Abstract
Purpose
To report the clinical efficacy of sorafenib and to evaluate biomarkers associated with sorafenib clinical benefit in the BATTLE program.
Patients and Methods
Patients with previously treated non-small–cell lung cancer (NSCLC) received sorafenib until progression or unacceptable toxicity. Eight-week disease control rate (DCR), progression-free survival (PFS), and overall survival (OS) were assessed. Prespecified biomarkers included K-RAS, EGFR, and B-RAF mutations, and EGFR gene copy number. Gene expression profiles from NSCLC cell lines and patient tumor biopsies with wild-type EGFR were used to develop a sorafenib sensitivity signature (SSS).
Results
105 patients were eligible and randomized to receive sorafenib. Among 98 patients evaluable for 8-week DCR, the observed DCR was 58.2%. The median PFS and OS were 2.83 (95% confidence interval [CI], 2.04-3.58) and 8.48 months (95% CI, 5.78-10.97), respectively. Eight-week DCR was higher in patients with wt-EGFR than patients with EGFR mutation (P=0.012), and in patients with EGFR gene copy number gain (FISH positive) versus patients FISH negative (P=0.048). In wt-EGFR tumors, the SSS was associated with improved PFS (median PFS 3.61 months in high SSS versus 1.84 months in low SSS, P=0.026) but not with 8-week DCR. Increased expression of fibroblast growth factor-1, NF-kB and hypoxia pathways were identified potential drivers of sorafenib resistance.
Conclusion
Sorafenib demonstrates clinical activity in NSCLC, especially with wt-EGFR. SSS was associated with improved PFS. These data identify subgroups that may derive clinical benefit from sorafenib and merit investigation in future trials. ClinicalTrials.gov: NCT00411671.
Keywords: multikinase inhibitor, non–small cell lung cancer, sorafenib, biomarkers, targeted treatment
Introduction
Despite advances in our understanding of cancer biology, lung cancer remains the leading cause of cancer-related death in the United States. In 2010, 222,520 people were diagnosed and 157,300 people died from lung cancer (1). Eighty-five percent of lung cancers are non-small cell lung cancer (NSCLC), with a 5-year survival rate of <5% in advanced disease (2). Until recently, treatment options for patients with advanced NSCLC have been limited to cytotoxic chemotherapy (3-6).
NSCLC is a complex disease comprising three major histologic subgroups: adenocarcinoma, squamous-cell, and large-cell carcinomas (7, 8). Its growth is dependent on dysregulation of multiple signaling pathways. New targeted therapies including tyrosine kinase inhibitors and monoclonal antibodies offer the ability to target critical pathways that control mechanisms of tumor growth. There are several activating driver mutations in NSCLC. The most common, K-RAS (20%-30% of cases) drives constitutive activation of downstream pathways including the mitogen-activated protein kinase (MAPK) pathway and is often associated with resistance to systemic therapies (9).
Sorafenib is a potent oral multi-targeted inhibitor of vascular endothelial growth factor 2 (VEGFR-2), RAF-kinases, platelet derived growth factor beta (PDGFR-β), and c-Kit and has antitumor activity in mutant-K-RAS NSCLC xenografts (10, 11). Single-agent sorafenib was active in several phase I and II trials in chemotherapy-refractory NSCLC (12-14). Sorafenib was the most clinically effective agent in (BATTLE Biomarker–integrated Approaches of Targeted Therapy for Lung Cancer Elimination) (15). However, the relative contribution of each potential target to sorafenib's antitumor activity in NSCLC is unknown, and no definitive predictive biomarkers of benefit have been reported. In this context, we developed a gene expression signature of sorafenib efficacy in vitro using a large panel of NSCLC cell lines and applied the signature in patients included in the BATTLE trial and treated with sorafenib, using gene expression profiles of core needle biopsies collected prospectively at baseline. This report presents an in-depth analysis of clinical outcomes and prespecified biomarkers for patients treated with sorafenib (15).
Patients and methods
Patient selection
Patients ≥18 years with confirmed biopsy-accessible advanced NSCLC (stage IIIB or IV, with disease progression), Eastern Cooperative Oncology Group (ECOG) performance status (PS) of 0-2, measurable disease by Response Evaluation Criteria in Solid Tumors (RECIST) (16), adequate organ function and at least one prior systemic treatment ≥28 days were eligible. Brain metastases had to be asymptomatic, with no systemic steroid use for >1 week, and stable >4 weeks after radiation. Clinically significant bleeding in the past month, prior hemoptysis or previous sorafenib was not allowed. Prior treatment with other targeted agents [epidermal growth factor receptor (EGFR), MEK, farnesyl transferase, RAF, or VEGF/VEGFR inhibitors] was permitted.
Once patients were consented for BATTLE and completed the appropriate procedures, they were adaptively randomized to one of 4 treatment arms: erlotinib, vandetanib, erlotinib/bexarotene, or sorafenib according to their baseline biomarker profile analysis of 11 prespecified individual biomarkers, clinical eligibility, and their prior therapy (15). A patient was excluded from the sorafenib arm if he/she had received prior sorafenib. Fresh tumor biopsies were mandatory for evaluation of prespecified biomarkers, and remaining tissues were used for biomarker discovery. All patients signed informed consent approved by the MD Anderson Cancer Center's institutional review board.
Treatment schedule
Sorafenib 400 mg twice/daily was administered orally (p.o) to patients in continuous 28-day cycles until evidence of tumor progression or intolerable drug-related toxicity. Doses were delayed or reduced for clinically significant treatment-related toxicities. Dose was reduced to 400 mg daily for patients with grade 3-4 toxicities, with the option of re-escalating to 400 mg twice/daily after resolution. If grade 3-4 toxicity persisted despite dose reduction, sorafenib was discontinued.
Assessment of efficacy and safety
The 8-week disease control rate (DCR) was the primary end-point of the trial. It has two advantages: it has been proposed as a short-term surrogate for overall survival by the Southwest Oncology Group (17), and it facilitates the use of adaptive randomization. It was compared with the historical 30% disease control rate in a similar population of patients (18). Treatment efficacy was defined as >0.80 probability of achieving > 30% disease control rate (15). Patients who completed one cycle of therapy were included for efficacy analysis and underwent imaging every two cycles to evaluate response and DCR. DCR was defined as the proportion of patients who did not meet RECIST criteria for progressive disease (PD) at or before the first follow-up imaging at 8 weeks. PFS and OS were measured from date of randomization until PD or death respectively.
