Abstract
Several classes of antihypertensive agents have been in clinical use, including diuretics, α-blockers, β-blockers, angiotensin converting enzyme (ACE) inhibitors, angiotensin II type 1 receptor blockers (ARB), and organic calcium channel blockers (CCBs). All these drugs are being currently used in the treatment of Hypertension & various disease conditions of the heart either alone or in combination. Cilnidipine is a new antihypertensive drug distinguished from other L-type Ca2+ channel blockers or even other antihypertensives, which will be useful for selection of antihypertensive drugs according to the pathophysiological condition of a patient.
1. Hypertension or high blood pressure
Hypertension is one of the most important risk factors for cardiovascular diseases, including ischemic and haemorrhagic stroke, dementia, ischemic heart disease, heart failure, vision loss, and kidney failure. Hypertension is a multifactorial and multifaceted disease in which elevated blood pressure is only one sign of multiple underlying physiological abnormalities.1–3
Hypertension or high blood pressure is a leading cause of death. The condition is often called a “silent killer” because its symptoms can go undetected until damage to the body has occurred. Because of this, it is one of the most significantly under-diagnosed and under-treated medical conditions all over the world. High blood pressure is usually a lifelong condition. High blood pressure can occur at any age but is particularly prevalent in people with a family history of high blood pressure, people who are overweight or obese, people with diabetes, and heavy drinkers.4,5
2. Antihypertensive drugs
Several classes of antihypertensive agents have been in clinical use, including diuretics, α-blockers, β-blockers, angiotensin converting enzyme (ACE) inhibitors, angiotensin II type 1 receptor blockers (ARB), and organic calcium channel blockers (CCBs). All these drugs are being currently used in the treatment of hypertension and various disease conditions of the heart either alone or in combination. They have specific indications, therapeutic efficacies and limitations for the treatment of an individual patient. A patient has to consume these medicines for lifetime accommodating and adjusting to all their side effects.6
Clinically, an important goal of antihypertensive therapy is to prevent the occurrence of cardiovascular complications. It has been suggested that increased sympathetic activity is the common link among many of the “non pressure-related” coronary risk factors in hypertension. More importantly hyperactivity of sympathetic nervous system often triggers hypertensive complications including ischemic heart disease, strokes, heart failure, and renal failure which show the importance of controlling sympathetic nerve activity in clinical practice. Sympathetic nerve activity is one of the major culprits implicated in the onset of hypertension. Julius7 reported that the occurrence of a hyperkinetic state, that is, one in which both cardiac output and heart rate are elevated, was five times more frequently observed in patients with borderline hypertension than in the normotensive population.
3. Calcium channel blockers (CCBs)
Calcium channel blockers (CCBs), comprising two subclasses – dihydropyridines and non-dihydropyridines – have been for many years one of the mainstays of hypertension therapy. Calcium channel blockers (CCBs) share a common mechanism of action. However, the manner in which they exert their pharmacological effects is different between subclasses. Dihydropyridine (DHP) CCBs tend to be more potent vasodilators than non-dihydropyridine (non-DHP) agents, whereas the latter have more marked negative inotropic effects. Both subclasses have a similar capacity to lower BP; however, non-DHPs appear to offer potential advantages in the management of patients with chronic kidney disease and diabetic nephropathy.8,9
