Abstract
Infants with congenital heart disease (CHD) have altered brain development. We characterized cortical folding, a critical part of brain development, in CHD infants and demonstrated an overall decrease in cortical surface area and cortical folding with regional alterations in the right lateral sulcus and left orbitofrontal region, cingulate region, and central sulcus. These abnormalities were present prior to surgery.
Keywords: Brain, magnetic resonance imaging, neonate, cardiac
Infants with congenital heart disease (CHD) requiring surgery in early infancy are at increased risk for impaired neurodevelopment. Magnetic resonance imaging (MRI) has demonstrated alterations in brain development and growth in this population [1, 2]. Recent advances in MRI techniques have improved characterization of brain development in the newborn and may provide insights into potential mechanisms of adverse neurological outcomes. One such technique is surface-based analysis, which depicts cortical folding and sulcation, a vital part of brain development. Measures of cortical growth appear to be predictive of neurocognitive outcome in later childhood [3], and preliminary data in a small number of infants with CHD revealed less curvedness and concavity within the operculum, with subtle differences existing between infants with hypoplastic left heart syndrome and transposition of the great arteries [4]. Surface-based analysis of the fetus has also suggested delayed cortical development across multiple regions of the brain in infants with hypoplastic left heart syndrome [5]. The aim of this study was to quantify global brain cortical folding and regional sulcal depth differences in infants with CHD prior to surgery. The hypothesis was that infants with CHD would exhibit reduced cortical folding compared with healthy, term-born infants.
Methods
Fifteen term infants with CHD requiring surgery in early infancy who were part of a larger prospective cohort from Starship Children’s Hospital in Auckland, NZ were included in the analysis. Control infants included twelve healthy term-born infants from Washington University in St. Louis. Informed consent was obtained from all parents, and the study was approved by the local ethics committees at both hospitals.
MRI scans were performed as previously described [6]. The T2-weighted image acquisitions were used in this analysis. For infants with CHD, coronal (2-mm slice thickness) and transverse (3-mm slice thickness) T2-weighted images were acquired. The coronal and transverse T2-weighted images were co-registered, averaged, and resampled to 1-mm isotropic spatial resolution. The control data were acquired at 1-mm isotropic resolution. Post-processing was the same for both the CHD and control population, allowing for comparison between the groups. Post-processing analysis was undertaken as described by Hill et al [7]. The images were aligned along the anterior and posterior commissures and cropped into left and right hemispheres. A semi-automated algorithm, LIGASE, was applied and segmentations were manually edited using CARET software (http://brainvis.wustl.edu) [8] to generate three-dimensional cortical mid-thickness surface reconstructions. Cortical surface area (CSA) was calculated from the cortical mid-thickness surface. A cerebral hull surface was then generated that runs along the margins of the gyri and does not dip into the sulci. The gyrification index (GI), the ratio of the cortical mid-thickness surface area to the cerebral hull surface area, was calculated for each hemisphere. Surfaces were registered to the PALS-term 12 version 2 atlas by using the “Core Six” landmarks: the central sulcus, the Sylvian fissure, the anterior portion of the superior temporal gyrus, the calcarine sulcus, and the dorsal and ventral portions of the medial wall, as previously described [7, 9]. All image processing was reviewed by a single rater (DA).
Statistical Analyses
Statistical analysis was conducted using SPSS version 19.0. Baseline characteristics were compared using a two-sample t-test for continuous variables and a Fisher’s exact test for comparing the proportion of males in each group. MRI measures of CSA and GI were analyzed using a two-sample t-test. Although there was not a difference in head circumference between the groups, brain size may be affected by head size and therefore, head circumference was controlled for in all analyses. An analysis of covariance was performed to control for head circumference. CSA and GI were the dependent variables, group (CHD vs. control) was a fixed factor, and head circumference was a covariate. Alpha of <0.05 was used as the level of significance for this portion of the analysis. Sulcal depth maps were computed for each hemisphere, where depth is the Euclidean distance between each vertex on the cortical mid-thickness surface and the nearest vertex on the cerebral hull surface [9]. Then a t-test was performed using methods similar to Hill et al [7], except that we used a two-sample t-test, rather than a paired t-test. Alpha of <0.025 per hemisphere was used as the level for significance for multiple comparisons correction.
Results
Fifteen infants with CHD were included in this study. Five infants had transposition of the great arteries, four had right-sided lesions requiring a Blalock-Taussig shunt, five had hypoplastic left heart syndrome requiring a Norwood procedure, and one infant had transposition of the great arteries with tricuspid atresia and interrupted aortic arch and underwent a Norwood procedure. Baseline characteristics between control and CHD infants were similar (Table). The CHD infants had a MRI an average of 5 days (range 2–9 days) after birth.