Toxicity was assessed at scheduled visits every 4 weeks while on therapy, and data were collected until the first follow-up visit 4 weeks after therapy discontinuation after which patients were followed every 3 months for 3 years for survival. Adverse events (AE) were defined by National Cancer Institute Common Terminology Criteria for Adverse Events (NCI-CTCAE) version 3.0.
Prespecified biomarker assessment
Mutations of EGFR (exons 18–21), K-RAS (exons 1, codons 12 and 13; and exon 2, codon 61), and B-RAF (exons 11 and 15) were assessed using DNA from micro dissected formalin-fixed paraffin-embedded tumor cells. DNA sequences were amplified by polymerase chain reaction (PCR) using primers as previously described (15) and PCR products were sequenced using Applied Biosystems PRISM dye terminator cycle sequencing method. All sequence variants were confirmed by independent PCR amplifications from two independent DNA extractions, and sequenced both directions (15).
EGFR copy number analysis was performed using fluorescence in situ hybridization (FISH) as previously described (19). Cases were classified into six FISH strata by frequency of cells with EGFR gene copy number in reference to chromosome 7 centromere as previously described (15). High polysomy and gene amplification were combined and reported as FISH positive; other categories were considered FISH negative (20).
Retrospective biomarker development: gene-expression profiling
Because patients with mutant-EGFR tumors and treated with sorafenib had a worse 8-week DCR compared to other agents used in BATTLE, a sorafenib sensitivity signature focused on the wild-type-EGFR tumors was trained in-vitro, using gene expression profiling of 68 wild-type-EGFR NSCLC cell lines with available sorafenib IC50 (Supplementary Table 1 and Supplementary Material and Methods). Spearman correlation of IC50 with each probe was computed for the whole genome. Fifty probes, corresponding to 47 genes were found significant with a false discovery rate of 0.5 and a P-value <0.0001 (Supplementary Table 2). Their effect was summarized by the first Principal Component (PC) and called sorafenib sensitivity signature (SSS).
When available, material obtained from baseline core needle biopsies was used to generate genome-wide gene expression profiles for biomarker discovery as detailed in Supplementary Material and Methods. Gene expression profiles were available in 101/255 (40%) patients who were randomized and evaluable in the BATTLE trial including 47/105 (45%) patients treated with sorafenib. We excluded 3 of the 47 profiles generated from samples with no tumor or malignant cells detected on the H&E control section (21). Among the 44 remaining patients, 7 had a tumor with EGFR mutation, leaving 37 patients in whom the SSS was tested.
The signature was then tested in 37 wild-type-EGFR tumors from sorafenib treated patients. Kaplan-Meier curves were used to estimate PFS in patients with high versus low first principal component (PC) based on the median of the first PC and compared with log-rank statistic. Detailed methods, including the Gene Set Enrichment Analysis, are in Supplementary Material and Methods. Raw gene-expression data, clinical information, mutational status, and sorafenib IC50 have been deposited in the National Institutes of Health GEO database at www.ncbi.nlm.nih.gov/geo under accession numbers GSE33072 (BATTLE patient samples) and GSE32036 (cell lines).
Statistical methods
Full statistical details for the BATTLE trial have been reported (15). The outcome-based adaptive randomization under a Bayesian hierarchical model was employed to intend to treat more patients into more effective treatments according to their biomarker profiles. Standard statistical methods were applied for the analysis, including Fisher's exact test for contingency tables, log-rank tests for survival data. Each randomized patient represented a unit of the analysis.
Results
Patient characteristics
Between November 2006 and October 2009, 255 patients were randomized among four BATTLE trials(15). A total of 105 patients were randomized to sorafenib, including 35 patients (33%) only eligible for this therapy because of prior treatments and/or failure to meet the eligibility for other trials. Patient characteristics are listed in Table 1. Median age was 62 years, 51% of patients were male, 75% were former/current smokers, and 89% had an ECOG PS of 0-1. Median number of prior therapy regimens for stage IV NSCLC was 2 (range, 1-6), 68% of patients received prior erlotinib, and 41% prior bevacizumab.
Table 1.