Dihydropyridines are among the most widely used drugs for the management of cardiovascular disease. Introduced in the 1960s, dihydropyridines have undergone several changes to optimize their efficacy and safety. Four generations of dihydropyridines are now available. The first-generation nicardipine and nifedipine have proven efficacy against hypertension. However, because of their short duration and rapid onset of vasodilator action, these drugs were more likely to be associated with adverse effects. The new second generation slow-release and short-acting preparations like benidipine, and efonidipine allowed better control of the therapeutic effect and a reduction in some adverse effects. The third-generation dihydropyridines, amlodipine and azelnidipine exhibit more stable pharmacokinetics, are less cardio-selective and, consequently, well tolerated in patients with heart failure. The fourth-generation highly lipophilic dihydropyridines, lercanidipine and lacidipine are now available which provide a real degree of therapeutic comfort in terms of stable activity, a reduction in adverse effects and a broad therapeutic spectrum, especially in myocardial ischemia and potentially in congestive heart failure.10
Ca2+ channel blockers have been categorized according to selectivity for the voltage-dependent Ca2+ channels in vascular smooth muscle against those in cardiac tissue,11 chemical class, and binding affinity to receptors in Ca2+ channels, chemical structure, or lipophilicities.12 In 1996, a useful classification was proposed to divide Ca2+ channel blockers into three groups – first, second, and third generation, which were fundamentally based on the effects on Ca2+ channel receptor-binding properties, tissue selectivity, and pharmacokinetic profile.13
4. Calcium channels and CCBs
Among antihypertensive drugs, calcium channel blockers, which inhibit L-type voltage-gated calcium channels, are potent vasodilators, and have been used as a first- or second-line drug. Dihydropyridine-class calcium channel blockers are categorized into three generations according to the length of activity, and long-acting calcium channel blockers cause less activation of sympathetic nervous system, and are reported to offer beneficial action compared with short-action agents. Furthermore, novel types of calcium channel blockers have been developed that possess the blocking action on other calcium channel subtypes (T- and N-type), and exert agent-specific action apart from their class effects, such as the effects on heart rate and renin/aldosterone release. These additional benefits conferred by T/N-type calcium channel blockade are anticipated to provide organ protective actions in the treatment of hypertension, in addition to the blood pressure-lowering effect of L-type calcium channel blockade. In conclusion, novel calcium channel blockers with sustained activity and T/N-type calcium channel-blocking action could provide more beneficial effects than classical blockers, and may expand the clinical utility of these agents.14
The voltage-gated calcium channel consists of 4 subunits, α1, α2-δ, β and γ. An α1 subunit is the dominant component of the calcium channels and constitutes pore structure for ion conduction. Ten different α1 subunits have been reported and each of them has specific distribution and ion conductance of its channels. These distinct subunits characterize the channel properties of L-, N-, T-, P-, Q- and R-type calcium channels.15,16 Of these channels, L-type calcium channels are the main targets of the CCB. Based on the chemical structure, CCBs are categorized into 3 subgroups; benzothiazepines (e.g., diltiazem and clenazem), phenylalkylamines (e.g., verapamil and gallopamil) and dihydropyridines (e.g., nifedipine, nicardipine, felodipine, amlodipine, aranidipine, azelnidipine, cilnidipine, efonidipine, manidipine and nilvadipine). The differences in chemical structures would provide heterogeneity in the action of these agents. All CCBs block the calcium influx by binding to the α1 subunit,17 and inhibit cell excitability. Although the CCB inhibits calcium currents through L-type calcium channels, some CCBs possess the ability to block other calcium channels (Fig. 1).
Fig. 1.
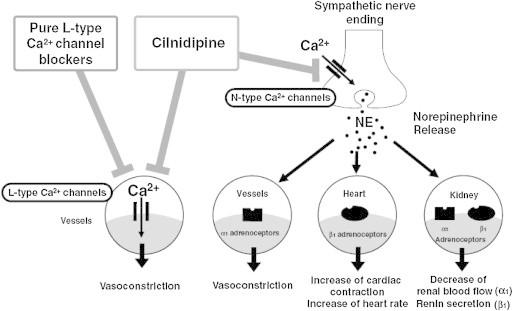
Diagrammatic representation of L/N dual action of cilnidipine.
Cilnidipine has been extensively studied by researchers in its preclinical and clinical development phases. Renoprotective, neuroprotective and cardioprotective effects of cilnidipine have been demonstrated in clinical practice or animal examinations. It is noticed that cilnidipine may have pleiotropic effects besides N-type Ca2+ channel-blocking action. Therefore, the inhibition of N-type Ca2+ channels may provide a new strategy for the treatment of cardiovascular diseases. This article reviews the current understanding of the pharmacological profile and clinical utility of cilnidipine as a unique antihypertensive drug.