Table 1.
Baseline characteristics and cortical folding measures.
| Controls (n=12) | CHD (n=15) | Unadjusted p value |
Adjusted p value* |
|
|---|---|---|---|---|
| Baseline Characteristics | ||||
| Gestational Age, wks: mean (SD) | 39.4 (0.91) | 39.1 (1.3) | 0.43 | - |
| Birth weight, kg: mean (SD) | 3.50 (0.38) | 3.33 (0.68) | 0.45 | - |
| Head circumference, cm: mean (SD) | 34.2 (1.1) | 34.8 (1.4) | 0.17 | - |
| Sex, male: n (%) | 7 (58) | 5 (33) | 0.26 | - |
| Ethnicity: | 0.002 | - | ||
| Caucasion: n (%) | 5 (42) | 8 (53) | ||
| African American: n (%) | 7 (58) | 0 | ||
| Maori: n (%) | 0 | 5 (33) | ||
| Other | 0 | 2 (14) | ||
| MRI measures | ||||
| Left CSA, cm2 mean (95% CI) | 316 (301–330) | 279 (266–292) | 0.06 | 0.001 |
| Right CSA, cm2 mean (95% CI) | 322 (306–337) | 287 (274–301) | 0.10 | 0.002 |
| Left GI: mean (95% CI) | 2.06 (2.00–2.11) | 1.88 (1.83–1.92) | 0.001 | <0.001 |
| Right GI: mean (95% CI) | 2.10 (2.03–2.16) | 1.93 (1.88–1.99) | 0.008 | 0.001 |
adjusted for head circumference at birth for CSA and GI using analysis of covariance. The mean (95% CI) values displayed in each MRI measure represent the values for the adjusted model
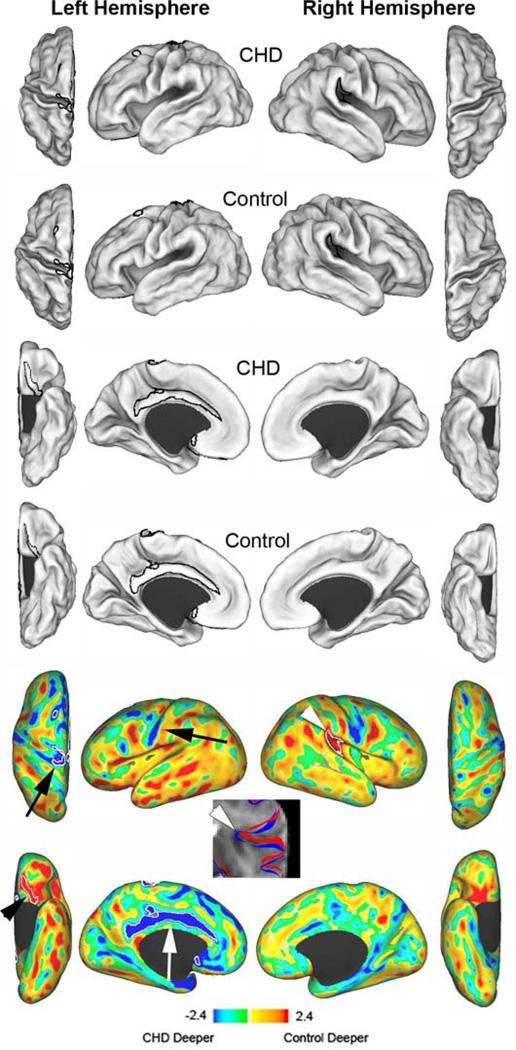
After controlling for head circumference, CHD infants had reduced CSA and GI compared with control infants for both the left and right hemispheres (Table). Individual cortical mid-thickness reconstructions are displayed in Figure 1. Regional sulcal depth analyses (Figure 2) demonstrated differences in the left hemisphere in the orbitofrontal region, superior portion of the central sulcus, and the cingulate gyrus. CHD infants had less sulcation in the orbitofrontal region, specifically within the olfactory sulcus. Regions in the dorsal prefrontal sulcus and the superior temporal sulcus showed a trend towards differences, with the sulcus for control infants deeper than CHD infants. For the right hemisphere, CHD infants displayed less sulcation in the posterior ascending limb of the lateral sulcus. In contrast, there were two regions that appeared deeper in CHD infants due to a simpler, broader cortical surface - the cingulate gyrus and the superior portion of the central sulcus.