Patient and tumor characteristics
| Characteristic | Pts treated with sorafenib (N = 105) | Pts used to test the SSS (N=37) | ||
|---|---|---|---|---|
| No. | % | No. | % | |
| Gender | ||||
| Male | 54 | 51.4 | 21 | 56.8 |
| Female | 51 | 48.6 | 16 | 43.2 |
| Age, years | ||||
| < 60 | 43 | 41.0 | 17 | 45.9 |
| ≥ 60 | 62 | 59.0 | 19 | 54.1 |
| Race/Ethnicity | ||||
| White | 86 | 81.9 | 33 | 89.2 |
| Black | 7 | 6.7 | 2 | 5.4 |
| Hispanic | 7 | 6.7 | 0.0 | 0.0 |
| Asian | 5 | 4.8 | 2 | 5.4 |
| Smoking | ||||
| Current | 6 | 5.7 | 0.0 | 0.0 |
| Former | 73 | 69.5 | 31 | 83.8 |
| Never | 26 | 24.8 | 6 | 16.2 |
| ECOG performance status | ||||
| 0 | 6 | 5.7 | 2 | 5.4 |
| 1 | 87 | 82.9 | 30 | 89.2 |
| 2 | 12 | 11.4 | 5 | 5.4 |
| Histology | ||||
| Adenocarcinoma | 71 | 67.7 | 29 | 78.4 |
| Squamous cell carcinoma | 14 | 13.3 | 3 | 8.1 |
| NSCLC otherwise unspecified | 16 | 15.2 | 5 | 13.5 |
| Other | 4 | 3.8 | 0.0 | 0.0 |
| Number of prior treatments for stage IV disease | ||||
| 1 | 25 | 23.8 | 9 | 24.3 |
| 2 | 33 | 31.4 | 12 | 32.4 |
| 3 | 24 | 22.9 | 11 | 29.7 |
| 4 | 16 | 15.2 | 3 | 8.1 |
| ≥5 | 7 | 6.7 | 2 | 5.5 |
| Previous erlotinib | ||||
| Yes | 71 | 67.6 | 22 | 59.5 |
| No | 34 | 32.4 | 15 | 40.5 |
| Previous bevacizumab | ||||
| Yes | 43 | 41.0 | 15 | 40.5 |
| No | 62 | 59.0 | 22 | 59.5 |
| EGFR mutation | ||||
| No | 72 | 85.7 | 37 | 100 |
| Yes | 12# | 14.3 | 0 | 0.0 |
| EGFR gene copy number | ||||
| FISH negative | 70 | 84.3 | 32 | 86.5 |
| FISH positive | 13* | 15.7 | 5 | 13.5 |
| K-RAS mutation | ||||
| No | 65 | 77.4 | 30 | 81.1 |
| Yes | 19# | 22.6 | 7 | 18.9 |
| B-RAF mutation | ||||
| No | 80 | 95.2 | 37 | 100 |
| Yes | 4# | 4.8 | 0 | 0.0 |
among 84 patients with available data;
among 83 patients with available data;
Abbreviations: SSS: sorafenib sensitivity signature; ECOG, Eastern Cooperative Oncology Group; NSCLC: non-small cell lung
Efficacy and safety
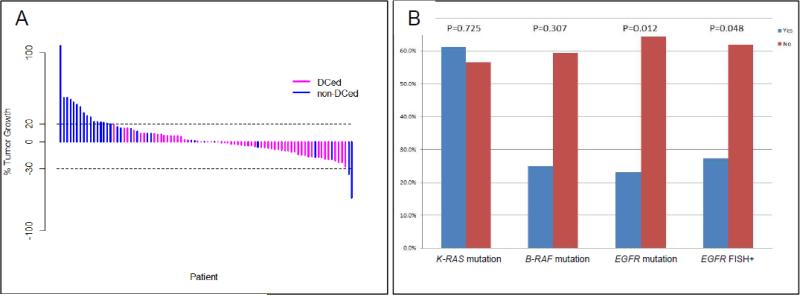
Among the 105 patients treated by sorafenib, 98 of them were evaluable for the primary endpoint 8-week disease control, including 57 patients (58.2%) with stable disease (SD), and median SD duration was 1.87 (range, 0.07-12.91) months. With a median follow-up of 9.43 months, median PFS was 2.83 (95% confidence interval [CI], 2.04-3.58) months and median OS was 8.48 (95% CI, 5.78-10.97) months. Figure 1A summarizes the maximum percentage reduction of target lesions in patients with disease control versus non-disease control. Eight-week DCR was 59.1%, 57.1%, and 55.6% for adenocarcinomas, squamous-cell carcinomas and other histologies respectively.
Figure 1.
Maximum percent reduction of target lesions in patients (N = 88) with disease control vs. non-disease control (A). Disease control rates at 8 weeks by K-RAS mutations, B-RAF mutations, EGFR mutation and EGFR gene copy number gain by FISH; FISH positive tumors included those with high polysomy and gene amplification as previously described (20) (B). Abbreviations: DCed: patients who did achieve 8-week disease control; non-DCed: patients who did not achieve 8-week disease control.
The most commonly reported treatment-related AEs among the 105 patients were hand-foot syndrome (HFS) (59.6%), fatigue (42.3%), rash (40.4%), diarrhea (38.5%), and weight loss (38.5%) (Supplementary Table 3). Overall, 45 patients (43%) had grade 3-4 treatment-related AEs and 101 patients (96%) experienced AEs of any grade. Median duration of treatment for all patients was 8 weeks with a median compliance rate of 98% of intended sorafenib dose. Twenty patients experienced dose reduction secondary to drug-related toxicity, the most common cause being HFS (50%). Three patients were re-escalated after dose reduction. The most common reason for treatment discontinuation was PD (56%). Out of the 20 patients who stopped therapy with sorafenib for reasons other than PD, 15 did so secondary to drug-related toxicity (hemoptysis: n=5; HFS: n=4; allergic reaction, hyperglycemia, cardiac ischemia, cerebrovascular accident, pleuritic pain, and intracerebral hemorrhage: each n=1). There were no significant differences in toxicities by histology. There were two deaths, both unrelated to treatment. One patient died from aspiration pneumonia, and one from sepsis.
Prespecified biomarkers and efficacy
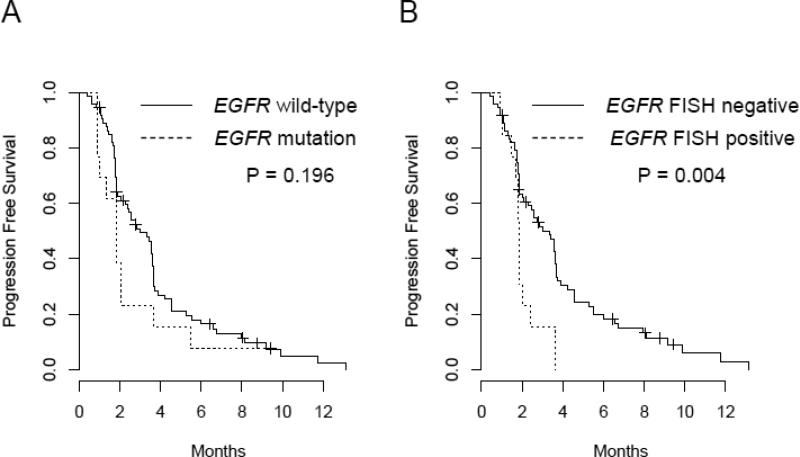
Table 2 summarizes 8-week DCRs by patient characteristics and the important biomarkers. Among the patients treated in sorafenib, 22.5% of them had tumors with K-RAS mutations, 5.0% with B-RAF mutations, 16.3% with EGFR mutations, and 13.8% were EGFR FISH positive. There was no EGFR or K-RAS mutation in tumors of 65% of the patients. Three patients had tumors with both EGFR and K-RAS mutations. Presence of K-RAS or B-RAF mutations were not statistically significantly associated with 8-week DCR (P=0.725 and P=0.307, respectively). Patients with EGFR-mutant tumors had significantly lower 8-week DCR compared to patients with wild-type tumors (23.1% versus 64.2%, P=0.012). This effect remained true after stratification by ECOG performance status and the number of prior treatments patients (Supplementary Table 4). In addition, patients with tumors EGFR FISH positive had significantly lower 8-week DCR compared with patients with EGFR FISH negative tumors (27.3% versus 61.8%, P=0.048). Figure 1B illustrated the 8-week DCR by K-RAS mutations, B-RAF mutations and EGFR FISH positivity. There were no associations identified with PFS and OS by K-RAS or B-RAF mutation status. Patients with EGFR wild-type tumors had longer PFS compared to patients with EGFR mutation, but not statistically significant. However, patients with EGFR FISH positive tumors had a statistically significant shorter compared to patients with EGFR FISH negative tumors (P=0.004). The median PFS was 3.35 (95%CI: 2.30, 3.68) months in patients with EGFR FISH negative, vs. 1.84 (95%CI: 1.68, NA) months in patient with EGFR FISH positive. The Kaplan-Meier curves for PFS by EGFR mutation status and EGFR FISH are presented in Figure 2.