Cilnidipine is a recently developed CCB, and possesses both L- and N-type calcium channels blocking activity. Since N-type calcium is distributed along the nerve and in the brain, cilnidipine is anticipated to exert specific action on nerve activity, such as inhibition of the sympathetic nervous system.18 Uneyama et al demonstrated that submicro molecular concentrations of cilnidipine effectively suppressed N-type Ca2+ channel currents in isolated sympathetic neurons.19
They further compared the inhibitory effect of various dihydropyridines on cardiac L-type Ca2+ channels in isolated ventricular myocytes with that on N-type Ca2+ channels in superior cervical ganglion neurons obtained from Wistar rats.20 In that study, all dihydropyridines, except cilnidipine, showed a small inhibitory effect at a concentration of 1 μM. Furthermore, it was noted that the selectivity for L-type/N-type of Ca2+ channels differed markedly among the compounds tested, where nifedipine showed high selectivity for L-type Ca2+ channels and cilnidipine blocked both L- and N-type of Ca2+ channels. The N-type channel-blocking action of cilnidipine has also been confirmed by Takahara A et al in IMR-32 human neuroblastoma cells.21 Cilnidipine inhibits N-type Ca2+ channels more potently than other Ca2+ channel blockers and several in vitro studies conducted by Nap A et al (2004) have demonstrated that cilnidipine attenuates norepinephrine release from sympathetic nerve endings.22,23 Furthermore, such effects have been observed in in vivo experiments using anesthetized rats24 and dogs.25 The cardioprotective action of cilnidipine has been analyzed in a rabbit model of myocardial infarction, in which cilnidipine decreased the myocardial interstitial norepinephrine levels during ischemia and reperfusion periods, leading to reduction of the myocardial infarct size and incidence of ventricular premature beats.26 Furthermore, in vivo experimental data have suggested that cilnidipine shows antianginal effects in the experimental model of vasopressin-induced angina and improvement of the ventricular repolarization abnormality in the canine model of long QT syndrome.27,28
Sympatholytic profiles of cilnidipine observed in both in vitro and in vivo, are also observed in clinical practice.19,24 In clinical studies, conducted by Nagahama S et al (2007) and Iimura O et al (1993) the antihypertensive effect of cilnidipine has been demonstrated in hypertensive patients,29 and also in patients with severe hypertension.30 In a study conducted in 2920 hypertensive patients, treatment with cilnidipine and angiotensin receptor blocker showed significant reductions in heart rate, particularly in those with a higher baseline heart rate ≥75 beats/min, whereas there were few adverse reactions associated with central nervous functions.29 Hoshide et al31 demonstrated that the reductions in heart rate were significantly greater in the cilnidipine group than the amlodipine group in a 24-h ambulatory blood pressure monitoring study with hypertensive patients. Furthermore, cilnidipine has been clinically demonstrated to be effective for morning hypertension and white-coat hypertension, which is closely associated with sympathetic nerve activation.32,33 The CANDLE trial34 and other clinical studies have demonstrated that cilnidipine improves left ventricular function.35
Hypertension is one of the most important risk factors for the progression of renal disease.36,37 In the follow-up study of the multiple risk factor intervention trial (MRFIT), a strong, graded relation between blood pressure and end-stage renal disease was identified.38 Moreover, chronic renal dysfunction,39 proteinuria or albuminuria40 are independent risk factors for cerebrovascular and cardiovascular diseases. Thus, the treatment of hypertension constitutes a crucial strategy to minimize the development of renal disease and to reduce the risk of cardiovascular events.
In clinical studies, Rose and Ikebukoro41 demonstrated that cilnidipine significantly decreased urinary albumin excretion without affecting serum creatinine concentration in hypertensive patients, which is comparable to the angiotensin converting enzyme inhibitor benazepril. Other studies conducted by Kojima S (2004) and Tsuchihashi T et al (2005) have shown that the renal protective effect of cilnidipine was greater than pure L-type Ca2+ channel blockers.42,43 Furthermore, the combination of cilnidipine and valsartan was shown to decrease the albumin/creatine ratio more markedly than valsartan alone.44 Recently, the multicenter, open labeled and randomized trial of Cilnidipine versus Amlodipine Randomized Trial for Evaluation in Renal disease (CARTER) has shown that cilnidipine is superior to amlodipine in preventing the progression of proteinuria in patients with hypertension and chronic renal disease when coupled with a renin–angiotensin system inhibitor.45 Recently, prevalence of cardiovascular disease and cardiovascular mortality have been suggested to be closely associated with renal function; namely, cardio–renal connection.46 Thus, the renal protective effects of cilnidipine may secondarily contribute to cardioprotection.
