HSIL outcome on a follow-up excisional procedure.
DISCUSSION
As a diagnostic entity, LSIL (or CIN1) presents two dilemmas to the practitioner. The first is its separation from CIN2 (HSIL), a distinction that is critical to management. An initial diagnosis of LSIL results in a one-year follow-up period22, 23. In contrast, a diagnosis of CIN2, depending on the circumstances and the patient's age, could result in an excisional procedure or a closer follow-up interval. Excisional procedures (Cone biopsy or LEEP) have been linked to a higher risk of premature delivery in subsequent pregnancies, therefore a difference in grading between CIN1 (LSIL) and CIN2 (HSIL) can impact patient welfare 24. A second dilemma is the follow-up risk of HSIL. Studies have estimated the risk of a HSIL outcome at two years or more after a diagnosis of LSIL to be from 4–13%, a figure influenced somewhat by the accuracy with which the initial and follow-up diagnoses are made5–9. Moreover, studies have demonstrated that initial colposcopy only detects approximately two thirds of HSIL lesions suggesting that this rate of HSIL following LSIL could be underestimated. 25 Thus, any strategy that would clarify the risk of a given LSIL being either classified as an HSIL or being followed by HSIL would be valuable, as this distinction cannot be consistently made. 26
This study presents findings that shed light on the above conundrums with the application of markers designed empirically to determine whether the lesion in question is linked to the SCJ. The rationale for this approach is the recent discovery of SCJ-specific cells in the cervix and evidence linking them to a large proportion of HSILs, adenocarcinomas in situ and malignancies16. This study uncovered a strong association between SCJ-specific biomarkers and high risk HPVs in both LSILs and HSILs and distinguished two forms of LSIL based on these markers. We proposed that direct infection of SCJ cells by carcinogenic HPVs resulted in trans-differentiation with an outgrowth of subjacent squamous cells (so-called top-down differentiation) leading to SIL, often high grade17. In contrast, infection of the ectocervical or squamous metaplastic epithelium usually resulted in LSILs16,17. This dichotomy of LSILs coupled with the low frequency of HSILs on the ectocervix (and vagina) points to two target cell types with distinctly different risks, the SCJ cells and keratinocytes either derived from the cervical transformation zone or the ectocervix/vaginal epithelium. This differential susceptibility to high grade morphology following carcinogenic HPV infection would explain the markedly different risks of vaginal and cervical HSIL and cancer 27. Indeed, according to epidemiologic studies, HPV-related lesions are ten to twenty times more common in the cervix than the vagina. 27
An observation of clinical significance was that certain SILs were diagnostically problematic and others were clearly not. All HSILs diagnosed by the panelists were corroborated by the original pathologist, in keeping with the fact that such lesions are not typically under-called in practice. Panelists disagreed on 6 SCJ+ LSILs. Moreover, disagreement over this group between the panel and the original pathologists was significant. Eighteen (46.2%) of 39 cases designated by the panel as SCJ+ LSILs were classified as HSIL by the original pathologists. Interestingly, most of these proceeded to excision and several were verified as HSIL by the panel. Those classified as LSIL by both panel and the original pathologist and followed had no HSIL outcomes. This indicates that SCJ+ LSILs are heterogeneous, with some behaving as LSIL while others might be either under-appreciated or inadequately sampled HSILs.
The more diagnostically problematic LSILs seem to be derived from a population of LSILs that usually exhibit full-thickness p16ink4 immunostaining, positivity for SCJ biomarkers and a high frequency of HPV16. In a prior study from our group, virtually all SCJ+ LSILs were carcinogenic HPV positive.16 Regarding the percentage of p16ink4 positive LSIL, the rates of positivity in this study (71%) are at the high end of estimates for LSIL.11,12,16 This begs the question of whether the high rate of p16ink4 positive LSILs in this study is due to a higher proportion of SCJ+ cases in the LSIL mix or the fact that the panel classified cases as LSIL that should have been classified as HSIL.
In contrast to SCJ+ LSILs, review and follow-up of LSILs lacking SCJ marker staining showed high agreement and very low risk of HSIL outcome. Not only did the panel agree on all 78 SCJ- LSILs but the original pathologist corroborated this diagnosis in every case (This high level of agreement might seem improbable, but it should be stressed that the original diagnoses, being made in a practice situation, are not usually made in a vacuum, involving residents and often, other consultants). In essence, SCJ markers, by their absence, identified LSILs likely to be corroborated by multiple observers. Moreover, virtually all were followed without surgery and none had an HSIL outcome on any diagnostic test (cytology or biopsy) during the follow-up period. Explanations include 1) the absence of SCJ markers identifies unequivocal LSILs, and 2) infections of metaplastic or ectocervical epithelium generate an immune response that protects the SCJ from subsequent infections. Importantly, these variables suggest that variations in HSIL outcome following LSIL biopsy across different studies could be influenced by the proportion of SCJ+ LSILs in the study.5–9 Interestingly, 6 individuals with SCJ- LSILs had a previous excisional procedure for HSIL, suggesting that subsequent infections following removal of the SCJ will be limited to residual metaplastic or ectocervical epithelium.