Table 2.
Disease control rates at 8 weeks by clinical, pathological, K-RAS mutations, B-RAF mutations, EGFR mutations and EGFR FISH
| Variable | 8-week DCR | P-value | |
|---|---|---|---|
| Gender | Female | 57.4% (27/47) | 0.890 |
| Male | 58.8% (30/51) | ||
| Age | >60 | 57.9% (33/57) | 0.949 |
| <=60 | 58.5% (24/41) | ||
| Race: white | No | 66.7% (8/12) | 0.756 |
| Yes | 57.0% (49/86) | ||
| Smoking | Current | 40.0% (2/5) | 0.549 |
| Former | 60.9% (42/69) | ||
| Never | 54.2% (13/24) | ||
| ECOG | 0 | 66.7% (4/6) | 0.095 |
| 1 | 61.7% (50/81) | ||
| 2 | 27.3% (3/11) | ||
| Histology | Adenocarcinoma | 59.1% (39/66) | 0.961 |
| Squamous cell carcinoma | 57.1% (8/14) | ||
| Other | 55.6% (10/18) | ||
| Number of prior treatments | 0-1 | 41.7% (10/24) | 0.059 |
| >=2 | 63.5% (47/71) | ||
| Previous erlotinib | No | 52.8% (19/36) | 0.410 |
| Yes | 61.3% (38/62) | ||
| Previous bevacizumab | No | 50.8% (30/59) | 0.071 |
| Yes | 69.2% (27/39) | ||
| EGFR mutation | No | 64.2% (43/67) | 0.012 |
| Yes | 23.1 (3/13) | ||
| EGFR FISH positive | No | 61.8% (42/68) | 0.048 |
| Yes | 27.3% (3/11) | ||
| K-RAS mutation | No | 56.5% (35/62) | 0.725 |
| Yes | 61.1% (11/18) | ||
| B-RAF mutation | No | 59.2% (45/76) | 0.307 |
| Yes | 25.0% (1/4) | ||
Abbreviations: RECIST: Response evaluation criteria in solid tumors. DCR: Disease control rates. PFS: Progression-free survival. OS: Overall survival.
Figure 2.
Progression-free survival by EGFR mutation status (A), and by EGFR gene copy number gain by FISH; FISH positive tumors included those with high polysomy and gene amplification as previously described (20) (B).
Retrospective biomarker development: the sorafenib sensitivity signature
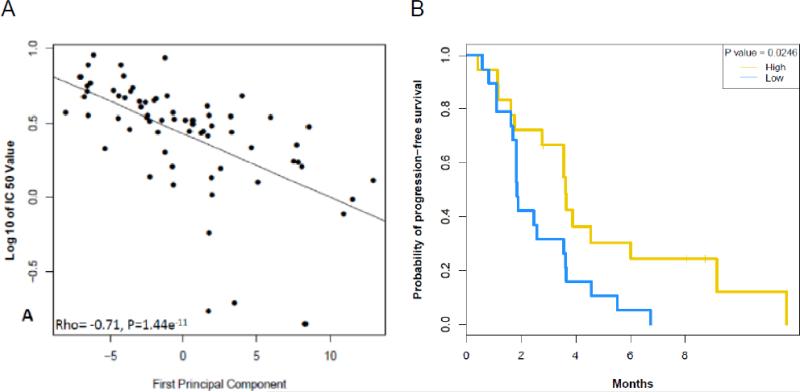
The SSS developed in NSCLC cell lines was then further evaluated. Figure 3A shows the correlation of the SSS with IC50 of sorafenib (rho=-0.71, P<0.0001). The list of 47 individual genes is provided as Supplementary Table 2. A heatmap of the 68 wild-type-EGFR NSCLC cell lines using the genes included in the SSS is shown is Supplementary Figure 1. We found groups of genes related to metabolism, MAPK signaling, membrane and nuclear (steroid receptors) and protein synthesis. Gene Set Enrichment Analysis showed that gene sets related to epithelial-tomesenchymal transition, NF-kB pathway and hypoxia were associated with resistance to sorafenib (Supplementary Results 1&2).
Figure 3.
Sorafenib sensitivity signature: (A) Scatter plot showing the correlation between Sorafenib signature and IC50 of sorafenib (rho = -0.71, P < 0.0001). (B) Kaplan-Meier curve for progression-free survival for the 37 patients with EGFR wild-type tumors and a high vs. low sorafenib sensitivity signature based on the median of the first principal component analysis.
Based on the median of the first PC analysis of the SSS trained in-vitro tested in tumor samples, the 8-week DCR was higher in patients with tumors with high-SSS (13/19, 68.4%) vs. those with low-SSS (10/18, 55.6%) although not reaching statistical significance (P=0.5077). Median PFS in the patients with high-SSS was 3.61 (95% CI, 2.76-NA) months vs. 1.84 (95% CI, 1.81-3.65) months in the low-SSS, P=0.0263 (Figure 3B). A heatmap of the 37 patients with wild-type-EGFR tumors using the genes included in the SSS is shown is Supplementary Figure 2.