Takashi Masuda, Misao N. Ogura, Tatsumi Moriya, et al (2011) have demonstrated the beneficial effects of L- and N-type calcium channel blocker on glucose and lipid metabolism and renal function in patients with hypertension and type II diabetes mellitus.47
A recent study by Fan et al (2011) indicates that cilnidipine relaxes human arteries through Ca2+ channel antagonism and increases production of nitric oxide by enhancement of endothelial nitric oxide synthase in the human internal thoracic artery.48
Ranjan Shetty, G Vivek et al (2013) have conducted a study in 27 patients of essential hypertension with amlodipine-induced ankle edema and concluded that therapy with cilnidipine resulted in complete resolution of amlodipine-induced edema in all the cases without significant worsening of hypertension or tachycardia. Cilnidipine is an acceptable alternative antihypertensive for patients with amlodipine-induced edema.49
Xu Guo-Liang et al (2012) conducted a meta-analysis of the efficacy and safety of cilnidipine in Chinese patients with mild to moderate essential hypertension, of 11 clinical trials and concluded that cilnidipine is a useful agent to treat mild to moderate essential hypertension.50
5. Conclusion
Cilnidipine is a promising 4th generation Ca2+ channel blocker with a rational pharmacological profile; i.e. dual L/N-type Ca2+ channel-blocking action. The blockade of N-type Ca2+ channels effectively suppresses neurohumoral regulation in the cardiovascular system, including sympathetic nervous system and renin–angiotensin–aldosterone system. Thus, cilnidipine is expected to be favorable for various types of complications of hypertension. The currently described information suggests that cilnidipine is a new type of antihypertensive drug distinguished from other L-type Ca2+ channel blockers or even other antihypertensives, which will be useful for selection of antihypertensive drugs according to the pathophysiological condition of a patient.
Conflicts of interest
All authors have none to declare.
References
- 1.Page I.H. The mosaic theory of arterial hypertension–its interpretation. Perspect Biol Med. 1967;10:325–333. doi: 10.1353/pbm.1967.0031. [DOI] [PubMed] [Google Scholar]
- 2.Frolich B.D. The first Irvine H. Page lecture. The mosaic of hypertension: past, present & future. J Hypertens Suppl. 1988;6:S2–11. doi: 10.1097/00004872-198812040-00002. [DOI] [PubMed] [Google Scholar]
- 3.Frolich E.D. Mechanisms contributing to high blood pressure. Ann Intern Med. 1983;98:709–714. doi: 10.7326/0003-4819-98-5-709. [DOI] [PubMed] [Google Scholar]
- 4.Chatzikyrkoyu C., Menne J., Haller H. Hypertension. Med Klin(Munich) 2009 Aug;104:614–621. doi: 10.1007/s00063-009-1133-4. [DOI] [PubMed] [Google Scholar]
- 5.Using Calcium Channel Blockers to Treat High Blood Pressure and Heart Diseases. 2011 March. pp. 01–20.ConsumerReportsHealth.org [Google Scholar]
- 6.Chobanian A.V., Bakris G.L., Black H.R. The seventh report of the Joint National Committee on prevention, detection, evaluation & treatment of high blood pressure: the JNC 7 report. JAMA. 2003;289:2560–2572. doi: 10.1001/jama.289.19.2560. [Pub Med 12748199] [DOI] [PubMed] [Google Scholar]
- 7.Julius S., Schork N., Schork A. Sympathetic hyperactivity in early stages of hypertension: the Ann Arbor data set. J Cardiovasc Pharmacol. 1988;12:S121–129. [PubMed] [Google Scholar]
- 8.Weir M.R. Calcium channel blockers: differences between subclasses. Am J Cardiovasc Drugs. 2007;7:5–15. doi: 10.2165/00129784-200707001-00003. [DOI] [PubMed] [Google Scholar]