Regarding the potential use of SCJ markers in SIL management, two points should be made. The first pertains to the use of SCJ markers to by themselves identify LSILs at higher risk of HSIL outcome. Here it is important to note that whether the original pathologist agreed or not with the panel diagnosis of LSIL, the rate of HPV16 positivity was equivalent (52.4% vs 55.5%). This means that the combination of SCJ+, HPV16 and strong p16 staining is still insufficient to guarantee a biopsy diagnosis of HSIL or an HSIL outcome. Because of this, we do not recommend SCJ staining (just as we do not recommend p16 immunostaining) to adjudicate the question of whether to upgrade a lesion from CIN1 to CIN2. The second point concerns the concept of using SCJ markers to - by their absence - identify LSILs conferring a very low risk of HSIL outcome. An important caveat here is that a small percentage of HSILs are SCJ- and presumably ectocervical in origin. However, the absence of HSIL outcomes in SCJ- LSILs raises the possibility that an LSIL/SCJ marker-negative combination, similar to an ASCUS/HPV-negative combination, could identify women requiring less intensive surveillance. This concept merits further study.
ACKNOWLEDGMENTS
We thank the Division of Gynecologic Oncology in the Department of Obstetrics and Gynecology and Reproductive Biology at Brigham and Women’s Hospital for their cooperation. MH is a Postdoctoral Researcher of the Belgian National Fund for Scientific Research and he thanks the Fonds Leon Fredericq for its support. This work was supported by Defense Advanced Research Projects Agency (DARPA) Project N66001-09-1-2121; National Institutes of Health Grants RC1 HL100767, R01-GM083348, and R21CA124688; the Singapore–Massachusetts Institute of Technology Alliance for Research and Technology; the European Research Council; Agence de Nationale; the Institute of Medical Biology; and the Genome Institute of Singapore of the Agency for Science, Technology and Research. This work was also supported by Department of Defense Grant W81XWH-10-1-0289 (to C.P.C.).
Footnotes
The authors declare no conflict of interest.
REFERENCES
- 1.Crum CP, Ikenberg H, Richart RM, et al. Human papillomavirus type 16 and early cervical neoplasia. N Engl J Med. 1984;310:880–883. doi: 10.1056/NEJM198404053101403. [DOI] [PubMed] [Google Scholar]
- 2.Durst M, Gissmann L, Ikenberg H, et al. papillomavirus DNA from a cervical carcinoma and its prevalence in cancer biopsy samples from different geographic regions. Proc Natl Acad Sci U S A. 1983;80:3812–3815. doi: 10.1073/pnas.80.12.3812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.De Villiers EM, Fauquet C, Broker TR, et al. Classification of papillomaviruses. Virology. 2004;324:17–27. doi: 10.1016/j.virol.2004.03.033. [DOI] [PubMed] [Google Scholar]
- 4.Koutsky LA, Ault KA, Wheeler CM, et al. A controlled trial of a human papillomavirus type 16 vaccine. N Engl J Med. 2002;347:1645–1651. doi: 10.1056/NEJMoa020586. [DOI] [PubMed] [Google Scholar]
- 5.Bansal N, Wright JD, Cohen CJ, et al. Natural history of established low grade cervical intraepithelial (CIN 1) lesions. Anticancer Res. 2008;28:1763–1766. [PubMed] [Google Scholar]
- 6.Chen EY, Tran A, Raho CJ, et al. Histological 'progression' from low (LSIL) to high (HSIL) squamous intraepithelial lesion is an uncommon event and an indication for quality assurance review. Mod Pathol. 2010;23:1045–1051. doi: 10.1038/modpathol.2010.85. [DOI] [PubMed] [Google Scholar]
- 7.Cox JT, Schiffman M, Solomon D. ASCUS-LSIL Triage Study (ALTS) Group. Prospective follow-up suggests similar risk of subsequent cervical intraepithelial neoplasia grade 2 or 3 among women with cervical intraepithelial neoplasia grade 1 or negative colposcopy and directed biopsy. Am J Obstet Gynecol. 2003;188:1406–1412. doi: 10.1067/mob.2003.461. [DOI] [PubMed] [Google Scholar]
- 8.Holowaty P, Miller AB, Rohan T, et al. Natural history of dysplasia of the uterine cervix. J Natl Cancer Inst. 1999;91:252–258. doi: 10.1093/jnci/91.3.252. [DOI] [PubMed] [Google Scholar]
- 9.Schlecht NF, Platt RW, Duarte-Franco E, et al. Human papillomavirus infection and time to progression and regression of cervical intraepithelial neoplasia. J Natl Cancer Inst. 2003;95:1336–1343. doi: 10.1093/jnci/djg037. [DOI] [PubMed] [Google Scholar]