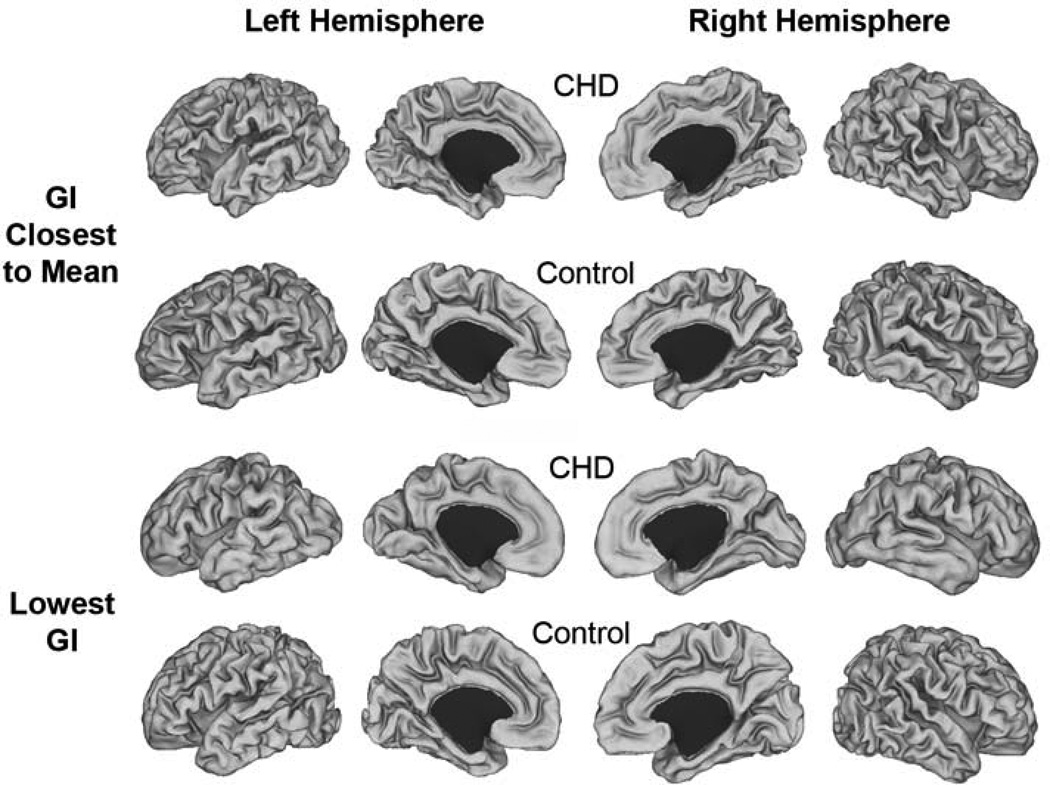
Figure 1.
Individual cortical mid-thickness surface reconstructions. Images represent individual surfaces from CHD and control infants whose GI was closest to the mean GI for the group (top two rows) and those who had the lowest GI in each group (bottom two rows).
Figure 2.
Cortical mid-thickness surface reconstructions and sulcal depth variations. The first two rows represent mean surface reconstructions for dorsal and lateral surfaces of CHD infants (first row) and term control infants (second row). The third (CHD infants) and fourth (control infants) rows represent the mean surface reconstructions for the ventral and medial surfaces. T maps (bottom two rows) represent the differences between the mean surfaces. Areas in yellow and red indicate regions where control infants were deeper; blue and green indicate regions where infants with CHD were deeper (or controls had broader gyri). White contours outline statistically significant differences between the two groups at p<0.025. The black arrowhead identifies the orbitofrontal region, the white arrow identifies the cingulate gyrus, the black arrows identify the central sulcus, and the white arrowhead identifies the superior ascending limb of the lateral sulcus. The T2-weighted image identifies the contours of the lateral sulcus in the right hemisphere for CHD infants (red) and control infants (blue). The CHD infants display reduced sulcation with a more open sulcus.
Of the 15 infants included in this study, seven (46%) had focal signal abnormalities in the white matter. None of the infants had any other signal abnormalities. There was no difference in CSA or GI between infants with or without focal signal abnormalities.
Discussion
This study demonstrates pre-operative alterations in global cerebral development in infants with CHD, including reductions in total cortical surface area and gyration. Such global reductions display regional variability, with greater alterations seen in the orbitofrontal region, cingulate and central sulcus of the left hemisphere. Alterations in the right hemisphere involve the posterior ascending limb of the lateral sulcus.