Discussion
BATTLE was designed to personalize NSCLC therapy. The reported correlatives, and the in-vitro discovery and tumor testing of a gene-expression signature that was associated with sorafenib clinical benefit advance the prospects for this personalized approach. The initial BATTLE paper reported the 8-week DCR for each treatment, whereas all other clinical outcome data comprised overall combined treatment groups (15). The present report provides the specifics on sorafenib efficacy and safety in association with a comprehensive biomarker analysis. PFS and OS, and 8-week DCR were better in the sorafenib trial comparing favorably with results of other targeted single agents in less-heavily pretreated populations (12, 13, 22, 23) as well as in other BATTLE studies (15). There were no differences in 8-week DCR, PFS, or OS by histology, consistent with previous sorafenib efficacy reports (12, 13). Safety analysis confirmed that sorafenib was well tolerated.
Analyses of the effects of prespecified predictive biomarkers (K-RAS mutations, B-RAF mutations, and EGFR aberrations) on treatment efficacy demonstrated that the presence or absence of K-RAS or B-RAF mutations did not correlate with patient outcome. However, EGFR mutations and EGFR FISH positive status predicted worse outcome. Unexpectedly, patients with mutant-K-RAS tumors had the best 8-week DCR (61%), and patients with mutant-EGFR tumors had the worst 8-week DCR (23.1%) and the shortest PFS (1.84 months). It is unclear if this difference in efficacy is due to differential effects of sorafenib or to different tumor natural history.
Our results have interesting implications due to the multiple mechanisms of action of sorafenib in NSCLC. K-RAS mutations have been linked to anti-EGFR therapy resistance (24), but little is known about their role in RAS-RAF pathway-directed lung cancer therapy. In fact different types of KRAS mutations may predict for different outcomes. We found through analysis of BATTLE biomarker data, that the K-RAS G12C/V mutation was associated with a decreased PFS compared with other K-RAS mutations (P=0.026) (25). In a small study of sorafenib selecting patients based on K-RAS mutations found three partial responses, three minor responses, and a median PFS of three months (95% CI, 2.2-3.8) in 10 patients with advanced, chemotherapy treated, and mutant-KRAS NSCLC. Investigators concluded that these results warranted K-RAS testing for subsequent sorafenib trials (14). A larger study of single agent sorafenib in 37 stage IV NSCLC patients, including 32% with mutant-K-RAS tumors, found no correlation between K-RAS mutations and PFS or OS (13). The MISSION trial, a randomized, double-blind, placebo-controlled multicenter phase III study compared sorafenib plus best supportive care (BSC) vs. BSC alone in unselected patients with nonsquamous cell carcinoma who were receiving third or fourth line therapy. Although the study did not meet its primary endpoint of overall survival (OS), median PFS was 84 days for sorafenib vs. 43 days for placebo (P < .0001), median time to progression was 89 vs. 43 days, respectively (P < .0001). Overall response rate was 4.9% vs. 0.9% (P < .001), and DCR was 47% vs. 25% (P < .0001) (26). A post hoc biomarker analysis performed in a small proportion of patients (107/703, 15%) suggested that EGFR mutation status had a positive interaction with OS. Of note, post study use of EGFR tyrosine kinase inhibitor (43% in the sorafenib arm vs. 18% in the placebo arm) may have biased overall outcome (27). Available data does not allow drawing a firm conclusion as of the role of EGFR and K-RAS mutation status to define a group of patients deriving the highest benefit from sorafenib. Given the biologic heterogeneity of tumors, utilizing a biologic signature rather than a single biomarker to identify patients likely to benefit from sorafenib is a rational next step. The SSS may provide this option if validated in future studies such as our ongoing BATTLE-2 program, which includes a sorafenib arm.
Our findings include the discovery (in-vitro) and testing (tumor samples) of a gene-expression signature that predicted sorafenib efficacy (PFS) in advanced EGFR wild-type NSCLC. Several approaches have been proposed for deriving gene expression signatures to predict clinical benefit of a drug: In-vitro- or clinically-derived signatures (28, 29) and sensitivity-based or pathway-based signatures (30, 31). The pathway-based approach is appealing in the sorafenib case because our data suggest that patients with wild-type EGFR tumors, including those with mutant K-RAS, may benefit from it. Preclinical studies of the expression of RAS pathway genes and knockdown of K-RAS using small-interfering RNA in NSCLC models have found that a RAS-pathway signature may be better than K-RAS-mutation status for measuring RAS dependence (31). We tested two independent K-RAS signatures developed either in-vitro or in lung adenocarcinoma patients (32). The latter was associated with K-RAS mutational status in BATTLE biopsies, however, neither was associated with outcome in sorafenib treated patients. This could be explained in part because sorafenib acts on multiple targets affecting different pathways.
The sensitivity-based approach we report was based on the hypothesis that gene-expression profiles may capture the effect of sorafenib on multiple pathways. Although several of the SSS genes were related to important functions in cancer, there were no correlations of specific genes or groups of genes with sorafenib sensitivity. Sorafenib is a multitargeted kinase with antitumor effects on tumor angiogenesis via VEGFR and PDGFR (33), therefore, high levels of pro-angiogenic factors including fibroblast growth factor-1 (34), the decay accelerating factor CD55 (35), PPAR-gamma (36), IGFBP-7, and gastrin-releasing peptide (37) are associated with sorafenib resistance. Together this suggests that tumors upregulating alternative pathways for promoting angiogenesis or protecting endothelial cells may be relatively resistant to sorafenib, due to their ability overcome blockade of the VEGFR and PDGFR pathways. The availability of two unique datasets comprising a large set of NSCLC cell lines and baseline core biopsies collected in a clinical trial allowed us to develop a SSS in-vitro and to test it in patient samples with associated outcome data. The signature was able to predict a better PFS in the sorafenib BATTLE (clinical) test set. These results provide a proof-of-principle of the feasibility of generating high-quality, high-throughput profiling from core biopsies within a clinical trial and the importance of this profiling for biomarker development.