- 10.Negri G, Vittadello F, Romano F, et al. p16INK4a expression and progression risk of low-grade intraepithelial neoplasia of the cervix uteri. Virchows Arch. 2004;445:616–620. doi: 10.1007/s00428-004-1127-9. [DOI] [PubMed] [Google Scholar]
- 11.Galgano MT, Castle PE, Atkins KA, et al. Using biomarkers as objective standards in the diagnosis of cervical biopsies. Am J Surg Pathol. 2010;34:1077–1087. doi: 10.1097/PAS.0b013e3181e8b2c4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Keating JT, Cviko A, Riethdorf S, et al. Ki-67, cyclin E, and p16INK4 are complimentary surrogate biomarkers for human papilloma virus-related cervical neoplasia. Am J Surg Pathol. 2001;25:884–891. doi: 10.1097/00000478-200107000-00006. [DOI] [PubMed] [Google Scholar]
- 13.Mirabello L, Schiffman M, Ghosh A, et al. Elevated methylation of HPV16 DNA is associated with the development of high grade cervical intraepithelial neoplasia. Int J Cancer. 2013;132:1412–1422. doi: 10.1002/ijc.27750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marsh M. Original site of cervical carcinoma; topographical relationship of carcinoma of the cervix to the external os and to the squamocolumnar junction. Obstet Gynecol. 1956;7:444–452. [PubMed] [Google Scholar]
- 15.Richart RM. Cervical intraepithelial neoplasia. Pathol Annu. 1973;8:301–328. [PubMed] [Google Scholar]
- 16.Herfs M, Yamamoto Y, Laury A, et al. A discrete population of squamocolumnar junction cells implicated in the pathogenesis of cervical cancer. Proc Natl Acad Sci U S A. 2012;109:10516–10521. doi: 10.1073/pnas.1202684109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Herfs M, Vargas SO, Yamamoto Y, et al. A novel blueprint for "top down" differentiation defines the cervical squamocolumnar junction during development, reproductive life and neoplasia. J Pathol. 2013;229:460–468. doi: 10.1002/path.4110. [DOI] [PubMed] [Google Scholar]
- 18.Nucci MR, Crum CP. Redefining early cervical neoplasia: recent progress. Adv Anat Pathol. 2007;14:1–10. doi: 10.1097/PAP.0b013e31802e0de7. [DOI] [PubMed] [Google Scholar]
- 19.Klaes R, Friedrich T, Spitkovsky D, et al. Overexpression of p16(INK4A) as a specific marker for dysplastic and neoplastic epithelial cells of the cervix uteri. Int J Cancer. 2001;92:276–284. doi: 10.1002/ijc.1174. [DOI] [PubMed] [Google Scholar]
- 20.Sano T, Oyama T, Kashiwabara K, et al. Expression status of p16 protein is associated with human papillomavirus oncogenic potential in cervical and genital lesions. Am J Pathol. 1998;153:1741–1748. doi: 10.1016/S0002-9440(10)65689-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Herfs M, Hubert P, Poirrier AL, et al. Proinflammatory cytokines induce bronchial hyperplasia and squamous metaplasia in smokers: implications for chronic obstructive pulmonary disease therapy. Am J Respir Cell Mol Biol. 2012;47:67–79. doi: 10.1165/rcmb.2011-0353OC. [DOI] [PubMed] [Google Scholar]
- 22.Wright TC, Jr, Massad LS, Dunton CJ, et al. 2006 consensus guidelines for the management of women with abnormal cervical cancer screening tests. Am J Obstet Gynecol. 2007;197:346–355. doi: 10.1016/j.ajog.2007.07.047. [DOI] [PubMed] [Google Scholar]
- 23.Wright TC, Jr, Massad LS, Dunton CJ, et al. 2006 consensus guidelines for the management of women with cervical intraepithelial neoplasia or adenocarcinoma in situ. Am J Obstet Gynecol. 2007;197:340–345. doi: 10.1016/j.ajog.2007.07.050. [DOI] [PubMed] [Google Scholar]
- 24.Samson SL, Bentley JR, Fahey TJ, et al. The effect of loop electrosurgical excision procedure on future pregnancy outcome. Obstet Gynecol. 2005;105:325–332. doi: 10.1097/01.AOG.0000151991.09124.bb. [DOI] [PubMed] [Google Scholar]
- 25.Gage JC, Hanson VW, Abbey K, et al. Number of cervical biopsies and sensitivity of colposcopy. Obstet Gynecol. 2006;108:264–272. doi: 10.1097/01.AOG.0000220505.18525.85. [DOI] [PubMed] [Google Scholar]
- 26.Darragh TM, Colgan TJ, Cox JT, et al. The Lower Anogenital Squamous Terminology Standardization Project for HPV-Associated Lesions: background and consensus recommendations from the College of American Pathologists and the American Society for Colposcopy and Cervical Pathology. J Low Genit Tract Dis. 2012;16:205–242. doi: 10.1097/LGT.0b013e31825c31dd. [DOI] [PubMed] [Google Scholar]
- 27.De Martel C, Ferlay J, Franceschi S, et al. Global burden of cancers attributable to infections in 2008: a review and synthetic analysis. Lancet Oncol. 2012;13:607–615. doi: 10.1016/S1470-2045(12)70137-7. [DOI] [PubMed] [Google Scholar]