Pre-operative alterations in brain development in infants with CHD have been previously demonstrated. Delay in neuronal and axonal development has been suggested in studies employing diffusion tensor imaging and magnetic resonance spectroscopy [2]. Additionally, smaller brain volumes and more immature measures of spectroscopy are present in the third trimester of pregnancy [1]. Fetuses with hypoplastic left heart syndrome had delayed cortical developmental in the superior frontal, postcentral, occipital, cingulate, calcarine, collateral, superior temporal, and sylvian regions [5]. Assessment of cortical folding after birth but before cardiac surgery has also suggested abnormalities of opercular folding and shape [4]. Our results - alterations in the orbitofrontal region, cingulate, central sulcus, and posterior ascending limb of the lateral sulcus - support these previous studies and expand on them by demonstrating more global alterations in cortical folding in a variety of cardiac lesions. These alterations are present before cardiac surgery and have regional variability. Further, they appear to be independent of “brain injury” as defined as signal abnormality on T1- and T2-weighted images. A potential explanation for this finding is that these folding abnormalities are caused by neuronal or axonal injury that could be associated with altered cerebral perfusion related to the underlying cardiac defect that alters cortical folding but is not visible as signal abnormality on MRI. Another potential explanation is that the folding abnormalities are unrelated to hypoxic/ischemic injury but share a common etiology, such as genetic, with CHD. These explanations are not mutually exclusive.
Infants with CHD are at significant risk for impairments in cognition, language, behavior, visuospatial, and motor function [10–12]. We demonstrated alterations within regions of the brain that are associated with these functions. The orbitofrontal region plays a role in behavior and emotion [13]; the cingulate cortex in behavior, emotion, memory, and executive function [14]; and the central sulcus in movement and sensory perception. The posterior ascending limb of the lateral sulcus potentially plays a role in tactile attention [15]. The alterations in the orbitofrontal region, cingulum, central sulcus, and lateral sulcus in infants with CHD may provide biological insights associated with these later outcomes. There was variation in how these regions differed from controls, with the CHD infants demonstrating deeper, broader and more simplistic sulcal patterns in the central sulcus and cingulate. The cingulate also demonstrated a more interrupted sulcal trajectory (see mean surfaces for infants with CHD compared with controls, Figure 2). This will require further characterization, but suggests that the deeper sulcal pattern in infants with CHD may represent atypical rather than just delayed cortical development. Control infants have deeper sulci in the orbitofrontal region and lateral sulcus, with trends in the dorsal prefrontal and superior temporal regions. A deepening of these regions occurs with typical brain development, thus more shallow sulci in these regions in infants with CHD may represent a less mature cortical pattern, as has been documented by spectroscopy and diffusion methods [2]. The superior temporal region is important in cognitive function and audiovisual multisensory integration in language development [16] and thus may provide insight to language impairments in these children. An increase in sample size, in addition to longitudinal evaluation at later ages, may assist in confirming the extent of this difference and whether it is transient or persists into childhood.
The limitations of this study relate to sample size, heterogeneity of the cardiac lesions and lack of follow-up data. It is possible that different types of lesions will affect brain development in different ways. The purpose of this pilot study was to describe the findings of a new analytic technique in a small group of infants. Another limitation is that our CHD and control infants were from different geographical populations (New Zealand and United States, respectively). Population-specific differences in sulcation have not been clearly characterized but may contribute to the alterations that we have identified. Perinatal cortical growth in preterm infants has been associated with subsequent cognitive outcome [3], but the relationship of these alterations in cortical surface based measures to subsequent outcomes in infants with CHD remains unknown.
Acknowledgments
The authors wish to acknowledge Jennifer Brockmeyer and Reginald Lee for their assistance in data processing and Jeffrey Neil, MD, PhD, for assistance in data interpretation.
Supported the National Heart Foundation of New Zealand, the Green Lane Research and Education Fund, the Auckland Medical Research Fund, the Doris Duke Distinguished Clinical Scientist Award, the National Institutes of Health under Ruth L. Kirschstein National Research Service Award (T32 HD043010), Eunice Kennedy Shriver National Institute Of Child Health & Human Development (Clinical and Translational Science Award UL1RR024992, P30HD062171, and RO1 HD061619).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare no conflicts of interest.