The SSS may serve as an additional biomarker to help defining a subgroup of patients with tumors wild-type for EGFR that may benefit most from sorafenib. However, a definitive conclusion on the value of this biomarker will require validation in a larger, independent group of wild-type EGFR tumors of sorafenib treated patients. Sorafenib was the most effective agent in BATTLE. These results led to include sorafenib therapy as one option in BATTLE-2. As in our previous BATTLE program, patients are adaptively randomized, based on K-RAS status, to 4 trial arms: erlotinib, erlotinib plus the AKT inhibitor MK-2206, MK-2206 plus the MEK inhibitor selumetinib, and sorafenib and the primary objective is 8-week DCR. The SSS is one of the promising biomarkers that will be tested in patients included in stage 1 of BATTLE-2 (38).
Few data are available on correlations between EGFR mutation status or copy number and sorafenib therapy outcomes, and further clinical and mechanistic studies are needed to confirm that EGFR mutations and high copy number are associated with a poor outcome of sorafenib therapy. The trial by Kelly et al. showed that EGFR mutation status did not correlate with any efficacy endpoint (13). More recently, a phase II randomized trial of erlotinib plus/minus sorafenib found an improved PFS in patients with wild-type-EGFR tumors in the combination group (HR, 0.56; 95% CI, 0.32-0.97) (39). A large proportion of the patients in our trial had previously received erlotinib (68%) or bevacizumab (41%). Patients with mutant-EGFR NSCLC acquire resistance to EGFR inhibitors, and 50% of these EGFR mutations are T790M (40, 41). An erlotinib study found a T790M mutation at progression in 58 (62%) of 93 patients with advanced NSCLC (42). Patients with a T790M mutation had a relatively favorable prognosis and more indolent disease progression compared with patients without T790M mutation. Reflecting only wild-type-EGFR NSCLC, our SSS results do not involve the T790M mutation. Other mechanistic hypotheses to explain these findings should be studied with careful attention to cross-talk of signaling pathways activated by exposure to targeted therapies.
Developments and forthcoming results of the validation studies of EGFR mutation status, EGFR FISH status and of the SSS in our ongoing BATTLE-2 trial will hopefully allow demonstrating the potential of sorafenib for becoming an option of personalized therapy in NSCLC patients.
Supplementary Material
Statement of Translational Relevance.
Sorafenib is an oral multi-targeted inhibitor that has shown some clinical activity in patients with non-resectable NSCLC who received at least one line of platinum-based chemotherapy. In our recently reported BATTLE trial, sorafenib was the most efficient drug in this setting. However, the relative contribution of each potential target to sorafenib's antitumor activity is unknown, and no definitive predictive biomarkers of benefit have been reported. In this study, we developed a gene expression signature of sorafenib efficacy in vitro using a large panel of NSCLC cell lines (Sorafenib Sensitivity Signature) and applied the signature in patients included in the BATTLE trial and treated with sorafenib, using gene expression profiles of core needle biopsies collected prospectively at baseline. We show that the in vitro Sorafenib Sensitivity Signature was associated with an improved progression-free survival in patients with EGFR wild-type NSCLC treated with sorafenib.
Acknowledgments
Funding: This research was supported by DoD grant W81XWH-6-1-0303, National Institutes of Health through MD Anderson's Cancer Center Support Grant CA016672, Bayer Healthcare Pharmaceuticals, Montville, NJ, and Onyx Pharmaceuticals, Richmond, CA.
Footnotes
Conflicts of interests: Consultant Advisory Role: GRB (Bayer), ESK (Bayer), RSH (Bayer); Research Funding: GRB (Bayer), ESK (Bayer), RSH (Bayer), JVH (Bayer), HTT (Bayer); other authors reported no conflicts
References
- 1.American Cancer Society Cancer Facts and Figures 2010 [cited; Available from. [Google Scholar]
- 2.Ginsberg R, Vokes E, Rosenzweig K. Non Small Cell Lung Cancer. In: DeVita V, Hellman S, Rosenberg S, editors. Cancer: Principles and Practice of Oncology. 6th ed. Lippincott Williams and Wilkins; Philadelphia: 2001. pp. 925–83. [Google Scholar]
- 3.Schiller JH, Harrington D, Belani CP, Langer C, Sandler A, Krook J, et al. Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med. 2002;346:92–8. doi: 10.1056/NEJMoa011954. [DOI] [PubMed] [Google Scholar]
- 4.Shepherd FA, Dancey J, Ramlau R, Mattson K, Gralla R, O'Rourke M, et al. Prospective randomized trial of docetaxel versus best supportive care in patients with non-small-cell lung cancer previously treated with platinum-based chemotherapy. J Clin Oncol. 2000;18:2095–103. doi: 10.1200/JCO.2000.18.10.2095. [DOI] [PubMed] [Google Scholar]
- 5.Fossella FV, DeVore R, Kerr RN, Crawford J, Natale RR, Dunphy F, et al. Randomized Phase III Trial of Docetaxel Versus Vinorelbine or Ifosfamide in Patients With Advanced Non–Small-Cell Lung Cancer Previously Treated With Platinum-Containing Chemotherapy Regimens. Journal of Clinical Oncology. 2000;18:2354–62. doi: 10.1200/JCO.2000.18.12.2354. [DOI] [PubMed] [Google Scholar]