- 9.Frishman W.H. Calcium channel blockers: differences between subclasses. Am J Cardiovasc Drugs. 2007;7:17–23. doi: 10.2165/00129784-200707001-00003. [DOI] [PubMed] [Google Scholar]
- 10.Aoum K., Berdeaux A. Dihydropyridines from the first to fourth generation: better effects and safety. Therapie. 2003 Jul–Aug;58:333–339. doi: 10.2515/therapie:2003051. [DOI] [PubMed] [Google Scholar]
- 11.Singh B.N. The mechanism of action of calcium antagonists relative to their clinical applications. Br J Clin Pharmacol. 1986;21:109S–121S. doi: 10.1111/j.1365-2125.1986.tb02860.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Spedding M., Paoletti R. Classification of calcium channels & the sites of action of drugs modifying channel function. Pharmacol Rev. 1992;44:363–376. [PubMed] [Google Scholar]
- 13.Toyooka T., Nayler W.G. Third generation calcium entry blockers. Blood Press. 1996;5:206–208. doi: 10.3109/08037059609079672. [DOI] [PubMed] [Google Scholar]
- 14.Ozawa Yuri, Hayashi Koichi, Kobori Hiroyuki. New generation calcium channel blockers in hypertensive treatment. Curr Hypertens Rev. 2006 May 1;2:103–111. doi: 10.2174/157340206776877370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ertel E.A., Campbell K.P., Harpold M.M. Nomenclature of voltage gated calcium channels. Neuron. 2000;25:533–535. doi: 10.1016/s0896-6273(00)81057-0. [Pub Med 10774722] [DOI] [PubMed] [Google Scholar]
- 16.Miljanich G.P., Ramchandran J. Antagonists of neuronal calcium channels: structure, function & therapeutic implications. Annu Rev Pharmacol Toxicol. 1995;35:707–734. doi: 10.1146/annurev.pa.35.040195.003423. [Pub Med 7598513] [DOI] [PubMed] [Google Scholar]
- 17.Hofmann F., Lacinova L., Klugbauer N. Voltage dependent calcium channels: from structure to function. Rev Physiol Biochem Pharmacol. 1999;139:33–87. doi: 10.1007/BFb0033648. [Pub Med 10453692] [DOI] [PubMed] [Google Scholar]
- 18.Fujii S., Kameyama K., Hosno M., Hayashi Y., Kitamura K. Effect of cilnidipine, a novel dihydropyridine calcium channel antagonist, on N type calcium channel in rat dorsal root ganglion neurons. J Pharmacol Exp Ther. 1997;280:1184–1191. [PubMed] [Google Scholar]
- 19.Uneyama H., Takahara A., Dohmoto H., Yashimoto R., Inoue K., Akaike N. Blockade of N type calcium current by cilnidipine (FRC 8653) in acutely dissociated rat sympathetic neurones. Br J Pharmacol. 1997;122:37–42. doi: 10.1038/sj.bjp.0701342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Uneyama H., Uchida H., Konda T., Yashimoto R., Akaike N. Selectivity of dihydropyridines for cardiac L type & sympathetic N type calcium channels. Eur J Pharmacol. 1999;373:93–100. doi: 10.1016/s0014-2999(99)00237-x. [DOI] [PubMed] [Google Scholar]
- 21.Takahara A., Fujita S., Moki K. Neuronal calcium channel blocking action of antihypertensive drug, cilnidipine in IMR-32 human neuroblastoma cells. Hypertens Res. 2003;26:743–747. doi: 10.1291/hypres.26.743. [DOI] [PubMed] [Google Scholar]
- 22.Nap A., Mathy M.J., Balt J.C., Pfaffendorf M., Van Zwieten P.A. The evaluation of N type channel blocking properties of cilnidipine and other voltage dependent calcium antagonists. Fundam Clin Pharmacol. 2004;18:309–319. doi: 10.1111/j.1472-8206.2004.00236.x. [DOI] [PubMed] [Google Scholar]
- 23.Hosono M., Fujii S., Hiruma T. Inhibitory effect of cilnidipine on vascular sympathetic neurotransmission & subsequent vasoconstriction in spontaneously hypertensive rats. Jpn J Pharmacol. 1995;69:127–134. doi: 10.1254/jjp.69.127. [DOI] [PubMed] [Google Scholar]