References
- 1.Limperopoulos C, Tworetzky W, McElhinney DB, Newburger JW, Brown DW, Robertson RL, Jr, et al. Brain volume and metabolism in fetuses with congenital heart disease: evaluation with quantitative magnetic resonance imaging and spectroscopy. Circulation. 2010;121:26–33. doi: 10.1161/CIRCULATIONAHA.109.865568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Miller SP, McQuillen PS, Hamrick S, Xu D, Glidden DV, Charlton N, et al. Abnormal brain development in newborns with congenital heart disease. N Engl J Med. 2007;357:1928–1938. doi: 10.1056/NEJMoa067393. [DOI] [PubMed] [Google Scholar]
- 3.Rathbone R, Counsell SJ, Kapellou O, Dyet L, Kennea N, Hajnal J, et al. Perinatal cortical growth and childhood neurocognitive abilities. Neurology. 2011;77:1510–1517. doi: 10.1212/WNL.0b013e318233b215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Awate SP, Yushkevich PA, Song Z, Licht DJ, Gee JC. Cerebral cortical folding analysis with multivariate modeling and testing: Studies on gender differences and neonatal development. Neuroimage. 2010;53:450–459. doi: 10.1016/j.neuroimage.2010.06.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clouchoux C, du Plessis AJ, Bouyssi-Kobar M, Tworetzky W, McElhinney DB, Brown DW, et al. Delayed Cortical Development in Fetuses with Complex Congenital Heart Disease. Cereb Cortex. 2012 doi: 10.1093/cercor/bhs281. [DOI] [PubMed] [Google Scholar]
- 6.Ortinau C, Beca J, Lambeth J, Ferdman B, Alexopoulos D, Shimony JS, et al. Regional alterations in cerebral growth exist preoperatively in infants with congenital heart disease. The Journal of thoracic and cardiovascular surgery. 2012;143:1264–1270. doi: 10.1016/j.jtcvs.2011.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hill J, Dierker D, Neil J, Inder T, Knutsen A, Harwell J, et al. A surface-based analysis of hemispheric asymmetries and folding of cerebral cortex in term-born human infants. J Neurosci. 2010;30:2268–2276. doi: 10.1523/JNEUROSCI.4682-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Van Essen DC, Drury HA, Dickson J, Harwell J, Hanlon D, Anderson CH. An integrated software suite for surface-based analyses of cerebral cortex. J Am Med Inform Assoc. 2001;8:443–459. doi: 10.1136/jamia.2001.0080443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Van Essen DC. A Population-Average, Landmark- and Surface-based (PALS) atlas of human cerebral cortex. Neuroimage. 2005;28:635–662. doi: 10.1016/j.neuroimage.2005.06.058. [DOI] [PubMed] [Google Scholar]
- 10.Bellinger DC, Wypij D, duPlessis AJ, Rappaport LA, Jonas RA, Wernovsky G, et al. Neurodevelopmental status at eight years in children with dextro-transposition of the great arteries: the Boston Circulatory Arrest Trial. J Thorac Cardiovasc Surg. 2003;126:1385–1396. doi: 10.1016/s0022-5223(03)00711-6. [DOI] [PubMed] [Google Scholar]
- 11.Majnemer A, Limperopoulos C, Shevell M, Rohlicek C, Rosenblatt B, Tchervenkov C. Developmental and functional outcomes at school entry in children with congenital heart defects. J Pediatr. 2008;153:55–60. doi: 10.1016/j.jpeds.2007.12.019. [DOI] [PubMed] [Google Scholar]
- 12.Majnemer A, Limperopoulos C, Shevell M, Rosenblatt B, Rohlicek C, Tchervenkov C. Long-term neuromotor outcome at school entry of infants with congenital heart defects requiring open-heart surgery. J Pediatr. 2006;148:72–77. doi: 10.1016/j.jpeds.2005.08.036. [DOI] [PubMed] [Google Scholar]
- 13.Kringelbach ML, Rolls ET. The functional neuroanatomy of the human orbitofrontal cortex: evidence from neuroimaging and neuropsychology. Prog Neurobiol. 2004;72:341–372. doi: 10.1016/j.pneurobio.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 14.Devinsky O, Morrell MJ, Vogt BA. Contributions of anterior cingulate cortex to behaviour. Brain. 1995;118(Pt 1):279–306. doi: 10.1093/brain/118.1.279. [DOI] [PubMed] [Google Scholar]
- 15.Burton H, Sinclair RJ. Attending to and remembering tactile stimuli: a review of brain imaging data and single-neuron responses. J Clin Neurophysiol. 2000;17:575–591. doi: 10.1097/00004691-200011000-00004. [DOI] [PubMed] [Google Scholar]
- 16.Beauchamp MS, Lee KE, Argall BD, Martin A. Integration of auditory and visual information about objects in superior temporal sulcus. Neuron. 2004;41:809–823. doi: 10.1016/s0896-6273(04)00070-4. [DOI] [PubMed] [Google Scholar]