- 6.Hanna N, Shepherd FA, Fossella FV, Pereira JR, De Marinis F, von Pawel J, et al. Randomized Phase III Trial of Pemetrexed Versus Docetaxel in Patients With Non–Small-Cell Lung Cancer Previously Treated With Chemotherapy. Journal of Clinical Oncology. 2004;22:1589–97. doi: 10.1200/JCO.2004.08.163. [DOI] [PubMed] [Google Scholar]
- 7.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 8.Molina JR, Yang P, Cassivi SD, Schild SE, Adjei AA. Non-small cell lung cancer: epidemiology, risk factors, treatment, and survivorship. Mayo Clin Proc. 2008;83:584–94. doi: 10.4065/83.5.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roberts PJ, Stinchcombe TE, Der CJ, Socinski MA. Personalized medicine in non-small-cell lung cancer: is KRAS a useful marker in selecting patients for epidermal growth factor receptor-targeted therapy? J Clin Oncol. 2010;28:4769–77. doi: 10.1200/JCO.2009.27.4365. [DOI] [PubMed] [Google Scholar]
- 10.Wilhelm SM, Carter C, Tang L, Wilkie D, McNabola A, Rong H, et al. BAY 43-9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res. 2004;64:7099–109. doi: 10.1158/0008-5472.CAN-04-1443. [DOI] [PubMed] [Google Scholar]
- 11.Carter CA, Chen C, Brink C, Vincent P, Maxuitenko YY, Gilbert KS, et al. Sorafenib is efficacious and tolerated in combination with cytotoxic or cytostatic agents in preclinical models of human non-small cell lung carcinoma. Cancer Chemother Pharmacol. 2007;59:183–95. doi: 10.1007/s00280-006-0257-y. [DOI] [PubMed] [Google Scholar]
- 12.Blumenschein GR, Jr., Gatzemeier U, Fossella F, Stewart DJ, Cupit L, Cihon F, et al. Phase II, multicenter, uncontrolled trial of single-agent sorafenib in patients with relapsed or refractory, advanced non-small-cell lung cancer. J Clin Oncol. 2009;27:4274–80. doi: 10.1200/JCO.2009.22.0541. [DOI] [PubMed] [Google Scholar]
- 13.Kelly RJ, Rajan A, Force J, Lopez-Chavez A, Keen C, Cao L, et al. Evaluation of KRAS mutations, angiogenic biomarkers, and DCE-MRI in patients with advanced non-small-cell lung cancer receiving sorafenib. Clin Cancer Res. 2011;17:1190–9. doi: 10.1158/1078-0432.CCR-10-2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smit EF, Dingemans AM, Thunnissen FB, Hochstenbach MM, van Suylen RJ, Postmus PE. Sorafenib in patients with advanced non-small cell lung cancer that harbor K-ras mutations: a brief report. J Thorac Oncol. 2010;5:719–20. doi: 10.1097/JTO.0b013e3181d86ebf. [DOI] [PubMed] [Google Scholar]
- 15.Kim ES, Herbst RS, Wistuba II, Lee JJ, Blumenschein GR, Tsao A, et al. The BATTLE Trial: Personalizing Therapy for Lung Cancer. Cancer Discovery. 2011;1:44–53. doi: 10.1158/2159-8274.CD-10-0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–16. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 17.Lara PN, Jr., Redman MW, Kelly K, Edelman MJ, Williamson SK, Crowley JJ, et al. Disease control rate at 8 weeks predicts clinical benefit in advanced non-small-cell lung cancer: results from Southwest Oncology Group randomized trials. J Clin Oncol. 2008;26:463–7. doi: 10.1200/JCO.2007.13.0344. [DOI] [PubMed] [Google Scholar]
- 18.Fossella FV, DeVore R, Kerr RN, Crawford J, Natale RR, Dunphy F, et al. Randomized phase III trial of docetaxel versus vinorelbine or ifosfamide in patients with advanced non-small-cell lung cancer previously treated with platinum-containing chemotherapy regimens. The TAX 320 Non-Small Cell Lung Cancer Study Group. J Clin Oncol. 2000;18:2354–62. doi: 10.1200/JCO.2000.18.12.2354. [DOI] [PubMed] [Google Scholar]
- 19.Tsao MS, Sakurada A, Cutz JC, Zhu CQ, Kamel-Reid S, Squire J, et al. Erlotinib in lung cancer - molecular and clinical predictors of outcome. N Engl J Med. 2005;353:133–44. doi: 10.1056/NEJMoa050736. [DOI] [PubMed] [Google Scholar]
- 20.Cappuzzo F, Hirsch FR, Rossi E, Bartolini S, Ceresoli GL, Bemis L, et al. Epidermal Growth Factor Receptor Gene and Protein and Gefitinib Sensitivity in Non-Small-Cell Lung Cancer. Journal of the National Cancer Institute. 2005;97:643–55. doi: 10.1093/jnci/dji112. [DOI] [PubMed] [Google Scholar]
- 21.Byers LA, Diao L, Wang J, Saintigny P, Girard L, Peyton M, et al. An epithelial mesenchymal transition gene signature predicts resistance to EGFR and PI3K inhibitors and identifies Axl as a therapeutic target for overcoming EGFR inhibitor resistance. Clin Cancer Res. 2013;19:279–90. doi: 10.1158/1078-0432.CCR-12-1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shepherd FA, Rodrigues Pereira J, Ciuleanu T, Tan EH, Hirsh V, Thongprasert S, et al. Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med. 2005;353:123–32. doi: 10.1056/NEJMoa050753. [DOI] [PubMed] [Google Scholar]
- 23.Kim ES, Hirsh V, Mok T, Socinski MA, Gervais R, Wu YL, et al. Gefitinib versus docetaxel in previously treated non-small-cell lung cancer (INTEREST): a randomised phase III trial. Lancet. 2008;372:1809–18. doi: 10.1016/S0140-6736(08)61758-4. [DOI] [PubMed] [Google Scholar]
- 24.Linardou H, Dahabreh IJ, Kanaloupiti D, Siannis F, Bafaloukos D, Kosmidis P, et al. Assessment of somatic k-RAS mutations as a mechanism associated with resistance to EGFR- targeted agents: a systematic review and meta-analysis of studies in advanced non-small-cell lung cancer and metastatic colorectal cancer. Lancet Oncol. 2008;9:962–72. doi: 10.1016/S1470-2045(08)70206-7. [DOI] [PubMed] [Google Scholar]