- 24.Takahara A., Koganei H., Takeda T., Iwata S. Antisympathetic & hemodynamic property of a dual L/N type calcium channel blocker cilnidipine in rats. Eur J Pharmacol. 2002;434:43–47. doi: 10.1016/s0014-2999(01)01521-7. [DOI] [PubMed] [Google Scholar]
- 25.Nagayama T., Yoshida M., Suzukikusaba M., Hisha H., Kimura T., Satoh S. Effect of cilnidipine, a novel dihydropyridine calcium channel blocker, on adrenal catecholamine secretion in anesthetized dogs. J Cardiovasc Pharmacol. 1998;32:479–484. doi: 10.1097/00005344-199809000-00020. [DOI] [PubMed] [Google Scholar]
- 26.Nagai H., Minatoguchi S., Chen X.H. Cilnidipine, an N+L type dihydropyridine calcium channel blocker, suppress the occurrence of ischemia/reperfusion arrhythmia in rabbit model of myocardial infarction. Hypertens Res. 2005;28:361–368. doi: 10.1291/hypres.28.361. [DOI] [PubMed] [Google Scholar]
- 27.Takahara A., Sugiyama A., Hashimoto K. Long term blockade of N type calcium channels reversed the ventricular electrical remodelling in the canine model of long QT syndrome. Circ J. 2006;70:575. [abstract] [Google Scholar]
- 28.Saitoh M., Sugiyam A., Nakamura Y., Hashimoto K. Antianginal effects of L type N type calcium channel blocker cilnidipine assessed using vasopressin induced experimental angina model in rats. J Pharmacol Sci. 2003;91 152 P (abstract) [Google Scholar]
- 29.Nagahama S., Norimatsu T., Maki T., Yasuda M., Tanaka S. The effect of combination therapy with L/N type calcium channel blocker cilnidipine, & an angiotensin II receptor blocker on blood pressure & heart rate in Japanese hypertensive patients: an observational study conducted in Japan. Hypertens Res. 2007;30:815–822. doi: 10.1291/hypres.30.815. [DOI] [PubMed] [Google Scholar]
- 30.Iimura O., Ishi M., Inagaki Y. Study of FRC-8653 (cilnidipine) in patients with severe hypertension. Jpn Pharmacol Ther. 1993;21:S155–S170. [Google Scholar]
- 31.Hoshide S., Kario K., Ishikawa J., Iguchi K., Shimada K. Comparison of the effects of cilnidipine and amlodipine on ambulatory blood pressure. Hypertens Res. 2005;28:1003–1008. doi: 10.1291/hypres.28.1003. [DOI] [PubMed] [Google Scholar]
- 32.Ashizawa N., Seto S., Shibata Y., Yano K. Bedtime administration of cilnidipine controls morning hypertension. Int Heart J. 2007;48:597–603. doi: 10.1536/ihj.48.597. [DOI] [PubMed] [Google Scholar]
- 33.Yamagishi T. Beneficial effect of cilnidipine on morning hypertension & white coat effect in patients with essential hypertension. Hypertens Res. 2006;29:339–344. doi: 10.1291/hypres.29.339. [DOI] [PubMed] [Google Scholar]
- 34.Chung W.J., Oh P.C., Ahn T.H. Effects of cilnidipine versus atenolol on left ventricular diastolic function and hypertrophy in essential hypertension – CANDLE trial. J Hypertens. 2008;26:459. [Google Scholar]
- 35.Ma Z.Y., Li L., Zhong X.Z. Cilnidipine improves left ventricular midwall function independently of blood pressure changes in Chinese patients with hypertension. J Cardiovasc Pharmacol. 2007;49:33–38. doi: 10.1097/FJC.0b013e31802bfdee. [DOI] [PubMed] [Google Scholar]
- 36.Domanski M., Mitchell G., Pfeffer M. Pulse pressure & cardiovascular disease related mortality: follow up study of the multiple risk factor intervention trial (MRFIT) JAMA. 2002;287:2677–2683. doi: 10.1001/jama.287.20.2677. [Pub Med 12020303] [DOI] [PubMed] [Google Scholar]
- 37.Vupputuri S., Batuman V., Muntner P. Effect of blood pressure on early decline in kidney function among hypertensive men. Hypertension. 2003;42:1144–1149. doi: 10.1161/01.HYP.0000101695.56635.31. [Pub Med 14597644] [DOI] [PubMed] [Google Scholar]