- 25.Ihle NT, Byers LA, Kim ES, Saintigny P, Lee JJ, Blumenschein GR, et al. Effect of KRAS oncogene substitutions on protein behavior: implications for signaling and clinical outcome. J Natl Cancer Inst. 2012;104:228–39. doi: 10.1093/jnci/djr523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Paz-Ares L HV, Zhang L, et al. Monotherapy administration of sorafenib in patients with non-small cell lung cancer: Phase III, randomized, double-blind, placebo-controlled MISSION trial.. ESMO Congress; September 29, 2012; 2012. Abstract LBA33. [Google Scholar]
- 27.Mok TSKP-AL, Wu Y-L, et al. Association between tumor EGFR and KRas mutation status and clinical outcomes in NSCLC patients randomized to sorafenib plus best supportive care (BSC) or BSC alone: Subanalysis of the phase III MISSION trial.. 2012 ESMO Congress; September 29, 2012; Abstract LBA9. [Google Scholar]
- 28.Gazdar AF, Girard L, Lockwood WW, Lam WL, Minna JD. Lung cancer cell lines as tools for biomedical discovery and research. Journal of the National Cancer Institute. 2010;102:1310–21. doi: 10.1093/jnci/djq279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ayers M, Symmans WF, Stec J, Damokosh AI, Clark E, Hess K, et al. Gene expression profiles predict complete pathologic response to neoadjuvant paclitaxel and fluorouracil, doxorubicin, and cyclophosphamide chemotherapy in breast cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2004;22:2284–93. doi: 10.1200/JCO.2004.05.166. [DOI] [PubMed] [Google Scholar]
- 30.Coldren CD, Helfrich BA, Witta SE, Sugita M, Lapadat R, Zeng C, et al. Baseline gene expression predicts sensitivity to gefitinib in non-small cell lung cancer cell lines. Mol Cancer Res. 2006;4:521–8. doi: 10.1158/1541-7786.MCR-06-0095. [DOI] [PubMed] [Google Scholar]
- 31.Loboda A, Nebozhyn M, Klinghoffer R, Frazier J, Chastain M, Arthur W, et al. A gene expression signature of RAS pathway dependence predicts response to PI3K and RAS pathway inhibitors and expands the population of RAS pathway activated tumors. BMC Med Genomics. 2010;3:26. doi: 10.1186/1755-8794-3-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Heymach JV, Saintigny P, Kim ES, Byers LA, Lee JJ, Coombes KR, et al. Gene expression signatures predictive of clinical outcome and tumor mutations in refractory NSCLC patients (pts) in the BATTLE trial (Biomarker-integrated Approaches of Targeted Therapy for Lung Cancer Elimination).. n: American Association of Cancer Research 102nd Annual Meeting; Orlando, FL Philadelphia, PA. 2011 Apr 2–6; AACR; 2011. 2011 Abstract nr LB-88. [Google Scholar]
- 33.Iyer R, Fetterly G, Lugade A, Thanavala Y. Sorafenib: a clinical and pharmacologic review. Expert Opin Pharmacother. 2010;11:1943–55. doi: 10.1517/14656566.2010.496453. [DOI] [PubMed] [Google Scholar]
- 34.Daniele G, Corral J, Molife LR, de Bono JS. FGF Receptor Inhibitors: Role in Cancer Therapy. Curr Oncol Rep. 2012;14:111–9. doi: 10.1007/s11912-012-0225-0. [DOI] [PubMed] [Google Scholar]
- 35.Mason JC, Lidington EA, Yarwood H, Lublin DM, Haskard DO. Induction of endothelial cell decay-accelerating factor by vascular endothelial growth factor: a mechanism for cytoprotection against complement-mediated injury during inflammatory angiogenesis. Arthritis Rheum. 2001;44:138–50. doi: 10.1002/1529-0131(200101)44:1<138::AID-ANR18>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 36.Benameur T, Tual-Chalot S, Andriantsitohaina R, Martinez MC. PPARalpha is essential for microparticle-induced differentiation of mouse bone marrow-derived endothelial progenitor cells and angiogenesis. PLoS One. 2010;5:e12392. doi: 10.1371/journal.pone.0012392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Levine L, Lucci JA, 3rd, Pazdrak B, Cheng JZ, Guo YS, Townsend CM, Jr., et al. Bombesin stimulates nuclear factor kappa B activation and expression of proangiogenic factors in prostate cancer cells. Cancer research. 2003;63:3495–502. [PubMed] [Google Scholar]
- 38.Papadimitrakopoulou V WI, Lee JJ, Tsao AS, Kalhor N, Fossella FV, Heymach JV, Alden CM, Gettinger SN, Coombes KR, Saintigny P, Tang X, Duffield E, Boyer J, Davis SE, Powis G, Mauro DJ, Rubin EH, Hong WK, Herbst RS. BATTLE-2: A biomarker-integrated targeted therapy study in previously treated patients with advanced non-small cell lung cancer (NSCLC). J Clin Oncol. 2013;31(suppl) doi: 10.1200/JCO.2015.66.0084. abstr TPS8118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Spigel DR, Burris HA, 3rd, Greco FA, Shipley DL, Friedman EK, Waterhouse DM, et al. Randomized, Double-Blind, Placebo-Controlled, Phase II Trial of Sorafenib and Erlotinib or Erlotinib Alone in Previously Treated Advanced Non-Small-Cell Lung Cancer. J Clin Oncol. 2011 doi: 10.1200/JCO.2010.30.7678. [DOI] [PubMed] [Google Scholar]
- 40.Kobayashi S, Boggon TJ, Dayaram T, Janne PA, Kocher O, Meyerson M, et al. EGFR mutation and resistance of non-small-cell lung cancer to gefitinib. N Engl J Med. 2005;352:786–92. doi: 10.1056/NEJMoa044238. [DOI] [PubMed] [Google Scholar]
- 41.Pao W, Miller VA, Politi KA, Riely GJ, Somwar R, Zakowski MF, et al. Acquired resistance of lung adenocarcinomas to gefitinib or erlotinib is associated with a second mutation in the EGFR kinase domain. PLoS Med. 2005;2:e73. doi: 10.1371/journal.pmed.0020073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Oxnard GR, Arcila ME, Sima CS, Riely GJ, Chmielecki J, Kris MG, et al. Acquired Resistance to EGFR Tyrosine Kinase Inhibitors in EGFR-Mutant Lung Cancer: Distinct Natural History of Patients with Tumors Harboring the T790M Mutation. Clin Cancer Res. 2011;17:1616–22. doi: 10.1158/1078-0432.CCR-10-2692. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.