- 38.Klag M.J., Whelton P.K., Randall B.L. Blood pressure and end stage disease in men. N Engl J Med. 1996;334:13–18. doi: 10.1056/NEJM199601043340103. [Pub Med 7494564] [DOI] [PubMed] [Google Scholar]
- 39.Manjunath G., Tighiouart H., Ibrahim H. Level of kidney function as a risk factor for atherosclerotic cardiovascular outcomes in the community. J Am Coll Cardiol. 2003;41:47–55. doi: 10.1016/s0735-1097(02)02663-3. [Pub Med 12570944] [DOI] [PubMed] [Google Scholar]
- 40.Hillege H l, Fidler V., Diercks G.F. Urinary albumin excretion predicts cardiovascular & non cardiovascular mortality in general population. Circulation. 2002;106:1777–1782. doi: 10.1161/01.cir.0000031732.78052.81. [Pub Med 12356629] [DOI] [PubMed] [Google Scholar]
- 41.Rose G.W., Ikebukoro H. Cilnidipine as effective as benazepril for control of blood pressure and proteinuria in hypertensive patients with benign nephrosclerosis. Hypertens Res. 2001;24:377–383. doi: 10.1291/hypres.24.377. [DOI] [PubMed] [Google Scholar]
- 42.Kojima S., Shida M., Yokoyama H. Comparison between cilnidipine and amlodipine besilate with respect to proteinuria in hypertensive patients with renal diseases. Hypertens Res. 2004;27:379–385. doi: 10.1291/hypres.27.379. [DOI] [PubMed] [Google Scholar]
- 43.Tsuchihashi T., Ueno M., Tominaga M. Antiproteinuric effect of N type calcium channel blocker, cilnidipine. Clin Exp Hypertens. 2005;27:583–591. doi: 10.1080/10641960500298558. [DOI] [PubMed] [Google Scholar]
- 44.Katayama K., Nomura S., Ishikawa H., Murata T., Koyabu S., Nakano T. Comparison between valsartan and valsartan plus cilnidipine in type II diabetics with normo- and microalbuminuria. Kidney Int. 2006;70:151–156. doi: 10.1038/sj.ki.5000349. [DOI] [PubMed] [Google Scholar]
- 45.Fujita T., Ando K., Nishimura H. For cilnidipine versus amlodipine randomised trial for evaluation of renal diseases(CARTER) study investigators. Antiproteinuric effect of calcium channel blocker cilnidipine added to rennin- angiotensin inhibition in hypertensive patients with chronic renal disease. Kidney Int. 2007;72:1543–1549. doi: 10.1038/sj.ki.5002623. [DOI] [PubMed] [Google Scholar]
- 46.Go A.S., Chertow G.M., Fan D., McCulloch C.E., Hsu C.Y. Chronic kidney disease and risks of death, cardiovascular events and hospitalization. N Engl J Med. 2004;351:1296–1305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 47.Takashi M., Misao O., Tatsumi M. Beneficial effects of L & N type calcium channel blocker on glucose and lipid metabolism & renal function in patients with hypertension and type II diabetes mellitus. Cardiovasc Ther. 2011;29:46–53. doi: 10.1111/j.1755-5922.2009.00126.x. @ 2010 Blackwell Publishing Ltd. [DOI] [PubMed] [Google Scholar]
- 48.Fan L. Dual actions of cilnidipine in human internal thoracic artery: inhibition of calcium channel & enhancement of endothelial nitric oxide synthase. J Thorac Cardiovasc Surg. April 2011;141:1063–1069. doi: 10.1016/j.jtcvs.2010.01.048. [DOI] [PubMed] [Google Scholar]
- 49.Shetty Ranjan, Vivek G. Excellent tolerance to cilnidipine in hypertensives with amlodipine induced edema. N Am J Med Sci. 2013 Jan;5:47–50. doi: 10.4103/1947-2714.106203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Guo-Liang Xu, Hui Xu, Hai-Di Wu, Ling Quin. A meta analysis of the efficacy & safety of cilnidipine in Chinese patients with mild to moderate essential hypertension. Afr J Pharm Pharmacol. 2012;6:2393–2399. [Google Scholar]


